Abstract
Introduction:
Slow-wave sleep (SWS) slow waves and sleep spindle activity have been shown to be crucial for memory consolidation. Recently, memory consolidation has been causally facilitated in human participants via auditory stimuli phase-locked to SWS slow waves.
Aims:
Here, we aimed to develop a new acoustic stimulus protocol to facilitate learning and to validate it using different memory tasks. Most importantly, the stimulation setup was automated to be applicable for ambulatory home use.
Methods:
Fifteen healthy participants slept 3 nights in the laboratory. Learning was tested with 4 memory tasks (word pairs, serial finger tapping, picture recognition, and face-name association). Additional questionnaires addressed subjective sleep quality and overnight changes in mood. During the stimulus night, auditory stimuli were adjusted and targeted by an unsupervised algorithm to be phase-locked to the negative peak of slow waves in SWS. During the control night no sounds were presented.
Results:
Results showed that the sound stimulation increased both slow wave (p = .002) and sleep spindle activity (p < .001). When overnight improvement of memory performance was compared between stimulus and control nights, we found a significant effect in word pair task but not in other memory tasks. The stimulation did not affect sleep structure or subjective sleep quality.
Conclusions:
We showed that the memory effect of the SWS-targeted individually triggered single-sound stimulation is specific to verbal associative memory. Moreover, the ambulatory and automated sound stimulus setup was promising and allows for a broad range of potential follow-up studies in the future.
Keywords: EEG, memory, acoustic stimulation, slow-wave sleep, auditory-evoked K-complex.
Statement of Significance
We developed an automated acoustic stimulation system that can facilitate learning. Precisely timed sounds were targeted to single slow waves recorded by EEG while healthy human participants were sleeping. The acoustic stimulation was able to increase the number of correctly recalled word pairs after the night sleep. Using several different memory tasks, we found that the memory effect is specific to verbal associative memory. The results provide important insights into the neural underpinnings of memory consolidation in sleep. In addition, our ambulatory and automated stimulus protocol opens new possibilities for clinical studies and further validation of the method.
INTRODUCTION
Recent evidence stresses the critical role of sleep in learning, in particular for memory consolidation.1 Being awake after the initial learning increases the risk of forgetting,2 whereas even a short nap protects memories against new conflicting experiences.3,4 However, the causal relationship between different sleep-related neural activities and different types of memory mechanisms is still being debated.
Current evidence supports the model of consolidation, where three different types of neural activities are hierarchically linked to each other.5 The memory material is replayed from the hippocampus to the neocortex via fast spindle activity,1 seen as minimum 500 ms bursts of 13–15 Hz activity in electroencephalography (EEG).6,7 In the hippocampus, the sleep spindles are phase-locked with high-frequency bursts (~80–100 Hz in humans), called hippocampal ripples.1,5 The sleep spindles are temporally grouped by large-scale cortical slow waves, the markers of slow-wave sleep (SWS).5,8 Support for this model has been found in both rodents and humans. Repetition of neural activity patterns related to awake experiences were shown to be replayed during SWS in the sensory cortical areas and hippocampus.9 In a combined EEG-fMRI study performed in humans, Bergmann et al.10 observed sleep spindle-related hemodynamic activity in SWS both in the hippocampus and in the task-specific cortical area, and reported that the strength of this network activity was related to memory recall performance. Larger scale dynamics of the consolidation mechanism, shown by Takashima et al.,11 revealed that memory consolidation-related hippocampal activity was systematically reduced at 1, 30, and 90 days after learning, while the cortical activity pattern was enhanced. The cortical activity pattern representing the memorized material not only strengthens over time, but is also distributed to wider neural networks.12,13 Thus, it seems that during the consolidation process the engram becomes gradually less dependent on the hippocampus, while storage of memorized material is distributed to the sensory areas of the neocortex (for another hypothesized model for sleep-related memory consolidation, see the study of Tononi and Cirelli14).
The neural memory consolidation process is sensitive to several types of sensory interference. During deep sleep, repetition of the learned material has been found to enhance the consolidation process.15,16 Similarly, memory consolidation can be excited by exposing a sleeping person to stimuli that is otherwise associated with the learning event, e.g., a smell.17,18 An alternative approach is to use sensory stimuli, such as acoustic stimulation, that are not directly associated with the learned material or learning event. Their possible influence on the mechanism is likely related to emergence of a K-complex (KC), which can both rise spontaneously and be evoked by a sensory stimulus (for review, see the study of Colrain19). An auditory stimulus presented during non-REM (NREM) sleep is the most common way to trigger a KC wave, but largely similar KCs can be triggered also by other stimulus modalities (visual, somatosensory).20
Ngo et al.21 used acoustic stimulation during SWS by presenting a train of sounds with a repetition rate at typical slow-wave activity (SWA) frequency. The results showed an increase in SWA, but no memory tasks were tested to show specific interference with a memory consolidation mechanism. Next Ngo et al.22 used a similar stimulus, now time-locked to spontaneous slow-wave onsets during SWS, and the acoustic stimulus was found to evoke additional slow waves as well as sleep spindle activity during the positive phases of slow waves. In addition, recall performance in a recall memory task was strikingly improved and correlated with sleep spindle activity enhancement. This finding of improving the memory consolidation process by slow wave-triggered acoustic stimulation has been replicated recently23 and even more advanced methods have been developed for accurate triggering.24
The idea of improving memory by a simple procedure during sleep is intriguing. However, in addition to safety issues, the procedure itself needs to be simplified and further validated. In the current study, we aimed at developing a simplified and automated protocol which could accurately target sound stimuli to single spontaneous slow waves during NREM sleep without the need for manual control. We elucidated the memory type specificity of sound stimulation by performing different memory tasks. Finally, we also evaluated the effects of sound stimulation on subjective sleep quality and mood.
METHODS
Participants
Fifteen healthy subjects (7 women, 8 men) with a mean age of 30.5 (range 23–42) years participated in the study. None of the participants had been diagnosed with sleep disorders or had medication that interferes significantly with sleep patterns. The participants were instructed to follow their regular circadian rhythm until all laboratory test nights were completed and to avoid alcohol for 3 days preceding every laboratory test night. All subjects gave their written informed consent before participation. The experiments were performed in accordance with the Declaration of Helsinki, and the study protocol was approved by the Research Ethics Committee of the Helsinki and Uusimaa Hospital District.
