Abstract
Solid tumor growth and metastasis require the interaction of tumor cells with the surrounding tissue, leading to a view of tumors as tissue-level phenomena rather than exclusively cell-intrinsic anomalies. Due to the ubiquitous nature of adipose tissue, many types of solid tumors grow in proximate or direct contact with adipocytes and adipose-associated stromal and vascular components, such as fibroblasts and other connective tissue cells, stem and progenitor cells, endothelial cells, innate and adaptive immune cells, and extracellular signaling and matrix components. Excess adiposity in obesity both increases risk of cancer development and negatively influences prognosis in several cancer types, in part due to interaction with adipose tissue cell populations. Herein, we review the cellular and noncellular constituents of the adipose “organ,” and discuss the mechanisms by which these varied microenvironmental components contribute to tumor development, with special emphasis on obesity. Due to the prevalence of breast and prostate cancers in the United States, their close anatomical proximity to adipose tissue depots, and their complex epidemiologic associations with obesity, we particularly highlight research addressing the contribution of adipose tissue to the initiation and progression of these cancer types. Obesity dramatically modifies the adipose tissue microenvironment in numerous ways, including induction of fibrosis and angiogenesis, increased stem cell abundance, and expansion of proinflammatory immune cells. As many of these changes also resemble shifts observed within the tumor microenvironment, proximity to adipose tissue may present a hospitable environment to developing tumors, providing a critical link between adiposity and tumorigenesis.
Introduction
Cancer is characterized by fundamental aberrations in cellular behavior, including the ability to multiply indefinitely in the absence of growth-promoting factors and a resistance to signals that normally result in programmed cell death (apoptosis) (160). In the case of solid tumors, carcinogenic transformation and cell proliferation are followed by establishment of a vascular supply, or tumor angiogenesis, which facilitates the delivery of oxygen and nutrients to the growing tumor (160). Subsequent invasion into and migration through surrounding tissues allows for the establishment of nearby satellite tumors or entry into the lymphatic or vascular systems for dissemination and secondary tumor formation (metastases) (160). Solid tumor growth and tissue invasion require the interaction of tumor cells with the surrounding tissue. It is well established that communication between cancer cells and the tissue-level context in which they reside, collectively referred to as the tumor “microenvironment,” is pivotal in determining whether a given tumor will exist in dormancy or progress to malignancy (410). The tumor microenvironment includes, but is not limited to, the tumor cells themselves, blood vessels (endothelial cells and pericytes), lymphatic vessels (lymphendothelial cells), adipocytes, fibroblasts, and various stem and progenitor cells (6) (Fig. 1). Also present is a wide variety of innate and adaptive immune cells, which can act as critical antitumor defenses or, alternatively, play central roles in tumor promotion. The tumor “stroma” is the connective, functionally supportive framework of the tumor, and by definition refers to a complex mixture of signaling molecules and extracellular matrix (ECM; for a list of abbreviations see Table 1) components, as well as the stromal cells (e.g., fibroblasts and pericytes) that produce and are embedded within them (44). However, the term “stroma” may also be used to collectively refer to all of the aforementioned cell types and secreted factors, as all are present within the cancer cell-adjacent tissue. Thus, considerable heterogeneity, both within the cancer cells themselves and among the interacting stromal cells, leads to a view of tumors as communities, and the process of tumorigenesis as a tissue-level phenomenon occurring in conjunction with intrinsic genetic deviations within individual cancer cells (380).
Figure 1. Tumors as communities.
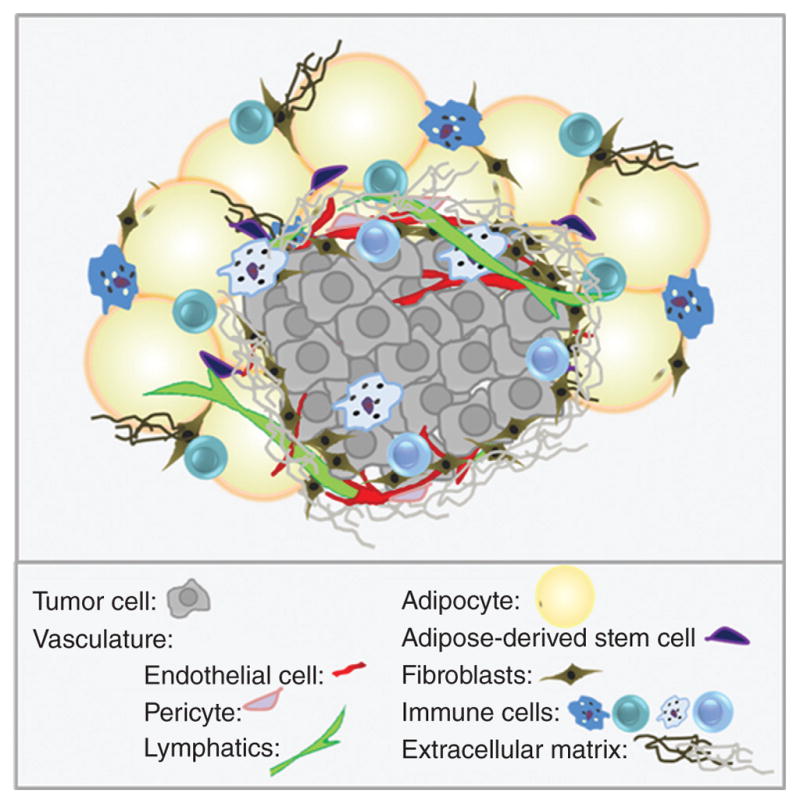
Tumor cells coexist with a variety of stromal and immune cells, and reside in a complex mixture of signaling molecules and extracellular matrix components. Adjacent adipose tissue may provide a hospitable environment to developing tumors.
Table 1.
Abbreviations Used in Text
| Abbreviation | Explanation |
|---|---|
| AMPK | AMP-activated protein kinase |
| Ang-2 | Angiopoietin-2 |
| APC | Antigen-presenting cell |
| ASC | Adipose stromal cell, Adipose-derived stem cell |
| ASCO | American Society of Clinical Oncology |
| α-SMA | Alpha smooth muscle actin |
| ATGL | Adipocyte triglyceride lipase |
| ATM | Adipose tissue macrophages |
| BAI | Body Adiposity Index |
| BMI | Body mass index |
| CAA | Cancer-associated adipocytes |
| CAFs | Cancer-associated fibroblasts |
| CCK | Cholecystokinin |
| CLS | Crown like structure |
| COX-2 | Cyclooxygenase-2 |
| CPT1 | Carnitine palmitoyltransferase 1 |
| DAMPs | Damage-associated molecular patterns |
| DCIS | Ductal carcinoma in situ |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transition |
| FACS | Fluorescence activated cell sorting |
| FGF-2 | Fibroblast growth factor 2 |
| GEMM | Genetically engineered mouse model |
| HGF | Hepatocyte growth factor |
| HIF-1, HIF-1α | Hypoxia-inducible factor, 1α subunit |
| IDC | Invasive ductal carcinoma |
| IGF-1 | Insulin-like growth factor-1 |
| IL-6 | Interleukin-6 |
| ILCs | Innate lymphoid cells |
| ILC2s | Innate lymphoid type 2 cells |
| LVD | Lymphatic vessel density |
| M1, M2 | Macrophage phenotypes |
| MCP-1/CCL2 | Monocyte-chemoattractant protein, also called CC chemokine ligand 2 |
| MMP | Matrix metalloprotease |
| MMTV-PyMT | Mouse mammary tumor virus, Polyoma middle T antigen |
| N1, N2 | Subtypes of tumor-associated neutrophils (see TAN) |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHANES | United States National Health and Nutrition Examination Survey |
| NK | cells Natural killer cells |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PD-1 | Programmed Death-1 |
| PDGF | Platelet-derived growth factor |
| PD-L1 | Programmed death-1 ligand |
| PGE2 | Prostaglandin E2 |
| PIN | Prostatic intraepithelial neoplasia |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| TAM | Tumor-associated macrophage |
| TAN | Tumor-associated neutrophil |
| TCR | T cell receptor |
| TDLU | Terminal ductal lobular unit |
| TEB | Terminal end bud |
| TGF-β | Transforming growth factor beta |
| Th1, Th2, | Th17 T helper cell subtypes |
| TNBC | Triple negative breast cancer |
| TNFα | Tumor necrosis factor alpha |
| Tregs | Regulatory T cells |
| VEGF | Vascular endothelial growth factor |
Due to the ubiquitous nature of adipose tissue, many types of solid tumors grow in proximate or direct contact with adipocytes and other adipose-associated cell populations. Although the specific nature of the reciprocal communication occurring between a developing tumor and adjacent adipose tissue is an area of active study, a growing body of literature indicates that these interactions with the local adipose milieu are important drivers of malignancy. Many of these studies have focused on dysregulated adipose and associated systemic metabolic dysfunction in the context of obesity, as there is now adequate evidence establishing a link between obesity/adiposity and elevated risk for, or accelerated progression of, several cancers. Following an overview of the adipose organ, we will briefly address epidemiologic links between obesity and cancer. Subsequently, we aim to provide the reader with an understanding of the recently described mechanistic links between cancer development or progression and adipose tissue per se, as opposed to obesity-associated systemic alterations such as metabolic dysfunction. Thus, although adipose dysfunction in obesity will be addressed frequently, we have chosen to emphasize the local physical and paracrine roles of adipose tissue in solid tumor development and malignancy by focusing on individual components of the adipose tissue microenvironment.
The Adipose Organ
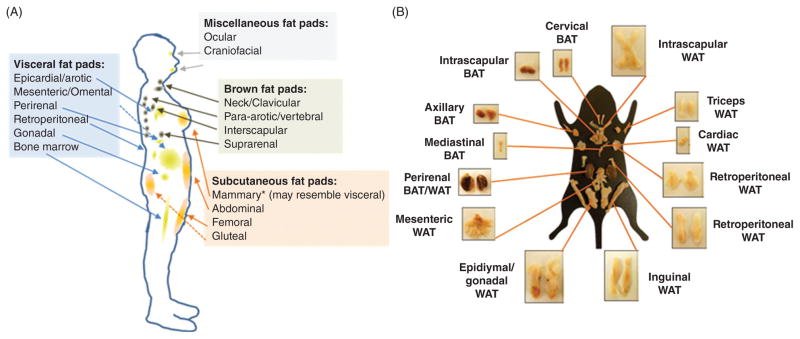
Adipose tissue is a type of loose connective tissue that was long considered to be largely physiologically inert, primarily storing energy in the form of lipids while cushioning and insulating the body. However, work over the past several decades has established that adipose tissue is also a substantial contributor to whole body endocrine signaling, modulating feeding behavior and total body energy expenditure, as well as hematopoiesis and lymphopoeisis, overall immune function, and reproduction (400, 402). Additionally, adipose tissue is now understood to contribute to the pathogenesis of a variety of regional and systemic diseases. The adipose tissue “organ” is in fact comprised of a variety of distinct adipose depots (Fig. 2), each of which differentially exerts systemic and regional control on overall energy metabolism and signaling based on location and adipose tissue subtype. Specifically, adipose depots can be divided according to anatomic location into subcutaneous, intramuscular, and visceral subtypes. Whole adipose depots, or specific regions within depots, may be further subclassified as white, brown, or beige depending on, among other factors, cellular mitochondrial content, with a higher relative number of mitochondria corresponding to a darker adipocyte hue. In humans, subcutaneous adipose tissue comprises ~80% of total body fat, and is contained primarily in the abdominal, gluteal, and femoral depots (216) (Fig. 2A). The breast fat pad is also a nontrivial contributor to total subcutaneous fat content in women. On the other hand, visceral depots represent approximately 5% to 20% of total body fat in normal weight (i.e., not overweight or obese) individuals (216). Visceral adipose tissue surrounds vital organs, and includes omental, mesenteric, and epiploic adipose, as well as the gonadal, epicardial, and retroperitoneal fat pads. Finally, numerous smaller depots, such as intramuscular, intraorbital, and bone marrow adipose, nourish and protect tissues throughout the body. While the majority of these depots are comprised of white adipose tissue—discussed further in the Adipocytes section—smaller brown and beige adipose tissue caches are also found in adults (147, 162). Importantly, due to similarities in the location and composition of adipose depots and endocrine function relative to humans, the laboratory mouse (Mus musculus) is a commonly used model for investigation of adipose tissue anatomy and physiology (Fig. 2B).
Figure 2. The adipose organ is comprised of several distinct adipose depots.
Adipose depot locations and subtypes in (A) humans and (B) mice [panel B adapted from (85) with permission].
Although adipocytes constitute approximately 90% of adipose tissue volume, the adipose tissue microenvironment is a rich ecosystem of additional stromal and vascular components (often referred to collectively as stromal-vascular fraction). The stromal-vascular compartment of human white adipose tissue includes endothelial cells (10–20% of cells), pericytes (3–5%), fibroblasts and other connective tissue cells (15–30%), and stem and progenitor cells (0.1%), which reside within a complex milieu of signaling molecules and ECM components (50) (Fig. 3). Adipose tissue also contains a rich and varied collection of innate and adaptive immune cells (macrophages, dendritic cells, mast cells, eosinophils, neutrophils, and lymphocytes; 25–45%) (50). However, the exact cellular proportions, degree of vascularity, ECM composition, metabolic characteristics, and secretory products of adipose tissue vary according to numerous factors, including depot location, sex, age, health status, and extent of adipose accumulation (216).
Figure 3.
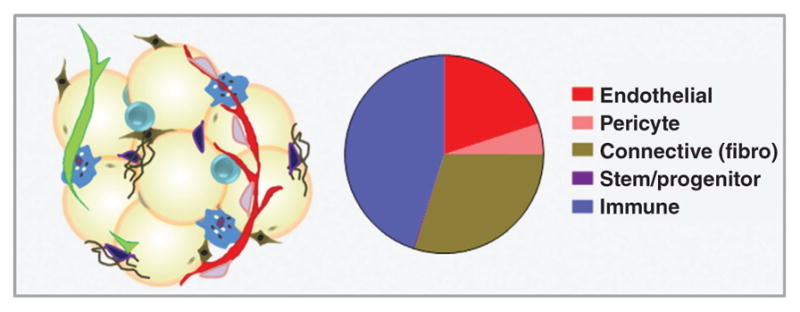
Approximate composition of human white adipose tissue stromal-vascular fraction (percent cellularity).
Obesity and Cancer
Adipose tissue exhibits an almost unlimited capacity to expand, a unique property that has received increased attention in recent years as obesity has moved to the forefront of global public health concerns. Overweight and obesity, defined by the World Health Organization (WHO) as abnormal or excessive adiposity that presents a risk to health, are frequently measured at the population level using the body mass index (BMI), an individual’s weight in kilograms divided by the square of his or her height in meters. However, it must be acknowledged that, at an individual level, the BMI formula can vary considerably by sex and race and says little about body composition, often underestimating adiposity (302,347). For this reason, additional measures specifically of adiposity, such as waist circumference or the Body Adiposity Index (BAI; [hip circumference (cm)/height (m)1.5−18]) developed by Bergman et al. (41), are sometimes used to correlate adiposity with disease risk.
Current status of the obesity epidemic, globally and in the United States
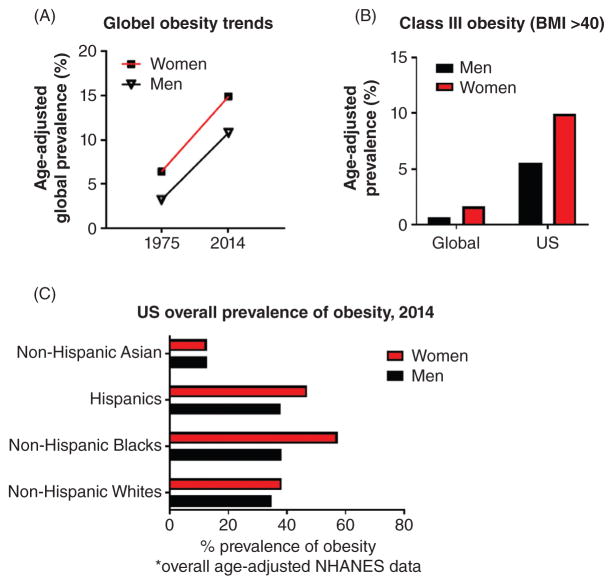
Since the recognition of obesity as a global epidemic in 1997 (54), increasing resources have been allocated to more completely understanding the prevalence, risk factors, and longterm consequences of this health hazard. For example, a recent quantitative meta-analysis analyzed 1698 population-based data sources, encompassing 186 countries and more than 19.2 million adult participants (9.9 million men and 9.3 million women), to evaluate trends in mean BMI over the last four decades (270). The authors reported a global increase in overall age-adjusted prevalence of obesity in men from 3.2% to 10.8%, and in women from 6.4% to 14.9%, between 1975 and 2014 (270) (Fig. 4A). An additional cross-sectional analysis of the United States National Health and Nutrition Examination Survey (NHANES) for the years 2013–2014 reports that the overall age-adjusted prevalence of obesity (again by BMI) among US adults (age 20+ years) has reached 37.7% (120). Moreover, among men and women in the US, obesity prevalence has now reached a staggering 35% and 40.4%, respectively (120). Furthermore, extreme obesity (or class 3 obesity, defined as BMI >40) in the United States is currently 9.9% for women and 5.5% for men (120), considerably higher than the global prevalence of 1.6% and 0.64%, respectively (270) (Fig. 4B). Importantly, a disproportionate burden of obesity and overweight is observed among women who self-identify as Hispanic or non-Hispanic black minorities; NHANES data indicate that the overall age-adjusted prevalence of obesity in non-Hispanic black and Hispanic women measures 57.2% and 46.9%, respectively, compared to 38.2% in non-Hispanic white women (120) (Fig. 4C). Finally, it should be noted that rising obesity rates are not restricted to adults. The prevalence of obesity in US children and adolescents ages 2 to 19 years old rose from approximately 10% during the 1988 to 1994 NHANES period to 17.0% in the 2011 to 2014 period, with extreme obesity more than doubling from approximately 2.5% to 5.8% (287).
Figure 4. Rising global and US obesity rates.
(A) Global age-adjusted prevalence of obesity in men and women, 1975 and 2014; (B) Class III obesity (BMI >40), globally and US; and (C) US obesity prevalence by race, ethnicity (270).
The obesity-cancer link
Cancer is currently the second leading cause of death in the United States, and is expected to surpass heart disease as the leading cause of death within the next few years (357). Approximately 40% to 60% of cancer patients are classified as overweight or obese (145, 320), and in 2004 it was estimated that overweight and obesity accounted for one in seven cancer deaths in men and one in five in women (56). Importantly, obesity is differentially associated with both increased risk of cancer development and increased risk of poorer cancer prognosis. Indeed, an association between obesity and increased risk of onset remains ambiguous for several cancer types for which there is strong support for an influence on outcome. With this caveat acknowledged, there is adequate evidence to support an association between obesity and increased risk of developing colorectal, post-menopausal breast, endometrial, kidney, esophageal, liver, gallbladder, pancreatic, and thyroid cancers, as well as non-Hodgkin’s lymphoma and myeloma (33, 55, 208, 210, 409). The American Society of Clinical Oncology (ASCO) has also acknowledged that obesity contributes to poorer cancer prognosis following diagnosis in a number of ways, including by impairing the delivery of systemic cancer therapies and by elevating risk of both tumor recurrence and development of additional primary malignancies (224). Interestingly, there is also a body of literature that supports a protective effect of obesity in overall survival for some cancer types, a finding known as the “obesity paradox.” Potential explanations for the obesity paradox emphasize methodological issues, such as unmeasured confounders and/or a reliance on BMI as a metric for obesity (219, 368). As mentioned previously, BMI is a rather crude mathematical estimate that does not capture important considerations such as percent adiposity, regional distribution of adiposity (e.g., android vs. gynoid obesity), or differences in lean mass. Gonzalez et al. reported that the use of body composition indices resulted in a disappearance of the obesity paradox in 175 cancer patients in which BMI was previously associated with a protective effect, emphasizing the importance of considering body composition in epidemiologic analyses of cancer outcomes (149). In fact, when body composition was included, loss of lean mass (sarcopenia) was a more important prognostic indicator than BMI for patients exhibiting cancer-associated cachexia, a systemic wasting syndrome frequently observed in end-stage cancer patients that is characterized by a rapid loss of both skeletal muscle and adipose tissue (149, 240). Thus, additional evidence is needed to determine whether isolated reports of the obesity paradox are simply artifactual or in fact clinically relevant.
Nevertheless, leading hypotheses seeking to explain observed connections between obesity and increased cancer morbidity and mortality emphasize factors such as metabolic disruption-induced growth factor dysregulation; higher levels of circulating adipokines and cytokines secreted by inflamed obese adipose tissue; and elevated production of estrogens by adipose tissue (90, 297). These hypotheses emphasize the role of adipose as an endocrine organ and obesity as a potential state of adipose endocrine dysfunction. However, growth and invasion of some solid tumors into adjacent adipose may promote tumor aggression even in the absence of obesity. For example, adipose tissue invasion at the tumor margin is associated with an increase in lymph node metastasis in patients with invasive breast carcinoma, irrespective of BMI (454). Thus, whether select adipose-mediated mechanisms of tumor promotion are merely exacerbated by obesity or are unique to a dysregulated obese adipose microenvironment in many cases remains to be determined. Moreover, the mechanisms whereby adipose accumulation increases risk of tumor onset and/or mediates tumor progression in adipose-adjacent cancers are multifactorial, complex, and likely tissue/organspecific, in part due to unique paracrine and physical interactions occurring between cancer cells and adjacent adipose tissue. In this review, we have especially highlighted the role of obesity in the development and progression of breast and prostate cancers due to the prevalence of these cancer types in the US population and their significant contributions to cancer-related mortality.
Breast and prostate cancers are the most frequently diagnosed cancers and the second leading causes of cancer-related death among US men and women, respectively (357). Due to their now recognized genetic and molecular heterogeneity, these cancer types have been shown to exhibit complex associations with obesity. For example, although the association between obesity and risk of postmenopausal breast cancer is now well established, the relationship between obesity and premenopausal breast cancer risk was controversial until studies began to consider molecular breast cancer subtypes. Specifically, recent work has clarified an association between obesity and premenopausal onset of triple-negative breast cancers (TNBCs), with differential risk according to race (12, 16, 67, 307, 405). Studies from our lab and others have also demonstrated that diet-induced obesity is associated with accelerated TNBC latency (time to development of a palpable tumor) in ovary-intact preclinical mouse models (18, 73, 375, 376). Additionally, in patients with confirmed breast cancers, obesity is associated with increased risk of breast cancer invasion (143, 272), development of distant metastases (111, 247, 294), tumor recurrence (42, 346), and mortality (2, 24, 55, 64, 84, 229, 420, 436) irrespective of molecular subtype. On the other hand, the role of obesity in risk of prostate cancer development remains equivocal (22, 32, 48, 283), in part because, similar to breast cancer, prostate cancer risk in obese individuals also appears to vary by race (32, 127). However, in confirmed prostate cancers, obesity is consistently associated with an elevated risk of cancer aggression (high Gleason scoring, a grading system used to inform the prognosis of men with prostate cancer) and prostate cancer-associated mortality (206, 463). Thus, rising obesity rates present an oncological crisis, both globally and within the United States.
Following a brief consideration of the anatomy of breast and prostate in humans and laboratory mice—a frequently used model in basic science and translational/pre-clinical cancer studies—potential mechanistic links between adipose tissue and breast and prostate cancer development or progression will be discussed in detail through a comprehensive examination of the available literature regarding adipose-cancer interactions in each organ.
Anatomy of the Breast and Prostate
The laboratory mouse remains the most widely used animal model for the study of cancer pathophysiology. Consequently, integration of experimental findings with studies of human disease requires an understanding of human and veterinary pathology and anatomy, as well as developmental, molecular, and cellular biology. While this level of detail is beyond the scope of this review, this section will provide a brief comparative biology overview of the breast and prostate in humans and mice as a backdrop for the studies reviewed in subsequent sections.
Mammary gland anatomy and adipose-cancer interaction in humans vs. mice
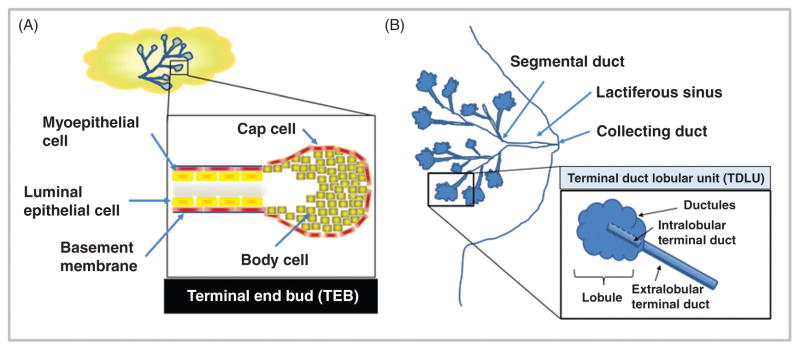
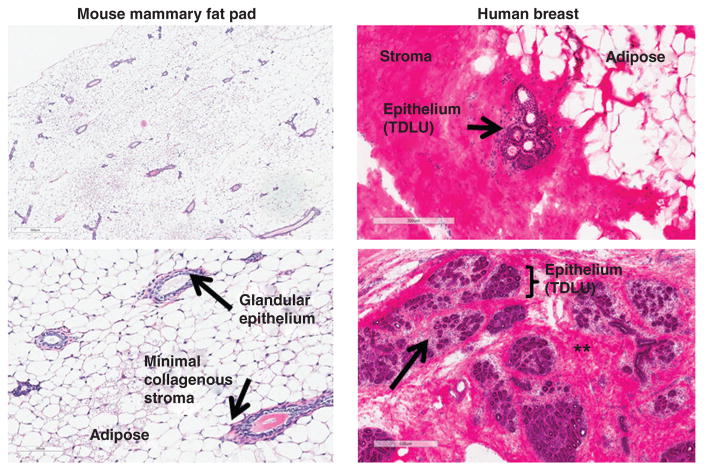
In both mice and humans, the mammary gland is a unique, dynamic organ that continuously undergoes anatomic and functional changes over the life course (180). In mice, the nascent mammary gland (“mammary tree”) consists of a network of epithelial ducts, each of which terminates in a stem cell-enriched structure called a terminal end bud (TEB; Fig. 5A). During sexual maturation, inductive hormonal and growth factor-derived signals stimulate the proliferation of ectodermal cells within these TEBs, driving ductal elongation and branching (168, 263, 361). The mature mammary epithelium continues to undergo further differentiation during later life stages such as pregnancy, lactation, and post-partum involution, or epithelial regression (98, 361). Development of the mammary tree and pregnancy/lactation-associated expansion and involution require remodeling of the surrounding stroma. In mice, mammary ductal-adjacent stroma is primarily comprised of adipose tissue, without a significant collagenous matrix layer (Fig. 6).
Figure 5. Comparison of mouse and human mammary gland anatomical structure.
(A) Murine ductal elongation and branching occur at the Terminal End Buds (TEBs). (B) The human mammary gland is extensively branched, culminating in the functional terminal ductal lobular unit (TDLU).
Figure 6. Comparison of mouse and human mammary gland histology.
Left: Adult mouse mammary fat pad from nulliparous C57BL/6 mouse (4× and 10×, H&E staining). Right: H&E-stained normal human breast tissue. Arrowhead and asterisks in right panel refer to loose intra- and dense interlobular stroma, respectively. Human histology images courtesy of Melissa Troester and the UNC Normal Breast Study (unpublished).
In comparison to mouse, the human mammary gland is a more extensively branching structure. Beginning at the nipple, the lactiferous sinus branches into segmental, or interlobular, ducts (Fig. 5B). Segmental ducts branch further into terminal ducts and lobules, which together comprise the functional unit of the human mammary gland, the terminal ductal lobular unit (TDLU). Immediately surrounding the TDLU is a loose intralobular stroma, referred to as “specialized stroma,” which contains abundant fibroblasts (Fig. 6) (98). Fibroblasts within the intra-lobular stroma exhibit phenotypic and functional differences from those found within inter-lobular stroma, including expression of select collagen isoforms (21) and ectoenzymes (20). Dense, collagenous inter-lobular stroma surrounds the entire human TDLU structure, forming a thick layer between the TDLU and adjacent adipose tissue. Surrounding the interlobular stroma is a large depot of subcutaneous adipose, comprising 7% to 56% of the volume of the adult breast (416).
