Abstract
Study Objectives
REM sleep behavior disorder (RBD) is a parasomnia affecting 33% to 46% of patients with Parkinson’s disease (PD). The existence of a unique and specific impaired cognitive profile in PD patients with RBD is still controversial. We extensively assessed cognitive functions to identify whether RBD is associated with more severe cognitive deficits in nondemented patients with PD.
Methods
One hundred sixty-two participants, including 53 PD patients with RBD, 40 PD patients without RBD, and 69 healthy subjects, underwent polysomnography, a neurological assessment and an extensive neuropsychological exam to assess attention, executive functions, episodic learning and memory, visuospatial abilities, and language.
Results
PD patients with RBD had poorer and clinically impaired performance in several cognitive tests compared to PD patients without RBD and healthy subjects. These two latter groups were similar on all cognitive measures. Mild cognitive impairment (MCI) diagnosis frequency was almost threefold higher in PD patients with RBD compared to PD patients without RBD (66% vs. 23%, p < .001). Moreover, subjective cognitive decline was reported in 89% of PD patients with RBD compared to 58% of PD patients without RBD (p = .024).
Conclusions
RBD in PD is associated with a more impaired cognitive profile and higher MCI diagnosis frequency, suggesting more severe and widespread neurodegeneration. This patient subgroup and their caregivers should receive targeted medical attention to better detect and monitor impairment and to enable the development of management interventions for cognitive decline and its consequences.
Keywords: Parkinson’s disease, REM sleep behavior disorder, Neuropsychology, Cognition, Mild cognitive impairment
Statement of Significance.
This study shows that REM sleep behavior disorder is a major risk factor for poorer cognitive performance and mild cognitive impairment in Parkinson’s disease (PD). Therefore, this subgroup of patients should be targeted in future clinical trials on the progression of cognitive decline in PD.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative condition characterized by motor and nonmotor symptoms. Cognitive decline and sleep dysfunction are among the most common of these symptoms, with major consequences for patients as well as relatives and caregivers.1 The main cognitive domains affected in PD are attention, executive functions, episodic learning and memory, and visuospatial abilities.2,3 Cross-sectional studies have reported a 30% prevalence of dementia in PD,2–5 while longitudinal studies have shown that 75% to 80% of PD patients may develop cognitive impairment within 15 to 20 years of disease onset.4,6 Clinical risk factors for dementia and cognitive decline that have been identified in PD include age, disease duration, mild cognitive impairment (MCI), subjective cognitive decline (SCD), REM sleep behavior disorder (RBD), and orthostatic hypotension.2,7,8
RBD is a parasomnia characterized by loss of REM sleep muscle atonia, resulting in undesirable motor activity during REM sleep as people “act out their dreams.” RBD prevalence in PD ranges from 33% to 46% when diagnosed with polysomnography (PSG).9,10 Although RBD is a risk factor for dementia in PD,7,11 there is a lack of consensus in the literature on the existence of a distinct cognitive profile in nondemented PD patients based on the presence of RBD.1 Indeed, some studies have associated the presence of RBD in PD with more severe cognitive impairment and higher MCI frequency,12–19 whereas others have not.20–26 However, most of these studies have methodological limitations that could explain the divergent results, including the use of screening tests with poor sensitivity to measure cognition,17,18,21–23,25 absence of PSG to diagnose RBD,12,14,16,18,19,21–23,25 small sample size,13–15,18,20,24 absence of MCI diagnosis,12,15–18,20–26 or absence of a healthy control group to better interpret the results.12,14,16–18,20–25
We previously reported neuropsychological findings in a small sample cohort of PD patients.13 In the present study, we used an extensive neuropsychological assessment to investigate cognition and PSG exams to confirm RBD in three PD cohorts (original, replication, and combined), and we included a healthy control group without cognitive impairment for comparison. We also investigated MCI diagnosis frequency in the combined PD cohort using the MCI diagnostic criteria proposed by the Movement Disorder Society Task Force.27 Moreover, we performed additional analyses to determine the frequency of SCD in PD-RBD compared to PD-nRBD and to explore the association between cognition, gender, and RBD onset in PD patients with RBD.
METHODS
Participants
One hundred eighty-six subjects participated in the study. PD patients were recruited at the Department of Neurology of the Montreal General Hospital and the Unité des troubles du mouvement André Barbeau of the Centre Hospitalier de l’Université de Montréal to participate in a study on sleep and cognition in PD. All PD patients but five (all idiopathic RBD patients seen in our sleep clinic who developed PD during the follow-up) were consecutive patients seen at their annual assessment in a movement disorder clinic and were referred by a neurologist (RBP, SC, or MP) for this study regardless of the patient’s sleep and cognitive complaints. Inclusion criteria for PD patients were: (1) a diagnosis of probable idiopathic PD confirmed by a neurologist specialized in movement disorders,28 (2) age from 40 to 80 years, and (3) at least 6 years of schooling (completed elementary school). Exclusion criteria were: (1) parkinsonism of other cause than PD; (2) presence of dementia according to the neuropsychological assessment and neurological exam; (3) a major psychiatric disorder (including major depression, schizophrenia, bipolar disorder) according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision;29 (4) a respiratory event index (apneas plus hypopneas) ≥ 20; (5) history of head injury, brain tumor, encephalitis, stroke, unstable hypertension, diabetes, or chronic obstructive pulmonary disease; and (6) abnormal electroencephalography (EEG) suggesting epilepsy. Dopaminergic medication was converted to levodopa dose equivalents. Details on medication are presented in Table 1. Control subjects without PD or cognitive impairment were recruited through a newspaper advertisement or by word of mouth and were subject to the same inclusion and exclusion criteria. The protocol was approved by a hospital–university ethics committee and participants gave their written informed consent to participate.
Table 1.
