Abstract
Purpose
To evaluate the role of infiltrating macrophages in murine models of single and double mutation head and neck tumors using a novel fluorine-19 (19F) MRI technology.
Methods
Tumor cell lines single-hit/SCC4 or double-hit/Cal27, with mutations of TP53 and TP53 & FHIT, respectively, were injected bilateral into flanks of (N=10) female mice. With tumors established, perfluorocarbon (PFC) nanoemulsion was injected intravenously, which labels in situ predominantly monocytes and macrophages. Longitudinal spin-density weighted 19F MRI data enabled quantification of the macrophage burden in tumor and surrounding tissue.
Results
The average number of 19F atoms within the tumors was twice as high in the Cal27 group compared to SCC4 (3.9×1019 and 2.0×1019 19F/tumor, respectively, p = 0.0034) two days post-contrast injection, signifying increased tumor associated macrophages in double-hit tumors. The difference was still significant ten days post-injection. Histology stains correlated with in vivo results, exhibiting numerous PFC labeled-macrophages in double-hit tumors and to a lesser extent in single-hit tumors.
Conclusion
This study helps establish 19F MRI as a method for quantifying immune cells in the tumor microenvironment allowing distinction between double- and single-hit head and neck tumors. This technique would be extremely valuable in the clinic for pre-treatment planning, prognostics and post-treatment surveillance.
Keywords: Head and neck cancer, inflammation, MRI, fluorine-19, perfluorocarbon
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a source of significant morbidity and mortality worldwide. Risk factors include human papillomavirus (HPV) status and tobacco/alcohol use (1,2). Current staging and clinical management of HNSCC patients are based on anatomic location, size, and metastatic spread (3). If patients undergo surgical excision without adjuvant therapy, management decisions are guided by lymph node invasion or presence/absence of bone invasion (4). Patients with locally advanced disease are treated aggressively with multimodal therapy including chemotherapy and radiotherapy along with surgical resection (5).
HPV is increasingly responsible for the growing incidence of oropharyngeal squamous cell carcinoma, representing 30% of HNSCC tumors (6). HPV-positive tumor status is usually associated with better response to therapy and survival and is currently the strongest prognostic factor for these patients (7). For HPV-negative tumors, no prognostic assays are widely accepted (4). We designed this project based on the findings of our prior work (8) querying The Cancer Genomic Atlas (TCGA) (7), which contains data on the largest collection of HNSCC tumor specimens. In this work (8) we found that among patients with HPV negative tumors, reduced survival outcomes associated with tumor protein 53 (TP53) mutation only occur in combination with loss of chromosome 3p. This results in reduction of median survival from 5 years for TP53 mutation to only 1.7 years for ‘double-hit’ mutations (TP53 and 3p) (8). Beyond genetic mutations, the reasons for poor prognoses are still unclear. Current hypotheses focus on decreased radiosensitivity of double-hit tumors and the effects of infiltrating host immune cells (9). Indeed, it has been shown that HNSCC tumors inhibit natural killer cells, T cells and antigen presenting cells while recruiting regulatory T cells and tumor-associated macrophages (TAMs) (6). TAM burden has been shown to be a predictor of lymph node involvement and metastasis and hence tumor aggressiveness (10), and TAMs are known to secrete matrix metalloproteinases (MMP) which promote angiogenesis and tumor progression (11).
To evaluate the role of TAMs in tumors with either TP53 mutation alone (single-hit) or TP53 mutation combined with 3p deletion (double-hit), we identified human HNSCC cell lines (Cal 27, SCC25, SCC15 and SCC4) from the American Type Culture Collection (ATCC) that have differential TP53 status and Fragile Histidine Triad Protein (FHIT) copy number as classified by the Cancer Cell Line Encyclopedia. FHIT, located at position 3p14.2, is the gene locus most highly correlated with the 3p deletion in our TCGA analysis. FHIT is a tumor suppressor gene that has been reported as frequently inactivated in HNSCC cell lines (12). Decreased FHIT expression has been shown to correlate with worse survival in patients with tongue cancer (13). In the 3p region, we found that copy number variation for FHIT correlates best with 3p deletion status. FHIT has been shown to be a tumor suppressor (14), and FHIT deficient mice show both increased incidence of spontaneous tumor formation as well as increased tumor formation in response to carcinogens (15). Interestingly, loss of FHIT expression has been shown to correlate with poor outcomes in patients with tongue cancer (12). Because previous in vivo ratiometric cell penetrating peptide (RACPP) experiments have shown that Cal27 xenografts have higher MMP activity than SCC4 xenografts (13), we expect higher TAM accumulation in double-hit Cal27 compared to the single-hit SCC4 xenografts.
Clinically, invasive biopsy is the gold standard for diagnosis and genomic analysis of HNSCC, but there are several disadvantages of biopsying friable tumor tissue adjacent to vital structures in the head and neck which include bleeding, damage to nerves/airway/vessels, tumor cell deposition along the biopsy tract among others (16). Prior work has validated the use of novel fluorine-19 (19F) magnetic resonance imaging (MRI) probe technologies for macrophage tracking in preclinical studies (17-19). In this approach, perfluorocarbon (PFC) nanoemulsion is injected intravenously, and the emulsion droplets are scavenged in situ by cells of the reticuloendothelial system (RES), particularly circulating monocytes, macrophages, neutrophils, and DCs (20). These labeled cells can home to inflammatory foci and can be imaged by 19F MRI with no endogenous background signal. PFC-labeled macrophages dominate MRI-visible 19F “hot-spots” and there is a linear correlation between 19F signal and macrophage burden which can be quantitated directly from the in vivo images (18,21). Our aim is to evaluate this new imaging technology to noninvasively differentiate between double- and single-hit tumors in a rodent model of head and neck tumor xenografts. Our results suggest that in situ PFC macrophage labeling and MRI is an effective non-invasive tool for assaying TAM burden and may be predictive of treatment outcomes and the appropriateness of neoadjuvant treatments.
