Tooth decay (dental caries) is a common cause of pain, impaired quality of life, and tooth loss in children and adults. It begins because of a compositional change in the microorganisms that colonize the tooth surface driven by repeated and sustained carbohydrate intake. Although several bacterial species are associated with tooth decay, Streptococcus mutans is the most common cause. Therefore, it is important to identify biological processes that contribute to the survival of S. mutans in the human mouth, with the aim of disrupting the processes with antimicrobial agents. We successfully applied Tn-seq to S. mutans, discovering genes that are required for survival, growth, and persistence, both in laboratory environments and in a mouse model of tooth decay. This work highlights new avenues for the control of an important human pathogen.
KEYWORDS: Streptococcus, antimicrobial targets, dental caries, essential genome, transposon sequencing
ABSTRACT
Transposon mutagenesis coupled with next-generation DNA sequencing (Tn-seq) is a powerful tool for discovering regions of the genome that are required for the survival of bacteria in different environments. We adapted this technique to the dental caries pathogen Streptococcus mutans UA159 and identified 11% of the genome as essential, with many genes encoding products required for replication, translation, lipid metabolism, and cell wall biogenesis. Comparison of the essential genome of S. mutans UA159 with those of selected other streptococci for which such information is available revealed several metabolic pathways and genes that are required in S. mutans, but not in some Streptococcus spp. We further identified genes that are essential for sustained growth in rich or defined medium, as well as for persistence in vivo in a rodent model of oral infection. Collectively, our results provide a novel and comprehensive view of the genes required for essential processes of S. mutans, many of which could represent potential targets for therapeutics.
IMPORTANCE Tooth decay (dental caries) is a common cause of pain, impaired quality of life, and tooth loss in children and adults. It begins because of a compositional change in the microorganisms that colonize the tooth surface driven by repeated and sustained carbohydrate intake. Although several bacterial species are associated with tooth decay, Streptococcus mutans is the most common cause. Therefore, it is important to identify biological processes that contribute to the survival of S. mutans in the human mouth, with the aim of disrupting the processes with antimicrobial agents. We successfully applied Tn-seq to S. mutans, discovering genes that are required for survival, growth, and persistence, both in laboratory environments and in a mouse model of tooth decay. This work highlights new avenues for the control of an important human pathogen.
INTRODUCTION
Dental caries is one of the most prevalent biofilm-related infectious diseases, with approximately 2.4 billion adults and 621 million children living with untreated caries as recently as 2010 (1). A polymicrobial disease, caries is associated with the opportunistic pathogen Streptococcus mutans (2, 3), although the strength of the association is influenced by geographic and socioeconomic factors (4). Aspects of the physiology of S. mutans that are thought to explain its substantial cariogenic potential include its capacity to form biofilms on teeth, to produce significant quantities of organic acids from many different dietary carbohydrates, to grow and metabolize at low pH, and to adapt rapidly to fluctuations in oxygen tension, pH, carbohydrate source and availability, and other environmental inputs (5, 6). In recent years, Cnm-positive strains of S. mutans, Cnm being a collagen-binding protein, have been associated with nonoral diseases, including infective endocarditis and cerebral microbleeds (7, 8). Despite extensive research on the many virulence-associated attributes of S. mutans, more comprehensive approaches are needed to develop a complete understanding of the biology of this microorganism, with the aim of developing highly effective prevention and treatment strategies.
Essential gene screens have provided a wealth of information for several important bacterial species (9–14). In these studies, mutant libraries, designed to include all the nonessential genes of a microorganism, are screened across diverse environments, and the genes that contribute to survival in particular conditions are identified. Genes that cannot be mutagenized are also discovered, defining the essential elements in the genome that are required for survival. Earlier work by Quivey et al. (15) made an important contribution to mutant screens in S. mutans by creating a gene-specific barcode library in S. mutans UA159. However, coverage of the genome was relatively low, as deletion constructs were not created for 530 genes (S. mutans UA159 has 1,960 protein-coding genes). Recently, transposon mutagenesis has been combined with next-generation DNA sequencing (11, 16). This technique, known as Tn-seq (or TraDIS), measures changes in the relative abundance of thousands of transposon mutants simultaneously as they are grown under one or more experimental conditions. Transposon insertions that become under- or overrepresented in the population indicate genes and pathways that are important or potentially dispensable, respectively, in specific conditions. Tn-seq technologies have been applied in Streptococcus pneumoniae (11), Streptococcus pyogenes (17), and Streptococcus agalactiae (18). It is only recently that this approach has been applied to the study of S. mutans (19), with a focus on genetic competence.
In the current study, we created high-coverage transposon libraries and grew them in in vitro and in vivo environments. After sequencing, analysis of transposon insertion sites allowed for the determination of the essential genome of S. mutans UA159. Genes required for growth in rich and defined media were discovered. Finally, we applied Tn-seq to identify genes required for persistent colonization in a mouse model for dental caries. This study provides the foundation to identify new molecular mechanisms required for the pathogenicity of S. mutans and for the discovery of new targets for anticaries agents.
RESULTS AND DISCUSSION
The essential genome of Streptococcus mutans UA159.
A Mariner in vitro transposition mutagenesis technique was used to create S. mutans transposon libraries (11, 20). The Mariner transposable element inserts randomly at TA dinucleotide sites, which makes it well suited for use in low-G+C bacteria, such as S. mutans (36.82% G+C). There are 147,733 TA sites (the transposon can insert in either DNA strand) randomly distributed throughout the S. mutans UA159 genome. Another feature of the transposable element is the location of an MmeI restriction site in the inverted repeat that helps to facilitate Tn-seq and mapping of transposons to their insertion sites. After transposon libraries are cultured, the DNA is extracted and digested with MmeI, leaving 15 to 20 bp of chromosomal DNA that can be sequenced after ligation to suitable primers. Two transposon libraries were made in S. mutans UA159, a Bratthall serotype c strain isolated in 1982 from a child with active caries. The libraries extracted after initial isolation on blood agar contained 66,728 or 144,816 transposon insertion mutants (determined after sequencing and alignment to unique TA sites), respectively. Genomic DNA was extracted from both libraries and sequenced to determine the locations of transposon insertions. After next-generation DNA sequencing, we obtained 20,137,898 and 13,186,867 reads per library. These reads were trimmed and aligned to the S. mutans UA159 genome to determine the number of transposon insertions per gene. There was a strong degree of similarity between the number of insertions in each gene between the two libraries (ρ = 0.88) (see Fig. S1A in the supplemental material).
Reproducibility among S. mutans UA159 transposon libraries is high. The total number of reads per gene (reads normalized to 1 million) per library is plotted for starter libraries selected on blood agar (A), rich media (B), and defined media (C). The number of reads per gene was highly correlated across the two transposon libraries. CPM, counts per million. Download FIG S1, PDF file, 0.3 MB (320.6KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
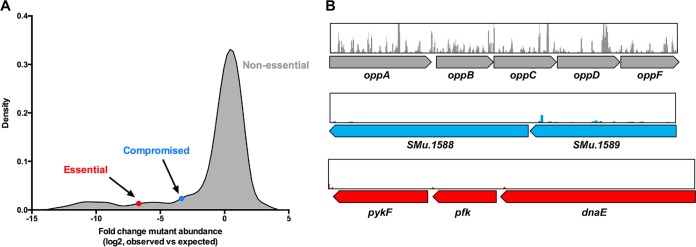
Multiple methods exist for determining essential genes from Tn-seq experiments, including annotation-dependent and -independent methods (21). Fundamentally, essential genes are defined as those that lack transposon insertions in a well-saturated transposon library that has been sequenced to saturation (each insertion has ≥50 reads on average) (21). It is known that certain genes are conditionally essential; that is, they may be required for growth in one environment, but not in others. For the purposes of this study, genes were designated essential under the conditions tested if they completely lacked transposon insertions or if the gene contained fewer than 1% of the insertions expected (based on gene size and TA site abundance; see Materials and Methods), similar to criteria applied in other Tn-seq studies (22–24). A total of 24 protein-coding genes (most often transposon related), 9 rRNA genes, and 8 tRNA genes were removed from the analysis because of multiple copies in the S. mutans UA159 genome (Table S1). Sixty-nine small genes (less than 100 bp) were also not included in the analysis (Table S1) on the basis that they have fewer than 10 TA sites, and therefore, transposon insertions could be underrepresented due to chance. Using these criteria, 203 genes were found to be essential for growth on blood agar, 53 of which contained no insertions and 150 had 100-fold or fewer insertions than expected (Fig. 1A and Table S2). Essential genes constituted 11% of the total annotated open reading frames (ORFs) in the S. mutans UA159 genome, within the range (5.3 to 22%) observed in other bacterial species (10, 17, 18, 25–28). A total of 101 genes contained 1 to 10% of the expected number of insertions. Insertions within these genes may compromise the fitness of transposon strains, but they are not essential for growth on blood agar. Examples of nonessential, compromised, and essential genes with mapped transposon insertions are shown in Fig. 1B (essential genes have very few or no transposon insertions present). Using the S. mutans core genome identified by Cornejo et al. (29), 177 of the 203 essential genes (87%) were part of the core genome, and the 26 remaining essential genes (13%) were in the accessory genome. Essential genes are expected to reside within the core genome, given that they encode proteins involved in fundamental biological functions and are therefore more likely to be conserved between strains (23, 30). Conversely, 100 to 300 noncore genes were estimated previously to differ between any two strains of S. mutans (29). When comparing the essential genomes of two strains of the same species, differences in essential gene content and metabolic pathways have been reported (26, 27). Clearly, accessory gene content is important and could alter gene-gene networks in such a way that genes that are essential in one strain become functionally redundant in another strain. Other essential functions of noncore genes may be related to coping with unique environmental conditions, associated with media and culture conditions, or from endogenously generated metabolic end products.