Demographic, Clinical, and Mood Characteristics: Combined PD Cohorts and Controls.
| Variables | Combined PD-RBD (A) | Combined PD-nRBD (B) | Controls (C) | p value; post hoc |
|---|---|---|---|---|
| (n = 53) | (n = 40) | (n = 69) | ||
| Sex men, n (%) | 40 (75) | 21 (53) | 38 (55) | .03; A > B*, A > C* |
| Age, years | 68.0 ± 8.4 | 63.2 ± 8.5 | 63.3 ± 10.3 | .01; A > B*, A > C** |
| Education, years | 14.5 ± 3.9 | 15.1 ± 3.0 | 14.5 ± 2.8 | ns |
| RBD duration, years | 4.1 ± 3.5 | — | — | — |
| PD duration since diagnosis, years | 6.1 ± 4.5 | 6.1 ± 4.3 | — | ns |
| Hoehn and Yahr stage | 2.5 ± 0.8 | 2.2 ± 0.9 | — | ns |
| UPDRS part III “on” | 23.1 ± 9.5 | 20.3 ± 9.8 | — | ns |
| REM tonic EMG, % | 61.3 ± 34.3 | 17.1 ± 23.9 | — | .000 |
| REM phasic EMG, % | 31.6 ± 20.9 | 14.3 ± 12.1 | — | .000 |
| Levodopa equivalent dosage, mg | 492.2 ± 402.3 | 383.7 ± 283.3 | — | ns |
| Levodopa use, n (%) | 42 (79) | 32 (80) | — | ns |
| Dopamine agonist use, n (%) | 25 (46) | 28 (78) | — | .004 |
| Antidepressant use, n (%) | 10 (19) | 7 (19) | — | ns |
| Antianxiolitic use, n (%) | 19 (35) | 7 (19) | — | ns |
| ISI scores | 11.6 ± 6.2 | 10.8 ± 7.8 | 6.6 ± 4.4 | .001; A > C***, B > C** |
| ESS scores | 9.3 ± 4.9 | 9.5 ± 4.9 | 6.9 ± 4.0 | .008; A > C*, B > C* |
| BDI-II scores | 10.9 ± 5.8 | 10.7 ± 7.5 | 5.9 ± 5.7 | .000; A > C***, B > C*** |
| BAI scores | 11.4 ± 8.7 | 9.6 ± 6.3 | 5.4 ± 5.4 | .001; A > C***, B > C* |
| MMSE scores | 28.0 ± 1.9 | 29.2 ± 0.9 | 29.3 ± 1.1 | .000; A < B***, A < C*** |
Results are expressed as mean ± standard deviation.
BAI = Beck Anxiety Inventory; BDI-II = Beck Depression Inventory second edition; EMG = electromyography; ESS = Epworth Sleepiness Scale; ISI = Insomnia Severity Index; MMSE = Mini-Mental State Examination; ns = not significant; PD = Parkinson’s disease; PD-RBD = PD with RBD; PD-nRBD = PD without RBD; RBD = REM sleep behavior disorder; UPDRS = Unified Parkinson’s Disease Rating Scale.
***p < .001; ** = p < .01; * = p < .05.
Procedure
All participants underwent one-night PSG recordings in the sleep laboratory. Sleep was recorded using a polygraph composed of two EEG electrodes: a central (C3/A2) and an occipital (O2/A1). A left and right electro-oculogram were used to measure eye movements and a submental electromyography (EMG) to measure muscle activity. Oral and nasal airflow and thoracic and abdominal movements were recorded, and oximetry was performed to exclude sleep apnea and hypopnea syndrome. Sleep stages were recorded according to a method developed for RBD patients and described in detail elsewhere.30 RBD was diagnosed by a sleep specialist (JM) according to the criteria of the International Classification of Sleep Disorders, Second Edition and PSG criteria.30,31 Percentages of REM tonic and phasic EMG activity were calculated using a previously published method.30
All patients underwent a detailed neurological examination (RBP), including administration of the Unified Parkinson’s Disease Rating Scale.32 The Beck Depressive Inventory, Second Edition,33 and Beck Anxiety Inventory34 were administrated to quantify depressive and anxiety symptom severity. The Epworth Sleepiness Scale (ESS)35 and Insomnia Severity Index36 were used to assess daytime sleepiness and insomnia symptom severity.
The neuropsychological assessment was divided into two 90-minute sessions and included measures of five cognitive domains: attention, executive functions, episodic verbal and nonverbal learning and memory, visuospatial abilities, and language (neuropsychological tests, variables, and normative data are presented in Supplementary Table 1). Test administration and scoring followed standard procedures.37 All patients took their usual medications prior to neuropsychological assessment. MCI diagnosis was established by a consensus between the neurologist and neuropsychologist according to the Movement Disorder Society Task Force criteria27 as: (1) a subjective cognitive complaint during the interview with the participant or spouse/caregiver, or a score > 25, or the response 3 (quite often) or 4 (very often) on at least one item on the Cognitive Failure Questionnaire (CFQ);38 (2) objective evidence of cognitive decline defined by performance at 1.5 standard deviations below the standardized mean on at least two variables in the same cognitive domain (Supplementary Table 1); and (3) the cognitive impairment does not significantly alter daily living activities and functioning. Impaired daily functioning was determined during the interview with patients and their relatives. Impairment was assessed in terms of decline in several activities, including managing finances, performing chores, preparing meals, shopping, driving, and using public transportation. MCI subtypes were defined as: amnestic MCI–single domain, nonamnestic MCI–single domain, amnestic MCI–multiple domain, or nonamnestic MCI–multiple domain. SCD was identified in PD patients without MCI by a subjective cognitive complaint during the interview with the participant or spouse/caregiver, or a score > 25, or the response 3 (quite often) or 4 (very often) on at least one item on the CFQ.38
Statistical Analysis
We first divided PD patients with and without RBD (PD-RBD and PD-nRBD) into the original cohort (previously published study),13 a replication cohort (new patients), and a combined cohort. Independent sample t-tests or nonparametric U Mann-Whitney tests (for not normally distributed variables) were applied to compare demographic, clinical, and mood variables between PD-RBD and PD-nRBD participants in the original, replication, and combined cohort, and univariate analyses of variance were applied to compare demographic, clinical, and mood variables between PD-RBD, PD-nRBD, and healthy controls. Subsequent univariate analyses of covariance with Bonferroni post hoc set at p < .05 were performed to assess cognitive performance between PD-RBD and PD-nRBD patients (original and replication cohorts) and between patients and controls in the combined cohort. Univariate analyses of covariance were also performed for additional analyses in the combined PD-RBD group to compare cognitive performance between gender (men vs. women), and RBD onset (prior vs. same time/after PD diagnosis), and in the combined cohorts to compare cognition between men only (PD-RBD vs. PD-nRBD vs. controls) and between women only (PD-RBD vs. PD-nRBD vs. controls). Certain cognitive variables were log transformed to ensure normality of distribution. Age and sex were included as covariates in the analysis of cognitive performance, for all three-cohort comparisons, given that they differed significantly between PD groups and were related to cognitive performance. Pearson’s or the nonparametric Spearman’s correlations were conducted between the percentages of REM sleep EMG activity (tonic or phasic) and cognitive variables in all PD patients. Pearson χ2 tests were used to compare proportions of men, medication users (levodopa, dopamine agonist, anxiolytic, and antidepressant), patients with clinically impaired cognitive performance (≥ 1.5 standard deviations below the standardized mean in PD-RBD vs. PD-nRBD), MCI diagnosis (PD-RBD vs. PD-nRBD), and SCD (PD-RBD without MCI vs. PD-nRBD without MCI). The association between RBD and each cognitive variable was defined as highly probable if all three cohorts (original, replication, and combined) showed a significant association with p < .05 for each cohort, probable if in two of three cohorts p < .05, possible if one cohort showed a significant association with p < .05, and probably not associated if all p values were p > .05. All analyses were computed using Statistical Package for the Social Sciences version 21 (Chicago, Illinois).