Methods
HNSCC cell lines
Human tongue squamous cell carcinoma cell lines (Cal27 & SCC4) from ATCC were used. The SCC4 cell line contained a missense TP53 mutation, and the Cal27 cell line contained both FHIT deletion and missense TP53 mutations (4). In detail, this prior work demonstrated homozygous loss of FHIT (FHIT-/-) for Cal-27 and FHIT heterozygosity (FHIT+/-) for SCC-4. The TP53 gene was mutated in both cell lines; SCC-4 contains a missense mutation (G→A) on nucleotide 7578479 of chromosome 17p, and CAL-27 contains a missense mutation (T→A) on nucleotide 7578271.
Mouse model of HNSCC
Animal protocols were approved by the University of California San Diego Institutional Animal Care and Use Committee (IACUC). Seven-week-old female Fox1nu nude mice (N=10) were obtained from Harlan/Envigo (Indianapolis, IN, USA) and divided into two experimental groups. Five million of either single-hit/ SCC4 or double-hit/Cal27 cells in 50% matrigel (Corning, Tewksbury, MA, USA) and 50% serum free DMEM (Gibco, Waltham, MA, USA) were injected in bilateral lower flanks of mice (N=5 in each group).
PFC emulsion
A sterile and injectable PFC nanoemulsion (VS-1000H, Celsense Inc., PA, USA) was used to label macrophages in situ. The nanoemulsion contains 30% (v/v) perfluoropolyether with a mean droplet size of 145 nm. The 19F magnetic resonance spectrum contains essentially a single major peak, with longitudinal (T1) and transverse (T2) relaxation times approximately T1 /T2 ∼ 450/180 ms at 11.7 Tesla. When PFC is administered intravenously, the nanoemulsion droplets are taken up by cells of the reticuloendothelial system, particularly monocytes and macrophages (22). After visible tumor growth to ∼8 mm, as measured by calipers, a single 0.2 ml dose of nanoemulsion (containing approximately 1.2×1021 F atoms) was injected intravenously via the lateral tail vein. For histology purposes, one animal in each group received a fluorescent version of the nanoemulsion (VS-1000H-DM-Red, Celsense) containing a bright fluorophore bound to the PFC droplet, with emission/excitation wavelengths equal to 596/615 nm.
In vivo MRI and quantification of 19F signal
Mice were subjected to longitudinal MRI on day 2 and 10 after the PFC injection. The MRI data were acquired using an 11.7 T Bruker BioSpec preclinical scanner (Bruker, Billerica, MA) with a dual-tuned 1H/19F birdcage volume coil (Bruker). During MRI, animals were anesthetized with 2% isoflurane in air, and core temperature was maintained at 37 °C using a heated pad. Reference capillaries with diluted PFC nanoemulsion (5% v/v) in 1% agarose were placed above the animal's torso in the image field of view (FOV). The 19F images were acquired using a RARE (Rapid Acquisition with Relaxation Enhancement) sequence with parameters TR/TE=1250/35 ms, RARE factor 16, matrix 64×64, FOV 30×30 mm2, slice thickness 1.5 mm, and 8-10 slices. The 1H anatomical images were also acquired using the RARE sequence with TR/TE=1000/18.5 ms, RARE factor 8, matrix 256×256, FOV 30×30 mm2, and the same slice package as the 19F data. Data were imported into Voxel Tracker® software (Celsense Inc., PA, USA), and 19F regions of interest (ROIs), or hot-spots, were manually outlined with guidance from the anatomical 1H images. ROIs of the 19F reference capillary and background noise were also delineated, where the external 19F capillary was used as an absolute 19F reference, The in vivo 19F signal in ROIs (i.e., apparent 19F atom number) was calculated using Voxel Tracker®, as previously reported (18,23) and quantitative analyses yielded the apparent number of fluorine atoms within the ROIs. For 19F image quantification, full-dynamic range data without thresholding were analyzed. Composite 19F/1H overlay images for display were also created; the 19F images were manually thresholded for visualization purposes to mask noise, and rendered in “red-hot” pseudo-color scale using Fiji software (24), while the 1H image remained in grayscale.
Histology and immunofluorescence
After in vivo MRI experiments, tumors and lymph nodes were excised and kept in OCT medium (VWR, Radnor, PA, USA) for histology cryosectioning to evaluate immune cell recruitment. Frozen sections were dried in the dark for 20 min at room temperature, fixed in 4% paraformaldehyde (Affimetrix, Santa Clara, CA) and then incubated with 2.5% BSA in PBS at room temperature for 15 min. Excess blocking reagent was discarded and the slides were incubated overnight in the dark at 4 °C with a rabbit anti-mannose receptor antibody (#64693, Abcam, Cambridge, MA, USA) diluted 1:200 in PBS BSA to bind a receptor present in macrophages. The slides were then washed three times with PBS and incubated for 30 min with Alexa Fluor 488 Goat anti-Rabbit IgG used at 1:200 dilution. Section nuclei were stained with Hoechst (#33342, 1:500 dilution, Thermo Fisher Scientific, Waltham, MA). The slides were washed with PBS three times, mounted using Vectashield (Vector), and photographed in a fluorescence microscope. Confocal images were acquired with a Leica SP5 2 confocal system (Leica Microsystems Inc.) coupled to a Leica DM 6000 CFS microscope and Leica LAS AF 1.8.2 software. Images were acquired using a ×63 immersion objective. Additionally, 5 μm sections (N=3) from six tumors in each group were subjected to immunohistochemistry staining using rabbit polyclonal antibody against CD68 (#125212, Abcam, Cambridge, MA, USA). CD68 stained macrophages were counted with three high power fields (HPF, 40×, N=108) per tumor by a pathologist, and an average macrophage count per tumor was calculated.
