Structured Abstract
Purpose of review
Skeletal muscle loss or sarcopenia is a frequent complication of cirrhosis that adversely affects clinical outcomes. Since skeletal muscle is the largest store of proteins in the body, proteostasis or protein homeostasis is required for maintenance of muscle mass. This review will focus on disordered skeletal muscle proteostasis in liver disease.
Recent findings
Increased skeletal muscle uptake of ammonia initiates responses that result in disordered proteostasis including impaired protein synthesis and increased autophagy. The cellular response to the stress of hyperammonemia (hyperammonemic stress response:HASR) involves the coordinated action of diverse signaling pathways targeting the molecular mechanisms of regulation of protein synthesis. Transcriptional upregulation of myostatin, a TGFβ superfamily member, results in impaired mTORC1 signaling. Phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α) also relates to decreased global protein synthesis rates and mTORC1 signaling. Ammonia also causes mitochondrial and bioenergetics dysfunction due to cataplerosis of α-ketoglutarate. Lowering ammonia, targeting components of HASR and regulating cellular amino acid levels can potentially restore proteostasis.
Summary
Signaling via myostatin and eIF2α phosphorylation causes decreases in protein synthesis and mTORC1 activity with a parallel mitochondrial dysfunction and increased autophagy contributing to proteostasis perturbations during skeletal muscle hyperammonemia of liver disease.
Keywords: integrated stress response, myostatin, cirrhosis liver, sarcopenia, eukaryotic initiation factor, cataplerosis, anaplerosis, signaling
Introduction
There is an extensive body of literature that loss of skeletal muscle mass or sarcopenia is a major and common complication in cirrhosis and results in decreased survival, lower quality of life, higher frequency of complications of cirrhosis, and worse post liver transplant outcomes[1–6]. In combination with decreased contractile function, loss of muscle mass results in deconditioning and frailty, that are now being recognized to contribute to poor clinical outcomes[7–9]. Even though the term sarcopenia (sarcos: flesh; penia: loss) is literally translated as loss of muscle mass, the term has been used in the aging literature to refer to not only loss of muscle mass, but also skeletal muscle function. Secondary sarcopenia has been suggested to refer to muscle loss in chronic diseases but most publications continue to use the term sarcopenia to refer to loss of skeletal muscle mass even though some use the term to refer to impaired muscle strength also. In this review, the term sarcopenia will refer to loss of skeletal muscle mass unless specifically stated to include impaired contractile function. The clinical importance of sarcopenia is increasingly recognized in the era of liver transplantation due to a variety of reasons that include the relatively low proportion of patients with liver disease who undergo liver transplantation, lack of recovery of muscle mass after transplantation and higher post liver transplantation complications in sarcopenic cirrhotics[1,4,5,7,10]. Despite the high significance, there are no effective therapies to date primarily due to limited understanding of the mechanisms that have resulted in an approach of deficiency replacement rather than targeted therapies. Most studies to date have used the principle of overcoming the anabolic resistance, a state where physiological stimuli including nutrients and physical activity do not result in the expected improvement in skeletal muscle mass or anabolism[11]. Since skeletal muscle is the largest protein store in the body a the balance between protein synthesis and proteolysis, components of protein homeostasis (proteostasis) is a major contributor to maintenance of muscle mass[12]. Protein homeostasis is controlled by the coordinated actions of several cellular processes, including protein synthesis and degradation. Studies in cirrhotic patients in the past based on whole body protein turnover studies or arteriovenous differences have reported conflicting results with increased, decreased or unaltered protein synthesis or breakdown. Clarity ensued once data from skeletal muscle biopsies from human subjects complemented by studies in rodent and cellular models of sarcopenia of liver disease were published in the past 2 decades[1,13]. In addition to better understanding of skeletal muscle proteostasis, the underlying molecular and metabolic perturbations also have allowed identification of novel, molecular and metabolic therapeutic targets. The present review will focus on recent advances in muscle proteostasis perturbations, potential mediators of the liver-muscle axis and the therapeutic possibilities that can help overcome the anabolic resistance of cirrhosis.
Skeletal muscle proteostasis in cirrhosis
Maintenance of skeletal muscle mass is a dynamic process that involves a balance between protein synthesis and proteolysis or protein breakdown[12]. Besides protein synthesis and proteolysis, other components that contribute to proteostasis include ribosomal mass and function, mitochondrial function to provide ATP, and the post translational modifications that occur in the endoplasmic reticulum that in the skeletal muscle is specialized to form the sarcoplasmic reticulum[12,14]. The process of protein synthesis is an energy intense process that involves a series of coordinated steps that involve assembly of the mRNAs with the ribosomal subunits for translation into proteins [15]. Current studies on skeletal muscle atrophy have focused primarily on the signaling responses with recent interest in the mitochondria, ribosomes and endoplasmic reticulum[11,14,16,17]. Muscle protein homeostasis is regulated primarily at the mRNA translation level and a number of proteolysis pathways that include the proteasome, autophagy, calpain and caspase dependent pathways. Proteostasis dysregulation with resultant cellular and tissue consequences contribute to sarcopenia.