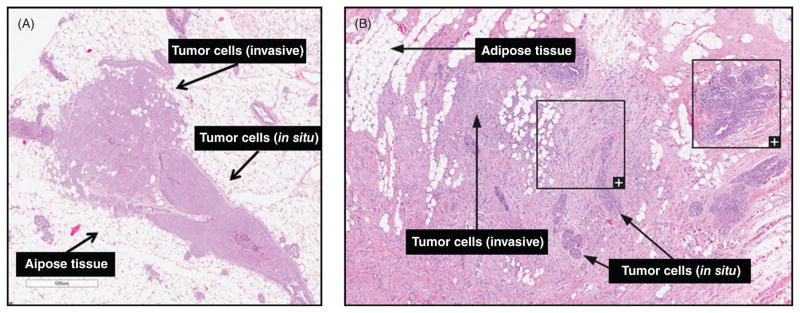
The most extreme example of tumor infiltration into adipose tissue is seen in breast cancer. Breast cancer most frequently begins in ductal epithelial cells, which proliferate to fill the ductal lumen and generate a precancerous lesion called ductal carcinoma in situ (DCIS). Subsequently, invasive ductal carcinoma (IDC) cells invade the mammary stromal compartment, encountering an area rich in adipose tissue. On the other hand, approximately 1 in 10 invasive breast cancers originate in the lobules, beginning as lobular carcinoma in situ and progressing to invasive lobular carcinoma. The lack of intra-lobular stroma in mice (98) and relatively thinner collagenous matrix means that tumor cell invasion in mouse models of breast cancer results in immediate encounter of adipocytes and other adipose cell populations (Fig. 7A), whereas human invasive breast carcinoma must invade through both intra- and interlobular stroma before directly encountering adipose tissue (Fig. 7B).
Figure 7. Adipose-breast cancer interactions in mice and humans.
(A) Early invasive lesions in H&E-stained mammary gland tissue from the C3(1)-TAg genetically engineered mouse model of spontaneous basal-like breast cancer (unpublished images). (B) Human breast cancer—female, 50 years, lobular carcinoma, grade 1, Elston-Ellis score 5. Image credit: The Human Protein Atlas (1,407).
Prostate gland anatomy and adipose-cancer interaction in humans vs. mice
Before progressing to a comparison of mouse and human prostate anatomy, it should be acknowledged that rat and canine models have generated important mechanistic knowledge in prostate cancer research, particularly in the context of the spontaneous development of prostate lesions (184). However, genetically engineered or xenografted mice remain the most commonly used model in prostate cancer research. For an overview and critique of currently available mouse models of human prostate cancer, the reader is directed to (151, 184).
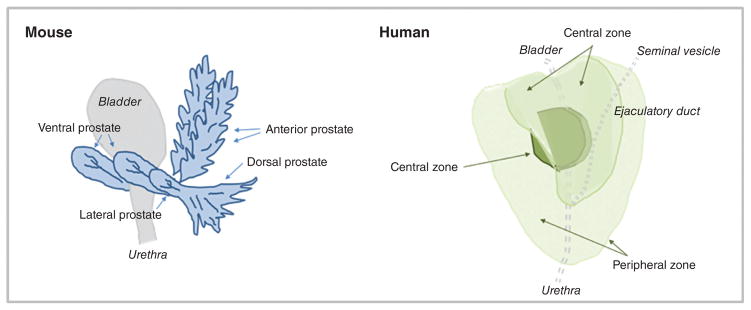
Like the mammary gland, the prostate exhibits important inter-species differences. In mice, the prostate is comprised of four lobes lying anterior and lateral to the urethra. These lobes are named after their spatial orientation (anterior, dorsal, ventral, and lateral lobes, see diagram in Fig. 8) and exhibit distinctive histology (184, 291). The glandular acini of the prostatic lobes are surrounded by a thin fibromuscular tunica, and are embedded in a loose connective tissue stroma with minimal smooth muscle cells and sparse collagen fibers (291). Individual mouse prostate lobes are surrounded by a delicate mesothelium-lined capsule, and are separated from each other by fibrous and adipose connective tissue (291).
Figure 8.
Anatomical comparison of mouse (left) and human (right) prostate glands.
In contrast to mice, the human male prostate does not have exterior lobation, but instead contains distinct glandular regions (a peripheral zone, a central zone, and a transition zone; see diagram in Fig. 8) (291), again with characteristic histology. Similar to the breast, a conspicuous histological difference between mouse and human prostate lies in the stromal component. In humans, the prostate gland bears an anterior, well-developed, nonglandular fibromuscular stromal region. Abundant adipose tissue is present surrounding most of the posterolateral aspects of the prostate (424), and is used as a marker of extraprostatic tissue in biopsy samples (49). This region of adipose is referred to in subsequent sections as periprostatic adipose. Intraprostatic adipose, when present, consists of a small focus of a few adipocytes, and is rarely observed histologically (49).
The most common type of prostate cancer is acinar adenocarcinoma, which originates from the glandular epithelium. Pre-neoplastic prostatic intraepithelial neoplasia (PIN) progresses to invasive adenocarcinoma, in which extension of prostatic carcinoma through the prostatic capsule (extraprostatic extension) and resulting interaction with the surrounding adipose is an indicator of malignant progression and advanced histopathological stage (378). The periprostatic adipose depot unambiguously contributes to prostate cancer malignancy (326, 386, 396). In fact, interaction with periprostatic adipose tissue has been suggested to be amore important determinant of cancer recurrence than an invasive phenotype (192). Analogous to breast cancer, recent advances in molecular phenotyping by The Cancer Genome Atlas Research Network have identified several genomically distinct molecular subtypes of prostate cancers (31). Whether these subtypes interact differentially with adjacent adipose remains to be determined.
Microenvironmental Links between Adipose Tissue and Cancer
Context matters: Extracellular matrix in adipose tissue and cancer
Adipocytes and other stromal cells are embedded in a loose, three-dimensional ECM, the noncellular tissue component that provides both structural and biochemical support to surrounding cells, such as cell adhesion, paracrine communication, and differentiation signals. Maintenance of the adipose tissue ECM—primarily comprised of fibronectin and collagens (373)—involves a variety of cell types, including fibroblasts, macrophages, adipocytes, and preadipocytes. Importantly, adipocyte function and survival is tightly regulated by both the molecular composition and mechanical properties of the surrounding ECM (239).
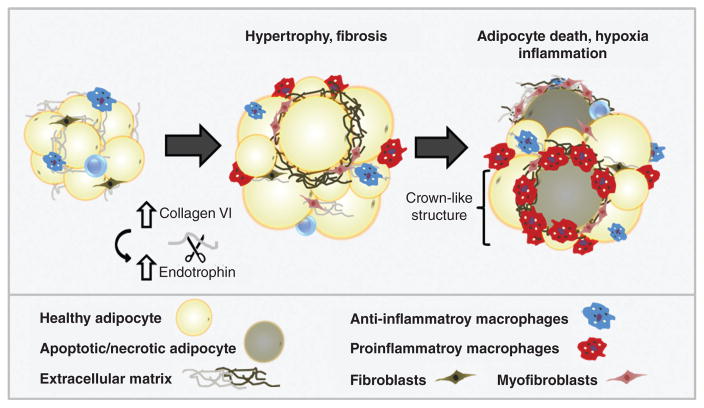
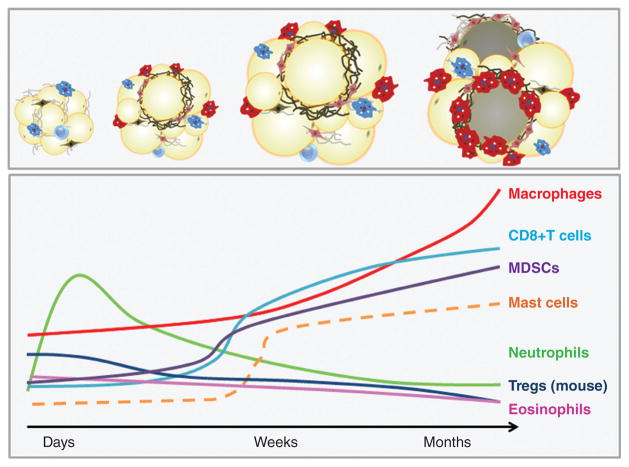
The structural flexibility of adipose tissue ECM facilitates transient volume changes in response to normal fluctuations in lipid stores throughout the feed-fast cycle. However, rapid adipocyte hypertrophy (increased adipocyte volume) during the development of obesity can result in intracellular or regional hypoxia. Reduced tissue oxygenation induces transcriptional programs in adipocytes and other stromal cells that ultimately lead to excess deposition of fibrillar ECM components such as collagens I, III, and VI and development of tissue fibrosis (373, 398). Indeed, adipose depots of obese subjects often exhibit greater total fibrosis, and particularly pericellular fibrosis around adipocytes, than lean individuals (95,363). Importantly, hypoxia-induced adipose tissue fibrosis is associated with onset of metabolic perturbations in adipocytes (199, 373), while dysregulation in visceral adipose function is linked to the pathogenesis of insulin resistance and type II diabetes mellitus (95, 158, 199). Furthermore, as adipocytes become encapsulated in a shell of rigid ECM, impaired cellular function also results in apoptosis and necrosis (277). Release of damage-associated molecular patterns (DAMPs) from dead and dying adipocytes and adjacent live adipocytes promotes recruitment of macrophages and other inflammatory cells; histologically, these macrophages can be observed within crown-like structures (CLS), foci of macrophages and other inflammatory cells surrounding dead and dying adipocytes (265). Macrophages are fully integrated into all stages of the fibrotic process through secretion of soluble mediators and cytokines such as transforming growth factor β1 (TGF-β1), platelet-derived growth factor (PDGF), and chemokines that attract and activate fibroblasts and collagen-producing myofibroblasts (373, 446).
Interestingly, while adipose tissue fibrosis in the context of obesity is well described, increased adipose ECM deposition, fibrosis, and immune cell infiltration are also observed in cancer-associated cachexia (35). Abdominal subcutaneous adipose depots of lean cachectic subjects bearing gastrointestinal cancers displayed extensive adipose ECM remodeling, including a dramatic increase in deposition of collagens I, III, and VI as well as elastin and fibronectin (11). These changes were associated with increased myofibroblast content and elevated activation of TGF-β/SMAD signaling pathways (11). As described later in the Adipocytes and adipocyte-cancer interactions section, cancer-associated cachexia is also associated with metabolic dysfunction in adipocytes, which may be mediated in part by ECM modifications.
Importantly, epithelial tissue homeostasis and tissue organization is also heavily dependent upon a dynamic dialogue with the surrounding ECM. Enhanced ECM stiffness triggers the process known as epithelial-to-mesenchymal transition (EMT), which is characterized by the loss of epithelial polarity, de-differentiation, and local invasion (271,313,340,442). Furthermore, disruption of ECM structure or misinterpretation of ECM-derived signals due to alterations in signaling receptor profiles is associated with development of a malignant phenotype in transformed epithelial cells (43, 141, 230). Hence, modifications in the adipose tissue ECM that provide a hospitable environment to developing tumors, such as enhanced stiffness in obese breast tissue, may provide a link between adipose tissue and tumorigenesis.
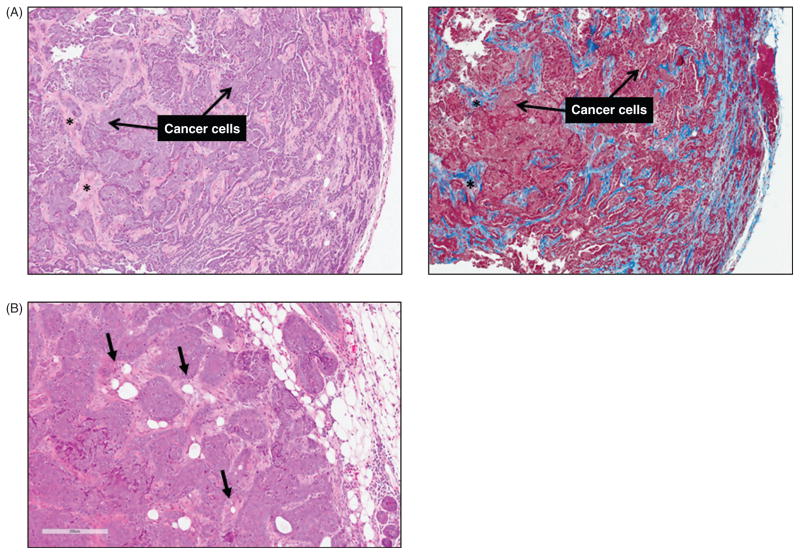
As discussed in later sections, chronic low-grade inflammation, macrophage infiltration, hypoxia, and aberrant wound healing responses, including an increase in myofibroblast and activated fibroblast content, are features of both the tumor and adipose tissue microenvironments (44, 101, 230). In tumors, chronic activation of the wound repair response leads to excess deposition of ECM components and accumulation of scar-like fibrotic tissue in a process known as desmoplasia, or the desmoplastic reaction (Fig. 9A). Desmoplasia is associated with poor outcomes in both breast and prostate cancers (23, 258), and can facilitate cancer progression by interfering with drug delivery. Thus, ECM remodeling and the resultant disturbances in cytoskeletal tension and mechanotransduction have emerged as important factors that promote neoplastic transformation, cancer malignancy, and cancer metastasis (44, 220, 230), and may provide another connection between adipose dysregulation and cancer.
Figure 9. Desmoplasia and cancer-associated adipocytes.
(A) Mammary tumors from C3(1)-TAg mice are stained with Hematoxylin/eosin (left) and Masson’s trichrome (right) (unpublished). In tumors, chronic activation of the wound-repair response results in desmoplasia, or excess collagenous extracellular matrix production, within tumors. Asterisks (*) indicate desmoplastic stroma. (B) Cancer-associated adipocytes (black arrows) at or near the tumor invasive front become smaller and exhibit decreased expression of adipocyte markers, while the number of fibroblast-like cells increases.
Adipose extracellular matrix composition and viscoelasticity: Influence on the normal breast and breast cancer
Mammographic density denotes the radiologic appearance of the breast, and is a metric of the fibroglandular (epithelial and nonfatty stromal) content in that tissue (322). A number of qualitative and quantitative methods have been developed to estimate mammographic density, including Breast Imaging Reporting and Data System (BI-RADS) categories, Wolfe’s parenchymal patterns, Tabar’s classification scheme, and numerous two- and three-dimensional image analysis techniques (452). Within heterogeneous breast tissue, tumors most frequently arise within the most mammographically dense regions of the breast, suggesting that denser fibroglandular tissue directly influences carcinogenesis (408). Indeed, regardless of the reporting method (322), high mammographic density is consistently and strongly associated with both elevated risk of breast cancer (51) and more aggressive tumor characteristics (453), even after adjustment for other risk factors such as age and BMI (178).
At the molecular level, high mammographic density reflects desmoplasia, a series of histological alterations including, but not limited to, the development of a dense, collagenous stroma rich in type I and/or type III collagen (88,126). Similar stromal changes are also observed in breast cancers (348), and are orchestrated by a heterogeneous, reactive population of so-called “cancer-associated fibroblasts” (CAFs). CAFs display remarkable plasticity, and frequently differentiate into myofibroblasts, a cell type exhibiting properties of both fibroblasts and smooth muscle cells (87,193,353). In nonmalignant tissue, myofibroblasts play an important role in wound healing responses, secreting a fibronectin- and collagen type I-rich ECM characterized by fibrillary architecture and increased cross-linking and density (344). They are also a predominant source of fibrogenic and/or inflammatory cytokines in fibrotic lesions (171). Despite the utility of this cell type to normal wound healing programs, however, the presence of myofibroblasts in tumors contributes to pathological desmoplasia (193), and may thereby promote cancer progression (198).
In addition to fibroblasts, local (adipose-derived) mesenchymal stem cells, bone marrow-derived mesenchymal stem cells, myeloid precursors, and cells derived from the epithelial-mesenchymal transition may also represent alternative sources of myofibroblasts in tumor stroma (93,251,304). Furthermore, in tumors growing in an adipose tissue-rich microenvironment, cancer cell-induced reprogramming of local adipocyte morphology, gene expression, and function has been observed to promote to adipocyte delipidation and atrophy/regression (46). This process occurs concurrently with the accumulation of fibroblast-like cells and a desmoplastic stroma; this synchronicity raises the possibility that some CAFs might be derived from dedifferentiated adipocytes (46) (Fig. 9B). However, as CAFs are a heterogeneous cell type, the extent to which their specific lineages determine their contribution to tumor progression remains inconclusive.
Although obesity is associated with reduced mammographic density, in part because fat is radiolucent, several studies have unveiled close links between chronically inflamed obese mammary adipose tissue and the development of fibrosis and associated ECM rigidity (301,344,372). Myofibroblasts are typically absent from normal, uninflamed breast tissue (401). However, Seo et al. showed that obesity elevated matrix rigidity in noncancerous breast tissue by enhancing myofibroblast content in mammary adipose (344). Distinct from tumors (65), these obesity-associated increases in myofibroblast content and matrix rigidity occurred in a transforming growth factor beta (TGFβ)-independent manner (344), suggesting that ECM composition and stiffness may be differentially regulated in benign obese and malignant breast tissue. The same study showed that adipose stromal cells (ASCs, also called adipose-derived stem cells) isolated from obese mice exhibited increased expression of α-smooth muscle actin (α-SMA, a myofibroblast marker), as well as increased fibronectin and a more fibrillar, partially unfolded, and stiffer ECM (344), implicating ASCs as a source of myofibroblasts in obesity. Furthermore, obese ASCs also exhibited enhanced proliferative capacity and secreted increased quantities of matrix components (344), thereby mimicking characteristics of tumor-associated stromal cells (65, 193). Consistent with these results, histologically normal breast tissue from obese patient mastectomies exhibited increased α-SMA staining and collagen fiber length and thickness relative to tissue from lean individuals (344). Obesity-associated increases in α-SMA levels also correlated with formation of CLS, further implicating macrophages in the development of mammary adipose tissue fibrosis (344).
Increased matrix rigidity in breast adipose tissue may be an important mediator of cancer initiation and progression in obese individuals. To test the effects of obesity and ECM on tumor cell behavior, Seo et al. cultured preinvasive human MCF10AT cells upon decellularized matrices produced by ASCs isolated from lean or obese mice. The authors reported that, relative to ECMs deposited by lean ASCs, obesity-associated ECMs increased MCF10AT cell motility and promoted the formation of disorganized three-dimensional acini, indicative of greater tumorigenic potential (344). Additionally, ECM generated by obese mammary ASCs significantly enhanced the proliferation of the highly invasive MDA-MB-231 cancer cell line by altering mechanotransduction through enhanced RhoA/ROCK-mediated cell contractility and YAP/TAZ transcription factor activity (344). Collectively, these results are suggestive of a relationship between obesity-associated mammary adipose tissue fibrosis and accelerated tumor initiation and/or proliferative capacity.
In addition to fibroblasts/myofibroblasts, adipocytes play a vital role in defining the ECM environment through secretion and processing of factors such as collagen VI, an ECM component with both structural and signaling roles that is highly enriched in adipose tissue (199, 300, 419). Excess adipocyte collagen VI expression in obesity is associated with adipose tissue fibrosis and metabolic dysregulation, while the absence of collagen VI in mouse models of obesity allowed for uninhibited adipocyte expansion and an improved metabolic phenotype (199). Increased adipocyte collagen VI expression is also associated with elevated local concentrations of the collagen VI α3 chain cleavage product, endotrophin, which has been identified as a driving factor in adipose tissue fibrosis, macrophage chemotaxis, and inflammation, and appears to mediate adipose metabolic dysregulation in obesity (Fig. 10) (300, 372). Unsurprisingly, increased collagen VI production also coincides with increased adipose tissue macrophage content (300, 301). To further illustrate parallels in the obese adipose and tumor microenvironments, collagen VI and its cleavage product have also been implicated in the initiation and progression of breast cancers. Collagen VI is abundantly expressed by breast cancer-associated adipocytes (discussed at greater length in the Adipocytes section), and its increased deposition in the ECM promotes tumorigenesis and malignant progression both in vitro and in vivo by inducing alterations in cancer cell signaling programs, gene expression patterns, and post-translational modifications (185,186). For example, treatment of MCF-7 human invasive breast cancer cells with collagen VI significantly elevated the activity of the oncogenic Akt-GSK3β–β-catenin–Tcf/Lef pathway, ultimately resulting in cyclin D1 protein stabilization and enhanced cell proliferation (185, 186). Accordingly, expression of the proto-oncogenes GSK3β and cyclin D1 in mammary tumors exhibited a steep immunohistochemical gradient, with increased staining intensities observed proximate to adipocytes. A similar gradient in collagen VI expression was also observed, further implicating adipocyte-derived collagen VI in the induction of mitogenic signaling pathways (186). In addition, adipocyte-derived endotrophin induces markers of EMT and acts as a potent adipokine that exerts growth-stimulatory and prosurvival effects on developing tumors (300). Furthermore, endotrophin overexpression in the breast tumor microenvironment is associated with increased rate of metastasis (300) and resistance to the platinum-based chemotherapeutic cisplatin (298). Thus, increased collagen VI deposition and endotrophin concentration in the extracellular milieu of obese adipose may influence both early tumor development and treatment outcomes.
Figure 10. Obesity-associated modifications in the adipose tissue microenvironment.
Adipose tissue expansion in obesity occurs in association with extracellular matrix changes such as fibrosis. Adipocyte hypertrophy and hypoxia trigger macrophage infiltration and crown-like structure formation, which further exacerbates development of fibrosis and inflammation.
Adipose extracellular matrix-derived factors: Direct effects on epithelial cells
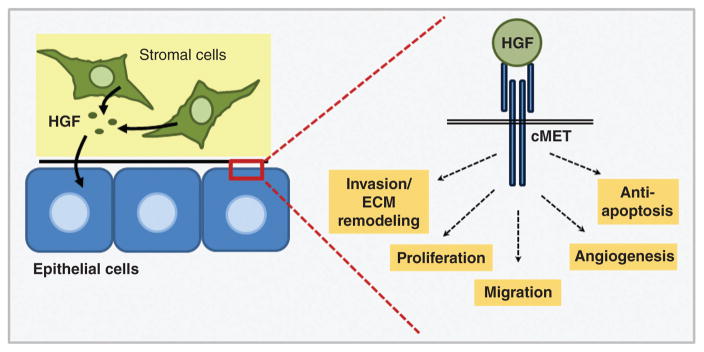
In addition to modulating composition and viscoelasticity of the breast ECM, stromal cells within the obese breast microenvironment secrete numerous soluble signaling mediators that have direct effects on epithelial cells. In particular, hepatocyte growth factor (HGF) is an excellent candidate for stromal-mediated breast cancer promotion in the context of obesity. Serum HGF is elevated in obese individuals and is reduced with weight loss (39, 172, 379), and HGF has been detected in both normal and malignant breast tissue (404). Although HGF is classified as an adipokine (421), it is produced by a number of breast cell types including stromal fibroblasts. HGF is the only known ligand for its receptor, cMET, and HGF signaling impacts the phenotypes of both early- and late-stage breast cancers. With respect to early-stage lesions, we have reported that treatment of premalignant basal-like breast cells with HGF-blocking antibodies inhibited 3D morphogenesis, reflecting a reduction in epithelial malignant potential (63). Importantly, basal-like breast cancer is a clinically intractable TNBC subtype that is more prevalent in obese individuals (12, 16, 67, 307, 405), and an HGF gene expression signature generated via treatment of pre-malignant breast cells with recombinant HGF was found to correlate with both basal-like subtype and poor survival in >700 breast cancer samples from three publically available datasets (63).
In advanced tumors, HGF signaling initiates an invasive growth program that promotes cell migration, invasion, proliferation, and angiogenesis (Fig. 11) (255). HGF is also elevated in the serum of breast cancer patients and correlates with advanced disease (63, 173, 174, 351). In support of this observation, our laboratory previously demonstrated that high fat diet-induced obesity increased HGF concentration and enhanced expression and activation of cMET in the mammary fat pad of C3(1)-T-antigen (TAg) mice, a unique genetically engineered mouse model (GEMM) of spontaneous basal-like breast cancer (152, 170, 376). We also reported that obesity increased HGF production by primary murine fibroblasts isolated from both normal mammary glands and tumors, and that CAFs isolated from obese animals induced epithelial cell migration in an HGF-dependent manner (376). Obesity-mediated regulation of HGF secretion from other stromal cell types such as adipocytes is currently under investigation.
Figure 11. HGF/cMET: an oncogenic signaling cascade.
HGF secretion by stromal cells such as fibroblasts, adipocytes, and macrophages initiates an invasive growth program in epithelial cells.
Adipose extracellular matrix in prostate cancer
Despite being a common feature of mouse models of prostate cancer, histologically conspicuous reactive stroma is much less prevalent in human prostate tumors (184). However, like the breast, induction of a myofibroblastic phenotype and degree of reactive stroma carry important prognostic value for prostate cancer malignancy (23, 365, 406). Notably, as the literature regarding the contribution of adipose tissue to breast cancer onset and progression has greatly outpaced that of prostate cancer, obesity-associated ECM modifications are currently better characterized in the mammary, relative to the periprostatic, fat pad. Additionally, conflicting data exist regarding the association between periprostatic fat density (measured by magnetic resonance imaging or computed tomography) and tumor aggressiveness in prostate cancer patients (413,414,441). Our literature search also revealed no publications reporting that periprostatic adipose tissue fibrosis occurs in obesity, but whether this is due to a lack of occurrence or a lack of examination is unknown. Furthermore, no studies investigating links between adipocyte-derived endotrophin and prostate cancer were available at the time of writing this review. Therefore, future obesity-prostate cancer studies may be informed by the sundry findings linking breast cancer and adipocyte-associated fibrosis, modifications in ECM dynamics, and endotrophin release.
Adipocytes and adipocyte-cancer interactions
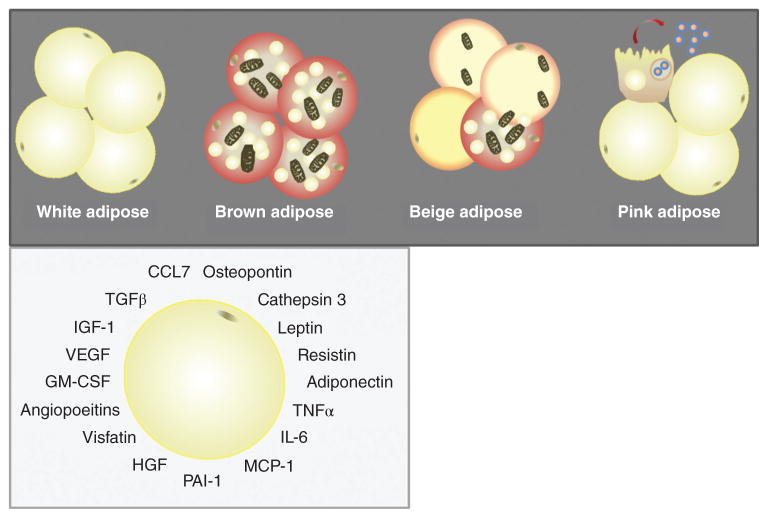
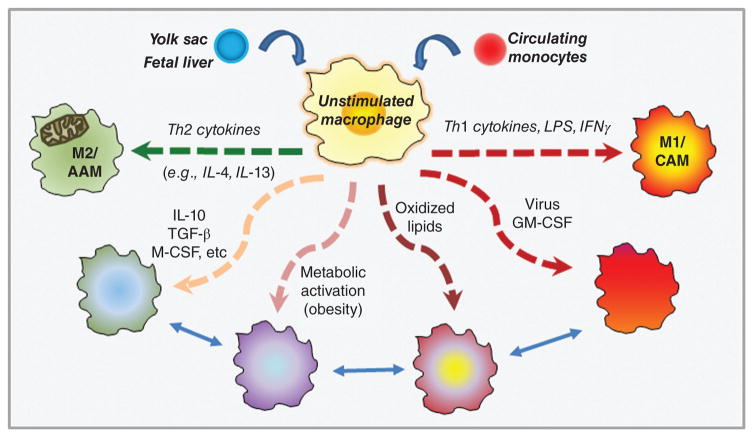
Adipocytes are specialized connective tissue cells that constitute a major cell type in both the normal-weight and obese breast. The majority of adipocytes in adult humans are white adipocytes, which contain a large, unilocular lipid droplet and are specialized for storage of neutral lipids. However, brown and/or beige adipocytes (also called “brite” or “inducible” adipocytes (147)) have also been reported in adults, and likely play important roles in thermogenesis (445). More recently, “pink” adipocytes have been described in murine mammary gland, arising exclusively during pregnancy and lactation due to a process wherein white adipocytes progressively transdifferentiate to acquire secretory, epithelial-like features (147). Adipocytes secrete a broad range of signaling molecules that exert local and/or systemic effects with the potential to influence tumor growth. Among the better studied adipocyte-derived factors are metabolic factors such as leptin, adiponectin, resistin, visfatin, and plasminogen activator inhibitor-1 (PAI-1); hematopoietic factors such as GM-CSF; growth factors such as angiopoietins, HGF, vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), and TGF-β; and a variety of cytokines, including interleukin-6 (IL-6) and TNF-α and the chemokine monocyte chemoattractant protein (MCP-1) [also referred to as chemokine (C-C motif) ligand 2 (CCL2)] (Fig. 12) (60, 391). Several of the aforementioned adipocyte-derived growth factors influence development of a tumor vascular supply (tumor angiogenesis), as discussed in the Endothelial Cells/Lymphendothelial Cells section below. Whereas leptin and adiponectin are considered true adipokines, many of the other signaling molecules, including resistin, visfatin, TNF-α, IL-6, MCP-1, and PAI-1, are not, as they are expressed by both adipocytes and immune cells populations such as macrophages, and play a variety of well-known roles in immunity (391). Thus, select functions for several of these signaling molecules will be discussed within the section titled Adipose
Figure 12. Adipocyte subtypes and secreted factors.
