Abstract
Study Objectives:
To characterize adolescents’ neurobehavioral changes during two cycles of restricted and recovery sleep and to examine the effectiveness of afternoon naps in ameliorating neurobehavioral deficits associated with multiple nights of sleep restriction.
Methods:
Fifty-seven healthy adolescents (aged 15–19 years; 31 males) participated in a parallel group study. They underwent two cycles of sleep restriction (5-hr time in bed [TIB] for five and three nights in the first and the second cycles, respectively; 01:00–06:00) and recovery (9-hr TIB for two nights per cycle; 23:00–08:00) intended to simulate the weekday sleep loss and weekend attempt to “catch up” on sleep. Half of the participants received a 1-hr nap opportunity at 14:00 following each sleep-restricted night, while the other half stayed awake. Sustained attention, sleepiness, speed of processing, executive function, and mood were assessed 3 times each day.
Results:
Participants who were not allowed to nap showed progressive decline in sustained attention that did not return to baseline after two nights of recovery sleep. Exposure to the second period of sleep restriction increased the rate of vigilance deterioration. Similar patterns were found for other neurobehavioral measures. Napping attenuated but did not eliminate performance decline. These findings contrasted with the stable performance of adolescents, given 9-hr TIB each night in our recent study.
Conclusions:
Adolescents’ neurobehavioral functions may not adapt to successive cycles of sleep curtailment and recovery. In sleep-restricted adolescents, weekend “catch-up sleep,” even when combined with napping during weekdays, is inferior to receiving a 9-hr sleep opportunity each night.
Keywords: adolescents, neurobehavioral functions, recovery sleep, repeated cycles, sleep restriction, sustained attention
Statement of Significance
During a school term, adolescents often expose themselves to repeated cycles of sleep curtailment on school nights and extended catch-up sleep over the weekend in an effort to maintain neurobehavioral function. Simulating 2 school weeks, the present study demonstrated that the negative effects of weekday sleep restriction on neurobehavioral function cumulated and were incompletely reversed by weekend catch-up sleep. Deficits were compounded by a second successive period of sleep restriction, indicating failure to adapt to successive cycles of sleep restriction. Afternoon naps were beneficial, but for the purpose of preserving neurobehavioral function, napping together with weekend catch-up sleep were still inferior to adequate and regular nocturnal sleep.
INTRODUCTION
Sleep curtailment in adolescents is prevalent in many societies.1 Approximately 75% of adolescents in the United States and over 90% in Asian countries receive less than the recommended 8–10 hr of sleep.2–4 Sleep curtailment in this demographic results from the tandem effects of later bedtime during late adolescence and early school start times.5 Factors contributing to later bedtimes include maturational delay in circadian rhythm,5,6 slower build up of homeostatic sleep pressure during wakefulness,7 reduced parental control over bedtime,8 increased academic pressure,9 and high levels of electronic media use.9,10
Adequate sleep is important for adolescent cognitive function,11,12 and sleep curtailment over multiple nights results in degraded neurobehavioral performance.13–15 Recently,14 we recorded neurobehavioral measures in adolescents when their sleep opportunity was reduced to 5 hr for 7 nights and found cumulative deficits in sustained attention, working memory/executive function, and speed of processing. Subjective alertness and positive mood were also impaired. Among the measures evaluated, sustained attention was most affected by multiple nights of sleep restriction. Critically, some neurobehavioral measures did not fully recover even after 2 nights of 9-hr sleep opportunity, raising the question of how they would respond on immediate reexposure to another bout of sleep restriction. This is relevant as many adolescents attempt to catch up on sleep on weekends,1 only to undergo sleep restriction again in the ensuing week.
Weekday naps are another measure often taken to offset sleepiness arising from nocturnal sleep curtailment: Approximately 30% of the adolescents in the United States and Australia reported napping at least 2 times per week.2,16 In adults, a nap can improve sustained attention17,18 and other cognitive functions17,19–21 that are degraded by a single night of sleep restriction. However, the effectiveness of napping in adolescents particularly following multiple nights of sleep curtailment has yet to be examined. Further, the cognitive benefits of napping have primarily been studied in the period immediately following waking and have rarely been tracked beyond late afternoon.
In this 15-day study, adolescent participants underwent a sleep schedule that simulated weekday sleep loss and the attempt to “catch up” with sleep over the weekend. We tracked changes in cognitive performance, subjective sleepiness, and mood during two successive cycles of sleep restriction and recovery to determine whether neurobehavioral deficits would be compounded by the second exposure to sleep restriction. We also examined the temporal evolution of behavior associated with daily 1-hr afternoon naps, taking measurements from afternoon to the following morning over multiple nights of sleep restriction.
METHODS
Participants
Fifty-seven adolescents (31 males; age = 15–19 years) participated in the Need for Sleep study 2 (NFS2). They were recruited through sleep education talks and recruitment campaigns in three high-ranking schools, advertisements on the laboratory and social networking websites as well as by word of mouth. All interested participants and their legal guardians were invited to attend a briefing session, during which written informed consent was obtained from both the participant and their legal guardian. The eligibility of each participant was based on the selection criteria which were the same in NFS114 and NFS2: 15–19 years of age, healthy; no sleep disorder, body mass index (BMI) ≤30, not habitual short sleepers (where short sleepers were identified as individuals having an actigraphically estimated average time in bed [TIB] of <6 hr and no sign of sleep extension for >1 hr on weekends), consumption of ≤5 cups of caffeinated beverages a day, and did not travel across >2 time zones 1 month prior to the experiment.
In this parallel group study, participants were randomized into the nap (n = 29) or the no nap group (n = 28). Twenty-six participants from NFS114 (11 males) served as the control group. These three groups were equivalent in age, gender distribution, BMI, habitual consumption of caffeinated beverages, morningness–eveningness preference,22 levels of daytime sleepiness,23 symptoms of chronic sleep reduction,24 and most self-reported25 and actigraphically assessed sleep parameters during term time (p > .10; Table 1). Despite the significant group difference in self-reported TIB on weekdays during term time (F = 3.36, p = .04), TIB was similar across groups when assessed with actigraphy (F = .87, p = .42).
