Key Points
Question
What is the optimal approach to testing for latent tuberculosis infection among residents born outside the United States with and without HIV, diabetes, and end-stage renal disease?
Findings
This Markov cohort simulation model study found that some type of latent tuberculosis infection testing and treatment with 3 months of rifapentine and isoniazid likely was associated with an incremental cost-effectiveness ratio below $100 000 per quality-adjusted life-year except for patients with end-stage renal disease. Strategies using interferon gamma release assay were likely preferred; tuberculin skin testing alone is likely dominated.
Meaning
It is cost-effective to test and treat non-US born residents for latent tuberculosis infection, although the choice of test depends on patient comorbidities and resources available.
Abstract
Importance
Testing for and treating latent tuberculosis infection (LTBI) is among the main strategies to achieve TB elimination in the United States. The best approach to testing among non-US born residents, particularly those with comorbid conditions, is uncertain.
Objective
To estimate health outcomes, costs, and cost-effectiveness of LTBI testing and treatment among non-US born residents with and without medical comorbidities.
Design, Setting, and Participants
Decision analytic tree and Markov cohort simulation model among non-US born residents with no comorbidities, with diabetes, with HIV infection, or with end-stage renal disease (ESRD) using a health care sector perspective with 3% annual discounting. Strategies compared included no testing, tuberculin skin test (TST), interferon gamma release assay (IGRA), confirm positive (initial TST, IGRA only for TST-positive results; both tests positive indicates LTBI), and confirm negative (initial IGRA, then TST for IGRA-negative; any test positive indicates LTBI). All strategies were coupled to treatment with 3 months of self-administered rifapentine and isoniazid.
Main Outcomes and Measures
Number needed to test and treat to prevent 1 case of TB reactivation, discounted quality-adjusted life-years (QALYs), discounted lifetime medical costs, and incremental cost-effectiveness ratios (ICERs).
Results
Improving health outcomes increased costs, with choice of test dependent on willingness to pay. Strategies ranked by ascending costs and benefits: no testing, confirm positive, TST, IGRA, and confirm negative. The ICERs varied by non–US born patient risk group: patients with no comorbidities, IGRA was likely cost-effective at $83 000/QALY; patients with diabetes, both confirm positive ($53 000/QALY) and IGRA ($120 000/QALY) were likely cost-effective; patients with HIV, confirm negative was clearly preferred ($63 000/QALY); and patients with ESRD, no testing was cost-effective. Increased LTBI prevalence and reduced return for TST reading improved IGRA’s relative performance. In 10 000 probabilistic simulations among non-US born patients with no comorbidities, with diabetes, and with HIV, some form of testing was virtually always cost-effective. These simulations highlight the uncertainty of test choice for non-US born patients with no comorbidities and non-US born patients with diabetes, but strategies including IGRA were preferred in over 60% of simulations for all non–US born populations except those with ESRD.
Conclusions and Relevance
Testing for and treating LTBI among non-US born residents with and without selected comorbidities is likely cost-effective except among those with ESRD in whom competing risks of death limit benefits. Strategies including IGRA fell below a $100 000/QALY willingness-to-pay threshold for non-US born patients with no comorbidities, patients with diabetes, and patients with HIV.
This cohort simulation model evaluates the use of tests and treatment of latent tuberculosis infection in residents born outside the United States.
Introduction
Most cases of tuberculosis (TB) in the United States occur among non-US born residents. Previous studies suggest that testing and treatment with isoniazid for latent TB infection (LTBI) among non–US born persons is cost-effective. Common comorbidities among non–US born persons, such as diabetes, end-stage renal disease (ESRD), and HIV infection, increase the risk of TB reactivation while carrying competing risks of death that may limit the health benefits of testing and treatment. Since the publication of previous cost-effectiveness analyses, the treatment regimen of rifapentine and isoniazid administered weekly for 3 months by directly observed therapy and self-administration was found to be safe, effective, and cost-effective among LTBI patients in the United States. The economic value of LTBI testing and 3 months of treatment with self-administered rifapentine and isoniazid in non–US born patients with medical comorbidities is not clear.
There are multiple LTBI testing strategies, including tuberculin skin tests (TSTs), interferon gamma release assays (IGRAs), and combinations of the 2 measures. Centers for Disease Control and Prevention guidance for testing and treatment of LTBI suggests that, in some patients, using 2 tests together to maximize the sensitivity of the LTBI testing algorithm is appropriate, but the long-term benefits and value of such combined testing approaches are not certain.
We used a decision-analytic Markov model to estimate clinical outcomes, costs, and cost-effectiveness of testing for and treatment of LTBI among non–US born persons in the United States. We considered testing with TST, IGRA, or in combination, and included 3 months of LTBI treatment with self-administered rifapentine and isoniazid. The results are intended to inform health care professionals seeking guidance on LTBI testing and policymakers seeking to recommend cost-effective interventions.