RESULTS
From the initial sample, 24 participants were excluded: 4 PD-RBD patients, 8 PD-nRBD patients, and 12 controls. Reasons for exclusion included sleep apnea (4 PD-RBD, 4 PD-nRBD, 3 controls), lower education (4 controls), atypical PD (4 PD-nRBD), and presence of MCI or dementia (5 controls). Of the remaining 162 participants, 53 were classified as PD-RBD, 40 were PD-nRBD, and 69 were healthy controls.
Demographic, Clinical, and Mood Characteristics
Results for the combined cohort are presented in Table 1, and results for the original and the replication cohorts are presented in the Supplementary Table 2. In both the original and combined cohort, the proportion of men was significantly higher in PD-RBD patients compared to PD-nRBD patients and controls. PD-RBD patients were also significantly older and had lower MMSE scores than PD-nRBD patients in the replication cohort, the combined cohort, and versus controls. In both the replication and combined cohorts, the proportion of PD-nRBD patients using a dopaminergic agonist was higher compared to PD-RBD patients. Of the three PD cohorts, PD-RBD patients had the highest REM tonic and phasic EMG activity. In the replication cohort, PD-RBD patients had a more advanced stage of PD. No significant between-group differences were found for education, PD duration (diagnosis), motor symptom severity, levodopa use and dosage, or antidepressant and anxiolytic use. No significant differences were found in the sleep and mood questionnaires between the two PD groups. However, both PD groups scored higher than controls on all questionnaires. No correlation was observed between questionnaire scores and cognitive test performance. The results were similar between the original and replication PD cohorts except for a younger age for the replication PD-nRBD cohort.
Neuropsychological Assessment
Cognitive Performance
Results for the three PD cohorts are presented in Table 2. PD-RBD patients performed worse than PD-nRBD in at least three cohorts (highly probable impaired) on tests assessing attention (Stroop Color-Word Test [III-II, time]), executive functions (Trail Making Test [part B, time and B–A, time]), and episodic verbal learning and memory (Rey Auditory-Verbal Learning Test [immediate recall]). PD-RBD patients performed worse than PD-nRBD in at least two cohorts (probable impaired) on tests assessing attention (Digit Span subtest [scaled score] and Stroop Color-Word Test [III-II, errors]), executive functions (Stroop Color-Word Test [IV-III, errors] and Verbal Fluency [semantic]), episodic verbal learning and memory (Rey Auditory-Verbal Learning Test [sum of trials 1 to 5]), and visuospatial abilities (Rey-O Complex Figure Test [copy] and Block Design subtest [scaled score]). PD-RBD patients also performed worse than PD-nRBD patients in only one cohort (possible impaired) on tests assessing executive functions (Verbal Fluency [letter]) and episodic verbal learning and memory (Rey Auditory-Verbal Learning Test [delayed recall]). No significant association was found between RBD and impaired cognitive performance for episodic nonverbal learning and memory and language. The results were similar between the original and replication PD cohorts except for poorer performance on visuospatial abilities for the original PD-RBD cohort.
Table 2.
Cognitive Performance: All PD Cohorts and Controls.
| Cognitive domains and tests | Original PD-RBD | Original PD-nRBD | p value | Replication PD-RBD | Replication PD-nRBD | p value | Combined PD-RBD (A) | Combined PD-nRBD (B) | Controls (C) | p value; post hoc |
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 21) | (n = 16) | (n = 32) | (n = 24) | (n = 53) | (n = 40) | (n = 69) | ||||
| Attention | ||||||||||
| Digit Span, scaled score | 9.7 ± 2.3 | 11.8 ± 2.4 | .01 | 9.6 ± 2.3 | 11.0 ± 3.0 | ns | 9.6 ± 2.3 | 11.3 ± 2.7 | 11.4 ± 3.2 | .003; A < B**, A < Cw** |
| Trail Making Test part A, time, sec | 57.3 ± 25.2 | 42.8 ± 16.8 | nsª | 54.8 ± 24.0 | 46.1 ± 20.0 | nsª | 55.8 ± 24.3 | 44.8 ± 18.6 | 37.4 ± 14.6 | .001ª; A > Cm*** |
| Stroop Color-Word Test | ||||||||||
| III-II time, sec | 74.8 ± 38.2 | 45.8 ± 14.2 | .03ª | 82.8 ± 38.7 | 49.5 ± 19.4 | .003ª | 79.5 ± 38.2 | 48.0 ± 17.4 | 47.4 ± 38.7 | .000ª; A > Bm,w***, A > Cm*** |
| III-II errors | 3.4 ± 4.2 | 0.8 ± 2.3 | .04ª | 3.0 ± 5.1 | 1.1 ± 2.9 | nsª | 3.2 ± 4.7 | 1.0 ± 2.7 | 0.6 ± 2.1 | .007ª; A > B*, A > Cm** |