A reduction in protein synthesis is not enough to result in muscle loss that also requires increased proteolysis. Unlike in muscle loss with immobilization and injury, muscle proteasome mediated proteolysis is not altered or decreased with liver disease[1,6]. Interestingly, muscle autophagy was significantly increased in liver disease[8,13]. These data provide compelling evidence that both arms of proteostasis, protein synthesis and proteolysis were perturbed with resultant sarcopenia in liver disease.
Signaling disturbances that result in perturbed proteostasis
Skeletal muscle protein synthesis is regulated by at least 2 critical factors: myostatin, a TGFβ superfamily member, and locally generated insulin like growth factor (IGF1) also called mechanogrowth factor[18]. Myostatin results in muscle loss primarily by signaling responses that impair the mammalian target of rapamycin complex 1 (mTORC1) and its downstream signaling responses. Canonical myostatin signaling involves binding to a type II TGFβ receptor, Activin IIBR that then heterodimerizes with a type 1 receptor activin like kinase 4/5. This ligand bound complex then initiates transcriptional regulation of genes via phosphorylation of Smad2/3. Increased myostatin results in inhibition of mTORC1, a positive regulator of protein synthesis, and increases autophagy but there is also data that myostatin also increases proteasome mediated proteolysis. However, during hyperammonemia, even though myostatin expression is increased, proteasomal proteolysis is not altered[1]. Following initial reports of increased skeletal muscle myostatin in human cirrhosis, cellular and rodent models of hyperammonemia and elevated circulating myostatin in cirrhosis, more recent data show that elevated circulating myostatin in cirrhosis is associated with hyperammonemia, decreased protein synthesis and lower overall survival[1,19]. As mentioned, of the various steps of protein synthesis, translation initiation is the most regulated step. A number of translation initiation factors in eukaryotes (eIFs) are involved in the steps of translation initiation[12]. Translation initiation involves binding of the mRNA 5′-m7G cap-structure to a protein complex referred to as the cap-binding complex or eIF4F. eIF4F consists of the cap-binding protein eIF4E, the scaffolding protein eIF4G and the ATP-dependent RNA helicase eIF4A, which unwinds the secondary structure in the 5′-untranslated region (5′-UTR) of the mRNA. The eIF4F-bound mRNA recruits the 43S initiation complex. The 43S complex consists of the 40S ribosomal subunit, the ternary complex (TC) containing an initiator tRNA molecule (eIF2•GTP•Met-tRNAiMet), and the multi-subunit initiation factor eIF3. The 43S initiation complex scans the 5′-UTR for the initiation codon. When the initiation AUG codon is recognized, the 40S subunit is joined by a 60S subunit to form an elongation-competent 80S ribosome. The recognition of the AUG initiation codon and the delivery of the initiator met-tRNA involve a GTP hydrolysis step, which leaves the eIF2 complex in the inactive GDP-bound state. The translation initiation factor eIF2B, regenerates an active TC via its guanine nucleotide exchange activity (GEF) and by recruiting a new initiator met-tRNA to the complex. The recycling of the TC is essential for continuing rounds of translation initiation[20]. During stress states, eIF2 phosphorylation at the α subunit (eIF2α) acts as an inhibitor of the eIF2B activity and stalls TC recycling, leading to global inhibition of protein synthesis[21].
In response to cellular stress, a diverse set of eIF2α kinases are activated that converge on the phosphorylation of eIF2α with translational repression and consequent decreased protein synthesis[22]. The 4 identified eIF2α kinases include: eIF2α kinase 1/heme regulated inhibitor (HRI) activated during heme deficiency, eIF2α kinase 2/protein kinase RNA activated (PKR) activated by double stranded RNA introduced during viral infection, eIF2α kinase 3/PKR like endoplasmic reticulum kinase (PERK) activated during endoplasmic reticulum stress, eIF2α kinase 4/general control non-derepressed 2 (GCN2). The eIF2α kinases recognize and phosphorylate Ser51 on eIF2α, a central step in the integrated stress response that inhibits global protein synthesis[22]. However, certain mRNA translation is upregulated including those for the transcription factor ATF4 and CHOP that restore cellular protein homeostasis[23]. Despite extensive work on the biology of integrated stress responses, and eIF2α phosphorylation, the regulation of the cellular stress response to hyperammonemia in liver disease are being dissected only recently[16].