Biosignal recording, stimulus material, and the stimulus system
The EEG, electrooculography, and electrocardiogram data were recorded by a wireless Enobio system (Neuroelectrics, Spain). Electrode contacts were prepared with abrasive paste (Abralyt2000, Easycap, Germany or Elektrodipasta, Berner, Finland). Ag/AgCl cup electrodes were attached beneath the hair with EC2 paste (Grass Technologies, USA). These EEG electrodes were placed in the standard locations of Fz, Cz, CPz, and Oz based on the 10/20 system. In the online recording, the Cz electrode acted as a common reference and the amplifier’s common mode feedback was connected to the electrode located at FCz. Additionally, single-use adhesive electrodes (Ambu, Denmark) were placed at Fpz (forehead), left and right mastoids, lower corner of the left eye (LOC), and upper corner of the right eye (ROC). The online algorithm controlling for acoustic stimulation utilized only these single-use electrodes attached to hair-free area. Other electrodes were used only to validate the data. Finally, a combined electrocardiogram/electromyograph electrode was attached to the left front shoulder. The data were hardware filtered to DC-250 Hz and sampled with a frequency of 500 Hz. The EEG data were transferred in real-time via Bluetooth from the bioamplifier to a tablet computer (Surface Pro 1, Microsoft Inc., USA), where the data were received by a custom-made C++ program and shared with the Matlab (Mathworks Inc., USA) program.
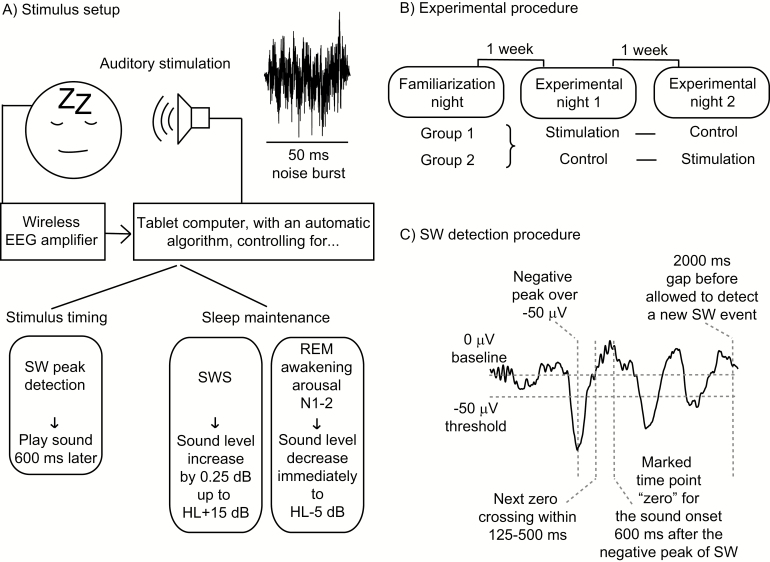
The auditory stimulus was generated by Matlab and comprised 50 ms long 1/f noise burst with 5 ms onset and offset fading (sampling frequency 44.1 kHz). Sounds were played via USB soundcard (Nuforce Icon uDAC2, Nuforce Inc., USA) and an active loudspeaker (Genelec 2029A, Genelec Oy, Finland) located 125 cm above the participants’ head. The stimulus timing was controlled by the C++ program running on the tablet computer and the triggering delay was calibrated and automatically compensated to zero before every recording. To detect the onsets of the slow waves the EEG signal on mastoid-referenced Fpz channel was bandpass filtered to 0.3–35 Hz. The algorithm then detected negative peaks of at least −50 µV whose next zero crossing was 125–500 ms ahead (corresponding to a wave frequency of 0.5–2 Hz, as the distance between negative peak and the next zero crossing is ¼ of a full cycle of pure sine wave; see Figure 1C). The sound onset was accurately 600 ms after the detected negative peak of slow wave (see Figures 1C and 4). After the detection of a slow wave, the algorithm waited for 2000 ms before being ready for a new detection.
Figure 1.
Visualization of the research procedure. In Figure 1C, the signal is the single trial data from the Fpz channel from one subject during the control night, during which no sounds were presented. HL = hearing level; SW = slow wave; SWS = slow-wave sleep.
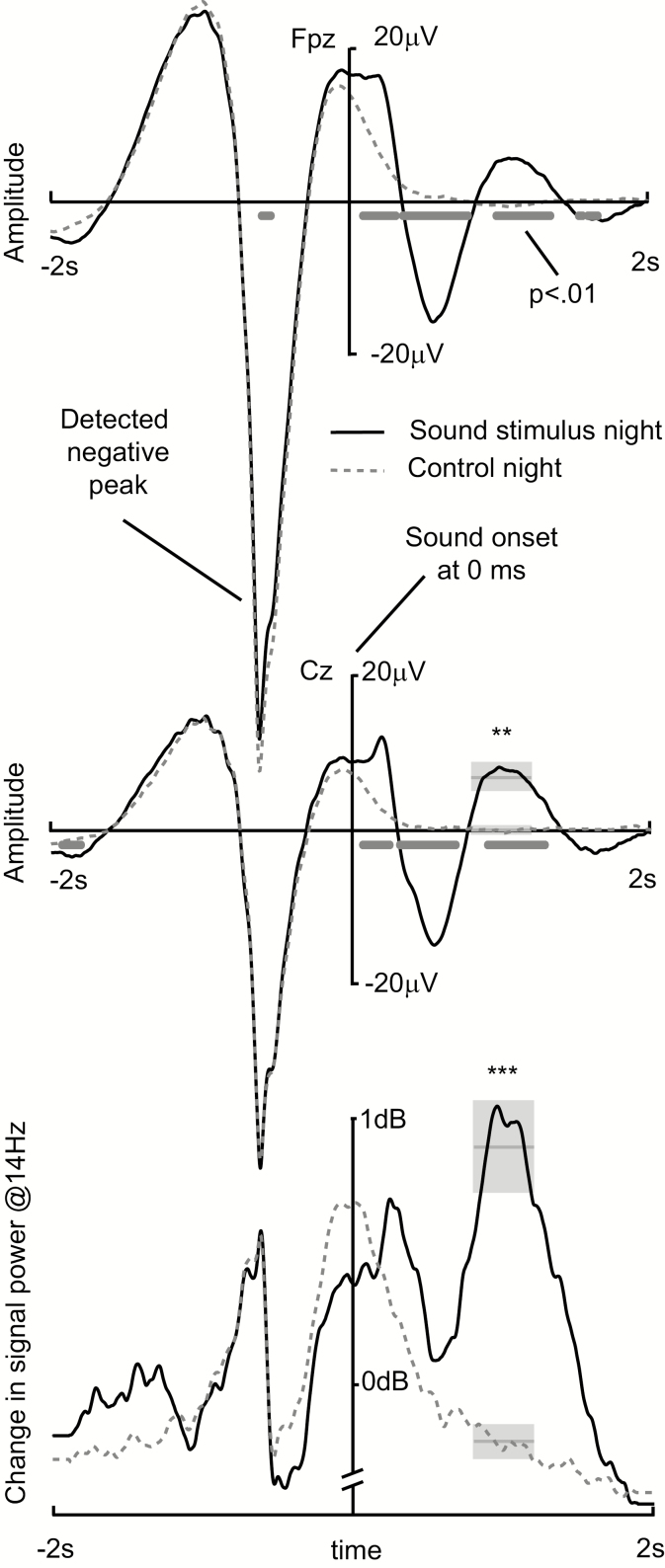
Figure 4.
