Abstract
The prevalence of health problems in the offspring of pregnant diabetic mothers has recently been verified. Therefore, the present study was designed to investigate the influence of dietary camel whey protein (CWP), administered as a supplement to streptozotocin (STZ)-induced diabetic pregnant mice, on the efficiency of the immune system of the offspring. Three groups of female mice (n = 10) were used: non-diabetic control mice, diabetic mice, and diabetic mice orally administered CWP during the pregnancy and lactation periods. We then tested the immune response of B and T cells in adult male offspring (n = 15 in each group) by using flow cytometry, western blotting, and ELISAs. Our data demonstrated that the offspring of diabetic dams exhibited several postpartum complications, such as significant aberrant overexpression of activating transcription factor-3 (ATF-3), significant elevation of the plasma levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and reactive oxygen species (ROS), marked decreases in the plasma levels of IL-2 and IL-7, significant inhibition of CCL21- and CXCL12-mediated chemotaxis of B- and T-lymphocytes, and a marked decrease in the proliferative capacity of antigen-stimulated B- and T-lymphocytes. Interestingly, administration of CWP to diabetic dams substantially restored the expression of ATF-3 and the levels of ROS, pro-inflammatory cytokines, IL-2, and IL-7 in the offspring. Furthermore, the chemotaxis of B- and T-lymphocytes toward CCL21 and CXCL12 and the proliferative capacities of these lymphocytes were restored in the male offspring of diabetic mice administered CWP. Our data provide evidence of a protective role of CWP in decreasing the tendency of the offspring of diabetic mothers to develop diabetes and related complications.
Keywords: antioxidant, camel whey protein, diabetes mellitus, free radicals, lymphocytes
Introduction
Diabetes mellitus (DM) is responsible for up to 7% of pregnancy complications; in maternity, it is the most frequent metabolic complication that elevates neonatal morbidity.1 The severity and time of onset of DM determine the consequences of maternal hyperglycemia on fetal development. Moreover, the pancreatic insulin output of the fetus is determined solely by the glucose levels in the maternal blood because insulin from the mother does not cross the placenta.2 The increase in fetal insulin output is motivated by high maternal serum glucose, thus resulting in elevated rates of macrosomia,3 which is a common complication associated with gestational diabetes mellitus (GDM). In addition, chronic fetal hypoxia in maternal DM may increase the inflammatory burden experienced by the fetus.4 Furthermore, interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) are expressed at higher levels in infants subjected to asphyxia and hypoxic ischemic encephalopathy associated with neuronal damage after such perinatal insults.5 TNF-α crosses the placenta and is elevated in maternal tissues,6 especially the uteri, of pregnant diabetic mice. The excessive elevation of cytokine release at the maternofetal interface results in the dysregulation of organogenesis and termination of the pregnancy. Inflammatory cytokines have been reported to affect neuronal development as well as the metabolism of neurotransmitters, owing to the increase in the expression levels of pro-inflammatory cytokines after infection.7
The pathogenesis of GDM, type 2 DM, involves oxidative stress8 and may contribute to the increased risk of schizophrenia observed in the offspring of diabetic mothers. Activating transcription factor-3 (ATF-3) is a stress-inducible gene, and its expression is induced in various tissues by different stress signals that are correlated with cellular damage, including β-cell apoptosis and several diabetic complications.9 Hyperglycemia causes the depletion of antioxidants and the generation of reactive oxygen species (ROS).10 Many studies have revealed the presence of increased oxidative stress in females with GDM. Cord blood samples taken from these mothers’ infants have indicated that this milieu is also shared with the fetus.11 Antioxidants play a critical role in sustaining the human body’s immunity to and protection from free radical damage. Free radical scavengers have been reported to decrease significantly in diabetic patients. In animal models, oxygen-free radicals also play an essential role in the progression of neuronal development, differentiation12 and synaptic plasticity. Changes in the balance of these signals cause variations in vital neuro-developmental processes. Additionally, the brain is particularly susceptible to oxidative damage, owing to its high oxygen consumption and poor antioxidant defenses.13
Camel whey protein (CWP) has been reported to modulate various immune functions, including lymphocyte activation and proliferation, cytokine secretion, and natural killer (NK) cell activity.14 Furthermore, whey peptides exhibit immunomodulatory activities, such as stimulating lymphocytes and increasing phagocytosis and the secretion of immunoglobulin A (IgA) from Peyer’s patches.15 It is also plausible that WP represents an effective cysteine delivery system for glutathione (GSH) replenishment in immunodeficiency diseases.16 Additionally, the antioxidant action of CWP prevents the manifestations of metabolic syndrome, including hyperglycemia, hyperlipidemia, and insulin resistance, which, in turn, inhibit the complications of DM.17 The current study sought to perform the first evaluation of the protective effects of CWP on the immune functions of lymphocytes in offspring of diabetic mothers as well as its beneficial effect in preventing these offspring from developing diabetes.
Materials and methods
Chemicals
Streptozotocin (STZ) was obtained from Sigma-Aldrich, St. Louis, MO, USA. STZ was dissolved in cold 0.01 M citrate buffer, pH 4.5, and was always prepared freshly and used within 5 min. CWP was prepared as previously described.18
Animals and experimental design
BALB/c mice weighing 25-30 g were purchased from the Theodor Bilharz Research Institute, Cairo, Egypt. All animal procedures were performed in accordance with the Declaration of Helsinki and the guidelines for the care and use of experimental animals established by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the National Institutes of Health (NIH) protocol. The animals were allowed to acclimate for two weeks before the commencement of the study. They were kept under standard laboratory conditions (25°C, 60–70% relative humidity and a 12-h light/dark cycle), housed in metal cages in a well-ventilated room, and fed a standard commercial chow diet and water. Thirty female mice were fasted for 20 h before the induction of diabetes by STZ. Female mice were rendered diabetic by five consecutive daily i.p. injections of STZ (60 mg/kg body weight) in 0.01 M citrate buffer (pH 4.5) starting two weeks before mating. Female mice were considered to serve as animal models of chronic diabetes if their blood glucose levels exceeded 250 mg/dl.19 Ten female non-diabetic control mice were injected with 0.01 M citrate buffer, pH 4.5. All female mice, including diabetic and non-diabetic mice, were mated with healthy male mice. Female diabetic mice were housed for two weeks before mating and CWP administration. After mating, the presence of spermatozoids in the vaginal smears indicated the first day of gestation. Pregnant mice were housed individually under the above-described conditions. To assess hyperglycemia during the gestation period, blood glucose levels were measured in blood samples obtained weekly after overnight fasting, by cutting off the tip of the tail of each mouse and squeezing it gently. Samples were collected starting from the day of STZ injection until two weeks after parturition by using a One Touch Ultra blood glucose meter (LifeScan, Paris, France). The animals were then assigned to three experimental groups (10 mice per group): Group 1: non-diabetic control dams administered distilled water (250 µL/mouse/day for one month through oral gavage); Group 2: diabetic mice administered distilled water (250 µL/mouse/day for one month through oral gavage); Group 3: diabetic mice administered non-denatured WP (100 mg/kg body weight dissolved in 250 µL/day for one month through oral gavage). The WP dose was established on the basis of the LD50.18
Blood samples
At the end of the experiment, after overnight fasting, the offspring at three months of age in each group were anesthetized with pentobarbital (60 mg/kg body weight). The abdominal cavity of each mouse was opened, and all of the blood was collected via the abdominal aorta. Serum was obtained by low-speed centrifugation (1000 × g for 20 min) and was immediately stored at −80°C for later cytokine profile analysis.
Determination of the serum triglyceride, ALT, AST, and creatinine levels
Serum triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine were measured with commercial kits (Labtest Diagnostica, Brazil) according to the manufacturer’s instructions.
