Abstract
Study objectives:
To investigate the temporal evolution of sleep EEG changes in adolescents across two cycles of sleep restriction and recovery simulating an intense school week and to examine the effect of an afternoon nap on nocturnal sleep.
Methods:
A parallel-group design, quasi-laboratory study was conducted in a student hostel. Fifty-seven adolescents (31 males, age = 15–19 years) were randomly assigned to nap or no nap groups. Participants underwent a 15-day protocol comprising two sleep restriction (5-hour time-in-bed [TIB]) and recovery (9-hour TIB) cycles. The nap group was also provided with a 1-hour nap opportunity at 14:00 following each sleep restriction night. Polysomnography recordings were obtained on nine nights and five nap episodes.
Results:
Naps reduced homeostatic sleep pressure on sleep restriction nights as evidenced by longer N2 latency and reduced total sleep time (TST), sleep efficiency (SE), and slow wave energy. Sleep debt accumulated in both groups, evidenced by increased TST, greater SE, and reduced wake after sleep onset on recovery compared to baseline nights. Changes were greater in the no nap group. Recovery sleep after the first cycle of sleep restriction did not restore sleep architecture to baseline in either group. SE, rapid eye movement (REM), and non-REM sleep increased, and N2 latency was reduced in the second sleep restriction period.
Conclusions:
Changes in sleep EEG induced by sleep restriction to 5-hour TIB for five nights were not eliminated after two nights of 9-hour recovery sleep. An afternoon nap helped but residual effects on the sleep EEG suggest that there is no substitute for adequate nocturnal sleep.
Keywords: Adolescents, sleep restriction, daytime nap, recovery sleep
Statement of Significance
Many adolescents presently sleep less on weekday nights than in the past. Daytime napping and weekend sleep extension are often adopted to remedy the negative effects of reduced nocturnal sleep. Two nights of 9-hour recovery sleep during simulated weekend nights were insufficient to return sleep architecture to baseline levels following an intense 5-hour time-in-bed school week. Residual effects of sleep loss were carried over to a second cycle of sleep restriction. A long 1-hour afternoon nap alleviated but did not eliminate sleep debt. These findings underscore the importance of adequate nocturnal sleep on adolescent sleep physiology.
INTRODUCTION
Adolescents undergo a period of rapid physical and psychological change, often accompanied by intense pressure to excel academically and keep up-to-date with their peers on social media. The resulting delay in bedtimes,1,2 together with early school start times, jointly lead to recurrent sleep loss during the school term. To combat the daytime sleepiness and cognitive deficits induced by sleep curtailment, many resort to napping. The National Sleep Foundation 2011 Sleep in America poll observed that approximately 61% of adolescents aged 13–18 years reported sleeping less than the recommended 8 hours of sleep,3,4 while 53% reported napping on school days.5 To catch up on lost sleep, many adolescents extend sleep on weekends.6
Existing research on sleep restriction has focused on acute and short-term changes in sleep and its architecture across a single cycle of multi-night sleep restriction. These studies have shown increases in homeostatic sleep drive following chronic sleep loss,7,8 together with increased sleep duration and intensity (measured by slow wave energy [SWE]) during recovery sleep.9
In contrast, little is known about the effects of recurrent sleep restriction and recovery commonly experienced by adolescents. There has been a steady decline in the number of adolescents reporting ≥7 hours sleep a night from 1991 to 2012 in the United States,10 and a quarter of adolescents sleep <6 hours.11 Elsewhere in the world, 54% of Taiwanese middle school adolescents sleep <6 hours on school nights,12 and 18.1% of South Korean adolescents sleep <5 hours.13 A separate Korean survey showed a mean sleep duration of just 4.86 hours in South Korean 12th graders.14 It may only be a matter of time before hitherto rare sleep restriction of <5 hours time in bed (TIB) becomes more pervasive. Only one study to-date has investigated the physiological effects of repeated cycles of sleep restriction and recovery in healthy adults.15 It found that stress responses were continually elevated throughout the sleep restriction cycles. It is still unclear how sleep architecture responds to repeated cycles of sleep restriction in adolescents.
Naps have been shown to benefit alertness and cognitive performance in adults following normal sleep,16 sleep deprivation,17 and one night of sleep restriction.18,19 However, naps can adversely affect nocturnal sleep if their timing and length are unfavorable.20 For example, a survey of 231 adolescents who habitually napped at least twice a week found that they went to bed later, woke up later, and obtained less nocturnal sleep on school nights than those who did not nap at all.6
In an earlier study on adolescents, we found that after seven nights of sleep restricted to 5 hours, the first recovery sleep episode had longer total sleep time (TST; consisting of increased N2 and rapid eye movement [REM] sleep) and reduced wake after sleep onset (WASO) relative to baseline. Incomplete recovery of neurobehavioral performance was still observed after the second recovery night (9-hour TIB per recovery night).21 Similar findings were also observed in a recent study on adolescents exposed to five nights of 5-hour sleep restriction followed by two recovery nights of 10-hour TIB.22 We recently demonstrated in this age group that these residual effects of sleep loss in the first sleep cycle would carry over and be aggravated in a second cycle of sleep restriction in the form of compounded neurobehavioral impairments.23
In the present work, we examined (1) how sleep architecture responds to repeated cycles of sleep restriction and recovery simulating an intense school week, (2) if a 1-hour afternoon nap could alleviate some of these changes, and (3) if napping in the afternoon would adversely affect nocturnal sleep in this setting.