Electroencephalography (EEG) results. Upper two curves show the group average of the EEG response in Fpz and Cz channels time locked to the negative peak of spontaneous slow wave. An automatic online algorithm was using the channel Fpz to detect slow waves and the channel Cz was used in group level offline statistics. Below the curves the time points of significant difference between conditions are shown based on paired samples t-test with noncorrected alpha level of 0.01. During the stimulus night the sound stimulus was presented at 600 ms after the slow-wave peak (time zero in this image), whereas during the control night the sound stimulus was missing. Lowest curve shows change in the 14 Hz oscillatory power from baseline (from −2 seconds to zero) in decibels. The grey boxes show the time window (width), mean (horizontal line in the middle), and its standard error (height of the box) of values extracted from the curves for statistical analyses. **p < .01, ***p < .001.
Independently of the stimulus-triggering mechanism, the stimulus loudness was controlled automatically to avoid waking the participants and to target the stimuli to SWS (see Figure 1A). The algorithm used mastoid-referenced LOC and ROC channels, in which beta activity (power in 18–45 Hz, where power increase indicates lighter sleep or awakening), eye movements (indicating REM sleep or awaking), and SWA (power in 0.5–6 Hz) were calculated.25 All indices were updated at 0.5-second time intervals and calculated in 2 s Hann windowed epochs. SWA-dominated sleep pattern (correlation coefficient larger than 0.5 and peak-to-peak difference more than 20 µV in SWA of LOC and ROC channels25,26) increased the sound volume from a minimum of 5 dB below the hearing threshold until the maximum level of 15 dB above the hearing threshold was reached (0.25 dB step after every 0.5 s interval). Eye movements (less than −0.75 correlation between LOC and ROC) and increase in beta activity (at least 5 times the median beta activity over previous 30 time windows) caused an immediate lowering of the sound level to the minimum.
Memory tests
To compare different memory systems, we used recall tests for semantically associated items, procedural sequences, and pictures with emotionally different contents. The memory tasks were semantic word pair task, face-name association task, finger tapping, and picture recognition task. All memory tests were performed by using the tablet computer and USB keyboard. Picture recognition and word pair tests were programmed in C# programming language, finger tapping task in Visual Basic, and face-name test was running on a PsychoPy version 1.80 programming environment.27
The word pair test included 240 semantically related word pairs (e.g., “muscle—tendon”), which were divided into two lists. Word pairs were translated and adapted from German, and the same stimuli have been used in several studies related to memory consolidation in sleep.22,28,29 Order of word pairs in the list was randomized for each subject, and both lists were equally often used on the stimulus night and on the control night across participants. In the learning phase, every word pair was visible for 4 s on the tablet screen, while the interstimulus interval (ISI) was 1 s. The first recall test was performed immediately after the learning phase. The first member of each word pair (in random order) was displayed, while the participants were asked to write its pair on the tablet. After the response, the correct answer was visible for 2 s independent of the correctness of the participant’s answer. The delayed recall task took place the next morning. The procedure was the same except that the correct answer was not presented. The memory performance was scored by counting together all correctly recalled words. The correct answers included responses that were clearly typos or inflectional errors (e.g., a plural form). Derivational mistakes were counted as errors (e.g., crime → criminality).
The face pictures for face-name associations test were chosen from The Center for Vital Longevity Face Database.30 The faces were for 18- to 29-year-old adults equally from both genders. The names were chosen from the most common first names in Finland for people born in 1970–1990. For each test night, 30 individually randomized face-name pairs were constituted in a way that gender of the face and the name matched. In the learning phase, each face was visible for 500 ms. After the face image, the written name was shown under the face image and also heard at a comfortable loudness. In the immediate recall, the face images were presented in random order, and participants were asked to write the recalled name on the table computer. After the response, the correct answer was heard. The morning recall was similar except that the correct answer was not heard. The answers were classified as correct, wrong, and missing.
In the finger tapping task, the participants placed four fingers (other than thumb) of the nondominant hand on the standard keyboard’s number buttons 1–4. Then they were given 5-unit-long digit series (e.g., “4-1-3-2-4”) that they were allowed to practice for 10 loops. Thereafter, they were instructed to repeat the series as many times as they could, as quickly and correctly as they could. The instruction was visible on the screen all the time. The task consisted of 6 blocks, each 30 s long with a 5 s break in between. The performance was scored by counting together all correctly repeated series in all 6 blocks. The test was repeated in the morning with the same digit series, but without the practicing loops. The task included 2 different sets of digit series, which were counterbalanced between the participants and test nights.
Pictures of the picture recognition task were obtained from the picture library of the International Affective Picture System (IAPS).31 For both nights, 119 pictures were used. Randomly presented images were visible for 4 s and ISI was 1 s. In an immediate recall, the participants answered with two response buttons whether or not they had seen the picture in the previous list of images. In addition to the original 119 learned images, the list included 119 new images. The order of pictures was randomized. The memory performance was scored by counting together correctly recognized pictures. Each picture had been rated in valence and arousal scales by 100 subjects.31 Thus, pictures were also divided into high versus low valence and into high versus low arousal categories based on the median value.