Statistics
All measurements are presented as mean ± standard error. A two-tailed T-test with unequal variances was performed to compare groups. The number of macrophages per tumor were correlated with total 19F signal per tumor using a Pearson's correlation coefficient test. P-values <0.05 are considered statistically significant.
Results
In vivo 19F MRI shows macrophage burden in the tumor
In vivo PFC labeling and subsequent 1H/19F MRI revealed several fluorine hotspots located in the abdomen of the mice. The hot-spots are displayed in the periphery of the tumors and in the adjacent lymph nodes (Fig. 1). Visually, there are more hotspots associated with the Cal27 (double-hit) tumors (Fig. 1A and C) versus the SCC4 tumors (single-hit) (Fig. 1B and D).
Figure 1.
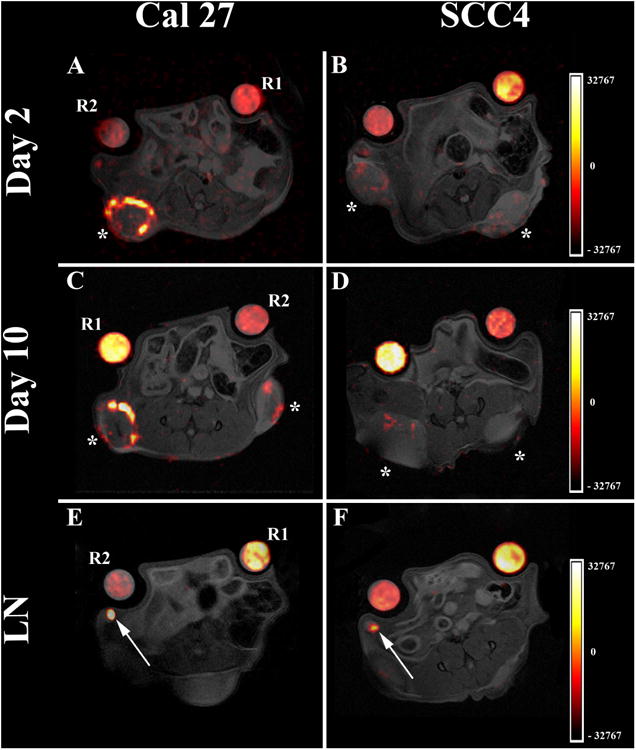
In vivo MRI displaying PFC distribution in the tumor periphery and draining lymph nodes. A. and C. Representative 1H/19F pseudo-color overlays of a mouse bearing Cal27 tumors showing significant macrophage infiltrates in the periphery of the tumors at day 2 and 10 respectively. B. and D. Representative 1H/19F overlays of a mouse bearing SCC4 tumors showing decreased macrophage burden at day 2 and 10 post PFC injection. E. and F. Corresponding 1H/19F pseudo-color overlays demonstrating fluorine hotspots (arrows) within local lymph nodes (LN) of the same animals in both Cal27 (E.) and SCC4 groups (F.). Tumors and lymph nodes were not visible in the same axial plane and therefore alternate slices were selected to show lymph node hotspots. Asterisks indicate the implanted tumors; R1 and R2 indicate tubes for reference quantification and the color scale is in arbitrary units.
Quantitatively, ROI analyses reveal that the total number of 19F atoms within the double-hit tumors is twice higher than the SCC4 single-hit tumors two days after PFC delivery (Fig. 2A, P = 0.0034). The difference between the double- and single-hit tumors remains significant at day 10 despite a mild decrease in overall signal over the course of 8 days (p =0.0063), showing that the imaging time window after agent injection is flexible. The number of macrophages per tumor moderately correlated to the fluorine signal (Pearson's r = 0.63, Fig. 2C). These quantitative differences support our hypothesis of increased TAM burden in the double-hit versus single-hit tumors. We also note that proximal lymph nodes (Fig. 1E and F, total 16 lymph nodes in 10 animals, Table 1) display 19F signals adjacent to the tumors, which is consistent with recruited immune cells aggregates within regional draining nodes. Quantitative analysis of the 19F within the lymph nodes show similar amounts of fluorine atoms in both double-hit and single-hit groups at day 2 (average of 3×1018 19F atoms, p = 0.3), which decrease over time (Fig. 2B, p = 0.07) in the double-hit group. The 19F signal within the lymph nodes for both double-hit and single-hit groups are lower than the corresponding tumors by a factor of ∼10. At the experimental endpoints, all animals were sacrificed, and the tumors and lymph nodes were excised and cryopreserved for histology.
Figure 2.