FIG 1 .
The S. mutans UA159 essential genome. (A) Density plot of the fold change in mutant abundance (observed versus expected reads). The blue circle indicates the point at which mutants are considered to display compromised fitness on blood agar, and the red circle indicates the point at which mutants are essential on blood agar. The majority of mutants are nonessential. (B) Example of the transposon insertion reads within genes that are considered nonessential (oppA to oppF), compromised (SMu.1588-SMu.89), and essential (pykF, pfk, and dnaE). Each peak represents a unique transposon insertion site, and the height of the peak relates to the number of sequencing reads for that site.
Genes removed prior to Tn-seq data analysis. Download TABLE S1, PDF file, 0.04 MB (39KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Observed versus expected transposon insertion reads. Download TABLE S2, PDF file, 0.9 MB (996.6KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
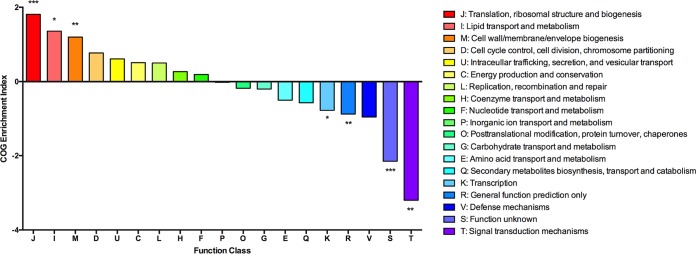
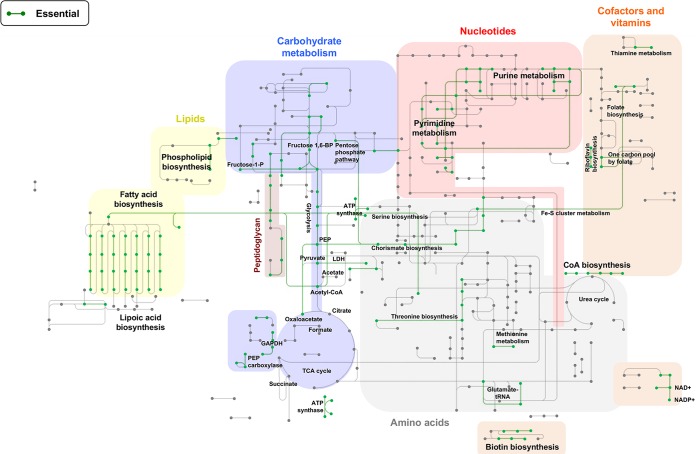
As an additional component of our analysis, essential genes were grouped according to Clusters of Orthologous Groups (COG) functional categories (31) to yield further insights into the functions of their gene products. For this analysis, we included genes that showed evidence of compromised fitness on blood agar (0.01- to 0.1-fold expected reads). Genes encoding products related to translation, lipid metabolism, and cell wall biogenesis were enriched among essential genes (COGs J, I, and M) (Fig. 2), consistent with essential genomes identified in other bacterial species (23, 27). Genes associated with signal transduction, transcription, general functions, or of unknown function were depleted (COGs K, R, S, and T), suggesting that these functions are less important for growth on blood agar. We also visualized metabolic pathways essential for S. mutans using the Kyoto Encyclopedia of Genes and Genomes (KEGG) mapper (32). A total of 194 of the 203 essential genes had KEGG numbers and were included in the analysis. As shown in Fig. 3, key cellular processes associated with the functions of genes included aminoacyl-tRNA biosynthesis, glycolysis, purine and pyrimidine metabolism, fatty acid metabolism, peptidoglycan biosynthesis, ribosome biogenesis, DNA replication and repair, and pathways involved in amino acid biosynthesis. These functions broadly fit into three major biological pathways: processing of genetic information, energy production, and maintenance of the cell envelope. Eleven of the essential genes are described as hypothetical proteins without a putative function, which is not surprising considering that even in Escherichia coli (strain K-12 W3110) more than 40% of the protein-coding genes have not been assigned a function (33). However, system-level approaches, such as Tn-seq, should lead to better annotation of microbial genomes by facilitating the association of phenotypes with uncharacterized genes. Finally, computational analyses of Tn-seq data sets have identified drug targets in other microbial pathogens (34). Thus, identification of essential genes in S. mutans and their metabolic processes may delineate putative therapeutic targets against caries.
FIG 2 .
Essential genes by Clusters of Orthologous Groups (COG) classification. Essential and compromised genes that are annotated with known functions are plotted according to the COG enrichment index. This index is calculated as the percentage of the essential genome made up of a COG divided by the percentage of the whole genome made up by the same COG. The log2 fold enrichment is displayed, and significant differences were calculated using the two-tailed Fisher’s exact test. If the COG enrichment index for a given COG is >0, it implies that a greater proportion of genes within that COG are essential than would be expected and vice versa for a COG enrichment index of <0. Log2 COG enrichment index values that are significantly different from expected are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01;***, P < 0.001.
FIG 3 .
The essential metabolic pathways of S. mutans UA159 for the initial library recovered on blood agar. Essential metabolic pathways are indicated by green lines. Shaded areas represent major metabolic processes (e.g., carbohydrate metabolism). We have also highlighted metabolic pathways that are essential (e.g., threonine biosynthesis). Abbreviations: TCA, tricarboxylic acid; CoA, coenzyme A; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LDH, lactate dehydrogenase; PEP, phosphoenolpyruvate; P, phosphate; BP, bisphosphate.
Conservation of essential genes among streptococci.