Table 1.
Characteristics for the Nap, No Nap, and Control Groups.
| Nap group | No nap group | Control group | F / χ2 | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| N | 29 | - | 28 | - | 26 | - | - | - |
| Age, years | 16.75 | 0.94 | 16.91 | 1.14 | 16.81 | 1.17 | 0.14 | 0.87 |
| Gender (% males) | 55.20 | - | 57.10 | - | 42.30 | - | 1.39 | 0.50 |
| Body mass index | 20.19 | 2.71 | 20.92 | 2.77 | 20.38 | 2.55 | 0.57 | 0.57 |
| Caffeinated drinks per day | 0.81 | 0.75 | 0.75 | 0.91 | 0.54 | 0.79 | 0.82 | 0.44 |
| Morningness-Eveningness Questionnaire score | 52.62 | 7.27 | 50.25 | 7.66 | 49.96 | 7.15 | 1.11 | 0.34 |
| Epworth Sleepiness Scale score | 6.57 | 2.86 | 6.52 | 2.57 | 6.19 | 3.57 | 0.12 | 0.88 |
| Chronic Sleep Reduction Questionnaire | ||||||||
| Total score | 33.62 | 4.12 | 34.21 | 5.07 | 33.81 | 5.13 | 0.11 | .89 |
| Shortness of sleep | 12.83 | 1.75 | 12.36 | 2.31 | 12.50 | 2.30 | 0.37 | .70 |
| Irritation | 6.28 | 1.51 | 6.36 | 1.50 | 6.77 | 1.58 | 0.81 | .45 |
| Loss of energy | 7.21 | 1.35 | 7.93 | 2.05 | 7.00 | 1.65 | 2.24 | .11 |
| Sleepiness | 7.31 | 1.23 | 7.57 | 1.60 | 7.54 | 1.75 | 0.25 | .78 |
| Pittsburgh Sleep Quality Index (term time) | ||||||||
| TIB on weekdays, hr | 6.50 | 0.90 | 6.52 | 0.72 | 5.94 | 1.14 | 3.36 | .04 |
| TIB on weekends, hr | 9.05 | 1.07 | 8.76 | 1.09 | 9.20 | 1.30 | 1.02 | .36 |
| TST on weekdays, hr | 6.05 | 0.91 | 6.13 | 0.73 | 5.78 | 1.15 | 1.02 | .36 |
| TST on weekends, hr | 8.57 | 1.03 | 8.40 | 1.02 | 9.04 | 1.30 | 2.38 | .10 |
| Global score | 5.28 | 1.89 | 5.39 | 2.25 | 4.58 | 2.58 | 1.03 | .36 |
| Actigraphy (term time) | ||||||||
| TIB on weekdays, hr | 6.20 | 1.03 | 6.44 | 0.99 | 6.09 | 0.85 | 0.87 | .42 |
| TIB on weekends, hr | 8.18 | 0.82 | 8.15 | 0.70 | 8.45 | 1.25 | 0.80 | .45 |
| TST on weekdays, hr | 5.43 | 0.95 | 5.69 | 0.89 | 5.37 | 0.73 | 1.00 | .37 |
| TST on weekends, hr | 7.31 | 0.86 | 7.23 | 0.63 | 7.53 | 1.14 | 0.77 | .47 |
| Sleep efficiency (%) | 88.00 | 4.98 | 88.51 | 4.10 | 88.45 | 4.66 | 0.10 | .90 |
Abbreviations: SD, standard deviation; TIB, time in bed; TST, total sleep time.
Study Protocol
NFS1 and NFS2 were conducted during the vacation period in 2014 and 2015, respectively. One week prior to the experimental session, participants adhered to a 9-hr sleep schedule (23:00–08:00) for circadian entrainment and to minimize the effect of prior sleep restriction on neurobehavioral functions and sleep. Napping was not allowed. Compliance was verified with actigraphy (mean ± standard error of mean of bedtime of the nap, no nap, and control groups: 23:00 ± 00:11, 23:00 ± 00:07, 23:02 ± 00:11, F = .62, P = .54; wake time: 08:02 ± 00:16, 08:03 ± 00:21, 07:53 ± 00:13, F = 2.40, p = .10).
