Abstract
Bone is the most common site of prostate cancer (PCa) metastasis. Once PCa cells metastasize to bone, the mortality rate of PCa patients increases significantly. Furthermore, bone metastases produce multiple skeletal complications, including bone pain, that impairs the patients’ quality of life. Effective therapies for bone metastatic disease are underdeveloped with most current therapies being primarily palliative with modest survival benefit. Although the exact mechanisms through which PCa metastasizes to bone are unclear, growing evidence suggests that the bone marrow microenvironment, particularly it’s hematopoietic activity, is a significant mediator of PCa’s bone tropism. Moreover, the bone microenvironment may regulate metastatic PCa cells between dormant and proliferative states. In this review, we discuss (1) how PCa cells interact with the bone microenvironment to establish bone metastases and (2) current and future potential treatments for PCa patients with bone metastases.
Keywords: prostate cancer, bone metastasis, bone targeted therapy, bone marrow microenvironment, skeletal complications
Introduction
The 5- and 10-year overall survival rates for prostate cancer in the setting of treated non-metastatic local regional disease are nearly 100% and 98%, respectively [1]. This high survival rate has brought attention to the possible over-diagnosis and over-treatment of prostate cancer, resulting in a shift of treatment strategies for patients with low-risk and low-volume prostate cancer. Specifically, patients may be offered active surveillance which entails close observation with frequent prostate-specific antigen (PSA) testing and scheduled biopsies [2]. However, not all prostate cancer patients fit this paradigm and some on active surveillance may progress to metastatic disease. Once prostate cancer metastasizes, the mortality rate of prostate cancer patients significantly increases.
Bone is the most frequent site of prostate cancer metastases with up to 90% of patients with advanced disease having bone metastases [3]. The exact mechanisms that favor bone metastasis versus other sites remain to be elucidated. The prostate is a highly-vascularized organ that has a vascular drainage to the bones of the vertebral column. Specifically, the prostatic venous plexus drains into the internal iliac vein, which connects to the vertebral venous plexus. Since the vertebral venous plexus is present throughout the entire spinal column, this may be one of the major routes of prostate cancer bone metastasis [4]. Bone metastatic cells commonly to target red marrow, which is typically found in trabecular bones [5]. The highly vascular trabecular structure of the bone provides a welcoming environment for bone metastatic cancer cells to colonize the bone including easy access to oxygen and other nutrients [6]. Besides anatomical properties of the bone itself and its relation to the prostate, intrinsic properties of primary tumor such as their unique gene expression profiles are believed to influence bone metastatic process. For example, high levels of cyclin A1 are observed in the bone metastatic lesion of prostate cancer patients which is consistent with the possibility that stem cell like prostate cancer cells, which overexpress cyclin A1, preferentially metastasize to the bone [7]. In addition, the expression of receptor activator of nuclear factor κ-B (NFκB) ligand (RANKL) in prostate cancer cells promoted their dissemination and colonization to the bone [8]. While these oncogenic properties of tumor cells are essential for metastatic progression, growing evidence has also suggested that the unique microenvironment of bone (e.g. the cells of hematopoietic origin and the cells that control bone remodeling) play important roles in prostate cancer bone metastasis [9–11].
Prostate cancer is no longer curable once bone metastases manifest. The median time to death after development of metastases is approximately 5 years [12]. Unfortunately, currently available treatments for bone metastasis are primarily palliative, providing improvement to the patient’s’ quality of life and may provide modest survival benefits. Bone metastases are not only incurable, but also very destructive. Bone metastases can lead to serious complications including hypercalcemia, incapacitating bone pain, pathological fracture, and spinal cord compression [13]. These can manifest as medical emergencies as spinal cord compression can lead to permanent paralysis and loss of limb function and mobility [13]. It is therefore urgent to develop effective therapies for improving both overall survival and quality of life of patients with bone metastases.
In this review, we will discuss the major mechanisms of prostate cancer bone metastasis with a focus on the interaction of prostate cancer with bone marrow microenvironment. Additionally, we will overview the current strategies and future directions in the treatments for prostate cancer patients with bone metastases.
Mechanisms of prostate cancer bone metastasis
Homing to the bone
After undergoing epithelial to mesenchymal transition (EMT), cancer cells begin to escape from the primary tumor site and enter blood circulation. To extravasate into a secondary site they must first adhere to endothelial cells. During their dissemination to the bone, prostate cancer cells interact with the bone marrow endothelial cells (BMECs). It has been demonstrated that E-selectin ligand on prostate cancer cells and E-selectin on endothelial cells contribute to the adhesion between the prostate cancer cells and BMECs [14–16]. CD44 expressed by prostate cancer cells has also been demonstrated to be involved in adhesion to endothelial cells by binding to vascular cell adhesion molecule 1 (VCAM-1), and this adhesion can be enhanced by interleukin-17 (IL-17), insulin, and insulin-like growth factor 1 (IGF1) [17]. Another adhesion molecule, the vitronectin receptor αvβ3 integrin, is involved in both the interaction with endothelial cells and the bone homing process of prostate cancer [18–20]. Consistent with this notion, αvβ3 integrin induces breast cancer bone metastasis, whereas the expression of αvβ3 integrin does not affect primary tumor growth [21]. Moreover, C-X-C motif chemokine ligand 12 (CXCL12), a chemokine secreted by bone marrow stromal cells or osteoblasts [22], enhances αvβ3 integrin expression in prostate cancer [23,20], suggesting the signal from the bone marrow microenvironment may influence bone metastatic ability of prostate cancer. These adhesion molecules, through facilitating binding of prostate cancer cells to BMECs, are important for the dissemination to bone.
In addition to regulating αvβ3 integrin expression, CXCL12 is one of the key factors to regulate hematopoietic stem cell (HSC) homing to the bone marrow [24]. Intriguingly, the mechanisms of prostate cancer bone dissemination are similar to the HSC homing. Both HSC and prostate cancer cells express the CXCL12 receptor C-X-C chemokine receptor type 4 (CXCR4) [25,26] and/or CXCR7 [27], and migrate towards CXCL12-expressing organs, including bone marrow. When the CXCL12/CXCR4 interaction is inhibited with a CXCR4 antagonist AMD3100, the initial colonization to the bone and the growth of prostate cancer are prevented [28]. However, the same treatment fails to diminish the established bone metastatic tumor [28]. This finding suggests that CXCL12/CXCR4 axis may be involved in an early dissemination process of prostate cancer, but not later metastatic growth.