Mediators of the liver-muscle axis
A number of metabolic, hormonal and cytokine perturbations have been reported in cirrhosis that can result in dysregulated muscle proteostasis[1]. Hyperammonemia, reduction in testosterone, reduced branched chain amino acids, endotoxemia and elevated circulating tumor necrosis factor α have all been suggested to contribute to muscle loss[24,25]. The focus of this review will be on ammonia mediated disturbed muscle proteostasis because hyperammonemia is a consistent abnormality in cirrhosis and therapies to lower ammonia are available for therapeutic application[8,26].
Ammonia is a cytotoxic compound that is generated from amino acid catabolism, purine turnover and gut microbial metabolism. Physiologically, ammonia disposal occurs in the hepatocytes via ureagenesis. In cirrhosis, a combination of hepatocellular dysfunction and portosystemic shunting result in elevated circulating ammonia. Hepatic encephalopathy is the best-studied clinical consequence of ammonia induced organ toxicity with morbidity and mortality[26]. Past reports of increased ammonia uptake by the skeletal muscle based on non-invasive methods were followed by direct quantification that showed increased skeletal muscle ammonia concentrations in patients with liver disease. Due to its cytotoxicity, increased ammonia in the muscle is converted to glutamate and glutamine that is subsequently exported to the plasma. It was thought that the skeletal muscle functioned as a metabolic sink for ammonia with very limited studies on its adverse effects on muscle phenotype or proteostasis. Studies in the past decade have however shown that ammonia induces a series of molecular and metabolic perturbations in the skeletal muscle that result in dysregulated proteostasis and sarcopenia[8,26]. Since ammonia is a simple chemical (4 elements, 3 hydrogen and 1 nitrogen atom), it is likely to initiate homeostatic biochemical responses to neutralize its toxicity. There is therefore great interest in identifying what we call as “interface molecules”, that are signaling proteins that detect metabolic perturbations to initiate a series of molecular responses. The mechanisms by which these biochemical responses initiate molecular perturbations are currently being dissected using integrated molecular-metabolic approaches[1].
Ammonia transcriptionally upregulates myostatin
The mechanisms by which ammonia uptake is increased in the muscle are currently being investigated[27]. Hyperammonemia increases nuclear translocation of p65NFkB that transcriptionally upregulates myostatin. Loss and gain of function studies in animal and cellular models have shown that hyperammonemia-induced myostatin results in impaired protein synthesis. Increased myostatin impairs mTORC1, a critical signaling molecule that increases protein synthesis and decreases autophagy. These provide novel mechanistic targets for therapy to overcome anabolic resistance and impaired proteostasis during hyperammonemia and sarcopenia in cirrhosis. Blocking myostatin and activating mTORC1 signaling are therefore potential interventions to restore proteostasis and reverse sarcopenia[1,6,18,19,28,29].
Cataplerosis of α-ketoglutarate and mitochondrial dysfunction
As mentioned above, ammonia disposal in non-hepatic tissue occurs by by its reaction with critical tricarboxylic acid (TCA) cycle intermediate α-ketoglutarate (αKG) to form glutamate and is catalyzed by the enzyme, glutamate dehydrogenase[30]. Glutamate reacts with another molecule of ammonia to generate glutamine, that is then exchanged for leucine (that enters the muscle) via the exchanger, SLC7A5[16,17,30]. This process of ammonia disposal results in loss of αKG (cataplerosis: loss of TCA cycle intermediates) that contributes to mitochondrial dysfunction. Consistently, during hyperammonemia, skeletal muscle TCA cycle intermediate concentrations are decreased with impaired mitochondrial respiration and ATP synthesis[17]. These data provide a mechanistic explanation for cirrhosis being a state of starvation with bioenergetic dysfunction that results in anabolic resistance. Since protein synthesis is a highly energy requiring cellular process[15], mitochondrial dysfunction with low ATP content contributes to dysregulated proteostasis. Since autophagy is increased during starvation to provide essential nutrients, mitochondrial dysfunction of hyperammonemia also increases autophagy. In addition to mitochondrial dysfunction due to cataplerosis of αKG, hyperammonemia also results in electron leak from Complex III of the electron transport chain in the mitochondrial inner membrane. Increased reactive oxygen species that are generated cause cellular injury and induce autophagy[17]. Uncompensated cataplerosis will result in loss of mitochondrial oxidative funciton and needs to be compensated by anaplerosis, or influx of TCA cycle intermediates. A number of anaplerotic influx points exist in the TCA cycle that will allow replenishment of αKG lost during hyperammonemia. Targeting cataplerosis or providing anaplerotic substrates to replenish the TCA cycle intermediates is therefore a promising therapeutic option[17].