Essential genes in S. mutans that are also essential in other streptococci may be effectively targeted by antimicrobial agents to control a variety of streptococcal infections, while genes that are essential only in S. mutans may be targeted for control of dental caries without perturbation of resident beneficial streptococci. Using the Database of Essential Genes (DEG) (http://www.essentialgene.org/) (35), we examined whether orthologs of essential genes of S. mutans UA159 were conserved in Streptococcus agalactiae, Streptococcus pneumoniae, Streptococcus pyogenes, and Streptococcus sanguinis. The conservation of essential genes between individual Streptococcus spp. and S. mutans ranged from 43% for S. pneumoniae (combination of strains R6 and Rx1) to 77% for S. agalactiae A909 (Table S3). More broadly, there were only 19 S. mutans essential genes without orthologs in any of the four streptococcal species. There was strong conservation across all four species for genes associated with fundamental cellular processes, including DNA replication, peptidoglycan synthesis, transcription and translation, lipid synthesis, and acetyl coenzyme A biochemical pathways. We also identified biological functions essential in S. mutans, but not in the other four streptococci. All genes involved in the shikimate pathway (aroABCDEGHK) were essential in S. mutans UA159, but not the other streptococci examined. The shikimate pathway has been identified as an attractive target for drug design against Mycobacterium tuberculosis, where it is also essential (36, 37). Genes for an arginine repressor (SMu.2093, argR), the l-lactate dehydrogenase enzyme (LDH) (SMu.1115, ldh), and the superoxide dismutase enzyme (SMu.629, sod) were also not essential in the other four streptococcal species. Hillman et al. (38) reported that LDH is essential for S. mutans (strain JH1000). Also, deletion of ldh in S. pneumoniae D39 led to distinct fitness defects and overaccumulation of pyruvate (39). However, the S. pneumoniae ldh mutant can maintain sufficient redox balance via alcohol dehydrogenase, thereby allowing ldh mutants to remain viable. Evidently, S. mutans is unable to achieve redox balance or survive toxic levels of pyruvate without LDH. The environmental niches of S. pneumoniae and S. mutans are distinct, and it is not surprising that the central metabolic processes are regulated in divergent ways. Notably, many commensal and potentially beneficial oral streptococci are members of the mitis group of streptococci, as is the pneumococcus. The connection between central carbon metabolism and S. mutans virulence is an important area on which to focus future Tn-seq studies.
Conservation of essential genes. Download TABLE S3, PDF file, 0.2 MB (164.4KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Metabolic requirements for survival in rich versus defined medium.
The initial selection of our transposon libraries on blood agar was designed to ensure that the maximum number of mutants was recovered. That is, blood agar is relatively nutritious, so the impact of gene deletions on auxotrophy should be reduced and growth for 48 h of well-diluted samples would increase the chances that even slow-growing colonies could be isolated. To begin to probe more deeply those genes needed for sustained growth in the laboratory, we cultured the mutant libraries in a rich liquid medium (brain heart infusion [BHI] broth) and a chemically defined medium (FMC [40]). Both media are used extensively to study S. mutans. We inoculated each medium with 107 CFU of the mutant library, passaged the cells for approximately 30 generations, and then isolated DNA from the populations for Tn-seq. Read counts were strongly correlated between the two libraries for both conditions (rich media, ρ = 0.89; defined media, ρ = 0.90) (Fig. S1). Analysis of transposon insertion reads, using the criteria that genes yielding fewer than 1% of the expected transposon reads were defined as essential, revealed that 295 genes were essential in rich medium and 319 were required in defined medium (Table S2). Compared to essential genes on blood agar, 96 and 123 genes were differentially represented when passaged in rich or defined medium, respectively. Of these genes, 76 genes were shared across the two conditions, but 20 were unique to rich medium and 47 were unique to defined medium. Of the 101 genes that displayed compromised fitness on blood agar, i.e., yielded 1 to 10% of the expected transposon insertions compared to the library prepassage, 50 and 53 genes were essential for growth in rich and defined medium, respectively. Implicit in the interpretation of these data is the assumption that all of the genes that are essential for growth on blood agar are also essential for growth in BHI broth or FMC.
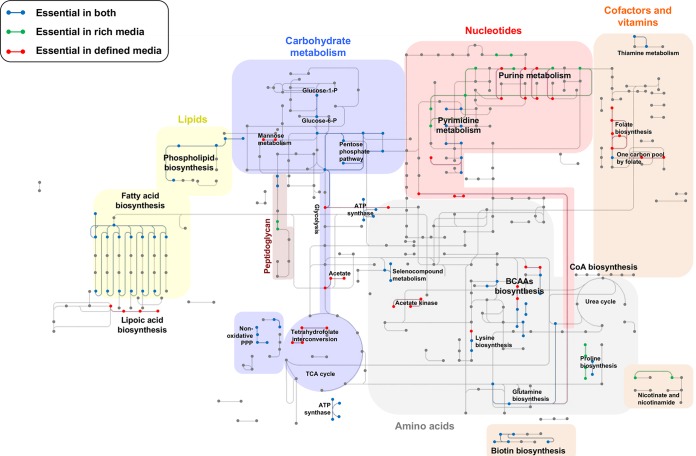
Using KEGG pathway analysis, we visualized the metabolic pathways that are essential in rich medium, defined medium, or both (Fig. 4). Metabolic pathways essential in both environments include processes involved in fatty acid biosynthesis, the pentose phosphate pathway, and biotin biosynthesis. Biological processes essential differentially in defined medium included lipoteichoic acid biosynthesis (dltABCD), folate biosynthesis (folADKP), and pathways involved in pyrimidine biosynthesis (pyrA and pyrAB). In rich medium, purine (purB and guaA) and proline (proC) metabolism are important. Differences in requirements for gene products appear to be consistent with differences in medium composition. For instance, we would predict that BHI broth is richer in folic acid than FMC, leading to an increased demand for tetrahydrofolate production by folADKP. Loss of mutants with insertions in the sucrose-6-phosphate hydrolase gene scrB (41) in rich medium may be due to the fact that although glucose is the dominant source of carbohydrate in BHI, this medium is contaminated with small amounts of sucrose and probably other carbohydrates (42); glucose is the sole carbohydrate in the FMC medium used here. In support of this concept, insertions within the glucose/mannose-specific phosphoenolpyruvate:sugar phosphotransferase system (manLMN) (43) were lost from the mutant pool during passage in defined medium compared to the initial library on blood agar and the library on rich medium.
FIG 4 .
Essential metabolic pathways of S. mutans in rich and defined media. Metabolic pathways essential in both rich and defined media (blue lines), essential only in rich medium (green lines), and essential only in defined medium (red lines) are shown. Shaded areas represent major metabolic processes (e.g., carbohydrate metabolism). We have also highlighted metabolic pathways that become essential in these environments (e.g., acetate metabolism). BCAAs, branched-chain amino acids; TCA, tricarboxylic acid; CoA, coenzyme A; P, phosphate; PPP, pentose phosphate pathway.
Although the clear majority (99.5%) of genes were either essential, compromised, or nonessential, a small subset of genes had increased transposon sequencing reads after passage in defined or rich media, and therefore, the mutations were interpreted to have enhanced the fitness of the strains. Alternatively, mutations within these genes may be overrepresented because they result in a phenotype with increased yields of DNA during DNA isolation versus other insertion mutants. Insertions in genes that encode proteins determining cell shape were notably overrepresented. In rich medium, overrepresented genes included mreC (SMu.20) and mreD (SMu.21), and in defined medium, mreC, mreD, pbp2b (SMu.597), and rodA (SMu.1279c) were overrepresented. Although not studied in S. mutans, the products of these genes contribute to peripheral peptidoglycan synthesis in S. pneumoniae, forming an “elongasome” consisting of MreC, MreD, RodA, RodZ, PBP2b, PBP1a, and GpsB (44). In this study, gpsB (SMu.471) was essential (on blood agar), and insertions within pbp1a (SMu.467) led to compromised fitness (in rich and defined media). The essentiality of genes that constitute the elongasome appears to be dependent on the species and strain. Indeed, in the coccus-shaped bacterium Staphylococcus aureus strain COL, mreC and mreD are dispensable and have no role in cell shape (45). In S. pneumoniae, mreC and mreD are dispensable in the unencapsulated strain R6 but required in the encapsulated strain D39 and the unencapsulated D39 Δcps mutant (46). Further evaluation of these cell-shape-determining genes in S. mutans is warranted because it appears that the regulation of this process might differ from regulation in previously studied bacteria.
Validation of the in vitro Tn-seq screen.
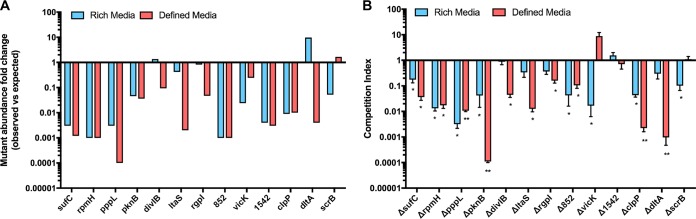
We selected a set of genes in which insertions caused fitness reductions (essential or compromised) in rich medium, defined medium, or both to validate the data set described above. A total of 13 genes were replaced with a nonpolar kanamycin cassette and competition assays against wild-type S. mutans UA159 were performed (see Materials and Methods). Briefly, a 1:1 ratio of exponentially growing cultures of the wild type and each of the mutant strains were harvested at the same optical density and inoculated together to approximately 1 × 106 CFU/ml in either rich or defined medium. After 20 h, equivalent to about 10 generations, mutant and wild-type S. mutans were serially diluted and plated. Of the strains with mutations in genes that were essential or mutant strains that showed compromised growth in Tn-seq screens in rich medium, 8 of 9 also displayed growth defects in the competition assays (Fig. 5). In defined medium, 9 out of 11 mutants were unable to compete against S. mutans UA159 (Fig. 5). Across both assays, 85% of the genes identified by Tn-seq screens to have reduced fitness also displayed reduced fitness during competition assays, validating our Tn-seq methodology. Other studies also reported similar levels of concurrence between fitness screens and subsequent validation (11, 47).