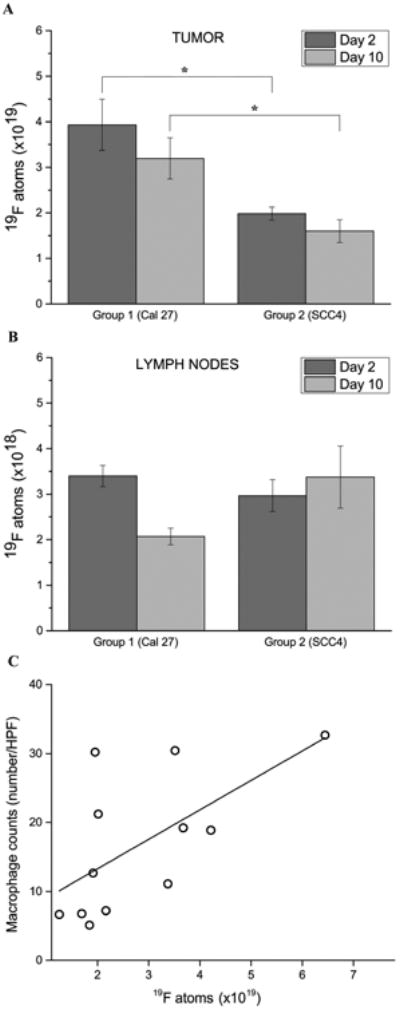
Quantitative analysis of macrophage burden in the tumors and draining lymph nodes from in vivo MRI data. A. 19F atom count is significantly higher in Cal27 tumors compared to SCC4 tumors at both time points (day 2 & 10 post PFC injection, asterisk indicates significance); this count is almost twice as high 2 days after PFC injection (3.94×1019 versus 1.98×1019 atoms respectively, p = 0.0034). B. Corresponding 19F atom count in the draining lymph nodes showing minor signal decrease for Cal27 tumors at day 10 compared to day 2 but no difference between the two groups at day 2; these differences were not significant, p>0.05. Overall, signal in the lymph nodes is lower than in the periphery of the corresponding tumors by a factor of ∼10. C. Plot showing moderate correlation (r = 0.63) between number of fluorine atoms per tumor and macrophage infiltration (N=108, high power fields, HPF).
Table 1.
Fluorine positive lymph nodes at Day 10. This table enumerates the number of individual (local & retroperitoneal) lymph nodes which demonstrated fluorine hot spots for both double hit (Cal 27) and single hit (SCC 4) groups 10 days post IV perfluorocarbon injection.
| Lymph nodes at Day 10 | |||
|---|---|---|---|
| Local LN | Retroperitoneal LN | ||
| Cal 27_Ml | 0 | 0 | |
| Cal 27_M2 | 2 | 0 | |
| Cal 27_M3 | 2 | 0 | |
| Cal 27_M4 | 0 | 2 | |
| Cal 27_M5 | 2 | 0 | |
| SCC4_M1 | 1 (R) | 0 | |
| SCC4_M2 | 1 (R) | 2 | |
| SCC4_M3 | 1 (R) | 1 (L) | |
| SCC4_M4 | 1 (R) | 0 | |
| SCC4M5 | 1 (R) | 0 | |
| LEGEND | |||
| 2 | Bilateral | ||
| 1 (R) or 1 (L) | Unilateral right or left | ||
| 0 | None | ||
Ex vivo analyses
Hematoxylin-eosin (H&E) and immunofluorescence staining was performed on cryosectioned tumors and lymph nodes after completion of the MRI experiments (Figs. 3-5). Co-registration of cell nuclei (Hoechst 33342) and macrophage mannose receptor (green) stains enabled qualitative visual assessment of macrophage burden in slide micrographs. The slides reveal the presence of fluorescent green signal in the periphery of both Cal27 double-hit and SCC4 single-hit tumors thus signifying presence of TAMs within tumors (Fig. 4), consistent with the 19F/1H MRI observations. The macrophage burden visually appears considerably higher for the Cal27 double-hit tumors (Fig. 4B). Importantly, the dual-mode MRI-fluorescent PFC agent tends to colocalize intracellularly with the green fluorescence depicting TAMs, consistent with macrophage-associated MRI hotspots (Fig. 4C). As expected, similar fluorescence signals are observed in the regional draining lymph nodes (Fig. 5B) confirming the local accumulation of fluorine containing macrophages, compared to a control lymph node showing minimal macrophage presence and no fluorine signal (Fig. 5C).
Figure 3.
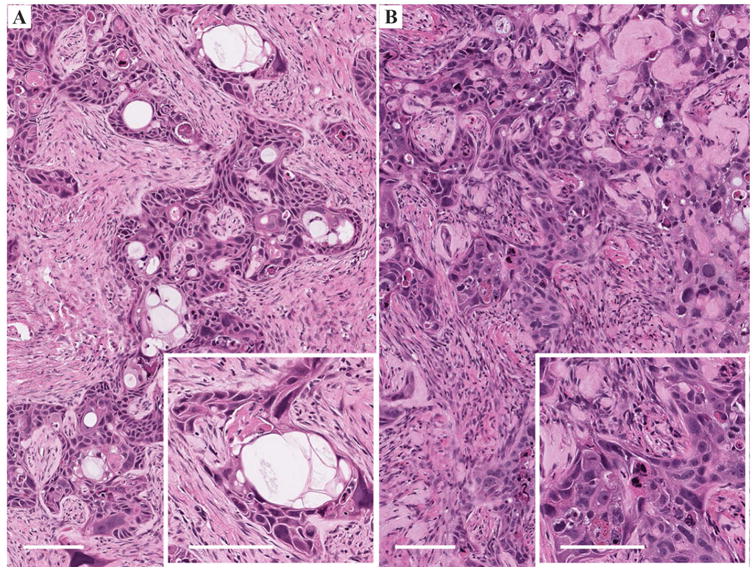
Histopathologic analyses of excised tumors stained with H&E. A. Moderately differentiated squamous cell carcinoma infiltrating a dense fibroblastic stroma. Numerous pseudocystic structures (white spaces) can be seen characteristic of this squamous cell carcinoma cell line. The inset shows a hyperintense multilocular structure containing cell debris. The proliferating cells are quite abnormal, depicting irregularly shaped and hyperchromatic nuclei. Bars represent 100 μm. B. Moderate to poorly differentiated squamous cell carcinoma infiltrating a hyalinized fibroblastic stroma. Squamous differentiation is focal. The inset shows the atypicity of the proliferating cells and the absence of clear keratinization. Bars represent 100 μm.