Methods
Overview
We developed a simulation model to investigate LTBI testing and treatment in 4 non-US born populations: with no comorbidities, with diabetes, with HIV, and with ESRD. Outcomes from the model include the number needed to test and treat (NNT) to prevent 1 case of reactivation TB, life expectancy, discounted quality-adjusted life expectancy, discounted lifetime medical costs, and incremental cost-effectiveness ratios (ICERs). All cost outcomes are reported in 2015 US dollars. The study was approved by Harvard University Institutional Review Board.
Model Structure
The model includes 2 components: a decision tree capturing outcomes relating to testing and a Markov model simulating the lifetime progression of a cohort of people. We developed the model using TreeAge software, version 2015 (TreeAge Corp).
Decision Tree
We modeled 5 testing strategies: no testing, TST, IGRA, confirm positive (patients with a positive TST given IGRA, with both positive resulting in LTBI diagnosis), and confirm negative (patients with a negative IGRA given TST, with either positive resulting in LTBI diagnosis). The branches of the decision tree capture the prevalence of LTBI, the probability of testing positive, and the probability of initiating treatment. Patients may be lost to follow-up before TST reading. At the ends of each branch in the decision tree, patients are separated into 4 groups to be “handed off” to the Markov model: LTBI with treatment, LTBI without treatment, no LTBI with treatment, and no LTBI without treatment.
Markov Model
The Markov model simulates long-term outcomes related to the natural history of LTBI, including progression to active TB (reactivation) and mortality from TB or other causes. The rate of TB reactivation decreases as the cohort ages. Across risk groups, a proportion with active TB develops severe TB disease, characterized by higher medical costs, hospitalization, and increased risk of death. Outpatient treatment of TB is associated with 6 months of increased mortality, decreased quality of life, and additional costs associated with treatment, while severe TB lasts for 9 months. Patients who survive TB are cured with no further risk of TB reactivation.
We modeled LTBI therapy as 3 months of self-administered rifapentine and isoniazid and assumed that LTBI treatment without toxic effects causes no change in quality of life. Completed LTBI therapy reduces the monthly probability of TB reactivation. Patients can withdraw from therapy due to toxic effects (adding 1 month of additional cost and quality-of-life decrement and possibility of death) or nonadherence. We assumed no partial protection for those who withdraw.
For each case of reactivation TB, we modeled additional TB cases due to transmission. Each secondary case results in additional cost and decreased life expectancy, estimated as the difference in outcomes between a healthy 35-year-old and a 35-year-old individual with active TB. We did not consider transmission beyond the first generation.
We modeled age- and sex-specific mortality from causes other than TB; non-US born patients with no comorbidities have the same competing risks of death as the US general population. We modeled elevated mortality and increased medical costs among the non-US born patients with diabetes, with HIV, and with ESRD cohorts. Despite a high prevalence of diabetes in the ESRD population, our model does not distinguish between those cohorts. The additional cost and risk of death from ESRD is greater than any other differences between these subpopulations.
Model Data
We conducted a meta-analysis to estimate the diagnostic performance of TST and IGRA (eAppendix in the Supplement). Using a Bayesian latent class model, we predicted test characteristics for each risk group, which are reported in Table 1.
Table 1. Cohort Description and Select Model Input Parameters for a Cost-effectiveness Analysis of Testing and Treating LTBI Among Non-US Born Residents.
| Variable | Base-Case Value | Range Evaluated |
|---|---|---|
| Proportion male | 0.490 | 0.40-0.60 |
| LTBI prevalence | 0.159 | 0.0-1.0 |
| Age at baseline, y | ||
| NC | 35 | 30-70 |
| HIV | 35 | 30-70 |
| Diabetes | 57 | 30-70 |
| ESRD | 58 | 30-70 |
| Life expectancy without LTBI | ||
| NC | 80 | 70-90 |
| HIV | 69 | 60-80 |
| Diabetes | 75 | 70-80 |
| ESRD | 65 | 60-70 |
| Cascade of testing and treatment | ||
| Return for TST read | 0.820 | 0.0-1.0 |
| Diagnosed who initiate treatment | 0.900 | 0.5-1.0 |
| Treatment completion | 0.783 | 0.5-1.0 |
| Hepatotoxicity | 0.005 | 0.0-0.01 |
| Mortality with hepatotoxicity | 0.001 | 0.0-0.002 |
| Lifetime risk of TB reactivation, untreated infection | ||
| NC | 0.038 | 0.01-0.1 |
| HIV | 0.100 | 0.05-0.2 |
| Diabetes | 0.031 | 0.01-0.1 |
| ESRD | 0.012 | 0.005-0.05 |
| Reduction in reactivation probability after complete therapy | 0.900 | 0.5-1.0 |
| Proportion with severe TB requiring hospitalization | 0.503 | 0.25-0.75 |
| Mortality with TB | 0.050 | 0.025-0.075 |
| Secondary cases | 0.250 | 0.1-1.0 |