METHODS
Participants
Fifty-seven adolescents took part in the Need for Sleep Study 2.23 They met the following screening criteria: (1) between 15 and 19 years of age, (2) no history of chronic medical conditions, psychiatric illness, or sleep disorders, (3) a body mass index (BMI) of ≤30 kg/m2, (4) daily consumption of fewer than five cups of caffeinated beverages, (5) no history of travel across more than two time zones 1 month prior to the experiment, and (6) not habitual short sleepers (i.e., those who had an average actigraphically-estimated TIB of <6 hours and showed no sign of sleep extension for >1 hour on weekend compared to weekday nights). The last criterion excluded individuals who slept <6 hours on both weekdays and weekends. Full details of the recruitment and screening criteria are detailed in Lo et al.23 The study was approved by the Institutional Review Board of the National University of Singapore and informed consent was obtained from both participants and their legal guardian.
Participants were subsequently randomized into nap (n = 29) or no nap (n = 28) groups. These groups were equivalent in age, gender distribution, proportion of habitual nappers (i.e., individuals who indicated that they napped at least once a week),24 BMI, habitual consumption of caffeinated beverages, morningness-eveningness preference,25 levels of daytime sleepiness,26 symptoms of chronic sleep reduction,27 subjective sleep quality,28 and self-reported and actigraphically estimated TIB and TST during term time (p’s > .12; Table 1).23
Table 1.
Characteristics of the Nap and No Nap Groups.
| Nap group | No nap group | t/ χ 2 | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| n | 29 | — | 28 | — | — | — |
| Age (y) | 16.75 | 0.94 | 16.91 | 1.14 | 0.55 | .59 |
| Gender (% males) | 55.20 | — | 57.10 | — | 0.02 | .88 |
| Habitual nappers (%) | 55.17 | — | 57.14 | — | 0.02 | .88 |
| Body mass index (kg/m2) | 20.19 | 2.71 | 20.92 | 2.77 | 1.01 | .32 |
| Caffeinated drinks per day | 0.81 | 0.75 | 0.75 | 0.91 | 0.27 | .79 |
| Morningness-Eveningness Questionnaire score | 52.62 | 7.27 | 50.25 | 7.66 | 1.20 | .24 |
| Epworth Sleepiness Scale score | 6.57 | 2.86 | 6.52 | 2.57 | 0.08 | .94 |
| Chronic Sleep Reduction Questionnaire | ||||||
| Total score | 33.62 | 4.12 | 34.21 | 5.07 | 0.48 | .63 |
| Shortness of sleep | 12.83 | 1.75 | 12.36 | 2.31 | 0.87 | .39 |
| Irritation | 6.28 | 1.51 | 6.36 | 1.50 | 0.20 | .84 |
| Loss of energy | 7.21 | 1.35 | 7.93 | 2.05 | 1.57 | .12 |
| Sleepiness | 7.31 | 1.23 | 7.57 | 1.60 | 0.69 | .49 |
| Pittsburgh Sleep Quality Index | ||||||
| TIB on weekdays (h) | 6.50 | 0.90 | 6.52 | 0.72 | 0.13 | .90 |
| TIB on weekends (h) | 9.05 | 1.07 | 8.76 | 1.09 | 1.02 | .31 |
| TST on weekdays (h) | 6.05 | 0.91 | 6.13 | 0.73 | 0.37 | .71 |
| TST on weekends (h) | 8.57 | 1.03 | 8.40 | 1.02 | 0.63 | .53 |
| Global score | 5.28 | 1.89 | 5.39 | 2.25 | 0.21 | .83 |
| Actigraphy | ||||||
| TIB on weekdays (h) | 6.20 | 1.03 | 6.44 | 0.99 | 0.86 | .40 |
| TIB on weekends (h) | 8.18 | 0.82 | 8.15 | 0.70 | 0.15 | .88 |
| TST on weekdays (h) | 5.43 | 0.95 | 5.69 | 0.89 | 1.04 | .30 |
| TST on weekends (h) | 7.31 | 0.86 | 7.23 | 0.63 | 0.39 | .70 |
| Sleep efficiency (%) | 88.00 | 4.98 | 88.51 | 4.10 | 0.42 | .68 |
SD = standard deviation; TIB = time in bed; TST = total sleep time.
One-Week Prestudy Protocol
One week prior to the study, participants were required to adhere to a 9-hour sleep schedule (23:00–08:00) to increase the likelihood of stable circadian entrainment and to minimize the effects of any prior sleep restriction. Napping was not allowed during this period. This was carried out in participants’ homes and compliance was verified using wrist-worn actigraphy (Actiwatch 2, Philips Respironics, Inc., Pittsburgh, PA) on the nondominant hand. Caffeine was not permitted 24 hours prior to the commencement of the study.
Two-Week Study Protocol
The experimental protocol during the 15-day period is shown in Figure 1. In the first two nights of the study (B1–B2), both nap and no nap participants were given a 9-hour nocturnal sleep opportunity (23:00–08:00) for adaptation (B1) and baseline characterization (B2). This was followed by two cycles of sleep restriction and recovery sleep. The first cycle started with five nights of 5-hour sleep restriction (M11–M15; 01:00–06:00) and ended with two nights of 9-hour recovery sleep opportunity (R11–R12; 23:00–08:00) simulating a typical school week. The second cycle began with three nights of sleep restriction (M21–M23) and ended with two nights of recovery sleep (R21–R22). Following all sleep-restricted nights, the nap group was given a 1-hour nap opportunity from 14:00–15:00 while the no nap group watched documentaries. All participants were housed in a student hostel and slept in darkened, twin-share, and air-conditioned rooms. Participants’ activities were closely monitored throughout the experiment to ensure that sleep did not occur outside scheduled sleep/nap times. Caffeine consumption was also prohibited.
Figure 1.
The 15-day experimental protocol is illustrated in a double raster plot. Both the nap and the no nap groups underwent one adaptation (B1) and one baseline (B2) night (9 h time-in-bed [TIB]), followed by the first cycle of sleep restriction for five nights (M11 to M15; 5 h TIB) and recovery sleep for two nights (R11 to R12; 9 h TIB). The second cycle consisted of three nights of sleep restriction (M21 to M23; 5 h TIB) and two nights of recovery sleep (R21 to R22; 9 h TIB). In addition, the nap group was given a 1 h sleep opportunity between 14:00 and 15:00 on days following a sleep-restricted night (gray triangles), when the no nap group stayed awake (white triangles). Asterisks mark nocturnal sleep and daytime nap episodes monitored with polysomnography.
