See Thompson and Woollams (doi:10.1093/brain/awx264) for a scientific commentary on this article.
Naming in aphasia can be facilitated by phonemic cueing, both as an immediate retrieval strategy and as a long-term treatment. Nardo et al. report that immediate and long-term facilitation of naming rely on common neural networks, while whole words and partial cues promote naming via different networks.
Keywords: aphasia, word retrieval, anomia treatment, phonemic cueing, picture naming
Abstract
See Thompson and Woollams (doi:10.1093/brain/awx264) for a scientific commentary on this article.
Previous research with aphasic patients has shown that picture naming can be facilitated by concurrent phonemic cueing [e.g. initial phoneme(s) of the word that the patient is trying to retrieve], both as an immediate word retrieval technique, and when practiced repeatedly over time as a long-term anomia treatment. Here, to investigate the neural mechanisms supporting word retrieval, we adopted—for the first time—a functional magnetic resonance imaging task using the same naming procedure as it occurs during the anomia treatment process. Before and directly after a 6-week anomia treatment programme, 18 chronic aphasic stroke patients completed our functional magnetic resonance imaging protocol—a picture naming task aided by three different types of phonemic cues (whole words, initial phonemes, final phonemes) and a noise-control condition. Patients completed a naming task based on the training materials, and a more general comprehensive battery of language tests both before and after the anomia treatment, to determine the effectiveness and specificity of the therapy. Our results demonstrate that the anomia treatment was effective and specific to speech production, significantly improving both patients’ naming accuracy and reaction time immediately post-treatment (unstandardized effect size: 29% and 17%, respectively; Cohen’s d: 3.45 and 1.83). Longer term gains in naming were maintained 3 months later. Functional imaging results showed that both immediate and long-term facilitation of naming involved a largely overlapping bilateral frontal network including the right anterior insula, inferior frontal and dorsal anterior cingulate cortices, and the left premotor cortex. These areas were associated with a neural priming effect (i.e. reduced blood oxygen level-dependent signal) during both immediate (phonemically-cued versus control-cue conditions), and long-term facilitation of naming (i.e. treated versus untreated items). Of note is that different brain regions were sensitive to different phonemic cue types. Processing of whole word cues was associated with increased activity in the right angular gyrus; whereas partial word cues (initial and final phonemes) recruited the left supplementary motor area, and right anterior insula, inferior frontal cortex, and basal ganglia. The recruitment of multiple and bilateral areas may help explain why phonemic cueing is such a successful behavioural facilitation tool for anomia treatment. Our results have important implications for optimizing current anomia treatment approaches, developing new treatments, and improving speech outcome for aphasic patients.
Introduction
Anomia—the inability to retrieve words an individual wants to say—is the most common symptom of aphasia post-stroke, often regardless of severity and lesion location (Goodglass, 1993; Crinion and Leff, 2007). The deficit typically persists in chronic aphasia, and constitutes a serious obstacle to patients’ speech, communication and effective functioning in everyday life (Code, 2003; Johansson et al., 2012). As such, anomia and its treatment is seen as a hallmark for aphasia recovery.
Functional imaging studies to date have mainly focused on naming performance as their primary outcome measure for aphasia treatment success (for a review, see Crinion and Leff, 2015). All report significant changes in brain activity following treatment with some degree of consistency. For some, left perilesional activation is deemed critical for aphasia recovery (Fridriksson, 2010; Rochon et al., 2010; Fridriksson et al., 2012; Abel et al., 2014), while others report significant bilateral changes (Fridriksson et al., 2007; Vitali et al., 2007; van Hees et al., 2014; Abel et al., 2015). In the case of speech production, the contribution of language homologue areas in the right hemisphere and the direction of these effects (i.e. increase versus decrease of task-dependent activation) remains hotly debated, particularly with respect to whether right frontal cortices—including Broca’s area homologue—play a beneficial (Blasi et al., 2002; Crinion and Price, 2005) or detrimental (Naeser et al., 2005; Winhuisen et al., 2005) role in recovery. Accompanying these functional brain changes within the language network itself (e.g. left inferior frontal and superior temporal cortices, as in Fridriksson et al., 2012), are reported changes in additional non-language cognitive networks (Fridriksson et al., 2007; van Hees et al., 2014), leading some authors to propose that successful aphasia (particularly speech) treatment requires recruitment of both language and domain-general networks to facilitate recovery (Vitali et al., 2007; Fridriksson, 2010; Rochon et al., 2010; Abel et al., 2014, 2015; Brownsett et al., 2014).
A key factor that could help explain the variability of reported aphasia recovery results and treatment effects is the nature of the therapy administered to drive the brain and behavioural change. Many studies have used confrontation picture naming as their functional MRI task and outcome measure of aphasia treatment. This methodological choice allows the neural correlates underlying the post-therapeutic outcome to be investigated. However, the treatment approaches used have varied widely across studies (e.g. semantic versus phonological, errorful versus errorless, etc.), with a wide range in dose (total hours range from 12 to 56); intensity (hours per week from 5 to 15); and number of items treated (from 30 to 80). When the functional MRI task (e.g. free-naming) is different from the task used in aphasia treatment (e.g. cued-naming, or spoken word-to-picture matching), the interpretation of the imaging results is complicated (i.e. it is not clear how therapy facilitates brain and behavioural change in these patients). To improve aphasic patients’ speech recovery and outcome we need to investigate, understand and optimize the therapeutic mechanisms themselves that are driving the brain and behavioural change. This constitutes the focus of our present study.
A striking feature of anomia is that phonemic cues immediately aid word retrieval in many patients. Patients who are unable to name a given item (e.g. ‘car’) find they can say the word perfectly when given an auditory cue (e.g. the initial phoneme /ka/ or whole word /ka:r/). The cues convey speech sound information about the word in question (Pease and Goodglass, 1978; Kendall et al., 2008). Pairing these cues with pictures of items a patient repeatedly practices naming can result in long-term (un-cued) naming improvement (for reviews see Howard, 1994; Maher and Raymer, 2004), and clinically meaningful speech gains (Raymer et al., 2007; Lambon Ralph et al., 2010; Best et al., 2013). It has been suggested that this phonemic cueing treatment approach relies upon the same processes that underlie priming in unimpaired speakers (Best et al., 2002; Nickels 2002), where phonemic cues prime the retrieval of a word’s correct phonological form (Miceli et al., 1996; Starreveld, 2000). In aphasic patients, the hypothesis is that naming improvements using this treatment approach rely on recruitment and priming of residual ‘normal’ naming neural networks (Madden et al., 2017). However, despite the longstanding use of phonemic cues to aid naming in clinical practice, surprisingly the neural mechanisms underlying this treatment approach have not been investigated to date.
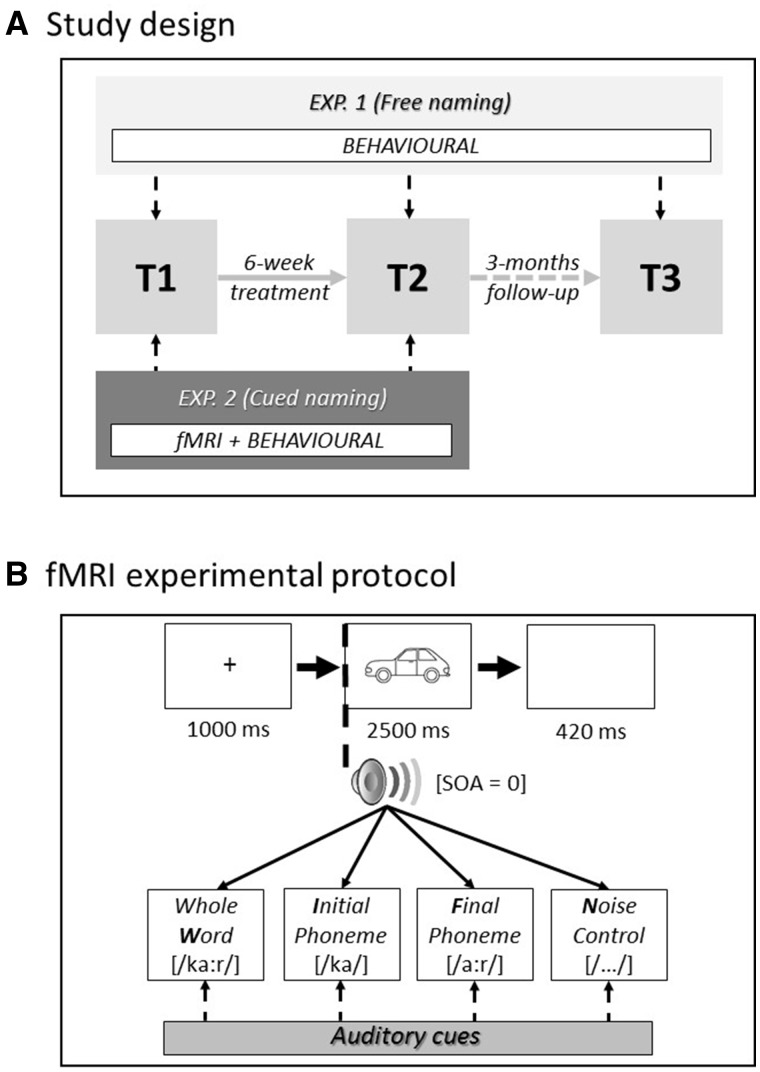
To do this, we designed two complementary experiments. The first experiment (Experiment 1: Free-naming; behavioural only) asked patients to perform a confrontation picture naming task, without the aid of any auditory cues at three time points: before (T1), directly after (T2), and 3 months after (T3) completion of an intensive, high-dose anomia treatment programme using the phonemic cued-naming approach and a large pool of items (see below).