FIG 5 .
Validation of Tn-seq fitness defects in rich and defined media. (A) Tn-seq results (rich and defined media) for 13 genes (observed versus expected Tn insertion reads) displayed as a bar graph. Values less than 1 are predictive of a gene that is required for optimal fitness in that environment. (B) Bar graph depicting the fitness of mutants compared to the fitness of the wild type using a 1 × 1 competition method. A competition index of >1 indicates that the mutant grew better than the wild type did. A competition index of <1 indicates that the mutant displayed reduced fitness compared to the wild-type strain. The genes and proteins they encode follow: sufC, ABC transporter involved in Fe-S cluster assembly; rpmH, 50S ribosomal protein L34; pppL, phosphoprotein phosphatase; pknB, serine/threonine phosphatase; divIB, cell division protein; ltaS, phosphoglycerol transferase; rgpI, glycosyltransferase 2-like protein; SMu.852, transcriptional regulator; vicK, histidine kinase; SMu.1542, diacylglycerol kinase; clpP, ATP-dependent Clp protease; dltA, d-alanine-d-alanyl carrier protein ligase; scrB, sucrose-6-phosphate hydrolase. *, P < 0.05; **, P < 0.01.
Fitness determinants in a mouse model of dental caries.
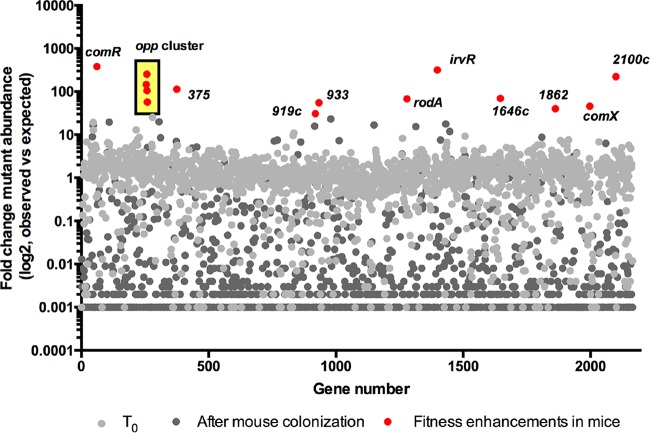
One powerful application of high-throughput transposon screens has been to identify genes that are important during infection. We used an established rodent dental caries model (48) to interrogate our S. mutans transposon libraries when challenged with colonization and survival in the oral cavity. Briefly, after antibiotic suppression of oral microbiota, 7-week-old BALB/cJ mice were placed on a carbohydrate-rich diet with sweetened drinking water (2% sucrose) and orally inoculated daily for five successive days with approximately 1 × 108 cells from either the library or with wild-type S. mutans UA159. The experiment was terminated after 3 weeks. Bacteria were collected from the mandibular molars, and DNA was isolated as outlined in Materials and Methods. Colonization of molars by S. mutans as a percentage of the total recovered bacterial population was similar for the transposon library and for wild-type S. mutans, as measured by quantitative PCR (qPCR) (24.4% ± 12.2% and 27.1% ± 12.8%, respectively). As a control, S. mutans was not detected from the molars of a third group consisting of uninoculated mice, even though total recovered bacteria from this group were equivalent to those from the other two groups (7.8 × 106 ± 2.7 × 106 from the uninoculated group, 6.5 × 106 ± 5.7 × 106 from the group inoculated with wild-type S. mutans, and 8.1 × 106 ± 6.3 × 106 from the group inoculated with streptococci from the transposon library). These results indicate our protocols were effective in preventing cross-contamination between animal groups and that total molar colonization by the transposon library and wild-type S. mutans were similar, averaging approximately 2.5 × 106 S. mutans per mouse. From the 16 mice inoculated with the transposon library, we obtained 3.6 μg of bacterial DNA from the mandibular molars, and this DNA was used to generate Tn-seq libraries. After next-generation DNA sequencing, we obtained a total of 9.28 × 107 sequenced reads, of which 8.98 × 107 remained after quality control and trimming of adapter and barcode sequences. Transposon insertion mutations were recovered from 1,725 of the 1,940 genes within the S. mutans UA159 genome (small and duplicate genes were removed from the analysis). After read analysis, negative selection (less than 1% of expected transposon reads) was observed for 79% of the S. mutans UA159 genome (Fig. 6 and Table S2) comparing the input library to the output library. In other Tn-seq studies, negative selection was also the dominant selective process in in vivo experiments (49, 50). Although it is surprising that >75% of the genes in S. mutans UA159 are required for colonization of the mouse oral cavity, there is obviously considerable selective pressure during the initial colonization of and then survival during competition with resurgent commensal bacteria for 3 weeks in the mouse oral cavity. Notably, mutant strains carrying transposon insertions in genes involved in genetic competence (i.e., comR, oppABCDF, irvR, and comX) showed an enhancement in recovery from the mouse oral cavity. Insertions within these genes were 50-fold (comX) to 400-fold (comR) higher than would be expected if Tn insertions within these genes had no effect on fitness (Fig. 5B). The “blooming” of these Tn insertions likely partly accounts for the low number of Tn insertions recovered from most other genes. Genetic competence is required for the internalization of extracellular DNA and has been linked to other behaviors, including stress tolerance and biofilm formation (51, 52). Both ComR and the Opp oligopeptide permease transporter are required for the activation of transcription of comX (often called sigX), encoding the alternative sigma factor that controls late competence gene activation (53, 54). IrvR is a regulator important for the development of genetic competence in S. mutans (55). In general, the fitness of strains carrying mutations in genes required for genetic competence has rarely been assessed in in vivo models. In one study, S. pneumoniae virulence was attenuated in a ΔcomX1 ΔcomX2 mutant, apparently due to loss of induction of the allolytic genes cbpD and cibAB (56). Orthologs of cbpD and cibAB are not present in the S. mutans UA159 genome. Notably, production of ComX was shown to lead to growth arrest and cell lysis in S. mutans (57), so fitness enhancements in competence-related mutants may account for the increases in recovery of these mutants. Alternatively, comRS and many of the competence genes are highly conserved across human isolates of S. mutans (58), so the possibility that the mouse caries model may not fully recapitulate the selective pressures found in a human host must be considered. One notable difference in our mouse model from the natural environment in humans is that we suppressed the endogenous flora with antibiotics prior to infecting with S. mutans. Thus, the competence genes may play important roles in competition with commensal bacteria.
FIG 6 .
Streptococcus mutans UA159 fitness determinants in a rodent caries model. Tn-seq results summarized using a scatterplot that displays the fold change in mutant abundance (observed versus expected reads). Genes containing transposon insertions that lead to fitness enhancements are highlighted in red. The complete list of insertions can be found in Table S2 in the supplemental material. SMu locus tags are shown without the SMu prefix in the figure (e.g., SMu.919c is shown as 919c).
Rodent models, more commonly involving rats, have been used to study dental caries for decades, and have made important contributions to the understanding of molecular components of the virulence of S. mutans (59–61). Potential limitations of in vivo Tn-seq experiments include infection bottlenecks (whereby most transposon mutants are lost during inoculation), complementation by other mutants (e.g., secreted products), the time point chosen for infection or collection of organisms, and differences in the microbiome of animals versus the human oral cavity. Bottlenecks are a major technical challenge for in vivo Tn-seq experiments (62). There was a significant bottleneck associated with this study (0.5% of the original inoculum was recovered), but mutants were recovered from 90% of the S. mutans UA159 genome in the 16 animals. Now that a protocol that allows for in vivo screening of libraries in the oral cavity has been established, it should be possible to further explore these in vivo phenotypes under different experimental conditions to ascertain how diet and other factors could influence the colonization, persistence, and virulence of S. mutans.