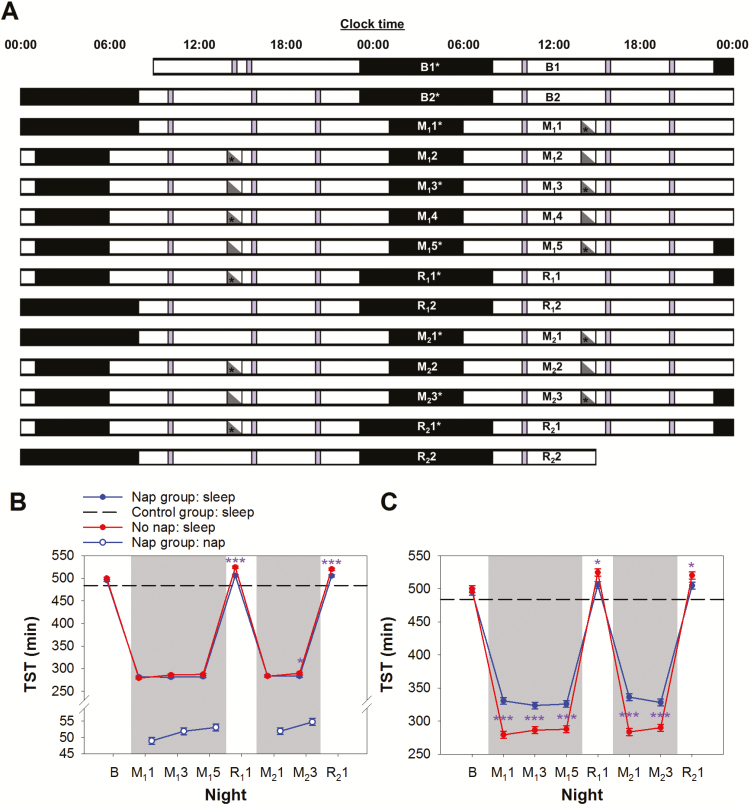
The 15-day protocol (Figure 1A) in NFS2 started with two baseline nights (B1 and B2) when all participants had 9-hr nocturnal sleep opportunities (23:00–08:00) for adaptation and baseline characterization. This was followed by two cycles of sleep restriction and recovery sleep. The first cycle began with five nights of 5-hr sleep opportunity (M11–M15; 01:00–06:00) and ended with two nights of 9-hr recovery sleep opportunity (R11–R12; 23:00–08:00). This protocol simulated the typical Monday to Friday school week, where sleep is usually restricted the night before school days (Sunday–Thursday) and extended on nights before weekend days (Friday and Saturday). The second cycle consisted of three nights of sleep restriction (M21–M23) and two nights of recovery sleep (R21–R22). After each sleep-restricted night, half of the participants (the nap group) had a 1-hr daytime nap opportunity from 14:00 to 15:00, while the rest (the no nap group) watched documentaries. The nap episodes began at 14:00 which corresponded to the time when many schools ended and the earliest time the students could have a siesta. The control group consisted of participants from NFS1 who had a 9-hr sleep opportunity (23:00–08:00) every night during a 14-day protocol.14
Figure 1.
Experimental protocol. (A) The 15-day experimental protocol is illustrated in a double raster plot. Both the nap and the no nap groups had two adaptation and baseline nights (B1 and B2; time-in-bed [TIB] = 9 hr), followed by the first cycle of sleep restriction for 5 nights (M11 to M15; TIB = 5 hr) and recovery sleep for 2 nights (R11 and R12; TIB = 9 hr). The second cycle consisted of 3 nights of sleep restriction (M21 to M23) and 2 nights of recovery sleep (R21 and R22). The nap group had a 1-hr nap opportunity between 14:00 and 15:00 on those days after a sleep restricted night (gray triangles), when the no nap group stayed awake (white triangles). Asterisks mark nocturnal sleep and daytime nap episodes monitored with polysomnography. A cognitive test battery (purple bars) was administered at 10:00, 15:45, and 20:00, except during the first and last days of the protocol. (B) Polysomnographically assessed total sleep time (TST) at night of the nap group (blue line and filled circles) and the no nap group (red line and filled circles) from baseline to the manipulation and recovery periods. The black dashed line represents the average TST of the control group from the Need for Sleep Study 1 when they were given a 9-hr sleep opportunity each night.29 TST during naps are indicated by blue open circles. (C) Sum of TST at night and during nap per 24-hr period. For (B) and (C), shaded areas represent the sleep restriction periods. The least square means and standard errors estimated with general linear mixed models are illustrated. ***p < .001; *p < .05 for contrasts between the no nap and the nap groups.
Male and female participants were housed in separate buildings in a boarding school throughout the protocol, with the nap and the no nap groups staying on different floors. All participants stayed in air-conditioned, twin-share bedrooms with en-suite bathrooms. Bedroom windows were fitted with blackout panels to ensure participants were not woken up prematurely by sunlight. Earplugs were also provided, and participants were allowed to adjust the temperature of their bedrooms to their personal comfort. Three main meals were served each day, with snacks being provided upon request. Caffeinated drinks, unscheduled sleep, and strenuous physical activities were prohibited. Outside scheduled sleep, meal, and cognitive testing times, participants spent the majority of their free time in a common room that was illuminated by natural and artificial lighting. They were allowed to read, study, play games that did not involve physical exertion, watch videos, and interact with the research staff and other participants. Participants were under constant supervision by the research staff. Throughout the study, sleep–wake patterns were monitored with wrist-worn actigraphy, except for the first day and for some participants, the first night (night B1) as well, when all the actiwatches were re-charged.
Other than the first and last day of the protocol, a computerized cognitive test battery was administered 3 times daily at 10:00, 15:45 (15:00 in NFS1), and 20:00 (Figure 1A). Each test battery lasted approximately 25 min and comprised seven tasks (further described subsequently). In this report, we focus on sustained attention, specifically the number of attention lapses in the Psychomotor Vigilance Task, because of the high sensitivity of this cognitive domain14,26,27 and measure28 to sleep deprivation.
Sleep was recorded polysomnographically on 9 selected nights for adaptation and baseline assessment and for characterization of the architecture of restricted and recovery sleep on selected nights in the first and second cycles (Figure 1A). Daytime nap was also recorded polysomnographically on selected days (Figure 1A).
Both studies were approved by the Institutional Review Board of the National University of Singapore and were conducted in accordance with the principles of the Declaration of Helsinki.
Cognitive Performance Test Battery
Each test battery comprised seven tasks in the following order: the Karolinska Sleepiness Scale (KSS),29 the Symbol Digit Modalities Test (SDMT),30 the verbal 1- and 3-back tasks,27 the Mental Arithmetic Test (MAT),31 the Positive and Negative Affect Scale (PANAS),32 and a 10-min Psychomotor Vigilance Task (PVT).33 The test battery lasted approximately 25 min. Hence, during the sleep restriction periods, although the afternoon test batteries started 45 min after the nap group had woken up, the PVT was not administered until another 15 min later, that is, 1 hr after the end of the nap episodes.
All the tasks were programmed in E-Prime 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA) and administered on identical laptop computers (Acer Aspire E11, Acer Inc, Taipei, Taiwan). Participants were required to wear earphones during testing to minimize distraction and for tone presentation during certain tasks.