Questionnaires
If the sound stimulus causes a measurable difference in sleep quality, the memory performance in the morning might be affected by overall difference in cognitive ability (performing the task is more difficult after a poorly slept night). To be able to investigate this possibility, we used the NASA-TLX test for subjective cognitive load.32 Participants marked on a six-dimension continuous scale how much effort the completion of memory tasks required. The effect of each test night was calculated by subtracting the evening value from the corresponding morning measure.
Subjective sleep quality was also directly assessed with a questionnaire every morning. The variable was formulated by calculating together the answers to two questions: (1) How did you sleep last night (1 = well, …, 4 = badly) and (2) Did you sleep worse or better than usually (1 = better, …, 5 = worse). We also asked if the participants had heard sounds during the night. This question was used to find out if the sound level adaptation worked and if the subjects correctly guess which night the sound stimulus was present compared with the control night.
To control for the mood-related variables and to measure possible qualitative changes in sleep, participants filled out a shortened version of the Profile of Mood States (POMS) questionnaire33 every evening and morning. The questionnaire includes 38 questions with a 5-point scale (0 = not at all, …, 4 = very much). The mood variables were calculated as a sum of 3–7 questions each (some of them reversed); tension-anxiety, fatigue, inattentivity, vigor-activity, depression, anger-hostility, inefficiency, and confusion. (Scales are slightly modified from the original short form of POMS partly due to Finnish translation. Also two additional scales were created: inattentivity [3 adjectives] and inefficiency [3 adjectives]. However, their relevance in light of the results of the current study is minimal.) The sleep effect was calculated by subtracting the evening score from the morning score of each sum scale.
Procedure
The participants visited the sleep laboratory (Brain and Work Research Center, Finnish Institute of Occupational Health, Helsinki, Finland) for three nights, all of them separated by one week (see Figure 1B). The facilities allowed three participants to be recorded simultaneously in separate isolated sleeping rooms with identical equipment. The purpose of the first night was to get participants familiarized with sleeping with equipment in the sleep laboratory. The other 2 nights were experimental test nights, and the order of them was randomized and balanced across the participants but also within the group of three simultaneously measured participants. During the stimulus night the sound stimuli were presented by the protocol described above. During the control night the stimulus algorithm only marked the detected slow waves in the data, but no sound was presented.
Participants arrived at the laboratory 3–4 hours before their typical sleep onset. Completion of memory tests and questionnaires took one hour and was typically performed between 9 and 10 pm. Tests were performed in acoustically isolated rooms without external distractors. Participants did not use any electronic equipment or watch displays (e.g., mobile phones, tablet computers, or television) in bed or just before sleeping. The hearing threshold was tested every evening with the stimulus sounds individually for each participant. It allowed us to control for the sound pressure level against the hearing level, but it also made the participants believe that the sounds were potentially played every night. Participants did not know beforehand that some of the nights would be without sound stimulus. When subjects went to the bed and lights were switched off, the algorithm controlling the detection of slow-wave events and sound stimulus was activated. Thereafter, the automatic sound level control took care of sound levels also during short awakenings. Subjects were monitored by research personnel for ethical safety reasons, but they did not interact with the algorithm, which autonomously determined deep sleep and detected slow-wave events. The wake-up time depended on the participant’s own sleeping rhythm, but the total sleeping time was at least 7 hours if possible. Usually waking up took place between 7 and 8 am. About 20–30 minutes after waking, the delayed recall memory tests were performed and the morning questionnaires completed. The morning test lasted typically about 30 minutes.
Data analysis
Data analyses were performed on data from the Cz channel, which was referenced digitally to the average of the mastoid electrodes. The evoked response analysis was time-locked to the spontaneous slow-wave negative peak detected by the online algorithm described above. Thus, for the control night, the averaged responses showed an average of spontaneous slow waves. Correspondingly, the stimulus night response included the average of the spontaneous slow waves and evoked activity caused by the sound stimulus, whose onset was 600 ms later than the negative peak of a spontaneous slow wave. For clearer presentation, all of the time axes in this paper have a zero at the time of sound stimulus onset or corresponding moment in the control night data. For statistical testing, the evoked response was quantified by calculating an average in the time window from 800 to 1200 ms. The time window of interest was chosen based on previous literature to cover the positive component of expected KC evoked by a sound stimulus (see also Figure 4). The sleep spindle activity was measured with wavelet transformation (3 cycles, Morlet base function), which was done using the EEGLAB toolbox in Matlab.34 The preliminary analysis was first performed to find an individual local maximum in the wavelet spectrum between 12 and 15 Hz. All subjects had a local spectrum maximum at approximately 14 Hz, and thus, this frequency point was chosen for the sleep spindle analysis. Spontaneous slow-wave event-related synchronization of sleep spindle activity was quantified by calculating the average within the time window of 800–1200 ms. To analyze the accuracy of the slow-wave event detection algorithm, the phase of SWA at the time of tone onset (or corresponding time in the control condition) was calculated. First EEG signal was band pass filtered to SWA frequency band (0.5–2 Hz), and then Hilbert transformed. Phase data were extracted at time point zero (tone onset) and circular means, standard deviations, and distributions were calculated in CircStat toolbox35 in Matlab.
Statistical analyses of different variables were performed with a paired t-test, with stimulus night and control night acting as a repeated factor. The normality of the variables was confirmed by the Kolmogorov–Smirnov test. In a picture recognition test, the effect of emotional components was tested with repeated measures ANOVA with the factors of Arousal (two levels, low and high) and Valence (two levels, low and high). All of the statistical testing was done using Sigmaplot software (version 11.0, Systat Software, Germany). The significance threshold of 0.05 was used and all the accurate p values were reported.
To validate the accuracy of an automatic sleep staging algorithm, an experienced professional sleep technologist manually scored the sleep data in 30 s segments to awake, REM, N1, N2, or N3, and marked all the arousals. The analysis was blinded so that the technologist did not know which data sets were which experimental conditions.