White adipocytes contain a large, unilocular lipid droplet and are specialized for storage of neutral lipids. Brown and/or beige adipocytes have increased mitochondrial content relative to white adipocytes and play important roles in thermogenesis. “Pink” adipocytes have been described in murine mammary gland, arising exclusively during pregnancy and lactation. Collectively, adipocytes secrete a broad range of signaling molecules.
Tissue Immune Populations in Cancer Development and Progression
Finally, although there are clear and important roles for leptin and adiponectin in tumorigenesis and malignancy, these roles have been reviewed extensively by others (137, 196, 284, 299, 421) and will be addressed only briefly within this review.
Adipocytes exhibit both short- and long-range interactions with cancer cells, and may be found in close proximity to tumors, along tumor margins, and within the tumor body. These cancer-associated adipocytes (CAAs; also referred to as peritumoral, intratumoral, or tumor-infiltrating adipocytes) influence tumor biology in a number of ways, including by promoting angiogenesis and inflammation (reviewed in 274, 423, 427). Although it is reasonable to hypothesize that proliferation and invasion of tumor cells into cancer-adjacent adipose may account for the presence of CAAs within the tumor body, the origin of CAAs in fact remains unclear. As explained in further detail in the section on Adipose-derived Stromal Cells below, several cell types may give rise to intratumoral CAAs.
In addition to indirect mechanisms of tumor growth promotion (e.g., stimulation of angiogenesis, production of proinflammatory cytokines), the proximity of CAA to growing tumors may also provide direct metabolic benefits to cancer cells. In the phenomenon known as metabolic symbiosis, cancer cells within hypoxic regions of a tumor undergo metabolic shifts that facilitate increased utilization of fuel sources such as lactate, glutamine, and fatty acids released by surrounding cells, including other cancer cells (8, 268) and adipocytes (241, 250). Lipid droplet size within mature white adipocytes is the net result of several processes, including fatty acid uptake or de novo fatty acid synthesis, esterification, and lipolysis. As mentioned previously, CAAs have been frequently observed to undergo delipidation. Interestingly, Nieman et al. showed that co-culture of primary omental adipocytes with ovarian cancer cells, which frequently metastasize to the omentum, induced lipolysis in adipocytes, upregulation of β-oxidation in cancer cells, and direct transfer of lipids between the two cell types (273). Notably, the transfer of lipids from adipocytes to cancer cells has also been observed in prostate cancer (139) and breast cancer (426). These findings indicate that active heterotypic cellular interactions between cancer cells and adipocytes induce metabolic symbiosis.
CAAs may also influence cancer cell phenotypes through the shedding of exosomes, small vesicular bodies released from cells as a form of short- or long-range communication. Lazar et al. (215) reported that exosome shedding by mature human adipocytes induced increased migratory and invasive behavior in melanoma cells, which grow in proximity to the hypodermal adipose layer. Proteomic analysis of adipocyte-derived exosome composition revealed enrichment for proteins involved in mitochondrial lipid metabolism, particularly fatty acid oxidation. Remarkably, their results suggested that these enzymes were incorporated and utilized by melanoma cells. Melanoma cells pretreated with exosomes exhibited increased ability to form lung metastases in mice and an increase in fatty acid oxidation without a concomitant change in glycolysis, indicating that augmentation of lipid oxidation pathways occurred in the absence of complete metabolic reprogramming. In further support of these findings, administration of the mitochondrial fatty acid oxidation inhibitors etomoxir or trimetazidine reversed exosome-induced enhancement of migration without affecting basal migration levels. Importantly, increasing adiposity in obese individuals enhanced both the number of exosomes released from adipocytes as well as the potency of their effect on melanoma cell migration. Collectively, these studies reveal important roles for adipocytes in regulating cancer cell migration and metastatic potential.
Adipocytes in the normal breast and breast cancer
Mouse models have revealed that adipocytes act as local regulators of normal mammary epithelial cell growth and function. Thus, mammary epithelial cells possess an inherent requirement to reside among adipocytes during embryonic and postnatal development, as well as throughout later life stages such as pregnancy, lactation, and involution (176). Indeed, using the novel FAT-ATTAC mouse, a model of inducible and reversible adipocyte loss developed by Scherer and colleagues, Landskroner-Eiger et al. showed that adipocytes play crucial roles in normal growth and development of mammary ductal epithelium (71, 209), contributing both to ductal branching morphogenesis during puberty and to maintenance of normal alveolar structures in adulthood (209).
Due to the proximity of the adipose pad to the mammary glandular organ, ductal tumor invasion results in interaction of breast cancer cells with adipocytes and other adipose stromal constituents (Fig. 6 and Fig. 7), with dramatic implications for tumor cell biology. Carter and Church reported that mature breast adipocytes, but not preadipocytes, increased motility of both normal and malignant breast epithelial cell lines through secretion of PAI-1 (62). Similarly, higher levels of CAA-specific IL-6 expression in human breast tumors were associated with larger tumor size and more extensive lymph node involvement (92). Moreover, coculture with adipocytes induced mesenchymal features in human breast cancer cells, including repolarization of vimentin and downregulation of E-cadherin, thereby promoting tumor cell invasion and metastasis (92). Furthermore, adipocytes cocultured with malignant breast epithelial cells exhibited the profound phenotypic changes associated with CAA, including delipidation and decreased expression of adipocyte markers (92). Hence, bidirectional communication between adipocytes and breast tumor cells also alters adipocyte biology.
For example, reminiscent of findings in melanoma (215), prostate (139), and ovarian cancers (273) (discussed in the Adipocytes and adipocyte-cancer interactions intro section above), following coculture of breast cancer cells with adipocytes Wang et al. reported increased lipolysis by adipocytes and concomitantly increased fatty acid oxidation by breast cancer cells (426). Importantly, the signal released by tumor cells to induce adipocyte delipidation was not identified, although IL-6 and β-adrenergic stimulation—factors previously implicated in lipolytic induction in cancer-associated cachexia (393)—were eliminated as potential candidates (426). Similar to Lazar et al. (215), Wang et al. reported that coculture with adipocytes increased both in vitro invasion toward a stimulus and formation of breast cancer lung metastases in vivo, each of which were restored to basal levels by administration of the fatty acid oxidation inhibitor etomoxir (426). In vitro etomoxir administration also reduced the morphological hallmarks of EMT. Remarkably, the increase in fatty acid oxidation by breast cancer cells appeared to be dependent on an upregulation of both adipocyte triglyceride lipase (ATGL) and the carnitine palmitoyltransferase 1 (CPT1) isoform CPT1A, enzymes not expressed at appreciable levels in noncancerous human breast epithelial cells. Short-hairpin (sh)RNA-mediated knockdown of CPT1A and ATGL reduced hallmarks of EMT and invasive potential, respectively.
In addition to oxidizing transferred fatty acids, breast cancer cells also esterified free fatty acid from adipocyte lipolysis (426), incorporating the newly synthesized triglyceride into lipid droplets within the cancer cells themselves. Breast cancer cell lipid droplet accumulation was supported by both in vitro coculture experiments employing radiolabeled palmitate and the observation of lipid droplet accumulation in breast cancer cells along the tumor margin in histological sections (i.e., in close proximity to adipocytes). Interestingly, despite increased fatty acid oxidation, breast cancer cells showed reduced ATP content and activation of AMP-activated protein kinase (AMPK). AMPK activation following coculture with adipocytes was associated with increased mitochondrial biogenesis and function, indicated by increased levels of PGC-1α and its associated transcription factor PPARα as well as an increase in the ratio of mitochondrial to genomic DNA. AMPK also inhibited acetyl-CoA carboxylase, the rate-limiting enzyme in fatty acid synthesis, ensuring uninterrupted flux of fatty acids into mitochondria. Furthermore, breast cancer cell fatty acid oxidation was determined to be uncoupled from ATP production and, unlike in melanoma (215), occurred with a concurrent increase in anaerobic glycolysis, consistent with activation of AMPK (426). Collectively, these findings provide new insight into mechanisms of metabolic symbiosis between adipocytes and cancer cells in breast tumors.
Interactions between cancer cells and adjacent adipose may also increase breast cancer stem cell abundance and facilitate metastatic progression. Picon-Ruiz et al. isolated human adipocyte stem cells and used adipogenic differentiation media to generate “immature” adipocytes. Coculture of these “immature” adipocytes with both primary breast cancer cells and established cancer lines conferred stem-like features to the epithelial cells, including elevated expression of the pluripotency markers Sox2, c-Myc, and Nanog (306). Coculture with adipocytes also increased mammosphere-forming capacity, indicating a more stem-like phenotype due to a greater ability to grow under nonadherent conditions. Furthermore, when co-cultured breast cancer lines were orthotopically injected into mouse models, the resulting tumors exhibited reduced latency, increased abundance of tumor-initiating cells, and an enhanced capacity to form distant metastases. Taken together, this study demonstrates that interactions between immature adipocytes and breast cancer cells drive initiation of highly metastatic cancers by enhancing epithelial cell tumor-initiating potential.
Due to the practice of autologous fat grafting as a method of breast reconstruction (oncoplastic surgery) following breast-conserving tumor excision, the impact of adipocytes on tumor malignancy may be a consideration for recurrence following treatment. Indeed, using a model of autologous fat grafting, Massa et al. reported increased proliferation of several breast cancer lines co-cultured with either induced adipocytes (i.e., differentiated from fibroblasts) or intact adipose tissue samples obtained from liposuction patients (245). However, a recently published prospective matched case-control analysis found no significant differences in locoregional recurrence in patients who received autologous fat grafting versus those who did not (249). Although cases and controls were matched for hormone receptor status in this study, no analysis was conducted to evaluate potential differences in recurrence by molecular tumor subtype, potentially due to the limited sample size and low locoregional event rate. Therefore, based on the aforementioned complex relationships between obesity status and risk of specific breast cancer subtypes, as well as the reported roles for adipocytes in regulating breast epithelial tumorigenicity and metastatic potential, additional studies are needed to address concerns regarding the potential risks associated with fat grafting in breast reconstructive surgery. Stratification by BMI and/or molecular tumor subtype may be necessary to fully assess the influence of fat grafting on breast cancer recurrence rates.
Adipocytes and prostate cancer
Bidirectional communication between adipocytes and prostate epithelial cells also influences prostate tumor biology, particularly with regard to chemokine activity. Chemokines, or chemotactic cytokines, are small secreted signaling proteins that induce directed, gradient-driven migration (chemotaxis) in nearby cells that express the appropriate chemokine receptor. The functions of chemokines in malignancy depend on both tumor characteristics and the specific chemokine in question, but are frequently associated with leukocyte infiltration as well as metastatic potential and site-specific spread of tumor cells (26). Adipose tissue-specific expression of many CC subfamily chemokines and their receptors is upregulated in human obesity (177). For example, Laurent et al. (211) identified a CCR3/CCL7 axis regulated by obesity, through which secretion of CCL7 by mature periprostatic adipocytes supported the directed migration of prostate cancer cells, thereby promoting cell migration toward the periprostatic fat pad and the spread of cancer cells outside of the prostate gland. This process appeared to be augmented in obesity by both enhanced secretion of CCL7 by hypertrophic adipocytes and increased expression of the CCL7 receptor, CCR3, by prostate cancer cells (211).
Adipocyte-derived CCL2 is also implicated in prostate cancer progression. Ito et al. reported that adipocyte-derived CCL2 directly stimulated prostate cancer cell proliferation, promoting invasion and migration through induction of MMP-2 activity and ultimately leading to enhanced tumorigenesis and metastasis (183). Importantly, increased production of CCL2 by bone marrow adipocytes and other stromal cells is also strongly implicated in the propensity of prostate cancer cells to metastasize preferentially to bone (161, 222). Adipocytes are an important component of the bone marrow microenvironment. Bone marrow adipocyte content increases with age, obesity, and obesity-associated metabolic pathologies (169, 197), suggesting a potential link between obesity and elevated rates of prostate cancer metastasis (161, 222).
Interestingly, prostate cancer-adipocyte crosstalk also appears to induce tumor-promoting changes in periprostatic adipocytes. Treatment of periprostatic adipose tissue organotypic explants with PC3 prostate carcinoma cell-conditioned medium activated a cancer-promoting secretory profile, including increased secretion of osteopontin, TNFα, and IL-6, and reduced production of adiponectin (328). These changes were not observed upon treatment of cells comprising the periprostatic adipose stromal vascular fraction (i.e., all stromal populations except adipocytes) with PC3 cell-conditioned medium, suggesting that the observed increase in protumorigenic factor production by explanted tissue was due specifically to tumor-mediated education of adipocytes (328). Indeed, adipocytes appear to be a major source of microenvironmental IL-6 in prostate cancer. Periprostatic adipose tissue harvested from patients undergoing radical prostatectomy secreted IL-6 at concentrations 375 times greater than that in patient-matched serum and correlated with histological grade (117). Additionally, Tang et al. (386) recently showed that co-culture of prostate cancer cells increased production of the cysteine protease cathepsin B by adipocytes. Further probing revealed that adipocyte co-culture induced secretion of the peptide hormone cholecystokinin (CCK) by prostate cancer cells, resulting in establishment of an autocrine/paracrine amplification loop in which CCK, acting through the CCK receptor CCKBR, induced expression of cancer stem cell markers such as CD49f and Sca-1 in prostate cancer cells and further production of cathepsin B by adipocytes. Importantly, cathepsin B has been shown to facilitate prostate cancer invasion and metastasis via degradation of ECM and basement membrane components (37, 252). Collectively, these studies demonstrate that prostate cancer cell-induced alterations in adipocyte function are important mediators of tumor progression. Figure 13 briefly summarizes adipocyte-cancer cell crosstalk findings.
Figure 13. Adipocytes promote tumor progression and metastasis.
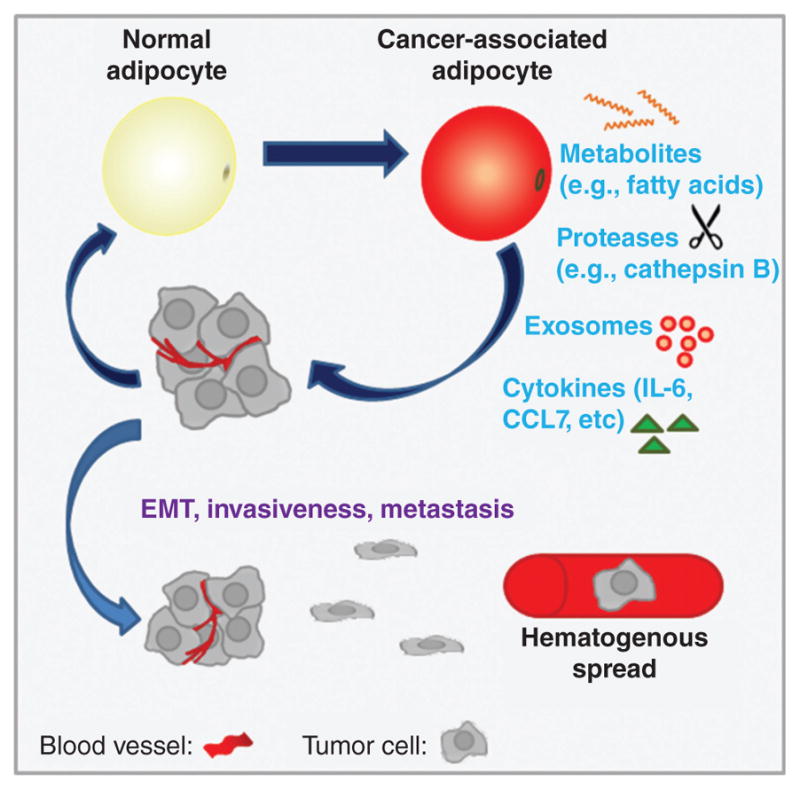
Adipocytes may provide metabolic substrates directly to cancer cells, or may indirectly influence cancer metabolism through exosome secretion. Adipocytes also secrete a variety of factors that promote tumor growth, EMT (epithelial-mesenchymal transition), acquisition of stem-like features, invasive behavior, and metastasis.
Adipocytes and adipose wasting in cancer-associated cachexia
An example of long-range adipocyte-tumor interactions can be observed in cancer-associated cachexia (referred to hereafter as cancer cachexia or simply cachexia). Cancer cachexia is a fatal energy-wasting syndrome that is estimated to be the immediate cause of death in approximately 20% to 40% of end-stage cancer patients (392). A key feature of cancer cachexia is white adipocyte “browning,” characterized by greatly increased levels of brown fat-mediated thermogenesis in white adipose depots (201, 303). Accordingly, cachectic patients exhibit irreversible, pathologically elevated basal energy expenditure levels, adipocyte lipolysis and adipose tissue wasting, rapid weight loss, and eventually, death (4, 78, 201, 303). Although prolonged systemic inflammation plays a well-established role in cachexia-associated adipose tissue wasting (4, 78), tumor-derived factors have also been shown to contribute to the pathophysiology of this syndrome. For example, in a murine model of Lewis lung carcinoma, Kir et al. (201) demonstrated that tumor-derived parathyroid hormone-related protein (PTHrP) induced the expression of thermogenesis-associated genes in adipose tissue, implying a crucial role for this hormone in energy expenditure and tissue wasting. Accordingly, administration of a PTHrP neutralizing antibody prevented cachexia-associated weight loss and ablated thermogenic gene expression in white and brown adipose tissue. Furthermore, compared to cancer patients lacking detectable levels of blood PTHrP, patients with detectable blood PTHrP levels exhibited significantly higher resting energy expenditure levels per kilogram of lean body mass, implying a clinically relevant association between this hormone and wasting.
On the other hand, Rohm et al. (332) reported that browning and associated thermogenesis in major white adipose depots was not the primary mechanism of adipose tissue wasting in mouse models of colon cancer-induced cachexia. Although the brown adipose-associated protein cell death activator (CIDEA) was upregulated in both brown and white adipose depots of cachectic mice relative to healthy controls, this upregulation occurred in the absence of changes in other proteins implicated in adipocyte browning and thermogenesis, such as uncoupling protein 1 (UCP-1). Furthermore, while increased free fatty acid release was observed in primary mouse adipocytes exposed to serum from cachectic mice, this increase in lipolysis was not associated with well-characterized lipolytic inducers such as increased expression of lipases (e.g., hormone-sensitive lipase and ATGL), or increased β-adrenergic receptor activation. Instead, CIDEA-mediated degradation of AMPK, evidenced by a reduction in AMPK protein and enzymatic activity, contributed to adipocyte metabolic dysfunction. For example, although increased lipolysis was observed, decreased AMPK activity also resulted in reduced inhibitory phosphorylation of acetyl-CoA carboxylase, suggesting the establishment of a futile cycle in adipocytes characterized by simultaneous increases in both lipolysis and lipogenesis. Microinjection of white adipose depots with a peptide designed to interfere with the AMPK-CIDEA interaction (termed AMPK–CIDEA-interfering peptide, or ACIP), followed by implantation of the cachexia-inducing colon cancer cell line C26, resulted in approximately 30% greater retention of adipose depot mass and greater adipocyte lipid droplet size compared to the contralateral control-injected depot. No significant effect was observed from ACIP injection into adipose depots of control, noncachectic mice, suggesting that the augmented AMPK-CIDEA interaction and downstream influences on lipid metabolism in adipocytes may be a cachexia-specific phenomenon.
These findings by Rohm et al. are particularly interesting in light of the reportedly opposite regulation of AMPK in breast cancer cells co-cultured with adipocytes that was highlighted in the previous section (426). Also interesting to note is the lack of a role for β-adrenergic signaling in either of these two studies (332, 426), as catecholamines are well-established regulators of lipolysis, while lipid mobilizing factor—a tumor-derived factor frequently implicated in cachexia (393, 394)—also signals through beta receptors. Thus, although the causes of cachexia are multifactorial and systemic, it is clear that adipocyte-cancer cell interactions are key players in the pathophysiology of this syndrome. Future work should seek to identify additional tumor-derived paracrine and hormonal signals that contribute to cachexia pathogenesis and progression.
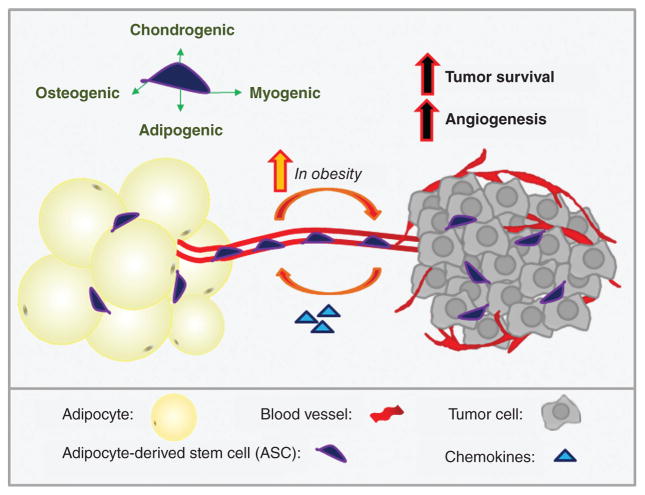
Adipose-derived stem cells
Human adipose tissue stroma is a rich source of multipotent mesenchymal stem cells, termed adipose stromal cells or adipose-derived stem cells (ASCs), that can differentiate toward the osteogenic, adipogenic, myogenic, and chondrogenic lineages (467). Interestingly, several recent studies suggest that ASC recruitment substantially contributes to stromal populations in both breast and prostate cancers. Due to the abundance of adipose tissue, as well as the minimally invasive procedures required to collect it, ASCs are a celebrated approach for tissue engineering and regenerative medicine. For example, lipoaspirate preparations may be “enriched” by the addition of ASCs to improve graft volume retention (204, 403). However, the findings described below suggest that caution may be advised in use of ASCs in patients with a history of cancer. Notably, factors such as age and menopausal status have been found to influence the proliferation and differentiation capacities of ASCs (47). Future studies on the impact of age on ASC recruitment to tumors will yield interesting findings.
Adipose-derived stem cells in breast cancer
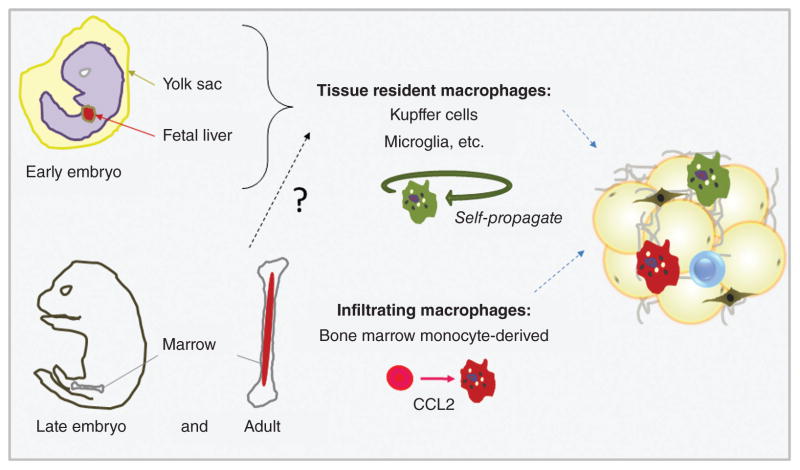
The varied stromal components of the tumor microenvironment must be recruited from either adjacent tissue or from distant precursor sources such as bone marrow. Kidd et al. (200) investigated the relative contribution of ASCs versus bone marrow-derived stem cells to stromal populations in mouse models of ovarian and breast cancers, and found that the majority (greater than 70%) of intratumoral myofibroblasts, pericytes, and endothelial cells were recruited from neighboring adipose tissue. However, CAF subpopulations were recruited from multiple distinct sources, with fibroblasts positive for fibroblast specific protein and fibroblast activation protein originating from bone marrow-derived mesenchymal stem cells, while α-smooth muscle actin+/chondroitin sulfate proteoglycan 4+ (α-SMA+/NG2+) CAFs were recruited from adjacent adipose. While the factors contributing to ASC recruitment to tumors are still ambiguous, Gehmert et al. have demonstrated that the PDGF-BB/PDGFR-β signaling pathway may be involved in ASC recruitment to breast cancers (140). Together these results imply that the diversity of the tumor microenvironment can be attributed, at least in part, to the heterogeneous origin of stromal constituents.
Although ASCs are primarily localized to fat depots, circulating ASCs have also been detected in obese individuals and cancer patients, with greater levels observed in obese patients bearing colon, prostate, or breast cancers (relative to lean) (142, 327, 367, 462). Additionally, relative to ASCs from lean adipose, ASCs isolated from obese adipose show enhanced potential to traffic to breast tumors in both humans and mice (366, 464). Zhang et al. (464) recently reported hematogenous seeding of breast and ovarian tumors by ASCs in obese mice, resulting in infiltration and subsequent differentiation to pericytes and intratumoral adipocytes/CAA. This process occurred in an obesity-dependent manner, with a sixfold increase in “shedding” of precursors from adipose depots in obesity contributing to tumor cell survival and angiogenesis. It will be interesting to note in future studies whether specific adipose depots shed ASCs to the circulation at different rates. Ultimately, these findings reinforce the need to more comprehensively evaluate the risk of breast cancer recurrence after autologous fat grafting, particularly in obese individuals.
Adipose-derived stem cells in prostate cancer
Similar to breast cancer, local and circulating ASCs have been reported in prostate cancer patients. Ribiero et al. observed higher levels of circulating ASCs in the blood of overweight or obese compared to lean prostate cancer patients (327). The authors also reported that periprostatic adipose tissue of prostate cancer patients bore significantly higher numbers of ASCs than nearby visceral adipose tissue, independent of BMI. Interestingly, increased recruitment of ASCs into prostate tumors in obesity has been reported, and was recently attributed to secretion of the chemokines CXCL1 and CXCL8 by cancer cells (Fig. 14) (462, 464). CXCL8 expression was restricted to malignant cells and was obesity-independent; on the other hand, secretion of CXCL1 by nonmalignant epithelium was exclusively observed in histological sections from obese individuals, while CXCL1 expression in tumor cells was found in a significantly higher percentage of tumor sections from obese as compared to lean patients (462). The extent to which periprostatic ASCs, as opposed to circulating ASCs released from other adipose depots, contribute to the cellular composition of prostate tumor stroma was not quantified in the highlighted studies and requires further investigation.
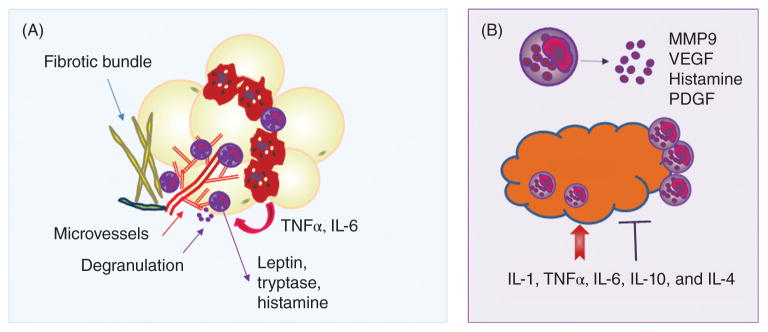
Figure 14. Obesity, cancer increase circulating ASCs.
Human adipose tissue stroma is a rich source of multipotent ASCs, which enter the circulation and traffic to other tissues. This “shedding” process is increased in obese and/or tumor-bearing individuals. Tumor chemokine secretion (e.g., CXCL1, CXCL8) is influenced by obesity and is implicated in ASC recruitment to developing tumors and differentiation into stromal populations such as fibroblasts, pericytes, and adipocytes.