The KSS29: Participants rated their current level of subjective sleepiness on a 9-point Likert-type scale (1: very alert; 9: very sleepy, great effort to keep awake).
The SDMT30: This 2-min task measures speed of processing. Participants were shown a key displaying 9 pairs of digits (from 1 to 9) and symbols. A symbol appeared below the key in each trial, requiring participants to respond by typing in the symbol’s corresponding digit as quickly as possible. If no response was detected after 15 s, a beeping tone was presented until a response was provided. The total number of correct trials was used as the critical measure.
The verbal 1- and 3-back tasks27: These tests assess working memory and executive function. Letters from the English alphabet were presented sequentially for 1 s, with a 3-s interstimulus interval. Participants had to decide if the current stimulus was identical to the letter shown 1 item (1-back) or 3 items (3-back) ago. The match to mismatch ratio was 8:24, and two nonparametric measures of sensitivity (A’) and response bias (B”D) were used to quantify performance. A’ is a measure of the ability to distinguish matches and mismatches and is computed using the hit rate (number of match trials correctly responded to × 100 / 8) and false alarm (fa) rate (number of mismatch trials incorrectly responded to × 100 / 24). A’ ranges from 0 to 1, with 0.5 indicating performance at chance level. B”D is a measure of the participant’s tendency toward liberal (B”D < 0) or conservative (B”D > 0) response behavior. The former favors more “match” responses and hence likely leads to higher hit and false alarm rates, while the latter favors “mismatch” responses and would therefore result in fewer hits and false alarms. Neutrality is centered at 0 (B”D = 0).
The two measures were derived with the following formula:
The MAT31: This 4-min task assesses speed of processing. Pairs of two-digit numbers were shown on the screen, and participants were required to add the numbers as quickly as possible. A lack of response after 15 s would result in a beeping tone. The critical measure was the total number of correct trials.
The PANAS32: Positive and negative affect was measured by the PANAS. Twenty adjectives were shown to the participants, 10 of which described a positive mood and 10 describing a negative mood. Participants were required to indicate how much they identified with each adjective using a 5-point Likert-type scale (1: very slightly; 5: extremely).
The PVT33: We used a 10-min PVT to measure sustained attention. A counter on the screen started counting at random intervals ranging from 2 to 10 s. Participants were required to respond to each stimulus onset as quickly as possible by pressing a key. If no response was detected after 10 s from stimulus onset, a beeping tone was presented. The number of lapses, defined as response times exceeding 500 ms, was used as a measure of sustained attention.
Actigraphy
An actiwatch (Actiwatch 2, Philips Respironics Inc., Pittsburgh, PA) was worn around the wrist of the nondominant hand (1) during term time for 1 week for screening purposes, (2) during the 1-week prestudy period to verify compliance with the 9-hr sleep schedule, and (3) during the 15-day protocol. Data were collected at 30-s resolution and scored with the Actiware software (version 6.0.2). Using a medium sensitivity algorithm (which defined waking as having an activity count of 40 or greater), total sleep time (TST) was calculated. Participants were also given a sleep diary to fill in when the actiwatch was worn at home. Based on their self-reported sleep–wake timing on the sleep diary and the event markers on the actogram, bedtimes and wake times were determined.
Polysomnography
A SOMNOtouch recorder (SOMNOmedics GmbH, Randersacker, Germany) was used to perform electroencephalography (EEG) from two channels (C3 and C4 in the international 10–20 system), referenced to the contralateral mastoids. Electrodes placed at Cz and FPz were used as the common ground and reference electrodes. We also used electrooculography (EOG) and submental electromyography (EMG). For EEG electrodes, impedance was kept below 5 kΩ, and for EOG and EMG electrodes, impedance was kept below 10 kΩ. Signal was sampled at 256 Hz and filtered between 0.2 and 35 Hz for EEG and between 0.2 and 10 Hz for EOG. Sleep scoring was performed using the FASST toolbox (http://www.montefiore.ulg.ac.be/~phillips/FASST.html). EEG signals were band-pass filtered between 0.2 and 25 Hz. Trained technicians visually scored the sleep data, following criteria set by the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events.34 Pulse oximetry was used in the first night (B1) to evaluate oxygen saturation and rule out undiagnosed obstructive sleep apnea. In this report, we will focus on TST. Findings regarding sleep macro- and micro-structure will be reported elsewhere.
Statistical Analyses
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). We used a general linear mixed model with PROC MIXED to determine the effects of group, day (day B2–R21), and the group × day interaction on cognitive performance, sleepiness, and mood, averaged across three test batteries each day. Performance in the evening test battery on day B1 (the fifth test battery the participants had done) was used as a covariate to control for group differences in baseline performance. To determine whether the effects of an afternoon nap were immediate (45 min to 1 hr after nap had ended), lasted into the evening, and were still observed the following morning, we applied the same statistical model to each time of the day separately.
Furthermore, we used similar statistical models to determine the effects of group, day (from night B2 to R21), and group × day interaction on polysomnography (PSG)-assessed TST so as to evaluate the effectiveness of our manipulation of sleep. PSG data from night B1 (i.e., the adaptation night) were not included in the analyses. We also investigated the change, if any, in PSG-assessed TST of nap episodes throughout the protocol (i.e., the effect of day). The least square means and standard errors estimated with PROC MIXED are plotted in the figures. Finally, we performed independent-samples t tests on the screening data to detect any group differences prior to the experiment.
RESULTS
Polysomnographically Assessed Sleep Duration
At baseline, the nap and the no nap groups had a TST of 495 and 500 min respectively—similar to the TST (484 min) of the control group averaged across their 9-hr sleep opportunities during the protocol35 (Figure 1B). TST dropped to about 280 min in the first sleep restriction night (p < .001).