The second experiment (Experiment 2: Cued-naming; conjoint behavioural-functional MRI) focused on the neural mechanisms implicated in word retrieval as it occurred during the therapeutic process at two time points: before (T1) and directly after (T2) the 6-week anomia treatment. Here, patients performed a picture naming task while in the functional MRI scanner, on a selected subset of items (both treated and untreated), presented concurrently with different auditory cue-types: three phonemic cues (whole words, initial phonemes, final phonemes) and one control condition (noise). This enabled us to investigate the neural mechanisms underlying: (i) immediate versus long-term cued facilitation of naming, in order to shed light on their relative nature; but also (ii) naming facilitation by different types of phonemic cues, to clarify whether they rely on shared or different neural processes. To our knowledge, the present study is the first to adopt a functional MRI task that directly mirrors the training task used in an anomia treatment programme. The comparison between cued and control items allowed us to investigate the immediate facilitation of naming, as per what was also happening in each cued-naming treatment session the patients completed. The comparison between treated and untreated items after treatment allowed us to characterize the consolidation of training utilizing these cues and long-term facilitation effects on naming performance.
We predicted that long-term word relearning and recovery would be correlated with increased efficiency and reduced blood oxygen level-dependent (BOLD) signal within the same bilateral residual speech network primed by phonemic cueing and facilitating immediate spoken word retrieval in our patients. Secondly, we predicted that different cue-types would have differential effects on naming performance (considering that they convey different amounts of information about the to-be-retrieved word), and would rely on differential activation within the bilateral residual speech network supporting spoken word retrieval in our brain damaged patients.
Materials and methods
Patients
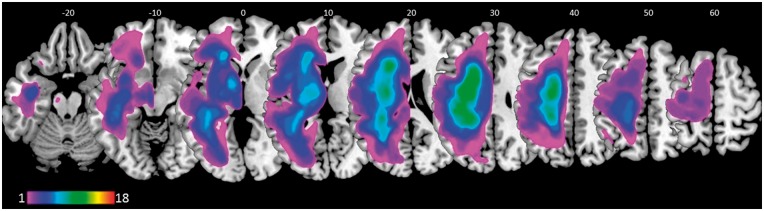
Eighteen right-handed native English speakers with acquired aphasia following a single left-hemisphere stroke participated in the study (see Fig. 1 for a lesion overlap map, Table 1 for demographic and clinical data, and Supplementary Table 1 for a description of lesion locations). All had normal hearing and no previous history of neurological or psychiatric disease, as well as no contraindications to MRI scanning. Inclusion criteria were: (i) anomia as determined by the Boston Naming Test (Kaplan et al., 1983; cut-off <56); (ii) good single word comprehension as assessed by the spoken words comprehension subtest of the Comprehensive Aphasia Test (Swinburn et al., 2005); (iii) relatively spared ability to repeat single monosyllabic words and non-words from the Psycholinguistic Assessments of Language Processing in Aphasia (Kay et al., 1992); (iv) absence of speech apraxia as determined by the Apraxia Battery for Adults (Dabul, 2000); and (v) spared or partially spared left inferior frontal cortex. All gave written informed consent to take part in the study, which was approved by the Central London Research Ethics Committee, and conducted in accordance with the ethical principles stated by the Declaration of Helsinki.
Figure 1.
Lesions overlap in our sample of patients. Colour range indicates the amount of overlap expressed as number of patients (colour bar). Numbers on the top represent z MNI coordinates of brain sections, displayed in neurological convention (i.e. L is L, R is R).
Table 1.
Demographic and clinical data of the patients
| Patient ID | Sex | Age | Lesion volume (cm3) | Months post-stroke | BNT | CAT | PALPA 9 | PALPA 8 | Hours of training |
|---|---|---|---|---|---|---|---|---|---|
| P1 | M | 64 | 171 | 78 | 47 | 15 | 20 | 6 | 40 |
| P2 | F | 49 | 44 | 17 | 12 | 15 | 21 | 6 | 31 |
| P3 | M | 54 | 294 | 78 | 14 | 11 | 10 | 0 | 77 |
| P4 | M | 41 | 234 | 65 | 28 | 14 | 24 | 8 | 116 |
| P5 | M | 49 | 144 | 57 | 34 | 15 | 17 | 2 | 50 |
| P6 | M | 66 | 109 | 61 | 52 | 15 | 24 | 6 | 63 |
| P7 | F | 44 | 82 | 72 | 34 | 14 | 24 | 10 | 59 |
| P8 | M | 54 | 95 | 34 | 35 | 15 | 24 | 8 | 70 |
| P9 | M | 67 | 341 | 47 | 42 | 14 | 24 | 9 | 85 |
| P10 | M | 41 | 75 | 8 | 23 | 13 | 23 | 8 | 89 |
| P11 | M | 63 | 139 | 264 | 51 | 15 | 24 | 9 | 81 |
| P12 | M | 47 | 314 | 52 | 16 | 15 | 22 | 6 | 77 |
| P13 | M | 56 | 150 | 40 | 1 | 14 | 18 | 2 | 61 |
| P14 | F | 60 | 104 | 121 | 27 | 13 | 22 | 7 | 120 |
| P15 | M | 41 | 114 | 18 | 42 | 14 | 21 | 3 | 43 |
| P16 | F | 21 | 155 | 33 | 18 | 15 | 20 | 3 | 108 |
| P17 | F | 47 | 161 | 53 | 9 | 9a | 12 | 0 | 76 |
| P18 | F | 43 | 165 | 5 | 21 | 15 | 23 | 1 | 67 |
| Mean (SD) | 50 (12) | 161 (84) | 61 (58) | 28 (15) | 14 (2) | 21 (4) | 5 (3) | 73 (25) | |
| Max score possible | 60 | 15 | 24 | 10 | |||||
BNT = Boston Naming Test; CAT = Comprehensive Aphasia Test (spoken words comprehension sub-test); PALPA = Psycholinguistic Assessments of Language Processing in Aphasia (PALPA 9 = monosyllabic words repetition, PALPA 8 = monosyllabic non-words repetition). aAlthough not normal, P17’s speech comprehension abilities were above chance, and all errors were semantic in nature (e.g. apple for pear).
Stimuli
In Experiment 1, stimuli consisted of 299 black and white line drawings of objects adapted from the International Picture-Naming Project (Szekely et al., 2004; http://crl.ucsd.edu/experiments/ipnp/index.html). All object names were monosyllabic, consonant-vowel-consonant in terms of phonological structure, and had high name agreement (i.e. at least 75% of test subjects produced the same target name). Monosyllabic words were used to minimize any effect of articulatory challenge.
In Experiment 2, a subset of 107 stimuli out of the 299 from Experiment 1 was used. In order to increase patients’ naming accuracy (and hence optimize the functional MRI design efficiency), this subset of stimuli was chosen by selecting items with the highest frequency ratings. Each picture was presented simultaneously with an auditory cue. Auditory cues were either: (i) a whole word cue (e.g. for the picture of a car, /ka:r/); (ii) an initial phoneme segment (e.g. /ka/); (iii) a final phoneme segment (/a:r/); or (iv) an unintelligible spectrally rotated noise-vocoded auditory control cue (noise), a condition successfully used in previous functional MRI studies (Scott et al., 2000; Narain et al., 2003; Obleser et al., 2007) See Supplementary material for full details on how the auditory cues were generated.
Anomia treatment
Patients were presented with 150 monosyllabic high frequency to-be-treated items taken from the pool of 299 used in the free-naming experiment (Experiment 1, previous section; 149 items were therefore untreated). Fifty-four items out of 150 were predetermined to be treated in all patients and also used as picture naming stimuli in the functional MRI scanner; whereas 53 of the untreated 149 items were used as naming stimuli in the scanner (Experiment 2). The remaining 96 to-be-treated items (150–54) and 96 untreated items (149–53) for each patient were determined on the basis of their individual pretreatment naming performance (in terms of accuracy) in Experiment 1. This ensured that each patient’s naming performance for the to-be-treated (96 + 54 = 150) and untreated (96 + 53 = 149) word lists were matched at baseline (i.e. that no bias occurred by chance between the two pools of items). These subject-specific items were not used in the functional MRI experiment (Experiment 2). Patients were given a laptop and asked to complete a minimum of 2 h of naming practice daily over a 6-week period. The pictures and auditory cues were presented using the ‘StepByStep’ aphasia treatment software (http://www.aphasia-software.com). The naming practice was designed to be completed in an error-reducing manner (Fillingham et al., 2003, 2006). For example, in naming a picture of a car the patient was asked to name it three times: (i) after a whole word auditory cue /ka:r/; (ii) after an initial phonemic cue /ka/; (iii) after a whole word cue again. Only then would the patient proceed to the next item to be named. Patients completed on average a total of 73 h of naming practice (Table 1). This is within one standard deviation (SD) of the mean total dose of treatment showing a positive impact on aphasic patients’ communicative ability, as found by Bhogal et al. (2003) in their meta-analysis of aphasia treatment studies.