| Costs, $ | ||
| TST | 7.870 | 5-15 |
| IGRA | 84.350 | 50-100 |
| Complete course of therapy | 582 | 300-1000 |
| Treatment for hepatotoxicity | 323 | 250-500 |
| Treatment for nonsevere active TB | 2900 | 1500-4500 |
| Treatment for severe active TB | 28 692 | 10 000-40 000 |
| Monthly health care costs, $ | ||
| NC | 106-1374 | 53-2061 |
| HIV | 2061 | 1030-3091 |
| Diabetes | 788-2056 | 394-3084 |
| ESRD | 3900-5168 | 1750-7752 |
| Utility measures | ||
| LTBIa | 1 | 0.99-1.0 |
| Hepatotoxicity | 0.750 | 0.6-1.0 |
| Active TB | 0.830 | 0.75-1.0 |
| Post-TB quality of lifea | 1 | 0.87-1.0 |
| TST sensitivity (%)b in non–US born patients | ||
| With no comorbidities | 71 | 0.5-1.0 |
| With HIV | 67 | 0.5-1.0 |
| With diabetes | 67 | 0.5-1.0 |
| With ESRD | 67 | 0.5-1.0 |
| IGRA sensitivity (%)b in non–US born patients | ||
| With no comorbidities | 79 | 0.5-1.0 |
| With HIV | 77 | 0.5-1.0 |
| With diabetes | 78 | 0.5-1.0 |
| With ESRD | 78 | 0.5-1.0 |
| TST specificity (%)b in non–US born patients | ||
| With no comorbidities | 89 | 0.5-1.0 |
| With HIV | 87 | 0.5-1.0 |
| With diabetes | 87 | 0.5-1.0 |
| With ESRD | 87 | 0.5-1.0 |
| IGRA specificity (%)b in non–US born patients | ||
| With no comorbidities | 99 | 0.5-1.0 |
| With HIV | 99 | 0.5-1.0 |
| With diabetes | 98 | 0.5-1.0 |
| With ESRD | 98 | 0.5-1.0 |
Abbreviations: D, diabetes; ESRD, end-stage renal disease; IGRA, interferon gamma release assay; LTBI, latent tuberculosis infection; NC, no comorbidities; TB, tuberculosis; TST, tuberculin skin test.
Model assumption.
eAppendix in the Supplement.
We selected model parameters from medical literature (Table 1). Miramontes et al reported LTBI prevalence in non–US born individuals of 15.9% using National Health and Nutrition Examination Survey data. We used an 18% failure of non–US born persons to return for TST reading reported by Desale et al.
We applied a rate of TB reactivation without LTBI treatment in the general non–US born population of 104 cases per 100 000 person-years at risk. For each decade after simulation start, we assumed a 10% reduction in the base reactivation rate due to self-cure. Monthly risk of reactivation was higher in populations with comorbidities. The non-US born patients with diabetes and non-US born patients with ESRD had 1.8 times the monthly rate of reactivation of non-US born patients with no comorbidities, while the baseline reactivation rate for non-US born patients with HIV was 3.5 times higher. Completed LTBI treatment confers a 90% reduction in the modeled rate of reactivation.
Medicare-allowable fees were applied as cost estimates for IGRA and TST ($84.35 vs $7.87). Therapy costs accrued monthly; patients who failed to complete the course did not incur the full cost of treatment ($582). Treatment-related toxic effects resulted in an additional $323 in medical costs, and the rare event of toxicity-induced death added $13 782. The cost of severe TB requiring hospitalization was roughly 10 times that of outpatient TB treatment ($28 692 vs $2900).
Quality of life with LTBI and during LTBI therapy was 1.0. Quality of life with treatment-related toxic effects was 0.75 during the month with toxic effects and 0.83 with active tuberculosis.
Statistical Analysis
The analysis assumed a health care sector perspective and lifetime horizon with a 3% annual discount to costs and benefits. We assumed a commonly cited willingness-to-pay threshold of $100 000 per quality-adjusted life-year (QALY) gained.
We conducted deterministic sensitivity analyses, systematically altering model parameters to observe their effect on conclusions. Parameters of interest included test characteristics, LTBI prevalence, age of cohort (which relates to remaining life expectancy and cumulative TB risk), and quality-of-life estimates. Second-order Monte Carlo simulation was used to conduct 10 000 probabilistic sensitivity analyses, and LTBI prevalence, test characteristics, and the rate of reactivation TB were incorporated. We sampled TST and IGRA test characteristics from joint distributions; both prevalence of LTBI and baseline rate of reactivation were sampled from uniform random distributions.
Results
Across all non–US born risk populations studied, testing and treatment for LTBI prevented TB cases, contributed to gains in QALYs, and increased cost (Table 2). No testing resulted in the worst health outcomes at the least cost. The confirm positive strategy provided greater health outcomes than no testing and was the next strategy to be least costly. Although TST provided better health outcomes than confirm positive, it did so at a greater cost per QALY than IGRA; therefore, TST was excluded from ICER calculations through the principle of extended dominance. IGRA provided greater health outcomes than the confirm positive strategy. The confirm negative strategy delivered the best health outcomes. Since the choice of strategy depends on payer willingness to pay by population, we present results for each risk population below (Figure 1).