Adipose and endothelial/lymphendothelial cells
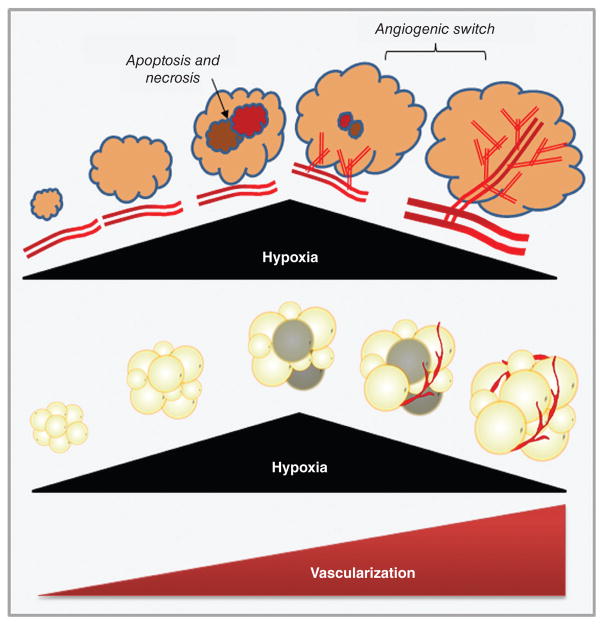
Vascularization mechanisms in adipose tissue and tumors
Expansion of adipose tissue during progression to obesity requires concomitant expansion of the adipose vascular bed through the process known as angiogenesis, the formation of new blood vessels from preexisting vessels. In fact, administration of antiangiogenic agents in models of both genetic and diet-induced obesity either prevented weight gain (385) or induced dose-dependent, reversible weight reduction and adipose tissue loss (52, 333). When expansion of the vasculature does not occur in proportion to the expansion of adipocyte volume (hypertrophy), cellular and/or regional hypoxia develops, resulting in activation of the transcriptional complex hypoxia-inducible factor 1 (HIF-1) through stabilization of the HIF-1α subunit. HIF-1-mediated upregulation of inflammatory and proangiogenic signaling pathways in adipocytes, endothelial cells, and immune cells induces vascular growth, facilitating further tissue expansion (216, 381, 434). In this way, the microenvironment during accumulation of adipose tissue resembles the tumor microenvironment during tumor vascularization (Fig. 15). The extensive list of signaling factors contributing to angiogenesis in both adipose tissue and tumors includes VEGF isoforms, angiopoietins 1 and 2, leptin, adiponectin, TNFα, fibroblast growth factor (FGF) isoforms, TGFβ, HGF, and cytokines such as IL-6 and IL-8 (60, 225, 266). Among these, the VEGF/VEGFR system—one of the best characterized and most potent of the known proangiogenic signaling pathways—is the main mediator of angiogenic activity in adipose tissue (115, 166, 225). The VEGF-A ligand in particular is abundantly expressed by adipocytes and other adipose stromal populations (166, 225). An additional shared factor of particular importance is angiopoietin-2, which signals through the receptor tyrosine kinase TIE2 to induce ECM degradation and disruption of endothelial-pericyte interactions during sprouting angiogenesis (157, 203). Importantly, several of the pro-angiogenic factors listed above, including multiple VEGF isoforms, leptin, HGF, and angiopoietin-2, are also elevated in the serum of obese subjects and are implicated in systemic effects of obesity on cancer progression (39, 253, 358).
Figure 15. Hypoxia & the angiogenic switch.
An extensive list of proangiogenic factors is involved in both induction of the angiogenic switch in developing solid tumors and expansion of adipose tissue during progression to obesity. As tumor cells proliferate or adipocytes hypertrophy, hypoxia develops and triggers stabilization of the HIF-1 complex, a transcription factor which promotes increased production of growth factors such as VEGF-A, FGF1, TGF-β, HGF, and angiopoietins 1 and 2. Additional proangiogenic factors include the adipokines leptin and adiponectin; cytokines such as TNFα, IL-6, and IL-8; and matrix metalloproteases, which degrade the extracellular matrix. Ultimately, increased vascularization alleviates regional hypoxia and facilitates further tissue expansion.
Similar to adipose tissue, growth of solid tumors is also heavily dependent upon synchronous expansion of their vascular beds. In early stage solid tumors, rapid proliferation leads to diffusion-limited hypoxia, wherein cells within the tumor mass end up at a distance from the surrounding vasculature that is beyond the diffusion limit of oxygen. Resulting hypoxia-induced apoptosis and necrosis limit further tumor growth unless an intratumoral vascular system is established. The shift in developing primary or metastatic tumors from avascular to vascularized is termed the “angiogenic switch,” and is a discrete and requisite step for exponential tumor growth and progression to malignancy (Fig. 15) (40,159,324). Accordingly, tumor microvessel density is a powerful and independent prognostic indicator for several human cancers, including breast, prostate, melanoma, ovarian, gastric, and colon cancers (324). However, in light of the myriad options for tumor vascularization described below, it is interesting to note that themicrovessel density in solid tumors is often lower than in their normal tissue counterparts (103).
New tumor vessel formation can occur through a number of nonmutually exclusive mechanisms, including sprouting and migration of endothelial cells (“classical” sprouting angiogenesis) or intussusceptive (nonsprouting) microvascular growth, a process in which tumor cells induce splitting and rapid remodeling of existing endothelial vessels (324). Remarkably, along with endothelial cells, tumor cells themselves may integrate into newly forming blood vessels, resulting in mosaicism (324). Tumor cells may also engage in a process known as vasculogenic mimicry, the arrangement of tumor cells into vascular channels, which anastomose with adjacent blood vessels (100, 234, 324). An additional mechanism for perfusion of tumors is vessel co-option, wherein tumor cells simply track alongside existing vessels for their own oxygen and nutrient gain, thereby exploiting nearby mature vessels in the host organ (324). Given that adipose tissue is one of the most vascularized tissues in the body (225, 423), it is unsurprising that co-option of adipose tissue vascular beds was recently shown to promote accelerated tumor growth and intratumoral vascularization (226).
Among other abnormal features, tumor vasculature is characterized by enhanced permeability, including transcellular holes and fenestrae, which drives further angiogenesis and increases nutrient and oxygen delivery, immune cell infiltration, and tumor cell extravasation during metastasis (25, 99). Similar to adipose tissue, the VEGF/VEGFR system—and particularly VEGF-A—is highly expressed in tumors and is a potent inducer of tumor vascular permeability (102). Given the extensive similarities of the pro-angiogenic signaling networks in adipose and tumors, it is unsurprising that the vasculature in these two tissue types is structurally similar. For example, adipose tissue capillaries also contain fenestrations, the presence of which depends upon a poorly understood synergistic relationship between VEGF, leptin, and FGF-2 signaling (59). It is tempting to speculate that the fenestrations within adipose vasculature may provide a convenient means of escape for tumor cells invading into adipose tissue.
In addition to hematogenous metastasis, a tumor cell can also escape from its primary location through lymphatic dissemination. In a number of cancer types, including breast cancer, melanoma, and prostate cancer, metastasis to the tumor draining lymph node(s), also referred to as the “sentinel” lymph node(s), is a common initial route for metastatic dissemination from solid tumors (7). For this reason, sentinel lymph node biopsy in newly detected and early-stage cancers is a frequent and evidence-based clinical practice required for staging of disease, determination of prognosis, and development of the treatment approach. In a process similar in principle to classical sprouting angiogenesis, secreted factors in some solid tumor types and other inflamed tissues can also initiate lymphangiogenesis, the formation of new lymphatic vessels from preexisting vessels. These newly formed lymphatic vessels exhibit morphological differences from those in their healthy tissue counterparts, including structural disorganization (7). Interestingly, tumor-associated lymphangiogenesis appears to involve both incorporation of bone marrow-derived endothelial progenitors and endothelial mimicry by CD11b+ tumor-associated macrophages, although there are conflicting reports regarding the extent to which the latter occurs (167, 323, 341, 468).
Although peritumoral lymphatic vessel density can act as a prognostic indicator in several cancer types, including cervical, colorectal, breast, and prostate cancers (79, 105, 135, 256, 334), several studies have suggested that intratumoral lymphatic vessels in solid tumors may be either collapsed due to intratumoral pressure, occluded by infiltrating tumor cells and therefore nonfunctional, or simply absent altogether (295, 399, 418, 439). Thus, the high frequency of cancer cell detection in regional lymph nodes implicates peripheral, peritumoral lymphatic vessels in mediating tumor metastasis in these tumor types (439, 440). However, results showing nonfunctional intratumoral lymphatic vessels have not been uniformly supported (360). Consequently, the role of tumor lymphangiogenesis and the relative contribution of intratumoral versus peritumoral lymphatics to lymph node metastasis remains controversial.
Adipose and breast cancer angiogenesis
In vivo tumor models have demonstrated the ability of breast tumors to obtain a blood supply through all of the aforementioned processes: vessel co-option, intussusceptive growth, vasculogenic mimicry, and classical sprouting angiogenesis (124, 343). Additional mechanisms have also been described for breast cancers, such as vasculogenesis and glomeruloid angiogenesis, albeit to a lesser extent (124). Nevertheless, remodeling of existing vessels appears to be the dominant mechanism for establishing new vasculature in human breast cancers (97, 123). In support of this assertion, Lim et al. (226) demonstrated that implantation of the E0771 murine mammary tumor line into either brown or white adipose tissue resulted in accelerated tumor growth rates and increased intratumoral vessel densities as compared to tumors grown subcutaneously. These results were attributed to co-option of preexisting adipose vascular beds, as tumor growth and vascularity reflected the differential degree of vascularity within the respective adipose types. Furthermore, adjacent adipose tissue fostered both reduced pericyte coverage and enhanced permeability, features associated with worse prognosis.
In obesity, both the increased abundance of white adipose and the resulting chronic inflammatory conditions of the microenvironment may promote tumor vascularization. Indeed, enhanced tumor angiogenesis in the context of obesity is observed in both mice and humans (18, 153, 218, 422, 464). In one compelling study, Arendt et al. (18) developed a novel humanized mouse model wherein human adipose stromal populations overexpressing CCL2 were injected into cleared mammary fat pads (cleared of endogenous mammary epithelium) to generate an obese-like microenvironment. Prior to tumor formation, the authors reported enhanced angiogenesis in CCL2-overexpressing mammary fat pads, which was shown to be mediated by elevated levels of macrophage recruitment and activation. Upon transplantation of transformed human breast epithelial cells, the obese-like microenvironment augmented macrophage-associated angiogenesis in early premalignant lesions as well as tumor-adjacent adipose following tumor formation, which induced the formation of larger and higher-grade tumors. Whether the observed tumor-promoting effects were due to specific macrophage phenotypes in “obese” versus lean mammary adipose or simply to an increase in macrophage numbers was not explored. Moreover, this study did not differentiate whether increased tumor-associated macrophage content in obesity was due to accelerated recruitment of bone marrow-derived macrophages or to co-option of nearby mammary adipose tissue macrophages. Nevertheless, similar results were reported by Cowen et al., who demonstrated that high-fat diet-induced obesity in the MMTV-PyMT model of spontaneous breast cancer resulted in mammary adipose tissue inflammation, enhanced macrophage recruitment, and increased mammary tumor vascular density (73).
As described in the previous section, obesity is also associated with elevated levels of circulating and infiltrating ASCs (464) which produce a range of proangiogenic factors, including VEGF and HGF (217). Our lab has demonstrated that inhibition of the HGF receptor, cMET, via the small molecule kinase inhibitor crizotinib significantly reduced tumor burden and tumor vascularity in both lean and obese C3(1)-TAg mice (74). Reversal of high fat diet-induced elevation of HGF/cMET expression in both normal mammary gland and tumors was also observed with weight loss, which significantly blunted the effects of obesity on both pre-neoplastic lesion formation (316) and tumor progression (377) (Fig. 16). Importantly, endothelial cell upregulation of cMET is one mechanism attributed to inherent or acquired resistance to anti-angiogenic therapies targeting VEGF (91, 355). In fact, the HGF/cMET pathway has been reported to act synergistically with VEGF (355, 371), and clinical trials investigating crizotinib alone [ClinicalTrials.gov: NCT 02101385 (342)] or in combination with anti-VEGF therapy [ClinicalTrials.gov: NCT 02074878 (36)] for the treatment of advanced TNBC are currently underway at the time of preparation of this review.1
Figure 16. Mammary HGF/cMET signaling in the in C3(1)-Tag mouse model of basal-like breast cancer.
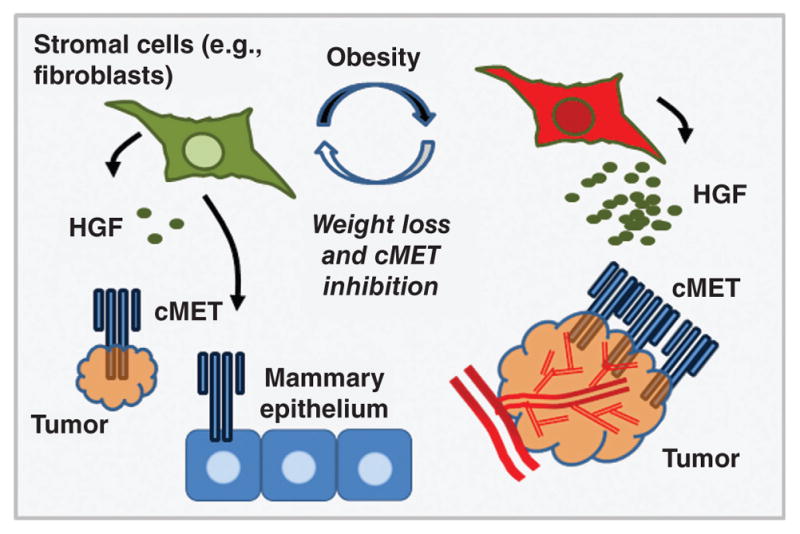
Obesity increased HGF production by stromal cells, promoting tumor growth and angiogenesis. HGF/cMET-mediated tumor promotion was reversible by weight loss or cMET inhibition.
However, one response to anti-angiogenic therapies is vessel pruning and regression, leading to intratumoral hypoxia. Such hypoxic conditions induce an influx of tumor-associated macrophages and other myeloid cells, triggering tumor revascularization and tumor relapse (248, 321, 329, 431). In addition, peritumoral adipose tissue is characterized by a dense macrophage infiltrate and a high degree of vascularization. Indeed, Wagner et al. demonstrated that inflamed, tumor-associated adipose tissue acts as a source of both vascular endothelium and activated proangiogenic macrophages, thereby fueling the growth of malignant cells (15, 422). Importantly, the presence of macrophages within adipose tissue increases considerably in obesity (429). Thus, obesity-associated mammary adipose inflammation and resulting macrophage infiltration and angiogenesis may contribute to tumor relapse following antiangiogenic therapies.
Adipose tissue and lymphangiogenesis in breast and prostate cancers
Lymphatic vessels in the normal breast are dispersed throughout the interlobular stroma and adipose tissue (418), the latter of which acts a source of molecules that directly affect the lymphatic endothelium. For example, the lymphangiogenic factors VEGF-C and VEGF-D are chemotactic for macrophages in mice, and their blockade in a diet-induced obesity model attenuated macrophage infiltration, adipose tissue inflammation, and onset of insulin resistance (194). An increase in circulating levels of pro-lymphangiogenic factors such as HGF and VEGF-C in obesity may also alter lymphatic vessel density or function by enhancing capillary permeability and inducing lymphendothelial hyperplasia (58,254,358). Indeed, obesity is associated with dysfunction of the adipose lymphatic system, including decreased lymph node size and number (430), reduced drainage of macromolecules (19), increased perilymphatic inflammation (281), and altered lymph node immune cell composition (430). These changes were recently attributed to the condition of obesity per se—specifically injury to lymphatic endothelial cells caused by inflamed adipose tissue—rather than the high fat diet used to generate the obese phenotype (136). Interestingly, using a model of Prox1 haploinsufficiency, Harvey et al. demonstrated that lymphatic vascular defects and resulting abnormal lymph leakage into surrounding tissues induced adult-onset obesity (163). A follow-up study by Escobedo, et al. further reported that the obese mutant phenotype of Prox1+/− mice could be rescued with tissue-specific restoration of Prox1 in lymphatic endothelial cells (110). Whether lymphatic vessel density is altered in peritumoral adipose, either normal or obese, has not been reported. However, Yamaguchi et al. observed a more than threefold increase in lymph node metastasis with adipose tissue invasion at the tumor margin in patients with invasive breast carcinoma (454).
The role of adipose tissue in prostate carcinoma angiogenesis and lymphangiogenesis is not well understood. However, as mentioned previously, ASCs are abundant in periprostatic adipose tissue (327) and are a source of lymphangiogenic factors (383). Indeed, implantation of ASCs has been used successfully in mice to induce lymphangiogenesis in a model of lymphedema (352, 459). Importantly, obesity may influence the degree of proangiogenic/lymphangiogenic factors released from the periprostatic adipose depot. Venkatasubramanian et al. reported that conditioned media generated via explant culture of human obese periprostatic adipose stimulated prostate cancer cell proliferation and angiogenesis to a significantly greater degree than explants from lean patients, providing a potential link between obesity and worse prostate cancer prognosis (417). Paradoxically, elevated leptin concentration in obese mouse models is associated with attenuated tumor cell proliferation and reduced angiogenesis and lymphangiogenesis in prostate cancers in vivo (259,325). Furthermore, rate of lymph node metastasis in patients with clinically localized prostate cancer does not appear to be altered by obesity (53). Thus, there are lingering questions surrounding the role of periprostatic adipose tissue in prostate tumor progression in both lean and obese individuals, particularly with regard to its influence on tumor angiogenesis.
Adipose Tissue Immune Populations in Cancer Development and Progression
Acute inflammatory responses, such as those that occur in the context of pathogen infections, are usually self-limiting and are characterized by an “acute inflammatory infiltrate” consisting primarily of neutrophils and sometimes eosinophils (72). However, when triggering factors persist or inflammatory resolution mechanisms fail, a shift occurs in the immune profile to a “chronic inflammatory infiltrate,” predominantly comprised of lymphocytes and mononuclear cells such as macrophages and dendritic cells. Chronic inflammation is consistently associated with increased risk of carcinogenesis and is a well-known hallmark of cancer (28, 72), leading Dvorak to describe tumors as “wounds that do not heal” (101). Solid tumors frequently contain a dense infiltrate of immune cells, including lymphocytes, neutrophils, macrophages and mast cells, each of which directly or indirectly influence the course of tumor progression. In fact, many of the changes that occur in the tumor microenvironment are largely orchestrated by immune cells (107, 310, 435). Chronic inflammation is also highly prevalent in obesity, and as discussed in previous sections, plays pivotal roles in adipose tissue (lymph)angiogenesis and development of fibrosis. Thus, the final section of this review will focus on adipose tissue immune populations. We will emphasize the changing immune profile during adipose accumulation and progression to obesity and the potential impact of these alterations on adipose-adjacent tumor progression. However, it should be noted that the immune profile of adipose tissue depends upon both the degree and the duration of adiposity, as well as a variety of other factors that are beyond the scope of this review, including physical activity, dietary intake, the microbiome, and certain therapeutics such as thiazolidinediones (188).
Healthy adipose tissue contains a wide variety of innate and adaptive immune cells, including macrophages, dendritic cells, mast cells, eosinophils, neutrophils, and lymphocytes, which collectively constitute ~25% to 45% of stromal cells in humans (50). In lean adipose, these “resident” immune cells maintain tissue homeostasis by clearing apoptotic cells, suppressing inflammation, and mediating basal ECM remodeling and angiogenesis in response to routine fluxes in caloric availability (51). However, during progression to obesity, rapid expansion of adipose tissue and associated adipocyte dysfunction trigger a dynamic infiltration of innate and adaptive immune populations (Fig. 17). These immune cells act as potent sources of inflammatory cytokines, chemokines, growth factors, and matrix-degrading enzymes such as matrix metalloproteases (MMPs), which rapidly remodel the tissue microenvironment and result in chronic low-grade, or “smoldering,” inflammation (373). A decrease in relative influence of select adipose resident populations known for their anti-inflammatory action (e.g., immunosuppressive macrophages, eosinophils, regulatory T cells, and innate lymphoid cells [ILC2s])may further exacerbate adipose inflammation in obesity and associated sequelae, thereby indirectly mediating differential immune responses during tumor-adipose interactions in lean versus obese individuals.
Figure 17. Summary of changes in immune cell profile during progression to obesity.
In the lean state, adipose tissue contains a variety of immunoregulatory cells such as M2-like tissue-resident macrophages, regulatory T cells, and eosinophils. Within days of exposure to an obesogenic diet neutrophils infiltrate adipose. Over weeks to months, an increase in CD8+ T cells, macrophages, and myeloid-derived suppressor cells (MDSCs) results in a mix of pro- and anti-inflammatory cells. In prolonged obesity, adipose mast cell content may also increase.
Despite a surge in research over the past 15 years on the roles of immune cells in adipose tissue biology, many fundamental lines of investigation remain incompletely understood. For example, a growing understanding of the complexity of innate lymphocyte subsets and their remarkable parallels with adaptive lymphocyte subsets (362) complicates interpretation of innate vs. adaptive influence. In addition, data regarding roles for select immune cell types, such as basophils, in adipose tissue remain in short supply. Notably, while comparing the immune response to tumor growth in lean and obese individuals many studies have failed to take into account co-morbidities associated with obesity, which may alter the immunometabolic milieu. For example, type II diabetes is a metabolic condition in which insulin resistance, often due to prolonged obesity and associated inflammation, results in hyperinsulinemia, hyperglycemia, and dyslipidemia. In addition to elevating risk of both cancer development and cancer mortality in several solid tumor types (146, 458), metabolic dysregulation in type II diabetics shifts availability of metabolic substrates such as glucose and fatty acids, which can alter immune cell number and behavior (66, 128, 189). Furthermore, medications prescribed for glucose control in type II diabetics, such as metformin, may have profound and confounding effects on antitumor immunity through suppression of inflammation in macrophages (165) or augmentation of the cytotoxic T cell response (104). With these caveats acknowledged, the increased presence of adipose inflammatory cells in obesity may provide a link between adipose tissue and the pathophysiology of adipose-associated cancers. Thus, when considering the effects of adipose tissue on cancer development, the potential for cross talk between adipose immune populations and the developing tumor is paramount. Due to a current dearth of literature addressing immune populations in periprostatic adipose, the structure for this final section of our review will diverge from the format above, which emphasized breast and prostate adipose pads individually, and instead focus more generally on literature regarding immune populations in a variety of adipose depots.
T cells in adipose and cancer
T cell diversity in the tumor microenvironment
Tlymphocytes, or Tcells, are central to cell-mediated immune responses and mediate exquisitely specific adaptive immune defenses within a given disease context, including cancer. Broadly speaking, T cells can be classified into CD4+ helper T (Th) and CD8+ cytotoxic T (Tc) cell subsets. CD4+ Th cells can be further subdivided into pro-inflammatory effector Th1 cells or immunoregulatory Th2 cells, which influence both generation and activity of CD8+ Tc cells and antigen-presenting cells (APCs), such as macrophages and dendritic cells, within the tumor microenvironment. Other T cell subsets include Th17 cells, γδT cells, and certain types of natural killer (NK) cells, the latter of which exhibit cytotoxic activity and play a role in antitumor immune defense. While each of these T cell subsets, along with other, less well-characterized populations, influence both tumor progression and adipose immunity (275, 466), a comprehensive review of T cell function in these contexts is beyond the scope of this review. However, several of the most well characterized subsets will be addressed below, with particular emphasis on how adiposity-associated alterations in CD8+ T cells and a subset of CD4+ T cells termed “classical T regulatory cells,” or Tregs, may contribute to cancer development in obese individuals.
CD8+ T cells and CD4+ Tregs generally exhibit opposing immunologic functions in both the tumor microenvironment and normal tissues. CD8+ Tc cells are a critical component of antitumor immune defense, directly killing tumor cells through release of cytotoxic granules containing perforin and granzyme B, and indirectly promoting tumor rejection by stimulating APC activity. On the other hand, Tregs are a subset of CD4+ T cells identified by expression of the cell surface markers CD4 and CD25 and the transcription factor forkhead box P3 (FOXP3), which acts as the master regulator of the Treg phenotype (122, 175). Tregs directly regulate the activity of other T cells through suppression of CD8+ Tc cell proliferation following T cell receptor (TCR) stimulation and activation of immune checkpoint pathways, which provide a critical defense against T cell-mediated responses to self-antigens (autoimmunity). Specifically, in T cells, the amplitude and duration of TCR-mediated immune responses are determined by immune checkpoint proteins, which exert co-stimulatory and/or inhibitory signals to effectively “tune” the immune response and curtail collateral tissue damage. For example, FOXP3-mediated constitutive expression of the immune checkpoint protein CTLA4 by Tregs inhibits development of self-reactive CD8+ Tc cells in secondary lymphoid organs such as lymph nodes (382). In peripheral tissue, including tumors, expression of the inhibitory checkpoint protein Programmed Death-1 (PD-1) by “exhausted” or chronically activated T cells impairs cell-mediated responses. Binding of ligands to the PD-1 receptor triggers T cell senescence, apoptosis, or conversion to a Treg phenotype (14,425), thereby attenuating cell-mediated immune responses (190). Additionally, Tregs potently suppress the function of other immune cells such as APCs, NK cells, and CD8+ Tc cells through production of cytokines including IL-10 and TGF-β (190, 275, 466). While these immune-regulatory functions provide a critical defense against rampant immune responses, by suppressing immunosurveillance and promoting immune tolerance in the tumor microenvironment Tregs actively prevent robust elimination of developing cancers. Accordingly, the density of Tregs in solid tumors is correlated with adverse clinical outcomes in melanoma, as well as ovarian, gastric, pancreatic, hepatic, breast, and prostate cancers (34,119,437).
Differential T cell content and activation in lean and obese adipose tissue: Links to cancer
In addition to their well-established roles in the tumor microenvironment, Tregs have also recently been shown to contribute to the maintenance of adipose tissue metabolic homeostasis. Feuerer et al. (116) demonstrated that nearly half of the CD4+ T cells in lean visceral adipose of male mice expressed FOXP3. In fact, visceral adipose in 30-week-old mice contained a greater abundance of Tregs than lymphoid tissues such as spleen and lymph nodes. Interestingly, these adipose-resident Tregs were frequently detected in CLS, which are typically associated with inflammatory cells. Expression profiling of isolated adipose Tregs revealed a distinct gene signature from that of “conventional” T cells from spleen and lymph nodes. Divergent transcription patterns in adipose Tregs included a relative increase in chemokines involved in leukocyte migration and extravasation and greatly elevated IL-10 expression (>100-fold) as compared to lymph node Tregs. Adipose-resident Tregs also exhibited limited TCR diversity relative to spleen or lymph node Tregs (116). Similarly, Yang et al. reported that adipose T cells displayed a TCR profile distinct from that of splenic T cells, further demonstrating that depot-specific microenvironments modulate lymphocyte phenotypes (455).
Feuerer et al. (116) also noted that the presence of Tregs in visceral adipose declined with increasing adiposity in three mouse models of obesity, although the abundance of lymphoid tissue Tregs was unaffected. Subsequent mechanistic studies employing Treg stimulation and depletion suggested that IL-10 secretion by Tregs dampens inflammation in adipose tissue, thereby safeguarding insulin sensitivity. A second study published the same year by Nishimura et al. (276) also reported a decrease in Treg content in obese murine visceral adipose, with a simultaneous and substantial increase in the presence of CD8+ Tc cells displaying markers of activated effector T cells. Of note, in obese mice the accumulation of CD8+ Tc cells preceded macrophage infiltration by 3 to 4 weeks, indicating that T cells may effect microenvironmental changes enabling macrophage recruitment (Fig. 17). An increase in CD8+ Tc cells, particularly within CLS, was also observed in subcutaneous adipose. Genetic or antibody-mediated depletion of CD8+ Tc cells during the course of high-fat feeding attenuated the onset of insulin resistance, prevented macrophage infiltration, and blunted obesity-associated increases in TNFα and IL-6 expression in whole adipose tissue; these phenotypes were “rescued” upon reintroduction of CD8+ Tc cells via adoptive transfer. CD8+ T cell depletion in established obesity similarly reduced the presence of proinflammatory macrophages and CLS density in adipose tissue. These findings were confirmed in vitro, as coculture of CD8+ T cells from obese adipose with macrophages induced significantly greater macrophage-specific TNFα expression than did CD8+ T cells from lean adipose. In sum, these studies illustrate that reduced Treg content and increased CD8+ T cell presence promote macrophage-specific expression of pro-inflammatory mediators, thereby contributing to adipose inflammation and metabolic dysfunction in obesity, both of which are drivers of tumor malignancy.
However, the nature of these reported shifts in T lymphocyte profiles of obese murine adipose has not been consistent in human studies. In fact, the opposite has been observed. In obese adults, the expression of Treg activation markers and Treg cytokines increased with increasing adiposity, particularly in subcutaneous as compared to visceral adipose (397, 461). One potential explanation for these observed increases in Treg activation relates to increased local estrogen concentration in adipose tissue of obese subjects. Indeed, Subbaramaiah et al. provided evidence that elevated cyclooxygenase-2 (COX-2)-induced prostaglandin E2 (PGE2) production by CLS-associated inflammatory cells mediates increased risk of breast cancer in obesity by inducing activity of aromatase in mammary adipose tissue (260, 370). Increased aromatase activity in adipose tissue increases the conversion of circulating androgens to estrogens, and thus is of particular concern for development of estrogen receptorpositive breast cancers in postmenopausal women, a population in which obesity is strongly linked to elevated risk of cancer (70, 412). Estrogen also exerts a positive effect on both expansion of Tregs and augmentation of their immunosuppressive activities (309, 411). Elevated PGE2 also induces FOXP3 expression and Treg function (29, 30, 349). Paradoxically, however, elevated aromatase and PGE2 levels are also present in adipose of obese mice. Thus, the significance of interspecies differences in obesity-associated Treg abundance and/or activation is unclear.
Interspecies differences in T cell content are not exclusive to Tregs. For example, although increases in Tc and Th1 cell content are frequently reported in murine models of obesity, the prevalence of these cell types in obese human adipose is controversial. Indeed, while Yang et al. reported that the stromal-vascular fraction of abdominal subcutaneous adipose from obese human subjects displayed an increased percentage of both CD4+ and CD8+ T cells compared to lean individuals (455), two additional studies profiling T cells in obese human adipose did not reach the same conclusions (397,461). Accordingly, although CD8+ T cells appear to contribute to adipose inflammation in mice, their role in human obese adipose remains ambiguous. Furthermore, in addition to identifying potentially critical cross-species differences in adipose T cell function, these results also suggest that, in humans, an increase in pro-inflammatory cell abundance in adipose occurs with a parallel protective response driven by Tregs. Should this be the case, an elevated presence of Tregs in human obese adipose may contribute to immunosuppression of anti-tumor responses in adipose-adjacent cancers.