Procedure
Experiments 1 and 2 were run in separate sessions, no more than 2 days apart at each of the testing time points (see Fig. 2 for details of study design and functional MRI experimental protocol, cf. Supplementary material). In both experiments, patients performed a picture naming task and were instructed to name each picture as quickly and as accurately as possible. Recordings of spoken responses were reviewed offline to score naming accuracy and determine trial-specific reaction time (RT) for each patient. We scored naming accuracy consistent with the standardized Comprehensive Aphasia Test guidelines (Swinburn et al., 2005): verbal, phonemic, neologistic, and dyspraxic errors were not accepted; dysarthric distortions were permitted provided it was clear that each phoneme within the word had been correctly selected.
Figure 2.
Study design (A) and functional MRI experimental protocol (B). fMRI = functional MRI; SOA = stimulus-onset asynchrony.
In Experiment 1, patients were asked to name 299 pictures without the aid of any auditory cue before (T1) and after (T2) the 6-week anomia treatment programme, plus at follow-up 3 months later (T3). In Experiment 2, patients performed an auditory-cued picture naming task in the scanner, at two time points (T1 and T2). Four functional runs were acquired within each scanning session at both time points. Each of the 107 picture stimuli was presented once within each functional run accompanied simultaneously (stimulus-onset-asynchrony = 0 ms) (Supplementary material) with one of four different cue-types (whole word, initial phoneme, final phoneme or noise-control). The order of pictures and accompanying cues was pseudo-randomized (i.e. avoiding more than three trials with the same cue-type), and the order of presentation was counterbalanced both within and across patients.
Behavioural analyses
To test the statistical significance of anomia treatment in Experiment 1 we conducted two repeated measures 2 × 2 ANOVAs (one on naming accuracy and one on RT) (Table 2 and Fig. 3A and B), with Time (T1, T2) and Treatment (treated items, untreated items) as within-subject variables. At T1, all items were untreated, but we kept them conceptually separated to check for any potential bias between the stimuli pools (Supplementary material). Hence, treated items at T1 are actually the about ‘to-be-treated’ items. We predicted a significant Time × Treatment interaction. The size of anomia treatment effects directly after treatment (T2 versus T1) and maintenance 3 months later (T3 versus T1) were quantified using both unstandardized and standardized (i.e. Cohen’s d) effect sizes (see Supplementary material for calculation).
Table 2.
Results of behavioural analyses in Experiment 1 and Experiment 2 (ANOVAs)
| EXPERIMENT 1 | ||||
|---|---|---|---|---|
| Accuracy | ||||
| F | DF-b | DF-w | P | |
| Time | 65.427 | 1 | 17 | <0.001 |
| Treatment | 69.163 | 1 | 17 | <0.001 |
| Time × Treatment | 29.109 | 1 | 17 | <0.001 |
| Reaction time | ||||
| F | DF-b | DF-w | P | |
| Time | 11.766 | 1 | 17 | <0.001 |
| Treatment | 29.170 | 1 | 17 | <0.001 |
| Time × Treatment | 23.859 | 1 | 17 | <0.001 |
| EXPERIMENT 2 | ||||
| Accuracy | ||||
| F | DF-b | DF-w | P | |
| Time | 14.377 | 1 | 17 | 0.001 |
| Treatment | 10.672 | 1 | 17 | 0.005 |
| Cueing | 29.050 | 3 | 51 | <0.001 |
| Time × Treatment | 24.279 | 1 | 17 | <0.001 |
| Time × Cueing | 1.266 | 3 | 51 | 0.296 |
| Treatment × Cueing | 3.701 | 3 | 51 | 0.017 |
| Time × Treatment × Cueing | 11.145 | 3 | 51 | <0.001 |
| Reaction time | ||||
| F | DF-b | DF-w | P | |
| Time | 12.275 | 1 | 17 | 0.003 |
| Treatment | 26.498 | 1 | 17 | <0.001 |
| Cueing | 61.631 | 3 | 51 | <0.001 |
| Time × Treatment | 17.216 | 1 | 17 | 0.001 |
| Time × Cueing | 3.170 | 3 | 51 | 0.032 |
| Treatment × Cueing | 3.998 | 3 | 51 | 0.012 |
| Time × Treatment × Cueing | 0.145 | 3 | 51 | 0.932 |
F = F-test; DF-b = degrees of freedom between; DF-w = degrees of freedom within; P = P-values.
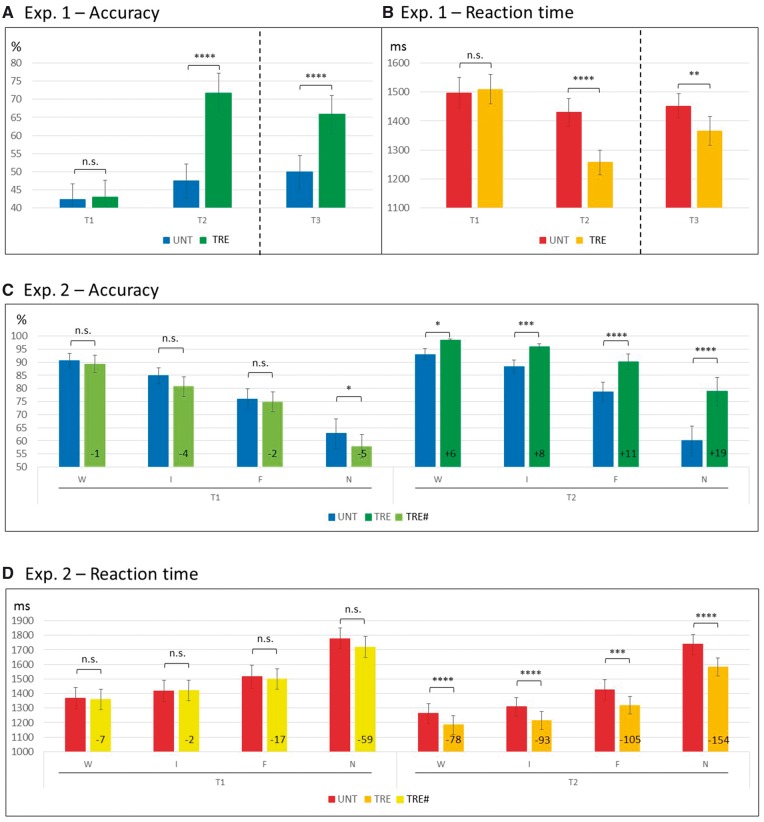
Figure 3.
Behavioural results of Experiment 1 (A–B) and Experiment 2 (C–D). Dispersions represent standard errors of the mean (SEM). Significance of post hoc comparisons: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005; ****P ≤ 0.001; n.s. = non-significant. For Experiment 1, ANOVAs reported are run across T1 and T2 only, for consistency with all other analyses (results at T3 are reported to show performance at follow-up). Note that (C) illustrates that the differences between untreated and ‘to-be-treated’ items at T1 were either non-significant or counter the predicted direction (i.e. UNT > TRE#), whereas at T2 all differences were significant and in line with the predicted direction (TRE > UNT). % = percentage of correct responses; F = final; I = initial; N = noise; TRE = treated items (at T1, TRE# = ‘to-be-treated' items); UNT = untreated items; W = word.
In Experiment 2, two repeated measures 2 × 2 × 4 ANOVAs were conducted (one on accuracy and one on correct RT) (Table 2 and Fig. 3C and D), with Time (T1, T2), Treatment (treated, untreated) and Cueing (whole word, initial phoneme, final phoneme, noise-control) as within-subject variables. Again, treated items at T1 are actually the about ‘to-be-treated’ items. Here, we focussed on both the effectiveness of the anomia treatment and the effect of cue-types, predicting significant interactions between Time and Treatment, and between Treatment and Cueing. Significance threshold for reported results was set to P < 0.05 throughout.
Imaging acquisition and analysis
Whole-brain imaging was performed on a 3 T Siemens TIM-Trio system (Siemens) at the Wellcome Trust Centre for Neuroimaging. T2*-weighted echo-planar images with BOLD contrast were acquired using a 12-channel head coil. Each image comprised 48 AC/PC-aligned axial slices with sequential ascending acquisition, slice thickness = 2 mm, inter-slice gap = 1 mm, in-plane resolution = 3 × 3 mm. Volumes were acquired with a repetition time = 3360 ms, and the first six volumes of each session were discarded to allow for T1 equilibrium effects. At each time point (T1 and T2), a total of 180 volume images (174 volumes of interest and six dummy scans) were acquired in four consecutive runs, each lasting ∼10 min. Prior to the first functional run of each scanning session, a gradient field map was acquired for each patient for later B0 field distortion correction of functional images. The same scanner and hardware were used for the acquisition of all images.