Table 2. Base-Case Resultsa.
| Variable | No Testing | Confirm Positive | TST | IGRA | Confirm Negative |
|---|---|---|---|---|---|
| Non-US Born Patient With No Comorbidities, Age 35 y | |||||
| Reactivation TB in total cohort, %b | 0.60 | 0.43 | 0.38 | 0.30 | 0.26 |
| NNT | 569 | 448 | 332 | 294 | |
| Incremental cost per person, $ | 47 | 30 | 50 | 42 | |
| Incremental QALY | 0.0013 | 0.0004 | 0.0006 | 0.0003 | |
| ICER ($/QALY) | 35 000 | Dominated | 83 000 | 147 000 | |
| Non-US Born Patient With Diabetes, Age 57 y | |||||
| Reactivation TB in total cohort, %b | 0.50 | 0.36 | 0.32 | 0.25 | 0.22 |
| NNT | 749 | 581 | 409 | 362 | |
| Incremental cost per person, $ | 47 | 35 | 54 | 47 | |
| Incremental QALY | 0.0009 | 0.0002 | 0.0005 | 0.0002 | |
| ICER | 53 000 | Dominated | 120 000 | 230 000 | |
| Non-US Born Patient With HIV, Age 35 y | |||||
| Reactivation TB in total cohort, %b | 1.59 | 1.17 | 1.04 | 0.82 | 0.72 |
| NNT | 237 | 182 | 130 | 114 | |
| Incremental cost per person, $ | 58 | 39 | 55 | 50 | |
| Incremental QALY | 0.0032 | 0.0010 | 0.0017 | 0.0008 | |
| ICER | 18 000 | Dominated | 35 000 | 63 000 | |
| Non-US Born Patient With ESRD, Age 58 y | |||||
| Reactivation TB in total cohort, %b | 0.20 | 0.15 | 0.13 | 0.10 | 0.09 |
| NNT | 1805 | 1399 | 984 | 871 | |
| Incremental cost per person, $ | 852 | 267 | 492 | 237 | |
| Incremental QALY | 0.0003 | 0.0001 | 0.0002 | 0.0001 | |
| ICER | 2 730 000 | Dominated | 2 933 000 | 3 546 000 | |
Abbreviations: ESRD, end-stage renal disease; ICER, incremental cost-effectiveness ratio; IGRA, interferon gamma release assay; NNT, number needed to test and treat to prevent 1 case of reactivation TB; QALY, quality-adjusted life-year; TB, tuberculosis; TST, tuberculin skin test.
All incremental values should be considered relative to the first column to the left, such that the total increase in mean cost per individual tested can be calculated by the sum of a row. Empty cells indicate not applicable.
Lifetime probability of reactivation TB is displayed for the entire subpopulation studied. Reactivation TB in total cohort represents the proportion of the cohort who will develop reactivation TB at some point. This figure is affected by prevalence, remaining life years, and reactivation probability. Although the non-US born patients with diabetes and non–US born-ESRD populations have an increased risk of reactivation on a monthly basis compared with non-US born patients with no comorbidities, competing risks of death result in a smaller proportion of each cohort progressing to active infection.
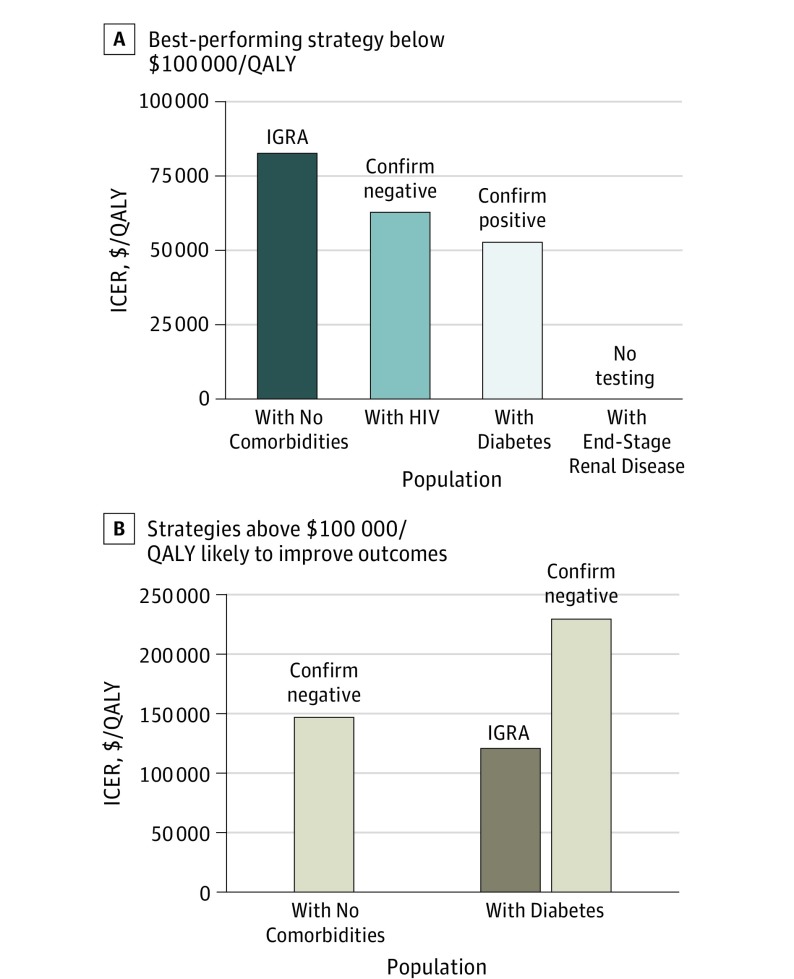
Figure 1. Primary Cost-effectiveness Results in Non–US Born Patients.