TST during afternoon naps increased from 49 min on day M11 to 55 min on day M23 (F = 5.41, p < .001; Figure 1B). Napping in the afternoon did not have any significant impact on the nap group’s TST in subsequent sleep-restricted nights (relative to night M11, p > .37; Figure 1B). Among all the sleep-restricted nights, the nap group slept less than the no nap group on only one night (M23; for 6.6 min, p < .05). Overall, during the sleep restriction periods, the nap group slept 37–53 min more each day (p < .001; Figure 1C), accumulating a smaller sleep debt. The latter was reflected in the smaller increase in TST during recovery nights (relative to baseline) in the nap group than the no nap group (night R11: 506 ± 5 vs. 525 ± 5 min; night R21: 505 ± 5 vs. 520 ± 6 min; both p < .05).
Sustained Attention During Successive Cycles of Sleep Restriction and Recovery: Data From the No Nap Group
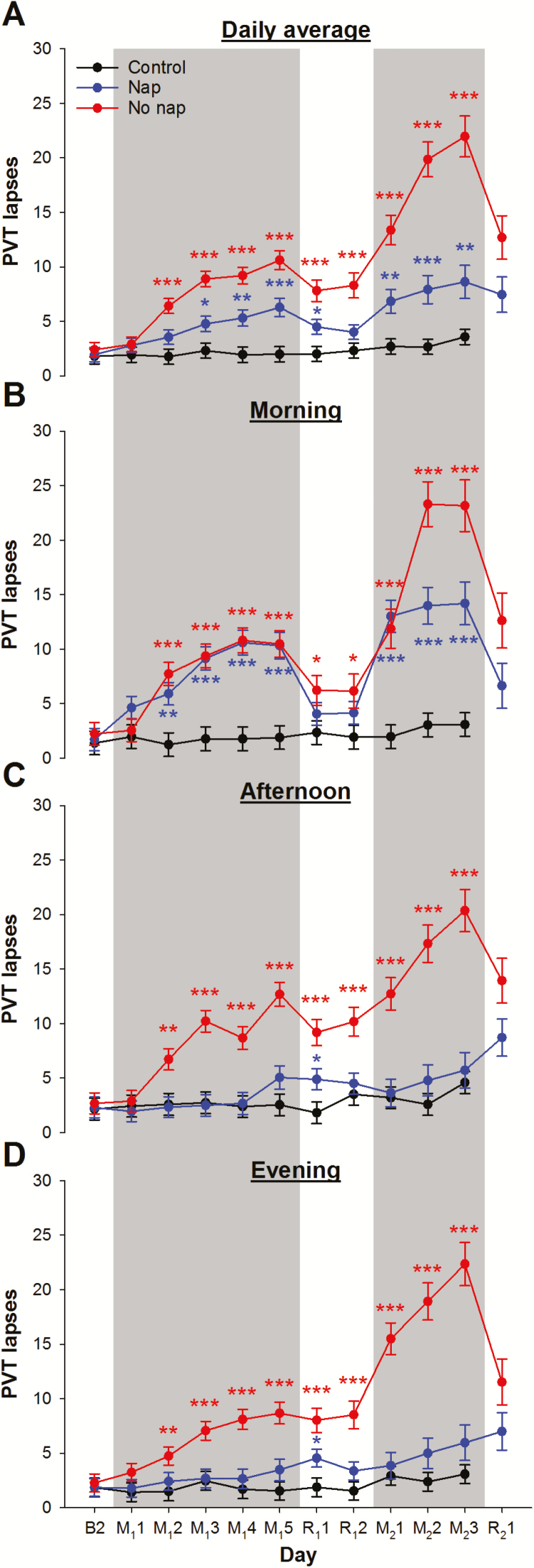
Changes in sustained attention, as indexed by the number of PVT lapses, differed significantly among the three groups (group × day interaction: F = 8.02, p < .001). While performance remained relatively stable for the control group, lapses increased in the no nap group after the second night of 5-hr sleep opportunity (akin to a “Tuesday,” p < .001; Figure 2A). The increase in lapses continued linearly until the end of the first sleep restriction period (like a “Friday”).
Figure 2.
Performance in the psychomotor vigilance task (PVT). The number of PVT lapses of the control group (black line), the nap group (blue line), and the no nap group (red line) (A) averaged across the three test batteries each day, in the (B) morning, (C) afternoon, and (D) evening from the days after the last baseline night (day B2), the first cycle of sleep restriction nights with nap– wake manipulation (day M11 to M15) and recovery nights (R11 and R12), to the second cycle (day M21 to M23; and R21). Shaded areas represent the sleep restriction periods. The least square means and standard errors estimated with general linear mixed models are illustrated. ***p < .001; ** p < .01; *p < .05 for contrasts of the no nap or the nap group with the control group.
After the first night of recovery sleep, the number of lapses in the no nap group dropped significantly (p = .004) but still remained above baseline (p < .001). Even a second night of recovery sleep did not offer additional benefit (p = .62). Thus, after two nights of recovery sleep, simulating extended sleep on weekends, sustained attention did not fully recover.
On reexposure to sleep restriction, the number of lapses in the no nap group not only increased relative to the end of the previous recovery period (i.e., “Monday” vs “the Sunday before,” p < .001) but was also worse than at the end of the first sleep restriction period (i.e., worse this “Monday” than last “Friday,” p = .02). Furthermore, the rate of deterioration was faster during the second period of sleep restriction than the first period. Finally, although the second recovery sleep period resulted in a prominent drop in the number of lapses (p < .001), sustained attention did not return to baseline levels, and remained at the level observed at the beginning of the second sleep restriction period (p = .57).