RESULTS
Memory tests
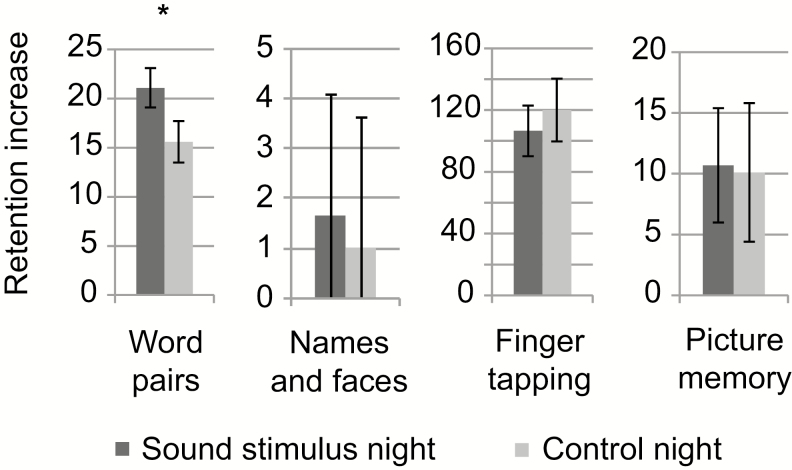
Cued recall performance in the word pair test improved during the sound stimulus night significantly more than during the control night [t(14) = 2.93; p = .01; see also Figure 2 and Supplementary Table S1]. On average, participants correctly recalled 21.1 (standard deviation [SD] 7.7) word pairs more on the morning after the sound stimulus night than in the evening, while during the sham stimulus night the overnight improvement was 15.6 (SD 8.1) words (large overall improvement was likely partly due to feedback during the evening measure). In the face-name association task, picture recognition task (for all pictures together), or serial finger tapping task, the improvement difference between test nights was not statistically significant (see Table 1). Also the emotional content in pictures did not modulate the effect of sound stimulus (interaction of sound stimulus and arousal: F(1,14) = 2.12, p = .17; interaction of sound stimulus and valence: F(1,14) = 0.39, p = .54).
Figure 2.
Memory performance test results. The unit of retention is the number of correctly recalled words in the word pair task, the number of correctly recalled names in the names and faces test, the number of correctly tapped sequences in the finger tapping test, and the number of correctly recognized pictures in the picture recognition test. In all tests, the test scores were calculated by subtracting the number of correctly recalled items on the evening test from the morning performance. *p < .05.
Table 1.
Results of Memory Tests.
| Sound mean | SD | SEM | No sound mean | SD | SEM | t(14) | p | |
|---|---|---|---|---|---|---|---|---|
| Word pairs | 21.1 | 7.7 | 2.0 | 15.6 | 8.1 | 2.1 | 2.93 | .01* |
| Names & faces | 1.7 | 2.4 | 0.6 | 1.0 | 2.6 | 0.7 | 1.32 | .21 |
| Serial finger tapping | 106.6 | 63.7 | 16.4 | 120.0 | 78.8 | 20.3 | −0.72 | .48 |
| Pictures | 10.7 | 18.1 | 4.7 | 10.1 | 21.9 | 5.7 | 0.15 | .88 |
*p < .05. SEM = standard error of mean; SD = standard deviation.
In this table, results of memory tests are shown for test night (sound) and control night (no sound) along with t-test results. The unit of retention is the number of correctly recalled words in the word pair task, the number of correctly recalled names in the names and faces task, the number of correctly tapped sequences in the finger tapping test, and the number of correctly recognized pictures in the picture recognition test. Test scores were calculated by subtracting the number of correctly recalled items on the evening test from the morning performance.
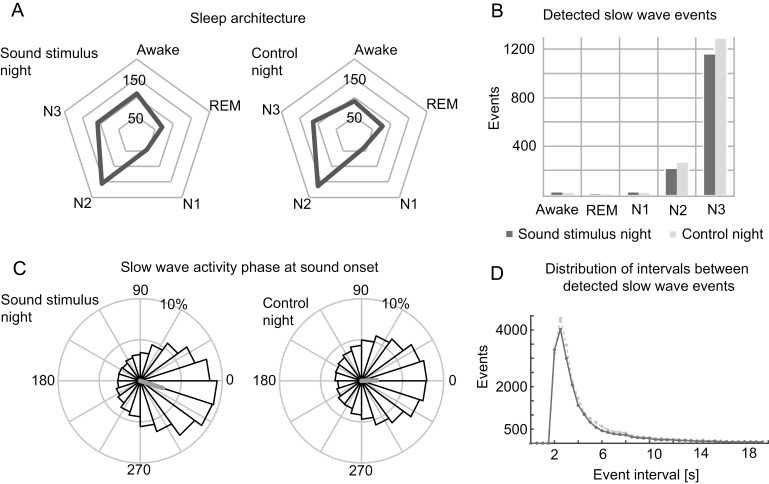
Stimulus targeting and sleep staging
The sleep characteristics did not differ between the test night and the control night (see Figure 3A and Supplementary Table S3). The correlation analyses between the sleep parameters and memory retention (28 correlation tests) found only one significant correlation with the amount of N2 sleep during the stimulus night (p = .04; see Supplementary Table S5). The automatic algorithm aimed at triggering individual stimulus sounds according to the detected slow-wave events in SWS. The comparison with manually scored sleep stages shows that about 83% of detected slow-wave events were found in N3, about 15% in N2, and a few single false alarms in some subjects in N1 or awake (see Figure 3B and Supplementary Table S4). On average, about 1400 tones were delivered over night for each subject. Based on the actual data, the minimum interval between two separate sounds was 2127 ms and the full distribution of interevent intervals for detected slow wave events is in Figure 3D. The average phase of SWA at the tone onset (or corresponding time point in control condition) over all the subjects was −18.0 (SD 67.4) degrees and 2.4 (SD 71.1) degrees for stimulus and control night, respectively. The distribution of phases is shown in Figure 3C (for all the subjects individually, see Supplementary Figure S1).
Figure 3.
Sleep architecture, accuracy of stimulation, and stimulation pattern. A: Manually scored mean amounts of different sleep stages in minutes during sound stimulus and control nights. B: Mean amount of automatically detected slow-wave events in each manually scored sleep stage. C: The distribution and circular mean phase of slow-wave activity over all the subjects at the time of sound onset or corresponding time in control night. D: Distribution of intervals between detected slow-wave events over all the subjects separately for sound stimulus and control nights.