Insulin level measurement
Plasma insulin levels were determined with commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, USA) according to the manufacturer’s instructions. The insulin concentration was then calculated using a standard insulin curve.
Western blot analysis
Skin and wound tissue biopsies were homogenized in lysis buffer (1% Triton X-100, 137mM NaCl, 10% glycerol, 1mM dithiothreitol, 10mM NaF, 2mM Na3VaO4, 5mM ethylenediaminetetraacetic acid, 1mM phenylmethylsulfonyl fluoride, 5ng/mL aprotinin, 5ng/mL leupeptin and 20mM Tris/HCl, pH 8.0), and the lysates were prepared as previously described.23 Fifty micrograms of total protein from the skin lysates was analyzed using SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis. Antibodies (Abs) directed against ATF-3 (1:500) and actin (1:4000) (Cell Signaling Technology, Paris, France) were used in combination with horseradish peroxidase-conjugated secondary Abs, and the proteins were visualized using an enhanced chemiluminescence (ECL, SuperSignal West Pico chemiluminescent substrate; Perbio, Bezons, France) detection system. The ECL signal was detected on Hyperfilm ECL. To quantify the band intensities, the films were scanned, saved as TIFF files, and analyzed using Image J.
Determination of ROS and plasma cytokines
ROS and cytokines were measured in samples that were stored at −80°C. Plasma cytokine (IL-1beta, IL-2, IL-6, IL-7, and TNF-α) levels were determined by ELISA using the mouse Bio-Plex cytokine assay kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions.20
In vitro chemotaxis assays
The chemokine-dependent migration of splenic lymphocytes was measured with an in vitro two-chamber migration assay (using Transwell plates that were purchased from Costar, Cambridge, MA, USA), and this was followed by flow cytometry analysis as previously described.21 All chemotaxis assays were performed in pre-warmed migration buffer (RPMI 1640 containing 1% FCS). A total of 600 µL of migration buffer alone or buffer supplemented with CCL21 (at 250 ng/mL; R&D Systems) or with CXCL12 (at 200 ng/mL; R&D Systems) was added to the lower chamber, and 105 cells in migration buffer were added to the upper chamber. The plates were then incubated for 3 h at 37°C; the input and transmigrated cells were centrifuged and then stained with PE-conjugated anti-CD3 or anti-CD19 mAbs for 30 min. The cells were then rinsed, fixed in 300 µL of 1× phosphate-buffered saline (PBS) + 1% formaldehyde and counted for 60 s via flow cytometry. The migration percentage was calculated as the percentage of input CD3+ T and CD19+ B cells that migrated to the lower chamber. For the calculation of the percentage of CCL21- and CXCL12-mediated migration, the percentage of CD3+ T and CD19+ B cell migration to the medium alone was subtracted from the percentage of T and B cell migration to the medium with chemokine.
CFSE proliferation assays
Peripheral blood mononuclear cells (PBMCs) were isolated from blood by using the Ficoll gradient method. Next, PBMCs were suspended at 20 × 106/mL in 1× PBS and stained with 0.63 mM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) for 8 min at room temperature. The reaction was stopped with FBS; the cells were rinsed three times in PBS and then resuspended at 2 × 106 cells/mL in prewarmed R-10 medium. CFSE-labeled cells were stimulated for six days with or without pokeweed mitogen (PWA) (final concentration, 20 ng/mL) at 37°C and 5% CO2. On day 6, the cells were stained with the surface antigens anti-CD3-PE and CD19-APC.
Statistical analysis
The data were analyzed for normality with the Anderson-Darling test and for homogeneity variances before further statistical analysis. The data are expressed as the mean ± standard error of the mean (SEM). Significant differences among the groups were analyzed with one- or two-way ANOVAs followed by Bonferroni’s test for multiple comparisons with PRISM statistical software (GraphPad Software). The data were also reanalyzed with one- or two-way ANOVAs followed by Tukey’s post-test with SPSS software, version 17. Differences were considered to be significant at P <0.05: *P <0.05 for diabetic versus control; #P <0.05 for diabetic + CWP versus non-diabetic control; +P <0.05 for diabetic + CWP versus diabetic.
Results
Gestational diabetes and the effects of CWP supplementation in diabetic mothers on maternofetal parameters and neonatal outcome
STZ-mediated induction of diabetes in female mice before mating caused marked hyperglycemia that was still detectable during the pregnancy and lactation periods (diabetic mothers: 372±14.1 mg/dl versus diabetic dams fed with CWP: 252±9.4 mg/dl, n = 10, +P <0.05). CWP supplementation in diabetic mothers significantly restored the levels of blood glucose and insulin, as compared with levels in control non-diabetic mothers (Table 1). The pregnancy success rate among the mothers was clearly decreased by diabetes induction (diabetic mothers: 60% versus 100% in control mothers and 90% in CWP-treated diabetic mothers). Diabetes induction prior to mating correlated with a decreased number of delivered neonates (41 pups born to diabetic dams versus 77 pups born to control dams). CWP supplementation during the pregnancy and lactation periods in diabetic mothers had a pronounced effect on the total number of delivered neonates (67 pups versus 41 pups to diabetic mothers without CWP supplementation). Additionally, there was an increase in the number of living pups at three months of age belonging to diabetic CWP-supplemented mothers compared with diabetic mothers (62 pups [34 male and 28 female] versus 32 pups [17 male and 15 female], respectively) (Table 1).
Table 1.
Blood glucose and insulin levels and the pregnancy outcomes of control and diabetic mothers.
The blood glucose levels were measured in the three groups of mice throughout the experimental period. The pooled data for ten mothers from each group are expressed as the mean value for each parameter ± SEM. * P <0.05 for diabetic vs. control; #P <0.05 for diabetic + CWP vs. control; +P <0.05 for diabetic + CWP vs. diabetic (ANOVA followed by Tukey’s post-test).
| Control mothers | Diabetic mothers | Diabetic mothers administered CWP | |||
|---|---|---|---|---|---|
| Parameters | |||||
| Blood glucose levels (mg/dl) | Before pregnancy | 3 days after vehicle or STZ injection |
167 ± 6.5 | 253 ± 9.4* | 248 ± 8.3†‡ |
| During pregnancy | 1st week | 148 ± 7.4 | 273 ± 11* | 249 ± 7.1†‡ | |
| 2nd week | 159 ± 9.1 | 297 ± 7.45* | 257 ± 6.6†‡ | ||
| 3rd week | 189 ± 10.3 | 343 ± 9.9* | 269 ± 8.4†‡ | ||
| After pregnancy | 7th day postpartum | 161 ± 8.8 | 372 ± 14.1* | 252 ± 9.4†‡ | |
| Blood insulin (ng/mL) | 3rd week after pregnancy | 13 ± 1.2 | 5.3 ± 0.7* | 8.68 ± 0.94†‡ | |
| Blood insulin (ng/mL) | 7th day postpartum | 12.7 ± 1.34 | 5.7 ± 0.64* | 10.8 ± 1.04‡ | |
| Total pregnancies | 10 | 10 | 10 | ||
| Successful pregnancies | 10 | 6 | 9 | ||
| Abortion (%) | 0 | 40 | 10 | ||
| Total deliverable neonates | 77 | 41 | 67 | ||
| Live pups | 74 | 32 | 62 | ||
| Dead pups | 3 | 9 | 5 | ||
| Mortality (%) | 3.9 | 21.95* | 7.46†‡ | ||
| Alive male offspring at 3 months of age | 44 | 17 | 34 | ||
| Alive female offspring at 3 months of age | 30 | 15 | 28 | ||
P <0.05 for diabetic vs. control.
P <0.05 for diabetic + CWP vs. control.
P <0.05 for diabetic + CWP vs. diabetic.