Hyperammonemic stress response
In addition to myostatin-mediated responses, hyperammonemia also induces a cellular stress response that is mediated by an eIF2α kinase, general control non-depressed 2 (GCN2) that is known to be activated during amino acid deficiency[16]. Cellular amino acid deficiency results in increased cytoplasmic uncharged tRNAs (not bound to their cognate amino acids) that in turn activate GCN2 and consequently its target, eIF2α with reduction in mRNA translation and protein synthesis[31]. In cellular systems of hyperammonemia, L-leucine supplementation reversed impaired protein synthesis, GCN2 activation, and eIF2α phosphorylation[16]. These data complement human studies with L-leucine enriched amino acid mixture reversing the molecular and metabolic perturbations in cirrhotic skeletal muscle[16]. Interestingly, despite activation of GCN2 and reversal by L-leucine supplementation, cellular concentration of leucine was unchanged in response to hyperammonemia that was consistent with previous data on muscle amino acid concentrations in cirrhosis. These data suggest that L-leucine in the skeletal muscle is partitioned into the mitochondrial compartment for metabolic utilization or remains in the cytoplasmic compartment and then into the lysosomes for activation of mTORC1 signaling via the Rag proteins. Consistently, mitochondrial leucine entry was higher during hyperammonemia in myotubes suggesting that GCN2 activation was due to compartmental partitioning of L-leucine[16].
The eIF2α kinases are stress specific and as mentioned above, hyperammonemia activated the GCN2 kinase [16,31,32]. Some mRNAs that are preferentially translated during cellular stress to promote recovery and resume protein synthesis include GADD34, a component of the protein phosphatase 1 (PP1) and ATF4, the master regulator of the stress-induced transcriptome[23,33,34]. Interestingly, in the well studied amino acid deficiency response, an integrated stress response is initiated via the GCN2-eIF2α phosphorylation axis with preferential increased translation of the ATF4 and GADD34 mRNAs[22]. However, during hyperammonemia, there is persistent GCN2 activation with absence of ATF4 and GADD34 translation with resultant persistent impaired protein synthesis and perturbed proteostasis[16,35]. Targeting these steps to restore muscle proteostasis is an attractive potential therapeutic strategy.
Other factors that contribute to disturbed anabolic resistance and dysregulated skeletal muscle proteostasis in cirrhosis
Even though hyperammonemia induced alterations in proteostasis, other metabolic, hormonal and cytokine perturbations in cirrhosis also result in impaired protein synthesis and increased autophagy[1]. These include a reduction in testosterone in men, endotoxemia, alterations in gut microbiome that promotes ammoniagenesis, alterations in plasma amino acid concentrations, decreased mobility and the consequence of complications of cirrhosis including infections, gastrointestinal bleeding, encephalopathy with poor oral intake and portal hypertensive enteropathy. These have been reviewed recently and the interested reader is referred to several recent publications in this area[1,3,29].
Treatment options based on dysregulated proteostasis
Given the proteostasis perturbations during hyperammonemia, a number of treatment options have been evaluated (Table 1). Stimulating protein synthesis using anabolic steroids including testosterone has been evaluated recently in male cirrhotics that showed improvement in muscle mass with a non-significant improvement in survival[24]. However, whether the molecular perturbations are reversed is not known. Previous studies on amino acid and dietary supplementation have also not been consistently effective[6]. Ammonia lowering therapies are currently approved for human use but have not been systematically evaluated for reversal of the molecular and metabolic perturbations and sarcopenia in human cirrhosis.
Table 1.
Potential molecular targets to restore proteostasis during hyperammonemic stress response
| Target ammonia | |
| Decrease ammoniagenesis: Gut microbiome, bind ammonia | |
| Ammonia uptake: Ammonia transporters (RhBG protein) | |
| Ammonia metabolism: branched chain amino acids, branched chain keto-analogs, cell permeable TCA cycle Intermediates | |
| Restore proteostasis | |
| Myostatin | Follistatin, myostatin antagonists |
| mTORC1 | L-leucine |
| Mitochondrial protection | Antioxidants, mitochondrial substrates |
| Autophagy | Exercise, L-leucine |
| Putative targets | ribosomes, sarcoendoplasmic reticulum |
Recently, long term ammonia lowering in cellular and a rat model of hyperammonemia was reported to reverse impaired protein synthesis, increased autophagy, and the molecular perturbations including increased myostatin, impaired mTORC1 signaling and increased phosphorylation of GCN2-eIF2α[13]. Whether these will benefit human subjects needs to be tested in prospective clinical trials. It must however be emphasized that even the most sophisticated methods to quantify muscle mass and function are relatively insensitive and small changes may not be detected. Long term, well-designed human studies are necessary to determine if ammonia lowering is an effective strategy to reverse perturbed proteostasis and translate into reversal of sarcopenia.