| Executive functions | ||||||||||
| Trail Making Test part B, time, sec | 157.6 ± 92.0 | 97.0 ± 59.3 | .01ª | 170.4 ± 89.2 | 99.3 ± 87.9 | .04ª | 165.3 ± 89.6 | 98.4 ± 76.8 | 95.9 ± 43.8 | .000ª; A > Bm,w***, A > Cm*** |
| Part B–part A, time, sec | 100.3 ± 79.0 | 54.2 ± 49.0 | .02ª | 115.4 ± 72.4 | 53.2 ± 77.9 | .001ª | 109.4 ± 74.7 | 53.6 ± 67.1 | 58.5 ± 37.8 | .000ª; A > Bm,w***, A > Cm** |
| Stroop Color-Word Test | ||||||||||
| IV-III time, sec | 31.2 ± 10.9 | 22.3 ± 16.7 | ns | 31.9 ± 33.7 | 22.1 ± 23.3 | ns | 31.8 ± 29.3 | 22.2 ± 21.7 | 14.2 ± 36.8 | nsª |
| IV-III, errors | 4.0 ± 4.3 | 2.9 ± 4.8 | nsª | 6.2 ± 5.9 | 2.1 ± 4.0 | .03ª | 5.7 ± 5.6 | 2.3 ± 4.2 | 1.9 ± 3.7 | .003ª; A > Bw*, A > Cw** |
| Verbal fluency | ||||||||||
| Semantic | 26.7 ± 6.8 | 35.3 ± 6.9 | .000 | 28.8 ± 7.8 | 36.9 ± 10.1 | ns | 27.9 ± 7.4 | 36.3 ± 8.9 | 36.5 ± 6.8 | .000; A < Bm***, A < Cm,w*** |
| Letter | 32.0 ± 12.4 | 38.3 ± 9.4 | ns | 30.9 ± 11.0 | 39.4 ± 10.7 | ns | 31.3 ± 11.5 | 38.9 ± 10.0 | 37.2 ± 11.1 | .008; A < B*, A < C* |
| Episodic learning and memory | ||||||||||
| Verbal | ||||||||||
| RAVLT | ||||||||||
| Sum of trials 1 to 5 | 38.0 ± 8.6 | 45.5 ± 5.9 | .04 | 37.3 ± 12.5 | 47.3 ± 12.8 | ns | 37.5 ± 11.1 | 46.6 ± 10.5 | 49.3 ± 8.6 | .000; A < Bm**, A < Cm*** |
| List B | 4.8 ± 1.5 | 5.1 ± 1.4 | ns | 3.8 ± 1.9 | 5.1 ± 2.0 | ns | 4.2 ± 1.8 | 5.1 ± 1.8 | 5.6 ± 1.9 | .002; A < Cw** |
| Immediate recall | 6.4 ± 3.0 | 9.1 ± 2.6 | .03 | 7.0 ± 3.5 | 10.3 ± 4.1 | .04 | 6.8 ± 3.3 | 9.8 ± 3.6 | 10.4 ± 2.7 | .000; A < Bm**, A < Cm,w*** |
| Delayed recall | 7.1 ± 3.1 | 9.8 ± 2.1 | ns | 7.4 ± 3.5 | 10.1 ± 4.0 | ns | 7.3 ± 3.3 | 10.0 ± 3.4 | 10.0 ± 3.1 | .008; A < B*, A < C** |
| Recognition | 12.8 ± 2.6 | 13.9 ± 1.2 | ns | 12.7 ± 2.3 | 14.0 ± 1.3 | ns | 12.7 ± 2.4 | 13.9 ± 1.3 | 14.1 ± 1.2 | .009; A < Cm** |
| Nonverbal | ||||||||||
| Rey-O Complex Figure Test | ||||||||||
| Immediate recall | 11.6 ± 6.0 | 14.3 ± 6.1 | nsª | 14.2 ± 6.9 | 17.4 ± 7.1 | nsª | 13.2 ± 6.6 | 16.1 ± 6.8 | 15.3 ± 6.1 | ns |
| Delayed recall | 11.4 ± 5.2 | 12.9 ± 6.4 | nsª | 12.8 ± 7.0 | 16.4 ± 7.9 | nsª | 12.2 ± 6.3 | 15.0 ± 7.4 | 15.5 ± 6.0 | ns |
| Visuospatial | ||||||||||
| Rey-O Complex Figure Test, copy | 26.5 ± 7.1 | 30.1 ± 3.4 | .04 | 28.2 ± 5.0 | 31.0 ± 4.0 | ns | 27.5 ± 5.9 | 30.6 ± 3.7 | 30.3 ± 4.0 | .02; A < B* |
| Block Design, scaled score | 8.1 ± 1.9 | 12.0 ± 2.4 | .000 | 9.5 ± 3.2 | 10.2 ± 3.8 | ns | 8.9 ± 2.8 | 10.9 ± 3.4 | 11.5 ± 3.2 | .000; A < B*, A < Cm*** |
| Bells test, omissions | 4.1 ± 2.8 | 3.2 ± 4.7 | nsª | 2.8 ± 2.9 | 2.7 ± 3.7 | nsª | 3.4 ± 2.9 | 2.9 ± 4.1 | 2.2 ± 2.8 | nsª |
| Language | ||||||||||
| Boston Naming Test | — | — | — | 28.7 ± 1.8 | 29.4 ± 0.8 | ns | 28.7 ± 1.8 | 29.4 ± 0.8 | — | ns |
| Vocabulary, scaled score | — | — | — | 10.2 ± 2.0 | 11.6 ± 2.4 | ns | 10.2 ± 2.0 | 11.6 ± 2.4 | — | ns |
| MMSE language score | 7.8 ± 0.5 | 7.9 ± 0.5 | ns | 7.7 ± 0.7 | 8.0 ± 0.2 | ns | 7.7 ± 0.6 | 7.9 ± 0.4 | 8.0 ± 0.1 | .01; A < C* |
Results are expressed as mean ± standard deviation. ªlog transformations.
Also statistically significant when comparing Menm and Womenw only in the combined cohorts.
MMSE = Mini-Mental State Examination; ns = not significant; PD = Parkinson’s disease; PD-RBD = PD with REM sleep behavior disorder; PD-nRBD = PD without RBD; RAVLT = Rey Auditory-Verbal Learning Test.
***p < .001; ** = p < .01; * = p < .05.
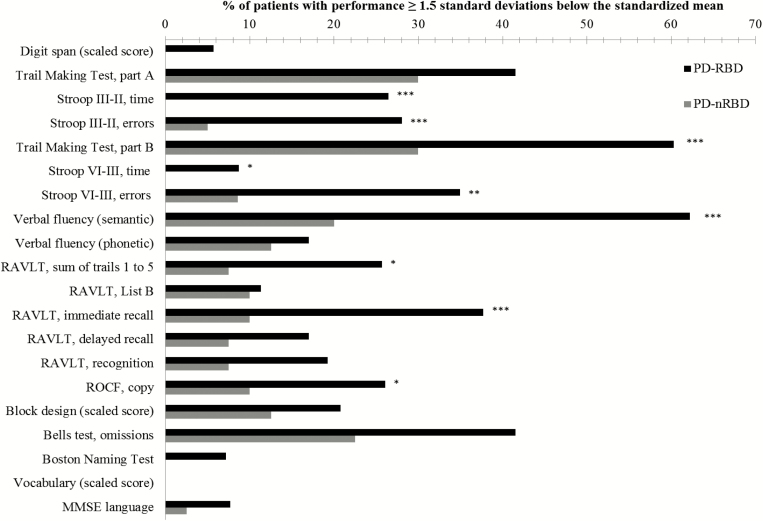
Additional analyses on the PD-RBD group revealed that men performed worse than women on the Rey Auditory-Verbal Learning Test (sum of trials 1 to 5, delayed recall, and recognition) and Bells test (Supplementary Table 3). Moreover, PD-RBD patients with RBD onset prior to PD diagnosis performed worse than PD-RBD patients with RBD onset at the same time/after PD diagnosis on the Verbal Fluency (letter) and Boston Naming Test (Supplementary Table 4). In addition, a higher proportion of RBD-PD patients had clinically impaired performance compared to PD-nRBD patients on the following cognitive tests: the Stroop Color-Word Test (III–II, time and errors; IV–III, time and errors), Trail Making Test (part B, time), Verbal Fluency (semantic), Rey Auditory-Verbal Learning Test (sum of trials 1 to 5, immediate recall), and Rey–O Complex Figure Test (copy) (Figure 1).