Figure 5.
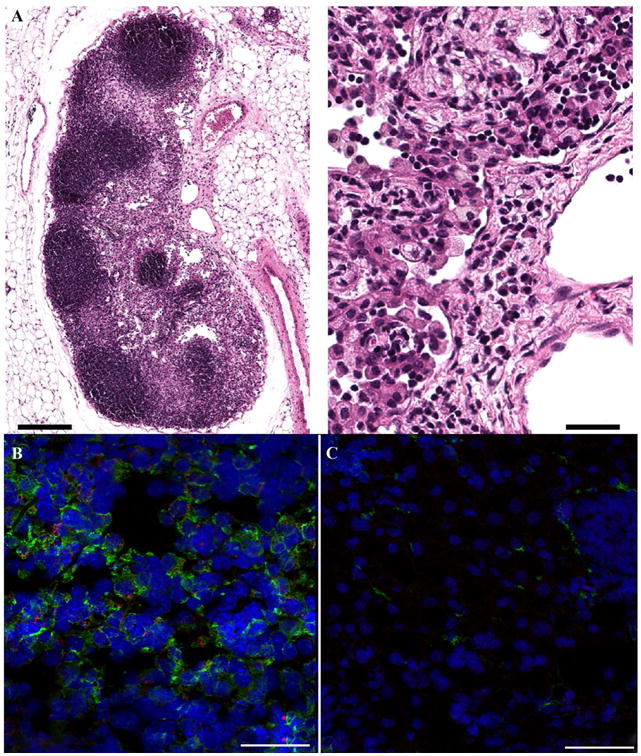
Histopathologic images of excised lymph nodes. A. H&E staining demonstrates numerous foamy macrophages within the excised lymph nodes. Left and right scale bars are 200 and 20 μm, respectively. B. Immunofluorescence staining demonstrates colocalization of macrophages (green) and intracellular dual-mode PFC (VS-1000H-DM Red) in lymph node, consistent with MRI findings (Fig. 1). Scale bar = 50 μm. Control lymph node in (C) displays minimal macrophage presence and an absence of PFC label. Scale bar = 50 μm.
Figure 4.
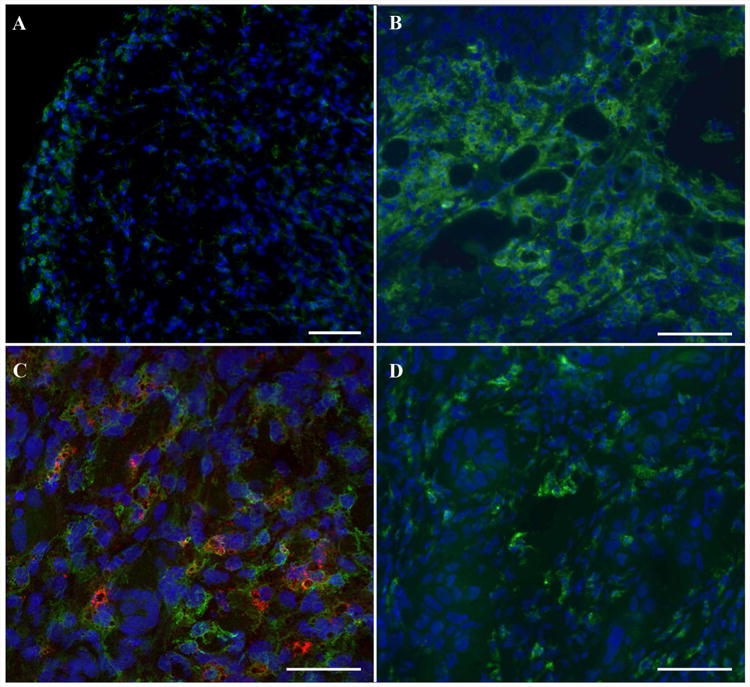
Immunofluorescence images of excised tumors. A. Low magnification immunofluorescence staining displays green fluorescent signal from TAMs (anti-mannose receptor, green), which confirming accumulation in the periphery of the tumors, and is consistent with the 19F MRI (Fig. 1). Scale bar is 100 μm. B. Qualitatively, numerous macrophages are observed within Cal27 tissue samples. C. Shows composite image of fluorescent dual-mode PFC (VS-1000H-DM Red) and macrophage (anti-mannose receptor, green) immunostaining and demonstrates intracellular uptake of PFC agent by macrophages in a Cal27 tissue sample. D. SCC4 tissue stains show scattered macrophages. Nuclei are stained with blue Hoechst 33342 stain. Scale bars (B-D) = 25 μm.
Discussion
To summarize, we show that PFC nanoemulsion and subsequent 1H/19F MRI allows for quantitative assessment of macrophage burden in tumors and adjacent lymph nodes in vivo. This imaging modality enables discrimination between double- and single-hit head and neck cancer xenografts based on differential TAM accumulation at the tumor periphery. These findings are in concordance with histopathologic observations. TAMs have been shown to accumulate at the tumor site and can be detected in vivo using in situ labeling techniques and MRI (25).