Limitations of Tn-seq analysis.
Here, we provide the first comprehensive overview of the essential genome of S. mutans UA159, but at the same time, we acknowledge the limitations of this technique. One, all transposons (Tn5, Tn7, Tn10, or Mariner) have insertion biases whereby the sequence of a region of the genome could lead to “cold spots.” Two, small genes can be misidentified as essential because of a lack of transposon insertions due to either too few insertion sites (TA dinucleotide sites in the case of Mariner) or a lack of transposon insertion density. We employed stringent criteria to disregard small genes. However, included within our analysis are genes that are classified as essential but that may be sufficiently small to be nonessential. A third issue relates to the initial selection of the library, whereby a limited number of genes are classified as essential but they are truly conditionally essential. For example, sod (SMu.629), encoding superoxide dismutase (SOD), was determined to be essential by our methodology but was previously shown to be nonessential in S. mutans under both aerobic and anaerobic environments (63). The absence of a growth defect for sod mutants in aerobic environments is somewhat surprising. SOD is employed by nearly all living organisms as an antioxidant defense and might be assumed to have increased prominence in facultative anaerobes, such as S. mutans. Our efforts to delete sod by PCR ligation mutagenesis in aerobic environments failed but were successful if the transformation was kept strictly anaerobic (Fig. S2A). The sod mutants had 1,000-fold-diminished competitiveness versus the wild type when grown in BHI in an aerobic environment (Fig. S2B). Therefore, sod essentiality is related to the conditions of initial selection, and this gene is not essential in all environments. As an aside, we observed evidence that extragenic suppression of the sod deletion may occur, which may further explain the discrepancies between our results and the previous report (63). Genes may also be classified as essential because strains lacking these genes display such a severe growth defect that they never replicate enough to be identified during library preparation and sequencing. Last, the polarity of the transposon insertion may also lead to incorrect recording of an essential gene, although this is thought to be a minor issue with the magellan6 transposon (11).
Validation of sodA as a conditionally essential gene. (A) PCR ligation mutagenesis (see Materials and Methods) was employed to replace the sodA gene with a nonpolar kanamycin cassette on both BHI (brain heart infusion) and blood agar, with incubation at 37°C in anaerobic, aerobic, and microaerophilic environments. Kanamycin-resistant colonies were obtained only if transformations were kept strictly anaerobic, except for one colony isolated in the microaerophilic conditions (white arrow). (B) Isolated sodA mutants (Kmr), kept strictly anaerobic during routine culture, were competed against wild-type S. mutans in both anaerobic (BHI) and aerobic (BHI, with shaking at 150 rpm) environments. A competition index of >1 indicates that the mutant grew better than the wild type did. A competition index of <1 indicates that the mutant displayed reduced fitness compared to the wild-type strain. Download FIG S2, PDF file, 2.2 MB (2.2MB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Limitations exist for other methods, including gold standard gene deletion libraries such as the Keio collection in Escherichia coli (9). In this comprehensive library, genes were denoted as nonessential but have since been shown to be essential. An example is holD, a gene denoted as nonessential in the Keio collection. However, it has been shown that ΔholD mutants accumulate suppressor mutations that override the essentiality of the gene (64). Therefore, a complete understanding of essential genes will likely require using multiple approaches, combining gene deletion libraries with modern technologies, such as Tn-seq. Furthermore, complete validation via essential gene knockdowns employing antisense RNA or clustered regularly interspaced short palindromic repeat (CRISPR) interference assays can also provide further confirmation of essential genes.
Conclusions.
We developed and validated Tn-seq technology for use in S. mutans using a Mariner minitransposon and next-generation DNA sequencing. We determined the essential genome for particular in vitro conditions and identified fitness determinants required for establishment and/or persistence in an animal model. Going forward, Tn-seq methods are sufficiently flexible for use under conditions to model processes important for the colonization, growth, stress tolerance, and persistence of genetically competent strains of S. mutans as well as related streptococci. In addition, Tn-seq may highlight metabolic pathways that are interesting candidates to disrupt oral colonization by S. mutans.
MATERIALS AND METHODS
Strains and growth conditions.
S. mutans UA159 and mutant strains are listed in Table S4 in the supplemental material. S. mutans strains were routinely cultured in brain heart infusion (BHI) broth at 37°C in a 5% CO2 microaerophilic environment. The chemically defined medium FMC (40) was used during Tn-seq and competition assay studies. Antibiotics were used at the following concentrations: kanamycin (1.0 mg/ml for S. mutans; 50 µg/ml for E. coli), spectinomycin (1.0 mg/ml for S. mutans; 50 µg/ml for E. coli) and ampicillin (100 µg/ml for E. coli). A list of strains and plasmids (Table S4) and oligonucleotide primers (Table S5) can be found in the supplemental material.
List of strains and plasmids used in this study. Download TABLE S4, PDF file, 0.05 MB (51.9KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotides used in this study. Download TABLE S5, PDF file, 0.1 MB (61.3KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Ethics statement.
The University of Florida IACUC committee approved all animal procedures (IACUC study 201509214).
Tn-seq experiments.
Transposon libraries were created following a previously published in vitro transposition technique with minor modifications (20). Genomic DNA from S. mutans UA159 was combined with the transposon Magellan6 and the transposase MarC9, and the resulting transposon DNA was repaired. This DNA was transformed into S. mutans, and transposon mutants were selected on blood agar containing spectinomycin with incubation for 2 days at 37°C in a 5% CO2 microaerophilic environment. Transposon mutants were collected and stored at −80°C. Two libraries were created in S. mutans UA159, and selection was carried out in rich medium, defined medium, and an in vivo mouse model. After selection, genomic DNA was obtained from each sample and digested with MmeI, prior to adapter ligation (see Table S5 for adapter sequences), as previously described (20). After Illumina sequencing, FASTQ sequencing files were processed on the University of Florida Research Computing Galaxy Instance. Files were split according to the sample barcode (Barcode Splitter version 1.0.0) and trimmed of the 8-nucleotide barcode (Trimmomatic version 0.32.2). The transposon sequence was removed (ACAGGTTGGATGATAA; Clip version 1.0.1), and the resulting 14- to 16-bp genomic DNA sequence was aligned to the S. mutans UA159 genome using Bowtie 2 (65). Importantly, 15% of the 3′ end of genes was removed from analysis, as insertions in this region may not interrupt gene function. Transposon insertion counts per gene were calculated using HTSeq-count (66). To determine gene essentiality, an annotation-dependent method was used (22). In short, the expected number of reads per gene was calculated based on gene size and transposon library size, assuming total randomness of Tn insertion (normalized to 1 million reads). The observed reads per gene (normalized to 1 million reads) was divided by the expected reads obtaining a ratio. For a gene, a ratio of 0.01 to 0.1 indicates compromised fitness, with a ratio less than 0.01 indicating essentiality of that gene.
Essential gene function and metabolic network analysis.
For the S. mutans UA159 genome, genes were assigned functions based on COG and KEGG annotations. Approximately 60% of S. mutans genes have been assigned a KEGG number. Five hundred eighty-nine S. mutans genes have not been assigned to a COG category. For each COG, the total number of genes related to that function (across the entire genome) and the total number of essential genes related to that function were counted in Excel. COG enrichment was determined using a previously described method (27). Metabolic networks were generated using KEGG Mapper (http://www.genome.jp/kegg/mapper.html) (32) and a previously described protocol (27).
Essential gene conservation.
We used the Database of Essential Genes (http://www.essentialgene.org) (35) to compare the essential genes of S. mutans UA159 to those of Streptococcus agalactiae, Streptococcus pneumoniae, Streptococcus pyogenes, and Streptococcus sanguinis. If a gene was not essential in these four species, then a comparison was made against all known essential genes (from the essential genomes of 46 bacterial species). For each essential gene, the protein sequence was used with default BLASTP parameters.
Gene mutagenesis.