Figure 1.
Percentage of patients with clinically impaired performance on neuropsychological tests. ***p < .001; **p < .01; *p < .05. MMSE = Mini-Mental State Examination; PD-RBD = Parkinson’s disease with REM sleep behavior disorder; PD-nRBD = PD without RBD; RAVLT = Rey Auditory-Verbal Learning Test; ROCF = Rey-O Complex Figure; Stroop components: II = Naming, III = Interference, IV = Flexibility.
Comparing combined PD cohort to healthy subjects (Table 2), PD-RBD patients performed worse than controls on tests assessing attention (all tests), executive functions (Trail Making Test [part B, time], Stroop Color-Word Test [IV-III, errors], and Verbal Fluency [semantic and letter]), episodic verbal learning and memory (Rey Auditory-Verbal Learning Test [all variables]), visuospatial abilities (Block Design subtest [scaled score]), and language (Mini-Mental State Examination [language score]). No significant differences were found between PD-nRBD patients and controls for all cognitive tests. Overall, most of the previous results remained significant when comparing men and women only but with slightly lower effects when comparing women, probably due to the smaller sample size (Table 2).
Correlations were performed for PD patients as one group between the percentage of REM sleep EMG activity and the cognitive variables (Table 3). Higher tonic EMG activity was associated with poorer performance on the Stroop Color-Word Test (III-II, time), Trail Making Test (part B, time and B–A, time), Rey Auditory-Verbal Learning Test (sum of trials 1 to 5), and Block Design subtest (scaled score). On the other hand, higher phasic EMG activity was related to poorer performance on the Stroop Color-Word Test (III-II, time), Trail Making Test (part B, time), Verbal Fluency (semantic), Rey Auditory-Verbal Learning Test (List B), and Block Design subtest (scaled score).
Table 3.
Correlations Between Cognitive Measures and REM Sleep EMG Features in all PD Patients.
| Neuropsychological tests | EMG tonic | EMG phasic |
|---|---|---|
| Attention | ||
| Digit Span, scaled score | 0.027 | −0.138 |
| Trail Making Test part A, time, secª | 0.169 | 0.213 |
| Stroop Color-Word Test | ||
| III-II, time, secª | 0.366*** | 0.288** |
| III-II, errorsª | 0.176 | 0.113 |
| Executive functions | ||
| Trail Making Test part B, time, secª | 0.277** | 0.222* |
| Part B–part A, time, secª | 0.286** | 0.205 |
| Stroop Color-Word Test | ||
| IV-III time, secª | 0.098 | −0.108 |
| IV-III, errorsª | 0.156 | 0.134 |
| Verbal fluency | ||
| Semantic | −0.217 | −0.279* |
| Letter | −0.181 | −0.115 |
| Episodic learning and memory | ||
| Verbal | ||
| RAVLT | ||
| Sum of trials 1 to 5 | −0.226* | −0.183 |
| List B | −0.107 | −0.272* |
| Immediate recall | −0.174 | −0.209 |
| Delayed recall | −0.161 | −0.184 |
| Recognition | −0.118 | 0.028 |
| Nonverbal | ||
| Rey-O Complex Figure Test | ||
| Immediate recall | −0.167 | −0.197 |
| Delayed recall | −0.130 | −0.184 |
| Visuospatial | ||
| Rey-O Complex Figure Test, copy | −0.194 | −0.163 |
| Block Design, scaled score | −0.324** | −0.251* |
| Bells test, omissionsª | 0.120 | 0.029 |
| Language | ||
| Boston Naming Test | −0.123 | −0.037 |
| Vocabulary, scaled score | 0.054 | −0.115 |
| MMSE language score | −0.096 | −0.087 |
ªSpearman correlations.
EMG = electromyography; MMSE = Mini-Mental State Examination; PD = Parkinson’s disease; RAVLT = Rey Auditory-Verbal Learning Test.
***p < .001; ** = p < .01; * = p < .05.
Mild Cognitive Impairment and Subjective Cognitive Decline
MCI diagnosis frequency and subtypes are presented in Table 4. MCI diagnosis was more frequent in PD-RBD patients (66%) compared to PD-nRBD patients (23%) (p < .001). Of the PD-RBD patients, 24% had MCI–single domain (9% amnestic MCI, 15% nonamnestic MCI), and 42% had MCI–multiple domain (23% amnestic MCI, 19% nonamnestic MCI). Of the PD-nRBD patients, 8% had the MCI–single domain subtype (3% amnestic MCI, 5% nonamnestic MCI) and 15% had MCI–multiple domain (7.5% amnestic MCI, 7.5% nonamnestic MCI). Three (33%) of the 9 PD-nRBD patients with MCI diagnosis had excessive REM sleep muscle tone without history or presence of movements during REM sleep during PSG (prodromal RBD). Moreover, SCD frequency was higher in PD-RBD patients without MCI (16 out of 18, 89%) compared to PD-nRBD patients without MCI (18 out of 31, 58%) (p = .024).
Table 4.
Mild Cognitive Impairment Diagnosis Frequency in PD Patients.
| MCI subtypes | Combined PD-RBD | Combined PD-nRBD |
|---|---|---|
| n = 53 | n = 40 | |
| MCI total, n | 35 | 9 |
| MCI–single domain, n | 13 | 3 |
| Amnesic MCI–single domain, n | 5 | 1 |
| Nonamnesic MCI–single domain, n | 8 | 2 |
| Executive, n | 8 | 2 |
| MCI–multiple domain, n | 22 | 6 |
| Nonamnesic MCI–multiple domain, n | 10 | 3 |
| Attention and executive, n | 3 | 1 |
| Attention and visuospatial, n | 0 | 0 |
| Executive and visuospatial, n | 2 | 2 |
| Attention, executive and visuospatial, n | 4 | 0 |
| Attention, executive and language, n | 1 | 0 |
| Amnesic MCI–multiple domain, n | 12 | 3 |
| + Attention, n | 1 | 0 |
| + Executive, n | 3 | 0 |
| + Attention and executive, n | 4 | 0 |
| + Attention and visuospatial, n | 0 | 1 |
| + Executive and visuospatial, n | 4 | 2 |
MCI = Mild cognitive impairment; PD-RBD = Parkinson’s disease with REM sleep behavior disorder; PD-nRBD = PD without RBD.