A, The best-performing strategy with an incremental cost-effectiveness ratio (ICER) below $100 000/quality-adjusted life-year (QALY). B, Strategies that are likely to improve health outcomes compared with the strategy indicated in panel A but are associated with ICERs above $100 000/QALY. ICERs are calculated against the next-best alternative strategy and are shown in 2015 US dollars per QALY gained. IGRA indicates interferon gamma release assay.
Non-US Born Patients With No Comorbidities Cohort
Among non-US born patients with no comorbidities, IGRA prevented 50% of lifetime TB reactivation compared with no testing (0.30% vs 0.60% in the total cohort), had an NNT of 332, and was associated with an ICER of $83 000/QALY. Confirm negative prevented 13% more TB reactivation cases than IGRA, had an NNT of 294, and was associated with an ICER of $147 000/QALY.
Non-US Born Patients With Diabetes Cohort
Among non-US born patients with diabetes, confirm positive prevented 28% of lifetime TB reactivation compared with no testing (0.36% vs 0.50% in the total cohort), had an NNT of 749, and was associated with an ICER of $53 000/QALY. IGRA prevented 50% of lifetime TB reactivation cases, had an NNT of 409, and was associated with an ICER of $120 000/QALY. Confirm negative prevented 56% of lifetime TB reactivation cases, was associated with the lowest NNT of 362, and had an ICER of $230 000/QALY.
Non-US Born Patients With HIV Cohort
Among non-US born patients with HIV, confirm negative prevented 55% of lifetime TB reactivation (0.72% vs 1.59%), had a relatively low NNT of 114, and was associated with an ICER of $63 000/QALY.
Non-US Born Patients With ESRD Cohort
For the non-US born patients with ESRD population, competing risks of death greatly limited remaining life expectancy and therefore also reduced the lifetime risk of TB reactivation. Testing for LTBI improved QALYs, but ICERs for all strategies were over $2 million/QALY gained.
Sensitivity Analysis
We varied the inputs to the model to test whether our conclusions were robust under different circumstances and whether any general patterns could be discerned to help inform the choice of testing strategy. In general, the base case conclusions were robust to changes in core model parameters.
LTBI Prevalence
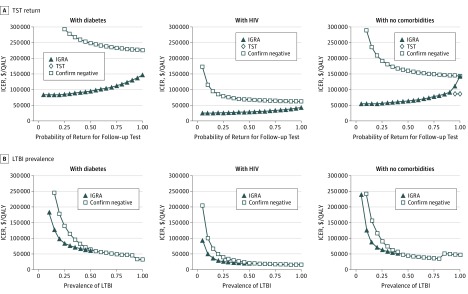
In populations with high LTBI prevalence, the confirm negative approach is most cost-effective (Figure 2). For example, confirm negative was the preferred strategy for non-US born patients with no comorbidities when prevalence was greater than 23% (base case, 15.9%) and it was preferred for non-US born patients with diabetes when the prevalence of LTBI was greater than 34%. Among the non-US born patients with ESRD population, no feasible LTBI prevalence resulted in testing being cost-effective.
Figure 2. Effect of Tuberculin Skin Test (TST) Return and Latent Tuberculosis Infection (LTBI) Prevalence on Cost-effectiveness Conclusions in Non–US Born Patients.
A, A 1-way sensitivity analysis demonstrating the effect of TST return on the cost-effectiveness conclusions for interferon gamma release assay (IGRA) testing and IGRA plus TST for sensitivity testing. B, An illustration of the effect of LTBI prevalence on cost-effectiveness conclusions for IGRA testing and confirm negative testing. In the confirm negative strategy, patients first underwent IGRA. If that test was positive, LTBI was diagnosed. If that test was negative, then the patient underwent TST. LTBI was ruled out only if both tests were negative. For each risk population, there is a unique LTBI prevalence above which IGRA requires more investment to gain the same amount of quality-adjusted life-years (QALYs) than confirm negative. Above this point, IGRA is excluded from consideration as a viable strategy and thus is not represented in the figure. The apparent discontinuity at high prevalence (>90% non–US born patients with diabetes, >80% non–US born individuals with no comorbidities) emerges when confirm negative is not only a cost-effective strategy but becomes more favorable than other, less costly strategies. Incremental cost-effectiveness ratios (ICERs) are calculated against the next-best alternative strategy and are shown in 2015 US dollars per QALY gained. End-stage renal disease was excluded from this figure because it is cost-ineffective.