As shown in Table 2, pups born to diabetic mothers typically had higher body weights at birth, indicating macrosomic neonates (2.9 ± 0.36 g), than did neonates born to diabetic dams administered CWP (1.29 ± 0.11 g) or those born to control mothers (1.23 ± 0.1 g). At three months of age, pups born to diabetic mothers maintained significant elevation in body weight compared with pups born to diabetic dams administered CWP or those born to control dams. The blood biochemical parameters and B- and T-lymphocyte chemotaxis and proliferation in 17 adult (at the age of three months) male offspring from each group were then monitored. At this age, offspring of diabetic mothers exhibited significant (*P <0.05) elevation in the levels of blood glucose accompanied by a significant (*P <0.05) decrease in insulin levels and blood lymphocyte counts, as compared with offspring of control mothers. Interestingly, CWP supplementation in diabetic mothers during the pregnancy and lactation periods restored the glucose levels, insulin levels, and the number of blood lymphocytes in the offspring. Nevertheless, there were no significant changes in the ALT, AST, and creatinine levels among the offspring of control, diabetic, and CWP-treated diabetic mothers.
Table 2.
Effects of gestational diabetes and CWP supplementation on the body weight and biochemical parameters of adult male offspring. The body weight at birth and at three months of age, blood parameters, and liver and kidney functions were measured in the offspring of the three groups of mice. The pooled data for 15 adult male offspring from each group are expressed as the mean value for each parameter ± SEM. * P < 0.05 for diabetic versus control; #P < 0.05 for diabetic + CWP versus control; +P < 0.05 for diabetic + CWP versus diabetic (ANOVA followed by Tukey’s post-test).
| Biochemical parameters | Offspring of control mothers | Offspring of diabetic mothers | Offspring of diabetic mothers administered CWP |
|---|---|---|---|
| Body weight (g) at birth | 1.23 ± 0.1 | 2.9 ± 0.36* | 1.29 ± 0.11† |
| At 3 months of age | |||
| Body weight (g) | 22 ± 2.6 | 27.4 ± 2.8* | 23.6 ± 2.9 |
| Blood glucose (mg/dl) | 153 ± 14.4 | 221 ± 21* | 189 ± 18†‡ |
| Insulin (ng/mL) | 11 ± 1.4 | 6.9 ± 1.1* | 9.98 ± 0.86† |
| WBC count (103/μL) | 14.2 ± 1.8 | 7.4 ± 0.8* | 11.8 ± 2.6† |
| Lymphocytes (%) | 75 ± 9.2 | 52 ± 6.2* | 71 ± 8.3†‡ |
| Monocytes (%) | 5 ± 0.6 | 4 ± 0.4 | 4 ± 0.7 |
| Neutrophils (%) | 19 ± 1.2 | 18 ± 0.88 | 18 ± 1.1 |
| Creatinine level (dg/mL) | 2.75 ± 0.3 | 2.8 ± 0.28 | 2.78 ± 0.39 |
| ALT activity (U/mL) | 50 ± 6.7 | 51 ± 6.1 | 49 ± 5.8 |
| AST activity (U/mL) | 47 ± 5.8 | 49 ± 6.5 | 45 ± 5.7 |
n = 15 male offspring/group.
P <0.05 for diabetic vs. control.
P <0.05 for diabetic + CWP vs. diabetic (ANOVA followed by Tukey’s post-test).
P <0.05 for diabetic + CWP vs. control.
Dietary CWP supplementation in diabetic mothers during pregnancy and lactation attenuates the expression of ATF-3 and reverses stress responses in adult male offspring
Oxidative stress is induced by the pro-inflammatory response, which activates transcription factors such as ATF-3. This reaction might be responsible for the prolonged impairment in the immune response. As a result, the expression of ATF-3 was analyzed in PBMCs isolated from the offspring of the three experimental mouse groups. A representative immunoblot illustrating one of the 15 experiments is shown (Figure 1a). Immunoblots for ATF-3 and total actin (loading control) levels in the PBMCs isolated from offspring of control non-diabetic mothers, offspring of diabetic mothers, and offspring of CWP-administered diabetic mice are also illustrated. The results showed that offspring of diabetic mothers exhibited a marked elevation in the expression of ATF-3 compared with offspring of control non-diabetic mothers. Additionally, the expression of ATF-3 was clearly decreased in offspring of CWP-treated diabetic mothers, because the immunoblots showed a very faint band compared with that observed for the offspring of diabetic mothers. The results of 15 individual male mice per group are shown for the expression of ATF-3 normalized against the total actin levels. The results represented in Figure 1b demonstrate that offspring of diabetic mothers, compared with the offspring of control and CWP-administered diabetic mothers, exhibited aberrant overexpression of ATF-3. When diabetic mothers were administered CWP during the pregnancy and lactation periods, their offspring showed significant amelioration in ATF-3 expression, which reached nearly normal expression levels.
Figure 1.
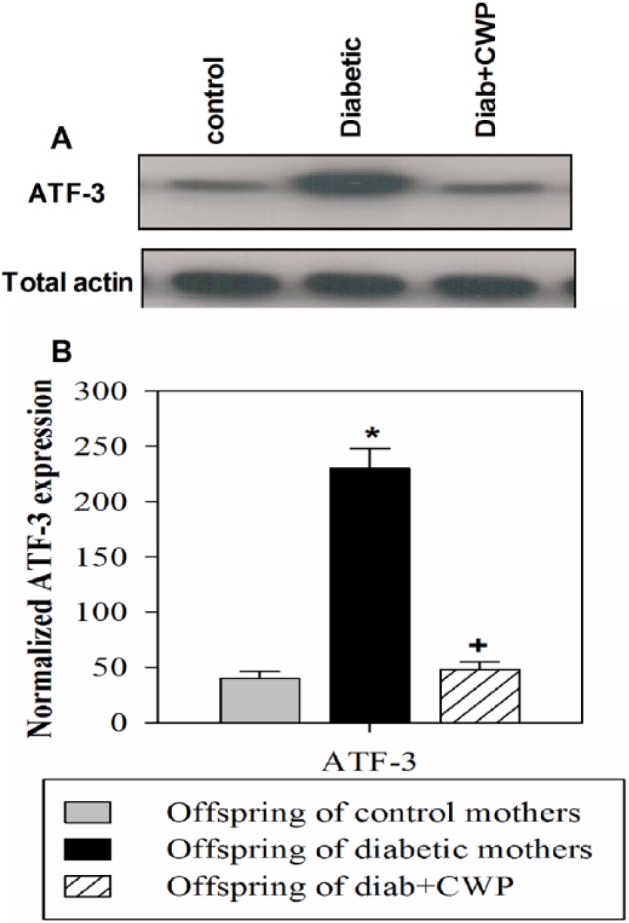
Influence of gestational diabetes and CWP supplementation on the expression of ATF-3 in adult male offspring. Western blot analysis was performed, and immunoblots for ATF-3 and total actin from one representative experiment are shown (a) for an offspring of a control non-diabetic mother, an offspring of a diabetic mother, and an offspring of a control CWP-treated diabetic mother. The normalized data from independent experiments (n = 15) are shown for ATF-3 expression (b) in the PBMCs of offspring of a non-diabetic mother (open gray bar), offspring of a diabetic mother (closed black bar), and offspring of a CWP-treated diabetic mother (hatched bar). The values represent the mean ± SEM. *P <0.05 for diabetic vs. control; #P <0.05 for diabetic + CWP vs. control; +P <0.05 for diabetic + CWP vs. diabetic (ANOVA followed by Tukey’s post-test).