CXCL12 expression derived from tumor microenvironment within the primary tumor has also been suggested to promote the prostate cancer bone metastases. Solid tumors are known to recruit bone marrow-derived mesenchymal stem cells (MSCs) to the primary site to enhance their metastatic potential [29]. CXCL16-expressing prostate cancer cells also recruit bone marrow-derived MSCs which express CXCR6 on their surface [30]. This CXCL16/CXCR6 interaction induces the transition of MSCs to cancer-associated fibroblasts, which is validated the high levels of CXCL12 expression. The CXCL12 released from the cancer-associated fibroblasts promotes the EMT of prostate cancer cells, resulting in the dissemination to the bone. On the other hand, prostate cancer cells also express CXCR6. The higher CXCR6 expression levels correlates with the higher Gleason scores, and prostate cancer cells migrate towards CXCL16 [31,32]. Interestingly, CXCL16 enhances binding of prostate cancer cells to BMECs by upregulating αvβ3 integrin expression in prostate cancer cells [31]. Additionally, the higher levels of CXCL16 are detected in bone samples obtained from prostate cancer patients with bone metastases [33,32]. It has also been recently suggested that factors, including exosome, released from the primary tumor educate bone marrow-derived cells to promote further metastases by establishing a pre-metastatic niche at distant organs [34–36]. In fact, exosome-derived miR-192 regulates bone colonization of lung cancer by influencing endothelial cells in the marrow, leading to enhanced tumor angiogenesis [37]. Interestingly, a greater number of oncosomes, tumor-derived exosomes, in the circulation is associated with the metastatic potential of prostate cancer [38,39]. These findings suggest that exosomes may also be involved in the dissemination process of prostate cancer to the bone. Further studies are warranted.
Once prostate cancer cells arrive in the bone, they take advantage of a unique environment where HSCs reside, known as the “HSC niche,” to survive. The HSC niche is important for the maintenance of the HSCs [24]. Prostate cancer cells compete for the occupancy of the HSC niche [40]. Once in the niche, the disseminated prostate cancer cells utilize the niche. Cell of the osteoblastic lineage compose a component of the HSC niche and have been shown to play a central role in the early bone colonization of cancer cells [41]. Disseminated prostate cancer cells bind to the osteoblastic HSC niche with the same mechanisms as HSCs. Both prostate cancer cells [42] and HSCs [43] express a receptor for Annexin II and bind to Annexin II expressed by the osteoblastic HSC niche. In addition, Annexin II regulates the CXCL12 expression of the HSC niche [44], and the Annexin II/CXCL12 interaction plays a crucial roles in the regulation of prostate bone metastasis [45]. Similar to HSCs [46], niche-associated disseminated prostate cancer cells are mobilized from the niche into the circulation when treated with AMD3100 [40]. In addition to CXCL12, stem cell factor (SCF), another osteoblast-derived factor that regulates HSC function [47], may promote prostate cancer bone metastasis. Indeed, circulating tumor cells [48] and bone metastatic samples [49] obtained from prostate cancer patients express a receptor for SCF, c-Kit or CD117, which is highly expressed in advanced prostate cancer. The effects of cabozantinib (XL184), a small molecule inhibitor which targets c-Kit, were evaluated in a phase II randomized discontinuation trial in men with castration-resistant bone metastatic prostate cancer [50]. In this study, cabozantinib significantly improved the median progression free survival (23.9 weeks) compared with placebo (5.9 weeks, hazard ratio, 0.12; p<0.001). Fifty-seven % of patients treated with cabozantinib had over 50% reduction in bone turnover markers (serum total alkaline phosphatase and plasma cross-linked C-terminal telopeptide of type I collagen), 68% of patients had improvement on bone scan including 12% of complete remission, and 67% of patients had beneficial effects on bone pain. Moreover, it has been proposed that the enhancement of susceptibility of bone metastatic cancer cells to chemotherapy by interfering with CXCL12/CXCR4 [51]. These findings suggest that the HSC niche can be a potential therapeutic target for prostate cancer bone metastasis.
Tumor dormancy within the bone
After colonizing bone, disseminated tumor cells must adopt to the new environment to enable long term survival [52]. In order to do so, cancer cells must control blood supply and escape from the immune system [53]. One mechanism to achieve this is cellular tumor dormancy [53]. In addition to dormancy at the cellular level, dormancy may occur at the tumor level when proliferation and apoptosis of the cells are balanced. This condition is called as “population-level tumor dormancy” (also known as “angiogenic dormancy” and/or “immune-mediated dormancy” [53]. The tumor microenvironment can influence both population-level dormancy and cellular dormancy [53,54].
Bone morphogenetic protein 7 (BMP7), a member of transforming growth factor (TGF)-β family, released from bone marrow stromal cells prevents bone metastatic growth by inducing prostate cancer stem cell dormancy [55]. This is achieved, in part, through BMP7’s ability to activate phosphorylation of p38, but not Erk in prostate cancer [55]. The ratio between phosphorylat ed p38 and phosphorylate ed Erk have been proposed a key regulator of the balance between the dormancy and proliferation of cancer cells [56,57]. Specifically, when phosphorylated p38 is greater than phosphorylated Erk, tumor cells tend to become dormant. Higher levels of BMP receptor type 2 (BMPR2), a receptor for BMP7, is observed in tumors obtained from prostate cancer patients without bone metastases, compared to those with bone metastases [55], suggesting that BMPR2 negatively correlates with prostate cancer bone metastatic progression. Intriguingly, it has been demonstrated that disseminated prostate cancer cells not only passively receive dormancy signals from bone marrow microenvironment, but also actively educate it to stay dormant [58]. To achieve this, dormant prostate cancer cells release secreted protein acidic and rich in cysteine (SPARC), resulting in increased BMP7 expression from bone marrow stromal cells. Furthermore, methylation in promoter regions epigenetically silences the SPARC expression in proliferating prostate cancer cells [58].