Effects of Napping on Sustained Attention: Data From the Nap Group
Overall, afternoon napping attenuated the cumulative deterioration in sustained attention (Figure 2A). Specifically, the nap group only started showing an increase in lapses from baseline after the third sleep restriction night (akin to a “Wednesday,” p = .004). The cumulative effects on sustained attention were also less severe in the nap group: At the end of the first sleep restriction period (i.e., “Friday”), the nap group had fewer lapses than the no nap group (p < .001). Nevertheless, two nights of recovery sleep was still insufficient to return performance to the baseline level (p < .001).
Reexposing the nap group to sleep restriction in the second cycle led to an increase in lapses from the previous recovery period (p = .02). However, in contrast to the no nap group, the number of lapses was not different from that observed at the end of the first sleep restriction period (i.e., the same this “Monday” as last “Friday,” p = .58). Additionally, subsequent deterioration in performance was less pronounced than for the no nap group (on M23, p < .001). Critically, despite evidence of benefit, sustained attention of the nap group remained poorer than that of the control group during most sleep restriction days (Figure 2A).
Temporal Evolution of Nap Benefit
Taking an afternoon nap had immediate benefits on sustained attention. During the afternoon PVT administered 1 hr after waking up from the nap, participants had fewer lapses relative to the test taken that morning (Figure 2B and C). Their performance was better than that of the no nap group (p < .001 except for the day after the first sleep restriction night) and was on par with the control group across both sleep restriction periods at this time of day (p > .09; Figure 2C). In contrast, after the second sleep restriction night, the no nap group consistently had more lapses than the control group in the afternoon (p < .003; Figure 2C). Interestingly, the significant gap in performance between the nap and the no nap groups was sustained through the evening tests (approximately 5 hr after nap had ended; p < .05; Figure 2D).
The nap benefit on sustained attention did not extend to the following morning during the first cycle of sleep restriction: The nap group had more lapses than the control group (P <.002) and in fact performed similar to the no nap group (p > .22; Figure 2B) from the second to the last day of sleep restriction. During the second cycle of sleep restriction, napping did confer some protective effect the following morning: The nap group had fewer lapses than the no nap group on the second and third days (p < .004; Figure 2B), although still more than the control group (p < .001).
Other Neurobehavioral Measures
The cumulative effect of repeated exposure to sleep restriction was also observed, albeit to a lesser extent, for other neurobehavioral measures. Specifically, the no nap group displayed greater deficits in working memory/executive function (A’ in the 1- and 3-back tasks; left panels of Figures S1A and S2A, respectively), and speed of processing (slower improvement in the MAT and the SDMT; Figure S3A) in the second sleep restriction period, relative to the first. They also exhibited a more prominent reduction in subjective alertness (KSS; Figure S4A) and positive mood (PANAS; left panel of Figure S5A). Strikingly, although working memory/executive function (i.e., A’ in the 3-back performance) did return to baseline level after two nights of recovery sleep, a faster rate of decline was observed upon reexposure to sleep restriction (left panel of Figure S2A).
As with PVT lapses, the other neurobehavioral measures also showed immediate benefits of afternoon napping (Figure S1-5C) that extended into the evening (Figure S1-5D). Also, we observed benefits to performance (i.e., better performance in the nap relative to the no nap group) in the following morning for subjective alertness at the end of the first sleep restriction period (Figure S4B) and for a working memory task with a high executive load in the second sleep restriction period (left panel of Figure S2B). In contrast, at this time of day, performance in a working memory task with a low executive load (left panel of Figure S1B) and two speed of processing tasks (Figure S3B) as well as mood (Figure S5B) received little protective effect from afternoon naps in the previous waking periods.
DISCUSSION
Contrary to the popular notion that adolescents are relatively resilient to the negative impact of sleep curtailment, we showed that sleep restriction to 5-hr TIB over five nights resulted in cumulative deterioration in adolescents’ cognitive functions, subjective alertness, and mood. We also showed that recovery was incomplete after 9-hr TIB for two simulated weekend nights. Critically, even neurobehavioral functions that had apparently “recovered” showed accelerated deterioration on reexposure to sleep restriction the following “school week” compared to the previous week. While daily 1-hr afternoon naps after each sleep-restricted night alleviated performance degradation, these deficits were not fully eliminated.
Cognitive Deficits are Compounded by a Second Consecutive Cycle of Sleep Restriction
In our previous study,14 we reported progressive deficits in neurobehavioral functions, particularly in sustained attention, in adolescents exposed to seven nights of partial sleep deprivation (5-hr TIB). Extending this work, here, even with the sleep restriction period shortened to five nights—the length of a typical school week—there was still incomplete recovery of sustained attention and other neurobehavioral functions following two nights of “weekend” recovery sleep. These findings are in line with two observations in adults: (1) progressive reduction in sustained attention, working memory/executive function, speed of processing, and subjective alertness following multiple nights of sleep restriction27,36–38 and (2) subsequent partial recovery of some neurobehavioral functions after an extended nocturnal sleep opportunity.36 Others have also found that adolescents who have been sleep-restricted for three to five nights have impaired cognitive performance15 and more frequent reports of sleepiness, inattention as well as problems with metacognition.13
Our novel finding that neurobehavioral functions deteriorate at an accelerated rate on exposure to a second sleep restriction period suggests the presence of residual effects of sleep restriction that were temporarily masked by recovery sleep. With two successive cycles of sleep restriction and recovery, we found no evidence for adolescents adapting to recurrent sleep loss. This contrasts with the finding of allostasis following sleep restriction in rodents,39 which would predict stable (albeit reduced) performance in the second period of sleep restriction as in the first.