Macrophages play a multidimensional role in cancer. TAMs serve several pro-tumoral functions including the expression of growth, angiogenesis and lymphangiogenesis factors. Moreover, TAMs release matrix proteases leading to the suppression of adaptive responses (26,27). Considering this, the 19F hotspots possibly represent the colonizing front of the tumor. M1 macrophage subtype demonstrate high capacity to present antigens and activate polarized type I T-cell responses resulting in cytotoxicity towards tumor cells. On the other hand, M2 macrophages have poor antigen presenting capacity, suppress inflammatory responses and Th1 adaptive immunity and thus contribute in hijacking the local immune system away from anti-tumor function (28). Tissue-resident macrophage number may be amplified through the recruitment and differentiation of circulating monocytes in the context of inflammation/tumor expansion (29). Many growth and differentiation factors like M-CSF, PGE2, IL-6 & IL-10 expressed in the tumor microenvironment have the potential to promote differentiation and polarization of recruited monocytes into TAMs of the M2 subtype (30). TAMs also exert immunosuppressive activity by releasing chemokines that preferentially attract T-cell subsets devoid of cytotoxic function and thus facilitate tumor progression and metastatic invasion (5). In situ labeling of TAMs with PFC does not permit M1/M2 phenotype discrimination.
Our results show increased accumulation of TAMs within the Cal27 double-hit tumors compared to the SCC4 single-hit tumors, thus confirming prior work that higher frequency of infiltrating TAM has been associated with poor prognosis in many human tumors particularly lymphoma, liver and breast cancers (26,31-33). These preliminary preclinical observations could potentially have significant clinical relevance. Prior work has shown double-hit HNSCC is less radiosensitive compared to single-hit tumors; the increased TAM accumulation in double-hit tumors could partially explain the observed aggressiveness (13). Patients with double-hit/more aggressive tumor genotype would not benefit from radiotherapy alone, thus different treatment options would be preferred that lead to increased survival, such as conjunction of radiotherapy with chemotherapy or inhibitors of the radioresistance pathway (34,35). Conversely, lower grade single-hit tumor patients could be steered toward radiotherapy and/or surgical resection (35,36).
In vivo 19F MRI also revealed high signal in local lymph nodes adjacent to both double-hit and single-hit tumors. Overall, signals in the lymph nodes were ten times lower than in the tumors. Histopathologic analyses further confirmed the presence of fluorine-labeled macrophages within these lymph nodes, compared to control lymph nodes from naïve mice which showed minimal macrophage presence. In situ PFC cell labeling is expected to only label a small portion of macrophages. Go et al. (5) reported similar results for gastric cancer, describing immune cell accumulation in the lymphatic vessels of metastatic lymph nodes. They hypothesized that TAMs induce lymphangiogenesis and enhance lymphatic spreading of cancer by secreting vascular endothelial growth factor (VEGF-C) (5). They also noted that the number of intranodal immune cells in non-metastatic nodes was higher in regions nearest to primary tumor, suggesting migration of macrophages from primary tumor to augment intranodal lymphangiogenesis (5). In addition, the chemokines produced by TAMs in the tumor may reach the draining lymph nodes, and thus the tumor may thereby change the microenvironment of the surrounding regional lymph nodes before colonization of tumor cells. Others have shown that the density of M2 macrophages in regional lymph nodes is associated with lymphangiogenesis and occult nodal metastasis of pancreatic, oral squamous cell and esophageal cancers (37,38). This would imply that the high 19F signals we observe adjacent to the tumors are regional lymph nodes. Since these 19F MRI-visible regional nodes indicate both local macrophage accumulation and potential spread of tumor, this information could be used for surgical planning to noninvasively map the regional draining lymph nodes associated with the tumor if these imaging techniques could be translated to humans, as discussed below.
Overall, using nanoparticulate imaging probes, there are two hypotheses regarding the in vivo sites for TAM labeling (39); the first includes extravasation of circulating probe through the leaky tumoral vessels which is then scavenged by local TAMs (40,41). The second hypothesis (42) suggests that reticuloendothelial cells, particularly monocytes, are labeled in the peripheral blood, which then migrate to the site of the cancer to serve a role of immune modulatory cells (29,39) We note that the plasma half-life of the PFC nanoemulsion formulation used is ∼9.5 hours in rodents (23); thus, there is ample time for either (or both) hypothesis(es) to be relevant. Our view is that the actual in vivo site where labeling occurs is not central to the interpretation of the 19F image data.
The 19F hotspots observed in this study were most likely dominated by labeled macrophages, but it is possible that other cells contributed to the observed 19F signal. Very small numbers of B lymphocytes, neutrophils, and dendritic cells will take up PFC nanoemulsion (42,43). Measuring the average amount of PFC uptake per cell (macrophage) in situ is not feasible, unlike the case of ex vivo labeling experiments, where apparent cell numbers at hotspots can be directly deduced from the in vivo imaging data after labeled cell infusion (44,45). However, it has previously been shown (18,21) that the apparent number of 19F atoms detected linearly correlates to the number of macrophages present and the inflammatory burden in vivo. Thus, relative TAM burden comparisons between tumors of different genotypes based on 19F atom counts is plausible. Generally, MRI-detectable macrophages labeled with PFC can persist for long time periods; for example, a recent study using the same imaging agent in a murine model of inflammatory bowel disease (46) displayed PFC-labeled macrophages in the bowel wall for 110 days. Over time, eventually the PFC enters the RES system, particularly Kupffer cells of the liver, and PFC slowly clears the body via lung exhalation (47,48).
Our results suggest that there is a clinical rationale for potentially developing 19F inflammation imaging for clinical diagnostic use. The PFC agent used in these studies was for preclinical use only, and there are currently no approved PFC agents for clinical inflammation imaging. However, we note that a PFC imaging agent with a similar composition to what has been used here has been employed in a first-in-man clinical trial to detect immunotherapeutic dendritic cell vaccines injected into colorectal cancer patients (49). However, in this study, a minuscule dose is delivered to the patient compared to what is needed for in situ cell labeling as described here, thus the clinical development pathway is more complex. Also, there are potential adverse effects of large PFC emulsion doses as observed in artificial blood substitute studies; side effects included headache, dizziness, nausea, vomiting, back and chest pain, but these were mostly mild (79.8% of patients) (50); moreover, the severity of these effects may be dependent upon the choice of molecular components for PFC nanoemulsion formulation.