Growth arrest specific 6 (GAS6) is a ligand for one of tyrosine kinase receptor families, the TAM receptor family (Tyro3, Axl, MerTK), and prostate cancer express all of the TAM receptors. The levels of GAS6 in skeletal organs influence induction of prostate cancer dormancy. Low levels of GAS6 are observed in the bone marrow of the hindlimb where more prostate cancer bone metastases are found, compared to the bone marrow of forelimb where higher levels of GAS6 are found [59]. The GAS6 receptor Axl, is upregulated in prostate cancer cells when they bind to Annexin II in the osteoblastic niche [10]. The increased Axl contributes to a dormant state and drug resistance of bone metastatic prostate cancer [10]. In addition, dormant disseminated prostate cancer cells express higher levels of Axl, compared to proliferating cells [60]. However, higher levels of Tyro3 are found in the proliferating prostate cancer cells [60]. Moreover, when the MerTK expression is down-regulated, prostate cancer become dormant by decreasing a ratio of phosphorylated Erk to phosphorylated p38 [61]. These findings suggest that GAS6 secreted by the osteoblastic HSC niche is also involved in the induction process of prostate cancer dormancy through its receptors.
Metastatic outgrowth within the bone
To establish clinically detectable metastasis, disseminated prostate cancer cells must exit from dormancy. This may occur after a decade or more of disease-free survival. Although bone metastases of prostate cancer are mainly thought to be osteoblastic, osteoclastic activity is also involved in bone metastatic progression and metastatic outgrowth. Bone is a unique organ that is actively remodeled. In normal physiology, to maintain bone remodeling, osteoblasts and osteoclasts influence one another. Once disseminated tumor cells come in the marrow environment, they interfere with this interaction. This feedback loop among disseminated tumor cells, osteoblasts, and osteoclasts is known as “vicious cycle”, which is believed to be a major mechanisms of bone metastatic outgrowth [62].
A major mediator of bone remodeling is RANKL. In the marrow, RANKL, mainly expressed by osteoblasts, osteocytes, and stromal cells, binds to its receptor RANK on the surface of osteoclast progenitors, resulting in osteoclast differentiation and bone resorption [63]. In addition, bone resorption promotes the release of growth factors that activate osteoblastogenesis, including TGF-β and BMPs, from the bone matrix [63]. To balance osteoclast activities, mature osteoblasts and stromal cells also secrete ostoprotegerin (OPG), a soluble decoy RANKL receptor, which prevents RANKL/RANK interaction [63]. It has been demonstrated that disseminated prostate cancer cells enhance RANKL expression of osteoblasts by expressing parathyroid hormone-related protein (PTHrP), leading to osteoclastogenesis [64]. Additionally, in the bone metastatic setting, osteoclasts highly express matrix metalloproteinase-7 (MMP7), which further promotes bone resorption by cleaving membrane binding RANKL on both osteoblasts and prostate cancer [65]. The inhibition of bone remodeling mediated by osteoclastogenesis associated with bone metastatic cancer cells is thought to be essential process for bone metastatic outgrowth, since it creates space for disseminated tumor cells to grow within the bone marrow. In similar fashion, the inhibition of osteoblastogenesis induces bone metastatic growth of prostate cancer. Dickkopf-1 (DKK-1), an inhibitor of Wnt (a major regulator for osteoblast differentiation), expressed by prostate cancer contributes to the inhibition of osteoblastogenesis, and consequently promotes prostate cancer growth in the marrow [66–68]. Interestingly, RANKL released from osteoblasts also stimulates the growth of prostate cancer through RANK on the prostate cancer [69–71]. Moreover, the RANKL secretion of osteoblasts and the RANK expression of prostate cancer are controlled by IL-6 derived from prostate cancer [71]. As a result, when the IL-6 signal and the RANK/RANKL axis are blocked, bone metastatic growth of prostate cancer is decreased [69–71].
Cancer-induced bone pain is one of the major complications associated with bone metastases [13]. Bone pain is mediated by both sensory and sympathetic nerve fibers that innervate into the marrow [72]. Aside from pain induction, the regulatory roles of central nerve systems in bone remodeling has been revealed [73–75]. For instance, it has been indicated that a sensory neuropeptide, calcitonin gene-related peptide (CGRP) promotes osteoblastic differentiation [74,75]. In addition, norepinephrine released from sympathetic nerve fibers prevents the proliferation of osteoblasts through β2-adrenergic receptor [74,75]. The β2-adrenergic receptor also induces RANKL expression in osteoblasts that leads to the osteoclast activation, [74,75]. Moreover, β2-and β3-adrenergic receptors are involved in the development of the HSC niche by controlling its CXCL12 expression [76–78]. Therefore, it has been hypothesized that nerve components within the marrow contributes to bone metastatic progression [79]. Interestingly, prostate cancer cells have a high affinity to nerve fibers [80,81], and perineural invasion in the primary tumor has been proposed as a predictor for prostate cancer bone metastasis [82,83]. These findings suggest that the crosstalk between prostate cancer and the central nervous system might be in part responsible for the bone metastatic process. It has been demonstrated that sympathetic nerve systems play a crucial role in prostate cancer bone metastasis. The high levels of β2-adrenergic receptor are observed in prostate cancer samples [84], and treatments with β-blockers lower bone metastatic lesion of prostate cancer [84,85]. Moreover, when autonomic nervous system is ablated, prostate cancer development and bone metastases are prevented [83]. Importantly, a recent retrospective study with over 3,500 prostate cancer patients who receive β-blockers demonstrated that β-blocker treatment improves mortality in high-risk or metastatic prostate cancer patients (adjusted sub-hazard ratio: 0.79; 95% confidence interval: 0.68–0.91; p=0.001) [86]. Furthermore, bone metastatic prostate cancer cells influence sensory neurons in the marrow. When prostate cancer cells reach to the bone, they enhance a sprouting of CGRP positive sensory neurons in the marrow, resulting in cancer-induced bone pain [87]. Since nerve components in the marrow in part assist the development of a favorable microenvironment for prostate cancer cells to establish bone metastasis, targeting the central nervous system can be an additional potential therapeutic option for prostate cancer patients with bone metastases.
Treatment strategies for men with bone metastases
Once prostate cancer has spread beyond the prostate to other parts of body such as bone, it becomes difficult to cure. Therefore, the goals of treatment shift from cure to palliative and disease control. Currently, nearly all prostate cancer patients with bone metastases receive analgesic medications, including opioids, due to painful skeletal-related events (SREs). However, opioid use has become a growing concern. Although opioids provide effective pain relief, the effects are usually partial and therefore the doses to achieve adequate analgesia for bone pain become progressively higher. This causes several side effects, including nausea, vomiting, constipation, pulmonary dysfunction, cognitive dysfunction, and addiction [88]. In addition, a recent study demonstrated that greater opioid requirement may be associated with inferior clinical outcome in advanced prostate cancer patients [89]. Since opioids do not treat the underlying cause of the pain, there is constant need to develop more effective treatments and/or strategies for addressing bone metastatic disease.