DISCUSSION
There is a lack of consensus in the literature on a cross-sectional association between RBD and poorer cognitive performance in nondemented individuals with PD.1 This is mainly due to methodological differences and limits between the studies. In the present study, we compared performance on a broad range of cognitive tests in two different cohorts of nondemented PD patients with and without RBD confirmed by PSG. We found highly probable or probable associations between the presence of RBD in PD and poorer performance on cognitive tests measuring attention, executive functions, episodic verbal learning and memory, and visuospatial abilities. Preliminary results in the PD-RBD group also show that men and patients with RBD onset prior to PD diagnosis are at higher risk of poorer cognitive performance. In the analyses using the combined PD cohort, we included a healthy control group to better interpret the results. We found that, in addition to the above-mentioned cognitive deficits, compared to controls, PD-RBD patients had poorer performance on cognitive test measuring delayed recall and recognition of verbal information, and language. PD patients without RBD had similar cognitive performance to controls on all cognitive tests. Two PSG manifestations of RBD, that is, excessive tonic and phasic EMG activity during REM sleep, were associated with poorer performance on several cognitive tests in PD. Moreover, using the proposed criteria for MCI diagnosis in PD,27 we found almost threefold higher frequency of MCI diagnosis in PD-RBD patients compared to PD patients without RBD. Taken together, these results indicate that RBD is strongly associated with cognitive impairment in PD.
Our results are consistent with other longitudinal studies suggesting that the presence of RBD in individuals with PD is an important clinical risk factor for the development of dementia.7,11,39,40 Another clinical risk factor for cognitive decline and dementia in the general population is SCD, which is characterized by a self-perception of a decline in cognitive performance in daily life in the absence of objective cognitive impairment measured by neuropsychological assessment.41 In PD, the presence of SCD has been poorly studied. One study reported that SCD in PD with normal cognition predicts future cognitive decline.8 We found a higher frequency of SCD in PD-RBD patients. Although this result should be validated in a larger cohort and its predictive value determined in a longitudinal design, our results strengthen the link between RBD and the risk of cognitive decline in PD, even in patients with normal cognition. Consequently, PD patients with cognitive impairment should be carefully screened clinically for the presence of RBD. This subgroup of patients should also be targeted in future clinical trials on the progression of cognitive decline in PD.
Cognitive impairment, determined by poorer cognitive performance or by the presence of MCI or dementia, is a well-known nonmotor feature in PD.2,27 Two distinct patterns of cognitive deficits have been identified in PD.27,42 The first pattern is more related to posterior cortically based cognitive deficits, probably reflecting nondopaminergic cortical dysfunction associated with explicit memory and visuospatial deficits. The second pattern is related more to frontostriatal cognitive deficits, reflecting dopaminergic dysfunctions associated with attention and executive deficits. It has been suggested that the dementia incidence in patients with PD was associated with posterior cortical deficits, whereas frontostriatal deficits were not.27,42 Nevertheless, patients with PD are commonly characterized by a range of heterogeneous cognitive impairments, even in early stage, suggesting that 15% of patients with PD can present with both patterns of cognitive deficits.42 In the present study, PD-RBD patients had poorer performance on cognitive tasks measuring working memory, visual search, mental flexibility, processing speed, cognitive inhibition, word retrieval and delayed recall of verbal information, and visuospatial organization compared to PD patients without RBD. Their cognitive profile reflects both frontostriatal and posterior cortical deficits.
The mechanisms underlying the association between cognitive impairment and RBD in PD remain to be determined. RBD has been associated with lesions of the brainstem regions involved in muscle atonia and motor control during REM sleep.43 However, much evidence now suggests that RBD is more than a simple sleep disorder, and that patients having RBD are at high risk for developing dementia.44 Moreover, cortical dysfunctions such as cortical hypometabolism, EEG slowing, and cortical thickness have also been identified in RBD patients without PD.45–47 Individuals with concomitant PD and RBD have more specific brain anatomical and functional changes compared to PD patients without RBD. In deed, several studies using quantitative EEG, event-related potentials, magnetic resonance imaging (voxel-based morphometry), and positron emission tomography ([11C] methylpiperidyl propionate acetylcholinesterase) have reported brain dysfunctions associated with the presence of RBD in PD.48–52 Ford et al.50 reported subtle changes in white matter integrity (widespread) and reductions in gray matter volume (posterior areas) in PD with clinical RBD. Recently, a study also found smaller volumes in the pontomesencephalic tegmentum, medullary reticular formation, hypothalamus, thalamus, putamen, amygdala, and anterior cingulate cortex in PD patient with clinical RBD.51 In a functional neuroimaging study, Kotagal et al.52 showed that the presence of clinical RBD in PD could be accounted for by progressive neocortical, limbic, cortical, and thalamic cholinergic denervation. Dysfunctions of cholinergic systems, namely the nucleus basalis of Meynert and pedunculopontine nucleus, and their projections to subcortical and cortical regions have been related to cognitive impairment in PD.53,54
Other studies have identified a distinct clinical subtype in PD related to the presence of RBD, with higher risk for dysautonomia, hallucinations, freezing and falls, symmetric disease, and a nontremor dominant subtype.55–57 Some of these nonmotor features have also been related to cholinergic impairment in PD.54 This suggests a common cholinergic mechanism between RBD, cognitive decline, and other nonmotor features in this population.
Interestingly, some of these previous studies reported that PD patients without RBD were similar to healthy controls except for motor impairment.48,55,56 This is similar to our results showing normal cognitive functioning in PD patients without RBD, suggesting that the presence of RBD in individuals with PD is associated with a more severe subtype of the disease, with a strong negative prognostic.40 Therefore, PD-RBD patients should be considered as a distinct PD subgroup, not only for neuroprotective trials or for examinations of the underlying pathophysiological mechanisms but also for the development of animal models of PD. It remains to determine whether RBD onset in the course of PD is the starting point for more rapid and pronounced neurodegeneration. Follow-up of these patients will be important to detect the emergence of cognitive decline, dysautonomia, hallucinations, and gait and balance difficulties and to provide medical attention and management to the patients and their caregivers.