Standard DNA manipulation techniques were used to engineer deletion strains (67). A PCR ligation mutagenesis method was used to replace genes with nonpolar kanamycin markers (68). For each gene deletion, primers A and B were designed to amplify 500 to 600 bp upstream of the coding sequence (with ca. 50 bp overlapping the coding sequence of the gene). Primers C and D were designed to amplify 500 to 600 bp downstream of the coding sequence (with ca. 50 bp overlapping the coding sequence of the gene). Primers B and C contain BamHI restriction enzyme sites for ligation of the AB and CD fragments to a nonpolar kanamycin cassette (lacking both a promoter and terminator) digested from plasmid pALH124 (69). The nonpolar kanamycin cassette was always inserted in the same direction as the open reading frame it was replacing. Transformants were selected on BHI agar containing kanamycin. Double-crossover recombination, without introduction of nearby secondary mutations, was confirmed by PCR and Sanger sequencing using primers E and F, away from the site of recombination.
Mouse model.
The mouse caries protocol was performed as previously described (48) with modifications. Inbred 5-week-old female specific-pathogen-free (SPF) BALB/cJ mice (The Jackson Laboratory) were allowed to acclimate for 2 days after shipping. Mice were then provided drinking water containing 0.8 mg/ml sulfamethoxazole and 0.16 mg/ml trimethoprim for 10 days to suppress indigenous oral bacteria. After a 3-day washout period with no antibiotics, mice were placed on a diet of irradiated powdered AIN-93G purified diet without fluoride, but with increased vitamins to compensate for irradiation (Envigo, Madison, WI). This diet contains 37.5% total sucrose, 24% corn starch, 17.7% protein as casein, and 4.45% maltodextrin. Mice were also given sterile water containing 2% sucrose. A total of 16 mice were inoculated with one of the transposon mutant libraries. Mice were inoculated each day for 5 days with ~1 × 108 cells in 100 µl of 1.5% carboxymethyl cellulose in sterile distilled water (dH2O). As additional controls, 16 mice were used as uninoculated controls, and a group of 8 mice were inoculated at the same time with wild-type S. mutans UA159. After 21 days, mice were euthanized by CO2 asphyxiation followed by cervical dislocation. Mandibles were aseptically extracted, dissected to isolate molar teeth, and then sonicated on ice in 1 ml sterile phosphate-buffered saline (PBS) using a Fisher F60 sonic dismembrator (100 W and 22.5 kHz) with six pulses (each pulse 10 s long) at 90-s intervals. To obtain linear standard curves, cells in quantitative PCR (qPCR) assays (see below), we added approximately 5 × 108 cells of S. mitis laboratory strain UF2 to each sonicate. S. mitis cells first underwent depurination (two 1-h incubations in 0.2 N HCl at 70°C) with extensive neutralization and washing in PBS. DNA was recovered from cell pellets obtained by centrifugation at 10,000 × g for 10 min at 4°C using the UltraClean microbial DNA isolation kit (Mo Bio Labs, Inc., Carlsbad, CA). Quantitative PCR was used to estimate total recovered bacteria and S. mutans. Primers (0.5 µM) specific for S. mutans were directed against the SMu.292 gene, a putative transcriptional regulator (forward primer, 5′-TGGCAAGTCCTGATGGTTTGAC-3′; reverse primer, 5′-GGAAGCGGAAGCTGTGATGAAC-3′). PCR mixtures (20 µl) were run on a Bio-Rad CFX96 thermocycler using SsoAdvanced Universal SYBR green supermix (Bio-Rad) as follows: (i) 3 min at 98°C; (ii) 40 cycles, with 1 cycle consisting of 15 s at 98°C and 45 s at 68°C. To estimate the total number of bacteria, we developed degenerate primers to conserved regions of the ubiquitous single-copy gene rpsL (30S ribosomal protein S12) (70) after ClustalW alignment (MacVector v.12) of sequences from 22 different species of oral and nonoral streptococci, two strains of Lactobacillus murinus (ASF361 and DSM20452), two species of Staphylococcus (Staphylococcus saprophyticus 772 and Staphylococcus xylosus HKUOPL8), three species of Corynebacterium (Corynebacterium pilosum CIP103422, Corynebacterium renale CIP52.96, and Corynebacterium mastitidis DSM44356), and Muribacter muris Ackerman 80-443-D. These organisms represent taxa, and include species, identified previously in SPF mice under biosafety level 2 (BSL2) conditions (48). Reaction conditions were similar to those for SMu.292 except the annealing temperature was 55°C for 30 cycles and with 2.5 µM primers (forward primer, 5′-CCKAAYTCNGCNYTNCGTAAR-3′; reverse primer, 5′-CGHACMCCHGGDARGTCYTT-3′). Standard curves were derived from DNA isolated from 103 to 108 S. mutans grown to mid-exponential phase in BHI. Cell numbers were determined from optical density at 600 nm (OD600) based on a growth curve of CFU versus OD600. Quantification cycle (Cq) values (in triplicate) were analyzed using CFX Manager software. The efficiencies, slopes, and r2 values for standard curves ranged from 97 to 105%, −3.274 to −3.323, and 0.985 to 0.998, respectively. As controls, primers to SMu.292 failed to generate a product when tested against DNA from bacteria isolated from oral swabs of mice prior to administration of antibiotics and grown overnight in BHI plus 2% yeast extract under anaerobic conditions. Also, both primer sets failed to produce a product against 5 × 108 S. mitis carrier cells after processing for DNA.
Competition assays.
For competition assays, strains were cultured in either rich (BHI) or defined (FMC) medium. An inoculum of 1 × 106 CFU/ml of the wild-type strain and an inoculum of each mutant strain to be tested were added to prewarmed medium and cultured for 20 h in a microaerophilic environment at 37°C. At both the start and end of the experiment, bacteria were serially diluted and plated onto BHI and BHI-kanamycin agar. Wild-type and mutant strains were enumerated (wild-type CFU were derived by subtracting mutant CFU from total CFU on BHI agar), and the competitive index was calculated using the following formula: (tend mutant CFU/tend wild-type CFU)/(tstart mutant CFU/tstart wild-type) where tend mutant CFU is the number of mutant CFU at the end of the experiment and tstart mutant CFU is the number of mutant CFU at the beginning of the experiment.
ACKNOWLEDGMENTS
We thank Andrew Camilli for providing the transposon sequencing plasmids pMalC9 and pMagellan6. We acknowledge Todd A. Atherly and Kacey B. Christian for excellent technical assistance.
This project was supported by NIDCR R01 DE013239 and DE025832.