The present findings should stimulate study into the broader significance of adverse effects of repeated cycles of sleep restriction on other aspects of adolescent health. For example, habitual sleep variability is associated with abdominal obesity in adolescents.40 Sleep curtailment also influences metabolic processes,41 gene expression,42 and inflammatory responses.43
A 60-Min Mid-Afternoon Nap Improves Neurobehavioral Functions in Sleep-Restricted Adolescents
The neurobehavioral functions of the nap group were, in general, superior to those of the no nap participants possibly due to the additional 37–53 min of sleep they had on the sleep-restricted days, which minimized the accrued sleep debt. This corroborates the cognitive benefits of napping found in adults after a night of sleep restriction.17–21 Previous work showed that these benefits could be observed almost immediately after napping.44 We found similar results, and further demonstrated that napping in the afternoon was still beneficial to neurobehavioral functions in the evening. This could possibly be attributed to the nap’s effect in reducing sleep propensity later in the day.45 However, afternoon naps did not have much benefit on cognitive functions the following morning, particularly in the first sleep restriction period. Interestingly, on exposure to sleep restriction for the second time, the nap group exhibited less deficit in some neurobehavioral functions than the no nap group, suggesting that afternoon naps may be beneficial at this time of day if sleep restriction is recurrent.
Limitations and Future Studies
Our experimental protocol had a few limitations. First, sleep restriction was achieved by both delaying bedtime and advancing wake times by 2 hr in order to align the mid-points for both the sleep episodes and the wake episodes throughout the protocol for minimizing shifting in circadian phase. However, a delay in circadian phase was still observed under such circumstances in earlier adult studies.27,46 Also, dissimilar to our protocol, adolescents may delay their bedtimes on weekends relative to weekdays so that the sleep timing becomes more in line with the maturational delay of their biological clock.5,6 Furthermore, in our protocol, as the test batteries were administered at the same clock times, participants were awake for 2 hr more at each neurobehavioral assessment during the sleep restriction period relative to the baseline and recovery periods. A longer duration of prior wakefulness before testing might accentuate neurobehavioral deficits associated with nocturnal sleep curtailment.
Second, to minimize the inconvenience caused to the participants during their vacation period, the second cycle consisted of only three nights of sleep restriction. However, exacerbated deficits were still found even in this relatively short period of sleep restriction, suggesting that the carryover effects from prior sleep restriction could be immediately observed upon reexposure to sleep loss. Future studies should use more cycles and investigate whether the human homeostatic sleep system shows allostatic responses, thereby allowing neurobehavioral functions to stabilize under conditions of chronic, recurrent sleep restriction.
Third, we shortened participants’ sleep opportunity to 5 hr in each sleep restriction night. This level of sleep restriction may be uncommon in Western countries but is common in Asia where more than half the world lives. A nationally representative survey in Korea has shown that 43% of the adolescents reported sleeping less than 6 hr each night.3 Furthermore, a survey on high school students in Singapore found that on average, the actigraphically estimated TST was below 5.5 hr during weekdays (unpublished data)—a sleep duration similar to the estimate from our sample (Table 1). As such, the severity and duration of sleep restriction used here has real-world relevance. Nevertheless, future studies should investigate the extent of neurobehavioral deficits with different TIBs during repeated cycles of sleep restriction and recovery.
Fourth, although changes in multiple cognitive tasks were tracked in the present study, other cognitive functions that are important for students’ academic performance, such as memory encoding and consolidation as well as creativity, were not studied. The impact of successive cycles of sleep restriction and recovery on these higher order cognitive functions remain to be evaluated in future studies.
Finally, here, the 1-hr nap opportunities for the nap group were placed at 14:00–15:00, and we found minimal impact of afternoon naps on nocturnal sleep in the subsequent nights. However, two surveys have pointed out that adolescents typically nap at 16:00,16 and 48% of adolescent nappers nap for at least 1 hr.2 Furthermore, data from young adults have revealed that the duration and timing of nap episodes can have a significant impact on nocturnal sleep. Specifically, a nap that starts at 18:00 and has an average TST of 75 min can increase sleep latency and shorten sleep in the subsequent night.47 Future studies should address whether late and long naps affect adolescents’ nocturnal sleep similarly and as a result alter the effectiveness of napping in alleviating neurobehavioral deficits48 during recurrent nocturnal sleep curtailment.
CONCLUSION
Sleep restriction for the duration of a school week has cumulative negative effects on cognitive functions, subjective alertness, and mood that cannot be completely reversed by weekend catch-up sleep. A second successive cycle of sleep restriction results in accelerated neurobehavioral impairment. While afternoon naps do confer some benefits, these together with weekend catch-up sleep appear to be less effective in optimizing neurobehavioral functions than having the recommended sleep duration every night. Hence, parents and clinicians should advise adolescents to get the recommended 8–10 hr of sleep each night for optimal performance. Furthermore, educators and policy makers should consider delaying school start time so as to increase nocturnal sleep opportunity for this age-group.49
SUPPLEMENTARY MATERIAL
Supplementary Material is available at Sleep online.
FUNDING
Financial support was provided by the National Medical Research Council, Singapore (NMRC/STaR/015/2013) and The Far East Organization.
DISCLOSURE STATEMENT
None declared.
This work was approved by the Institutional Review Board of the National University of Singapore. All participants and their legal guardian provided written, informed consent.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for Benny Chin Seah Koh for his assistance in participant recruitment, and James Cousins, Jesisca Tandi, Wei Shan Cher, Pearlynne Chong, Bindiya Lakshmi Raghunath, Shin Wee Chong, Karen Sasmita, Nicholas Chee, Wanzheng Zhu, Xuan Kai Lee, Ken Wong, and James Teng for their efforts in data collection and processing. This work was supported by the National Medical Research Council, Singapore (NMRC/STaR/015/2013) and The Far East Organization.