Radiotherapy
Radiotherapy offers another palliative pain control option for cancer patients with bone metastases. It has been demonstrated that palliative radiation provides overall pain relief to 50–80% of patients with cancer-induced bone pain [90]. A single fraction of local radiotherapy, repeated if necessary, appears to be the most effective when bone metastatic lesion is single or small. Recent randomized trials revealed that 8Gy in a single fraction is not inferior to, but less toxic than 20–30Gy in multiple fractions [91,92].
When bone metastases are found in multiple lesions, systemic radiotherapy is generally considered. Historically, β emitter based radiopharmaceuticals, Strontium-89 and Samarium-153, have been used as palliative treatment for metastatic castration-resistant prostate cancer patients. Although these two agents result in pain relief, neither of them have an impact on survival [93]. An α emitter Radium-223 (Ra-223) is the first and only Food and Drug Administration (FDA)-approved radioisotope for castration-resistant prostate cancer patients with bone metastases. Ra-223, by mimicking calcium, binds to hydroxyapatite enriched in the bone matrix at the area of high bone turnover associated with bone metastases, or osteoblastic bone metastatic lesion [94]. Thereafter, the local emission of high energy α particles from Ra-233 is thought to induce DNA damage and cell death in bone metastatic cancer cells [94]. Ra-223 has a very high energy deposit, but a very limited path range (< 100μm) because of its atomic weight [95]. Interestingly, Ra-223 provides substantial pain relief to prostate cancer patients with bone metastases, with only minimal myelotoxicity [96]. Therefore, Ra-223 appears to be safe and well tolerated. More importantly, Ra-223 also significantly improves mobility of bone metastatic patients. A phase III double blind randomized trial in symptomatic metastatic castration-resistant prostate cancer (ALSYMPCA) reported that Ra-223 treatment not only improves bone pain, but also prolongs overall survival, compared to placebo treatment (median survival: 14.9 months vs. 11.3 months; hazard ratio: 0.70; 95% confidence interval: 0.58–0.83; p<0.001) [97]. This significant improvement in overall survival encourages use of Ra-223 as combination or adaptive treatment strategies for prostate cancer patients with bone metastases. An international, early access, open- label, single-arm phase IIIb trial revealed that combining Ra-223 with other conventional treatments for advanced prostate cancer (e.g. abiraterone, enzalutamide, abiraterone plus enzalutaide, or denosumab) is associated with significantly longer overall survival than Ra-223 alone [98]. Therefore, Ra-223 appears to be a promising treatment for bone metastatic disease, however there are still financial hurdles that need to be overcome. Currently, in the US, 6 cycles of Ra-223 treatments cost about $70,000 [99].
Bone-targeting therapy
As stated above, the osteoclastogenesis plays a major role in development of skeletal metastasis. Therefore, targeting the osteoclast activity seems to be an ideal treatment strategy for bone metastatic disease, and this strategy contributes to delay the onset of SREs. Nitrogen-containing bisphosphonates are widely used for prevention and treatment of number of osteolytic conditions, including osteoporosis and bone metastases [100]. Bisphosphonates inhibit bone resorption by preventing hydroxyapatite breakdown by binding to hydroxyapatite crystals at the area of active bone turnover [100]. In addition, although clear mechanisms yet remain unknown, it has been suggested that bisphosphonates also directly induce osteoclasts apoptosis [101,102]. Several different generations of bisphosphonates (e.g. non-nitrogen-containing bisphosphonates, nitrogen-containing bisphosphonates) exist, but only the third-generation bisphosphonate, zoledronic acid show clinical benefit for advanced prostate cancer patients. A placebo-controlled randomized trial in metastatic castration-resistant prostate cancer patients showed that zoledronic acid reduce the incidence of SREs (44.2% vs. 33.2%, p=0.021) [103].
A human monoclonal antibody against RANKL, denosumab, has been emerged as an alternative osteoclast targeting agent [104]. Denosumab can inhibit osteoclast activity mediated by osteoblasts within the bone metastatic lesion by blocking the RANK/RANKL interaction. A phase III, randomized trials comparing denosumab and zoledronic acid, in metastatic castration-resistant prostate cancer patients indicated that denosumab extend the time to first SREs from 17.1 months to 20.7 months (hazard ratio, 0.82; 95% confidence interval: 0.71–0.95; p=0.0002 for noninferiority and p=0.008 for superiority) [105]. Another phase III, randomized trials in castration-resistant prostate cancer patients with no sign of bone metastasis, but with rising prostate-specific antigen (PSA), demonstrated that denosumab increase bone metastasis-free survival by a median of 4.2 months over placebo (hazard ratio, 0.85; 95% confidence interval: 0.73–0.98; p=0.028), and delay the onset of SREs [106]. However, in the same study, no differences in overall survival were observed between denosumab vs. placebo-treated patients [106].
Additionally, another potential bone-targeting reagent, OsteoDex has been recently developed. OsteoDex is a bi-functional macromolecular polybisphosphonate has osteoclast inhibitory function with anti-tumor ability [107,108]. A phase I safety study were performed in castration-resistant prostate cancer patients revealed no serious adverse effects, including renal toxicity [107]. Currently, a phase II trial testing the effect of osteodex on skeletal related pain in prostate cancer patients with bone metastases is underway (ClinicalTrias.gov: NCT02825628).
Conclusions and unanswered questions
Although bone marrow provides a fertile microenvironment for metastatic tumor cells, its role in the process of bone metastasis is largely unknown. The studies discussed in this article suggested that microenvironment for HSCs has a significant influence on the bone metastatic progression of prostate cancer (Figure 1). It has been evident that bone metastatic prostate cancer cells mimic hematopoietic cells during its dissemination to the bone to establish hold in the marrow, and that once in the marrow, disseminated prostate cancer cells parasitize the microenvironment for HSCs to survive within bone marrow coopting mechanisms that keep HSCs viable. These findings suggest that a specific component of the bone marrow microenvironment (e.g. HSC niche) can serve as a potential therapeutic target for bone metastatic disease.