REFERENCES
- 1.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. 2015. Global burden of untreated caries: a systematic review and metaregression. J Dent Res 94:650–658. doi: 10.1177/0022034515573272. [DOI] [PubMed] [Google Scholar]
- 2.Richards VP, Alvarez AJ, Luce AR, Bedenbaugh M, Mitchell ML, Burne RA, Nascimento MM. 2017. Microbiomes of site-specific dental plaques from children with different caries status. Infect Immun 85:e00106-17. doi: 10.1128/IAI.00106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. 2012. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One 7:e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson I, Witkowska E, Kaveh B, Lif Holgerson P, Tanner ACR. 2016. The microbiome in populations with a low and high prevalence of caries. J Dent Res 95:80–86. doi: 10.1177/0022034515609554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajdić D, McShan WM, McLaughlin RE, Savić G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe BA, Ferretti JJ. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A 99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemos JA, Burne RA. 2008. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154:3247–3255. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano K, Nomura R, Ooshima T. 2008. Streptococcus mutans and cardiovascular diseases. Jpn Dent Sci Rev 44:29–37. doi: 10.1016/j.jdsr.2007.09.001. [DOI] [Google Scholar]
- 8.Nakano K, Hokamura K, Taniguchi N, Wada K, Kudo C, Nomura R, Kojima A, Naka S, Muranaka Y, Thura M, Nakajima A, Masuda K, Nakagawa I, Speziale P, Shimada N, Amano A, Kamisaki Y, Tanaka T, Umemura K, Ooshima T. 2011. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat Commun 2:485. doi: 10.1038/ncomms1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu P, Ge X, Chen L, Wang X, Dou Y, Xu JZ, Patel JR, Stone V, Trinh M, Evans K, Kitten T, Bonchev D, Buck GA. 2011. Genome-wide essential gene identification in Streptococcus sanguinis. Sci Rep 1:125. doi: 10.1038/srep00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Opijnen T, Bodi KL, Camilli A. 2009. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods 6:767–772. doi: 10.1038/nmeth.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsyth RA, Haselbeck RJ, Ohlsen KL, Yamamoto RT, Xu H, Trawick JD, Wall D, Wang L, Brown-Driver V, Froelich JM, Kedar GC, King P, McCarthy M, Malone C, Misiner B, Robbins D, Tan Z, Zhu Z-Y, Carr G, Mosca DA, Zamudio C, Foulkes JG, Zyskind JW. 2002. A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol Microbiol 43:1387–1400. doi: 10.1046/j.1365-2958.2002.02832.x. [DOI] [PubMed] [Google Scholar]
- 13.Salama NR, Shepherd B, Falkow S. 2004. Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. J Bacteriol 186:7926–7935. doi: 10.1128/JB.186.23.7926-7935.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akerley BJ, Rubin EJ, Novick VL, Amaya K, Judson N, Mekalanos JJ. 2002. A genome-scale analysis for identification of genes required for growth or survival of Haemophilus influenzae. Proc Natl Acad Sci U S A 99:966–971. doi: 10.1073/pnas.012602299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quivey RG, Grayhack EJ, Faustoferri RC, Hubbard CJ, Baldeck JD, Wolf AS, MacGilvray ME, Rosalen PL, Scott-Anne K, Santiago B, Gopal S, Payne J, Marquis RE. 2015. Functional profiling in Streptococcus mutans: construction and examination of a genomic collection of gene deletion mutants. Mol Oral Microbiol 30:474–495. doi: 10.1111/omi.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langridge GC, Phan MD, Turner DJ, Perkins TT, Parts L, Haase J, Charles I, Maskell DJ, Peters SE, Dougan G, Wain J, Parkhill J, Turner AK. 2009. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res 19:2308–2316. doi: 10.1101/gr.097097.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Breton Y, Belew AT, Valdes KM, Islam E, Curry P, Tettelin H, Shirtliff ME, El-Sayed NM, McIver KS. 2015. Essential genes in the core genome of the human pathogen Streptococcus pyogenes. Sci Rep 5:9838. doi: 10.1038/srep09838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooven TA, Catomeris AJ, Akabas LH, Randis TM, Maskell DJ, Peters SE, Ott S, Santana-Cruz I, Tallon LJ, Tettelin H, Ratner AJ. 2016. The essential genome of Streptococcus agalactiae. BMC Genomics 17:406. doi: 10.1186/s12864-016-2741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields RC, O’Brien G, Maricic N, Kesterson A, Grace M, Hagen SJ, Burne RA. 2017. Genome-wide screens reveal new gene products that influence genetic competence in Streptococcus mutans. J Bacteriol 200:e00508-17. doi: 10.1128/JB.00508-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Opijnen T, Camilli A. 2010. Genome-wide fitness and genetic interactions determined by Tn-seq, a high-throughput massively parallel sequencing method for microorganisms. Curr Protoc Microbiol Chapter 1:Unit 1E.3. doi: 10.1002/9780471729259.mc01e03s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao MC, Abel S, Davis BM, Waldor MK. 2016. The design and analysis of transposon insertion sequencing experiments. Nat Rev Microbiol 14:119–128. doi: 10.1038/nrmicro.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valentino MD, Foulston L, Sadaka A, Kos VN, Villet RA, Santa Maria J, Lazinski DW, Camilli A, Walker S, Hooper DC, Gilmore MS. 2014. Genes contributing to Staphylococcus aureus fitness in abscess- and infection-related ecologies. mBio 5:e01729-14. doi: 10.1128/mBio.01729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein BA, Tenorio EL, Lazinski DW, Camilli A, Duncan MJ, Hu LT. 2012. Identification of essential genes of the periodontal pathogen Porphyromonas gingivalis. BMC Genomics 13:578. doi: 10.1186/1471-2164-13-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonough E, Lazinski DW, Camilli A. 2014. Identification of in vivo regulators of the Vibrio cholerae xds gene using a high-throughput genetic selection. Mol Microbiol 92:302–315. doi: 10.1111/mmi.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SA, Gallagher LA, Thongdee M, Staudinger BJ, Lippman S, Singh PK, Manoil C. 2015. General and condition-specific essential functions of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 112:5189–5194. doi: 10.1073/pnas.1422186112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner KH, Wessel AK, Palmer GC, Murray JL, Whiteley M. 2015. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc Natl Acad Sci U S A 112:4110–4115. doi: 10.1073/pnas.1419677112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayanan AM, Ramsey MM, Stacy A, Whiteley M. 2017. Defining genetic fitness determinants and creating genomic resources for an oral pathogen. Appl Environ Microbiol 83:e00797-17. doi: 10.1128/AEM.00797-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong Y-C, Abd El Ghany M, Naeem R, Lee K-W, Tan Y-C, Pain A, Nathan S. 2016. Candidate essential genes in Burkholderia cenocepacia J2315 identified by genome-wide TraDIS. Front Microbiol 7:1288. doi: 10.3389/fmicb.2016.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornejo OE, Lefébure T, Bitar PDP, Lang P, Richards VP, Eilertson K, Do T, Beighton D, Zeng L, Ahn S-J, Burne RA, Siepel A, Bustamante CD, Stanhope MJ. 2013. Evolutionary and population genomics of the cavity causing bacteria Streptococcus mutans. Mol Biol Evol 30:881–893. doi: 10.1093/molbev/mss278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan IK, Rogozin IB, Wolf YI, Koonin EV. 2002. Essential genes are more evolutionarily conserved than are nonessential genes in bacteria. Genome Res 12:962–968. doi: 10.1101/gr.87702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatusov RL, Galperin MY, Natale DA, Koonin EV. 2000. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res 28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. 2012. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlasblom J, Zuberi K, Rodriguez H, Arnold R, Gagarinova A, Deineko V, Kumar A, Leung E, Rizzolo K, Samanfar B, Chang L, Phanse S, Golshani A, Greenblatt JF, Houry WA, Emili A, Morris Q, Bader G, Babu M. 2015. Novel function discovery with GeneMANIA: a new integrated resource for gene function prediction in Escherichia coli. Bioinformatics 31:306–310. doi: 10.1093/bioinformatics/btu671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mobegi FM, van Hijum SAFT, Burghout P, Bootsma HJ, de Vries SPW, van der Gaast-de Jongh CE, Simonetti E, Langereis JD, Hermans PWM, de Jonge MI, Zomer A. 2014. From microbial gene essentiality to novel antimicrobial drug targets. BMC Genomics 15:958. doi: 10.1186/1471-2164-15-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo H, Lin Y, Gao F, Zhang C-T, Zhang R. 2014. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res 42:D574–D580. doi: 10.1093/nar/gkt1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parish T, Stoker NG. 2002. The common aromatic amino acid biosynthesis pathway is essential in Mycobacterium tuberculosis. Microbiology 148:3069–3077. doi: 10.1099/00221287-148-10-3069. [DOI] [PubMed] [Google Scholar]