In addition to influencing Treg-mediated immunosuppression, obesity may also impair T cell-mediated antitumor responses through systemic mechanisms. For example, obesity reportedly accelerates age-associated declines in immune function, including thymic atrophy. The thymus is a specialized primary lymphoid organ located in the mediastinum that houses maturing T lymphocytes. Beginning at puberty, the thymus undergoes involution, or atrophy, exhibiting fibrotic and fatty changes that culminate in its replacement by adipose tissue (96). Following thymic involution, the peripheral T cell pool is primarily maintained independently of thymic lymphopoiesis, such as by expansion of existing T cell populations; however, it should be noted that some studies in humans have reported that the aged thymus retains a limited capacity to produce naïve T cells (387). Eventually, the age-related decline in naïve T cell production, in combination with steady exposure to antigenic challenge and resulting expansion of effector-memory T cells, depletes the naïve T cell pool and reduces diversity of the TCR repertoire (432). Thus, these processes reduce the capability of the adaptive immune system to respond to new antigenic challenges, increasing susceptibility to infection, autoimmune responses, and cancer. Importantly, Yang et al. (456) reported that prolonged obesity in mice increased perithymic adipose tissue content, reduced thymocyte counts, and enhanced thymocyte apoptosis relative to lean animals, each of which are associated with thymic aging. Similarly, increased frequencies of CD4+ and CD8+ effector-memory cells in subcutaneous adipose of obese mice, concomitant with a notable decrease in TCR diversity and depletion of the CD4+ and CD8+ naïve T cell pools, further supported an acceleration of the immune aging process. Moreover, splenic T cells isolated from obese mice exhibited reduced expression of pro-inflammatory mediators important for antitumor immune defenses, including interferon-γ and TNFα. Finally, in humans, analysis of mature thymus-derived T cells demonstrated that increasing adiposity significantly correlated with a reduction in thymic output in overweight and obese middle-aged subjects. These obesity-related restrictions in TCR diversity and T cell function may account for reports of impaired adaptive immunity in obese patients (195, 296) and suggests a reduced capacity to mount an effective antitumor immune response.
Recent clinical successes with tumor immunotherapies targeting the PD-1 immune checkpoint pathway have increased interest in the regulation of this pathway in the context of obesity. As described above, PD-1 expression by T cells is an important driver of immunosuppression and reduced cytotoxic T cell response in the tumor microenvironment (68), prompting development of PD-1-targeting monoclonal antibodies (e.g., pembrolizumab and nivolumab) for clinical use. Recently, Shirakawa et al. reported B cell-dependent accumulation of CD4+ T cells constitutively expressing PD-1 within visceral adipose of obese mice and human omental adipose from obese patients (354), further suggesting that tumor-adjacent adipose in obese individuals may present an immunosuppressive environment. In light of the accelerated thymic aging and naïve T cell depletion reported in obese patients, it will be interesting to see whether adipose contributes to increased PD-1+ T-cell content in the solid tumor microenvironment.
Macrophages and myeloid-derived suppressor cells
Macrophage ontogeny and activation
Macrophages, or “big eaters,” are myeloid-lineage immune cells typically classified within the innate immune system, yet bridge innate and adaptive immunity through extensive interactions with adaptive immune cells such as T cells. Conventionally, macrophages have been classified according to the “M1/M2” dichotomy, wherein “M1” polarized, or “classically activated,” macrophages are proinflammatory, and “M2” polarized, or “alternatively activated,” macrophages are anti-inflammatory. M1 macrophages are generated in vitro upon exposure to Th1 cytokines (e.g., IFN-γ) or stimuli such as bacteria and lipids (191, 337, 338). In contrast, M2 macrophages are most commonly generated by culture in the presence of Th2 cytokines such as IL-4 and/or IL-13 (150). However, a variety of other compounds may also be used for M2 macrophage polarization, including TGF-β, IL-10, glucocorticoid hormones, M-CSF, and PGE2 (236). Importantly, a lack of standardized nomenclature and macrophage polarization strategy (267), coupled with the multifarious nature of tissue macrophages and their exquisite ability to respond to context-dependent cues (138), has resulted in a tremendous influx of literature about the respective roles of M1 vs. M2 macrophage subsets in disease that is often contradictory and difficult to reconcile (242). Furthermore, while much of our understanding of the M1 and M2 phenotypes have come from animal and in vitro studies, genomic profiling of human and mouse macrophages treated with M1 or M2 stimuli revealed that only approximately 50% of macrophage polarization markers are shared across both species (243). With these caveats acknowledged, despite their utility to in vitro research, truly polarized macrophages are rare in vivo. Instead, tissue macrophages display a diverse array of functional phenotypes and often express one or more markers of both M1 and M2 subtypes, resulting in a mixed phenotype with specific expression and function varying by tissue type and timing of residence, as discussed below (Fig. 18) (82, 212, 262, 451).
Figure 18. Macrophage activation as a spectrum.
Unstimulated macrophages can be polarized in vitro to generate M1 (right) or M2 macrophages (left) using single cytokines or cytokine and other stimuli cocktails. However, tissue macrophages are exquisitely plastic, often expressing one or more markers of both M1 and M2 subtypes. Thus, tissue macrophage activation lies along a spectrum, resulting in mixed phenotype with specific expression and function varying by tissue type and timing of residence.
Over the past few decades, macrophage ontogeny studies have revealed multiple origins for what are now referred to as “tissue resident” macrophage populations (for two excellent reviews on macrophage ontogeny the reader is referred to (144, 212)). During primitive hematopoiesis in early embryonic development, macrophages arise in the blood islands of the yolk sac from an erythromyeloid precursor, differentiating to macrophages without passing through a monocyte stage (148,244). These early embryonic macrophages are followed by a second wave derived from fetal monocytes and originating in the fetal liver (148, 244). Collectively, macrophages within these waves of early hematopoiesis populate tissues throughout the body and develop specialized functions based on their tissue of residence (e.g., microglia in the brain, Kupffer cells of the liver, etc.) (144, 212). Tissue-resident macrophages persist through adulthood and, in most tissues, self-maintain through local proliferation without significant contribution from circulating monocytes (exceptions include the intestine and the dermis) (81, 164). Only in later stages of embryonic development and postnatally do macrophages develop from bone marrow-derived circulating monocytes, which are recruited to tissues as needed when insults arise (38, 52).
Although the embryonic origin of many specialized tissue macrophage populations has been identified, the precise origin of adipose tissue macrophages (ATMs), and the degree to which resident ATM populations are replaced by circulating monocytes, remains unclear (Fig. 19). In one recent study, Franklin et al. demonstrated that ablation of the CCL2 receptor, CCR2, significantly reduced mammary fat pad macrophage content in lean mice (125); CCL2 mediates egress of monocytes from bone marrow and thereby augments the abundance of circulating monocytes (345). This study by Franklin and colleagues therefore suggests that mammary-specific ATMs in lean mice are replenished throughout adulthood by circulating monocytes. Whether this replenishment also occurs in other lean adipose depots under physiologic conditions has not been reported.
Figure 19. Adipose tissue macrophage ontogeny.
Lineage tracing studies have revealed multiple embryonic sources for tissue-resident macrophages (e.g., Kupffer cells, microglia) including the yolk sac and fetal liver. However, the contribution of bone marrow monocyte-derived macrophages to tissue-resident populations remains ambiguous. Moreover, the relative contribution of yolk sac, fetal liver, and bone marrow-derived macrophages within adipose tissue depots has not been established, although the overall proportion of inflammatory, bone-marrow derived macrophages increases in obese adipose.
Macrophage content and phenotypes in obesity
Macrophages are the most highly represented immune cells in adipose tissue, and their numbers increase considerably in both visceral and subcutaneous adipose in obesity. However, the increased presence of ATMs in obesity appears to arise from multiple tissue sources. For example, using bone marrow transplant studies employing CD45.2-expressing recipient mice and syngeneic CD45.1-expressing donor mice, Weisberg et al. reported that adipose-infiltrating macrophages in obesity had differentiated from bone marrow-derived, circulating monocytes (429). However, Amano et al. demonstrated that elevated CCL2 in visceral adipose drove local proliferation of macrophages in obesity, which contributed to ATM accumulation (13). Local ATM proliferation was also observed by Hasse et al., with live imaging of adipose explants showing that macrophages expressing M2-identifying markers underwent mitosis within CLS, followed by migration to interstitial spaces between adipocytes (154). Moreover, in vivo proliferation in a subset of bone marrow-derived macrophages has also been described, a surprising finding as bone marrow-derived macrophages were long believed to be terminally differentiated and thus nonproliferative (80). Importantly, however, recruitment of bone marrow-derived macrophages and local ATM proliferation need not be mutually exclusive, and future studies should examine obese ATM ontogeny in a longitudinal fashion.
Regardless of their tissue of origin, the increased presence of macrophages in obese adipose tissue can be best observed histologically as an increase in CLS formation. Indeed, Weisberg et al. demonstrated that macrophage influx and CLS formation in both mice and humans were significantly correlated with both adipocyte diameter and BMI (429). Time course studies probing the changing immune profile in obesity report that this macrophage accumulation occurs subsequent to neutrophil and T cell infiltration (106,276). However, there is variability in both the reported timing of macrophage influx and the degree of infiltration across adipose depots. For example, Elgazar-Carmen et al. observed an increase in CLS formation in murine visceral adipose tissue as early as 3 weeks into high fat feeding, which increased in density over time until the study endpoint at 16 weeks of diet exposure (106). On the other hand, Nishimura et al. reported that the presence of macrophages in the stromal-vascular fraction of visceral adipose tissue did not increase until 10 to 12 weeks of high-fat feeding (276). These temporal differences may be due to variation in the age at which obesity was induced and the dietary composition used to generate adiposity (i.e., both the percent kilocalories obtained from lipids as well as the lipid profile), as each are important considerations in diet-induced obesity studies. Nevertheless, although the initial timing of macrophage infiltration varies across studies, macrophage accumulation continues with prolonged obesity, with ATMs eventually comprising up to 50% of adipose stromal-vascular cells (75, 292, 429, 449). Due to sexual dimorphism in mice with regard to degree of adiposity in response to high-fat feeding, as well as differential contribution of adipose depots to obesity-associated metabolic dysregulation, many obesity studies have preferentially quantified changes in macrophage content in abdominal adipose depots of male mice (i.e., inguinal and periepididymal). However, we and others have also demonstrated obesity-associated CLS formation in the mammary fat pad of female mice, as well as human breast adipose tissue (260, 369, 370, 374).
As mentioned in previous sections of this review, obese adipose tissue frequently exhibits elevated levels of proinflammatory cytokines such as TNFα and IL-6. Although obese adipocytes have been shown to contribute to the secretion of these factors (130), macrophages and other stromal-vascular cells are thought to be the primary source of proinflammatory mediators in both mice (429) and humans (76, 112). Following initial reports of adipose macrophage influx in 2003 (429, 449), early characterization of ATMs reported the appearance of a CD11c-expressing population of ATMs in adipose tissue of obese, but not lean, mice (131,231), as well as a phenotypic switch in the collective ATM population from an anti-inflammatory (M2) polarized state in lean animals to a pro-inflammatory (M1) state in obese animals (231). Importantly, however, more recent research indicates that the nature of ATM phenotypes in obesity is more dynamic and complex than originally expected. For example, the pro-inflammatory phenotype of CD11c-expressing ATMs appears to be malleable, and may be modulated by degree of insulin sensitivity in obese animals (221). In addition, more extensive profiling of ATMs in obese adipose of mice and humans has revealed that these cells harbor a “mixed” pro-and anti-inflammatory phenotype (350, 460). For example, in human abdominal subcutaneous adipose, ATMs accumulating in CLS expressed both CD11c and the commonly used M2 marker mannose receptor C type 1 (CD206), as well as both pro- and anti-inflammatory interleukins (IL-1β, IL-6, IL-8, and IL-10) (433). These results are further supported by Nakajima et al., who reported accumulation of ATMs expressing both CD11c and CD163, the latter of which is commonly associated with M2-like macrophages, in abdominal visceral and subcutaneous adipose of obese subjects (269). Shaul et al. (350) also described a mixed M1/M2 phenotype in obese murine CD11c+ visceral ATMs, suggesting phenotypic and functional similarities between murine and human ATMs in obesity. Interestingly, in the latter study, these mixed phenotype ATMs exhibited a shift toward a more M2-like transcriptional profile as obesity progressed.
Due to the phenotypic overlap between ATMs and canonical M1- and M2-polarized macrophages, the precise stimuli that activate ATMs, as well as the specific surface marker profile of this cell population, have only recently been described. Using a membrane proteomics approach, Kratz et al. (205) described a unique, “metabolically activated” phenotype in visceral ATMs from obese mice, which displayed surface markers distinct from those of classically activated macrophages generated in vitro. When these metabolically activated ATMs were recapitulated in vitro by exposure to conditions characteristic of the metabolic syndrome (high glucose, insulin, and palmitate), they were further found to exhibit increased surface expression of M2-associated lipid metabolizing proteins, but not other M2-defining markers. Metabolically activated ATMs also exhibited increased PPARγ activation, as well as a strong and selective induction of protein sequestome-1/p62, a scaffold protein with a variety of signaling roles including activation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (205, 261). Importantly, PPARγ is a transcription factor crucial in the generation of the M2-like macrophage phenotype, while the NF-κB transcription factor family mediates several aspects of the M1 inflammatory response. Ablation of PPARγ or p62 in metabolically activated macrophages increased expression of several proinflammatory mediators, indicating that PPARγ and/or p62 attenuate proinflammatory responses in ATMs in obesity (205). Moreover, Ferrante and colleagues (450) observed elevated lysosome biogenesis and lipid metabolism in visceral adipose ATMs from obese mice relative to lean, without concomitant activation of inflammatory pathways. In fact, the authors suggested that the driving force for the chronic low-grade inflammation observed in obesity may simply be the increased density of macrophages in obese adipose, rather than a shift in the inflammatory potential of individual macrophages. Thus, questions remain regarding our understanding of ATM phenotype and degree of plasticity within adipose tissue.
Adipose tissue macrophages: Connections to cancer
Increased ATM content in obesity suggests a clear inflammatory link between obese adipose and initiation of adipose-adjacent cancers. For example, as mentioned previously, macrophage infiltration into obese breast adipose tissue and resulting inflammation are linked to increased risk of mammary carcinogenesis (260, 370). Additionally, in various sections of this review we have addressed roles for ATMs in development of adipose tissue fibrosis (373, 446), which may influence early stages of tumor initiation. Macrophages are also highly represented within the body and margins of many solid tumor types, and directly promote progression of both early and established tumors (317,438). Indeed, macrophages are implicated in every aspect of tumor progression, including induction of the angiogenic switch (227); generation of an immunosuppressive environment (236); ECM degradation to facilitate invasion and migration of tumor cells into surrounding tissue; and physical participation in tumor cell metastasis (315, 438). Macrophages have also been shown to negatively influence response to anticancer therapies in breast and prostate cancers (86,89,109,235,356). Accordingly, in human breast tumors, degree of macrophage infiltration is an independent prognostic indicator strongly associated with high vascular grade, reduced relapse-free survival, and decreased overall survival (57, 218, 438).
Differences in phenotype and trophic potential between embryonic-resident, locally proliferating, and bone marrow-derived ATM populations may influence tumor development and ATM participation in the tumor microenvironment. Collectively, macrophages found both along the solid tumor periphery and within the tumor mass are referred to as tumor-associated macrophages (TAMs). Studies investigating the origins of TAMs in mice have reported that circulating bone marrow-derived monocytes are the primary source of TAMs in syngeneically grafted (264) and spontaneously arising mammary tumors (125), as well as in breast cancer pulmonary metastases (314). Furthermore, Franklin et al. reported that monocyte-derived TAMs in the MMTV-PyMT mouse model of spontaneous breast cancer proliferate within the tumor site and are phenotypically and functionally distinct from the resident mammary tissue macrophages present before tumor development (125). Together these observations argue against recruitment of local tissue-resident macrophage populations. However, studies probing TAM ontogeny have investigated this question exclusively in lean animals. The term “tissue-resident macrophages” is often used to refer to embryonic macrophages, but may also refer to any macrophages residing in a given tissue before an insult induces recruitment of bone marrow-derived inflammatory monocytes. Both expansion of adipose tissue in obesity and the presence of a developing tumor act as inflammatory insults; thus, the marked increase in ATM content in obesity, as well as their variable tissues of origin and distinct phenotypic differences from macrophages in lean adipose, requires an evaluation of the ATM-TAM relationship in the context of obesity.
Similarities between adipose tissue macrophages and tumor-associated macrophages
In a similar vein to ATMs, discrepancies exist between reports of the defining “TAM phenotype.” Conventionally, TAMs have been described as resembling alternatively activated M2 macrophages (108, 236, 237). However, large-scale transcriptome analyses of TAMs in breast cancer suggest that TAMs collectively exhibit a mixed phenotype, expressing both M1-like and M2-like markers (288). Interestingly, this same study also showed that the gene signature of breast TAMs resembled that of fetal macrophages, with increased abundance of transcripts for genes regulating angiogenesis, tissue remodeling, and immune response (288). On the other hand, Franklin et al. recently reported that TAMs in the MMTV-PyMT model of metastatic, luminal-B breast cancer did not resemble M2-like macrophages, nor were they dependent upon tumor-elicited Th2 immune response (125). Together these studies indicate that, at least in breast cancer, TAMs are highly heterogeneous, and their phenotypes depend on tumor type, subtype, and location within the tumor (i.e., margins vs. periphery and extent of hypoxia) (315). Alterations in TAM phenotypes may also occur over the course of tumor development and progression, as Qian and Pollard have described a shift in TAMs throughout tumorigenesis from an “inflammatory” type during tumor initiation to an anti-inflammatory, M2-like trophic type in later stages of tumor progression (315). As mentioned previously and discussed further in the following section on Myeloid-derived suppressor cells, a similar shift has been described in ATMs over the course of prolonged obesity (350).
Shared characteristics between the tumor and obese adipose microenvironments, such as fibrosis, elevated ECM stiffness, angiogenesis, and regional hypoxia, may foster similarities between ATMs and TAMs. In particular, transient hypoxia activates the NF-κB transcription factor family (388). While numerous molecules are involved in generating inflammation, NF-κB has long been considered to lie at the center of the inflammatory response. However, due to the plurality of NF-κB family members, as well as the sheer number of combinatorial interactions within canonical and noncanonical signaling pathways, NF-κB activation can have both pro- and anti-inflammatory effects. Inflammatory mediators controlled by canonical NF-κB signaling include the TNF superfamily, IL-1β, IL-6, several chemokines, COX-2, 5-lipooxygenase, MMPs, VEGF, and cell surface adhesion molecules (3). Some of these gene products also activate NF-κB, with TNFα being a particularly potent stimulus (3). On the other hand, noncanonical NF-κB activities, such as regulation of IL-10 and TGF-β, play a role in inflammation resolution (213, 214). As discussed throughout this review, many of these signaling mediators also contribute to tumor malignancy through a variety of mechanisms, including growth promotion, matrix degradation, and tumor angiogenesis. In fact, NF-κB signaling is a known mediator of the tumor promoting activities of both early-stage, proinflammatory TAMs, and late-stage immunosuppressive TAMs (45, 155, 156, 293). A study by Mayi et al. (246) provided direct evidence underscoring the similarities between ATMs and TAMs. Specifically, ATMs from obese individuals expressed several of the same cancer-promoting genes as TAMs, including angiogenic factors, chemokines, cytokines, proteases, and growth factors. In fact, many of these protumoral genes, including VEGF-C and CXCL12, were expressed to an equal or greater extent in obese ATMs compared with TAMs (246), and are known targets of noncanonical NF-κB signaling (232). Taken together, these findings indicate that chronically activated NF-κB signaling and dysregulated immune responses are likely unifying themes between ATMs and TAMs.
Myeloid-derived suppressor cells
For reasons that are not well understood, abnormalities in myelopoiesis under conditions of prolonged inflammation such as chronic infections and cancer generate a poorly differentiated group of myeloid-lineage cells collectively termed myeloid-derived suppressor cells (MDSCs) (132). MDSCs include immature monocytes, neutrophils, dendritic cells, and macrophages, and are defined by their expression of the myeloid lineage markers CD11b and Gr1 and their potently immunosuppressive properties (132,133,311). Although they are comprised of multiple myeloid cell types, MDSCs are frequently described as immature macrophages. However, MDSCs in mice are reported to lack markers of mature macrophages such as major histocompatibility complex II (MHCII) and/or F4/80 (311, 447).
Factors implicated in promoting the egress of MDSCs from bone marrow, as well as their arrest in an immature state and their immunosuppressive nature, include PGE2, IL-6, TNFα, IL-1β, and VEGF (311). Of these factors, PGE2 is a particularly potent inducer of MDSCs that triggers upregulation of arginase metabolism, thereby suppressing T cell function (285,330,359). Several of these signaling molecules are in turn produced by MDSCs, resulting in a positive feedback loop of MDSC recruitment. Notably, as discussed in various sections throughout this review, each of these factors is also elevated in obese adipose tissue, and increased MDSC content in adipose tissue of obese mice has recently been reported. Indeed, Xia et al. (447) demonstrated that increased MDSC content in peripheral tissues (e.g., adipose and liver) of obese mice acted as an important safeguard of insulin sensitivity in both genetic and diet-induced models of obesity. Depletion of Gr1-expressing cells exacerbated symptoms of glucose intolerance and increased the presence of CD8+ T cells in adipose tissue. On the other hand, adoptive transfer of MDSCs improved fasting glucose and insulin levels in obese mice and reduced levels of circulating proinflammatory cytokines. Interestingly, the onset of MDSC accumulation coincided with previously reported windows of CD8+ T cell and proinflammatory macrophage recruitment, supporting the putative role of MDSCs in suppression of a rampant inflammatory response. Accordingly, the percentage of CD11b+ Gr1+ MDSCs in adipose tissue increased with the duration of obesity (447). Factors contributing to the accumulation of adipose MDSCs in obesity are poorly understood, but may include development of insulin resistance or increased local concentrations of estrogen and IGF-1, each of which have been found to influence MDSC biology (289). Importantly, influx of MDSCs into adipose in prolonged obesity may provide a partial explanation for reports of a shift in overall ATM phenotype over the course of obesity from pro-inflammatory M1-like to that of more immunosuppressive M2-like macrophages (350). For example, isolated MDSCs cultured with media conditioned by explanted obese adipose tissue displayed a greater shift toward an M2-like macrophage profile than MDSCs exposed to lean adipose explant-conditioned media (447). Future studies should examine the extent to which MDSCs in obese adipose differentiate to M2-like macrophages in vivo.
While the presence of MDSCs in obese adipose tissue is a relatively recent finding, a large body of literature supports the immunosuppressive functions of MDSCs within the tumor microenvironment. However, similarities in marker expression and immunosuppressive activation states may complicate a clear distinction between TAMs and MDSCs. Moreover, MDSCs can also differentiate into mature TAMs upon entry into the tumor microenvironment (207). Functional similarities between MDSCs and certain TAM subsets have also been documented. For example, MDSCS suppress the function of critical antitumor defense cells (e.g., CD8+ cytotoxic T cells and NK cells) through expression of cytokines such as IL-10 and TGF-β and through arginine metabolism via the enzymes arginase-1 or inducible nitric oxide synthase (iNOS) (311). Interestingly, simultaneous expression of arginase-1 and iNOS is a hallmark of MDSCs that is rarely observed in other immune cells (311).
As described in the T lymphocytes section above, activation of the PD-1 pathway in T cells is a critical checkpoint promoting immunosuppression in the tumor microenvironment (395). Prima et al. reported that coculture of bone marrow-derived myeloid cells with bladder tumor cells elevated production of PGE2 by both MDSCs and TAMs, and induced expression of the PD-1 ligand, programmed death-1 ligand (PD-L1), in these populations in a PGE2-dependent manner (312). PD-1 and its ligands PD-L1 and PD-L2 were also more highly expressed in prostate tumors of obese mice compared to those from lean animals (457). Importantly, hypoxia-induced HIF-1 activation in TAMs was also recently shown to control TAM-specific PD-L1 expression (279). Whether regional hypoxia in obese adipose and resulting HIF-1 activation increases PD-L1 expression in ATM remains to be seen. However, the presence of MDSCs in prolonged obesity, as well as their influence on ATM activation, further suggests that adipose-adjacent cancers in obese individuals may encounter an environment conducive to suppressed immunosurveillance.
Neutrophils
Neutrophils infiltrate adipose tissue early in progression to obesity
Neutrophils are the most abundant white blood cells in human circulation and are typically the first immune cells recruited in response to infection or sterile tissue injury. Upon arrival, neutrophils secrete a variety of proinflammatory cytokines and participate in presentation of antigen to, and activation of, T cells, while helping to recruit additional inflammatory cells such as macrophages (443). In lean animals, neutrophils represent a small fraction of total adipose tissue immune cells (<1%) (114). However, Elgazar-Carmon and colleagues (106) demonstrated that transient neutrophil infiltration into visceral adipose depots occurs early during the course of adipose tissue expansion in diet-induced obesity models, suggesting induction of an acute inflammatory response. Indeed, neutrophils accumulated in visceral (peri-epididymal) adipose of male mice as early as 3 days after initiating high-fat feeding—well before weight gain—with a corresponding increase in the neutrophil enzyme myeloperoxidase. Maximal myeloperoxidase was detected within 3 to 7 days, followed by a slow decline and return to baseline levels within 2 to 3 weeks of high-fat feeding, and neutrophils were no longer detectable histologically at 16 weeks on diet. Talukdar et al. (384) also reported a rapid and dramatic increase in adipose tissue neutrophil content by 3 days of high fat feeding. This increase was maintained for up to 90 days by FACS analysis of immune cells within the epididymal adipose stromal-vascular fraction of obese male mice, with a corresponding increase in neutrophil elastase mRNA. However, the exact adipose tissue-derived chemoattractant(s) that mediate neutrophil recruitment so early during the course of adipose tissue expansion remain unclear, as adipocyte hypertrophy and death do not typically occur until several weeks into diet-induced obesity studies. In either case, once inflammation is established, neutrophils in inflamed adipose engage in bidirectional interactions with macrophages, dendritic cells, natural killer cells, lymphocytes, and mesenchymal stem cells, with important implications for adipose metabolic homeostasis. For example, neutrophil elastase appears to be an important mediator in the development of obesity-associated insulin resistance in response to adipose inflammation, signaling through Toll-like receptor 4 and downstream NF-kB activation to influence both recruitment and inflammatory activation state of infiltrating immune cells in obesity, including neutrophils themselves (384).
Tumor-associated neutrophils
Within the tumor microenvironment neutrophils exhibit varied content and multiple phenotypes, and have been found to exert both pro- and antitumoral effects. Similar to the M1/M2 dichotomy long used for macrophages, tumor associated neutrophils (TANs) have been described as either “N1” (anti-tumoral) or “N2” (protumoral) (Fig. 20) (129). The N1 neutrophil profile is reported to be promoted by increased levels of interferon-β (305) and pro-inflammatory cytokines such as IL-1β and TNF-α (290,305), while transforming growth factor β (TGF-β) is an important determinant of the N2 phenotype (129). Relative to N2 neutrophils, N1 neutrophils display elevated oxygen radical-dependent cytotoxicity and increased expression of the chemokine CCL3 and the cell adhesion molecule ICAM (129), which recruit additional inflammatory cells and act to increase adherence and extravasation, respectively. These proinflammatory N1 neutrophils promote CD8+ cytotoxic T cell recruitment and activation by producing T-cell attracting chemokines and proinflammatory cytokines (339). The N2 subpopulation can be distinguished morphologically, with less pronounced segmentation of the nuclei than N1 neutrophils and elevated expression of proangiogenic mediators including chemokines (CXCR4, CCL2), growth factors (VEGF), and remodeling factors such as MMP9 (38, 290). Neutrophil-derived MMP9 was shown to contribute to the angiogenic switch in early-stage pancreatic adenocarcinoma (282). Additionally, tumors formed by highly disseminating variants of prostate carcinoma recruited elevated levels of MMP9-positive TAN, which correlated with tumor cell dissemination and increased levels of angiogenesis and intravasation (38). N2 neutrophils are also immunosuppressive; elevated expression of the enzyme arginase-1 by N2 neutrophils contributes to depletion of arginase within the tumor microenvironment, inhibiting T-cell receptor expression and antigen-specific T-cell responses (331).
Figure 20. Tumor-Associated Neutrophils have N1 and N2-like phenotypes.