Functional data were preprocessed (see Supplementary material for details) and analysed using Statistical Parametric Mapping software (SPM12; www.fil.ion.ucl.ac.uk/spm) running under Matlab 2015a (MathWorks, Natick, MA). Statistical analyses were first performed in a subject-specific fashion. Nine conditions per each time point (i.e. four cue-types × two treatment levels, plus incorrect responses) were modelled separately as events convolved with the SPM canonical haemodynamic response function. We used the presentation of the concurrent picture and auditory cue as the onset of the event to model the preparatory naming response. Movement realignment parameters were included as covariates of no interest. The resulting stimulus-specific parameter estimates were calculated for all brain voxels using the General Linear Model. At the second level, 16 conditions of interest were modelled (four cue-types × two treatment levels × two time points, discarding incorrect responses, and merging sessions across T1 and T2), modelling subjects as a random factor. Significance threshold was set to P < 0.05 (FWE-corrected for multiple comparisons across the whole brain or within a region of interest, see below). Anatomical labelling was determined by using the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002).
Results
Experiment 1: Free-naming
Accuracy
Results showed a significant Time × Treatment interaction (Table 2, Fig. 3A and Supplementary Table 2). As predicted, naming of treated items was more accurate at T2 (72%) than T1 (43%), and this unstandardized effect size (29%) was significantly greater (P < 0.001) than the difference (5%) between untreated items at T2 (47%) and T1 (42%; P = 0.006). There were also significant main effects of Time and Treatment (both driven by improvements for treated items at T2). Treatment effects remained significant at T3 (Fig. 3A), indicating that naming gains were maintained 3 months later. Cohen’s d-values indicated large anomia treatment effect sizes: 3.45 for the immediate post-treatment effects (comparison T2 versus T1), and 1.83 for longer-term naming changes (T3 versus T1).
Reaction time
Results here mirrored the accuracy results, with a significant Time × Treatment interaction (Table 2 and Fig. 3B). Naming of treated items was faster at T2 (1257 ms) than T1 (1510 ms), and this unstandardized effect size (17%) was significantly greater (P < 0.001) than the difference (4%) between untreated items at T2 (1430 ms) than T1 (1496 ms; P = 0.206). Again, there was a significant main effect of both Time (faster at T2 than T1) and Treatment (driven by the treatment effect at T2). Here Cohen’s d-values were smaller than the effects on naming accuracy (−0.88, i.e. reduced RT post-treatment for the comparison T2 versus T1; and −0.45 for T3 versus T1).
In summary, our anomia treatment approach using phonemic cues resulted in significant, effective and long-lasting (i.e. maintained 3 months later) improvements in patients’ naming accuracy and efficiency (RT) that was greater for treated items. There was no evidence to support a naming accuracy versus speed trade-off in treatment gains. Importantly, none of the indices of treatment outcome (i.e. differences between treated items at different time points) correlated with variables such as age, months post-stroke, hours of training, and lesion volume (Supplementary Table 3).
Experiment 2: Cued-naming behavioural data
Accuracy
As predicted—and consistent with Experiment 1—the Time × Treatment interaction (see Table 2 and Fig. 3C for ANOVAs results) showed that naming of treated items was more accurate at T2 (91%) than T1 (76%), and this unstandardized effect size (15%) was significantly greater (P < 0.001) than the difference between untreated items at T2 (80%) and T1 (79%). There were also significant main effects of Time (more accurate at T2 than T1) and Treatment (driven by the treatment effects at T2).
There was also a significant main effect of Cueing. Across time points, accuracy was highest for word cues (93%) and least for no cues (65%). Time × Treatment × Cueing interaction showed that cueing modulated the effect of treatment on naming accuracy (i.e. treated versus untreated items) at T2 compared to T1. This also resulted in a significant Treatment × Cueing interaction, whereas the Time × Cueing interaction was not significant.
Having factored out any potential bias between the two pools of naming stimuli (‘to-be-treated’ versus untreated items) at T1 (cf. Fig. 3C and Supplementary material), we focus on post hoc analyses of naming differences at T2. Naming for treated (relative to untreated) items improved as follows for each cue-type: whole words from 93% to 99% (+6%), initial phonemes from 88% to 96% (+8%), final phonemes from 79% to 90% (+11%), noise-control from 60% to 79% (+19%; all P < 0.05). Note the probable ceiling effects observed here where at T2 the degree of naming improvement post-treatment across cue-types was inversely proportional to the degree of possible improvement from T1 scores (i.e. the more room there was for improvement, the more they improved with treatment).
Reaction time
These results mirrored the naming accuracy results with one exception. Here, the Time × Treatment × Cueing interaction was not significant. The Time × Treatment interaction (Table 2 and Fig. 3D) showed that naming of treated items was faster at T2 (1326 ms) than T1 (1499 ms), and this unstandardized effect size (12%) was significantly greater (P < 0.001) than the difference between untreated items at T2 (1433 ms) and T1 (1519 ms). The Time × Cueing interaction showed that RT was modulated differently by cue-types at T1 and T2. The Treatment × Cueing interaction showed that reductions in RT following treatment were significantly modulated by cue-types.
Again, having factored out any potential bias between the two pools of stimuli (‘to-be-treated’ versus untreated items) at T1 (cf. Fig. 3D and Supplementary material), we focus on post hoc analyses of RT differences at T2. At T2 naming RT for treated (relative to untreated) items improved as follows for each cue-type: whole words 78 ms (+6%), initial phonemes 93 ms (+7%), final phoneme 105 ms (+7%), noise-control 154 ms (+9%; all P < 0.005). Consistent with the improvements observed in naming accuracy post-treatment, the naming gains in RT were visible for all treated items across all cue-types, and cueing effects were observed irrespective of whether they were used in treatment or not. Results also showed significant main effects of Time (T2 faster than T1), Treatment (at T2), and Cueing (fastest for word cues and slowest for no cues).
Overall, our behavioural results from Experiment 2 on a cued-naming task replicated those from Experiment 1 on free-naming task. There was a significant positive effect of the anomia treatment on the patients’ naming performance, improving both accuracy and RT for treated items more than untreated items. Furthermore, Experiment 2 showed that the effect of treatment was greatest in the noise-control naming trials. These cues had no priming effects on naming performance, so data from these trials are most similar to free-naming (i.e. non-facilitated) performance (Experiment 1).
Neuroimaging data
Time
First, we assessed whether the simple effect of time elapsed between the two measures (T1, T2) had any impact on brain activity. Both contrasts T1 > T2 and T2 > T1 did not show any significant results even though accuracy was higher and RT was faster at T2.
Immediate facilitation of naming (cueing)
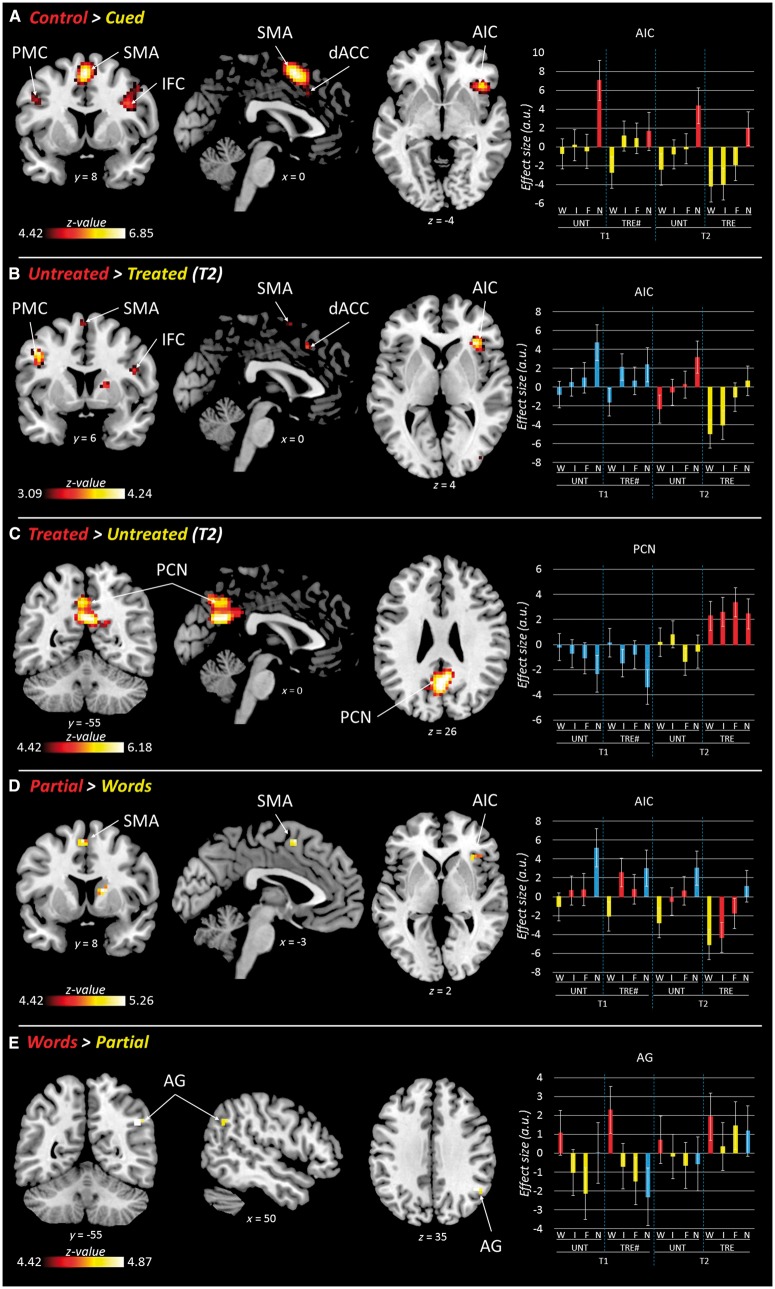
Second, we identified the neural network associated with the immediate facilitation effects of phonemic cues paired during naming by contrasting items paired with (i) whole word, initial and final phoneme cues (‘Cued’); and (ii) noise-control cue (‘Control’). The contrast Control > Cued resulted in a significant neural priming effect, i.e. reduced BOLD response for cued items as compared to noise-control items. Bilateral reductions were observed in the dorsal anterior cingulate cortex, supplementary motor area, premotor cortex (precentral gyri), and opercular inferior frontal cortex. Right lateralized reductions were observed in the anterior insula, extending into the adjacent orbital and triangular inferior frontal cortex, plus in the posterior superior temporal sulcus extending into the adjacent superior and middle temporal cortices (Table 3 and Fig. 4A). These results defined a functional ‘cueing network’ supporting naming performance. The reverse contrast (Cued > Control) did not show any significant result, although a sub-threshold peak was identified in the precuneus (Table 3).