Figure 1. Crosstalk between bone metastatic prostate cancer and bone marrow microenvironment.
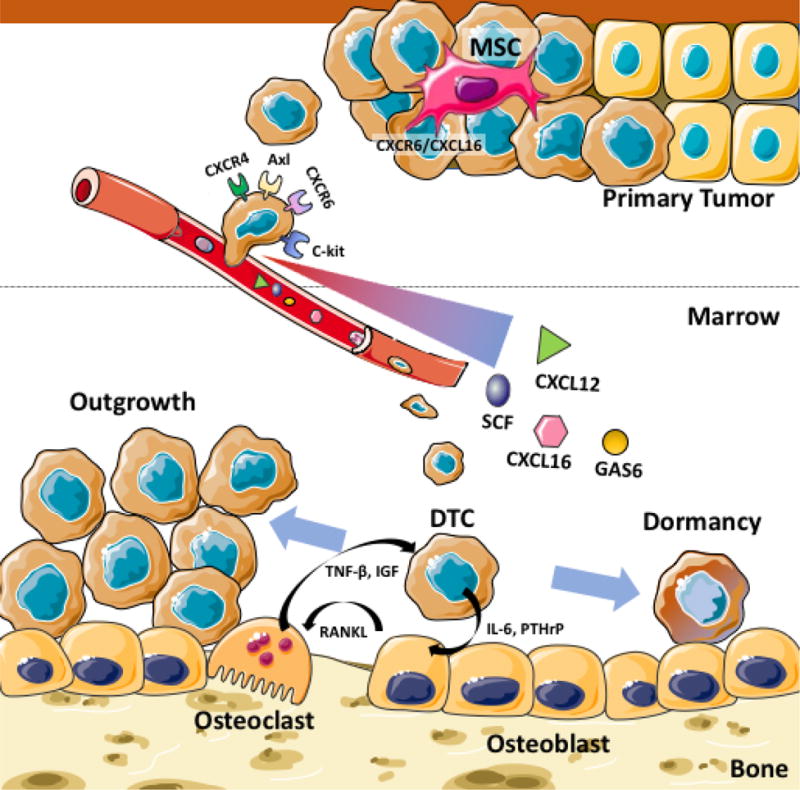
The bone marrow microenvironment is involved in the multiple steps of bone metastasis development. Bone marrow-derived mesenchymal stem cells (MSCs) promote primary prostate cancer to disseminate to bone using the CXCL16/CXCR6 axis. The circulating tumor cells that express the high level of CXCR4, CXCR6, c-Kit and Axl migrate towards bone based on a gradient of the chemokines and ligands (CXCL12, CXCL16, SCF, and GAS6) released from the bone marrow. Once in the bone marrow, the disseminated tumor cells (DTCs) are believed to become dormant by directly interacting with the bone marrow niche (e.g. osteoblast). At the same time, the DTCs influence the osteoblasts and osteoclasts to create a favorable microenvironment to accelerate later outgrowth.
In addition to pursuing therapeutic targets, palliative strategies are critical for bone metastases patient management. Pain management is vital in improving treatments for patients with advanced prostate cancer, since patients with bone metastases often suffer from significant painful complications, and cancer bone pain can cause substantially impair quality of life. Nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids are widely used analgesics for cancer bone pain, but can have serious side effects and abuse and addiction of these analgesics are a growing concern. Although bone-targeting agents (bisphosphonate, denosumab) and/or external beam radiotherapy are the standards of care for prevention or delay of SREs, these treatment strategies are not curative. Therefore, new approaches to cure bone metastatic prostate cancer are urgently needed. Immunotherapy has been recently investigated as an alternative therapeutic strategy. Subgroup analysis within the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) study found that Sipuleucel-T (Provenge), the first FDA approved immunotherapy for advanced prostate cancer reduces the risk of death of patients with greater than 10 bone metastases [109]. This finding warrants further investigation into the roles of checkpoint blockade inhibitors (e.g. an anti-PD1 antibody nivolumab and an anti-CTLA 4 antibody ipilimumab) in prostate cancer management. Combination therapies may offer the efficacy of the agents we discussed while minimizing their toxicity. Indeed, the combination of Ra-223 with hormone therapy, immunotherapy, chemotherapy, or bone-targeting therapy have been testing in the clinical setting [110–112].
Acknowledgments
This work is directly supported by National Cancer Institute Grants CA163124 (Y. Shiozawa) and P01 CA093900 (E. Keller), Department of Defense (W81XWH-14-1-0403 and PC160455, Y. Shiozawa), the Wake Forest School of Medicine Internal Pilot Funding (Y. Shiozawa), and the Wake Forest Baptist Comprehensive Cancer Center Internal Pilot Funding (Y. Shiozawa). Y Shiozawa is supported as the Translational Research Academy which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420. This work is also supported by the National Cancer Institute’s Cancer Center Support Grant award number P30CA012197 issued to the Wake Forest Baptist Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflict of interest:
The authors declare that there are no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Morash C, Tey R, Agbassi C, Klotz L, McGowan T, Srigley J, Evans A. Active surveillance for the management of localized prostate cancer: Guideline recommendations. Can Urol Assoc J. 2015;9(5–6):171–178. doi: 10.5489/cuaj.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 4.Batson OV. The Function of the Vertebral Veins and Their Role in the Spread of Metastases. Ann Surg. 1940;112(1):138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahim F, Hajizamani S, Mortaz E, Ahmadzadeh A, Shahjahani M, Shahrabi S, Saki N. Molecular regulation of bone marrow metastasis in prostate and breast cancer. Bone Marrow Res. 2014;2014:405920. doi: 10.1155/2014/405920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazo IB, von Andrian UH. Adhesion and homing of blood-borne cells in bone marrow microvessels. J Leukoc Biol. 1999;66(1):25–32. doi: 10.1002/jlb.66.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Miftakhova R, Hedblom A, Semenas J, Robinson B, Simoulis A, Malm J, Rizvanov A, Heery DM, Mongan NP, Maitland NJ, Allegrucci C, Persson JL. Cyclin A1 and P450 Aromatase Promote Metastatic Homing and Growth of Stem-like Prostate Cancer Cells in the Bone Marrow. Cancer Res. 2016;76(8):2453–2464. doi: 10.1158/0008-5472.CAN-15-2340. [DOI] [PubMed] [Google Scholar]
- 8.Chu GC, Zhau HE, Wang R, Rogatko A, Feng X, Zayzafoon M, Liu Y, Farach-Carson MC, You S, Kim J, Freeman MR, Chung LW. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr Relat Cancer. 2014;21(2):311–326. doi: 10.1530/ERC-13-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zalucha JL, Jung Y, Joseph J, Wang J, Berry JE, Shiozawa Y, Taichman RS. The Role of Osteoclasts in Early Dissemination of Prostate Cancer Tumor Cells. J Cancer Stem Cell Res. 2015;3 doi: 10.14343/jcscr.2015.3e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta KJ, Taichman RS. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12(2):116–127. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, Cher ML. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2006;66(1):32–48. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- 12.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 13.Tsuzuki S, Park SH, Eber MR, Peters CM, Shiozawa Y. Skeletal complications in cancer patients with bone metastases. Int J Urol. 2016;23(10):825–832. doi: 10.1111/iju.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok JL. Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer Res. 2004;64(15):5261–5269. doi: 10.1158/0008-5472.CAN-04-0691. [DOI] [PubMed] [Google Scholar]