Polysomnography
Sleep was recorded using portable EEG recording devices (SOMNOtouch RESP, SOMNOmedics GmbH, Germany). Recordings were obtained on nine nocturnal sleep episodes (B1, B2, M11, M13, M15, R11, M21, M23, and R21) and five daytime nap episodes (M11, M13, M15, M21, M23; note that the daytime nap labels correspond to afternoons following the respective sleep restriction night). B1 recordings, which were performed to allow participants to adapt to new sleeping conditions, were not used in the present analysis. EEG was recorded from two main channels (C3 and C4 in the international 10–20 system of electrode placement) referenced to the contralateral mastoids. The common ground and reference electrode were placed at Cz and Fpz, respectively. Electrooculography (EOG; right and left outer canthi) and submental electromyography (EMG) were also used for sleep stage classification. Signals were sampled at 256 Hz and band-pass filtered between 0.2 and 35 Hz (EEG and EOG) or 1–128 Hz (EMG).
Sleep Staging and EEG Spectral Analysis
Sleep scoring was performed in 30-second epochs using the FASST toolbox.29 Scoring was performed by trained technicians following the criteria set by the AASM Manual for the Scoring of Sleep and Associated Events.30 The following sleep parameters (in minutes) were computed for each polysomnographic record: TST, N2 latency (time from lights off to N2 sleep onset), duration of individual sleep stages (N1, N2, and N3), total non-rapid eye movement (NREM) and rapid eye movement (REM) sleep, and WASO. Sleep efficiency (SE) was also computed as a percentage of TST to TIB. Plots for each parameter were obtained for (1) nocturnal sleep, (2) daytime nap, and (3) total nocturnal sleep + daytime nap episodes. For sleep macrostructure, 29 nap and 28 no nap participants entered the final analyses.
As both sleep duration and SWE have been shown to increase following chronic sleep restriction,9 we additionally computed SWE by integrating power in the delta band summed across all NREM epochs. This method is preferable to using average SWA measures as the rate of SWA dissipation decreases throughout the night, confounding analyses with different TIBs.
EEG recordings were visually inspected to identify artifact-free 5-second epochs. Recordings containing more than 10% artifacts (from epochs scored as sleep) were excluded from further analyses (12% of nocturnal sleep and 3% of nap records). Participants with unusable B2 recordings were also removed from further analyses, leaving 27 nap and 26 no nap participants for the final EEG spectral analyses.
EEG spectral analysis was then performed on non-overlapping 5-second epochs using custom routines written in Matlab R2012a (The MathWorks, Inc. Natick, MA). Analysis was conducted primarily using C3/A2, unless data from C4/A1 was assessed as having fewer artifacts (2.5% of all records). For each epoch, power spectral density estimates were computed using Welch’s modified periodogram method31 (Hamming window; 0.2 Hz bin resolution) and integrated from 0.6 to 4 Hz using the trapezoidal rule for integral approximation to obtain to SWE measures per epoch. SWE was then summed across all NREM epochs and expressed as a percentage of total SWE in the baseline night. In addition, to investigate temporal dynamics of SWE dissipation across nocturnal sleep episodes, we computed total SWE for each hour after N2 sleep onset for either (1) the first 7 hours of nocturnal sleep on 9-hour TIB nights (B2, R11, and R21) or (2) the first 4 hours of nocturnal sleep on 5-hour TIB nights (M11, M13, M15, M21, and M23), which was the longest sleep duration common to all subjects. These values were expressed as a percentage of total SWE in the first hour of the baseline night (B2).
Statistical Analysis
Statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC). A general linear mixed model with PROC MIXED was used to investigate the effects of group (nap, no nap), night (for nocturnal sleep; day for daytime nap), and group × night (or day) interactions on macrostructure and SWE measures. Differences of least square means were used to determine significant differences (p < .05) (1) between groups for nocturnal sleep and total nocturnal sleep + daytime nap parameters, (2) within group with respect to baseline (B2) parameters, and (3) within group (nap group only) with respect to M11 for daytime nap parameters. All data are presented as mean ± standard error of the mean.
In addition, we also investigated if habitual napping affected nocturnal and daytime sleep parameters.
RESULTS
Sleep Measures During the 1-Week Prestudy Period
During the 1-week prestudy period, participants complied with the 23:00–08:00 sleep schedule (Table 2). Average actigraphically assessed TST was 7.88 ± 0.50 hours. No significant group differences in bed time, wake time, TIB, and TST were observed (p’s > .63; Table 2).
Table 2.
Actigraphically Assessed Sleep Timing and Duration During the 1-Week Prestudy Period.
| Nap group | No nap group | t | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Bed time (hh:mm) | 23:00 | 00:12 | 23:00 | 00:07 | 0.20 | .85 |
| Wake time (hh:mm) | 08:02 | 00:16 | 08:03 | 00:21 | 0.14 | .89 |
| Time in bed (h) | 9.02 | 0.28 | 9.04 | 0.28 | 0.27 | .78 |
| Total sleep time (h) | 7.85 | 0.55 | 7.92 | 0.45 | 0.48 | .63 |
SD = standard deviation.
Sleep Macrostructure
Macrostructure parameters for (1) nocturnal sleep, (2) daytime nap, and (3) nocturnal sleep + daytime nap episodes are plotted in Figure 2A–I (left, middle, and right panels, respectively). A summary of main and interaction effects of group (nap, no nap) and night (or day) on these parameters is provided in Table 3. In addition, main and interaction effects of habitual napping, group (nap, no nap), and night (or day) on (1) nocturnal sleep, and (2) daytime nap parameters are reported in Supplementary Table S1.