(A) Neutrophil content and phenotype is both pro- and anti-tumoral with cytokines such as IFNβ, IL-1β, TNF-α activating the N1 or proinflammatory phenotype and TGF-B driving the N2 immunomodulatory phenotype. The N1 neutrophil releases reactive oxygen species (ROS) and proteins that increase cell recruitment and extravasation [ICAM and CCL3 (MIP-1-alpha)]. N1 neutrophils support cytotoxic CD8+ T cell activity. N2 neutrophils have a less segmented nucleus than typical and secretes many angiogenic and immunosuppressive mediators, expressing arginase 1 for example. ROS secreted by both N1 and N2 may both promote genotoxicity in tumor initiation, or in contrast, can be cytotoxic to growing tumors. The timing and phenotype of neutrophil influx in obesity and tumor progression warrants further study. (B) Neutrophils infiltrate adipose early during progression to obesity. Neutrophil production of ROS, for example, through myeloperoxidase (MPO) expression, contributes to oxidative stress and fibrotic changes.
Adipose tissue neutrophils and cancer
Potentially due to the minimal presence of neutrophils in lean adipose, very few studies have addressed the influence of adipose tissue on neutrophils specifically in tumors that are adipose-adjacent or adipose-invading. Wagner et al. reported that melanoma cell lines implanted within white adipose tissue of lean mice showed significantly greater infiltration of CD11b+ cells than tumors implanted at a site distant from adipose (422). Although these cells were initially described as monocytes and/or macrophages, CD11b is expressed by multiple myeloid lineage cells, including neutrophils (415). Furthermore, inflamed peritumoral adipose exhibited increased expression of proinflammatory cytokines and chemotactic factors implicated in both macrophage and neutrophil recruitment, including CXCL1, macrophage-inflammatory protein-2 (MIP-2), and CCL2 (422). In obese adipose, neutrophils likely contribute to both tumor initiation and tumor progression. In addition to facilitating recruitment of additional inflammatory cells, neutrophils participate in establishment of the mutagenic pro-inflammatory microenvironment associated with cancer initiation. Indeed, neutrophil-derived reactive oxygen species and myeloperoxidase are genotoxic, and are recognized mutagens in certain tumor types, such as lung cancer (202). Furthermore, the skewed cytokine profile of inflamed obese adipose, such as elevated CCL2, may influence recruitment of neutrophils to developing tumors.
Alternatively, tumor-adjacent adipose may impinge upon the phenotype of TANs. Incio et al. (179) reported that pancreatic tumors from obese animals contained higher concentrations of adipocyte-derived IL-1β than those from lean animals, resulting in increased TAN recruitment, TAN-induced activation of pancreatic stellate cells, and enhanced deposition of fibrillary collagen (i.e., desmoplasia). Obesity was also associated with greater tumor weight, which was reverted to lean levels by TAN depletion. Importantly, tumor formation in this study was induced, via orthotopic cell injection or tumor fragment implant, following 10 weeks on a high fat diet—a period during which, as illustrated above, the presence of neutrophils in visceral adipose depots is elevated (106, 384). Reversion of tumor growth rate was only observed when TAN depletion was initiated on day 1 following tumor induction, as opposed to day 7 (179). Thus, it is unclear whether neutrophils recruited from the visceral adipose, as opposed to newly trafficked peripheral blood neutrophils, were the primary contributors to induction of the desmoplastic response.
Taken together, the balance of N1/N2 TAN subtypes is an important factor in tumor progression, and future studies should consider the functions of adipose tissue neutrophils in initiation and/or progression of adipose-adjacent or adipose-invading tumors in obese individuals. Notably, although the presence of neutrophils in visceral adipose is clearly enhanced in early stage obesity, it is important to acknowledge that the time course studies described earlier regarding neutrophil adipose infiltration used exclusively male mice, and therefore it is unknown to what extent, or when, neutrophils also infiltrate the obese mammary fat pad.
Mast cells
Mast cell content and activation states in adipose tissue
An understudied immune cell in both adipose and tumor biology is the mast cell. Historically described as mediators of allergic hypersensitivity reactions (77), mast cells are found in virtually all tissues and are frequently classified into one of two subtypes: those residing in connective tissues, which express both tryptase and chymase, and those residing in mucosal tissues, which express only tryptase (181). However, similar to other immune cells, mast cells exhibit plasticity based on microenvironmental conditions, and thus several phenotypic subtypes may exist (134).
Accumulation of mast cells in visceral white adipose in obesity has been reported in both mice (10, 228) and humans (94,228), with documented heterogeneity across specific adipose depots. Altintas et al. found that mast cell density in the epididymal fat pad of male mice increased up to 230-fold under conditions of prolonged obesity, with mast cells intermingled with macrophages in the interstitial spaces between adipocytes (10). A similar study published the same year by the same group also found dramatically increased mast cell infiltration in mesenteric and perirenal adipose, but no significant obesity-induced changes in mast cell density in inguinal subcutaneous adipose (9). However, Liu et al. reported increased numbers of mast cells in abdominal subcutaneous adipose tissue from obese human subjects, as well as significantly elevated serum tryptase levels, relative to lean individuals (228). Many of these mast cells were found in association with microvessels (228), implicating mast cells in the regulation of endothelial cell biology and angiogenesis in adipose tissue. Interestingly, increased serum tryptase levels were not found in obese children and adolescents, suggesting an adult-specific window of susceptibility to adipose-mast cell interactions (428).
Degree of mast cell activation is also affected by obesity. Divoux et al. (94) reported that mast cells isolated from omental and subcutaneous adipose depots of obese subjects exhibited a more activated state than mast cells isolated from lean subjects, secreting increased levels of pro-inflammatory cytokines, chemokines, and growth factors. Histological sections also revealed that mast cells in obese subjects preferentially localized to fibrotic bundles or proximate to endothelial vessels, and showed increased degranulation relative to those in lean tissue (Fig. 21A). Collectively, these results suggested that mast cells in obesity harbor a pro-inflammatory profile, a phenotype that was recapitulated by culture of mast cells in a 3D matrix designed to mimic fibrotic conditions. Furthermore, a positive correlation was observed between mast cell density and both fasting glucose and glycated hemoglobin, suggesting a role for mast cells in altered glycemic status in obese subjects. Finally, Zhou et al. recently showed that mast cells in both white adipose and bone marrow of obese mice express elevated levels of leptin, potentially in response to increased regional concentrations of IL-6 or TNFα in obesity (465).
Figure 21. Mast cells: Unappreciated players in adipose and tumor biology.
(A) Mast cell content in adipose tissue increases with obesity, with mast cells localized to blood vessels and/or within fibrotic bundles. Obesity is also associated with increased mast cell degranulation, an indicator of a mast cell activation. (B) In cancer, mast cells contribute to tumor progression through release of proangiogenic factors (MMP9, VEGF), immunosuppressive mediators (histamine), or growth factors such as PDGF. Mast cells also secrete cytokines that may promote (arrow) or inhibit (line) tumor progression. Mast cell influence on tumor progression appears to be dependent upon mast cell localization as peri-versus intratumoral.
Similar to the other immune cell populations described above, mast cells have been ascribed both pro- and antitumoral roles. Tumor promotion by mast cells has been attributed to secretion of proangiogenic factors such as MMP9 and VEGF, immunosuppression through release of histamine, or growth promotion by mitogenic factors including PDGF (390). Mast cells also secrete IL-1, TNFα, IL-6, IL-10, and IL-4 (390), each of which plays complex—and sometimes controversial—roles in solid tumor biology (17,27,113,118,223,286). Thus, below we consider the potential relevance of adipose mast cells to cancer progression with regard to potential changes induced with increased adiposity and prolonged obesity.
Mast cells in breast cancer
In breast cancer, mast cell tryptase levels are linked to angiogenesis and lymphangiogenesis (238, 318), lymph node metastasis (448), and myofibroblast differentiation (233). Samoszuk et al. (336) reported elevated serum tryptase in the blood of breast cancer patients as compared to healthy controls, as well as mast cell infiltration and mast cell tryptase expression adjacent to or within the stroma of every breast cancer patient sample examined, including DCIS specimens. Interestingly, in patients with invasive breast cancers, tryptase was found more frequently as extracellular deposits, suggesting mast cell degranulation, whereas in patients with early stage breast cancer, tryptase was located intracellularly, within intact mast cells (336).
Remarkably, mast cell activation state and influence on the course of tumor development appear to also depend upon their localization within the tumor microenvironment (Fig. 21B). For example, correlation between mast cell density and lymphatic microvessel density varied based on breast cancer subtype and peritumoral versus intratumoral mast cell location (318). As discussed in an earlier section of this review, peritumoral lymphatic vessel density is a prognostic indicator in several cancer types, including cervical, colorectal, breast, and prostate cancers (79, 105, 135, 256, 334). Indeed, peritumoral mast cell density was significantly positively correlated with lymphatic density in luminal A and basal-like breast carcinomas; on the other hand, intratumoral mast cell density showed a low inverse correlation with lymphatic density in both luminal A and HER2+ breast cancer subtypes, yet a positive correlation with basal-like carcinomas (318). In addition, Rajput et al. investigated over 4,000 clinically annotated tissue microarrays from invasive breast cancer patients with long-term follow-up, and reported that intratumoral mast cell infiltration was a strong marker of favorable prognosis independent of age, tumor grade, tumor size, lymph node status, and ER or HER2 status (319). Future work should address the molecular significance of the differential prognostic implications based on mast cell localization observed across breast cancer subtypes.
Mast cells in prostate cancer
Mast cell location also appears to influence prognosis in prostate cancers. Nonomura et al. reported that increased peritumoral mast cell count was associated with reduced recurrence-free survival and higher Gleason scores in prostate cancer patients treated with radical prostatectomy, irradiation therapy, or androgen deprivation therapy (280). Androgen deprivation therapy, also called castration therapy, is the gold standard for treatment of patients with metastatic prostate cancer. However, despite high initial response rates, nearly all men eventually develop progressive disease, referred to as “castration-resistant” prostate cancer. Johansson et al. (187) found that androgen deprivation therapy increased mast cell recruitment to the peritumoral tissue compartment of locally relapsing human prostate tumors, but not to the tumor itself. Peritumoral mast cells promoted tumor growth and tumor angiogenesis, which were further exacerbated by mast cell degranulation. Moreover, patients with higher peritumoral mast cell density had higher Gleason scores and significantly shorter cancer-specific survival, while patients with low numbers of intratumoral mast cells exhibited the same patterns. Low intratumoral mast cell count was also associated with high tumor stage, higher tumor cell proliferation index, and metastatic spread (187). Similar results have been reported by others, with poorest outcomes in prostate cancer patients lacking intratumoral mast cells (121). These studies raise several important questions: how different are peritumoral vs. intratumoral mast cells, and what are the factors determining which phenotype develops? Are these factors tumor-intrinsic or determined by the surrounding tissue, particularly adipose tissue?
Impact of obesity on peritumoral mast cells
Given consistent reports regarding the increased mast cell content and altered mast cell activation state in obese adipose, we were surprised to find not a single publication addressing the impact of obesity or adipose tissue on the density or phenotype of peritumoral mast cells. In fact, the only study found even peripherally linking mast cells in adipose tissue to cancer outcomes addressed the frequency of metastatic ovarian cancer colonization within “milky spots,” vascularized accumulations of mononuclear cells in human omental adipose that include mast cells (69). It must also be noted that BMI was not included as a variable in any of the aforementioned studies addressing mast cell function in breast and prostate tumors.
Increased adipose tissue mast cell density in obesity suggests the potential for elevated peritumoral mast cell concentrations in adipose-infiltrating tumors of obese individuals. However, although increases in adipose mast cell density have been reported in visceral adipose tissue of obese mice (10) and abdominal subcutaneous adipose of obese patients (228), whether obesity influences mast cell density in breast subcutaneous or periprostatic adipose tissue has not been reported. Ishijima et al. demonstrated that mast cells influence preadipocyte-adipocyte transition under both physiological and pathological conditions (182), suggesting a possible role for mast cells in adipose expansion. Furthermore, adipose tissue hematopoietic progenitor cells contain a population committed to the mast cell lineage, allowing white adipose tissue to act as a reservoir for mast cells that traffic to other tissues such as skin and, potentially, developing tumors (308). Thus, considering the differential associations between peritumoral vs. intratumoral mast cells and cancer outcomes, future studies should investigate the positioning and granulation status of peritumoral mast cells in relation to adipose tissue in lean and obese patients.
Eosinophils
Eosinophils are granulocytes typically associated with allergy and asthma that play key immunoregulatory roles in antigen presentation, suppression of inflammation, and maintenance of metabolic homeostasis (5, 83). Under physiologic conditions, circulating eosinophils are rare. However, eosinophils comprise ~4% to 5% of cells in the stromal-vascular fraction of lean adipose (444). Indeed, Wu et al. (444) demonstrated that eosinophils are the primary source of IL-4 in adipose tissue, as ~ 90% of IL-4-expressing cells recovered from visceral adipose of lean mice were eosinophils. Interestingly, they also noted an inverse relationship between adiposity and adipose eosinophil content in both genetic and diet-induced models of obesity. Furthermore, mice engineered to be eosinophil-deficient developed significantly greater adiposity and impaired glucose tolerance in response to high-fat diet feeding. These results were attributed to impaired eosinophil-mediated maintenance of alternatively activated, anti-inflammatory macrophages, which are generated upon exposure to IL-4 and/or IL-13 and are generally considered to be protective against diet-induced obesity and associated metabolic dysregulation. Subsequent studies have revealed that visceral adipose eosinophil populations, and thus alternatively activated macrophages, are in turn dependent upon innate lymphoid type 2 cells (ILC2s) through their production of IL-13 and IL-5, an eosinophil colony stimulating factor (257). In light of their direct or indirect anti-inflammatory effects, it is tempting to speculate that the presence of eosinophils and ILC2s in lean adipose, and their relative absence in inflamed obese adipose, may be a contributing factor to the differential cancer risk profile in lean versus obese individuals.
In light of the role of eosinophils in the maintenance of alternatively activated macrophages in normal, uninflamed adipose, it may seem surprising that these cells appear to promote proinflammatory macrophage polarization in tumors. Accordingly, an E1/E2 classification scheme analogous to the macrophage M1/M2 and T helper cell Th1/Th2 subsets has been proposed (335). Eosinophil peroxidase enhances TNF-α and hydrogen peroxide release by human monocyte-derived macrophages, suggesting that paracrine signaling between eosinophils and macrophages within the tumor microenvironment may be relevant in promoting activity of certain tumoricidal TAM populations (364). In agreement with this proposition, injection of exogenous eosinophils in a mouse melanoma model reportedly reprogrammed TAMs toward a pro-inflammatory, tumoricidal phenotype, a result attributed to increased production of eosinophil-derived IFN-γ (61). However, it should be noted that while eosinophils facilitate tumor rejection in numerous cancer models, increased levels of circulating eosinophils are associated with poor prognosis in some hematologic malignancies, such as non-Hodgkin’s and T cell lymphomas (335). Therefore, future research should systematically address relationships between local and circulating eosinophil content, site-specific tumor promotion vs. rejection, and eosinophil-mediated modulation of macrophage polarization.
Although little research has addressed these functions in the context of obesity, it is clear that eosinophils also facilitate anti-tumor immune reactions independent of their effects on macrophage polarization. For example, Carretero et al. (61) recently reported that eosinophil-mediated production of the chemoattractants CCL5, CXCL9, and CXCL10 promoted cytotoxic T cell recruitment in developing melanomas. Antibody-mediated depletion of eosinophils reduced CD8+ T cell infiltration, impaired tumor rejection, and severely reduced animal survival. Moreover, injection of melanoma cells together with exogenous eosinophils resulted in tumor vessel normalization, as evidenced by reduced permeability, enhanced perfusion, and reduced tumor hypoxia, alterations sometimes associated with reduced tumor aggression and more efficient vascular delivery of chemotherapies. In addition to their effects on other immune cells within the tumor microenvironment, eosinophils may also exhibit direct cytotoxicity. Tepper et al. (389) reported that mouse melanoma and plasmacytoma cells engineered to express IL-4 exhibited reduced or absent tumorigenicity in transplant studies due to elicitation of an inflammatory infiltrate comprised predominantly of macrophages and cytotoxic eosinophils. Accordingly, administration of a monoclonal antibody with granulocyte-specific cytotoxicity depleted eosinophils and restored tumorigenicity of IL-4-producing cells. However, these results were called into question by a subsequent study in which eosinophil-deficient IL-5-knockout mice showed similar degrees of IL-4-expressing melanoma rejection wildtype animals, a phenotype attributed to a neutrophil-mediated response (278). Ultimately, the conflicting results of these two studies indicate that the putative cytotoxic functions of eosinophils in anti-tumor immunity warrant further study. Moreover, additional investigation into eosinophil content in lean vs. obese adipose and their potential influence on adipose-tumor interactions should yield interesting findings.
Conclusion
Although adipocytes comprise the bulk of adipose tissue volume, adipose also contains a rich variety of stromal and vascular cells, as well as matrix and signaling components, which together constitute the adipose tissue microenvironment. A growing body of literature indicates that reciprocal, heterotypic interactions between developing tumors and the local adipose milieu influence the course of solid tumor progression. Herein, we have provided an overview of interactions between select adipose tissue components and developing adipose-adjacent cancers, emphasizing breast and prostate cancers and the known or potential impact of changes that occur in the adipose tissue microenvironment during progression to obesity. As described throughout this review, obesity-associated adipose modifications often resemble aberrations observed within the tumor microenvironment. For example, similar to tumors, dysregulated obese adipose tissue is characterized by chronic low-grade inflammation, macrophage infiltration, hypoxia, and aberrant wound healing responses, including an increase in myofibroblast and activated fibroblast content. Obese adipose tissue is also a harbor for soluble mediators of cancer development, including metabolites, exosomes, cytokines, growth factors, and extracellular matrix scaffolding proteins, which collectively provide a critical link between adiposity and tumorigenesis. Thus, we posit that adipose-adjacent epithelium in obese individuals encounters an environment particularly conducive to tumor initiation and progression.
Despite a recent increase in research regarding the contributions of adipose tissue in cancer development, many questions still remain. For example, the identities of many adipose-derived microenvironmental signaling mediators that modify tumor biology are largely unknown. Furthermore, while immune cells in both adipose tissue and cancer biology have been characterized individually, few studies have attempted to quantify recruitment of immune cells originating in adipose tissue adjacent to tumors. This potential for recruitment becomes especially important in the context of obesity, wherein adipose tissue immune cell content is greatly increased, yet the relative immune composition and phenotype shifts dramatically. Thus, the extent to which specific adipose-derived cell lineages contribute to tumor development and/or progression remains inconclusive. Ultimately, given the rising global prevalence of obesity, a better understanding of the molecular interactions between adipose tissue components and tumor cells is critical for the identification of novel targets for prevention and/or treatment of obesity-associated cancers.
Didactic Synopsis.
Major teaching points
Solid tumor growth requires the interaction of tumor cells with the surrounding tissue, leading to a view of tumors as communities rather than exclusively tumor cells.
Adipose tissue, or fat, plays important roles in cancer risk and outcome because many tumors grow close to or in direct contact with adipose.
The adipose community—or microenvironment—includes adipocytes and adipose-associated stromal and vascular components, such as fibroblasts and other connective tissue cells, stem cells, endothelial cells, innate and adaptive immune cells, and extracellular signaling and matrix components.
Herein, we review the cellular and noncellular parts of the adipose “organ” and the mechanisms by which varied microenvironmental components contribute to tumor development, with emphasis on obesity.
Obesity dramatically modifies the adipose tissue microenvironment in numerous ways, which intriguingly resemble shifts observed within the tumor microenvironment.
Understanding neighboring adipose is critical in tumorigenesis.
Acknowledgments
The authors thank Dr. Melissa Troester, PhD, from the University of North Carolina, Chapel Hill for providing human breast histology through the UNC Normal Breast Study. We further show our gratitude to Drs. Laura Bowers, PhD, and Stephen Hursting, PhD, also from the University of North Carolina, Chapel Hill, for sharing mouse mammary histology. Finally, the authors would like to acknowledge Kathryn Hobbs and Ottavia Zattra for their help with formatting and editing during manuscript preparation.
Footnotes
During peer review and publication of the present review, clinical trial NCT 02074878 was terminated due to poor accrual.
References
- 1.The Human Protein Atlas, www.proteinatlas.org. Human breast cancer - Female, 50 years, lobular carcinoma, grade 1, Elston-Ellis score 5. Image data available at the following URL: v16.proteinatlas.org/learn/dictionary/cancer/breast+cancer+4.
- 2.Abrahamson PE, Gammon MD, Lund MJ, Flagg EW, Porter PL, Stevens J, Swanson CA, Brinton LA, Eley JW, Coates RJ. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1871–1877. doi: 10.1158/1055-9965.EPI-06-0356. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Gehlot P. Inflammation and cancer: How friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agustsson T, Ryden M, Hoffstedt J, van Harmelen V, Dicker A, Laurencikiene J, Isaksson B, Permert J, Arner P. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007;67:5531–5537. doi: 10.1158/0008-5472.CAN-06-4585. [DOI] [PubMed] [Google Scholar]
- 5.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: A new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht I, Christofori G. Molecular mechanisms of lymphangiogenesis in development and cancer. Int J Dev Biol. 2011;55:483–494. doi: 10.1387/ijdb.103226ia. [DOI] [PubMed] [Google Scholar]
- 8.Allen E, Mieville P, Warren CM, Saghafinia S, Li L, Peng MW, Hanahan D. Metabolic symbiosis enables adaptive resistance to antiangiogenic therapy that is dependent on mTOR signaling. Cell Rep. 2016;15:1144–1160. doi: 10.1016/j.celrep.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, Reiser J, Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J Lipid Res. 2011;52:480–488. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altintas MM, Rossetti MA, Nayer B, Puig A, Zagallo P, Ortega LM, Johnson KB, McNamara G, Reiser J, Mendez AJ, Nayer A. Apoptosis, mastocytosis, and diminished adipocytokine gene expression accompany reduced epididymal fat mass in long-standing diet-induced obese mice. Lipids Health Dis. 2011;10:198. doi: 10.1186/1476-511X-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves MJ, Figuerêdo RG, Azevedo FF, Cavallaro DA, Neto NIP, Lima JDC, Matos-Neto E, Radloff K, Riccardi DM, Camargo RG, De Alcântara PSM, Otoch JP, Junior MLB, Seelaender M. Adipose tissue fibrosis in human cancer cachexia: The role of TGFβ pathway. BMC Cancer. 2017;17:190. doi: 10.1186/s12885-017-3178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, Hainaut P. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: A systematic review and dose-response meta-analysis. Obes Rev. 2013;14:665–678. doi: 10.1111/obr.12028. [DOI] [PubMed] [Google Scholar]
- 13.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amarnath S, Mangus CW, Wang JCM, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, Levine BL, June CH, Medin JA, Fowler DH. The PDL1-PD1 axis converts human Th1 cells into regulatory T cells. Sci Transl Med. 2011;3:111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amor S, Iglesias-de la Cruz MC, Ferrero E, Garcia-Villar O, Barrios V, Fernandez N, Monge L, Garcia-Villalon AL, Granado M. Peritumoral adipose tissue as a source of inflammatory and angiogenic factors in colorectal cancer. Int J Colorectal Dis. 2016;31:365–375. doi: 10.1007/s00384-015-2420-6. [DOI] [PubMed] [Google Scholar]
- 16.Anderson GL, Neuhouser ML. Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res (Phila) 2012;5:515–521. doi: 10.1158/1940-6207.CAPR-12-0091. [DOI] [PubMed] [Google Scholar]
- 17.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 18.Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, Kuperwasser C. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013;73:6080–6093. doi: 10.1158/0008-5472.CAN-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arngrim N, Simonsen L, Holst JJ, Bulow J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: A possible link between obesity and local tissue inflammation [quest] Int J Obes. 2013;37:748–750. doi: 10.1038/ijo.2012.98. [DOI] [PubMed] [Google Scholar]
- 20.Atherton AJ, O’Hare MJ, Buluwela L, Titley J, Monaghan P, Paterson HF, Warburton MJ, Gusterson BA. Ectoenzyme regulation by phenotypically distinct fibroblast sub-populations isolated from the human mammary gland. J Cell Sci. 1994;107(Pt 10):2931–2939. doi: 10.1242/jcs.107.10.2931. [DOI] [PubMed] [Google Scholar]
- 21.Atherton AJ, Warburton MJ, O’Hare MJ, Monaghan P, Schuppan D, Gusterson BA. Differential expression of type XIV collagen/undulin by human mammary gland intralobular and interlobular fibroblasts. Cell Tissue Res. 1998;291:507–511. doi: 10.1007/s004410051020. [DOI] [PubMed] [Google Scholar]
- 22.Augustin H. Obesity and prostate cancer: An ambiguous relationship. Eur J Cancer. 2007;43:1114–1116. doi: 10.1016/j.ejca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9:4792–4801. [PubMed] [Google Scholar]
- 24.Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: A review of the recent literature. Curr Nutr Rep. 2014;3:9–15. doi: 10.1007/s13668-013-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol. 2013;3:211. doi: 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 27.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 28.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 29.Baratelli F, Lee JM, Hazra S, Lin Y, Walser TC, Schaue D, Pak PS, Elashoff D, Reckamp K, Zhang L, Fishbein MC, Sharma S, Dubinett SM. PGE(2) contributes to TGF-β induced T regulatory cell function in human non-small cell lung cancer. Am J Transl Res. 2010;2:356–367. [PMC free article] [PubMed] [Google Scholar]
- 30.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 31.Barlow J, Hirschberg Jensen V, Jastroch M, Affourtit C. Palmitate-induced impairment of glucose-stimulated insulin secretion precedes mitochondrial dysfunction in mouse pancreatic islets. Biochem J. 2015;473:487–496. doi: 10.1042/BJ20151080. [DOI] [PubMed] [Google Scholar]
- 32.Barrington WE, Schenk JM, Etzioni R, Arnold KB, Neuhouser ML, Thompson IM, Jr, Lucia MS, Kristal AR. Difference in association of obesity with prostate cancer risk between US African American and non-Hispanic white men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA Oncol. 2015;1:342–349. doi: 10.1001/jamaoncol.2015.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basen-Engquist K, Chang M. Obesity and cancer risk: Recent review and evidence. Curr Oncol Rep. 2011;13:71–76. doi: 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 35.Batista ML, Jr, Henriques FS, Neves RX, Olivan MR, Matos-Neto EM, Alcantara PS, Maximiano LF, Otoch JP, Alves MJ, Seelaender M. Cachexia-associated adipose tissue morphological rearrangement in gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle. 2016;7:37–47. doi: 10.1002/jcsm.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baylor Breast Care Center BCoM. CRIZENT: Crizotinib and Sunitinib in Metastatic Breast Cancer. 2015 ClinicalTrials.gov identifier, NCT02074878. [Google Scholar]
- 37.Beckham TH, Lu P, Cheng JC, Zhao D, Turner LS, Zhang X, Hoffman S, Armeson KE, Liu A, Marrison T, Hannun YA, Liu X. Acid ceramidase-mediated production of sphingosine 1-phosphate promotes prostate cancer invasion through upregulation of cathepsin B. Int J Cancer. 2012;131:2034–2043. doi: 10.1002/ijc.27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179:1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell LN, Ward JL, Degawa-Yamauchi M, Bovenkerk JE, Jones R, Cacucci BM, Gupta CE, Sheridan C, Sheridan K, Shankar SS, Steinberg HO, March KL, Considine RV. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metab. 2006;291:E843–E848. doi: 10.1152/ajpendo.00174.2006. [DOI] [PubMed] [Google Scholar]
- 40.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 41.Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, Xiang AH, Watanabe RM. A better index of body adiposity. Obesity (Silver Spring) 2011;19:1083–1089. doi: 10.1038/oby.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biglia N, Peano E, Sgandurra P, Moggio G, Pecchio S, Maggiorotto F, Sismondi P. Body mass index (BMI) and breast cancer: Impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol. 2013;29:263–267. doi: 10.3109/09513590.2012.736559. [DOI] [PubMed] [Google Scholar]