This study includes certain limitations. First, the PD-RBD group was older and contained a higher proportion of men compared to the PD-nRBD and control groups. Although we controlled for age and gender and performed additional analyses with gender as the independent variable, we cannot discount potential age or gender effects on some of our results. Moreover, the proportion of patients taking dopaminergic agonists was also higher in the PD-nRBD compared to the PD-RBD group. The chronic cognitive effects of dopaminergic agonists in PD remain controversial,58 and further studies are needed to better understand their impacts on PD in terms of cognition and RBD status. Second, there is neither a systematic definition of SCD nor a standardized method to measure SCD in PD populations. Our results should therefore be confirmed in larger PD populations using appropriate SCD assessment methods. Finally, although cognitive performance was similar between PD-nRBD and healthy subjects, future studies may find differences in larger-sized samples, or differences in cognitive functions that were not deeply examined in the present study (eg, language, planning, working memory, and procedural learning).
In conclusion, RBD in PD is associated with higher risks for cognitive deficits. In addition, the presence of RBD in PD increases the risk of a MCI diagnosis and SCD, which are associated with cognitive decline and dementia development in PD. Future studies attempting to identify a distinct cognitive profile in PD with RBD should use a greater variety of tests to more deeply assess all the language components (ie, naming, reading, writing, understanding, and pragmatism) and higher executive functions (ie, planning, problem solving) as well as procedural learning.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
ETHICS COMMITTEE APPROVAL
All procedures were performed at the Centre for Advanced Research in Sleep Medicine at the Hôpital du Sacré-Coeur de Montréal (Quebec, Canada). The hospital’s ethical committee approved the study, and all subjects signed a written consent before participating.
FUNDING
RB Postuma received personal compensation for travel and speaker fees from Biotie, Biospective, Boehringer, Roche, Novartis Canada and Teva Neurosciences, and is funded by grants from the Canadian Institutes of Health Research (CIHR), Parkinson Society of Canada, W. Garfield Weston Foundation, Webster Foundation, and Fonds de Recherche du Québec - Santé (FRQ-S). J Montplaisir receives research support from GlaxoSmithKline, Merck Pharma, and the CIHR, Parkinson Society of Canada, and W. Garfield Weston Foundation. He holds a Canada Research Chair in Sleep Medicine. M. Panisset receives research support from the NIH, W. Garfield Weston Foundation, and Medtronic. He serves on advisory boards for Allergan and Merz. S Chouinard receives research support from Abbvie. JF Gagnon receives research support from the CIHR, W. Garfield Weston Foundation, Parkinson Society of Canada, and FRQ-S. He holds a Canada Research Chair in Cognitive Decline in Pathological Aging. N Jozwiak, V Latreille, and PA Bourgouin have nothing to disclose.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Canadian Institutes of Health Research and Fonds de Recherche du Québec—Santé.
REFERENCES
- 1. Gagnon JF, Postuma RB, Lyonnais-Lafond G. Cognition and the sleep–wake cycle in Parkinson’s disease. In: Videnovic A, & Högl B, eds. Disorders of Sleep and Circadian Rhythms in Parkinson’s Disease.Vienna, Austria: Springer-Verlag; 2015: 183–194. [Google Scholar]
- 2. Dubois B, Burn D, Goetz C et al. . Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007; 22(16): 2314–2324. [DOI] [PubMed] [Google Scholar]
- 3. Aarsland D, Brønnick K, Fladby T. Mild cognitive impairment in Parkinson’s disease. Curr Neurol Neurosci Rep. 2011; 11(4): 371–378. [DOI] [PubMed] [Google Scholar]
- 4. Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol. 2010; 20(3): 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riedel O, Klotsche J, Spottke A et al. . Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson’s disease. J Neurol. 2010; 257(7): 1073–1082. [DOI] [PubMed] [Google Scholar]
- 6. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008; 23(6): 837–844. [DOI] [PubMed] [Google Scholar]
- 7. Anang JB, Gagnon JF, Bertrand JA et al. . Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014; 83(14): 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong JY, Sunwoo MK, Chung SJ et al. . Subjective cognitive decline predicts future deterioration in cognitively normal patients with Parkinson’s disease. Neurobiol Aging. 2014; 35(7): 1739–1743. [DOI] [PubMed] [Google Scholar]
- 9. Sixel-Döring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. 2011; 77(11): 1048–1054. [DOI] [PubMed] [Google Scholar]
- 10. Gagnon JF, Bédard MA, Fantini ML et al. . REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology. 2002; 59(4): 585–589. [DOI] [PubMed] [Google Scholar]
- 11. Nomura T, Inoue Y, Kagimura T, Nakashima K. Clinical significance of REM sleep behavior disorder in Parkinson’s disease. Sleep Med. 2013; 14(2): 131–135. [DOI] [PubMed] [Google Scholar]
- 12. Sinforiani E, Zangaglia R, Manni R et al. . REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson’s disease. Mov Disord. 2006; 21(4): 462–466. [DOI] [PubMed] [Google Scholar]
- 13. Gagnon JF, Vendette M, Postuma RB et al. . Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Ann Neurol. 2009; 66(1): 39–47. [DOI] [PubMed] [Google Scholar]
- 14. Erro R, Santangelo G, Picillo M et al. . Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. J Neurol. 2012; 259(9): 1808–1813. [DOI] [PubMed] [Google Scholar]
- 15. Marques A, Dujardin K, Boucart M et al. . REM sleep behaviour disorder and visuoperceptive dysfunction: a disorder of the ventral visual stream? J Neurol. 2010; 257(3): 383–391. [DOI] [PubMed] [Google Scholar]
- 16. Chahine LM, Xie SX, Simuni T et al. . Longitudinal changes in cognition in early Parkinson’s disease patients with REM sleep behavior disorder. Parkinsonism Relat Disord. 2016; 27: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gong Y, Xiong KP, Mao CJ et al. . Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behavior disorder. Sleep Med. 2014; 15(6): 647–653. [DOI] [PubMed] [Google Scholar]
- 18. Wang G, Wan Y, Wang Y et al. . Visual hallucinations and associated factors in Chinese patients with Parkinson’s disease: roles of RBD and visual pathway deficit. Parkinsonism Relat Disord. 2010; 16(10): 695–696. [DOI] [PubMed] [Google Scholar]
- 19. Zhang JR, Chen J, Yang ZJ et al. . Rapid eye movement sleep behavior disorder symptoms correlate with domains of cognitive impairment in parkinson’s disease. Chin Med J (Engl). 2016; 129(4): 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plomhause L, Dujardin K, Duhamel A et al. . Rapid eye movement sleep behavior disorder in treatment-naïve Parkinson disease patients. Sleep Med. 2013; 14(10): 1035–1037. [DOI] [PubMed] [Google Scholar]