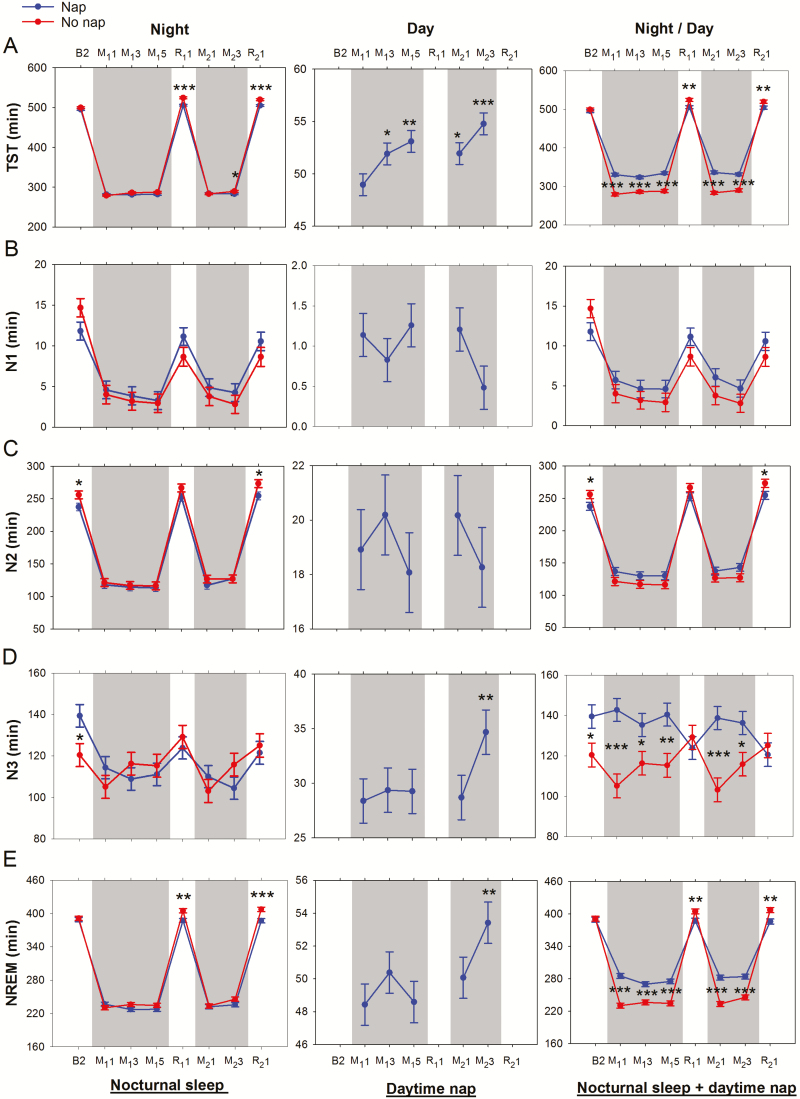
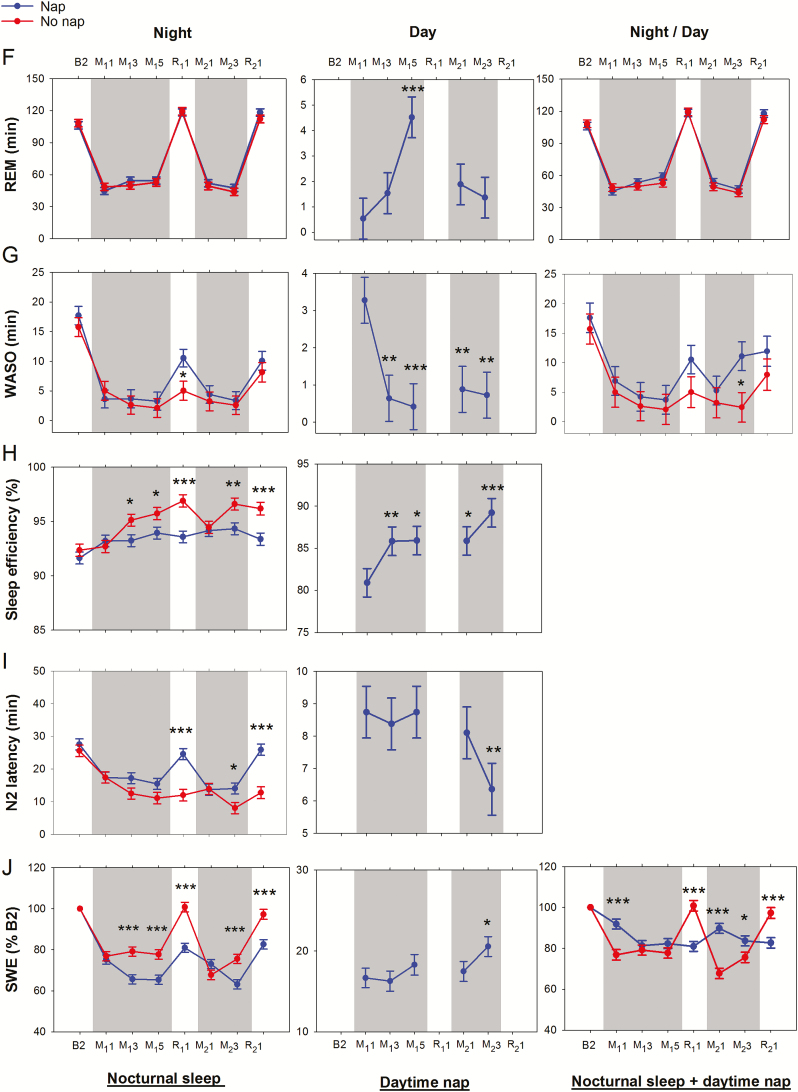
Figure 2.