- 43.Bissell MJ, Kenny PA, Radisky DC. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: The role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biswas SK, Lewis CE. NF-kappaB as a central regulator of macrophage function in tumors. J Leukoc Biol. 2010;88:877–884. doi: 10.1189/jlb.0310153. [DOI] [PubMed] [Google Scholar]
- 46.Bochet L, Lehuede C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le Gonidec S, Couderc B, Escourrou G, Valet P, Muller C. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–5668. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 47.Bodle JC, Teeter SD, Hluck BH, Hardin JW, Bernacki SH, Loboa EG. Age-related effects on the potency of human adipose-derived stem cells: Creation and evaluation of superlots and implications for musculoskeletal tissue engineering applications. Tissue Engineering Part C, Methods. 2014;20:972–983. doi: 10.1089/ten.tec.2013.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boehm K, Sun M, Larcher A, Blanc-Lapierre A, Schiffmann J, Graefen M, Sosa J, Saad F, Parent ME, Karakiewicz PI. Waist circumference, waist-hip ratio, body mass index, and prostate cancer risk: Results from the North-American case-control study Prostate Cancer&Environment Study. Urol Oncol. 2015;33:494.e491–497. doi: 10.1016/j.urolonc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Bostwick DG, Cheng L. Urologic Surgical Pathology. St. Louis, MO: Elsevier Health Sciences; 2008. [Google Scholar]
- 50.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells:Ajoint statement of the International Federation for Adipose Therapeutics (IFATS) and Science and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102:1224–1237. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 53.Briganti A, Karakiewicz PI, Chun FKH, Suardi N, Gallina A, Abdollah F, Freschi M, Doglioni C, Rigatti P, Montorsi F. Obesity does not increase the risk of lymph node metastases in patients with clinically localized prostate cancer undergoing radical prostatectomy and extended pelvic lymph node dissection. Int J Urol. 2009;16:676–681. doi: 10.1111/j.1442-2042.2009.02330.x. [DOI] [PubMed] [Google Scholar]
- 54.Caballero B. The global epidemic of obesity: An overview. Epidemiol Rev. 2007;29:1–5. doi: 10.1093/epirev/mxm012. [DOI] [PubMed] [Google Scholar]
- 55.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 56.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 57.Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, Au A, Baehner F, Chen Y, Malaka DO. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011;128:703–711. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 59.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically. Proc Natl Acad Sci U S A. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 62.Carter JC, Church FC. Mature breast adipocytes promote breast cancer cell motility. Exp Mol Pathol. 2012;92:312–317. doi: 10.1016/j.yexmp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Casbas-Hernandez P, D’Arcy M, Roman-Perez E, Brauer HA, McNaughton K, Miller SM, Chhetri RK, Oldenburg AL, Fleming JM, Amos KD. Role of HGF in epithelial–stromal cell interactions during progression from benign breast disease to ductal carcinoma in situ. Breast Cancer Res. 2013;15:R82. doi: 10.1186/bcr3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chandler EM, Saunders MP, Yoon CJ, Gourdon D, Fischbach C. Adipose progenitor cells increase fibronectin matrix strain and unfolding in breast tumors. Phys Biol. 2011;8:015008. doi: 10.1088/1478-3975/8/1/015008. [DOI] [PubMed] [Google Scholar]
- 66.Chang CH, Pearce EL. Emerging concepts of T cell metabolism as a target of immunotherapy. Nat Immunol. 2016;17:364–368. doi: 10.1038/ni.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, Cook LS, Tang MT, Porter PL, Hill DA, Wiggins CL, Li CI. Body mass index and risk of luminal, HER2-overexpressing, and triple negative breast cancer. Breast Cancer Res Treat. 2016;157:545–554. doi: 10.1007/s10549-016-3825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J Clin Invest. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark R, Krishnan V, Schoof M, Rodriguez I, Theriault B, Chekmareva M, Rinker-Schaeffer C. Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am J Pathol. 2013;183:576–591. doi: 10.1016/j.ajpath.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, Green J. Adipose tissue: A vital in vivo role in mammary gland development but not differentiation. Dev Dyn. 2002;223:459–468. doi: 10.1002/dvdy.10065. [DOI] [PubMed] [Google Scholar]
- 72.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cowen S, McLaughlin SL, Hobbs G, Coad J, Martin KH, Olfert IM, Vona-Davis L. High-fat, high-calorie diet enhances mammary carcinogenesis and local inflammation in MMTV-PyMT mousemodel of breast cancer. Cancers. 2015;7:1125–1142. doi: 10.3390/cancers7030828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cozzo AJ, Sundaram S, Zattra O, Qin Y, Freemerman AJ, Essaid L, Darr DB, Montgomery SA, McNaughton KK, Ezzell JA, Galanko JA, Troester MA, Makowski L. cMET inhibitor crizotinib impairs angiogenesis and reduces tumor burden in the C3(1)-Tag model of basal-like breast cancer. Springerplus. 2016;5:348. doi: 10.1186/s40064-016-1920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Curat CA, Miranville A, Sengenès C, Diehl M, Tonus C, Busse R, Bouloumié A. From blood monocytes to adipose tissue-resident macrophages: Induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 76.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 77.da Silva EZ, Jamur MC, Oliver C. Mast cell function: A new vision of an old cell. J Histochem Cytochem. 2014;62:698–738. doi: 10.1369/0022155414545334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233–238. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- 79.Datta K, Muders M, Zhang H, Tindall DJ. Mechanism of lymph node metastasis in prostate cancer. Future Oncol. 2010;6:823–836. doi: 10.2217/fon.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davies LC, Rosas M, Jenkins SJ, Liao C, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow-derived and tissue resident macrophage-lineages proliferate at key stages during inflammation. Nat Commun. 2013;4 doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol. 2011;41:2155–2164. doi: 10.1002/eji.201141817. [DOI] [PubMed] [Google Scholar]
- 82.Davies LC, Taylor PR. Tissue-resident macrophages: Then and now. Immunology. 2015;144:541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2:1–8. doi: 10.1158/2326-6066.CIR-13-0196. [DOI] [PubMed] [Google Scholar]
- 84.Dawood S, Broglio K, Gonzalez-Angulo AM, Kau SW, Islam R, Hortobagyi GN, Cristofanilli M. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res. 2008;14:1718–1725. doi: 10.1158/1078-0432.CCR-07-1479. [DOI] [PubMed] [Google Scholar]
- 85.de Jong JM, Larsson O, Cannon B, Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab. 2015;308:E1085–E1105. doi: 10.1152/ajpendo.00023.2015. [DOI] [PubMed] [Google Scholar]
- 86.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 87.DeWever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 88.DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen YY, Fontenay GV, Berman HK, Gauthier ML, Zhao J, Hu D, Marx JJ, Tjoe JA, Ziv E, Febbraio M, Kerlikowske K, Parvin B, Tlsty TD. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov. 2012;2:826–839. doi: 10.1158/2159-8290.CD-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 91.Ding S, Merkulova-Rainon T, Han ZC, Tobelem G. HGF receptor upregulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood. 2003;101:4816–4822. doi: 10.1182/blood-2002-06-1731. [DOI] [PubMed] [Google Scholar]
- 92.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 93.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 94.Divoux A, Moutel S, Poitou C, Lacasa D, Veyrie N, Aissat A, Arock M, Guerre-Millo M, Clement K. Mast cells in human adipose tissue: Link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab. 2012;97:E1677–E1685. doi: 10.1210/jc.2012-1532. [DOI] [PubMed] [Google Scholar]
- 95.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker J-D, Bedossa P, Clément K. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dixit VD. Thymic fatness and approaches to enhance thymopoietic fitness in aging. Curr Opin Immunol. 2010;22:521–528. doi: 10.1016/j.coi.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Djonov V, Andres A-C, Ziemiecki A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc Res Tech. 2001;52:182–189. doi: 10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 98.Dontu G, Ince TA. Of mice and women: A comparative tissue biology perspective of breast stem cells and differentiation. J Mammary Gland Biol Neoplasia. 2015;20:51–62. doi: 10.1007/s10911-015-9341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dudley AC. Tumor endothelial cells. Cold Spring Harb Perspect Med. 2012;2:a006536. doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dunleavey JM, Xiao L, Thompson J, Kim MM, Shields JM, Shelton SE, Irvin DM, Brings VE, Ollila DW, Brekken RA, Dayton PA, Melero-Martin JM, Dudley AC. Vascular channels formed by subpopulations of PECAM1+ melanoma cells. Nat Commun. 2014;5:5200. doi: 10.1038/ncomms6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 102.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 103.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: Implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- 104.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Gohary YM, Metwally G, Saad RS, Robinson MJ, Mesko T, Poppiti RJ. Prognostic significance of intratumoral and peritumoral lymphatic density and blood vessel density in invasive breast carcinomas. Am J Clin Pathol. 2008;129:578–586. doi: 10.1309/2HGNJ1GU57JMBJAQ. [DOI] [PubMed] [Google Scholar]
- 106.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 107.Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16:447–462. doi: 10.1038/nrc.2016.54. [DOI] [PubMed] [Google Scholar]
- 108.Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE(2) catabolism in myeloid cells. J Leukoc Biol. 2010;88:839–848. doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Escamilla J, Schokrpur S, Liu C, Priceman SJ, Moughon D, Jiang Z, Pouliot F, Magyar C, Sung JL, Xu J, Deng G, West BL, Bollag G, Fradet Y, Lacombe L, Jung ME, Huang J, Wu L. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 2015;75:950–962. doi: 10.1158/0008-5472.CAN-14-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, Oliver G. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85096. pii: e85096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ewertz M, Jensen M-B, Gunnarsdóttir KÁ, Højris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 112.Fain JN, Bahouth SW, Madan AK. TNFalpha release by the nonfat cells of human adipose tissue. Int J Obes Relat Metab Disord. 2004;28:616–622. doi: 10.1038/sj.ijo.0802594. [DOI] [PubMed] [Google Scholar]
- 113.Fernandez-Garcia B, Eiro N, Miranda MA, Cid S, Gonzalez LO, Dominguez F, Vizoso FJ. Prognostic significance of inflammatory factors expression by stroma from breast carcinomas. Carcinogenesis. 2016;37:768–776. doi: 10.1093/carcin/bgw062. [DOI] [PubMed] [Google Scholar]
- 114.Ferrante AW. The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15:34–38. doi: 10.1111/dom.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 116.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Petricoin EF, Zaldivar F, Santos R, Tyson DR, Ornstein DK. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J Urol. 2009;182:1621–1627. doi: 10.1016/j.juro.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 118.Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol. 2014;26:38–47. doi: 10.1016/j.smim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Flammiger A, Weisbach L, Huland H, Tennstedt P, Simon R, Minner S, Bokemeyer C, Sauter G, Schlomm T, Trepel M. High tissue density of FOXP3+ T cells is associated with clinical outcome in prostate cancer. Eur J Cancer. 2013;49:1273–1279. doi: 10.1016/j.ejca.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 120.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fleischmann A, Schlomm T, Kollermann J, Sekulic N, Huland H, Mirlacher M, Sauter G, Simon R, Erbersdobler A. Immunological microenvironment in prostate cancer: High mast cell densities are associated with favorable tumor characteristics and good prognosis. The Prostate. 2009;69:976–981. doi: 10.1002/pros.20948. [DOI] [PubMed] [Google Scholar]
- 122.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 123.Fox SB, Gatter KC, Bicknell R, Going JJ, Stanton P, Cooke TG, Harris AL. Relationship of endothelial cell proliferation to tumor vascularity in human breast cancer. Cancer Res. 1993;53:4161–4163. [PubMed] [Google Scholar]
- 124.Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res. 2007;9:1–11. doi: 10.1186/bcr1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Freedland SJ, Aronson WJ. Examining the relationship between obesity and prostate cancer. Rev Urol. 2004;6:73–81. [PMC free article] [PubMed] [Google Scholar]
- 128.Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, Macintyre AN, Goraksha-Hicks P, Rathmell JC, Makowski L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289:7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 131.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425–426. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 135.Gao P, Zhou GY, Yin G, Liu Y, Liu ZY, Zhang J, Hao CY. Lymphatic vessel density as a prognostic indicator for patients with stage I cervical carcinoma. Hum Pathol. 2006;37:719–725. doi: 10.1016/j.humpath.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 136.Garcia Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, Gardenier JC, Savetsky IL, Aschen SZ, Nitti MD, Mehrara BJ. Obesity but not high-fat diet impairs lymphatic function. Int J Obes (Lond) 2016;40:1582–1590. doi: 10.1038/ijo.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 138.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene expression profiles and transcriptional regulatory pathways underlying mouse tissue macrophage identity and diversity. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J Lipid Res. 2007;48:1846–1856. doi: 10.1194/jlr.M700131-JLR200. [DOI] [PubMed] [Google Scholar]
- 140.Gehmert S, Gehmert S, Prantl L, Vykoukal J, Alt E, Song YH. Breast cancer cells attract the migration of adipose tissue-derived stem cells via the PDGF-BB/PDGFR-beta signaling pathway. Biochem Biophys Res Commun. 2010;398:601–605. doi: 10.1016/j.bbrc.2010.06.132. [DOI] [PubMed] [Google Scholar]
- 141.Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: Insights from imaging. Histochem Cell Biol. 2008;130:1105–1118. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ghosh S, Hughes D, Parma DL, Ramirez A, Li R. Association of obesity and circulating adipose stromal cells among breast cancer survivors. Mol Biol Rep. 2014;41:2907–2916. doi: 10.1007/s11033-014-3146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gillespie EF, Sorbero ME, Hanauer DA, Sabel MS, Herrmann EJ, Weiser LJ, Jagielski CH, Griggs JJ. Obesity and angiolymphatic invasion in primary breast cancer. Ann Surg Oncol. 2010;17:752–759. doi: 10.1245/s10434-009-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 145.Gioulbasanis I, Martin L, Baracos VE, Thezenas S, Koinis F, Senesse P. Nutritional assessment in overweight and obese patients with metastatic cancer: Does it make sense? Ann Oncol. 2015;26:217–221. doi: 10.1093/annonc/mdu501. [DOI] [PubMed] [Google Scholar]
- 146.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: A consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Giralt M, Villarroya F. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology. 2013;154:2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 148.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: New insights provided by body composition. Am J Clin Nutr. 2014;99:999–1005. doi: 10.3945/ajcn.113.071399. [DOI] [PubMed] [Google Scholar]
- 150.Gordon S. The macrophage: Past, present and future. Eur J Immunol. 2007;37(Suppl 1):S9–S17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 151.Grabowska MM, DeGraff DJ, Yu X, Jin RJ, Chen Z, Borowsky AD, Matusik RJ. Mouse models of prostate cancer: Picking the best model for the question. Cancer Metastasis Rev. 2014;33:377–397. doi: 10.1007/s10555-013-9487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Green JE, Shibata M-A, Yoshidome K, Liu M-l, Jorcyk C, Anver MR, Wigginton J, Wiltrout R, Shibata E, Kaczmarczyk S. The C3 (1)/SV40 T-antigen transgenic mouse model of mammary cancer: Ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene. 2000;19:1020–1027. doi: 10.1038/sj.onc.1203280. [DOI] [PubMed] [Google Scholar]
- 153.Gu J-W, Young E, Patterson SG, Makey KL, Wells J, Huang M, Tucker KB, Miele L. Postmenopausal obesity promotes tumor angiogenesis and breast cancer progression in mice. Cancer Biol Ther. 2011;11:910–917. doi: 10.4161/cbt.11.10.15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haase J, Weyer U, Immig K, Kloting N, Bluher M, Eilers J, Bechmann I, Gericke M. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57:562–571. doi: 10.1007/s00125-013-3139-y. [DOI] [PubMed] [Google Scholar]
- 155.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: The multifaceted role of NF-kappaB. Blood. 2009;113:3139–3146. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hakanpaa L, Sipila T, Leppanen VM, Gautam P, Nurmi H, Jacquemet G, Eklund L, Ivaska J, Alitalo K, Saharinen P. Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. Nat Commun. 2015;6:5962. doi: 10.1038/ncomms6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 160.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 161.Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Bone marrow fat: Linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev. 2014;33:527–543. doi: 10.1007/s10555-013-9484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Harms M, Seale P. Brown and beige fat: Development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 163.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. NatGenet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 164.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hattori Y, Hattori K, Hayashi T. Pleiotropic benefits of metformin: Macrophage targeting its anti-inflammatory mechanisms. Diabetes. 2015;64:1907–1909. doi: 10.2337/db15-0090. [DOI] [PubMed] [Google Scholar]
- 166.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 167.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 168.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1:467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 169.Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, Podgorski I. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4:2108–2123. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, Van Dyke T, Perou CM. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: One function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hiratsuka A, Adachi H, Fujiura Y, Yamagishi S-I, Hirai Y, Enomoto M, Satoh A, Hino A, Furuki K, Imaizumi T. Strong association between serum hepatocyte growth factor and metabolic syndrome. J Clin Endocrinol Metab. 2005;90:2927–2931. doi: 10.1210/jc.2004-1588. [DOI] [PubMed] [Google Scholar]
- 173.Ho-Yen CM, Green AR, Rakha EA, Brentnall AR, Ellis IO, Kermorgant S. C-Met in invasive breast cancer: Is there a relationship with the basal-like subtype? Cancer. 2014;120:163–171. doi: 10.1002/cncr.28386. [DOI] [PubMed] [Google Scholar]
- 174.Ho-Yen CM, Jones JL, Kermorgant S. The clinical and functional significance of c-Met in breast cancer: A review. Breast Cancer Res. 2015;17:1–11. doi: 10.1186/s13058-015-0547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 176.Hovey RC, Aimo L. Diverse and active roles for adipocytes during mammary gland growth and function. J Mammary Gland Biol Neoplasia. 2010;15:279–290. doi: 10.1007/s10911-010-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93:3215–3221. doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- 178.Huo CW, Chew G, Hill P, Huang D, Ingman W, Hodson L, Brown KA, Magenau A, Allam AH, McGhee E, Timpson P, Henderson MA, Thompson EW, Britt K. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015;17:79. doi: 10.1186/s13058-015-0592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, Babykutty S, Huang Y, Jung K, Rahbari NN, Han X, Chauhan VP, Martin JD, Kahn J, Huang P, Desphande V, Michaelson J, Michelakos TP, Ferrone CR, Soares R, Boucher Y, Fukumura D, Jain RK. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6:852–869. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: Cell fate specification, stem cells and the microenvironment. Development. 2015;142:1028–1042. doi: 10.1242/dev.087643. [DOI] [PubMed] [Google Scholar]
- 181.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ishijima Y, Ohmori S, Ohneda K. Mast cell deficiency results in the accumulation of preadipocytes in adipose tissue in both obese and non-obese mice. FEBS Open Bio. 2013;4:18–24. doi: 10.1016/j.fob.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ito Y, Ishiguro H, Kobayashi N, Hasumi H, Watanabe M, Yao M, Uemura H. Adipocyte-derived monocyte chemotactic protein-1 (MCP-1) promotes prostate cancer progression through the induction of MMP-2 activity. The Prostate. 2015;75:1009–1019. doi: 10.1002/pros.22972. [DOI] [PubMed] [Google Scholar]
- 184.Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R, Simons BW, Ward JM, Robinson BD, Chu GC, Loda M, Thomas G, Borowsky A, Cardiff RD. Animal models of human prostate cancer: The Consensus Report of the New York Meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res. 2013;73:2718–2736. doi: 10.1158/0008-5472.CAN-12-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C, Lisanti MP, Pestell RG, Scherer PE. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 186.Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J, Chopra N, Russell RG, Sasisekharan R, Trock BJ, Lippman M, Calvert VS, Petricoin EF, 3rd, Liotta L, Dadachova E, Pestell RG, Lisanti MP, Bonaldo P, Scherer PE. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Johansson A, Rudolfsson S, Hammarsten P, Halin S, Pietras K, Jones J, Stattin P, Egevad L, Granfors T, Wikstrom P, Bergh A. Mast cells are novel independent prognostic markers in prostate cancer and represent a target for therapy. Am J Pathol. 2010;177:1031–1041. doi: 10.2353/ajpath.2010.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Johnson AR, Milner JJ, Makowski L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Johnson AR, Qin Y, Cozzo AJ, Freemerman AJ, Huang MJ, Zhao L, Sampey BP, Milner JJ, Beck MA, Damania B, Rashid N, Galanko JA, Lee DP, Edin ML, Zeldin DC, Fueger PT, Dietz B, Stahl A, Wu Y, Mohlke KL, Makowski L. Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Mol Metab. 2016;5:506–526. doi: 10.1016/j.molmet.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kapoor J, Namdarian B, Pedersen J, Hovens C, Moon D, Peters J, Costello AJ, Ruljancich P, Corcoran NM. Extraprostatic extension into periprostatic fat is a more important determinant of prostate cancer recurrence than an invasive phenotype. J Urol. 2013;190:2061–2066. doi: 10.1016/j.juro.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 193.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Karaman S, Hollmen M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, Wolfrum C, Detmar M. Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Mol Metab. 2015;4:93–105. doi: 10.1016/j.molmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 2010;235:1412–1424. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- 196.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: A systematic review. Br J Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Keto CJ, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL, Freedland SJ. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: Results from the SEARCH database. BJU Int. 2012;110:492–498. doi: 10.1111/j.1464-410X.2011.10754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Khamis ZI, Sahab ZJ, Sang QX. Active roles of tumor stroma in breast cancer metastasis. Int J Breast Cancer. 2012;2012:574025. doi: 10.1155/2012/574025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, Andreeff M, Marini FC. Origins of the tumor microenvironment: Quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Knaapen AM, Gungor N, Schins RP, Borm PJ, Van Schooten FJ. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis. 2006;21:225–236. doi: 10.1093/mutage/gel032. [DOI] [PubMed] [Google Scholar]
- 203.Kobayashi H, DeBusk LM, Lin PC. Angiopoietin/Tie2 signaling regulates tumor angiogenesis. In: Teicher BA, Ellis LM, editors. Antiangiogenic Agents in Cancer Therapy. Totowa, NJ: Humana Press; 2008. pp. 171–187. [Google Scholar]
- 204.Kolle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman ML, Thomsen C, Dickmeiss E, Drzewiecki KT. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382:1113–1120. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 205.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kristal AR, Gong Z. Obesity and prostate cancer mortality. Future Oncol. 2007;3:557–567. doi: 10.2217/14796694.3.5.557. [DOI] [PubMed] [Google Scholar]
- 207.Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 208.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Martin-Hirsch P, Tsilidis KK. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ (Clinical research ed) 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Landskroner-Eiger S, Park J, Israel D, Pollard JW, Scherer PE. Morphogenesis of the developing mammary gland: Stage-dependent impact of adipocytes. Dev Biol. 2010;344:968–978. doi: 10.1016/j.ydbio.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Laurent V, Guerard A, Mazerolles C, Le Gonidec S, Toulet A, Nieto L, Zaidi F, Majed B, Garandeau D, Socrier Y, Golzio M, Cadoudal T, Chaoui K, Dray C, Monsarrat B, Schiltz O, Wang YY, Couderc B, Valet P, Malavaud B, Muller C. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lawrence T, Fong C. The resolution of inflammation: Anti-inflammatory roles for NF-kappaB. Int J Biochem Cell Biol. 2010;42:519–523. doi: 10.1016/j.biocel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 214.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 215.Lazar I, Clement E, Dauvillier S, Milhas D, Ducoux-Petit M, LeGonidec S, Moro C, Soldan V, Dalle S, Balor S, Golzio M, Burlet-Schiltz O, Valet P, Muller C, Nieto L. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: A novel mechanism linking obesity and cancer. Cancer Res. 2016;76:4051–4057. doi: 10.1158/0008-5472.CAN-16-0651. [DOI] [PubMed] [Google Scholar]
- 216.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lee TJ, Bhang SH, Yang HS, La WG, Yoon HH, Shin JY, Seong JY, Shin H, Kim BS. Enhancement of long-term angiogenic efficacy of adipose stem cells by delivery of FGF2. Microvasc Res. 2012;84:1–8. doi: 10.1016/j.mvr.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 218.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 219.Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: A review. Curr Oncol Rep. 2016;18:56. doi: 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, Amidi A, Watkins SM, Nguyen U, Olefsky JM. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, McCauley LK. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69:1685–1692. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li Z, Chen L, Qin Z. Paradoxical roles of IL-4 in tumor immunity. Cell Mol Immunol. 2009;6:415–422. doi: 10.1038/cmi.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, Fabian CJ, Gucalp A, Hershman DL, Hudson MM, Jones LW, Kakarala M, Ness KK, Merrill JK, Wollins DS, Hudis CA. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lijnen HR. Angiogenesis and obesity. Cardiovasc Res. 2008;78:286–293. doi: 10.1093/cvr/cvm007. [DOI] [PubMed] [Google Scholar]
- 226.Lim S, Hosaka K, Nakamura M, Cao Y. Co-option of pre-existing vascular beds in adipose tissue controls tumor growth rates and angiogenesis. Oncotarget. 2016;7(25):38282–38291. doi: 10.18632/oncotarget.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 228.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Loi S, Milne RL, Friedlander ML, McCredie MR, Giles GG, Hopper JL, Phillips KA. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1686–1691. doi: 10.1158/1055-9965.EPI-05-0042. [DOI] [PubMed] [Google Scholar]
- 230.Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Madge LA, May MJ. Classical NF-kappaB activation negatively regulates noncanonical NF-kappaB-dependent CXCL12 expression. J Biol Chem. 2010;285:38069–38077. doi: 10.1074/jbc.M110.147207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mangia A, Malfettone A, Rossi R, Paradiso A, Ranieri G, Simone G, Resta L. Tissue remodelling in breast cancer: Human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology. 2011;58:1096–1106. doi: 10.1111/j.1365-2559.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 234.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro:Vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J ExpMed. 2015;212:435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 237.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 238.Marech I, Ammendola M, Sacco R, Capriuolo GS, Patruno R, Rubini R, Luposella M, Zuccala V, Savino E, Gadaleta CD, Ribatti D, Ranieri G. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: Possible translational significance. BMC Cancer. 2014;14:534. doi: 10.1186/1471-2407-14-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 241.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Power surge: Supporting cells “fuel” cancer cell mitochondria. Cell Metab. 2012;15:4–5. doi: 10.1016/j.cmet.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 242.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 244.Mass E, Ballesteros I, Farlik M, Halbritter F, Günther P, Crozet L, Jacome-Galarza CE, Händler K, Klughammer J, Kobayashi Y, Gomez-Perdiguero E, Schultze JL, Beyer M, Bock C, Geissmann F. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353:aaf4238. doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Massa M, Gasparini S, Baldelli I, Scarabelli L, Santi P, Quarto R, Repaci E. Interaction between breast cancer cells and adipose tissue cells derived from fat grafting. Aesthet Surg J. 2016;36:358–363. doi: 10.1093/asj/sjv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mayi TH, Daoudi M, Derudas B, Gross B, Bories G, Wouters K, Brozek J, Caiazzo R, Raverdi V, Pigeyre M, Allavena P, Mantovani A, Pattou F, Staels B, Chinetti-Gbaguidi G. Human adipose tissue macrophages display activation of cancer-related pathways. J Biol Chem. 2012;287:21904–21913. doi: 10.1074/jbc.M111.315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mazzarella L, Disalvatore D, Bagnardi V, Rotmensz N, Galbiati D, Caputo S, Curigliano G, Pelicci PG. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur J Cancer. 2013;49:3588–3597. doi: 10.1016/j.ejca.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 248.McIntyre A, Harris AL. Metabolic and hypoxic adaptation to antiangiogenic therapy: A target for induced essentiality. EMBO Mol Med. 2015;7:368–379. doi: 10.15252/emmm.201404271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mestak O, Hromadkova V, Fajfrova M, Molitor M, Mestak J. Evaluation of oncological safety of fat grafting after breast-conserving therapy: A prospective study. Ann Surg Oncol. 2016;23:776–781. doi: 10.1245/s10434-015-4908-2. [DOI] [PubMed] [Google Scholar]
- 250.Meyer KA, Neeley CK, Baker NA, Washabaugh AR, Flesher CG, Nelson BS, Frankel TL, Lumeng CN, Lyssiotis CA, Wynn ML, Rhim AD, O’Rourke RW. Adipocytes promote pancreatic cancer cell proliferation via glutamine transfer. Biochem Biophys Rep. 2016;7:144–149. doi: 10.1016/j.bbrep.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Micallef L, Vedrenne N, Billet F, Coulomb B, Darby IA, Desmoulière A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis & Tissue Repair. 2012;5:S5. doi: 10.1186/1755-1536-5-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Miyake H, Hara I, Eto H. Serum level of cathepsin B and its density in men with prostate cancer as novel markers of disease progression. Anticancer Res. 2004;24:2573–2577. [PubMed] [Google Scholar]
- 253.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 254.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 255.Mizuno S, Nakamura T. HGF-MET cascade, a key target for inhibiting cancer metastasis: The impact of NK4 discovery on cancer biology and therapeutics. Int J Mol Sci. 2013;14:888–919. doi: 10.3390/ijms14010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mohammed RA, Ellis IO, Mahmmod AM, Hawkes EC, Green AR, Rakha EA, Martin SG. Lymphatic and blood vessels in basal and triple-negative breast cancers: Characteristics and prognostic significance. Mod Pathol. 2011;24:774–785. doi: 10.1038/modpathol.2011.4. [DOI] [PubMed] [Google Scholar]