Evoked and induced responses in EEG
EEG data showed a response evoked by the slow wave-locked sound stimulation [see Figure 4, t(14) = 3.82, p = .002]. Also the increase of activity in the sleep spindle frequency band was significant [t(14) = 6.32, p < .001]. The linear correlation between sleep spindle activity increase and word pair recall performance did not significantly explain the variance [F(1,13) = 0.68, p = .42].
Questionnaires
The results showed no difference in subjective cognitive load caused by memory tasks (see Table 2) or in subjective sleep quality [t(14) = 0.49, p = .63]. Furthermore, participants could not reliably remember afterwards if they heard sounds between the hearing threshold test in the evening and awakening [t(14) = 1.00, p = .34]. All mood-related scales improved during the night regardless of the condition, but for the tension-anxiety scale the change was smaller after the sound stimulus night [t(14) = 2.56, p = .02; see Table 3]. However, already the baseline of the tension-anxiety scale differed between the nights (p = .05, see Supplementary Table S2), whereas the same score in the morning did not differ between the nights. Further, the tension-anxiety baseline scores did not correlate with word pair memory task performance improvement (r = .052, p = .86, and r = .043, p = .88 for stimulus and control nights, respectively).
Table 2.
Results of Questionnaire for Experienced Cognitive and Physical Task Load.
| Sound mean | SD | SEM | No sound mean | SD | SEM | t(14) | p | |
|---|---|---|---|---|---|---|---|---|
| Mental load | −20.5 | 19.1 | 4.9 | −14.1 | 15.2 | 3.9 | −1.19 | .26 |
| Physical load | 0.0 | 8.4 | 2.2 | −1.7 | 13.1 | 3.4 | 0.39 | .70 |
| Temporal load | 4.0 | 15.9 | 4.1 | 0.1 | 16.3 | 4.2 | 1.09 | .29 |
| Performance | −9.6 | 10.5 | 2.7 | −10.1 | 19.6 | 5.1 | 0.07 | .94 |
| Effort | −6.4 | 20.2 | 5.2 | −10.9 | 19.1 | 4.9 | 0.98 | .34 |
| Frustration | −7.8 | 16.9 | 4.4 | −17.7 | 31.2 | 8.1 | 1.43 | .18 |
SEM = standard error of mean; SD = standard deviation.
Table 3.
Results of Mood Questionnaire.
| Sound mean | SD | SEM | No sound mean | SD | SEM | t(14) | p | |
|---|---|---|---|---|---|---|---|---|
| Tension anxiety | −0.2 | 1.3 | 0.3 | −1.2 | 1.0 | 0.3 | 2.56 | .02* |
| Fatigue | −1.1 | 1.6 | 0.4 | −1.2 | 2.4 | 0.6 | 0.10 | .92 |
| Inattentivity | −0.5 | 1.0 | 0.3 | −1.1 | 2.4 | 0.6 | 1.02 | .33 |
| Vigor activity | −0.1 | 2.2 | 0.6 | −0.9 | 2.5 | 0.7 | 1.05 | .31 |
| Depression | −0.8 | 1.8 | 0.5 | −0.5 | 1.1 | 0.3 | −0.54 | .60 |
| Anger hostility | −0.2 | 1.7 | 0.4 | −1.2 | 1.4 | 0.4 | 1.71 | .11 |
| Inefficiency | −0.5 | 0.9 | 0.2 | −1.0 | 1.6 | 0.4 | 1.10 | .29 |
| Confusion | −1.0 | 1.5 | 0.4 | −0.8 | 1.7 | 0.4 | −0.36 | .72 |
*p < .05. SEM = standard error of mean; SD = standard deviation.
In this table, results of experienced cognitive and physical task load caused by the memory tests are shown for test night (sound) and control night (no sound) along with t-test results. The effect of each night was calculated by subtracting the evening value from the corresponding morning measure.
In this table, results of POMS mood questionnaire are shown for test night (sound) and control night (no sound) along with t-test results. Test scores were calculated by subtracting the evening score from the morning score of each sum scale.
DISCUSSION
In this study, we aimed at developing an automated auditory stimulation protocol for SWS. Starting with the basic parameters (short pink noise bursts as stimulus triggered by negative peaks of slow waves during SWS and semantically associated word pairs as memory task) adopted from the study of Ngo et al.,22 the protocol was refined: the stimulus protocol was simplified by using individually triggered single sounds and the procedure was automated. These steps will be necessary for the application of the procedure outside laboratory conditions. The improvement in the cued memory recall performance after the sound stimulus was observed also in our study, thereby supporting the previous findings22 and further showing that slow wave-locked sound stimulus during SWS can interact with the memory consolidation process.
The single-sound protocol used in this study had two important advantages. First, as we found that the single-sound alone was able to produce memory consolidation effects, we may hypothesize, that also in the previous studies the first sound after the spontaneous slow wave was mainly causing the memory effects. This may explain also the recent finding23 in which no differences in memory consolidation between trains of two or more stimulation sounds were identified. However, although a single stimulation sound improved the memory consolidation, it remains unresolved if two or more sounds in a sequence increase refractoriness and if more precise targeting of the stimulus to EEG phase could result in more robust effects.24
Second, the single-sound stimulus method enabled more precise analysis of the evoked EEG responses in the absence of overlapping responses from consecutive sound stimuli. The neurophysiological data showed large EEG responses evoked by the sound stimulus, and a burst of activity at the sleep spindle frequency range during the scalp-positive phase of the evoked slow wave. The simplest explanation could be that the evoked slow wave can naturally trigger sleep spindles (see the study of Steriade8 for review), which then induce memory consolidation. Slow waves and KCs have often been thought to reflect different phenomena, but recent evidence suggests that their neural origins may be related.36 Bellesi et al.36 proposed a hypothetical model in which nonlemniscal thalamic networks are able to give rise to a large-scale neural chain reaction, which is recorded as a slow wave in EEG. Via corticothalamic connections, this slow wave is able to trigger sleep spindles,8 which are further temporally clustering hippocampal ripples.5
The memory task of semantically associated item pairs has also previously been used in studying memory consolidation in sleep (see e.g., the study of Payne et al.37), and is potentially sensitive to changes in the hippocampus-dependent consolidation process. In this study, we demonstrated a memory effect that was specific to the word pair task (verbal associative memory): the performance in the other memory tasks including semantically associated items of human faces and names were not improved by sound stimulation during SWS. Lack of effect in the latter task may be explained by the difference in the learned material. Face-name task required integration of both verbal and visual stimulus material, whereas the word pair test included only verbal material. It is also possible that in the face-name test task difficulty and the floor effect affected results, while the trend of improvement in the recall performance suggests that the optimized version of the faces and names task should be further tested in future studies (see Figure 2 and Table 1). We also found a positive correlation between the amount of N2 sleep and memory retention in word pair task (for stimulus night only; see Supplementary Table S5), but as the amount of N2 sleep did not differ between the nights (Supplementary Table S3), it is difficult to assess its relevance to the memory performance.