Table 3.
Functional MRI results
| Contrast | Control > Cued | Untreated > Treated | ||||||
|---|---|---|---|---|---|---|---|---|
| Region | x y z | P(FWE) | K | Z | x y z | P(SVC) | K | Z |
| R anterior insular cortex | 42 23 −4 | <0.001 | 112 | 6.22 | 33 26 5 | 0.003 | 108 | 4.17 |
| R/L supplementary motor area | 0 8 56 | <0.001 | 271 | 6.85 | −3 5 65 | 0.082 | 163 | 3.25 |
| R dorsal anterior cingulate cortex | 9 20 35 | 5.74 | 3 20 44 | 0.022 | 3.67 | |||
| L dorsal anterior cingulate cortex | −6 20 38 | 5.55 | −6 17 35 | 0.944 | 1 | 1.68 | ||
| R inferior frontal gyrus (opercular) | 42 11 29 | <0.001 | 109 | 6.12 | 45 8 23 | 0.022 | 87 | 3.66 |
| R precentral gyrus | 48 8 44 | 5.02 | − | − | − | − | ||
| L inferior frontal gyrus (opercular) | −42 5 29 | <0.001 | 25 | 5.14 | − | − | − | − |
| L precentral gyrus | −45 5 38 | 4.92 | −45 5 38 | 0.007 | 25 | 3.98 | ||
| R middle temporal cortex | 60 −46 11 | 0.003 | 10 | 5.01 | − | − | − | − |
| Contrast | Cued > Control | Treated > Untreated | ||||||
| Region | x y z | P(unc.) | K | Z | x y z | P(FWE) | K | Z |
| R precuneus | 6 −55 29 | 0.003 | 4 | 2.70 | 6 −55 26 | <0.001 | 349 | 6.18 |
| L precuneus | − | − | − | − | −3 −58 41 | 5.78 | ||
| Contrast | Partial > Words | Initial > Final | ||||||
| Region | x y z | P(FWE) | K | Z | x y z | P(unc.) | K | Z |
| R anterior insular cortex | 33 26 2 | 0.004 | 9 | 4.93 | − | − | − | − |
| R inferior frontal gyrus (triangular) | 42 26 2 | 4.71 | − | − | − | − | ||
| L supplementary motor area | −3 8 56 | 0.003 | 11 | 5.06 | − | − | − | − |
| R caudate/putamen | 15 5 11 | <0.001 | 25 | 5.26 | − | − | − | − |
| Contrast | Words > Partial | Final > Initial | ||||||
| Region | x y z | P(FWE) | K | Z | x y z | P(unc.) | K | Z |
| R angular gyrus | 45 −52 32 | 0.004 | 9 | 4.87 | − | − | − | − |
| Contrast | Final > Words | Initial > Words | ||||||
| Region | x y z | P(FWE) | K | Z | x y z | P(unc.) | K | Z |
| R anterior insula | 33 23 2 | 0.012 | 4 | 4.68 | 33 26 2 | 0.001 | 225 | 3.84 |
| R inferior frontal cortex (triangular) | 48 26 17 | 0.020 | 2 | 4.77 | 45 26 5 | 3.36 | ||
| L supplementary motor area | −3 8 56 | <0.001 | 30 | 5.53 | −3 8 56 | 0.001 | 4 | 3.20 |
| R caudate/putamen | 15 5 11 | <0.001 | 29 | 5.46 | 15 5 11 | 0.001 | 225 | 3.66 |
| Contrast | Words > Final | Words > Initial | ||||||
| Region | x y z | P(FWE) | K | Z | x y z | P(unc.) | K | Z |
| R angular gyrus | 45 −55 32 | 0.028 | 1 | 4.73 | 51 −55 35 | 0.001 | 96 | 4.01 |
R = right; L = left; x y z = MNI coordinates; K = cluster size; Z = z-scores; FWE = family-wise error corrected P-values; SVC = small-volume corrected P-values within the volume of interest resulting from the contrast ‘Control > Cued'; unc. = uncorrected P-values (reported for completeness).
Figure 4.
Functional MRI results. (A) Reductions in BOLD response related to immediate facilitation of naming. (B) Reductions in BOLD response related to long-term facilitation of naming. (C) Increased BOLD response for treated (as compared to untreated) items. (D) Activations related to partial (i.e. initial and final phonemes) cues processing. (E) Activations related to whole word cues processing. Results are displayed at P < 0.05 (FWE-corr.), except in (B), where they are displayed at P < 0.001 (unc.) for cluster extent, without correction at cluster-level (to allow for small-volume correction). Red and yellow bars refer to the conditions compared (red > yellow, as in the title of each contrast). Blue bars are conditions not included in the contrasts. a.u. = arbitrary unit; AG = angular gyrus; AIC = anterior insular cortex; Control = control trials (i.e. noise); Cued = cued trials (i.e. word, initial, and final); dACC = dorsal anterior cingulate cortex; F = final; I = initial; IFC = inferior frontal cortex; N = noise; Partial = partial cues (i.e. initial and final phonemes); PCN = precuneus; PMC = premotor cortex; SMA = supplementary motor area; TRE = treated items (TRE# = ‘to-be-treated' items); UNT = untreated items; W = word; Words = whole word cues; x y z = MNI coordinates of brain sections. Sections are displayed in neurological convention.
Long-term facilitation of naming (treatment)
Third, we characterized the long-term facilitation effects of anomia treatment on naming (i.e. effect of treated versus untreated items at T2 only). The contrast Untreated > Treated did not show any significant activations at the whole-brain level. When the statistical threshold was lowered (P < 0.001 unc.) and a small-volume correction applied within the ‘cueing network’ identified with the orthogonal contrast Control > Cued, the following regions were identified: right anterior insula, dorsal anterior cingulate cortex, and opercular inferior frontal cortex; plus left premotor cortex (Table 3 and Fig. 4B). The effect of treatment in these areas mirrored that seen for cueing—i.e. a reduction in BOLD response when naming response was facilitated by treatment (or cueing). The reverse contrast Treated > Untreated showed a significant cluster of activation located in the precuneus and the adjacent posterior cingulate cortex (Fig. 4C), but no significant activation within the ‘cueing network’.
Partial cues versus whole-word cues (within-cues)
Fourth, we investigated whether the different auditory cue-types that contained varying amounts of phonemic/semantic information—i.e. whole words, initial phonemes, final phonemes—had a differential effect on brain activity during picture naming, considering that differential behavioural effects of cue-types were identified in terms of naming accuracy and RT between them (Fig. 3C and D). The contrasts Initial > Final and Final > Initial did not show any significant results, so we grouped Initial and Final cues together (i.e. ‘Partial’) for further analyses. The contrast Partial > Words identified significant clusters of activation in the left supplementary motor area, and right anterior insula and triangular inferior frontal cortex (within the ‘cueing network’), as well as in the right basal ganglia (Table 3 and Fig. 4D). The contrast Words > Partial showed a significant activation in the right angular gyrus (Table 3 and Fig. 4E). Importantly, results were replicated when initial and final cues were compared with words separately (i.e. Initial > Words and Final > Words, and vice-versa; cf. Table 3).
In summary, neuroimaging results showed a substantial overlap between the neural mechanisms implicated in immediate and long-term facilitation of picture naming in chronic aphasic stroke patients. Furthermore, different auditory cues facilitating picture naming recruited different hubs within the residual naming network with whole words activating right angular gyrus, and partial cues bilateral frontal regions.
Relationship between naming performance and brain activity
As a final step, we investigated whether and how individual patients’ change in brain activity (BOLD response: Experiment 2) following anomia treatment was related to their change in free-naming performance (Experiment 1). More specifically, we tested—for successfully named items only—whether the change in BOLD response for noise-cued pictures (Experiment 2) correlated with a change in un-cued naming RT (speed; Experiment 1). The noise-control cues had no priming effects on naming performance so data from these trials are most similar to free-naming (i.e. non-facilitated) performance (Experiment 1). First, we extracted the adjusted BOLD response values (noise-control condition only) on an individual basis from each of the peak regions identified with the contrast Untreated > Treated shown to be sensitive to anomia treatment (right anterior insula, inferior frontal cortex and dorsal anterior cingulate cortex, plus left premotor cortex) (Table 3). Then, we computed correlations between individual differences in BOLD response for treated and untreated items at T2 (T2_UNT-TRE) and the corresponding differences between naming RT scores (in Experiment 1).