- 15.Dimitroff CJ, Descheny L, Trujillo N, Kim R, Nguyen V, Huang W, Pienta KJ, Kutok JL, Rubin MA. Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Res. 2005;65(13):5750–5760. doi: 10.1158/0008-5472.CAN-04-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barthel SR, Wiese GK, Cho J, Opperman MJ, Hays DL, Siddiqui J, Pienta KJ, Furie B, Dimitroff CJ. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci U S A. 2009;106(46):19491–19496. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Zhang Q, Liu S, Parajuli KR, Qu Y, Mei J, Chen Z, Zhang H, Khismatullin DB, You Z. IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through CD44-VCAM-1 interaction. Prostate. 2015;75(8):883–895. doi: 10.1002/pros.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper CR, Chay CH, Pienta KJ. The role of alpha(v)beta(3) in prostate cancer progression. Neoplasia. 2002;4(3):191–194. doi: 10.1038/sj/neo/7900224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barthel SR, Hays DL, Yazawa EM, Opperman M, Walley KC, Nimrichter L, Burdick MM, Gillard BM, Moser MT, Pantel K, Foster BA, Pienta KJ, Dimitroff CJ. Definition of molecular determinants of prostate cancer cell bone extravasation. Cancer Res. 2013;73(2):942–952. doi: 10.1158/0008-5472.CAN-12-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engl T, Relja B, Marian D, Blumenberg C, Muller I, Beecken WD, Jones J, Ringel EM, Bereiter-Hahn J, Jonas D, Blaheta RA. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia. 2006;8(4):290–301. doi: 10.1593/neo.05694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8(2):R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, McCauley LK, Taichman RS. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38(4):497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67(1):61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 24.Shiozawa Y, Taichman RS. Getting blood from bone: an emerging understanding of the role that osteoblasts play in regulating hematopoietic stem cells within their niche. Exp Hematol. 2012;40(9):685–694. doi: 10.1016/j.exphem.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–1837. [PubMed] [Google Scholar]
- 26.Cojoc M, Peitzsch C, Trautmann F, Polishchuk L, Telegeev GD, Dubrovska A. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. Onco Targets Ther. 2013;6:1347–1361. doi: 10.2147/OTT.S36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283(7):4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 28.Conley-LaComb MK, Semaan L, Singareddy R, Li Y, Heath EI, Kim S, Cher ML, Chinni SR. Pharmacological targeting of CXCL12/CXCR4 signaling in prostate cancer bone metastasis. Mol Cancer. 2016;15(1):68. doi: 10.1186/s12943-016-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 30.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, Wang J, Jin T, Zhang H, Dai J, Krebsbach PH, Keller ET, Pienta KJ, Taichman RS. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh R, Kapur N, Mir H, Singh N, Lillard JW, Jr, Singh S. CXCR6-CXCL16 axis promotes prostate cancer by mediating cytoskeleton rearrangement via Ezrin activation and alphavbeta3 integrin clustering. Oncotarget. 2016;7(6):7343–7353. doi: 10.18632/oncotarget.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y, Wang J, Xu Y, Koch AE, Cai Z, Chen X, Galson DL, Taichman RS, Zhang J. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol Cancer Res. 2008;6(4):546–554. doi: 10.1158/1541-7786.MCR-07-0277. [DOI] [PubMed] [Google Scholar]
- 33.Ha HK, Lee W, Park HJ, Lee SD, Lee JZ, Chung MK. Clinical significance of CXCL16/CXCR6 expression in patients with prostate cancer. Mol Med Rep. 2011;4(3):419–424. doi: 10.3892/mmr.2011.446. [DOI] [PubMed] [Google Scholar]
- 34.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21(2):139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valencia K, Luis-Ravelo D, Bovy N, Anton I, Martinez-Canarias S, Zandueta C, Ormazabal C, Struman I, Tabruyn S, Rebmann V, De Las Rivas J, Guruceaga E, Bandres E, Lecanda F. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol. 2014;8(3):689–703. doi: 10.1016/j.molonc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, Moses MA, Demichelis F, Lisanti MP, Wu H, Klagsbrun M, Bhowmick NA, Rubin MA, D’Souza-Schorey C, Freeman MR. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181(5):1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, True LD, Rubin MA, Adam RM, Beroukhim R, Demichelis F, Freeman MR. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009;69(13):5601–5609. doi: 10.1158/0008-5472.CAN-08-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, Zhao H, Zhao Z, Du S, Tao J, Lee B, Westbrook TF, Wong ST, Jin X, Rosen JM, Osborne CK, Zhang XH. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27(2):193–210. doi: 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105(2):370–380. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung Y, Wang J, Song J, Shiozawa Y, Wang J, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110(1):82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung Y, Shiozawa Y, Wang J, Patel LR, Havens AM, Song J, Krebsbach PH, Roodman GD, Taichman RS. Annexin-2 is a regulator of stromal cell-derived factor-1/CXCL12 function in the hematopoietic stem cell endosteal niche. Exp Hematol. 2011;39(2):151–166 e151. doi: 10.1016/j.exphem.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung Y, Wang J, Lee E, McGee S, Berry JE, Yumoto K, Dai J, Keller ET, Shiozawa Y, Taichman RS. Annexin 2-CXCL12 interactions regulate metastatic cell targeting and growth in the bone marrow. Mol Cancer Res. 2015;13(1):197–207. doi: 10.1158/1541-7786.MCR-14-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taichman RS, Emerson SG. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells. 1998;16(1):7–15. doi: 10.1002/stem.160007. [DOI] [PubMed] [Google Scholar]