Effects of repeated cycles of sleep restriction and recovery on sleep macrostructure across experimental nights. Shaded gray regions indicate periods of sleep restriction. Mean ± SEM of the nap (blue line and filled circles) and the no nap (red line and filled circles) groups were plotted for (A) total sleep time (TST), (B) N1 sleep, (C) N2 sleep, (D) N3 sleep, (E) non-rapid eye movement (NREM) sleep, (F) rapid eye movement (REM) sleep, (G) wake after sleep onset (WASO), (H) sleep efficiency, (I) N2 latency, and (J) slow wave energy (SWE) for nights where polysomnography was recorded (B2, M11, M13, M15, R11, M21, M23, R21). Plots were obtained for nocturnal sleep (left panel), daytime nap (middle panel), and total nocturnal sleep + daytime nap (right panel). Asterisks indicate significant differences between groups for each night (nocturnal sleep and total nocturnal sleep + daytime nap plots), and within the nap group with respect to M11 (daytime nap plots; *p < .05, **p < .01, ***p < .001).
Table 3.
Main and Interaction Effects of Group (Nap, No Nap) and Night on Sleep Parameters for (a) Nocturnal Sleep, (b) Daytime Nap, and (c) Total Nocturnal Sleep + Daytime Nap Episode.
| Nocturnal sleep | Daytime nap | Total nocturnal sleep + daytime nap | |||||
|---|---|---|---|---|---|---|---|
| FGroup | FNight | FGroup × Night | FDay | FGroup | FNight/Day | FGroup × Night/Day | |
| TST | 10.45** | 7598.94*** | 7.11*** | 5.41*** | 101.83*** | 1430.3*** | 32.68*** |
| N1 | 0.48 | 38.20*** | 1.44 | 1.64 | 1.65 | 32.28*** | 1.65 |
| N2 | 1.80 | 665.33*** | 1.90 | 0.67 | 0.74 | 509.92*** | 7.52*** |
| N3 | 0.00 | 12.72*** | 4.27*** | 3.98** | 7.72** | 1.20 | 9.42*** |
| NREM sleep | 4.13* | 1814.88*** | 4.57*** | 3.15* | 29.43*** | 696.26*** | 32.63*** |
| REM sleep | 0.17 | 345.57*** | 0.83 | 3.98* | 0.36 | 297.67*** | 0.88 |
| WASO | 1.58 | 23.94*** | 0.98 | 3.90** | 4.08 | 6.88*** | 0.63 |
| SE | 10.62** | 10.19*** | 4.1*** | 4.25** | — | — | — |
| N2 latency | 11.06** | 22.83*** | 7.05*** | 2.57* | — | — | — |
| SWE | 16.66*** | 82.88*** | 11.29*** | 2.47 | 0.94 | 27.62*** | 23.70*** |
Asterisks indicate the threshold of significance (*p < .05, **p < .01, ***p < .001).
NREM = non-rapid eye movement; REM = rapid eye movement; SE = sleep efficiency; SWE = slow wave energy; TST = total sleep time; WASO = wake after sleep onset.
Critically, all participants in the nap group were able to nap in the 1-hour sleep opportunity provided, and habitual napping did not significantly affect most sleep parameters (with the exception of nocturnal REM, which was slightly higher in the nap group [p = .01; Supplementary Table S1]).
No Nap Group
Relative to the baseline night, sleep in the first restriction period was characterized by reduced TST (in particular N1, N2, and REM sleep), reduced WASO, and shorter N2 latencies (p’s < .001; Figure 2A–C, F–G, I). Accumulation of sleep debt across sleep restriction nights was evidenced by progressive increase in TST (in particular, N3) and reduction in N2 latency from M11 to M13 (p’s < .02). This trend continued into R11 where there was increased TST (NREM and REM sleep), reduced WASO, greater SE, and shorter N2 latency (relative to baseline, p’s < .002).
Incomplete recovery after two recovery nights (R11–R12), resulted in carry over effects of sleep loss into the second cycle of sleep restriction: SE was greater on M21 and M23 versus M11 and M13, respectively (p = .01 and p = .03; Figure 2H), and N2 latency was shorter on M23 versus M13 (p = .03; Figure 2I). Increases in TST (in particular, N3) and reductions in N2 latency were also observed from M21 to M23 (p’s < .01). On R21, TST (in particular N2 sleep) and SE were greater, and WASO and N2 latency were shorter compared to baseline (p’s < .001; Figure 2A, C, G–I). The longer duration of NREM sleep (Figure 2E), together with the same amount of REM sleep (Figure 2F), relative to baseline (p < .001 and 0.32 respectively) suggest the prioritization of NREM sleep when sleep pressure is increased.32
Nap Group
Prior to the introduction of the nap manipulation on day M11, sleep opportunities were equivalent for the nap and the no nap groups in all nights, including B2 and M11. Although there were initial group differences on night B2 in N2 and N3 sleep (18.50 min and 18.95 min respectively; Figure 2C and D; p = .03 and .02), there were no significant group differences in TST, sleep stage duration, WASO, SE, or N2 latency (p’s > .23) on night M11 (Figure 2A–I). The nap group, like the no nap group, demonstrated reductions in TST (both NREM and REM) and WASO, and shorter N2 latencies during the first sleep restriction night (p’s < .001). However, these changes appeared to stabilize (no further change across sleep restriction nights), unlike gradual increases in TST and reductions in N2 latency that were observed in the no nap group. Notably, TST increased from approximately 279 to 290 minutes from night M11 to M23 (p = .0004; Figure 2A) while N2 latency decreased from 17 to 8 minutes (P < .0001; Figure 2I) in the no nap group.