Patients’ difference in BOLD response following treatment significantly correlated with treatment-induced improvements in naming efficiency in the right anterior insula (r = 0.51, P = 0.031) and right inferior frontal cortex (r = 0.57, P = 0.013), but not in the right dorsal anterior cingulate cortex and left premotor cortex. These correlations were positive: patients with the greatest right frontal decreases in BOLD response following treatment also had the greatest improvement in naming RT (biggest change in naming speed, i.e. faster) (Fig. 5).
Figure 5.
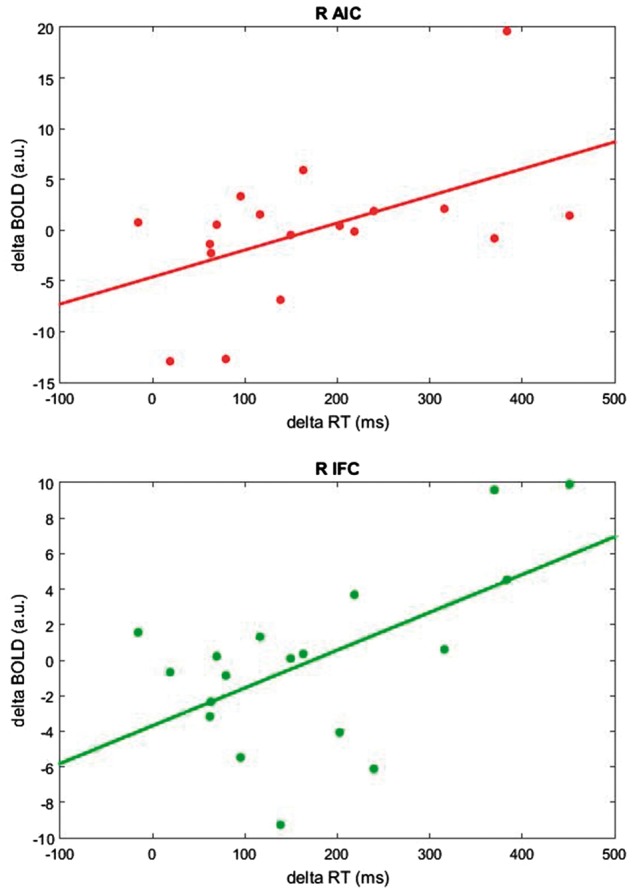
Correlation between behaviour and brain activity in the right anterior insular cortex (A) and in the right inferior frontal cortex (B). Plots show the relationship between naming efficiency (computed as the difference between mean RT: untreated-treated items at T2) and corresponding changes in BOLD response (i.e. untreated-treated) extracted during the noise-control condition. A greater improvement in naming efficiency (delta RT) is associated with greater changes in BOLD response (delta BOLD). R AIC = right anterior insular cortex; R IFC = right inferior frontal cortex; a.u. = arbitrary unit.
Discussion
In this study we aimed to understand, for the first time, how speech production in aphasic patients is supported by neural mechanisms both during anomia treatment (i.e. the therapeutic process itself), and following anomia treatment (i.e. longer-term speech outcome). To address this, we delivered a high-dose, cued-naming anomia treatment programme to a group of chronic aphasic stroke patients (Experiment 1) and—in the same patients—utilized a functional MRI cued-naming paradigm that mirrored the therapy approach, before and directly after the treatment (Experiment 2). We found significant treatment-specific effects, both in terms of naming improvements (RT and accuracy) and brain activity (BOLD signal), with immediate facilitation of naming performance and longer-term facilitation of naming (post-treatment) supported by the same bilateral residual neural network. Furthermore, patients’ treatment outcome (free-naming efficiency – RT) was directly related to neural priming (decreases in BOLD signal) in right frontal cortices. These data suggest that language homologue regions in the right hemisphere play an active and facilitatory role in chronic aphasic stroke patients’ anomia treatment response. Interestingly, naming accompanied by whole word cues activated the right angular gyrus, while partial cues (initial and final phonemic cues) activated bilateral frontal regions.
Like our first experiment (Experiment 1), there have been many behavioural studies focused on changes in aphasic patients’ speech performance (naming) following anomia treatment using a cued-naming approach, i.e. treatment outcome changes (Raymer et al., 2007; Kendall et al., 2008; Lambon Ralph et al., 2010; Best et al., 2013). This approach to anomia treatment works, even in the chronic stage post-stroke, and when given a high dose patients can make significant long-lasting speech gains (Bhogal et al., 2003; Brady et al., 2016; also cf. Breitenstein et al., 2017). However, treatment effects are item-specific with little-to-no generalization of improvements when naming untrained items (i.e. only naming of treated items improves). Consistent with this, we found significant treatment effects for treated items only, with long-lasting maintenance of naming gains observed 3 months post-treatment. The size of our treatment effects was large and arguably greater and/or longer-lasting than those reported in previous neuroimaging studies (cf. Fridriksson et al., 2007; Vitali et al., 2007; Rochon et al., 2010; Abel et al., 2014; van Hees et al., 2014; Dignam et al., 2016). This is likely due to the higher dose of treatment we delivered (73 h on average) and the larger pool of items we treated (n = 150).
Surprisingly, none of these studies have investigated how their adopted anomia treatments work at a brain systems level and lead to the observed speech change, i.e. brain plasticity underlying word retrieval during—and as a longer-term consequence of—therapy. In our study we intended to directly address this question. To do this, we used a functional MRI task (Experiment 2) based on the same approach as used in our behavioural therapy (Experiment 1). This enabled us to identify brain regions sensitive to our anomia treatment approach both immediately (during) and directly after (T2) the intervention. We found that immediate and long-term naming facilitation relied on a common bilateral neural network. This included in the right hemisphere the anterior insula, inferior frontal cortex, and dorsal anterior cingulate cortex; and in the perilesional left hemisphere the premotor cortex and supplementary motor area.
That the right hemisphere was consistently recruited during successful picture naming both during and directly post-treatment in our chronic aphasic patients is striking. There has long been a debate in the literature about the role of the right hemisphere in speech and language recovery. While some authors have claimed that its involvement is detrimental (Naeser et al., 2005; Winhuisen et al., 2005), others have argued that it might be beneficial (Blasi et al., 2002; Crinion and Price, 2005). More recently, it has been shown that language production outcome in chronic aphasic patients is associated with structural changes in language homologue areas in the right hemisphere (Wan et al., 2014; Pani et al., 2016; Xing et al., 2016).
The primary supporting evidence of an inhibitory role of the right hemisphere in aphasic patients’ spoken language function comes from neurostimulation studies. Here, low frequency repetitive (i.e. inhibitory) transcranial magnetic stimulation applied to the right inferior frontal cortex (Broca’s area homologue) has been associated with improved naming abilities (Martin et al., 2004; Naeser et al., 2005; for a meta-analysis, see also Ren et al., 2014). While the contradictions in the literature about this still need to be solved, it has been suggested that the mechanisms elicited by neurostimulation might not be as straightforward as previously outlined, inviting us to interpret this with caution. Indeed, issues such as the nature of the relationship between inhibitory and excitatory balance within a reorganizing bi-hemispheric language network, the relationship between short- and long-term effects of neurostimulation, and the interplay between specific subparts of the language network and their right hemisphere homologues are still rather unclear (Turkeltaub, 2015).
The set of prefrontal areas we identified included not only regions that might be considered right homologues of well-known speech and language network hubs (inferior frontal cortex, anterior insula, premotor cortex—especially related to phonological processing) (Bamiou et al., 2003; Liakakis et al., 2011), but also regions involved in executive processes and domain-general or multiple-demand systems (dorsal anterior cingulate cortex and supplementary motor area) (Duncan, 2010; Fedorenko et al., 2013; Fedorenko, 2014; Hertrich et al., 2016). Within our study design, we cannot tease apart the different role each of these regions contributed to naming. However, it is interesting to note that these regions were consistently modulated by task difficulty, i.e. hard versus easy naming conditions, as indexed by RT and accuracy. For example, for each of the functional MRI contrasts: Control > Cued, Untreated > Treated, and Partial > Words, BOLD activity within these regions was higher (Table 3).
In contrast, a posterior hub (precuneus) was associated with increased activation for the ‘easier’ naming conditions (Treated > Untreated; Cued > Control). The precuneus is a core hub of the so-called ‘default mode network’ (Raichle et al., 2001; Greicius et al., 2003), whose activity has been shown to be anti-correlated to task engagement (McKiernan et al., 2003; Pfefferbaum et al., 2011). Interestingly, in most previous studies of aphasic patients its activity has been systematically reported to be modulated by behavioural changes following therapeutic interventions (Fridriksson et al., 2007; Vitali et al., 2007; Menke et al., 2009; Fridriksson, 2010; Rochon et al., 2010; Abel et al., 2014, 2015; van Hees et al., 2014), although typically as a secondary or complementary result. Given the sensitivity of this neural structure to task engagement and its possible implication in treatment response, further investigation on the role of the precuneus in language recovery may be of interest in future studies.