- 48.Kerr BA, Miocinovic R, Smith AK, West XZ, Watts KE, Alzayed AW, Klink JC, Mir MC, Sturey T, Hansel DE, Heston WD, Stephenson AJ, Klein EA, Byzova TV. CD117(+) cells in the circulation are predictive of advanced prostate cancer. Oncotarget. 2015;6(3):1889–1897. doi: 10.18632/oncotarget.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiesner C, Nabha SM, Dos Santos EB, Yamamoto H, Meng H, Melchior SW, Bittinger F, Thuroff JW, Vessella RL, Cher ML, Bonfil RD. C-kit and its ligand stem cell factor: potential contribution to prostate cancer bone metastasis. Neoplasia. 2008;10(9):996–1003. doi: 10.1593/neo.08618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Corn PG, Vogelzang NJ, Small EJ, Harzstark AL, Gordon MS, Vaishampayan UN, Haas NB, Spira AI, Lara PN, Jr, Lin CC, Srinivas S, Sella A, Schoffski P, Scheffold C, Weitzman AL, Hussain M. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiozawa Y, Pienta KJ, Taichman RS. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res. 2011;17(17):5553–5558. doi: 10.1158/1078-0432.CCR-10-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghajar CM. Metastasis prevention by targeting the dormant niche. Nat Rev Cancer. 2015;15(4):238–247. doi: 10.1038/nrc3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam HM, Vessella RL, Morrissey C. The role of the microenvironment-dormant prostate disseminated tumor cells in the bone marrow. Drug Discov Today Technol. 2014;11:41–47. doi: 10.1016/j.ddtec.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, Wilber A, Watabe K. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208(13):2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63(7):1684–1695. [PubMed] [Google Scholar]
- 57.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12(4):863–879. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma S, Xing F, Liu Y, Wu K, Said N, Pochampally R, Shiozawa Y, Lin HK, Balaji KC, Watabe K. Secreted Protein Acidic and Rich in Cysteine (SPARC) Mediates Metastatic Dormancy of Prostate Cancer in Bone. J Biol Chem. 2016;291(37):19351–19363. doi: 10.1074/jbc.M116.737379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung Y, Shiozawa Y, Wang J, McGregor N, Dai J, Park SI, Berry JE, Havens AM, Joseph J, Kim JK, Patel L, Carmeliet P, Daignault S, Keller ET, McCauley LK, Pienta KJ, Taichman RS. Prevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironment. Neoplasia. 2012;14(5):429–439. doi: 10.1596/neo.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taichman RS, Patel LR, Bedenis R, Wang J, Weidner S, Schumann T, Yumoto K, Berry JE, Shiozawa Y, Pienta KJ. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One. 2013;8(4):e61873. doi: 10.1371/journal.pone.0061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cackowski FC, Eber MR, Rhee J, Decker AM, Yumoto K, Berry JE, Lee E, Shiozawa Y, Jung Y, Aguirre-Ghiso JA, Taichman RS. Mer Tyrosine Kinase Regulates Disseminated Prostate Cancer Cellular Dormancy. J Cell Biochem. 2016 doi: 10.1002/jcb.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cook LM, Shay G, Araujo A, Aruajo A, Lynch CC. Integrating new discoveries into the “vicious cycle” paradigm of prostate to bone metastases. Cancer Metastasis Rev. 2014;33(2–3):511–525. doi: 10.1007/s10555-014-9494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Body JJ, Casimiro S, Costa L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol. 2015;12(6):340–356. doi: 10.1038/nrurol.2015.90. [DOI] [PubMed] [Google Scholar]
- 64.Sottnik JL, Keller ET. Understanding and targeting osteoclastic activity in prostate cancer bone metastases. Curr Mol Med. 2013;13(4):626–639. doi: 10.2174/1566524011313040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B, Shirai T, Matrisian LM, Futakuchi M. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7(5):485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate. 2008;68(13):1396–1404. doi: 10.1002/pros.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thudi NK, Martin CK, Murahari S, Shu ST, Lanigan LG, Werbeck JL, Keller ET, McCauley LK, Pinzone JJ, Rosol TJ. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate. 2011;71(6):615–625. doi: 10.1002/pros.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai J, Hall CL, Escara-Wilke J, Mizokami A, Keller JM, Keller ET. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008;68(14):5785–5794. doi: 10.1158/0008-5472.CAN-07-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armstrong AP, Miller RE, Jones JC, Zhang J, Keller ET, Dougall WC. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate. 2008;68(1):92–104. doi: 10.1002/pros.20678. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107(10):1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng Y, Basel D, Chow SO, Fong-Yee C, Kim S, Buttgereit F, Dunstan CR, Zhou H, Seibel MJ. Targeting IL-6 and RANKL signaling inhibits prostate cancer growth in bone. Clin Exp Metastasis. 2014;31(8):921–933. doi: 10.1007/s10585-014-9680-3. [DOI] [PubMed] [Google Scholar]
- 72.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 73.Patel MS, Elefteriou F. The new field of neuroskeletal biology. Calcif Tissue Int. 2007;80(5):337–347. doi: 10.1007/s00223-007-9015-3. [DOI] [PubMed] [Google Scholar]
- 74.Elefteriou F, Campbell P, Ma Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif Tissue Int. 2014;94(1):140–151. doi: 10.1007/s00223-013-9752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elefteriou F. Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci. 2005;62(19–20):2339–2349. doi: 10.1007/s00018-005-5175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 77.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elefteriou F. Role of sympathetic nerves in the establishment of metastatic breast cancer cells in bone. Journal of Bone Oncology. 2016;5(3):132–134. doi: 10.1016/j.jbo.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, Rowley D. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14(23):7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 81.Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, Rowley D. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49(3):213–223. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- 82.Ciftci S, Yilmaz H, Ciftci E, Simsek E, Ustuner M, Yavuz U, Muezzinoglu B, Dillioglugil O. Perineural invasion in prostate biopsy specimens is associated with increased bone metastasis in prostate cancer. Prostate. 2015;75(15):1783–1789. doi: 10.1002/pros.23067. [DOI] [PubMed] [Google Scholar]