- 37.Reichau S, Jiao W, Walker SR, Hutton RD, Baker EN, Parker EJ. 2011. Potent inhibitors of a shikimate pathway enzyme from Mycobacterium tuberculosis: combining mechanism- and modeling-based design. J Biol Chem 286:16197–16207. doi: 10.1074/jbc.M110.211649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hillman JD, Chen A, Duncan M, Lee SW. 1994. Evidence that l-(+)-lactate dehydrogenase deficiency is lethal in Streptococcus mutans. Infect Immun 62:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaspar P, Al-Bayati FAY, Andrew PW, Neves AR, Yesilkaya H. 2014. Lactate dehydrogenase is the key enzyme for pneumococcal pyruvate metabolism and pneumococcal survival in blood. Infect Immun 82:5099–5109. doi: 10.1128/IAI.02005-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terleckyj B, Willett NP, Shockman GD. 1975. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun 11:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayakawa M, Aoki H, Kuramitsu HK. 1986. Isolation and characterization of the sucrose 6-phosphate hydrolase gene from Streptococcus mutans. Infect Immun 53:582–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng L, Burne RA. 2013. Comprehensive mutational analysis of sucrose-metabolizing pathways in Streptococcus mutans reveals novel roles for the sucrose phosphotransferase system permease. J Bacteriol 195:833–843. doi: 10.1128/JB.02042-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abranches J, Chen Y-YM, Burne RA. 2003. Characterization of Streptococcus mutans strains deficient in EIIAB Man of the sugar phosphotransferase system. Appl Environ Microbiol 69:4760–4769. doi: 10.1128/AEM.69.8.4760-4769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massidda O, Nováková L, Vollmer W. 2013. From models to pathogens: how much have we learned about Streptococcus pneumoniae cell division? Environ Microbiol 15:3133–3157. doi: 10.1111/1462-2920.12189. [DOI] [PubMed] [Google Scholar]
- 45.Tavares AC, Fernandes PB, Carballido-López R, Pinho MG. 2015. MreC and MreD proteins are not required for growth of Staphylococcus aureus. PLoS One 10:e0140523. doi: 10.1371/journal.pone.0140523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Land AD, Winkler ME. 2011. The requirement for pneumococcal MreC and MreD is relieved by inactivation of the gene encoding PBP1a. J Bacteriol 193:4166–4179. doi: 10.1128/JB.05245-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Opijnen T, Dedrick S, Bento J. 2016. Strain dependent genetic networks for antibiotic-sensitivity in a bacterial pathogen with a large pan-genome. PLoS Pathog 12:e1005869. doi: 10.1371/journal.ppat.1005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Culp DJ, Robinson B, Cash MN, Bhattacharyya I, Stewart C, Cuadra-Saenz G. 2015. Salivary mucin 19 glycoproteins: innate immune functions in Streptococcus mutans-induced caries in mice and evidence for expression in human saliva. J Biol Chem 290:2993–3008. doi: 10.1074/jbc.M114.597906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skurnik D, Roux D, Aschard H, Cattoir V, Yoder-Himes D, Lory S, Pier GB. 2013. A comprehensive analysis of in vitro and in vivo genetic fitness of Pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. PLoS Pathog 9:e1003582. doi: 10.1371/journal.ppat.1003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao B, Vorwerk H, Huber C, Lara-Tejero M, Mohr J, Goodman AL, Eisenreich W, Galán JE, Hofreuter D. 2017. Metabolic and fitness determinants for in vitro growth and intestinal colonization of the bacterial pathogen Campylobacter jejuni. PLoS Biol 15:e2001390. doi: 10.1371/journal.pbio.2001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seaton K, Ahn S-J, Sagstetter AM, Burne RA. 2011. A transcriptional regulator and ABC transporters link stress tolerance, (p)ppGpp, and genetic competence in Streptococcus mutans. J Bacteriol 193:862–874. doi: 10.1128/JB.01257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y-H, Tang N, Aspiras MB, Lau PCY, Lee JH, Ellen RP, Cvitkovitch DG. 2002. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J Bacteriol 184:2699–2708. doi: 10.1128/JB.184.10.2699-2708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fontaine L, Goffin P, Dubout H, Delplace B, Baulard A, Lecat-Guillet N, Chambellon E, Gardan R, Hols P. 2013. Mechanism of competence activation by the ComRS signalling system in streptococci. Mol Microbiol 87:1113–1132. doi: 10.1111/mmi.12157. [DOI] [PubMed] [Google Scholar]
- 54.Mashburn-Warren L, Morrison DA, Federle MJ. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol Microbiol 78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niu G, Okinaga T, Zhu L, Banas J, Qi F, Merritt J. 2008. Characterization of irvR, a novel regulator of the irvA-dependent pathway required for genetic competence and dextran-dependent aggregation in Streptococcus mutans. J Bacteriol 190:7268–7274. doi: 10.1128/JB.00967-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu L, Lin J, Kuang Z, Vidal JE, Lau GW. 2015. Deletion analysis of Streptococcus pneumoniae late competence genes distinguishes virulence determinants that are dependent or independent of competence induction. Mol Microbiol 97:151–165. doi: 10.1111/mmi.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wenderska IB, Lukenda N, Cordova M, Magarvey N, Cvitkovitch DG, Senadheera DB. 2012. A novel function for the competence inducing peptide, XIP, as a cell death effector of Streptococcus mutans. FEMS Microbiol Lett 336:104–112. doi: 10.1111/j.1574-6968.2012.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer SR, Miller JH, Abranches J, Zeng L, Lefebure T, Richards VP, Lemos JA, Stanhope MJ, Burne RA. 2013. Phenotypic heterogeneity of genomically-diverse isolates of Streptococcus mutans. PLoS One 8:e61358. doi: 10.1371/journal.pone.0061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. 1993. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun 61:3811–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Culp DJ, Quivey RQ, Bowen WH, Fallon MA, Pearson SK, Faustoferri R. 2005. A mouse caries model and evaluation of aqp5−/− knockout mice. Caries Res 39:448–454. doi: 10.1159/000088179. [DOI] [PubMed] [Google Scholar]
- 61.Tanzer JM, Freedman ML, Fitzgerald RJ, Larson RH. 1974. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun 10:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abel S, Abel zur Wiesch P, Davis BM, Waldor MK. 2015. Analysis of bottlenecks in experimental models of infection. PLoS Pathog 11:e1004823. doi: 10.1371/journal.ppat.1004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujishima K, Kawada-Matsuo M, Oogai Y, Tokuda M, Torii M, Komatsuzawa H. 2013. dpr and sod in Streptococcus mutans are involved in coexistence with S. sanguinis, and PerR is associated with resistance to H2O2. Appl Environ Microbiol 79:1436–1443. doi: 10.1128/AEM.03306-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Durand A, Sinha AK, Dard-Dascot C, Michel B. 2016. Mutations affecting potassium import restore the viability of the Escherichia coli DNA polymerase III holD mutant. PLoS Genet 12:e1006114. doi: 10.1371/journal.pgen.1006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anders S, Pyl PT, Huber W. 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sambrook J, Russell D. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 68.Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods 49:193–205. doi: 10.1016/S0167-7012(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 69.Ahn S-J, Burne RA. 2006. The atlA operon of Streptococcus mutans: role in autolysin maturation and cell surface biogenesis. J Bacteriol 188:6877–6888. doi: 10.1128/JB.00536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lang JM, Darling AE, Eisen JA. 2013. Phylogeny of bacterial and archaeal genomes using conserved genes: supertrees and supermatrices. PLoS One 8:e62510. doi: 10.1371/journal.pone.0062510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reproducibility among S. mutans UA159 transposon libraries is high. The total number of reads per gene (reads normalized to 1 million) per library is plotted for starter libraries selected on blood agar (A), rich media (B), and defined media (C). The number of reads per gene was highly correlated across the two transposon libraries. CPM, counts per million. Download FIG S1, PDF file, 0.3 MB (320.6KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genes removed prior to Tn-seq data analysis. Download TABLE S1, PDF file, 0.04 MB (39KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Observed versus expected transposon insertion reads. Download TABLE S2, PDF file, 0.9 MB (996.6KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Conservation of essential genes. Download TABLE S3, PDF file, 0.2 MB (164.4KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Validation of sodA as a conditionally essential gene. (A) PCR ligation mutagenesis (see Materials and Methods) was employed to replace the sodA gene with a nonpolar kanamycin cassette on both BHI (brain heart infusion) and blood agar, with incubation at 37°C in anaerobic, aerobic, and microaerophilic environments. Kanamycin-resistant colonies were obtained only if transformations were kept strictly anaerobic, except for one colony isolated in the microaerophilic conditions (white arrow). (B) Isolated sodA mutants (Kmr), kept strictly anaerobic during routine culture, were competed against wild-type S. mutans in both anaerobic (BHI) and aerobic (BHI, with shaking at 150 rpm) environments. A competition index of >1 indicates that the mutant grew better than the wild type did. A competition index of <1 indicates that the mutant displayed reduced fitness compared to the wild-type strain. Download FIG S2, PDF file, 2.2 MB (2.2MB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of strains and plasmids used in this study. Download TABLE S4, PDF file, 0.05 MB (51.9KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotides used in this study. Download TABLE S5, PDF file, 0.1 MB (61.3KB, pdf) .
Copyright © 2018 Shields et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.