Following treatment, only activation change in right anterior insula/inferior frontal cortex correlated significantly with improved naming efficiency (Fig. 5). This indicates a specific involvement of these regions in our patients’ recovery. We interpret this result as a consequence of our anomia treatment: the repeated pairing of cue and picture during treatment primed these right hemisphere regions (decreased BOLD signal) and facilitated more efficient word retrieval (faster and more accurate naming). This treatment approach made a naming task that was hard for the patients at the outset easier by utilizing and optimizing their residual right hemisphere speech networks.
Furthermore, similar neural mechanisms were involved in immediate and long-term facilitation of word retrieval. A significant neural priming effect during naming (i.e. reduced BOLD response) was observed for (i) cued items compared to control items; as well as for (ii) treated items compared to untreated items (i.e. as a consequence of the treatment undertaken). This suggests that neural priming mechanisms within the naming network underlie the patients’ immediate facilitation (faster RT) when naming cued pictures. Treatment (mass practice) then consolidated these mechanisms leading to further neural priming within the same network and faster naming RT when patients named the treated items (T2). This is consistent with cognitive models of speech production proposing that the phonemic cueing approach used in anomia treatment relies upon the same processes underlying cued-picture naming priming effects found in healthy speakers (Best et al., 2002; Nickels, 2002). Phonemic cues presented concurrently with a picture to-be-named prime the retrieval of a correct phonological word form, reduce lexical selection demands, and result in faster naming responses (Miceli et al., 1996).
Indeed, different auditory cues had differential effects on the patients’ immediate naming performance and right hemisphere brain activation patterns. This suggests that they may have been facilitating residual speech functions by tapping into different underlying neural and/or cognitive mechanisms. When given partial cues (initial and final phonemes), picture naming was more demanding/harder (as indexed by slower and less accurate responses) than naming with whole word cues (cf. Fig. 4D). Partial cues activated bilateral frontal regions, while whole word cues activated the right angular gyrus (Fig. 4E). Interestingly, this pattern was reversed in the case of treated items, whereby partial cues elicited a BOLD response similar to whole words (cf. Fig. 4D and E).
By definition, partial cues share only part of the phonological information of the target word, so that patients still need to retrieve the lexical, phonological and semantic representations of the target word. These cues probably act by priming phonologically similar words, thereby reducing competition among antagonist lexical representations (Aristei et al., 2012; Vitkovitch and Cooper, 2012; Melinger and Abdel Rahman, 2013; Britt et al., 2016; but see Mahon et al., 2007 and Navarrete et al., 2014 for alternative views), eventually improving word search and retrieval in patients. In healthy subjects, the left inferior frontal cortex has been reported to be implicated in phonological processing (Poldrack et al., 1999), word retrieval (Grabowski et al., 1998; Sharp et al., 2005), and articulatory planning (Price, 2010). On the other hand, recent neurostimulation studies have shown that the right inferior frontal cortex is also crucially implicated in phonological processing and object naming (Hartwigsen et al., 2010; Sollmann et al., 2014). In aphasic patients, perilesional areas in the inferior frontal cortex have been shown to play a vital role in aphasia recovery (Fridriksson, 2010; Fridriksson et al., 2012). Moreover, the right homologue of Broca’s area was found activated during word retrieval in patients with lesions to the left inferior frontal cortex (Perani et al., 2003).
In contrast, whole word cues provide the full lexical, phonological and semantic forms of the target word. As such, it could be argued that it is an ‘easier’ naming condition, and indeed patients may not have been using lexical retrieval processes per se, but rather word repetition processes to complete the task (Nozari et al., 2010). In healthy subjects, the left angular gyrus has been implicated in semantic processing (Mechelli et al., 2007; Price, 2010; Seghier et al., 2010), language comprehension and sentence processing (Sakai et al., 2001; Dronkers et al., 2004), and verbal working memory (Clark et al., 2001). In aphasic patients, lesions in the left angular gyrus were associated with impaired sentence comprehension and verbal working memory (Newhart et al., 2012), whereas in patients with extensive damage to the language network in the left hemisphere the right angular gyrus was found activated during semantic processing (Sims et al., 2016).
In summary, the present study aimed to understand—for the first time—the neural mechanisms underlying the phonemic cueing therapeutic process involved in anomia treatment, and post-treatment longer-term speech outcome in chronic aphasic patients. Our main result shows that immediate facilitation of naming performance using a cued-naming approach, and long-term facilitation of naming post-treatment are supported by a common bilateral residual neural network, including the anterior insula, inferior frontal cortex, and dorsal anterior cingulate cortex in the right hemisphere; the premotor cortex and supplementary motor area in the left hemisphere.
We have presented a new technique for evaluating the effect of anomia treatment in aphasia patients. The novel protocol we provide can be used as a framework for brain and behavioural plasticity in the damaged brain from which researchers and clinicians can optimize current anomia treatment approaches, develop new treatments, and ultimately improve speech outcome for aphasic patients. Future studies in our lab utilizing this approach will aim to investigate individual patients’ responses and make predictions about the therapeutic outcome. We hope this will lead to better patients’ stratification (e.g. identification of good candidates for this approach), optimizing both treatment path and outcome.
Funding
D.N. and J.C. are supported by a Wellcome Trust Senior Research Fellowship in Clinical Science (106161/Z/14/Z) awarded to J.C. Data collection was supported by an MRC Clinical Scientist Fellowship (G0701888) awarded to J.C.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- BOLD
blood oxygen level-dependent
- RT
reaction time
- T1
pretreatment measure
- T2
post-treatment measure
- T3
follow-up measure 3 months after treatment
References
- Abel S, Weiller C, Huber W, Willmes K. Neural underpinnings for model-oriented therapy of aphasic word production. Neuropsychologia 2014; 57: 154–65. [DOI] [PubMed] [Google Scholar]
- Abel S, Weiller C, Huber W, Willmes K, Specht K. Therapy-induced brain reorganization patterns in aphasia. Brain 2015; 138: 1097–112. [DOI] [PubMed] [Google Scholar]
- Aristei S, Zwitserlood P, Abdel Rahman R. Picture-induced semantic interference reflects lexical competition during object naming. Front Psychol 2012; 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamiou DE, Musiek FE, Luxona LM. The insula (Island of Reil) and its role in auditory processing: literature review. Brain Res Brain Res Rev 2003; 42: 143–54. [DOI] [PubMed] [Google Scholar]
- Best W, Greenwood A, Grassly J, Herbert R, Hickin J, Howard D. Aphasia rehabilitation: does generalisation from anomia therapy occur and is it predictable? A case series study. Cortex 2013; 49: 2345–57. [DOI] [PubMed] [Google Scholar]
- Best W, Herbert R, Hickin J, Osborne F, Howard D. Phonological and orthographic facilitation of word-retrieval in aphasia: immediate and delayed effects. Aphasiology 2002; 16: 151–68. [Google Scholar]
- Bhogal SK, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke 2003; 34: 987–93. [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron 2002; 36: 159–70. [DOI] [PubMed] [Google Scholar]
- Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev 2016; 6: CD000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C, Grewe T, Flöel A, Ziegler W, Springer L, Martus P, et al. FCET2EC study group. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet 2017; 389: 1528–38. [DOI] [PubMed] [Google Scholar]
- Brownsett SL, Warren JE, Geranmayeh F, Woodhead Z, Leech R, Wise RJ. Cognitive control and its impact on recovery from aphasic stroke. Brain 2014; 137: 242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt AE, Ferrara C, Mirman D. Distinct effects of lexical and semantic competition during picture naming in younger adults, older adults, and people with aphasia. Front Psychol 2016; 7: 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CR, Moores KA, Lewis A, Weber DL, Fitzgibbon S, Greenblatt R, et al. Cortical network dynamics during verbal working memory function. Int J Psychophysiol 2001; 42: 161–76. [DOI] [PubMed] [Google Scholar]
- Code C. The quantity of life for people with chronic aphasia. Neuropsychol Rehabil 2003; 13: 379–90. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Leff AP. Recovery and treatment of aphasia after stroke: functional imaging studies. Curr Opin Neurol 2007; 20: 667–73. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Leff AP. Using functional imaging to understand therapeutic effects in poststroke aphasia. Curr Opin Neurol 2015; 28: 330–7. [DOI] [PubMed] [Google Scholar]
- Crinion J, Price CJ. Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain 2005; 128: 2858–71. [DOI] [PubMed] [Google Scholar]
- Dabul B. Apraxia Battery for Adults. Austin, TX: PRO-ED; 2000. [Google Scholar]
- Dignam J, Copland D, Rawlings A, O'Brien K, Burfein P, Rodriguez AD. The relationship between novel word learning and anomia treatment success in adults with chronic aphasia. Neuropsychologia 2016; 81: 186–97. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition 2004; 92: 145–77. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 2010; 14: 172–9. [DOI] [PubMed] [Google Scholar]