The notion of performing 19F MRI has been around for at least 40 years (51). Certainly, technical barriers associated with implementation of 19F MRI on a clinical scanner are surmountable, as the resonant frequency of 19F differs from 1H by only ∼6%. Many current generations of clinical scanners can be adapted to detect 19F, with minor hardware/software modifications (45). The sensitivity loss for the detection of labeled TAMs at lower clinical field strengths (3 T) compared to the preclinical MRI scanner (11.7 T) used in this study will depend on multiple factors such as field strength, coil configuration, voxel size and pulse sequence used, amongst other factors (52). One of the rate limiting factors in the clinical adoption of 19F-based technologies is the current availability of 19F-capable scanners in a hospital setting.
Conclusions
To conclude, we demonstrate the use of a novel non-invasive method to quantify apparent macrophage burden within head and neck tumors and local lymph nodes based on the use of PFC nanoemulsions and 19F MRI detection. We show that this technology can discriminate between single- and double-hit tumors with characteristic macrophage burden patterns, as well as sentinel lymph node involvement. Such an imaging tool would be very beneficial in a clinical setting to tailor personalized therapy without unnecessary biopsies.
Acknowledgments
We thank Monica Valeria Estrada for histopathology assistance. This work was funded by the National Institutes of Health grants R01-EB017271, R01-134633, 1TL1 TR001443 and the California Institute for Regenerative Medicine grant LA1-C12-06919. E.T.A is a Founder, member of the scientific advisory board and shareholder at Celsense, Inc.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Sanderson RJ, Ironside JA. Squamous cell carcinomas of the head and neck. BMJ. 2002;325(7368):822–827. doi: 10.1136/bmj.325.7368.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 4.Saber CN, Gronhoj Larsen C, Dalianis T, von Buchwald C. Immune cells and prognosis in HPV-associated oropharyngeal squamous cell carcinomas: Review of the literature. Oral Oncol. 2016;58:8–13. doi: 10.1016/j.oraloncology.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Go Y, Tanaka H, Tokumoto M, Sakurai K, Toyokawa T, Kubo N, Muguruma K, Maeda K, Ohira M, Hirakawa K. Tumor-associated macrophages extend along lymphatic flow in the pre-metastatic lymph nodes of human gastric cancer. Ann Surg Oncol. 2016;23(2):S230–235. doi: 10.1245/s10434-015-4458-7. [DOI] [PubMed] [Google Scholar]
- 6.Russell S, Angell T, Lechner M, Liebertz D, Correa A, Sinha U, Kokot N, Epstein A. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol. 2013;5(3):24. [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross AM, Orosco RK, Shen JP, Egloff AM, Carter H, Hofree M, Choueiri M, Coffey CS, Lippman SM, Hayes DN, et al. Multi-tiered genomic analysis of head and neck cancer ties TP53 mutation to 3p loss. Nat Genet. 2014;46(9):939–943. doi: 10.1038/ng.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B, Han SY, Wang X, Ottey M, Potoczek MB, Dicker A, Huebner K, Wang Y. Involvement of the Fhit gene in the ionizing radiation-activated ATR/CHK1 pathway. J Cell Physiol. 2005;202(2):518–523. doi: 10.1002/jcp.20139. [DOI] [PubMed] [Google Scholar]
- 10.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4(1):66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang JC, Chen JS, Lee CH, Chang JJ, Shieh YS. Intratumoral macrophage counts correlate with tumor progression in colorectal cancer. J Surg Oncol. 2010;102(3):242–248. doi: 10.1002/jso.21617. [DOI] [PubMed] [Google Scholar]
- 12.Lee JI, Soria JC, Hassan K, Liu D, Tang X, El-Naggar A, Hong WK, Mao L. Loss of Fhit expression is a predictor of poor outcome in tongue cancer. Cancer Res. 2001;61(3):837–841. [PubMed] [Google Scholar]
- 13.Raju SC, Hauff SJ, Lemieux AJ, Orosco RK, Gross AM, Nguyen LT, Savariar E, Moss W, Whitney M, Cohen EE, et al. Combined TP53 mutation/3p loss correlates with decreased radiosensitivity and increased matrix-metalloproteinase activity in head and neck carcinoma. Oral Oncol. 2015;51(5):470–475. doi: 10.1016/j.oraloncology.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996;84(4):587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 15.Zanesi N, Fidanza V, Fong LY, Mancini R, Druck T, Valtieri M, Rudiger T, McCue PA, Croce CM, Huebner K. The tumor spectrum in FHIT-deficient mice. Proc Natl Acad Sci U S A. 2001;98(18):10250–10255. doi: 10.1073/pnas.191345898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oetter N, Knipfer C, Rohde M, von Wilmowsky C, Maier A, Brunner K, Adler W, Neukam FW, Neumann H, Stelzle F. Development and validation of a classification and scoring system for the diagnosis of oral squamous cell carcinomas through confocal laser endomicroscopy. J Transl Med. 2016;14(1):159. doi: 10.1186/s12967-016-0919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flogel U, Su S, Kreideweiss I, Ding Z, Galbarz L, Fu J, Jacoby C, Witzke O, Schrader J. Noninvasive detection of graft rejection by in vivo (19) F MRI in the early stage. Am J Transplant. 2011;11(2):235–244. doi: 10.1111/j.1600-6143.2010.03372.x. [DOI] [PubMed] [Google Scholar]