In the first sleep restriction period, TST during the nap episodes increased from approximately 49 minutes on day M11 to 53 minutes on day M15 (p = .002; Figure 2A). This was driven by a small increase in REM sleep (4.0 ± 1.1 minutes; Figure 2F) and decrease in WASO (2.9 ± 0.8 minutes; Figure 2G). The nap episodes comprised mainly of N2 and N3 sleep, and no significant change in the duration of these two stages was found (p’s > .46; Figure 2C and D).
When the total of nocturnal sleep and daytime napping during the first sleep restriction period was considered, the additional sleep opportunity during the daytime led the nap participants to have longer TST (37.3–51.4 minutes, p’s < .001; Figure 2A) and N3 duration (19.0–37.6 minutes, p’s < .05; Figure 2D) than their no nap counterparts. As a result of the smaller amount of sleep debt accumulated across the first five sleep restriction nights, the nap group experienced shorter TST, higher WASO, lower SE, and longer N2 latency (p’s < .05; Figure 2A, G–I) compared to the no nap group in the first recovery night. Nevertheless, in the nap group, sleep architecture features reflecting residual effects of sleep restriction (increased TST in particular N2 and REM sleep, reduced WASO, and greater SE compared to baseline; p’s < .01; Figure 2A, C, F–H) were still evident on the first recovery night.
In the second sleep restriction period, the nap group continued to show residual effects of prior sleep loss: REM sleep was 7.3 minutes longer on night M21 versus M11 (Figure 2F), and N2 sleep was 12.7 minutes longer on night M23 versus M13 (Figure 2C). This was accompanied by changes in daytime napping parameters: (1) increased TST on day M21 and M23 versus M11 and M13 (p’s = .03; Figure 2A), (2) N3 duration on day M23 versus M13 (p = .03; Figure 2D), as well as (3) reduced N2 latency on M23 versus M13 (p = .02; Figure 2I).
These findings show incomplete recuperation after the first recovery period, and further elevation of sleep debt in the second sleep restriction period despite additional daytime nap opportunities.
Slow Wave Energy
In both nap and no nap groups, SWE was reduced with respect to baseline on all sleep restriction nights, even when the sum of total nocturnal sleep and daytime nap SWE was considered (p’s < .003; Figure 2J). During the recovery nights, however, total nocturnal sleep + daytime nap SWE returned to baseline levels in the no nap group (p’s > .37) but remained below baseline levels in the nap group (p’s < .0001), likely due to a reduction in sleep pressure from the afternoon nap taken prior to the first recovery nights in each cycle.
Plots for NREM SWE by group (nap, no nap) and hour across nocturnal sleep episodes are also shown in Figure 3. In all nocturnal sleep episodes, there was a main effect of hour into night (p’s < .001) where SWE decreased as sleep progressed. Concerning group and group × hour interactions on SWE, there were no significant differences on those nights before the nap manipulation was introduced (i.e., B2 and M11; p’s > .20).
Figure 3.
Slow wave energy (SWE) by hour (normalized to SWE in the first hour [H1] of the baseline night [B2]) from the onset of N2 sleep. Only NREM epochs were considered. Values indicate mean ± SEM of the nap (blue line and filled circles) and the no nap (red line and filled circles) groups for (1) the first 7 hours of nocturnal sleep on 9-hour TIB nights (B2, R11, and R21), and (2) the first 4 hours of nocturnal sleep on 5-hour TIB nights (M11, M13, M15, M21, and M23). Asterisks indicate significant differences between groups (*p < .05, **p < .01, ***p < .001).
However, both group and group × hour interactions were significant on M13, M15, and R11 (p’s < .02), where the no nap group showed elevated SWE, particularly in the first few hours of sleep, compared to the nap group. In the first hour of sleep on M13 and M15, SWE in the no nap group was about 9% greater than its corresponding baseline value, indicating elevated sleep pressure. Following two nights of recovery sleep, SWE of both groups remained comparable on M21 (no main effect of group and group × hour; p’s > .36), but became elevated again in the no nap group on M23 and R21 (significant group and group × hour interactions; p’s < .007).
DISCUSSION
Adolescent self-reported sleep duration has been on the decline in recent years.10 This trend, if left unchecked could lead to a slew of academic, social, and health issues.1,33,34 The present study aimed to investigate changes in sleep physiology arising from repeated cycles of multinight sleep restriction to 5-hour TIB simulating an intense school week followed by 2 recovery nights of 9-hour TIB. Our findings reveal that multiple nights of sleep restriction bring about cumulative changes in sleep architecture indicative of a buildup in sleep debt that are aggravated early in a second cycle of sleep restriction. A 1-hour afternoon nap each day resulted in reduced homeostatic sleep pressure. However, two nights of 9-hour TIB recovery sleep simulating a weekend were insufficient to restore sleep EEG to baseline levels even in the nap group. Finally, we demonstrated that having a 1-hour mid-afternoon nap following each night of 5-hour TIB did not perturb nocturnal sleep and may have instead contributed to stabilizing sleep debt accrued across days.
Sleep Debt Accumulated Across Sleep Restriction Nights in Both Nap and No Nap Groups, but Was Greater in the No Nap Group
Sleep macrostructure differences in the recovery nights compared to baseline (B2) in both groups were observed in the form of increased N2 sleep, REM sleep, and SE and reduced WASO. Additionally, the no nap group displayed increased TST, NREM sleep, and reduced N2 latency and N1 sleep. These transitions to deeper and longer sleep in the recovery nights are indicative of compensatory responses activated to overcome elevated sleep pressure accumulated over multiple nights of sleep restriction. Even across nap episodes within the nap group, increases in TST, N3 sleep, SE, and SWE and decreases in WASO were observed, still rising at the end of the second sleep restriction period.
There was greater accumulation of sleep debt in the no nap group as evidenced by more pronounced changes in NREM sleep, WASO, SE, N2 latency, and SWE in the first recovery night, and onward into the second sleep restriction period. A 1-hour mid-afternoon nap appears to have stabilized EEG features of sleep debt accumulated across multiple nights.