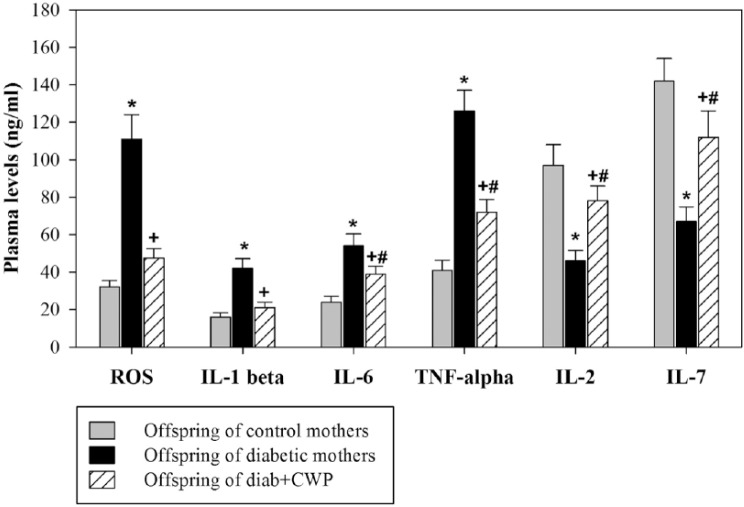
CWP supplementation protects blood lymphocytes from pro-inflammatory cytokines and free radicals and restores IL-2 and IL-7 levels in offspring
We investigated whether the decreased lymphocyte numbers in the offspring of diabetic mothers were due to the exhausted/stunned status of the lymphocytes that affected the survival and maintenance of peripheral lymphocytes. Levels of ROS (IL-1β, IL-6, TNF-α), IL-2, and IL-7 were determined in freshly isolated blood. The levels of ROS were markedly elevated in the blood of offspring belonging to diabetic mothers compared with those of control mothers (Figure 2). Nevertheless, the ROS levels were significantly restored in the blood of offspring reared by diabetic mothers administered CWP (n = 15). Similarly, pro-inflammatory cytokine plasma levels were significantly increased in the offspring of diabetic dams compared with those in CWP-treated diabetic dams or those in control mothers. Additionally, IL-2 and IL-7 plasma levels were significantly decreased in the offspring of diabetic mothers compared with those of control mothers. Interestingly, in diabetic mothers administered CWP during pregnancy and lactation, compared with diabetic mothers, their offspring exhibited a pronounced restoration of the levels of ROS, pro-inflammatory cytokines, and IL-2 and IL-7.
Figure 2.
CWP decreases the plasma levels of ROS and pro-inflammatory cytokines and restores the levels of IL-2 and IL-7 in adult male offspring. The plasma cytokine profile and ROS levels were measured in freshly isolated blood from offspring (aged three months) of control (gray bars), diabetic (black bars) and diabetic mothers administered CWP (hatched bars), and the data are expressed as the mean ± SEM. *P <0.05 for diabetic vs. control; #P <0.05 for diabetic + CWP vs. control; +P <0.05 for diabetic + CWP vs. diabetic (ANOVA followed by Tukey’s post-test).
Dietary CWP supplementation in diabetic mouse dams during pregnancy and lactation restores B- and T-cell chemotaxis to CCL21 and CXCL12
Normally, B- and T-lymphocytes migrate from the blood to the secondary lymphoid organs, where they recognize antigens, proliferate, and differentiate into effector and memory cells. The chemotactic response of peripheral B- and T-lymphocytes to CCL21 and CXCL12 was assessed. Then, cells that migrated to the medium without chemokines and cells that migrated to the medium with CCL21 or CXCL12 were stained with anti-CD19 or anti-CD3. The cells were counted for 60 s using flow cytometry, and the numbers of CD19+ B cells and CD3+ T cells that migrated to the medium with or without CCL21 and CXCL12 were divided by the number of input cells to determine the percentage of B- and T-cell chemotaxis. The percentage of cell migration to the medium was subtracted from the percentage of cell migration to chemokines to determine the specific cell migration to chemokines (Figure 3). The results from 15 separate experiments demonstrated that the percentages of B cells (Figure 3a) and T cells (Figure 3b) that migrated specifically toward CCL21 and CXCL12 were significantly decreased in offspring of diabetic mothers compared with those of control mothers. When the diabetic mothers were administered CWP during pregnancy and lactation, their offspring, compared with those of diabetic mothers, exhibited a significant restoration in the chemotactic activities of B and T cells to CCL21 and CXCL12.
Figure 3.
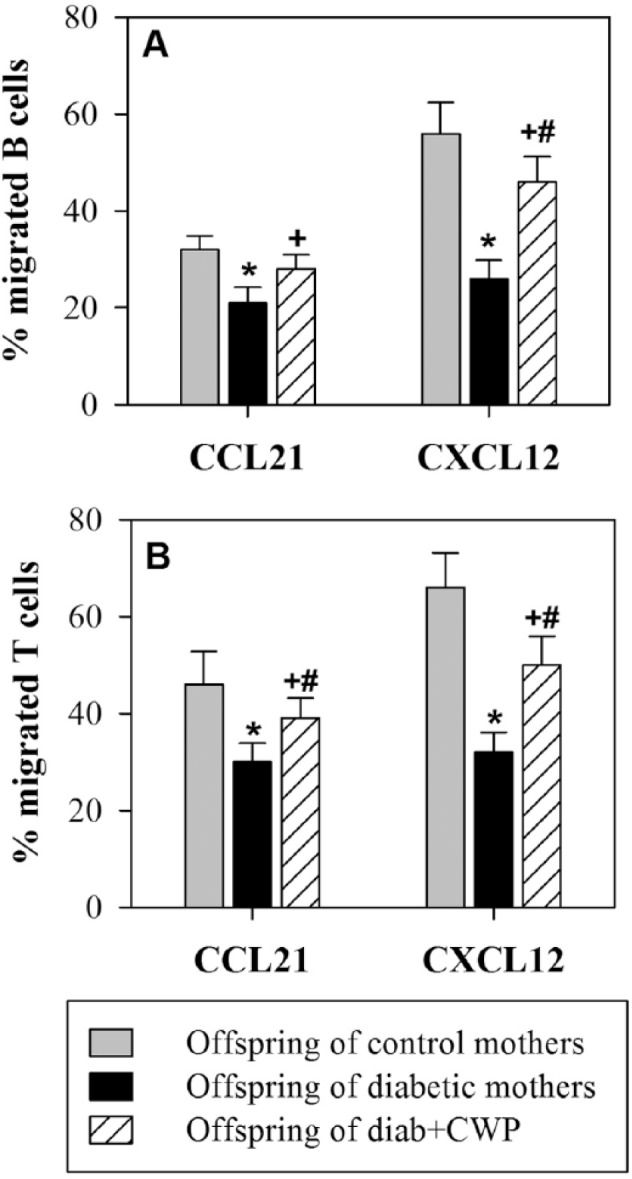
Effects of gestational diabetes and CWP supplementation on the chemotaxis of B- and T-lymphocytes in adult male offspring. The chemotaxis of B-lymphocytes (a) and T-lymphocytes (b) toward CCL21 and CXCL12 was measured in freshly isolated blood from offspring (aged three months) of control (gray bars), diabetic (black bars) and diabetic mothers administered CWP (hatched bars), and the data are expressed as the mean percentage of migrated cells ± SEM. *P <0.05 for diabetic vs. control; #P <0.05 for diabetic + CWP vs. control; +P <0.05 for diabetic + CWP vs. diabetic (ANOVA followed by Tukey’s post-test).