A single dose of a leucine enriched amino acid mixture was also reported in a small group of patients with alcoholic cirrhosis to revers impaired mTORC1 signaling, phosphorylation of eIF2α, and restore the molecular responses of dysregulated proteostasis[35]. Protein synthesis in response to the amino acid mixture was similar to that in healthy controls. However, whether these benefits are maintained in response to long-term replacement is currently unknown.
In addition to nutrient and nutriceutical interventions, exercise is another approach to improve proteostasis[1,36,37]. Both resistance and endurance exercise have been reported to be beneficial in small, short term clinical studies with limited readouts. It must be reiterated that ammonia results in contractile dysfunction and the exercising muscles generate ammonia both of which may adversely affect the beneficial response to exercise in cirrhosis[38,39]. Recently, barriers to nutritional supplements and physical activity have been reported that can be targeted for improving proteostasis since exercise promotes protein homeostasis[40]. Rigorous data are needed to determine the molecular responses to exercise in cirrhosis and interventions to improve mitochondrial function, substrate utilization and contractile responses may be necessary to complement physical activity alone.
Newer approaches including targeting myostatin[28], providing cell permeable TCA cycle intermediates, specifically α-ketoglutarate[17], protecting against mitochondrial reactive oxygen species generation, and inhibiting eIF2α phosphorylation and signaling are exciting areas that need to be determined in preclinical followed by clinical studies.
Conclusions
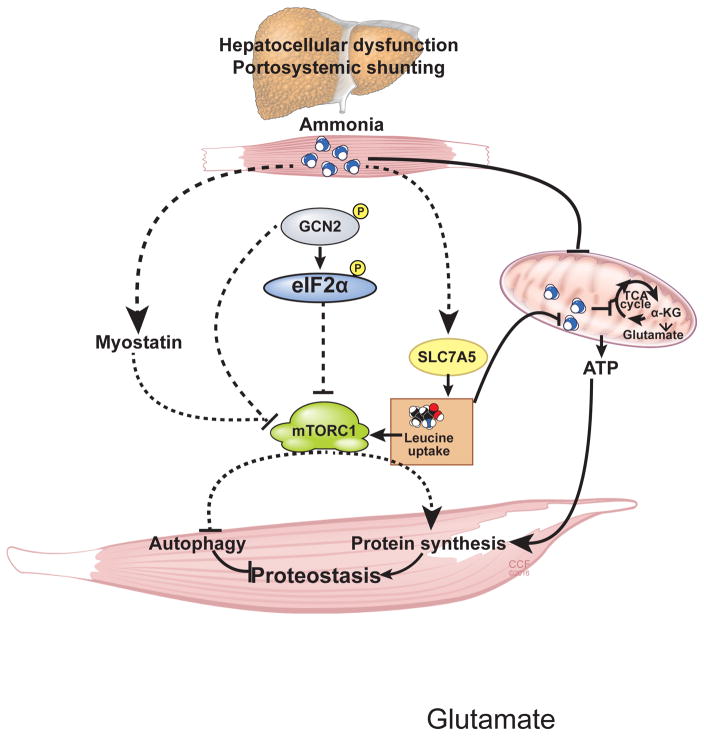
Despite a number of abnormalities in cirrhosis, hyperammonemia is observed consistently and contributes to both impaired protein synthesis and increased autophagy dependent proteolysis. Signaling abnormalities that result in dysregulated proteostasis include myostatin dependent impaired mTORC1 signaling and hyperammonemic stress response characterized by a GCN2 mediated eIF2α phosphorylation and translational repression with a modified cellular stress response. Additional contributors to disordered proteostasis include reduction in ATP synthesis and increased reactive oxygen species due to cataplerosis of αKG and impaired mitochondrial function. Long-term ammonia lowering therapy and leucine enriched amino acid mixture hold promise as therapeutic options (Figure 1).
Figure 1.
A number of metabolic and molecular perturbations that contributes to ammonia induced dysregulated proteostasis and the potential treatments targeting these abnormalities can reverse sarcopenia in liver disease.
Key points.
Hyperammonemia is a consistent abnormality in cirrhosis and results in decreased muscle protein synthesis and increased autophagy with resultant loss of muscle mass or sarcopenia.
Myostatin, a TGFβ superfamily member is transcriptionally upregulated during hyperammonemia and impairs protein synthesis and may increase proteolysis.
A novel cellular stress response mediated via the GCN2-eIF2α phosphorylation axis with decreased protein synthesis may cross talk with impaired mTORC1 signaling.
Mitochondrial dysfunction due to cataplerosis of αKG with decreased ATP generation and increased reactive oxygen species is an additional abnormality that contributes to both decreased protein synthesis and increased autophagy.
Lowering ammonia and providing a large dose of leucine to satisfy the metabolic demand may restore proteostasis during hyperammonemia.