- 83.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 84.Palm D, Lang K, Niggemann B, Drell TLT, Masur K, Zaenker KS, Entschladen F. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118(11):2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 85.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D’Agostino R, Jr, Danial N, Datta SR, Kulik G. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. 2013;123(2):874–886. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grytli HH, Fagerland MW, Fosså SD, Taskén KA. Association between use of β-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014;65(3):635–641. doi: 10.1016/j.eururo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 87.Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30(44):14649–14656. doi: 10.1523/JNEUROSCI.3300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–120. [PubMed] [Google Scholar]
- 89.Zylla D, Gourley BL, Vang D, Jackson S, Boatman S, Lindgren B, Kuskowski MA, Le C, Gupta K, Gupta P. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119(23):4103–4110. doi: 10.1002/cncr.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, Howell D, Konski A, Kachnic L, Lo S, Sahgal A, Silverman L, von Gunten C, Mendel E, Vassil A, Bruner DW, Hartsell W, American Society for Radiation O Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 91.Chow E, van der Linden YM, Roos D, Hartsell WF, Hoskin P, Wu JS, Brundage MD, Nabid A, Tissing-Tan CJ, Oei B, Babington S, Demas WF, Wilson CF, Meyer RM, Chen BE, Wong RK. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164–171. doi: 10.1016/S1470-2045(13)70556-4. [DOI] [PubMed] [Google Scholar]
- 92.Hartsell WF, Scott CB, Bruner DW, Scarantino CW, Ivker RA, Roach M, 3rd, Suh JH, Demas WF, Movsas B, Petersen IA, Konski AA, Cleeland CS, Janjan NA, DeSilvio M. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 93.El-Amm J, Aragon-Ching JB. Targeting Bone Metastases in Metastatic Castration-Resistant Prostate Cancer. Clin Med Insights Oncol. 2016;10(Suppl 1):11–19. doi: 10.4137/CMO.S30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harrison MR, Wong TZ, Armstrong AJ, George DJ. Radium-223 chloride: a potential new treatment for castration-resistant prostate cancer patients with metastatic bone disease. Cancer Manag Res. 2013;5:1–14. doi: 10.2147/CMAR.S25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bruland OS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12(20 Pt 2):6250s–6257s. doi: 10.1158/1078-0432.CCR-06-0841. [DOI] [PubMed] [Google Scholar]
- 96.Nilsson S, Larsen RH, Fossa SD, Balteskard L, Borch KW, Westlin JE, Salberg G, Bruland OS. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005;11(12):4451–4459. doi: 10.1158/1078-0432.CCR-04-2244. [DOI] [PubMed] [Google Scholar]
- 97.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall’Oglio M, Franzen L, Coleman R, Vogelzang NJ, O’Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland OS, Sartor O, Investigators A Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 98.Saad F, Carles J, Gillessen S, Heidenreich A, Heinrich D, Gratt J, Levy J, Miller K, Nilsson S, Petrenciuc O, Tucci M, Wirth M, Federhofer J, O’Sullivan JM, Radium-223 International Early Access Program I Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17(9):1306–1316. doi: 10.1016/S1470-2045(16)30173-5. [DOI] [PubMed] [Google Scholar]
- 99.Vuong W, Sartor O, Pal SK. Radium-223 in metastatic castration resistant prostate cancer. Asian J Androl. 2014;16(3):348–353. doi: 10.4103/1008-682X.127812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15(8):1467–1476. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- 102.Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001;28(5):465–473. doi: 10.1016/s8756-3282(01)00412-4. [DOI] [PubMed] [Google Scholar]
- 103.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B, Zoledronic Acid Prostate Cancer Study G A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 104.Paller CJ, Carducci MA, Philips GK. Management of bone metastases in refractory prostate cancer–role of denosumab. Clin Interv Aging. 2012;7:363–372. doi: 10.2147/CIA.S27930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, Miller K, Sieber P, Karsh L, Damiao R, Tammela TL, Egerdie B, Van Poppel H, Chin J, Morote J, Gomez-Veiga F, Borkowski T, Ye Z, Kupic A, Dansey R, Goessl C. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thellenberg-Karlsson C, Nyman C, Nilsson S, Blom R, Marquez M, Castellanos E, Holmberg AR. Bone-targeted Novel Cytotoxic Polybisphosphonate Conjugate in Castration-resistant Prostate Cancer: A Multicenter Phase 1 Study. Anticancer Res. 2016;36(12):6499–6504. doi: 10.21873/anticanres.11249. [DOI] [PubMed] [Google Scholar]
- 108.Alaiya A, Fox J, Bobis S, Matic G, Shinwari Z, Barhoush E, Marquez M, Nilsson S, Holmberg AR. Proteomic analysis of soft tissue tumor implants treated with a novel polybisphosphonate. Cancer Genomics Proteomics. 2014;11(1):39–49. [PubMed] [Google Scholar]
- 109.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 110.Nilsson S. Radionuclide Therapies in Prostate Cancer: Integrating Radium-223 in the Treatment of Patients With Metastatic Castration-Resistant Prostate Cancer. Curr Oncol Rep. 2016;18(2):14. doi: 10.1007/s11912-015-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morris MJ, Higano CS, Scher HI, Sweeney C, Antonarakis ES, Shevrin DH, Ryan CJ, Loriot Y, Fizazi K, Pandit-Taskar N, Garcia-Vargas JE, Lyseng K, Bloma M, Carrasquillo JA. Effects of radium-223 dichloride (Ra-223) with docetaxel (D) vs D on prostate-specific antigen (PSA) and bone alkaline phosphatase (bALP) in patients (pts) with castration-resistant prostate cancer (CRPC) and bone metastases (mets): a phase 1/2a clinical trial. 2015;33(suppl) [Google Scholar]
- 112.O’Sullivan JGS, Heidenreich A, Heinrich D, Gratt J, Lévy J, et al. Effects of concomitant use of abiraterone and/or enzalutamide with radium-223 on safety and overall survival in metastatic castration-resistant prostate cancer (mCRPC) patients treated in an international early access program (EAP) European society for medical oncology; Vienna, Austria: 2015. [Google Scholar]