REFERENCES
- 1. Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011; 12(2): 110–118. [DOI] [PubMed] [Google Scholar]
- 2. National Sleep Foundation. Sleep in America poll: Teens and Sleep. Washington, DC:National Sleep Foundation; 2006. [Google Scholar]
- 3. Do YK, Shin E, Bautista MA, Foo K. The associations between self-reported sleep duration and adolescent health outcomes: what is the role of time spent on Internet use? Sleep Med. 2013; 14: 195–200. [DOI] [PubMed] [Google Scholar]
- 4. Ohida T, Osaki Y, Doi Y, et al. An epidemiologic study of self-reported sleep problems among Japanese adolescents. Sleep. 2004; 27(5): 978–985. [DOI] [PubMed] [Google Scholar]
- 5. Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58(3): 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crowley SJ, Van Reen E, LeBourgeois MK, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014; 9: e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005; 28(11): 1446–1454. [DOI] [PubMed] [Google Scholar]
- 8. Short MA, Gradisar M, Wright H, Lack LC, Dohnt H, Carskadon MA. Time for bed: parent-set bedtimes associated with improved sleep and daytime functioning in adolescents. Sleep. 2011; 34(6): 797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005; 115(1 Suppl): 250–256. [DOI] [PubMed] [Google Scholar]
- 10. Bartel KA, Gradisar M, Williamson P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev. 2015; 21: 72–85. [DOI] [PubMed] [Google Scholar]
- 11. Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatr Clin North Am. 2011; 58(3): 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Bruin EJ, Van Run C, Staaks J, Meijer AM. Effects of sleep manipulation on cognitive functioning of adolescents: A systematic review. Sleep Med Rev. 2016. doi:10.1016/j.smrv.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 13. Beebe DW, Fallone G, Godiwala N, et al. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. J Child Psychol Psychiatry. 2008; 49(9): 915–923. [DOI] [PubMed] [Google Scholar]
- 14. Lo JC, Ong JL, Leong RL, Gooley JJ, Chee MW. Cognitive Performance, Sleepiness, and Mood in Partially Sleep Deprived Adolescents: The Need for Sleep Study. Sleep. 2016; 39(3): 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003; 74(2): 444–455. [DOI] [PubMed] [Google Scholar]
- 16. Gradisar M, Wright H, Robinson J, Paine S, Gamble A. Adolescent napping behavior: Comparisons of school week versus weekend sleep patterns. Sleep Biol Rhythms. 2008; 6:183–186. [Google Scholar]
- 17. Brooks A, Lack L. A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep. 2006; 29(6): 831–840. [DOI] [PubMed] [Google Scholar]
- 18. Gillberg M, Kecklund G, Axelsson J, Akerstedt T. The effects of a short daytime nap after restricted night sleep. Sleep. 1996; 19(7): 570–575. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi M, Arito H. Maintenance of alertness and performance by a brief nap after lunch under prior sleep deficit. Sleep. 2000; 23(6): 813–819. [PubMed] [Google Scholar]
- 20. Tietzel AJ, Lack LC. The short-term benefits of brief and long naps following nocturnal sleep restriction. Sleep. 2001; 24(3) :293–300. [DOI] [PubMed] [Google Scholar]
- 21. Tietzel AJ, Lack LC. The recuperative value of brief and ultra-brief naps on alertness and cognitive performance. J Sleep Res. 2002; 11(3): 213–218. [DOI] [PubMed] [Google Scholar]
- 22. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 23. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991; 14(6): 540–545. [DOI] [PubMed] [Google Scholar]
- 24. Meijer AM. Chronic sleep reduction, functioning at school and school achievement in preadolescents. J Sleep Res. 2008; 17(4): 395–405. [DOI] [PubMed] [Google Scholar]
- 25. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 26. Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010; 136(3): 375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lo JC, Groeger JA, Santhi N, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One. 2012; 7(9): e45987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990; 52(1–2): 29–37. [DOI] [PubMed] [Google Scholar]
- 30. Smith A. Symbol Digit Modalities Test. Los Angeles: Western Psychological Services; 1991. [Google Scholar]
- 31. Klein KE, Wegmann HM, Athanassenas G, Hohlweck H, Kuklinski P. Air operations and circadian performance rhythms. Aviat Space Environ Med. 1976; 47(3): 221–230. [PubMed] [Google Scholar]
- 32. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988; 54(6): 1063–1070. [DOI] [PubMed] [Google Scholar]
- 33. Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985; 17(6): 652–655. [Google Scholar]
- 34. Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: Rules, terminology, and technical specification. first ed Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 35. Ong JL, Lo JC, Gooley JJ, Chee MWL. EEG across multiple nights of sleep restriction and recovery in adolescents: the Need for Sleep Study Sleep. 2016; 39(6): 1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010; 33(8): 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003; 12(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 38. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003; 26(2): 117–126. [DOI] [PubMed] [Google Scholar]
- 39. Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007; 104(25): 10697–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. He F, Bixler EO, Liao J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015; 16(12): 1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc Natl Acad Sci U S A. 2011; 108(Suppl 3): 15609–15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Möller-Levet CS, Archer SN, Bucca G, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A. 2013; 110(12): E1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010; 24(5): 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hayashi M, Watanabe M, Hori T. The effects of a 20 min nap in the mid-afternoon on mood, performance and EEG activity. Clin Neurophysiol. 1999; 110(2): 272–279. [DOI] [PubMed] [Google Scholar]
- 45. Horne J, Anderson C, Platten C. Sleep extension versus nap or coffee, within the context of ‘sleep debt’. J Sleep Res. 2008; 17(4): 432–436. [DOI] [PubMed] [Google Scholar]
- 46. Rogers NL, Dinges DF. Interaction of chronic sleep restriction and circadian system in humans. J Sleep Res. 2008; 17(4): 406–411. [DOI] [PubMed] [Google Scholar]
- 47. Werth E, Dijk DJ, Achermann P, Borbély AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996; 271(3 Pt 2): R501–510. [DOI] [PubMed] [Google Scholar]
- 48. Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009; 18(2): 272–281. [DOI] [PubMed] [Google Scholar]
- 49. Adolescent Sleep Working Group, Committee on Adolescence, and Council on School Health. School start times for adolescents. Pediatrics. 2014; 134(3): 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.