Novel therapeutic approaches include myostatin antagonists, anaplerotic substrates and inhibitors of the negative effects of eIF2α phosphorylation on protein synthesis.
Acknowledgments
We would like to thank our colleagues and collaborators both in our laboratories and outside our institutions for their contributions that have resulted in this work.
Financial Support: This work was supported in part by the generous grant support from the National Institutes of Health to the authors, the details of which have been stated.
Footnotes
Conflicts of Interest. The authors have been funded in part by grants from the National Institutes of Health.
References
- 1**.Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232–1244. doi: 10.1016/j.jhep.2016.07.040. This comprehensive review summarizes many of the molecular mechanisms, therapeutic options and potential reasons for lack of effective therapies for sarcopenia in cirrhosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanai T, Shiraki M, Ohnishi S, Miyazaki T, Ideta T, Kochi T, Imai K, Suetsugu A, Takai K, Moriwaki H, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res. 2016;46:743–751. doi: 10.1111/hepr.12616. [DOI] [PubMed] [Google Scholar]
- 3*.Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, Merli M, Riggio O. Sarcopenia Is Risk Factor for Development of Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt Placement. Clin Gastroenterol Hepatol. 2017;15:934–936. doi: 10.1016/j.cgh.2016.10.028. This group has published in the past that sarcopenia in cirrhosis increases the risk of encepahlopathy, another major consequence of hyperammonemia. This work is of high significance to hepatologists and those interested in the clinical consequences of sarcopenia in cirrhosis. [DOI] [PubMed] [Google Scholar]
- 4.Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, de Vos M, Papadimitriou K, Thorburn D, O’Beirne J, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113–121. doi: 10.1002/jcsm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Bhanji RA, Carey EJ, Yang L, Watt KD. The Long Winding Road to Transplant: How Sarcopenia and Debility Impact Morbidity and Mortality on the Waitlist. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2017.04.004. Even though this is a review, this is recommended for anyone interested in the consequences of sarcopenia in patients on the liver transplant waiting list. Sarcopenia adversely affects patient outcomes while on the waiting list increasing wait list morbidity and mortality. [DOI] [PubMed] [Google Scholar]
- 6.Dasarathy S. Cause and management of muscle wasting in chronic liver disease. Curr Opin Gastroenterol. 2016;32:159–165. doi: 10.1097/MOG.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CW, Feng S, Covinsky KE, Hayssen H, Zhou LQ, Yeh BM, Lai JC. A Comparison of Muscle Function, Mass, and Quality in Liver Transplant Candidates: Results From the Functional Assessment in Liver Transplantation Study. Transplantation. 2016;100:1692–1698. doi: 10.1097/TP.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Chen HW, Dunn MA. Muscle at Risk: The Multiple Impacts of Ammonia on Sarcopenia and Frailty in Cirrhosis. Clin Transl Gastroenterol. 2016;7:e170. doi: 10.1038/ctg.2016.33. This brief summary of how ammonia affects the skeletal muscle summarizes many of the recent publications in the field with a clinical perspective on its significance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, Delitto A. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl. 2016;22:1324–1332. doi: 10.1002/lt.24506. Even though dysregulated proteostasis results in sarcopenia or loss of muscle mass, ammonia also adversely impacts contractile function. This work provides support for the high clinical significance of impaired contractile function and consequent deconditioning in cirrhotic patients and lays the foundation for the urgent need for effective therapeutic interventions. [DOI] [PubMed] [Google Scholar]
- 10.Golse N, Bucur PO, Ciacio O, Pittau G, Sa Cunha A, Adam R, Castaing D, Antonini T, Coilly A, Samuel D, et al. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl. 2017;23:143–154. doi: 10.1002/lt.24671. [DOI] [PubMed] [Google Scholar]
- 11.Chaillou T. Impaired ribosome biogenesis could contribute to anabolic resistance to strength exercise in the elderly. J Physiol. 2017;595:1447–1448. doi: 10.1113/JP273773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Anthony TG. Mechanisms of protein balance in skeletal muscle. Domest Anim Endocrinol. 2016;56(Suppl):S23–32. doi: 10.1016/j.domaniend.2016.02.012. This is an elegant review of skeletal muscle proteostasis that explains the process of protein homeostasis with a focus on protein synthesis and proteolysis. Additional components of proteostasis include folding, secretion, and storage and the entire process is a dynamic, energy intense process involving multiple subcellular organelles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Kumar A, Davuluri G, Silva RNE, Engelen M, Ten Have GAM, Prayson R, Deutz NEP, Dasarathy S. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology. 