Two Nights of 9-Hour Recovery Sleep may be Insufficient to Reverse the Electrophysiological Effects of 5 Nights of 5-Hour Sleep Restriction
This study was designed to simulate a typical school week—with five nights of 5-hour sleep restriction on school nights followed by two nights of 9-hour recovery sleep during a weekend—and investigate the EEG dynamics when school starts again in the ensuing week. When nocturnal sleep in the second sleep restriction cycle was compared with the first, there were indications that recovery was not complete and residual effects of sleep loss carried over to the second cycle. In our prior work investigating the effects of seven nights of sleep restriction, recovery was also shown to be incomplete by the third night,21 in contrast to adults who show full recovery in terms of restitution to baseline sleep stage durations by the first or third recovery night,7,8,32,35 even with seven nights of 3-hour TIB.8 While no direct comparison can be made with adults here, these findings reinforce the notion that adolescents may not habituate to recurrent episodes of partial sleep loss and that lost sleep may not be adequately compensated by two nights of 9-hour TIB (akin to a weekend), even with a 1-hour afternoon nap following each sleep-restricted night.
The accentuation of EEG changes in the second sleep restriction cycle ought to serve as a wake-up-call to adolescents who subject themselves to draconian sleep schedules for an entire school term involving many more cycles of sleep restriction and potentially incomplete recovery.
A 1-Hour Afternoon Nap Does Not Alter Nocturnal Sleep Architecture in These Sleep-Restricted Participants
Long naps that contain slow wave sleep or naps taken late in the day can lead to increased alertness in the evening due to reduced homeostatic sleep pressure, leading to decreased nocturnal sleep later that night.36 In turn, this could increase the likelihood of napping the following day, leading to a vicious cycle of short sleep and increased daytime napping.37 However, in this study, we showed that there was comparable nocturnal NREM sleep, REM sleep, and WASO between the nap and the no nap groups on sleep restriction nights. When differences were observed between groups (eg, TST and N2 latency on M23, and SE on most sleep restriction nights), these were due to changes across nights in the no nap group rather than in the nap group, suggesting that this was due to greater sleep debt in the no nap group rather than reduced sleep capacity in the nap group.
Prior work36,38 has advocated shorter duration naps (e.g., 10-minute), out of concern that of sleep inertia would impair performance and alertness soon after awakening if those naps were to contain a greater amount of slow wave sleep. Although we did not compare effects of differing nap durations, it seems reasonable to state that if performance immediately after napping is not crucial, a 1-hour afternoon nap may be preferable to shorter naps given that reduction in sleep debt and sleepiness, and gains in neurobehavioral functions23 may be accrued without interference with nocturnal sleep.
Study Limitations
In this study, sleep restriction and recovery nights were experimentally set at 5 and 9 hours, respectively. This level of sleep restriction may seem severe to some, but is commonly observed in Asian adolescents.13,14 Adolescents in our sample had actigraphically estimated habitual TIBs of 6.32 ± 1.02 hours (mean ± standard deviation) on weekdays and 8.17 ± 0.76 hours on weekends during term time. Habitual TST was 5.56 ± 0.92 hours on weekdays and 7.27 ± 0.76 hours on weekends. These levels of weekday sleep are far below the recommended 8 hours of sleep prescribed for this age group3 and less than the sleep duration reported in other North American, European, and Asian countries.39 Whether our findings can be generalized to adolescents from other countries who habitually sleep longer remain to be addressed.
In addition, our findings could differ for less severe levels of nightly sleep restriction, or greater weekend recovery durations—an issue future studies should investigate. Adolescents may also choose to take additional naps on weekends, which could allow for complete recovery before the next cycle of sleep restriction. A 2 pm nap time might also be unrealistic for students who would be in class at this time. Future work could look into the effect of later nap times.
We were also only able to track EEG changes across two sleep restriction and recovery cycles. As sleep debt appeared to be cumulating in both nap and no nap groups in the second cycle of sleep restriction, future studies should investigate if these changes continue to accrue over more cycles of sleep restriction and recovery or if an allostatic change in sleep homeostasis would instead be observed.
CONCLUSION
The present study underscores the importance of adequate nocturnal sleep in adolescents. Sleep debt accumulated during an intense school week may not be fully compensated by extending sleep on weekend nights and may intensify upon re-exposure to sleep restriction. Afternoon naps can be taken to alleviate sleep debt under conditions of severe sleep restriction without compromising nocturnal sleep architecture but these do not restore sleep to baseline, especially if sleep restriction is repeated the following week.
SUPPLEMENTARY MATERIAL
Supplementary data are available at SLEEP online.
FUNDING
This was not an industry supported study. Financial support was provided by the National Medical Research Council, Singapore (NMRC/STaR/015/2013) and The Far East Organization. The authors have indicated no financial conflicts of interest. This work was approved by the Institutional Review Board of the National University of Singapore (B-15–116). All participants provided written informed consent.
Supplementary Material
ACKNOWLEDGEMENTS
Benny Chin Seah Koh assisted with participant recruitment, Su Mei Lee and Lydia Teo co-ordinated logistics, James Cousins, Jesisca Tandi, Wei Shan Cher, Pearlynne Chong, Bindiya Lakshmi Raghunath, Shin Wee Chong, Karen Sasmita, Nicholas Chee, Wanzheng Zhu, Xuan Kai Lee, Kian Foong Wong, and James Teng helped with data collection and processing. JLO and JCL contributed equally to this work.