At no plausible LTBI prevalence among non–US born persons was the no testing strategy preferred for risk populations other than ESRD. Among the non-US born patients with no comorbidities cohort, when prevalence is between 2.5% and 12.5%, confirm positive is the preferred option (ICER, $38 000/QALY); if the prevalence is below 2.5%, then no testing is preferred. Similarly, among the non-US born patients with diabetes cohort, confirm positive had an ICER of less than $100 000/QALY unless prevalence is less than 4.5%, at which point no testing is preferred. Among the non-US born patients with HIV cohort, IGRA remained cost-effective even at a prevalence of less than 1%.
TST Specificity
With modest improvement in specificity, TST became a cost-effective strategy. For example, when TST specificity among the non-US born patients with no comorbidities cohort was greater than 92.5% (base case, 88.6%), TST became the preferred strategy, and the ICER for IGRA compared with TST was just over the willingness-to-pay threshold ($103 000/QALY). However, decreases in TST specificity resulted in IGRA becoming the preferred choice. For example, in the non-US born patients with diabetes cohort with TST specificity less than 64%, IGRA became the preferred strategy, with an ICER compared with confirm positive of $100 000/QALY.
TST Return Rate
Only substantial improvements in rates of follow-up made TST cost-effective (Figure 2). For example, among the non-US born patients with no comorbidities cohort when more than 91.5% of individuals returned (base case, 82%), TST became the preferred strategy (ICER compared with confirm positive, $86 000/QALY). When return for TST in the non-US born patients with diabetes cohort was less than 58%, IGRA was the cost-effective strategy.
Age
Some form of testing and treatment was cost-effective regardless of age. In younger persons, the cumulative lifetime risk of reactivation is higher than in older persons; therefore, more sensitive testing algorithms became economically attractive. For example, when we assumed the non-US born patients with diabetes population was younger (35 years), IGRA became the preferred strategy (ICER, $59 000/QALY compared with confirm positive). Likewise, a further reduction in age among non-US born patients with diabetes to 30 years resulted in confirm negative being the preferred strategy (ICER, $100 000/QALY).
Quality of Life With LTBI and Post-TB
If living with LTBI carried any associated disutility (0.99), then confirm negative became clearly preferred for all groups other than non-US born patients with ESRD. If recovering from TB conveyed permanent pulmonary sequelae associated with modest disutility, strategies using IGRA were preferred for all groups other than non-US born patients with ESRD. Confirm negative was preferred when the health state utility of cured TB was less than 0.97 among non-US born patients with no comorbidities and when it was less than 0.87 for the non-US born patients with diabetes cohort.
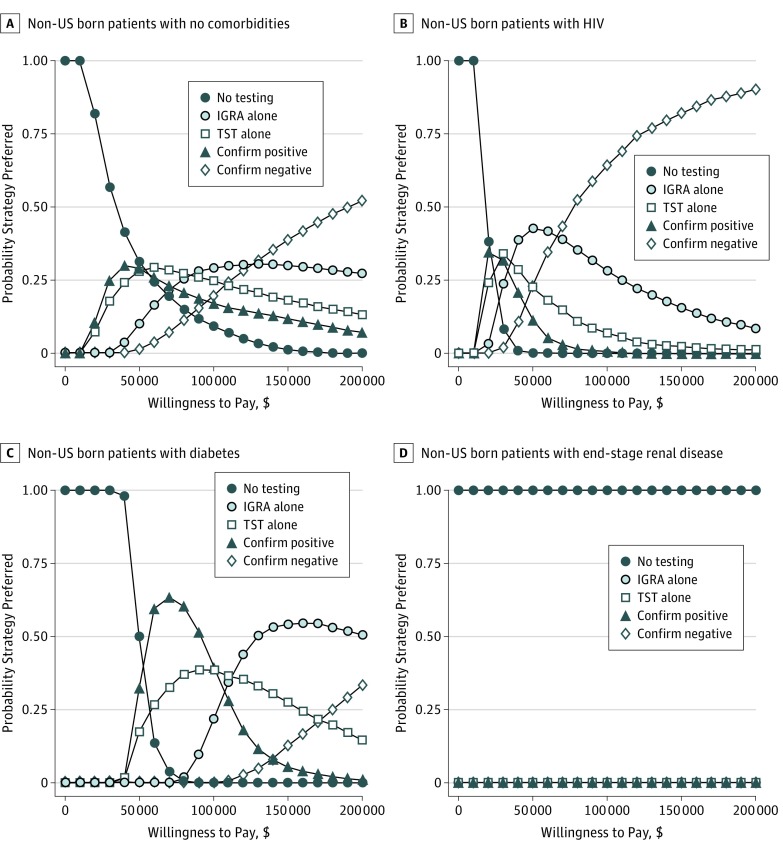
Probabilistic Sensitivity Analysis
Among the non-US born patients with no comorbidities cohort, some form of LTBI testing was cost-effective in more than 90% of simulations (Figure 3). IGRA was the preferred strategy in 29% of simulations, confirm negative was preferred for 20%, confirm positive was preferred in 17% of simulations, and TST was the preferred strategy in 25% of simulations.
Figure 3. Cost-effectiveness Acceptability Curves Representing the Proportion of Simulations for Which Each Strategy Was Preferred at a Given Willingness-to-Pay Threshold.