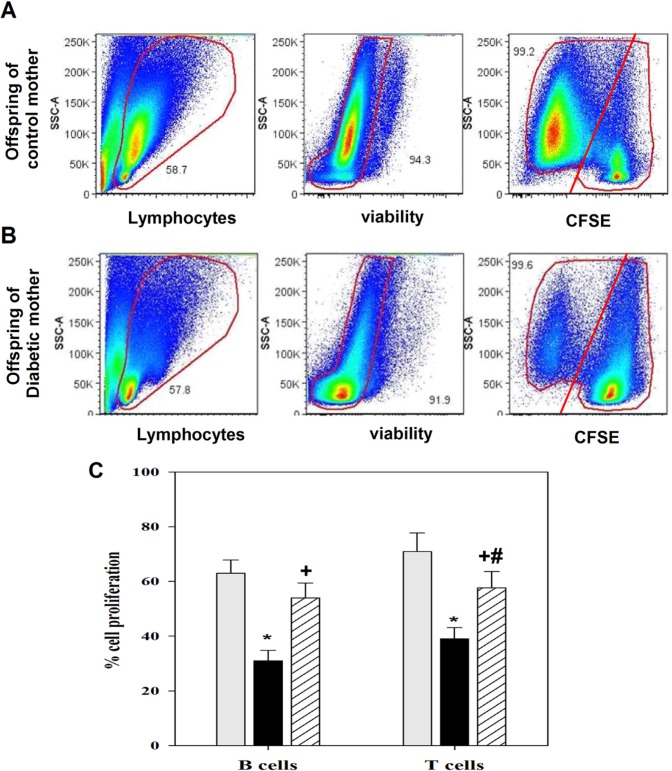
Treatment of diabetic mouse dams with CWP enhances antigen stimulation and B- and T-lymphocyte proliferation in the offspring
Defective B- and T-lymphocyte proliferation after antigen recognition may lead to impaired adaptive immunity and, in some cases, immunodeficiency. Diminished IL-2 and IL-7 levels may result in decreased lymphocyte proliferation; therefore, the proliferative capacities of B- and T-lymphocytes after mitogen stimulation (PWM) were examined by using a CFSE dilution assay. PBMCs were isolated from the offspring (at three months of age) of the three groups of mouse dams, and these cells were then labeled with CFSE. The CFSE-labeled cells were stimulated with PWM; the control cells were not stimulated. The cells were then grown for six days in cell culture medium. After six days in culture, the cells were stained with surface antigens anti-CD3-PE and CD19-APC. Then, their proliferative capacity was analyzed using flow cytometry. The plots were first gated for lymphocytes according to the forward and side scatter and then for viable cells to exclude dead cells. One representative experiment is shown to demonstrate the method used to analyze CFSE-stained B (after gating for viable lymphocytes and then for the CD19+ population) and T cells (after gating for viable lymphocytes and then for the CD3+ population) in the offspring of control non-diabetic mothers (Figure 4a) and diabetic mothers (Figure 4b). The results from 15 separate experiments in each group confirmed that stimulation with PWM significantly (*P <0.05) decreased the percentage of proliferating B- and T-lymphocytes by two-fold in the diabetic group relative to the control group (Figure 4c). Interestingly, in diabetic mothers administered CWP during pregnancy and lactation, compared with diabetic mothers, the offspring exhibited a significant restoration in the B- and T-cell proliferative capacities, to near-normal levels.
Figure 4.
CWP supplementation restores B- and T-lymphocyte proliferative capacities after antigen stimulation. PBMCs were isolated from the blood of offspring (aged three months) of control, diabetic, and diabetic mice administered CWP and were assessed for their proliferative capacity by using CFSE dilution assays and flow cytometry in response to PWM after six days of stimulation. (a, b) In offspring of control (a) or diabetic mothers (b), dot plots were gated on lymphocytes (left column) and then on viable cells to exclude dead cells (middle column) and on CFSE-labeled T- or B-lymphocytes (right column). In the right column, the cell numbers in the left side represent the percentage of CFSE-lo (proliferating cells) within the cell population, whereas cell numbers on the right side represent the percentage of CFSE-hi (undivided cells). One representative experiment is shown. (c) The data from offspring (n = 15) of control mothers (gray bars), diabetic (black bars) mothers, and diabetic mothers administered CWP (hatched bars) are expressed as the mean ± SEM percentages of CFSE-lo in B- and T-lymphocytes. *P <0.05 for diabetic vs. control; #P <0.05 for diabetic + CWP vs. control; +P <0.05 for diabetic + CWP vs. diabetic (ANOVA followed by Tukey’s post-test).
Discussion
WP has many bio-active properties, and its peptide hydrolysates modulate various immune functions, including the activation and proliferation of lymphocytes.14 The antioxidant action of CWP improves immune function and prevents hyperglycemia, hyperlipidemia, and insulin resistance, which, in turn, decreases the complications of DM.17 Natural antioxidants play an essential role in boosting the immune system through mechanisms dependent on oxidative stress, which appears to be responsible for many disorders, including autoimmune diseases. Thus, the beneficial effects of different antioxidants against insecticide-induced immunological and histological damage, as well as their protective anti-diabetic effects, have previously been demonstrated.22–26 Moreover, several studies have indicated the effects of other natural antioxidants (CWP and bee propolis) as immune modulators in promoting healing of diabetic wounds in experimental animal models.18,27–32 In addition, natural antioxidants isolated from snake and ant venoms enhance normal lymphocyte functions and exert antitumor effects in different human and animal cancer cells.33–37
In the present study, diabetes resulted in fewer neonates born to diabetic mothers than to control mothers. Additionally, diabetes induction in the mothers resulted in macrosomic pups with several postpartum complications and a decreased number of delivered neonates. In contrast, CWP supplementation in diabetic mothers during pregnancy and lactation markedly increased the total number of delivered neonates and decreased the number of macrosomic pups.
Interestingly, the present study demonstrated that dietary CWP supplementation in diabetic mothers during pregnancy and lactation significantly restored the levels of glucose and insulin as well as the number of blood lymphocytes in the offspring. In agreement with data presented in this study, WP has been reported to have insulin-tropic effects in non-diabetic and diabetic conditions that are related its amino acid content.38 Amino acids stimulate insulin release from pancreatic β-cells.39 In addition to the insulinotropic action of amino acids, WP produces an elevation in the plasma glucose-dependent insulinotropic polypeptide after ingestion.38 Previous studies have shown that camel protein decreases blood glucose levels in diabetic rats.40 Agrawal et al.41 have confirmed the hypoglycemic role of camel milk in STZ-induced diabetic animal models. In agreement with these results, it has been reported that STZ-induced diabetic mice treated with CWP exhibit a decline in blood glucose levels with higher levels of insulin.18 In this regard, our results suggested that the insulin-tropic effects of CWP in the offspring of diabetic mothers might be related to insulin receptor activation and function. Our suggestion is inconsistent with that of Abdulrahman et al.,42 who have reported that camel milk affects downstream signaling via activated ERK1/2 and potentiates the insulin-induced activation of ERK1/2, thus reflecting the engagement of the growth factor receptor-bound protein 2 (Grb2) and insulin receptor signaling protein (IRS1) pathways.
Similarly, WP has been found to be a lymphocytic mitogen43 because WP contains substantial cysteine, which is a potential factor that controls the lymphocyte number in the blood. Furthermore, the T-cell response to the WP is significantly higher than its response to concanavalin A, which is a T-cell mitogen.44 Mercier et al.45 have verified that WP stimulates the in vitro proliferation of murine spleen lymphocytes because it possesses many bio-active properties.46 In addition, Rusu et al.47 have described WP’s modulation of various immune functions, including lymphocyte activation, and proliferation.