- 21. Bugalho P, da Silva JA, Neto B. Clinical features associated with REM sleep behavior disorder symptoms in the early stages of Parkinson’s disease. J Neurol. 2011; 258(1): 50–55. [DOI] [PubMed] [Google Scholar]
- 22. Lavault S, Leu-Semenescu S, Tezenas du Montcel S, Cochen de Cock V, Vidailhet M, Arnulf I. Does clinical rapid eye movement behavior disorder predict worse outcomes in Parkinson’s disease? J Neurol. 2010; 257(7): 1154–1159. [DOI] [PubMed] [Google Scholar]
- 23. Yoritaka A, Ohizumi H, Tanaka S, Hattori N. Parkinson’s disease with and without REM sleep behaviour disorder: are there any clinical differences? Eur Neurol. 2009; 61(3): 164–170. [DOI] [PubMed] [Google Scholar]
- 24. Benninger D, Waldvogel D, Bassetti CL. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology. 2008; 71(12): 955–956. [DOI] [PubMed] [Google Scholar]
- 25. Lee JE, Kim KS, Shin HW, Sohn YH. Factors related to clinically probable REM sleep behavior disorder in Parkinson disease. Parkinsonism Relat Disord. 2010; 16(2): 105–108. [DOI] [PubMed] [Google Scholar]
- 26. Sixel-Döring F, Trautmann E, Mollenhauer B, Trenkwalder C. Rapid eye movement sleep behavioral events: a new marker for neurodegeneration in early Parkinson disease? Sleep. 2014; 37(3): 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Litvan I, Goldman JG, Tröster AI et al. . Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012; 27(3): 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. National Collaborating Centre for Chronic Conditions. Parkinson’s Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care.London: Royal College of Physicians of London; 2006: 22–47. [PubMed] [Google Scholar]
- 29. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.4th ed Text revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 30. Montplaisir J, Gagnon JF, Fantini ML et al. . Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010; 25(13): 2044–2051. [DOI] [PubMed] [Google Scholar]
- 31. American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and Coding Manual.2nd ed Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 32. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003; 18(7): 738–750. [DOI] [PubMed] [Google Scholar]
- 33. Beck AT, Steer RA, Brown GK.. The Beck Depression Inventory.2nd ed San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 34. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988; 56(6): 893–897. [DOI] [PubMed] [Google Scholar]
- 35. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991; 14(6): 540–545. [DOI] [PubMed] [Google Scholar]
- 36. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011; 34(5): 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strauss E, Sherman EM, Spreen O.. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary.3rd ed New York: Oxford University Press; 2006. [Google Scholar]
- 38. Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982; 21(1): 1–16. [DOI] [PubMed] [Google Scholar]
- 39. Anang JB, Nomura T, Romenets SR, Nakashima K, Gagnon JF, Postuma RB. Dementia predictors in Parkinson disease: a validation study. J Parkinsons Dis. 2017; 7(1): 159–162. [DOI] [PubMed] [Google Scholar]
- 40. Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 2015; 72(8): 863–873. [DOI] [PubMed] [Google Scholar]
- 41. Molinuevo JL, Rabin LA, Amariglio R et al. ; Subjective Cognitive Decline Initiative (SCD-I) Working Group. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017; 13(3): 296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams-Gray CH, Evans JR, Goris A et al. . The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009; 132(11): 2958–2969. [DOI] [PubMed] [Google Scholar]
- 43. Jennum P, Christensen JA, Zoetmulder M. Neurophysiological basis of rapid eye movement sleep behavior disorder: informing future drug development. Nat Sci Sleep. 2016; 8: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iranzo A, Fernández-Arcos A, Tolosa E et al. . Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014; 9(2): e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vendette M, Montplaisir J, Gosselin N et al. . Brain perfusion anomalies in rapid eye movement sleep behavior disorder with mild cognitive impairment. Mov Disord. 2012; 27(10): 1255–1261. [DOI] [PubMed] [Google Scholar]
- 46. Rahayel S, Montplaisir J, Monchi O et al. . Patterns of cortical thinning in idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2015; 30(5): 680–687. [DOI] [PubMed] [Google Scholar]
- 47. Rodrigues Brazète J, Montplaisir J, Petit D et al. . Electroencephalogram slowing in rapid eye movement sleep behavior disorder is associated with mild cognitive impairment. Sleep Med. 2013; 14(11): 1059–1063. [DOI] [PubMed] [Google Scholar]
- 48. Gagnon JF, Fantini ML, Bédard MA et al. . Association between waking EEG slowing and REM sleep behavior disorder in PD without dementia. Neurology. 2004; 62(3): 401–406. [DOI] [PubMed] [Google Scholar]
- 49. Gaudreault PO, Gagnon JF, Montplaisir J et al. . Abnormal occipital event-related potentials in Parkinson’s disease with concomitant REM sleep behavior disorder. Parkinsonism Relat Disord. 2013; 19(2): 212–217. [DOI] [PubMed] [Google Scholar]
- 50. Ford AH, Duncan GW, Firbank MJ et al. . Rapid eye movement sleep behavior disorder in Parkinson’s disease: magnetic resonance imaging study. Mov Disord. 2013; 28(6): 832–836. [DOI] [PubMed] [Google Scholar]
- 51. Boucetta S, Salimi A, Dadar M, Jones BE, Collins DL, Dang-Vu TT. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson’s disease. Sci Rep. 2016; 6: 26782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kotagal V, Albin RL, Müller ML et al. . Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. 2012; 71(4): 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Müller ML, Bohnen NI. Cholinergic dysfunction in Parkinson’s disease. Curr Neurol Neurosci Rep. 2013; 13(9): 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord. 2011; 26(14): 2496–2503. [DOI] [PubMed] [Google Scholar]
- 55. Postuma RB, Montplaisir J, Lanfranchi P et al. . Cardiac autonomic denervation in Parkinson’s disease is linked to REM sleep behavior disorder. Mov Disord. 2011; 26(8): 1529–1533. [DOI] [PubMed] [Google Scholar]
- 56. Romenets SR, Gagnon JF, Latreille V et al. . Rapid eye movement sleep behavior disorder and subtypes of Parkinson’s disease. Mov Disord. 2012; 27(8): 996–1003. [DOI] [PubMed] [Google Scholar]
- 57. Videnovic A, Marlin C, Alibiglou L, Planetta PJ, Vaillancourt DE, Mackinnon CD. Increased REM sleep without atonia in Parkinson disease with freezing of gait. Neurology. 2013; 81(12): 1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Poletti M, Bonuccelli U. Acute and chronic cognitive effects of levodopa and dopamine agonists on patients with Parkinson’s disease: a review. Ther Adv Psychopharmacol. 2013; 3(2): 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.