Probabilistic sensitivity analysis was performed on reactivation rate, LTBI prevalence, and test characteristics in non–US born persons with no comorbidities (A), those with HIV (B), individuals with diabetes (C), and those with end-stage renal disease (D). IGRA indicates interferon gamma release assay; TST, tuberculin skin test.
For the non-US born patients with diabetes cohort, some form of testing was cost-effective in all simulations. Confirm positive was the preferred strategy in 39.5% of the simulations, and IGRA was preferred in 21.8%; TST was the preferred strategy in 38.7% of the simulations.
For the non-US born patients with HIV cohort, confirm negative was the preferred strategy in 63% of simulations, IGRA was preferred 31% of the time, and TST was preferred in 6% of the cases. Confirm positive was preferred in fewer than 1% of the simulations. Finally, for the non-US born patients with ESRD cohort, no testing was the preferred strategy in 100% of the simulations.
Discussion
We used simulation modeling to project the long-term outcomes and cost-effectiveness of strategies for testing and treating LTBI among non–US born individuals with and without common comorbidities. First, we found that testing for LTBI in non–US born individuals followed by 3 months of self-administered rifapentine and isoniazid therapy prevents reactivation TB, improves health outcomes, and is cost-effective compared with no LTBI testing or treatment. The exception is for non-US born patients with ESRD, where competing risks of death substantially reduce the benefits of treatment such that no testing is cost-effective.
Second, we demonstrated that TST alone–one common approach to testing–is likely not the best use of limited TB control resources among non–US born persons. In over 60% of the simulations for the non-US born patients with no comorbidities and non-US born patients with diabetes and in 94% of simulations for the non-US born patients with HIV cohort, some sequence of testing with IGRA was preferred. Both lower specificity and loss to follow-up associated with TST reduce its cost-effectiveness. If only testing cost is considered in the design of TB control strategies, TST might be selected despite the fact that IGRA provides better outcomes per dollar spent. Tuberculosis control programs can use these results to identify effective candidate strategies.
Third, we found that, among non-US born patients with HIV in whom both the prevalence of LTBI and the risk of reactivation are high, testing algorithms should maximize test sensitivity to approach treatment for all cases of LTBI. Among non-US born patients with HIV, the confirm negative algorithm suggested in current Centers for Disease Control and Prevention guidance is economically attractive, assuming a $100 000 willingness-to-pay threshold.
Among the non-US born patients with no comorbidities and non-US born patients with diabetes, the choice of how to use IGRA—either alone or in combination with TST to maximize either specificity or sensitivity—is more complex. Our probabilistic sensitivity analyses results highlight the level of uncertainty in this decision. Some sequence of IGRA testing was preferred in the majority of simulations, but each algorithm that used IGRA had a similar probability of being preferred at a willingness-to-pay threshold of less than $100 000/QALY. The single-variable sensitivity analyses, however, shed some light on the dynamics behind this uncertainty and should help decision makers to navigate this obstacle. The cost-effectiveness of TST is highly dependent on its specificity and return rate. For non-US born patients with no comorbidities, IGRA provides a 1-step testing algorithm that has good health outcomes and whose ICER is below a $100 000 willingness-to-pay threshold; for non-US born patients with diabetes, the ICER for IGRA was $120 000/QALY, just over a $100 000 willingness-to-pay threshold. Although a strict interpretation recommends confirm positive as the preferred strategy for the non-US born patients with diabetes population, the sensitivity of this conclusion to key parameters, such as LTBI prevalence and TST return, suggests that it is not definite. When policymakers consider additional factors that were not explicitly modeled, such as patient convenience and the desire to publish simple, effective guidance applicable to all non–US born individuals, evidence mounts to recommend 1-step IGRA testing for both the non-US born patients with no comorbidities and non-US born patients with diabetes groups. With additional available resources or a prevalence of LTBI approaching the high end of estimates, it is likely reasonable to follow-up a negative IGRA with a TST for added sensitivity.
Limitations
It is notable that LTBI testing and treatment among non-US born patients with ESRD was not cost-effective. Among patients undergoing dialysis, competing risks of death limit life expectancy, which reduces the remaining years at risk for developing TB and limits the benefit of preventing TB. One limitation of our study is that the model only considered 1 generation of TB transmission. While we did not find that LTBI treatment was likely to be cost-effective among non–US born patients with ESRD, another rationale for TB testing in dialysis centers might be to prevent TB outbreaks in hospitals and ensure infection control.
There are additional limitations to this study. The prevalence of LTBI and the test characteristics of TST and IGRA are impossible to directly observe and difficult to estimate without a reference standard for LTBI diagnosis. One strength of our analysis is the synthesis of multiple data sources to inform TST and IGRA sensitivity and specificity. We used second-order Monte Carlo simulation to test the robustness of base-case conclusions and transparently demonstrate the quantitative effect of such uncertainty on our conclusions. Similarly, the rate of TB reactivation over decades of remaining life is uncertain. We modeled decreasing reactivation over time, consistent with observational data, and also explored the impact of reactivation rates in probabilistic sensitivity analyses. In addition, if we were to implement a societal framework through which to consider costs and benefits, the additional costs to patients due to the need to return for a TST reading may further reduce the appeal of strategies using TST.