Additionally, diabetes contributes to prolonged inflammation and impairment of the immune response, owing to elevated levels of pro-inflammatory cytokines.48 Therefore, targeting inflammatory mediators has been suggested as an effective strategy to improve the immune response and modulate inflammation in diabetic patients. The data in this study demonstrated a significant increase in the levels of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α plasma in offspring of diabetic mothers. This effect is supported by the elevation of IL-1β, IL-6, and TNF-α in many diseases.49,50 TNF-α is a key mediator of immune and inflammatory responses; it crosses the placenta and is elevated in maternal tissues, including the uteri of pregnant diabetic women.51 A previous study has reported that elevated levels of TNF-α significantly contribute to the pathological alterations observed in several inflammatory diseases.52 Consequently, the different diabetic complications observed are probably mediated by the raised levels of pro-inflammatory cytokines. In contrast, the results from this study showed that CWP caused a significant restoration of the pro-inflammatory cytokine plasma levels. The present results are consistent with earlier studies showing that WP significantly decreases early changes in the inflammatory cytokines IL-1β, TNF-α, and IL-6 during the acute phase of the inflammatory response in diabetes.53 Moreover, WP limits prolonged inflammation and modulates the immune response during the progress of wound healing in diabetic animals.54 The present study suggests that the decreased pro-inflammatory cytokines in offspring of diabetic mothers administered CWP during pregnancy and lactation is correlated with a decrease in the body weight compared with that of diabetic mice. Our results are inconsistent with the results of Haus et al.,55 who have reported that obese type 2 diabetes contributes to insulin resistance by activating inflammatory mediators such as TNF-α. However, high levels of multiple chemokine ligands and receptors have been observed in the adipose tissues of obese subjects and are associated with increased inflammation.56
Diabetic complications are caused by free radical action that damages cellular components such as lipids, proteins and DNA.57 Increased oxidative stress may contribute to DM and the development of vascular and neurologic complications of the disease.58 The present study aimed to investigate the mechanistic role of oxidative stress in the diabetic offspring. We observed that offspring of diabetic mothers showed upregulated expression of ATF-3 compared with offspring of control non-diabetic mothers. ATF-3 is highly expressed in vascular endothelial cells in atherosclerotic lesions and is associated with cell death.59 We have previously reported that ATF-3 expression is upregulated in diabetic wounds and plays a crucial role in the oxidative stress-mediated impairment of cellular differentiation.60 Additionally, ATF3 mediates the switch from an activated to a repressive state of cell growth and causes altered expression levels of cell cycle regulators in diabetic angiopathy.61 Hence, the expression of ATF-3 was markedly decreased in offspring of CWP-treated diabetic mothers. This difference may be related to the protective effects of CWP, possibly as a result of its antioxidant activity62 and chelating effects on toxicants.63 Camel milk possesses high levels of vitamins that are beneficial in preventing tissue injury associated with toxic agents such as STZ.64 Additionally, camel milk, owing to its high mineral content, may act as an antioxidant, thereby removing free radicals.65
In the present study, the plasma levels of IL-2 and IL-7 were significantly decreased in the offspring of diabetic mothers, and we observed a decrease in the proliferative capacity of antigen-stimulated B- and T-lymphocytes, thus providing important evidence of impaired immune function. Furthermore, offspring of CWP-treated diabetic mothers exhibited significant restoration of IL-2 and IL-7 levels and the proliferation of B- and T-lymphocytes. This proliferative effect of CWP enhanced and maintained an efficient immune response by lymphocytes during GD. IL-2 has been shown to promote T-lymphocyte survival and proliferation and to be consistently decreased in several diseases, thus indicating defective T-cell function. Moreover, IL-7 plays an essential role in the maintenance of T cells after antigen stimulation.66 T-cell survival may be impaired in the absence of IL-7.67 Remarkably, the acute homeostatic proliferation of memory T cells depends at least partially on endogenous IL-7 levels.68 Moreover, IL-7 has several essential roles in B-cell development, including promoting the proliferation and survival of B-cell progenitors and the maturation of B cells.69 Impeded T- and B-lymphocyte capacity is related to the abnormal activation of the immune system and has been shown to contribute to immunodeficiency.70
CXCL12 and CCL21 participate in naive T- and B-cell recruitment to the extra-follicular area in secondary lymphoid organs via their lymphocyte receptors.71 The data in this study showed the percentages of chemotactic B and T cells were significantly decreased in offspring of diabetic mothers. In contrast, offspring of CWP-treated diabetic mothers exhibited a significant elevation in the percentages of chemotactic B and T cells. In agreement with these results, CD34+ cells isolated from diabetic patients are defective in migration to CXCL12.72 Moreover, the CXCR4/CXCL12 signaling pathway has been shown to protect non-obese diabetic mice from autoimmune diabetes.73 In this context, CCR7 and CXCR4 have been shown to be involved in the recruitment of blood-borne leukocytes to sites of inflammation.74 Reports have shown that bovine WP enhances innate immunity by elevating neutrophil chemotaxis.75 However, WP treatment has been suggested to improve in the B- and T-cell chemotaxis efficiency in diabetic mice.18
Conclusion
On the basis of the literature, we hypothesized that CWP treatment might significantly improve complications associated with diabetes, including impairment of immune functions. Our results indicated that CWP is a potential treatment for both diabetes complications and immune impairment of offspring of diabetic mothers. The improvement induced by CWP was mediated by significant decreases in ATF-3 expression, which, in turn, led to a decrease in the levels of pro-inflammatory cytokines and ROS. In addition, we demonstrated positive significant restorative effects of CWP regarding the plasma levels of IL-2 and IL-7, CCL21- and CXCL12-mediated chemotaxis of B- and T-lymphocytes, and the proliferative capacity of antigen-stimulated B- and T-lymphocytes. Therefore, CWP is a promising active ingredient that may offer multiple potential therapeutic modalities in different clinical settings. The efficacy of CWP, however, should be measured for different disease states.
Footnotes
Declaration of conflicting interests: The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at king Saud University for its funding this Research group NO (RG-1435-019).