- 18.Kadayakkara DK, Ranganathan S, Young WB, Ahrens ET. Assaying macrophage activity in a murine model of inflammatory bowel disease using fluorine-19 MRI. Laboratory Investigation. 2012;92(4):636–645. doi: 10.1038/labinvest.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong J, Narsinh K, Morel PA, Xu H, Ahrens ET. In vivo quantification of inflammation in experimental autoimmune encephalomyelitis rats using fluorine-19 magnetic resonance imaging reveals immune cell recruitment outside the nervous system. PLoS One. 2015;10(10):e0140238. doi: 10.1371/journal.pone.0140238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flaim SF. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Artif Cells Blood Substit Immobil Biotechnol. 1994;22(4):1043–1054. doi: 10.3109/10731199409138801. [DOI] [PubMed] [Google Scholar]
- 21.Ahrens ET, Young WB, Xu H, Pusateri LK. Rapid quantification of inflammation in tissue samples using perfluorocarbon emulsion and fluorine-19 nuclear magnetic resonance. BioTechniques. 2011;50(4):229–234. doi: 10.2144/000113652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacoby C, Temme S, Mayenfels F, Benoit N, Krafft MP, Schubert R, Schrader J, Flogel U. Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: image reconstruction, biological half-lives and sensitivity. NMR Biomed. 2014;27(3):261–271. doi: 10.1002/nbm.3059. [DOI] [PubMed] [Google Scholar]
- 23.Hitchens TK, Ye Q, Eytan DF, Janjic JM, Ahrens ET, Ho C. F-19 MRI Detection of Acute Allograft Rejection with In Vivo Perfluorocarbon Labeling of Immune Cells. Magn Reson Med. 2011;65(4):1145–1154. doi: 10.1002/mrm.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C, Rao J, Tikhomirov GA, Wendland MF, Corot C, et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res. 2011;17(17):5695–5704. doi: 10.1158/1078-0432.CCR-10-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 28.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 29.Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2 doi: 10.1038/npjbcancer.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 31.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 33.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 34.Perri F, Pacelli R, Della Vittoria Scarpati G, Cella L, Giuliano M, Caponigro F, Pepe S. Radioresistance in head and neck squamous cell carcinoma: biological bases and therapeutic implications. Head Neck. 2015;37(5):763–770. doi: 10.1002/hed.23837. [DOI] [PubMed] [Google Scholar]
- 35.Suh Y, Amelio I, Guerrero Urbano T, Tavassoli M. Clinical update on cancer: molecular oncology of head and neck cancer. Cell Death Dis. 2014;5:e1018. doi: 10.1038/cddis.2013.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corvo R. Evidence-based radiation oncology in head and neck squamous cell carcinoma. Radiother Oncol. 2007;85(1):156–170. doi: 10.1016/j.radonc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, Sakoda M, Iino S, Ishigami S, Ueno S, et al. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas. 2013;42(1):155–159. doi: 10.1097/MPA.0b013e318254f2d1. [DOI] [PubMed] [Google Scholar]
- 38.Wehrhan F, Buttner-Herold M, Hyckel P, Moebius P, Preidl R, Distel L, Ries J, Amann K, Schmitt C, Neukam FW, et al. Increased malignancy of oral squamous cell carcinomas (oscc) is associated with macrophage polarization in regional lymph nodes - an immunohistochemical study. BMC Cancer. 2014;14:522. doi: 10.1186/1471-2407-14-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58(14):1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc Res. 1989;37(1):77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 41.Mason RP, Antich PP, Babcock EE, Constantinescu A, Peschke P, Hahn EW. Non-invasive determination of tumor oxygen tension and local variation with growth. Int J Radiat Oncol Biol Phys. 1994;29(1):95–103. doi: 10.1016/0360-3016(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 42.Flogel U, Ding Z, Hardung H, Jander S, Reichmann G, Jacoby C, Schubert R, Schrader J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118(2):140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hertlein T, Sturm V, Kircher S, Basse-Lusebrink T, Haddad D, Ohlsen K, Jakob P. Visualization of abscess formation in a murine thigh infection model of staphylococcus aureus by F-19-magnetic resonance imaging (MRI) Plos One. 2011;6(3) doi: 10.1371/journal.pone.0018246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med. 2007;58(4):725–734. doi: 10.1002/mrm.21352. [DOI] [PubMed] [Google Scholar]
- 45.Ahrens ET, Zhong J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed. 2013;26(7):860–871. doi: 10.1002/nbm.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin SH, Kadayakkara DK, Bulte JW. In vivo 19F MR imaging cell tracking of inflammatory macrophages and site-specific development of colitis-associated dysplasia. Radiology. 2017;282(1):194–201. doi: 10.1148/radiol.2016152387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowe KC. Engineering blood: Synthetic substitutes from fluorinated compounds. Tissue Eng. 2003;9(3):389–399. doi: 10.1089/107632703322066570. [DOI] [PubMed] [Google Scholar]
- 48.Riess JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2005;33(1):47–63. doi: 10.1081/bio-200046659. [DOI] [PubMed] [Google Scholar]
- 49.Ahrens ET, Helfer BM, O'Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014;72(6):1696–1701. doi: 10.1002/mrm.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noveck RJ, Shannon EJ, Leese PT, Shorr JS, Flaim KE, Keipert PE, Woods CM. Randomized safety studies of intravenous perflubron emulsion. II. Effects on immune function in healthy volunteers. Anesth Analg. 2000;91(4):812–822. doi: 10.1097/00000539-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Holland GN, Bottomley PA, Hinshaw WS. 19F magnetic resonance imaging. J Magn Reson. 1977;28(1):133–136. [Google Scholar]
- 52.Hashemi RH, Bradley WG. MRI the basics. LW & W. 1997 [Google Scholar]