REFERENCES
- 1. Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011; 58(3): 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartel KA, Gradisar M, Williamson P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev. 2015; 21: 72–85. [DOI] [PubMed] [Google Scholar]
- 3. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016; 12(6): 785–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015; 1(1): 40–43. [DOI] [PubMed] [Google Scholar]
- 5. National Sleep Foundation. Communications Technology in the Bedroom. Washington, DC: National Sleep Foundation; http://www.sleepfoundation.org/sites/default/files/sleepinamericapoll/SIAP_2011_Summary_of_Findings.pdf Accessed August 23, 2016. [Google Scholar]
- 6. Gradisar M, Wright H, Robinson J, Paine S, Gamble A. Adolescent napping behavior: comparisons of school week versus weekend sleep patterns. Sleep Biol Rhythms. 2008; 6(3): 183–186. [Google Scholar]
- 7. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003; 26(2): 117–126. [DOI] [PubMed] [Google Scholar]
- 8. Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003; 12(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 9. Banks S, van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010; 33(8): 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keyes KM, Maslowsky J, Hamilton A, Schulenberg J. The great sleep recession: changes in sleep duration among US adolescents, 1991-2012. Pediatrics. 2015; 135(3): 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts RE, Roberts CR, Duong HT. Sleepless in adolescence: prospective data on sleep deprivation, health and functioning. J Adolesc. 2009; 32(5): 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen MY, Wang EK, Jeng YJ. Adequate sleep among adolescents is positively associated with health status and health-related behaviors. BMC Public Health. 2006; 6(1): 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Do YK, Shin E, Bautista MA, Foo K. The associations between self-reported sleep duration and adolescent health outcomes: what is the role of time spent on Internet use? Sleep Med. 2013; 14(2): 195–200. [DOI] [PubMed] [Google Scholar]
- 14. Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005; 115(1 Suppl): 250–256. [DOI] [PubMed] [Google Scholar]
- 15. Simpson NS, Diolombi M, Scott-Sutherland J, et al. Repeating patterns of sleep restriction and recovery: do we get used to it? Brain Behav Immun. 2016; 58: 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayashi M, Watanabe M, Hori T. The effects of a 20 min nap in the mid-afternoon on mood, performance and EEG activity. Clin Neurophysiol. 1999; 110(2): 272–279. [DOI] [PubMed] [Google Scholar]
- 17. Lumley M, Roehrs T, Zorick F, Lamphere J, Roth T. The alerting effects of naps in sleep-deprived subjects. Psychophysiology. 1986; 23(4): 403–408. [DOI] [PubMed] [Google Scholar]
- 18. Takahashi M, Arito H. Maintenance of alertness and performance by a brief nap after lunch under prior sleep deficit. Sleep. 2000; 23(6): 813–819. [PubMed] [Google Scholar]
- 19. Gillberg M, Kecklund G, Axelsson J, Akerstedt T. The effects of a short daytime nap after restricted night sleep. Sleep. 1996; 19(7): 570–575. [DOI] [PubMed] [Google Scholar]
- 20. Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009; 18(2): 272–281. [DOI] [PubMed] [Google Scholar]
- 21. Ong JL, Lo JC, Gooley JJ, Chee MW. EEG changes across multiple nights of sleep restriction and recovery in adolescents: the need for sleep study. Sleep. 2016; 39(6): 1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agostini A, Carskadon MA, Dorrian J, Coussens S, Short M. An experimental study of adolescent sleep restriction during a simulated school week: changes in phase, sleep staging, performance and sleepiness. J Sleep Res. 2016; 26(2): 227–235. [DOI] [PubMed] [Google Scholar]
- 23. Lo JC, Lee SM, Teo LM, Lim J, Gooley JJ, Chee MW. Neurobehavioral impact of successive cycles of sleep restriction with and without naps in adolescents. Sleep. 201. 7; 40(2): zsw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Milner CE, Fogel SM, Cote KA. Habitual napping moderates motor performance improvements following a short daytime nap. Biol Psychol. 2006; 73(2): 141–156. [DOI] [PubMed] [Google Scholar]
- 25. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976; 4(2): 97–110. [PubMed] [Google Scholar]
- 26. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991; 14(6): 540–545. [DOI] [PubMed] [Google Scholar]
- 27. Meijer AM. Chronic sleep reduction, functioning at school and school achievement in preadolescents. J Sleep Res. 2008; 17(4): 395–405. [DOI] [PubMed] [Google Scholar]
- 28. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 29. Leclercq Y, Schrouff J, Noirhomme Q, Maquet P, Phillips C. fMRI artefact rejection and sleep scoring toolbox. Comput Intell Neurosci. 2011; 2011: 598206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leclercq Y, Schrouff J, Noirhomme Q, Maquet P, Phillips C. fMRI artefact rejection and sleep scoring toolbox. Comput Intell Neurosci. 2011; 2011: 598206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iber C, Ancoli-Israel S, Chesson A, Quan SF.The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification. Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 31. Welch PD. The use of the fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE T Acoust Speech 1967; 15(2): 70–73. [Google Scholar]
- 32. Brunner DP, Dijk DJ, Borbély AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993; 16(2): 100–113. [DOI] [PubMed] [Google Scholar]
- 33. Beebe DW, Field J, Milller MM, Miller LE, LeBlond E. Impact of multi-night experimentally-induced short sleep on adolescent performance in a simulated classroom. Sleep. 2017; 40(2): zsw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Owens J; Adolescent Sleep Working Group; Committee on Adolescence Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014; 134(3): e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009; 32(2): 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brooks A, Lack L. A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep. 2006; 29(6): 831–840. [DOI] [PubMed] [Google Scholar]
- 37. Jakubowski KP, Hall MH, Lee L, Matthews KA. Temporal relationships between napping and nocturnal sleep in healthy adolescents. Behav Sleep Med 2016. –. http://dx.doi.org/10.1080/15402002.2015.1126595. Accessed August 23, 2016.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tietzel AJ, Lack LC. The short-term benefits of brief and long naps following nocturnal sleep restriction. Sleep. 2001; 24(3): 293–300. [DOI] [PubMed] [Google Scholar]
- 39. Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011; 12(2): 110–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.