- Fedorenko E. The role of domain-general cognitive control in language comprehension. Front Psychol 2014; 5: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci USA 2013; 110: 16616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingham JK, Hodgson C, Sage K, Lambon Ralph MA. The application of errorless learning to aphasic disorders: a review of theory and practice. Neuropsychol Rehabil 2003; 13: 337–63. [DOI] [PubMed] [Google Scholar]
- Fillingham JK, Sage K, Lambon Ralph MA. The treatment of anomia using errorless learning. Neuropsychol Rehabil 2006; 16: 129–54. [DOI] [PubMed] [Google Scholar]
- Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci 2010; 30: 11558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Moser D, Bonilha L, Morrow-Odom KL, Shaw H, Fridriksson A, et al. Neural correlates of phonological and semantic-based anomia treatment in aphasia. Neuropsychologia 2007; 45: 1812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. Neuroimage 2012; 60: 854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H. Understanding aphasia. San Diego: Academic Press; 1993. [Google Scholar]
- Grabowski TJ, Damasio H, Damasio AR. Premotor and prefrontal correlates of category-related lexical retrieval. Neuroimage 1998; 7: 232–43. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 2003; 100: 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Price CJ, Baumgaertner A, Geiss G, Koehnke M, Ulmer S, et al. The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: evidence from dual-site TMS. Neuropsychologia 2010; 48: 3155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertrich I, Dietrich S, Ackermann H. The role of the supplementary motor area for speech and language processing. Neurosci Biobehav Rev 2016; 68: 602–10. [DOI] [PubMed] [Google Scholar]
- Howard D. The treatment of acquired aphasia. Philos Trans R Soc Lond B Biol Sci 1994; 346: 113–20. [DOI] [PubMed] [Google Scholar]
- Johansson MB, Carlsson M, Sonnander K. Communication difficulties and the use of communication strategies: from the perspective of individuals with aphasia. Int J Lang Commun Disord 2012; 47: 144–55. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kay J, Coltheart M, Lesser R. PALPA: Psycholinguistic Assessments of Language Processing in Aphasia. Hove, East Sussex: Psychology Press; 1992. [Google Scholar]
- Kendall DL, Rosenbek JC, Heilman KM, Conway T, Klenberg K, Gonzalez Rothi LJ, et al. Phoneme-based rehabilitation of anomia in aphasia. Brain Lang 2008; 105: 1–17. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Snell C, Fillingham JK, Conroy P, Sage K. Predicting the outcome of anomia therapy for people with aphasia post CVA: both language and cognitive status are key predictors. Neuropsychol Rehabil 2010; 20: 289–305. [DOI] [PubMed] [Google Scholar]
- Liakakis G, Nickel J, Seitz RJ. Diversity of the inferior frontal gyrus—A meta-analysis of neuroimaging studies. Behav Brain Res 2011; 225: 341–7. [DOI] [PubMed] [Google Scholar]
- Madden EB, Robinson RM, Kendall DL. Phonological treatment approaches for spoken word production in aphasia. Semin Speech Lang 2017; 38: 62–74. [DOI] [PubMed] [Google Scholar]
- Maher LM, Raymer AM. Management of anomia. Top Stroke Rehabil 2004; 11: 10–21. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Costa A, Peterson R, Vargas KA, Caramazza A. Lexical selection is not by competition: a reinterpretation of semantic interference and facilitation effects in the picture-word interference paradigm. J Exp Psychol Learn Mem Cogn 2007; 33: 503–35. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Theoret H, Tormos JM, Nicholas M, Kurland J, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang 2004; 25: 181–91. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci 2003; 15: 394–408. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Josephs O, Lambon Ralph MA, McClelland JL, Price CJ. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Hum Brain Mapp 2007; 28: 205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melinger A, Abdel Rahman R. Lexical selection is competitive: evidence from indirectly activated semantic associates during picture naming. J Exp Psychol Learn Mem Cogn 2013; 39: 348–64. [DOI] [PubMed] [Google Scholar]
- Menke R, Meinzer M, Kugel H, Deppe M, Baumgärtner A, Schiffbauer H, et al. Imaging short- and long-term training success in chronic aphasia. BMC Neurosci 2009; 10: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli G, Amitrano A, Capasso R, Caramazza A. The treatment of anomia resulting from output lexical damage: analysis of two cases. Brain Lang 1996; 52: 150–74. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang 2005; 93: 95–105. [DOI] [PubMed] [Google Scholar]
- Narain C, Scott SK, Wise RJ, Rosen S, Leff A, Iversen SD, et al. Defining a left-lateralized response specific to intelligible speech using fMRI. Cereb Cortex 2003; 13: 1362–8. [DOI] [PubMed] [Google Scholar]
- Navarrete E, Del Prato P, Peressotti F, Mahon BZ. Lexical retrieval is not by competition: evidence from the blocked naming paradigm. J Mem Lang 2014; 76: 253–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhart M, Trupe LA, Gomez Y, Cloutman L, Molitoris JJ, Davis C, et al. Asyntactic comprehension, working memory, and acute ischemia in Broca's area versus angular gyrus. Cortex 2012; 48: 1288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels L. Therapy for naming disorders: revisiting, revising, and reviewing. Aphasiology 2002; 16: 935–79. [Google Scholar]
- Nozari N, Kittredge AK, Dell GS, Schwartz MF. Naming and repetition in aphasia: steps, routes, and frequency effects. J Mem Lang 2010; 63: 541–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J, Zimmermann J, Van Meter J, Rauschecker JP. Multiple stages of auditory speech perception reflected in event-related fMRI. Cereb Cortex 2007; 17: 2251–7. [DOI] [PubMed] [Google Scholar]
- Pani E, Zheng X, Wang J, Norton A, Schlaug G. Right hemisphere structures predict poststroke speech fluency. Neurology 2016; 86: 1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease DM, Goodglass H. The effects of cuing on picture naming in aphasia. Cortex 1978; 14: 178–89. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, et al. A fMRI study of word retrieval in aphasia. Brain Lang 2003; 85: 357–68. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Müller-Oehring E, Shankaranarayanan A, Alsop DC, et al. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cereb Cortex 2011; 21: 233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 1999; 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 2010; 1191: 62–88. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA 2001; 98: 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymer AM, Ciampitti M, Holliway B, Singletary F, Blonder LX, Ketterson T, et al. Semantic-phonologic treatment for noun and verb retrieval impairments in aphasia. Neuropsychol Rehabil 2007; 17: 244–70. [DOI] [PubMed] [Google Scholar]
- Ren CL, Zhang GF, Xia N, Jin CH, Zhang XH, Hao JF, et al. Effect of low-frequency rTMS on aphasia in stroke patients: a meta-analysis of randomized controlled trials. PLoS One 2014; 9: e102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon E, Leonard C, Burianova H, Laird L, Soros P, Graham S, et al. Neural changes after phonological treatment for anomia: an fMRI study. Brain Lang 2010; 114: 164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai KL, Hashimoto R, Homae F. Sentence processing in the cerebral cortex. Neurosci Res 2001; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain 2000; 123: 2400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J Neurosci 2010; 30: 16809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Cutler A, Wise RJ. Lexical retrieval constrained by sound structure: the role of the left inferior frontal gyrus. Brain Lang 2005; 92: 309–19. [DOI] [PubMed] [Google Scholar]
- Sims JA, Kapse K, Glynn P, Sandberg C, Tripodis Y, Kiran S. The relationships between the amount of spared tissue, percent signal change, and accuracy in semantic processing in aphasia. Neuropsychologia 2016; 84: 113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollmann N, Tanigawa N, Ringel F, Zimmer C, Meyer B, Krieg SM. Language and its right-hemispheric distribution in healthy brains: an investigation by repetitive transcranial magnetic stimulation. Neuroimage 2014; 102: 776–88. [DOI] [PubMed] [Google Scholar]
- Starreveld PA. On the interpretation of auditory context effects in word production. J Mem Lang 2000; 42: 497–525. [Google Scholar]
- Swinburn K, Porter G, Howard D. The Comprehensive Aphasia Test. Hove, UK: Psychology Press; 2005. [Google Scholar]
- Szekely A, Jacobsen T, D'Amico S, Devescovi A, Andonova E, Herron D, et al. A new on-line resource for psycholinguistic studies. J Mem Lang 2004; 51: 247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE. Brain stimulation and the role of the right hemisphere in aphasia recovery. Curr Neurol Neurosci Rep 2015; 15: 72. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–89. [DOI] [PubMed] [Google Scholar]
- van Hees S, McMahon K, Angwin A, de Zubicaray G, Copland DA. Neural activity associated with semantic versus phonological anomia treatments in aphasia. Brain Lang 2014; 129: 47–57. [DOI] [PubMed] [Google Scholar]
- Vitali P, Abutalebi J, Tettamanti M, Danna M, Ansaldo AI, Perani D, et al. Training-induced brain remapping in chronic aphasia: a pilot study. Neurorehabil Neural Repair 2007; 21: 152–60. [DOI] [PubMed] [Google Scholar]
- Vitkovitch M, Cooper E. My word! Interference from reading object names implies a role for competition during picture name retrieval. Q J Exp Psychol 2012; 65: 1229–40. [DOI] [PubMed] [Google Scholar]
- Wan CY, Zheng X, Marchina S, Norton A, Schlaug G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca's aphasia. Brain Lang 2014; 136: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke 2005; 36: 1759–63. [DOI] [PubMed] [Google Scholar]
- Xing S, Lacey EH, Skipper-Kallal LM, Jiang X, Harris-Love ML, Zeng J, et al. Right hemisphere grey matter structure and language outcomes in chronic left hemisphere stroke. Brain 2016; 139: 227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.