To investigate the memory type specificity of the current (memory consolidation facilitation) protocol, we used two memory tasks that were not linked to associative pairs of semantic items. The first control task was the procedural finger tapping task, which included continuous repeating of short five-item motor sequences. These sequences were indeed consolidated by sleep, and the average overnight increase in performance was 113 sequences. However, the sound stimulus did not facilitate this consolidation. This is in line with previous findings, which have stressed the role of REM sleep29 as well as stage 2 sleep38 and slow-frequency spindle activity39 in consolidation of procedural schemas. The second control task involved remembering pictures. For the emotional content in pictures, the result was expected. REM sleep has been shown to be more important for emotional content consolidation than NREM sleep, and thus, the acoustic stimulation targeted to the SWS only did not affect neural processes during REM. However, we found no improvement in overall picture recognition performance. This is somewhat surprising, as both word pairs and pictures are thought to be stored within the declarative memory system. The lack of effect in picture recognition task could be explained by three important differences between the picture recognition task and the word pair task. First, picture memory overall has been demonstrated to be less dependent on consolidation in sleep.40,41 Second, the word pair learning sequence included feedback, which allowed participants to rememorize the correct word pair, and it is likely that repetition is critical when the memory consolidation system values different memory items depending on their estimated importance. Additional evidence supporting this view was given by our pilot study, in which the word pair consolidation effect disappeared when the feedback in the learning phase was missing.42 The third important difference between the picture recognition and word pair tasks was in the recalling test. In the word pair task, the participants saw a cue (the first member of the word pair), which they used to recall the other word that was paired with it. In contrast, the picture recognition task was simply to answer whether the picture had been seen before or not. In these two memory tasks, the recall strategy may involve different neural strategies, and thus, it is possible that only the cued recall task benefits from the consolidation boost given by sound stimulation. This hypothesis requires closer scrutiny.
In addition to exploring the effects of sound stimulation on memory consolidation, we used questionnaires to examine its possible effects on metacognitive ratings, subjective sleep quality, and mood. We did not find significant effects on cognitive load or subjective sleep quality, but there was a difference in a mood measure of the tension-anxiety scale; on the night with acoustic stimulation the decrease in the tension-anxiety factor was smaller than on the control night. This may indicate that the regulation mechanism of sleep for this specific mood component was disturbed with putative input from serotonergic neurons in raphe nuclei, which are involved in memory consolidation, sleep cycle regulation, and mood disorders (for review, see the study by Zhao et al.43). However, by chance, the baseline value of tension-anxiety scale was lower before the stimulus night than before the control night, which may contribute to the result (Supplementary Table S1). Taking this into account, and that none of the other mood measures were sensitive to sound stimulation, dispute the reliability of the result on acoustic stimulation’s capability to disrupt the mood regulation during sleep. To ensure that the observed baseline difference in the tension-anxiety scale was unrelated to memory test results, we used correlation analysis, which did not show any connection between these two effects.
Finally, an important aim was to improve the reliability of the sleep stimulation procedure by increasing its automatization and relieving the procedure of manual overnight monitoring of sleep stages. To this end, we successfully demonstrated that the automated method was as efficient as the manual procedure used in the previous studies. The algorithm automatically (1) detected the SWS phases, (2) faded the sound stimulus smoothly in, thus minimizing the possibility of waking the subject, (3) detected single slow-wave event onsets for accurate timing of stimulation, and (4) disabled the stimulation immediately following arousal, awakening, lightening of sleep, or shifts in REM sleep. The automatic stimulation mechanism ensured that subjects did not accidentally hear sounds, and thus, increased the reliability by reducing possible placebo bias. Indeed, subjects did not know which night they were presented with sounds, despite the fact that many of them woke naturally at some point during the night. The stimulation setup consisted of a wireless mini-sized EEG amplifier, a tablet computer controlling the online analysis and stimulation, and a loudspeaker, thus being maximally ambulatory and compatible with out-of-lab use. Most importantly, the algorithm used the EEG signal only from disposable adhesive electrodes which were attached to hair-free areas (forehead, mastoids, and corners of eyes). These electrodes can be easily attached by a person to her/himself and the signal is still relatively reliable without EEG caps or other additional attaching techniques. In addition, based on visual inspection of the EEG curves of Cz and Fpz channels in Figure 4, it is possible to note that the negative peak seems enhanced in Fpz, whereas sound evoked slow-wave amplitudes look quite similar in both channels. This suggests that the forehead channel is not only more practical, but also detection of the negative peak of SW might be more reliable (due to higher signal to noise ratio). The most critical limitation of our study is the relatively low number of subjects investigated. While a group size of 15 subjects is common in cognitive neuroscience studies, generalization of the results would benefit from a larger subject pool. The automatic and ambulatory stimulation setup developed in this study can be used in achieving this goal.
SUPPLEMENTARY MATERIALS
Supplementary materials are available at SLEEP online.
FUNDING
Research was funded by the Finnish Funding Agency for Innovation (Tekes), project 2310/31/2012.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
Research site was Finnish Institute of Occupational Health, Helsinki, Finland. We thank sleep technologists Riitta Velin and Nina Lapveteläinen for crucial help in data collection.