2017;65:2045–2058. doi: 10.1002/hep.29107. This is the first preclinical study to evaluate skeletal muscle proteostasis responses including the molecular regulatory pathways during hyperammonemia and its partial reversal in response to 4 weeks of ammonia lowering using clinically available therapies. This work will be of interest to clinicians and scientists evaluating how ammonia regulates protein metabolism in the skeletal muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol (1985) 2015;119:321–327. doi: 10.1152/japplphysiol.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kafri M, Metzl-Raz E, Jona G, Barkai N. The Cost of Protein Production. Cell Rep. 2016;14:22–31. doi: 10.1016/j.celrep.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Davuluri G, Krokowski D, Guan BJ, Kumar A, Thapaliya S, Singh D, Hatzoglou M, Dasarathy S. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J Hepatol. 2016;65:929–937. doi: 10.1016/j.jhep.2016.06.004. This is the first description of the hyperammonemic stress response (HASR), a unique cellular signaling response to ammonia. The HASR shares some characteristics of an amino acid deficiency and other responses similar to that of endoplasmic reticulum stress, without activation of PERK, the classical response to ER stress and aggregated proteins. Interestingly, even though the molecular responses suggest an intracellular amino acid deficiency and the dysregulated proteostasis responds to L-leucine supplementation, direct measurement of cellular amino acids are not deficient. These data provide evidence of a metabolic and molecular roles of L-leucine in different cellular compartments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Davuluri G, Allawy A, Thapaliya S, Rennison JH, Singh D, Kumar A, Sandlers Y, Van Wagoner DR, Flask CA, Hoppel C, et al. Hyperammonaemia-induced skeletal muscle mitochondrial dysfunction results in cataplerosis and oxidative stress. J Physiol. 2016;594:7341–7360. doi: 10.1113/JP272796. This work is a detailed characterization of how the skeletal muscle that is not capable of generating urea metabolizes ammonia. Detailed functional studies using high sensitivity respirometry show how cataplerosis adversely affects muscle mitochondrial oxidation, ATP generation and evaluates an anaplerotic substrate to reverse these processes. Even though flux studies to determine the cataplerosis/anaplerosis are not evaluated, the large number of studies lay the foundation for future potential interventions to reverse skeletal muscle bioenergetic dysfunction during hyperammonemai. Since proteostasis is an energy dependent process, reduced mitochondrial function and ATP content in the muscle in response to ammonia is likely to contribute to dysregulated proteostasis during hyperammonemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JL, Colgan TD, Walton KL, Gregorevic P, Harrison CA. The TGF-beta Signalling Network in Muscle Development, Adaptation and Disease. Adv Exp Med Biol. 2016;900:97–131. doi: 10.1007/978-3-319-27511-6_5. [DOI] [PubMed] [Google Scholar]
- 19*.Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, Yuri Y, Hasegawa K, Nakano C, Nishimura T, et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J Cachexia Sarcopenia Muscle. 2017 doi: 10.1002/jcsm.12212. This is the first longitudinal study in a large cohort of cirrhotics that showed that baseline myostatin was correlated with blood ammonia concentrations and long term survival. An important advance in the field of sarcopenia in cirrhosis especially because serum myostatin is a potential biomarker for prognosis and can be used as a measure of response to interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashiwagi K, Ito T, Yokoyama S. Crystal structure of eIF2B and insights into eIF2-eIF2B interactions. FEBS J. 2017;284:868–874. doi: 10.1111/febs.13896. [DOI] [PubMed] [Google Scholar]
- 21.Majumder M, Mitchell D, Merkulov S, Wu J, Guan BJ, Snider MD, Krokowski D, Yee VC, Hatzoglou M. Residues required for phosphorylation of translation initiation factor eIF2alpha under diverse stress conditions are divergent between yeast and human. Int J Biochem Cell Biol. 2015;59:135–141. doi: 10.1016/j.biocel.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. Little is known about how the skeletal muscle responds to cellular stress. Ammonia is a major cellular stressor in the muscle and this comprehensive review summarizes the current state of knowledge on how cells in general respond to different stresses. The role of the eukaryotic initiation factor alpha subunit and its upstream regulators and downstream targets are summarized. This work provides a comprehensive understanding on cellular adaptation to stressors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol. 2016;65:906–913. doi: 10.1016/j.jhep.2016.06.007. Even though not strictly ammonia or proteostasis related, this provides a novel approach to improve muscle mass by targeting androgen receptor dependent proteostasis in the muscle. An interesting study for future designs of clinical trials but the lack of molecular markers is a limitation. [DOI] [PubMed] [Google Scholar]