- 257.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Moorman AM, Vink R, Heijmans HJ, van der Palen J, Kouwenhoven EA. The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur J Surg Oncol. 2012;38:307–313. doi: 10.1016/j.ejso.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 259.Moreira Â, Pereira SS, Machado CL, Morais T, Costa M, Monteiro MP. Obesity inhibits lymphangiogenesis in prostate tumors. Int J Clin Exp Pathol. 2014;7:348–352. [PMC free article] [PubMed] [Google Scholar]
- 260.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Moumen M, Chiche A, Cagnet S, Petit V, Raymond K, Faraldo MM, Deugnier MA, Glukhova MA. The mammary myoepithelial cell. Int J Dev Biol. 2011;55:763–771. doi: 10.1387/ijdb.113385mm. [DOI] [PubMed] [Google Scholar]
- 264.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 265.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 266.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 267.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nakajima EC, VanHouten B. Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol Carcinog. 2013;52:329–337. doi: 10.1002/mc.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nakajima S, Koh V, Kua LF, So J, Davide L, Lim KS, Petersen SH, Yong WP, Shabbir A, Kono K. Accumulation of CD11c+CD163+ adipose tissue macrophages through upregulation of intracellular 11beta-HSD1 in human obesity. J Immunol. 2016;197:3735–3745. doi: 10.4049/jimmunol.1600895. [DOI] [PubMed] [Google Scholar]
- 270.NCD Risk Factor Collaboration (NCD-RisC) a. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: Tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, Thomson CA, Caan BJ, Tinker LF, Urrutia RP, Knudtson J, Anderson GL. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015;1:611–621. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes supports tumorigenesis and metastasis() Biochim Biophys Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 276.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 277.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Noffz G, Qin Z, Kopf M, Blankenstein T. Neutrophils but not eosinophils are involved in growth suppression of IL-4-secreting tumors. J Immunol. 1998;160:345–350. [PubMed] [Google Scholar]
- 279.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nonomura N, Takayama H, Nishimura K, Oka D, Nakai Y, Shiba M, Tsujimura A, Nakayama M, Aozasa K, Okuyama A. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of prostate cancer. Br J Cancer. 2007;97:952–956. doi: 10.1038/sj.bjc.6603962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, Gardenier JC, Savetsky IL, Aschen SZ, Nitti MD, Mehrara BJ. Obesity but not high-fat diet impairs lymphatic function. Int J Obes (Lond) 2016;40:1582–1590. doi: 10.1038/ijo.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.O’Malley RL, Taneja SS. Obesity and prostate cancer. Can J Urol. 2006;13(Suppl 2):11–17. [PubMed] [Google Scholar]
- 284.Obeid S, Hebbard L. Role of adiponectin and its receptors in cancer. Cancer Biol Med. 2012;9:213–220. doi: 10.7497/j.issn.2095-3941.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 286.Oft M. IL-10: Master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2014;2:194–199. doi: 10.1158/2326-6066.CIR-13-0214. [DOI] [PubMed] [Google Scholar]
- 287.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Okwan-Duodu D, Umpierrez GE, Brawley OW, Diaz R. Obesity-driven inflammation and cancer risk: Role of myeloid derived suppressor cells and alternately activated macrophages. Am J Cancer Res. 2013;3:21–33. [PMC free article] [PubMed] [Google Scholar]
- 290.Oliveira AG, Guabiraba R, Teixeira MM, Menezes GB. Trends in Stem Cell Proliferation and Cancer Research. Dordrecht, Netherlands: Springer; 2013. Tumor-associated neutrophils; pp. 479–501. [Google Scholar]
- 291.Oliveira DSM, Dzinic S, Bonfil AI, Saliganan AD, Sheng S, Bonfil RD. The mouse prostate: A basic anatomical and histological guideline. Bosn J Basic Med Sci. 2016;16:8–13. doi: 10.17305/bjbms.2016.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW, Krakoff J. Macrophage content in subcutaneous adipose tissue: Associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes. 2009;58:385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ortega RA, Barham W, Sharman K, Tikhomirov O, Giorgio TD, Yull FE. Manipulating the NF-κB pathway in macrophages using mannosylated, siRNA-delivering nanoparticles can induce immunostimulatory and tumor cytotoxic functions. Int J Nanomedicine. 2016;11:2163–2177. doi: 10.2147/IJN.S93483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Osman MA, Hennessy BT. Obesity correlation with metastases development and response to first-line metastatic chemotherapy in breast cancer. Clin Med Insights Oncol. 2015;9:105–112. doi: 10.4137/CMO.S32812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 296.Paich HA, Sheridan PA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens MG, Noah TL, Weir SS, Beck MA. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring) 2013;21:2377–2386. doi: 10.1002/oby.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Park J, Morley TS, Scherer PE. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol Med. 2013;5:935–948. doi: 10.1002/emmm.201202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Park J, Scherer PE. Leptin and cancer: From cancer stem cells to metastasis. Endocr Relat Cancer. 2011;18:C25–C29. doi: 10.1530/ERC-11-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest. 2012;122:4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94:5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Pasco JA, Holloway KL, Dobbins AG, Kotowicz MA, Williams LJ, Brennan SL. Body mass index and measures of body fat for defining obesity and underweight: A cross-sectional, population-based study. BMC Obesity. 2014;1:9. doi: 10.1186/2052-9538-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 304.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 306.Picon-Ruiz M, Pan C, Drews-Elger K, Jang K, Besser AH, Zhao D, Morata-Tarifa C, Kim M, Ince TA, Azzam DJ, Wander SA, Wang B, Ergonul B, Datar RH, Cote RJ, Howard GA, El-Ashry D, Torne-Poyatos P, Marchal JA, Slingerland JM. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b-mediated malignant progression. Cancer Res. 2016;76:491–504. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- 307.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137:307–314. doi: 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- 308.Poglio S, De Toni-Costes F, Arnaud E, Laharrague P, Espinosa E, Casteilla L, Cousin B. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;28:2065–2072. doi: 10.1002/stem.523. [DOI] [PubMed] [Google Scholar]
- 309.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170:85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 310.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012;144:250–268. doi: 10.1016/j.clim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 312.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A. 2017;114:1117–1122. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast tumor metastasis. Nat Aust. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Qin Y, Sundaram S, Essaid L, Chen X, Miller SM, Yan F, Darr DB, Galanko JA, Montgomery SA, Major MB, Johnson GL, Troester MA, Makowski L. Weight loss reduces basal-like breast cancer through kinome reprogramming. Cancer Cell Int. 2016;16:26. doi: 10.1186/s12935-016-0300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Quail D, Joyce J. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Raica M, Cimpean AM, Ceausu R, Ribatti D, Gaje P. Interplay between mast cells and lymphatic vessels in different molecular types of breast cancer. Anticancer Res. 2013;33:957–963. [PubMed] [Google Scholar]
- 319.Rajput AB, Turbin DA, Cheang MC, Voduc DK, Leung S, Gelmon KA, Gilks CB, Huntsman DG. Stromal mast cells in invasive breast cancer are a marker of favourable prognosis: A study of 4,444 cases. Breast Cancer Res Treat. 2008;107:249–257. doi: 10.1007/s10549-007-9546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ramos Chaves M, Boleo-Tome C, Monteiro-Grillo I, Camilo M, Ravasco P. The diversity of nutritional status in cancer: New insights. Oncologist. 2010;15:523–530. doi: 10.1634/theoncologist.2009-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rapisarda A, Melillo G. Role of the hypoxic tumor microenvironment in the resistance to anti-angiogenic therapies. Drug Resist Updat. 2009;12:74–80. doi: 10.1016/j.drup.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Razzaghi H, Troester MA, Gierach GL, Olshan AF, Yankaskas BC, Millikan RC. Mammographic density and breast cancer risk in White and African American Women. Breast Cancer Res Treat. 2012;135:571–580. doi: 10.1007/s10549-012-2185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 324.Ribatti D, Vacca A. Angiogenesis. New York, NY: Springer; 2008. Overview of angiogenesis during tumor growth; pp. 161–168. [Google Scholar]
- 325.Ribeiro AM, Andrade S, Pinho F, Monteiro JD, Costa M, Lopes C, Aguas AP, Monteiro MP. Prostate cancer cell proliferation and angiogenesis in different obese mice models. Int J Exp Pathol. 2010;91:374–386. doi: 10.1111/j.1365-2613.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ribeiro R, Monteiro C, Cunha V, Oliveira M, Freitas M, Fraga A, Príncipe P, Lobato C, Lobo F, Morais A, Silva V, Sanches-Magalhães J, Oliveira J, Pina F, Mota-Pinto A, Lopes C, Medeiros R. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J Exp Clin Cancer Res. 2012;31:32. doi: 10.1186/1756-9966-31-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ribeiro R, Monteiro C, Silvestre R, Castela A, Coutinho H, Fraga A, Principe P, Lobato C, Costa C, Cordeiro-da-Silva A, Lopes JM, Lopes C, Medeiros R. Human periprostatic white adipose tissue is rich in stromal progenitor cells and a potential source of prostate tumor stroma. Exp Biol Med (Maywood) 2012;237:1155–1162. doi: 10.1258/ebm.2012.012131. [DOI] [PubMed] [Google Scholar]
- 328.Ribeiro RJ, Monteiro CP, Cunha VF, Azevedo AS, Oliveira MJ, Monteiro R, Fraga AM, Principe P, Lobato C, Lobo F, Morais A, Silva V, Sanches-Magalhaes J, Oliveira J, Guimaraes JT, Lopes CM, Medeiros RM. Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile. Cell Physiol Biochem. 2012;29:233–240. doi: 10.1159/000337604. [DOI] [PubMed] [Google Scholar]
- 329.Rivera LB, Bergers G. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol. 2015;36:240–249. doi: 10.1016/j.it.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 332.Rohm M, Schafer M, Laurent V, Ustunel BE, Niopek K, Algire C, Hautzinger O, Sijmonsma TP, Zota A, Medrikova D, Pellegata NS, Ryden M, Kulyte A, Dahlman I, Arner P, Petrovic N, Cannon B, Amri EZ, Kemp BE, Steinberg GR, Janovska P, Kopecky J, Wolfrum C, Bluher M, Berriel Diaz M, Herzig S. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat Med. 2016;22:1120–1130. doi: 10.1038/nm.4171. [DOI] [PubMed] [Google Scholar]
- 333.Rupnick MA, Panigrahy D, Zhang C-Y, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Saad RS, Kordunsky L, Liu YL, Denning KL, Kandil HA, Silverman JF. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol. 2006;19:1317–1323. doi: 10.1038/modpathol.3800651. [DOI] [PubMed] [Google Scholar]
- 335.Sakkal S, Miller S, Apostolopoulos V, Nurgali K. Eosinophils in cancer: Favourable or unfavourable? Curr Med Chem. 2016;23:650–666. doi: 10.2174/0929867323666160119094313. [DOI] [PubMed] [Google Scholar]
- 336.Samoszuk M, Corwin MA. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int J Cancer. 2003;107:159–163. doi: 10.1002/ijc.11340. [DOI] [PubMed] [Google Scholar]
- 337.Sampey BP, Freemerman AJ, Zhang J, Kuan PF, Galanko JA, O’Connell TM, Ilkayeva OR, Muehlbauer MJ, Stevens RD, Newgard CB, Brauer HA, Troester MA, Makowski L. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PloS One. 2012;7:e38812. doi: 10.1371/journal.pone.0038812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity (Silver Spring) 2011;19:1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 340.Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol. 2011;3:a003228. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, Demory A, Falkowska-Hansen B, Kurzen H, Ugurel S, Geginat G, Arnold B, Goerdt S. Lymphatic endothelium specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 342.Schneider B. Randomized controlled trial of genomically girected therapy in patients with triple negative breast cancer. 2016 ClinicalTrials.gov identifier, NCT02101385. [Google Scholar]
- 343.Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol. 2005;23:1782–1790. doi: 10.1200/JCO.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 344.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, Mohanan S, Morris PG, Du B, Zhou XK, Vahdat LT, Verma A, Elemento O, Hudis CA, Williams RM, Gourdon D, Dannenberg AJ, Fischbach C. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 346.Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 347.Shah NR, Braverman ER. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Shao ZM, Nguyen M, Barsky SH. Human breast carcinoma desmoplasia is PDGF initiated. Oncogene. 2000;19:4337–4345. doi: 10.1038/sj.onc.1203785. [DOI] [PubMed] [Google Scholar]
- 349.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 350.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet–induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sheen-Chen SM, Liu YW, Eng HL, Chou FF. Serum levels of hepatocyte growth factor in patients with breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:715–717. doi: 10.1158/1055-9965.EPI-04-0340. [DOI] [PubMed] [Google Scholar]
- 352.Shimizu Y, Shibata R, Shintani S, Ishii M, Murohara T. Therapeutic lymphangiogenesis with implantation of adipose-derived regenerative cells. J Am Heart Assoc. 2012;1:e000877. doi: 10.1161/JAHA.112.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, Itoh H, Manabe I, Sekai M, Hamazaki Y, Fukuda K, Minato N, Sano M. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 2016;126:4626–4639. doi: 10.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, Feng J, Stewart AE, Hu-Lowe DD, Christensen JG. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70:10090–10100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 356.Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–2479. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 358.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 359.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 360.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 361.Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer. 2003;3:832–844. doi: 10.1038/nrc1212. [DOI] [PubMed] [Google Scholar]
- 362.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Spessotto P, Dri P, Bulla R, Zabucchi G, Patriarca P. Human eosinophil peroxidase enhances tumor necrosis factor and hydrogen peroxide release by human monocyte-derived macrophages. Eur J Immunol. 1995;25:1366–1373. doi: 10.1002/eji.1830250535. [DOI] [PubMed] [Google Scholar]
- 365.Stewart DA, Cooper CR, Sikes RA. Changes in extracellular matrix (ECM) and ECM-associated proteins in the metastatic progression of prostate cancer. Reprod Biol Endocrinol. 2004;2:2. doi: 10.1186/1477-7827-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Strong AL, Semon JA, Strong TA, Santoke TT, Zhang S, McFerrin HE, Gimble JM, Bunnell BA. Obesity-associated dysregulation of calpastatin and MMP-15 in adipose-derived stromal cells results in their enhanced invasion. Stem Cells. 2012;30:2774–2783. doi: 10.1002/stem.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Strong AL, Strong TA, Rhodes LV, Semon JA, Zhang X, Shi Z, Zhang S, Gimble JM, Burow ME, Bunnell BA. Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res. 2013;15:R102. doi: 10.1186/bcr3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Strulov Shachar S, Williams GR. The obesity paradox in cancer-moving beyond BMI. Cancer Epidemiol Biomarkers Prev. 2017;26:13–16. doi: 10.1158/1055-9965.EPI-16-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 370.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, Hudis CA, Dannenberg AJ. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 371.Sulpice E, Ding S, Muscatelli-Groux B, Berge M, Han ZC, Plouet J, Tobelem G, Merkulova-Rainon T. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell. 2009;101:525–539. doi: 10.1042/BC20080221. [DOI] [PubMed] [Google Scholar]
- 372.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, Goncalves Marangoni R, Nicoloro SM, Czech MP, Varga J, Ploug T, An Z, Scherer PE. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, Troester MA. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat. 2012;131:1003–1012. doi: 10.1007/s10549-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Sundaram S, Freemerman AJ, Galanko JA, McNaughton KK, Bendt KM, Darr DB, Troester MA, Makowski L. Obesity-mediated regulation of HGF/c-Met is associated with reduced basal-like breast cancer latency in parous mice. PLoS One. 2014;9:e111394. doi: 10.1371/journal.pone.0111394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Sundaram S, Freemerman AJ, Johnson AR, Milner JJ, McNaughton KK, Galanko JA, Bendt KM, Darr DB, Perou CM, Troester MA, Makowski L. Role of HGF in obesity-associated tumorigenesis: C3(1)-TAg mice as a model for human basal-like breast cancer. Breast Cancer Res Treat. 2013;142:489–503. doi: 10.1007/s10549-013-2741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sundaram S, Le TL, Essaid L, Freemerman AJ, Huang MJ, Galanko JA, McNaughton KK, Bendt KM, Darr DB, Troester MA, Makowski L. Weight loss reversed obesity-induced HGF/c-Met pathway and basal-like breast cancer progression. Front Oncol. 2014;4:175. doi: 10.3389/fonc.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sung MT, Eble JN, Cheng L. Invasion of fat justifies assignment of stage pT3a in prostatic adenocarcinoma. Pathology. 2006;38:309–311. doi: 10.1080/00313020600820914. [DOI] [PubMed] [Google Scholar]
- 379.Swierczynski J, Korczynska J, Goyke E, Adrych K, Raczynska S, Sledzinski Z. Serum hepatocyte growth factor concentration in obese women decreases after vertical banded gastroplasty. Obes Surg. 2005;15:803–808. doi: 10.1381/0960892054222678. [DOI] [PubMed] [Google Scholar]
- 380.Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer. 2015;15:473–483. doi: 10.1038/nrc3971. [DOI] [PubMed] [Google Scholar]
- 381.Tahergorabi Z, Khazaei M. The relationship between inflammatory markers, angiogenesis, and obesity. ARYA Atheroscler. 2013;9:247. [PMC free article] [PubMed] [Google Scholar]
- 382.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Takeda K, Sowa Y, Nishino K, Itoh K, Fushiki S. Adipose-derived stem cells promote proliferation, migration, and tube formation of lymphatic endothelial cells in vitro by secreting lymphangiogenic factors. Ann Plast Surg. 2015;74:728–736. doi: 10.1097/SAP.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 384.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in high fat diet fed mice via secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Tam J, Duda DG, Perentes JY, Quadri RS, Fukumura D, Jain RK. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: Role of local versus bone marrow-derived endothelial cells. PLoS One. 2009;4:e4974. doi: 10.1371/journal.pone.0004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Tang KD, Liu J, Jovanovic L, An J, Hill MM, Vela I, Lee TK, Ma S, Nelson C, Russell PJ, Clements JA, Ling MT. Adipocytes promote prostate cancer stem cell self-renewal through amplification of the cholecystokinin autocrine loop. Oncotarget. 2016;7:4939–4948. doi: 10.18632/oncotarget.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 388.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-κB in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 390.Theoharides TC, Conti P. Mast cells: The Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 391.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 392.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 393.Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiology (Bethesda) 2005;20:340–348. doi: 10.1152/physiol.00019.2005. [DOI] [PubMed] [Google Scholar]
- 394.Tisdale MJ. Cancer cachexia. Curr Opin Gastroenterol. 2010;26:146–151. doi: 10.1097/MOG.0b013e3283347e77. [DOI] [PubMed] [Google Scholar]
- 395.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PDL1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Toren P, Venkateswaran V. Periprostatic adipose tissue and prostate cancer progression: New insights into the tumor microenvironment. Clin Genitourin Cancer. 2014;12:21–26. doi: 10.1016/j.clgc.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 397.Travers RL, Motta AC, Betts JA, Bouloumié A, Thompson D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int J Obes (Lond) 2015;39:762–769. doi: 10.1038/ijo.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 399.Trojan L, Michel MS, Rensch F, Jackson DG, Alken P, Grobholz R. Lymph and blood vessel architecture in benign and malignant prostatic tissue: Lack of lymphangiogenesis in prostate carcinoma assessed with novel lymphatic marker lymphatic vessel endothelial hyaluronan receptor (LYVE-1) J Urol. 2004;172:103–107. doi: 10.1097/01.ju.0000128860.00639.9c. [DOI] [PubMed] [Google Scholar]
- 400.Trottier MD, Naaz A, Li Y, Fraker PJ. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci U S A. 2012;109:7622–7629. doi: 10.1073/pnas.1205129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Trujillo KA, Heaphy CM, Mai M, Vargas KM, Jones AC, Vo P, Butler KS, Joste NE, Bisoffi M, Griffith JK. Markers of fibrosis and epithelial to mesenchymal transition demonstrate field cancerization in histologically normal tissue adjacent to breast tumors. Int J Cancer. 2011;129:1310–1321. doi: 10.1002/ijc.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 403.Tsekouras A, Mantas D, Tsilimigras DI, Ntanasis-Stathopoulos I, Kontos M, Zografos GC. Adipose-derived stem cells for breast reconstruction after breast surgery—preliminary results. Case Reports Plast Surg Hand Surg. 2017;4:35–41. doi: 10.1080/23320885.2017.1316201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Tuck AB, Park M, Sterns EE, Boag A, Elliott BE. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol. 1996;148:225–232. [PMC free article] [PubMed] [Google Scholar]
- 405.Turkoz FP, Solak M, Petekkaya I, Keskin O, Kertmen N, Sarici F, Arik Z, Babacan T, Ozisik Y, Altundag K. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON. 2013;18:335–341. [PubMed] [Google Scholar]
- 406.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: Induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 407.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 408.Ursin G, Hovanessian-Larsen L, Parisky YR, Pike MC, Wu AH. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res. 2005;7:R605–R608. doi: 10.1186/bcr1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: International evaluation of the evidence. Eur J Cancer Prev. 2002;11(Suppl 2):S94–S100. [PubMed] [Google Scholar]
- 410.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Valor L, Teijeiro R, Aristimuno C, Faure F, Alonso B, de Andres C, Tejera M, Lopez-Lazareno N, Fernandez-Cruz E, Sanchez-Ramon S. Estradiol-dependent perforin expression by human regulatory T-cells. Eur J Clin Invest. 2011;41:357–364. doi: 10.1111/j.1365-2362.2010.02414.x. [DOI] [PubMed] [Google Scholar]
- 412.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 413.van Roermund JG, Hinnen KA, Tolman CJ, Bol GH, Witjes JA, Bosch JL, Kiemeney LA, van Vulpen M. Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU Int. 2011;107:1775–1779. doi: 10.1111/j.1464-410X.2010.09811.x. [DOI] [PubMed] [Google Scholar]
- 414.van Roermund JGH, Bol GH, Alfred Witjes J, Ruud Bosch JLH, Kiemeney LA, van Vulpen M. Periprostatic fat measured on computed tomography as a marker for prostate cancer aggressiveness. World J Urol. 2010;28:699–704. doi: 10.1007/s00345-009-0497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.van Spriel AB, Leusen JHW, van Egmond M, Dijkman HBPM, Assmann KJM, Mayadas TN, van deWinkel JGJ. Mac-1 (CD11b/CD18) is essential for Fc receptor–mediated neutrophil cytotoxicity and immunologic synapse formation. Blood. 2001;97:2478–2486. doi: 10.1182/blood.v97.8.2478. [DOI] [PubMed] [Google Scholar]
- 416.Vandeweyer E, Hertens D. Quantification of glands and fat in breast tissue: An experimental determination. Ann Anat. 2002;184:181–184. doi: 10.1016/S0940-9602(02)80016-4. [DOI] [PubMed] [Google Scholar]
- 417.Venkatasubramanian PN, Brendler CB, Plunkett BA, Crawford SE, Fitchev PS, Morgan G, Cornwell ML, McGuire MS, Wyrwicz AM, Doll JA. Periprostatic adipose tissue from obese prostate cancer patients promotes tumor and endothelial cell proliferation: a functional and MR imaging pilot study. Prostate. 2014;74:326–335. doi: 10.1002/pros.22756. [DOI] [PubMed] [Google Scholar]
- 418.Vleugel MM, Bos R, van der Groep P, Greijer AE, Shvarts A, Stel HV, van derWall E, van Diest PJ. Lack of lymphangiogenesis during breast carcinogenesis. J Clin Pathol. 2004;57:746–751. doi: 10.1136/jcp.2003.014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Vogel WF. Collagen-receptor signaling in health and disease. Eur J Dermatol. 2001;11:506–514. [PubMed] [Google Scholar]
- 420.von Drygalski A, Tran TB, Messer K, Pu M, Corringham S, Nelson C, Ball ED. Obesity is an independent predictor of poor survival in metastatic breast cancer: Retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. Int J Breast Cancer. 2011;2011:523276. doi: 10.4061/2011/523276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 422.Wagner M, Bjerkvig R, Wiig H, Melero-Martin JM, Lin RZ, Klagsbrun M, Dudley AC. Inflamed tumor-associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis. Angiogenesis. 2012;15:481–495. doi: 10.1007/s10456-012-9276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Wagner M, Dudley AC. A three-party alliance in solid tumors: Adipocytes, macrophages and vascular endothelial cells. Adipocyte. 2013;2:67–73. doi: 10.4161/adip.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, Menon M, Montorsi F, Myers RP, Rocco B, Villers A. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57:179–192. doi: 10.1016/j.eururo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 425.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Wang YY, Attane C, Milhas D, Dirat B, Dauvillier S, Guerard A, Gilhodes J, Lazar I, Alet N, Laurent V, Le Gonidec S, Biard D, Herve C, Bost F, Ren GS, Bono F, Escourrou G, Prentki M, Nieto L, Valet P, Muller C. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2:e87489. doi: 10.1172/jci.insight.87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Wang YY, Lehuede C, Laurent V, Dirat B, Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P, Muller C. Adipose tissue and breast epithelial cells: A dangerous dynamic duo in breast cancer. Cancer Lett. 2012;324:142–151. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 428.Ward BR, Arslanian SA, Andreatta E, Schwartz LB. Obesity is not linked with increased whole-body mast cell burden in children. J Allergy Clin Immunol. 2012;129:1164–1166.e1164. doi: 10.1016/j.jaci.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D, Mehrara BJ. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. 2013;8:e70703. doi: 10.1371/journal.pone.0070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Weng N. Aging of the immune system: How much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Werno C, Menrad H, Weigert A, Dehne N, Goerdt S, Schledzewski K, Kzhyshkowska J, Brune B. Knockout of HIF-1alpha in tumor-associated macrophages enhances M2 polarization and attenuates their pro-angiogenic responses. Carcinogenesis. 2010;31:1863–1872. doi: 10.1093/carcin/bgq088. [DOI] [PubMed] [Google Scholar]
- 435.Whiteside TL. The role of immune cells in the tumor microenvironment. Cancer Treat Res. 2006;130:103–124. doi: 10.1007/0-387-26283-0_5. [DOI] [PubMed] [Google Scholar]
- 436.Widschwendter P, Friedl TW, Schwentner L, DeGregorio N, Jaeger B, Schramm A, Bekes I, Deniz M, Lato K, Weissenbacher T, Kost B, Andergassen U, Jueckstock J, Neugebauer J, Trapp E, Fasching PA, Beckmann MW, Schneeweiss A, Schrader I, Rack B, Janni W, Scholz C. The influence of obesity on survival in early, high-risk breast cancer: Results from the randomized SUCCESS A trial. Breast Cancer Res. 2015;17:129. doi: 10.1186/s13058-015-0639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010;127:748–758. doi: 10.1002/ijc.25464. [DOI] [PubMed] [Google Scholar]
- 438.Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: Unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2 doi: 10.1038/npjbcancer.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Williams CS, Leek RD, Robson AM, Banerji S, Prevo R, Harris AL, Jackson DG. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J Pathol. 2003;200:195–206. doi: 10.1002/path.1343. [DOI] [PubMed] [Google Scholar]
- 440.Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789–9798. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- 441.Woo S, Cho JY, Kim SY, Kim SH. Periprostatic fat thickness on MRI: Correlation with Gleason score in prostate cancer. AJR Am J Roentgenol. 2015;204:W43–W47. doi: 10.2214/AJR.14.12689. [DOI] [PubMed] [Google Scholar]
- 442.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford, England) 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 444.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Wynn TA, Barron L. Macrophages: Master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286:23591–23599. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Xiang M, Gu Y, Zhao F, Lu H, Chen S, Yin L. Mast cell tryptase promotes breast cancer migration and invasion. Oncol Rep. 2010;23:615–619. doi: 10.3892/or_00000676. [DOI] [PubMed] [Google Scholar]
- 449.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Yaffe MJ. Mammographic density. Measurement of mammographic density. Breast Cancer Res. 2008;10:209. doi: 10.1186/bcr2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Yaghjyan L, Colditz GA, Collins LC, Schnitt SJ, Rosner B, Vachon C, Tamimi RM. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J Natl Cancer Inst. 2011;103:1179–1189. doi: 10.1093/jnci/djr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Yamaguchi J, Ohtani H, Nakamura K, Shimokawa I, Kanematsu T. Prognostic impact of marginal adipose tissue invasion in ductal carcinoma of the breast. Am J Clin Pathol. 2008;130:382–388. doi: 10.1309/MX6KKA1UNJ1YG8VN. [DOI] [PubMed] [Google Scholar]
- 455.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: Implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Yang S, Zhang Q, Liu S, Wang AR, You Z. PD-1, PD-L1 and PD-L2 expression in mouse prostate cancer. Am J Clin Exp Urol. 2016;4:1–8. [PMC free article] [PubMed] [Google Scholar]
- 458.Yeh HC, Platz EA, Wang NY, Visvanathan K, Helzlsouer KJ, Brancati FL. A prospective study of the associations between treated diabetes and cancer outcomes. Diabetes Care. 2012;35:113–118. doi: 10.2337/dc11-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Yoshida S, Hamuy R, Hamada Y, Yoshimoto H, Hirano A, Akita S. Adipose-derived stem cell transplantation for therapeutic lymphangiogenesis in a mouse secondary lymphedema model. Regen Med. 2015;10:549–562. doi: 10.2217/rme.15.24. [DOI] [PubMed] [Google Scholar]
- 460.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive proinflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 461.Zeyda M, Huber J, Prager G, Stulnig TM. Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity (Silver Spring) 2011;19:743–748. doi: 10.1038/oby.2010.123. [DOI] [PubMed] [Google Scholar]
- 462.Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-Ning-Tapia EM, Troncoso P, Davis J, Pettaway C, Ward J, Frazier ML, Logothetis C, Kolonin MG. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat Commun. 2016;7:11674. doi: 10.1038/ncomms11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Zhang X, Zhou G, Sun B, Zhao G, Liu D, Sun J, Liu C, Guo H. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncol Lett. 2015;9:1307–1312. doi: 10.3892/ol.2014.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–5208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]
- 465.Zhou Y, Yu X, Chen H, Sjoberg S, Roux J, Zhang L, Ivoulsou AH, Bensaid F, Liu CL, Liu J, Tordjman J, Clement K, Lee CH, Hotamisligil GS, Libby P, Shi GP. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 2015;22:1045–1058. doi: 10.1016/j.cmet.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 467.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One. 2009;4:e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]