References
- 1. Metzger BE. (1991) Summary and recommendations of the Third International workshop–Conference on Gestational Diabetes Mellitus. Diabetes 40: 197–201. [DOI] [PubMed] [Google Scholar]
- 2. Kelly L, Evans L, Messenger D. (2005) Controversies around gestational diabetes. Canadian Family Physician 51: 688–695. [PMC free article] [PubMed] [Google Scholar]
- 3. Ballard JL, Rosenn B, Khoury JC, et al. (1993) Diabetic fetal macrosomia: Significance of disproportionate growth. Journal of Pediatrics 122: 115–119. [DOI] [PubMed] [Google Scholar]
- 4. Loukovaara M, Leinonen P, Teramo K, et al. (2004) Fetal hypoxia is associated with elevated cord serum C-reactive protein levels in diabetic pregnancies. Biology of the Neonate 85: 237–242. [DOI] [PubMed] [Google Scholar]
- 5. Silveira RC, Procianoy RS. (2003) Interleukin-6 and tumor necrosis factor-alpha levels in plasma and cerebrospinal fluid of term newborn infants with hypoxic-ischemic encephalopathy. Journal of Pediatrics 143: 625–629. [DOI] [PubMed] [Google Scholar]
- 6. Saliba E, Henrot E. (2001) Inflammatory mediators and neonatal brain damage. Biology of the Neonate 79: 224–227. [DOI] [PubMed] [Google Scholar]
- 7. Mehler MF, Kessler JA. (1997) Hematol-ymphopoeitic and inflammatory cytokines in neural development. Trends in Neuroscience 20: 357–365. [DOI] [PubMed] [Google Scholar]
- 8. Kowalski J, Blada P, Kucia K, et al. (2001) Neuroleptics normalize increased release of interleukin-1 beta and tumor necrosis factor-alpha from monocytes in schizophrenia. Schizophrenia Research 50: 169–175. [DOI] [PubMed] [Google Scholar]
- 9. Yao JK, Reddy RD, van Kammen DP. (2001) Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs 15: 287–303. [DOI] [PubMed] [Google Scholar]
- 10. Nakagomi S, Suzuki Y, Namikawa K, et al. (2003) Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. Journal of Neuroscience 23: 5187–5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biri A, Onan A, Devrim E, et al. (2006) Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta 27: 327–332. [DOI] [PubMed] [Google Scholar]
- 12. Allen RG, Venkatraj VS. (1992) Oxidants and antioxidants in development and differentiation. Journal of Nutrition 122: 631–635. [DOI] [PubMed] [Google Scholar]
- 13. Mahadik SP, Yao JK. (2006) Phospholipids in schizophrenia. In: Lieberman JA, Stroup TS, Perkins DO. (eds) The American Psychiatric Publishing Textbook of Schizophrenia. Washington, DC: American Psychiatric Publishing, Inc., pp. 117–135. [Google Scholar]
- 14. Gauthier SF, Pouliot Y, Saint-Sauveur D. (2006) Imunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. International Dairy Journal 16: 1315–1323. [Google Scholar]
- 15. Beaulieu J, Dupont C, Lemieux P. (2006) Whey proteins and peptides: Beneficial effects on immune health. Therapy 3: 1–10. [Google Scholar]
- 16. Micke P, Beeh KM, Schlaak JF, et al. (2001) Oral supplementation with whey proteins increases plasma glutathione levels of HIV-infected patients. European Journal of Clinical Investigation 31: 171–178. [DOI] [PubMed] [Google Scholar]
- 17. Agrawal RP, Jain S, Shah S, et al. (2011) Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. European Journal of Clinical Nutrition 65: 1048–1052. [DOI] [PubMed] [Google Scholar]
- 18. Badr G, Mohany M, Metwalli A. (2011) Effects of undenatured whey protein supplementation on CXCL12- and CCL21-mediated B and T cell chemotaxis in diabetic mice. Lipids in Health Disease 10: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tie L, Yang HQ, An Y, et al. (2012) Ganoderma lucidum polysaccharide accelerates refractory wound healing by inhibition of mitochondrial oxidative stress in type 1 diabetes. Cellular Physiology and Biochemistry 29: 583–594. [DOI] [PubMed] [Google Scholar]
- 20. Sayed D, Badr G, Maximous D, et al. (2010) HLA-G and its relation to proliferation index in detection and monitoring breast cancer patients. Tissue Antigens 75: 40–47. [DOI] [PubMed] [Google Scholar]
- 21. Badr G, Saad H, Waly H, et al. (2010) Type I interferon (IFN-alpha/beta) rescues B-lymphocytes from apoptosis via PI3Kdelta/Akt, Rho-A, NFkappaB and Bcl-2/Bcl(XL). Cell Immunol. 263(1): 31–40. [DOI] [PubMed] [Google Scholar]
- 22. Mohany M, El-Feki M, Refaat I, et al. (2012) Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. Journal of Toxicology Sciences 37: 1–11. [DOI] [PubMed] [Google Scholar]
- 23. Mohany M, El-Feki M, Refaat I, et al. (2012) Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. J Toxicol Sci. 37(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 24. Badr G, Mohany M. (2011) Maternal perinatal undernutrition attenuates T-cell function in adult male rat offspring. Cell Physiol Biochem. 27(3–4): 381–90. [DOI] [PubMed] [Google Scholar]
- 25. Badr G, Mahmoud MH, Farhat K, et al. (2013) Maternal supplementation of diabetic mice with thymoquinone protects their offspring from abnormal obesity and diabetes by modulating their lipid profile and free radical production and restoring lymphocyte proliferation via PI3K/AKT signaling. Lipids in Health Disease 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Badr G, Al-Sadoon MK, Abdel-Maksoud MA, et al. (2012) Cellular and molecular mechanisms underlie the anti-tumor activities exerted by Walterinnesia aegyptia venom combined with silica nanoparticles against multiple myeloma cancer cell types. PLoS One. 7(12): e51661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Badr G, Ebaid H, Mohany M, et al. (2012) Modulation of immune cell proliferation and chemotaxis towards CC chemokine ligand (CCL)-21 and CXC chemokine ligand (CXCL)-12 in undenatured whey protein-treated mice. Journal of Nutritional Biochemistry 23: 1640–1646. [DOI] [PubMed] [Google Scholar]
- 28. Badr G, Al-Sadoon MK, Rabah DM, et al. (2013) Snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles induce apoptosis and growth arrest in human prostate cancer cells. Apoptosis. 18(3): 300–14. [DOI] [PubMed] [Google Scholar]
- 29. Badr G. (2012) Supplementation with undenatured whey protein during diabetes mellitus improves the healing and closure of diabetic wounds through the rescue of functional long-lived wound macrophages. Cellular Physiology and Biochemistry 3: 571–582. [DOI] [PubMed] [Google Scholar]
- 30. Badr G. (2013) Camel whey protein enhances diabetic wound healing in a streptozotocin-induced diabetic mouse model: The critical role of β-Defensin-1, -2 and -3. Lipids in Health Disease 12: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al Ghamdi AA, Badr G, Hozzein WN, et al. (2015) Oral supplementation of diabetic mice with propolis restores the proliferation capacity and chemotaxis of B and T lymphocytes towards CCL21 and CXCL12 by modulating the lipid profile, the pro-inflammatory cytokine levels and oxidative stress. BMC Immunology 16: 54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Hozzein WN, Badr G, Al Ghamdi AA, et al. (2015) Topical application of propolis enhances cutaneous wound healing by promoting TGF-beta/Smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cellular Physiology and Biochemistry 37: 940–954. [DOI] [PubMed] [Google Scholar]
- 33. Badr G, Garraud O, Daghestani M, et al. (2012) Human breast carcinoma cells are induced to apoptosis by samsum ant venom through an IGF-1-dependant pathway, PI3K/AKT and ERK signaling. Cellular Immunology 273: 10–16. [DOI] [PubMed] [Google Scholar]
- 34. Badr G, Al-Sadoon MK, El-Toni AM, et al. (2012) Walterinnesia aegyptia venom combined with silica nanoparticles enhances the functioning of normal lymphocytes through PI3K/AKT, NFκB and ERK signaling. Lipids in Health Disease 11: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Badr G, Al-Sadoon MK, Rabah DM. (2013) Therapeutic efficacy and molecular mechanisms of snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles in the treatment of breast cancer- and prostate cancer-bearing experimental mouse models. Free Radical Biology & Medicine 65: 175–189. [DOI] [PubMed] [Google Scholar]
- 36. Al-Sadoon MK, Rabah DM, Badr G. (2013) Enhanced anticancer efficacy of snake venom combined with silica nanoparticles in a murine model of human multiple myeloma: Molecular targets for cell cycle arrest and apoptosis induction. Cellular Immunology 284: 129–138. [DOI] [PubMed] [Google Scholar]
- 37. Sayed D, Al-Sadoon MK, Badr G. (2012) Silica nanoparticles sensitize human multiple myeloma cells to snake (Walterinnesia aegyptia) venom-induced apoptosis and growth arrest. Oxid Med Cell Longev. 2012: 386286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nilsson M, Holst JJ, Björck IME. (2007) Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. American Journal of Clinical Nutrition 85: 996–1004. [DOI] [PubMed] [Google Scholar]