- 25.Kitajima Y, Takahashi H, Akiyama T, Murayama K, Iwane S, Kuwashiro T, Tanaka K, Kawazoe S, Ono N, Eguchi T, et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J Gastroenterol. 2017 doi: 10.1007/s00535-017-1370-x. [DOI] [PubMed] [Google Scholar]
- 26.Dasarathy S, Mookerjee RP, Rackayova V, Rangroo Thrane V, Vairappan B, Ott P, Rose CF. Ammonia toxicity: from head to toe? Metab Brain Dis. 2017;32:529–538. doi: 10.1007/s11011-016-9938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Takeda K, Takemasa T. Expression of ammonia transporters Rhbg and Rhcg in mouse skeletal muscle and the effect of 6-week training on these proteins. Physiol Rep. 2015:3. doi: 10.14814/phy2.12596. The first evidence that skeletal muscle in mammals express ammonia transporters and these can be regulated provides a potential therapeutic target. Even though this study did not focus on ammonia, it does provide evidence that ammonia can be transported into the muscle during hyperammonemic states. An important paper for anyone interested in ammonia transport into the skeletal muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen PR, Lee K. INVITED REVIEW: Inhibitors of myostatin as methods of enhancing muscle growth and development. J Anim Sci. 2016;94:3125–3134. doi: 10.2527/jas.2016-0532. [DOI] [PubMed] [Google Scholar]
- 29.Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016;43:765–777. doi: 10.1111/apt.13549. [DOI] [PubMed] [Google Scholar]
- 30**.Holecek M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition. 2017;41:80–85. doi: 10.1016/j.nut.2017.04.003. This comprehensive review summarizes the current perspectives on amino acids and ammonia metabolism in liver disease. This is perhaps one of the best reviews on the physiological and biochemical relevance of branched chain amino acids in ammonia metabolism. [DOI] [PubMed] [Google Scholar]
- 31.Anda S, Zach R, Grallert B. Activation of Gcn2 in response to different stresses. PLoS One. 2017;12:e0182143. doi: 10.1371/journal.pone.0182143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lageix S, Zhang J, Rothenburg S, Hinnebusch AG. Interaction between the tRNA-binding and C-terminal domains of Yeast Gcn2 regulates kinase activity in vivo. PLoS Genet. 2015;11:e1004991. doi: 10.1371/journal.pgen.1004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan BJ, Krokowski D, Majumder M, Schmotzer CL, Kimball SR, Merrick WC, Koromilas AE, Hatzoglou M. Translational control during endoplasmic reticulum stress beyond phosphorylation of the translation initiation factor eIF2alpha. J Biol Chem. 2014;289:12593–12611. doi: 10.1074/jbc.M113.543215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krokowski D, Jobava R, Guan BJ, Farabaugh K, Wu J, Majumder M, Bianchi MG, Snider MD, Bussolati O, Hatzoglou M. Coordinated Regulation of the Neutral Amino Acid Transporter SNAT2 and the Protein Phosphatase Subunit GADD34 Promotes Adaptation to Increased Extracellular Osmolarity. J Biol Chem. 2015;290:17822–17837. doi: 10.1074/jbc.M114.636217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, Schulze JM, Barnes D, McCullough AJ, Engelen MP, et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. The first human study that direclty quantified skeletal muscle proteostasis in cirrhosis and resposne to an oral leucine enriched branched chain amino acid mixture. However, limitations include the short term evaluation of response since it is not known if these benefits of amino acid supplementation are maintained over time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kappus MR, Mendoza MS, Nguyen D, Medici V, McClave SA. Sarcopenia in Patients with Chronic Liver Disease: Can It Be Altered by Diet and Exercise? Curr Gastroenterol Rep. 2016;18:43. doi: 10.1007/s11894-016-0516-y. [DOI] [PubMed] [Google Scholar]
- 37.Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S, Calleja JL, Banares R, Garcia-Pagan JC, Mesonero F, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 38*.McDaniel J, Davuluri G, Hill EA, Moyer M, Runkana A, Prayson R, van Lunteren E, Dasarathy S. Hyperammonemia results in reduced muscle function independent of muscle mass. Am J Physiol Gastrointest Liver Physiol. 2016;310:G163–170. doi: 10.1152/ajpgi.00322.2015. Even though the effects of ammonia are best studied in the context of dysregulated proteostasis, this work in a comprehensive array of models showed impaired contractile function during hyperammonemia. This is important for investigators studying contractile function and exercise in cirrhosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieuwoudt S, Mulya A, Fealy CE, Martelli E, Dasarathy S, Naga Prasad SV, Kirwan JP. In vitro Contraction Protects Against Palmitate-Induced Insulin Resistance in C2C12 Myotubes. Am J Physiol Cell Physiol. 2017 doi: 10.1152/ajpcell.00123.2017. ajpcell 00123 02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ney M, Gramlich L, Mathiesen V, Bailey RJ, Haykowsky M, Ma M, Abraldes JG, Tandon P. Patient-perceived barriers to lifestyle interventions in cirrhosis. Saudi J Gastroenterol. 2017;23:97–104. doi: 10.4103/1319-3767.203357. [DOI] [PMC free article] [PubMed] [Google Scholar]