REFERENCES
- 1. Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013; 93(2): 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004; 55: 235–269. [DOI] [PubMed] [Google Scholar]
- 3. Ellenbogen JM, Hulbert JC, Jiang Y, Stickgold R. The sleeping brain’s influence on verbal memory: boosting resistance to interference. PLoS One. 2009; 4(1): e4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alger SE, Lau H, Fishbein W. Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiol Learn Mem. 2012; 98(2): 188–196. [DOI] [PubMed] [Google Scholar]
- 5. Staresina BP, Bergmann TO, Bonnefond M, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015; 18(11): 1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003; 7(5): 423–440. [DOI] [PubMed] [Google Scholar]
- 7. American Academy of Sleep Medicine. A Technologist’s Handbook for Understanding and Implementing The AASM Manual for the Scoring of Sleep and associated Events. American Academy of Sleep Medicine, Westchester, IL; 2007. [Google Scholar]
- 8. Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006; 137(4): 1087–1106. [DOI] [PubMed] [Google Scholar]
- 9. Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007; 10(1): 100–107. [DOI] [PubMed] [Google Scholar]
- 10. Bergmann TO, Mölle M, Diedrichs J, Born J, Siebner HR. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage. 2012; 59(3): 2733–2742. [DOI] [PubMed] [Google Scholar]
- 11. Takashima A, Petersson KM, Rutters F, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci USA. 2006; 103(3): 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durrant SJ, Cairney SA, Lewis PA. Overnight consolidation aids the transfer of statistical knowledge from the medial temporal lobe to the striatum. Cereb Cortex. 2013; 23(10): 2467–2478. [DOI] [PubMed] [Google Scholar]
- 13. Payne JD, Kensinger EA. Sleep leads to changes in the emotional memory trace: evidence from FMRI. J Cogn Neurosci. 2011; 23(6): 1285–1297. [DOI] [PubMed] [Google Scholar]
- 14. Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014; 81(1): 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antony JW, Gobel EW, O’Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012; 15(8): 1114–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guerrien A, Dujardin K, Mandai O, Sockeel P, Leconte P. Enhancement of memory by auditory stimulation during postlearning REM sleep in humans. Physiol Behav. 1989; 45(5): 947–950. [DOI] [PubMed] [Google Scholar]
- 17. Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007; 315(5817): 1426–1429. [DOI] [PubMed] [Google Scholar]
- 18. Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009; 326(5956): 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colrain IM. The K-complex: a 7-decade history. Sleep. 2005; 28(2): 255–273. [DOI] [PubMed] [Google Scholar]
- 20. Riedner BA, Hulse BK, Murphy MJ, Ferrarelli F, Tononi G. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. Prog Brain Res. 2011; 193: 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ngo HV, Claussen JC, Born J, Mölle M. Induction of slow oscillations by rhythmic acoustic stimulation. J Sleep Res. 2013; 22(1): 22–31. [DOI] [PubMed] [Google Scholar]
- 22. Ngo HV, Martinetz T, Born J, Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013; 78(3): 545–553. [DOI] [PubMed] [Google Scholar]
- 23. Ngo HV, Miedema A, Faude I, Martinetz T, Mölle M, Born J. Driving sleep slow oscillations by auditory closed-loop stimulation-a self-limiting process. J Neurosci. 2015; 35(17): 6630–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santostasi G, Malkani R, Riedner B, et al. Phase-locked loop for precisely timed acoustic stimulation during sleep. J Neurosci Methods. 2016; 259: 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Virkkala J, Hasan J, Värri A, Himanen SL, Müller K. Automatic sleep stage classification using two-channel electro-oculography. J Neurosci Methods. 2007; 166(1): 109–115. [DOI] [PubMed] [Google Scholar]
- 26. Virkkala J, Leminen M, Saure E, Huotilainen M, Paunio T. (2015, 28 Aug). Online wireless sleep analysis and auditory sleep stimulation. Paper presented at the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milano, Italy. [Google Scholar]
- 27. Peirce JW. PsychoPy–Psychophysics software in Python. J Neurosci Methods. 2007; 162(1–2): 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marshall L, Mölle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004; 24(44): 9985–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997; 9(4): 534–547. [DOI] [PubMed] [Google Scholar]
- 30. Minear M, Park DC. A lifespan database of adult facial stimuli. Behav Res Methods Instrum Comput. 2004; 36(4): 630–633. [DOI] [PubMed] [Google Scholar]
- 31. Lang P, Bradley M, Cuthbert B. International Affective Picture System (IAPS): Digitized photographs, instruction manual and affective ratings. Technical Report. University of Florida, FL, USA: 2005. [Google Scholar]
- 32.Development of NASA-TLX (Task Load Index): Results of empirical and theoretical research. Hart SG, Staveland LE. In Hancock PA, Meshkati N (Eds). (1988). Human mental workload., (pp. 139–183). Oxford, England: North-Holland, xvi, 382 pp. [Google Scholar]
- 33. Pollock V, Cho DW, Reker D, Volavka J. Profile of Mood States: the factors and their physiological correlates. J Nerv Ment Dis. 1979; 167(10): 612–614. [DOI] [PubMed] [Google Scholar]
- 34. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004; 134(1): 9–21. [DOI] [PubMed] [Google Scholar]
- 35. Berens P. CircStat : a MATLAB toolbox for circular statistics. J Stat Softw. 2009; 31: 1–21. [Google Scholar]
- 36. Bellesi M, Riedner BA, Garcia-Molina GN, Cirelli C, Tononi G. Enhancement of sleep slow waves: underlying mechanisms and practical consequences. Front Syst Neurosci. 2014; 8: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Payne JD, Tucker MA, Ellenbogen JM, et al. Memory for semantically related and unrelated declarative information: the benefit of sleep, the cost of wake. PLoS One. 2012; 7(3): e33079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002; 35(1): 205–211. [DOI] [PubMed] [Google Scholar]
- 39. Nishida M, Nakashima Y, Nishikawa T. Slow sleep spindle and procedural memory consolidation in patients with major depressive disorder. Nat Sci Sleep. 2016; 8: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atienza M, Cantero JL. Modulatory effects of emotion and sleep on recollection and familiarity. J Sleep Res. 2008; 17(3): 285–294. [DOI] [PubMed] [Google Scholar]
- 41. Sterpenich V, Albouy G, Boly M, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007; 5(11): e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saure E, Huotilainen M. Unenaikaisen äänistimulaation vaikutus muistissa säilymiseen [master’s thesis]. Faculty of Behavioural Sciences, University of Helsinki, Helsinki, Finland; 2014. [Google Scholar]
- 43. Zhao H, Zhang BL, Yang SJ, Rusak B. The role of lateral habenula-dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav Brain Res. 2015; 277: 89–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.