- 39. Schmid R, Schusdziarra V, Schulte-Frohlinde E, et al. (1989) Role of amino acids in stimulation of postprandial insulin, glucagon, and pancreatic polypeptide in humans. Pancreas 4: 305–314. [DOI] [PubMed] [Google Scholar]
- 40. Kamal AM, Salama OA, El-Saied KM. (2007) Changes in amino acids profile of camel milk protein during the early lactation. International Journal of Dairy Science 2: 226–234. [Google Scholar]
- 41. Agrawal RP, Sahani MS, Tuteja FC, et al. (2005) Hypoglycemic activity of camel milk in chemically pancreatectomized rats—an experimental study. International Journal of Diabetes in Developing Countries 25: 75–79. [Google Scholar]
- 42. Abdulrahman AO, Ismael MA, Al-Hosaini K, et al. (2016) Differential effects of camel milk on insulin receptor signaling toward understanding the insulin-like properties of camel milk. Frontiers in Endocrinology 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Middleton N, Jelen P, Bell G. (2004) Whole blood and mononuclear cell glutathione response to dietary whey protein supplementation in sedentary and trained male human subjects. International Journal of Food Sciences and Nutrition 55: 131–141. [DOI] [PubMed] [Google Scholar]
- 44. Wong CW, Watson DL. (1995) Immunomodulatory effects of dietary whey proteins in mice. Journal of Dairy Research 62: 359–368. [DOI] [PubMed] [Google Scholar]
- 45. Mercier A, Gauthier SF, Fliss I. (2004) Immunomodulating effects of whey proteins and their enzymatic digests. International Dairy Journal 14: 175–183. [Google Scholar]
- 46. Ballard KD, Bruno RS, Seip RL, et al. (2009) Acute ingestion of a novel whey-derived peptide improves vascular endothelial responses in healthy individuals: a randomized, placebo controlled trial. Nutrition Journal 22: 8–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rusu D, Drouin R, Pouliot Y, et al. (2010) A bovine whey protein extract stimulates human neutrophils to generate bioactive IL-1Ra through a NF-kappaB- and MAPK-dependent mechanism. Journal of Nutrition 140: 382– 391. [DOI] [PubMed] [Google Scholar]
- 48. Bufalo MC, Bordon-Graciani AP, Conti BJ, et al. (2014) The immunomodulatory effect of propolis on receptors expression, cytokine production and fungicidal activity of human monocytes. Journal of Pharmacy and Pharmacology 66: 1497–1504. [DOI] [PubMed] [Google Scholar]
- 49. Chiesa CG, Pellegrini T, De Luca M, et al. (2003) Umbilical cord interleukin 6 levels are elevated in term neonatres with perinatal asphyxia. European Journal of Clinical Investigation 33: 352–358. [DOI] [PubMed] [Google Scholar]
- 50. Badr G, Waly H, Eldien HM, et al. (2010) Blocking type I interferon (IFN) signaling impairs antigen responsiveness of circulating lymphocytes and alters their homing to lymphoid organs: protective role of type I IFN. Cell Physiol Biochem. 26(6): 1029–40. [DOI] [PubMed] [Google Scholar]
- 51. McLachlan KA, O’Neal D, Jenkins A, et al. (2006) Do adiponectin, TNF alpha leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes during and after pregnancy. Diabetes/Metabolism Research and Reviews 22: 131–138. [DOI] [PubMed] [Google Scholar]
- 52. Ibrahim HM, El-Elaimy IA, Saad Eldien HM, et al. (2013) Blocking type I interferon signaling rescues lymphocytes from oxidative stress, exhaustion, and apoptosis in a streptozotocin-induced mouse model of type I diabetes. Oxid Med Cell Longev. 2013: 148725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ebaid H, Salem A, Sayed A, et al. (2011) Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids in Health Disease 10: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ebaid H, Ahmed OM, Mahmoud AM, et al. (2013) Limiting prolonged inflammation during proliferation and remodeling phases of wound healing in streptozotocin-induced diabetic rats supplemented with camel undenatured whey protein. BMC Immunology 14: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haus JM, Kashyap SR, Kasumov T, et al. (2009) Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Holland WL, Bikman BT, Wang LP, et al. (2011) Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. Journal of Clinical Investigation 121: 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Golbidi S, Laher I. (2010) Antioxidant therapy in human endocrine disorders. Medical Science Monitor 16: 9–24. [PubMed] [Google Scholar]
- 58. Elgazzar MA. (2007) Thymoquinone suppresses in vitro production of IL-5 and IL-13 by mast cells in response to lipopolysaccharide stimulation. Inflammation Research 56: 345–351. [DOI] [PubMed] [Google Scholar]
- 59. Nawa T, Nawa MT, Adachi MT, et al. (2002) Expression of transcriptional repressor ATF3/LRF1 in human atherosclerosis: Colocalization and possible involvement in cell death of vascular endothelial cells. Atherosclerosis 16: 281–291. [DOI] [PubMed] [Google Scholar]
- 60. Badr G, Hozzein WN, Badr BM, et al. (2016) Bee venom accelerates wound healing in diabetic mice by suppressing activating transcription factor-3 (ATF-3) and inducible nitric oxide synthase (iNOS)-mediated oxidative stress and recruiting bone marrow-derived endothelial progenitor cells. Journal of Cellular Physiology 231: 2159–5171. [DOI] [PubMed] [Google Scholar]
- 61. Okamoto A, Iwamoto Y, Maru Y. (2006) Oxidative stress-responsive transcription factor ATF3 potentially mediates diabetic angiopathy. Molecular and Cellular Biology 26: 1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shori AB. (2013) Nutritional and therapeutical values of chickpea water extract enriched yogurt made from cow and camel milk. American Journal of Drug Discovery and Development 3: 47–59. [Google Scholar]
- 63. Al-Humaid AI, Mousa HM, El-Mergawi RA, et al. (2010) Chemical composition and antioxidant activity of dates and dates-camel-milk mixtures as a protective meal against lipid peroxidation in rats. American Journal of Food Technology 5: 22–30. [Google Scholar]
- 64. Kedziora-Kornatowska K, Szram S, Kornatowski T, et al. (2003) Effect of vitamin E and vitamin C supplementation on antioxidative state and renal glomerular basement membrane thickness in diabetic kidney. Nephron. Experimental Nephrology 95: 134–143. [DOI] [PubMed] [Google Scholar]
- 65. Ozdemir G, Inanc F. (2005) Zinc may protect remote ocular injury caused by intestinal ischemia reperfusion in rats. Tohoku Journal of Experimental Medicine 206: 247–251. [DOI] [PubMed] [Google Scholar]
- 66. Jameson SC. (2005) T cell homeostasis: Keeping useful T cells alive and live T cells useful. Seminars in Immunology 3: 231–237. [DOI] [PubMed] [Google Scholar]
- 67. Schluns KS, Kieper WC, Jameson SC, et al. (2000) Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nature Immunology 5: 426–432. [DOI] [PubMed] [Google Scholar]
- 68. Tan JT, Ernst B, Kieper WC, et al. (2002) Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. Journal of Experimental Medicine 195: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kikuchi K, Kasai H, Watanabe A, et al. (2008) IL-7 specifies B cell fate at the CLP to pre-proB transition stage by maintaining EBF expression. Journal of Immunology 181: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Koesters SA, Alimonti JB, Wachihi C, et al. (2006) IL-7Ralpha expression on CD4+ T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. European Journal of Immunology 36: 336–365. [DOI] [PubMed] [Google Scholar]
- 71. Gunn MD, Kyuwa S, Tam C, et al. (1999) Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. Journal of Experimental Medicine 189: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Segal MS, Shah R, Afzal A, et al. (2006) Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes 55: 102–109. [PubMed] [Google Scholar]
- 73. Aboumrad E, Madec AM, Thivolet C. (2007) The CXCR4/CXCL12 (SDF-1) signaling pathway protects non-obese diabetic mouse from autoimmune diabetes. Clinical and Experimental Immunology 148: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Blades MC, Manzo A, Ingegnoli F, et al. (2002) Stromal cell-derived factor 1 (CXCL12) induces human cell migration into human lymph nodes transplanted into SCID mice. Journal of Immunology 168: 4308. [DOI] [PubMed] [Google Scholar]
- 75. Rusu D, Drouin R, Pouliot Y, et al. (2009) A bovine whey protein extract can enhance innate immunity by priming normal human blood neutrophils. Journal of Nutrition 139: 386–393. [DOI] [PubMed] [Google Scholar]