Conclusions
This study highlights the need for observational research about the relative performance of tests for LTBI as well as the necessity of less costly, better-performing tests. A single test with improved characteristics and a lower cost than that of IGRA could reduce investment needed in terms of patient and provider time and cost and make universal testing for non–US born patients even more attractive.
Targeted testing and treatment for LTBI remains a cornerstone of the US TB elimination plan. We found that LTBI testing and treatment among non–US born persons with comorbidities, including diabetes and HIV, prevents cases of TB, improves quality-adjusted life expectancy, and is cost-effective. Targeted testing and treatment for LTBI among non–US born people with and without common comorbidities, using IGRAs and 3 months of self-administered rifapentine and isoniazid, are effective and provide good value for the resources invested.
eAppendix. Meta-analysis
References
- 1.Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2014. https://www.cdc.gov/tb/statistics/reports/2014/pdfs/tb-surveillance-2014-report_updated.pdf. Published October 2015. Accessed January 15, 2017.
- 2.Linas BP, Wong AY, Freedberg KA, Horsburgh CR Jr. Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med. 2011;184(5):590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oza-Frank R, Narayan KMV. Overweight and diabetes prevalence among US immigrants. Am J Public Health. 2010;100(4):661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterling TR, Villarino ME, Borisov AS, et al. ; TB Trials Consortium PREVENT TB Study Team . Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155-2166. [DOI] [PubMed] [Google Scholar]
- 5.Bliven-Sizemore EE, Sterling TR, Shang N, et al. ; TB Trials Consortium . Three months of weekly rifapentine plus isoniazid is less hepatotoxic than nine months of daily isoniazid for LTBI. Int J Tuberc Lung Dis. 2015;19(9):1039-1044, i-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shepardson D, Marks SM, Chesson H, et al. Cost-effectiveness of a 12-dose regimen for treating latent tuberculous infection in the United States. Int J Tuberc Lung Dis. 2013;17(12):1531-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belknap R, Borisov AS, Holland DP, et al. Adherence to once-weekly self-administered isoniazid and rifapentine for latent tb infection: iAdhere [abstract 827LB]. Conference on Retroviruses and Opportunistic Infections. http://www.croiconference.org/sessions/adherence-once-weekly-self-administered-inh-and-rifapentine-latent-tb-iadhere. Accessed August 28, 2017.
- 8.Beckles GL, Chou C-F; Centers for Disease Control and Prevention (CDC) . Diabetes—United States, 2006 and 2010. MMWR Suppl. 2013;62(3):99-104. [PubMed] [Google Scholar]
- 9.Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deuffic-Burban S, Atsou K, Viget N, Melliez H, Bouvet E, Yazdanpanah Y. Cost-effectiveness of QuantiFERON-TB test vs tuberculin skin test in the diagnosis of latent tuberculosis infection. Int J Tuberc Lung Dis. 2010;14(4):471-481. [PubMed] [Google Scholar]
- 11.Mazurek GH, LoBue PA, Daley CL, et al. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001;286(14):1740-1747. [DOI] [PubMed] [Google Scholar]
- 12.Mazurek GH, Zajdowicz MJ, Hankinson AL, et al. Detection of Mycobacterium tuberculosis infection in United States Navy recruits using the tuberculin skin test or whole-blood interferon-gamma release assays. Clin Infect Dis. 2007;45(7):826-836. [DOI] [PubMed] [Google Scholar]
- 13.Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. [DOI] [PubMed] [Google Scholar]
- 14.Podany AT, Bao Y, Swindells S, et al. ; AIDS Clinical Trials Group A5279 Study Team . Efavirenz pharmacokinetics and pharmacodynamics in HIV-infected persons receiving rifapentine and isoniazid for tuberculosis prevention. Clin Infect Dis. 2015;61(8):1322-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterling TR, Scott NA, Miro JM, et al. ; Tuberculosis Trials Consortium; AIDS Clinical Trials Group for the PREVENT TB Trial (TBTC Study 26ACTG 5259); investigators of the TB Trials Consortium; AIDS Clinical Trials Group for the PREVENT TB Trial are listed in the Supplement, item 17 . Three months of weekly rifapentine and isoniazid for treatment of Mycobacterium tuberculosis infection in HIV-coinfected persons. AIDS. 2016;30(10):1607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Managing drug interactions in the treatment of HIV-related tuberculosis 2014. https://www.cdc.gov/tb/publications/guidelines/tb_hiv_drugs/recommendations04.htm. Updated September 22, 2014. Accessed January 15, 2017.
- 17.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167(11):1145-1151. [DOI] [PubMed] [Google Scholar]
- 18.Schackman BR, Fleishman JA, Su AE, et al. The lifetime medical cost savings from preventing HIV in the United States. Med Care. 2015;53(4):293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United States Renal Data System 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD; 2015. [Google Scholar]
- 20.Forbes SP, Di M, Salomon JA, Linas BP, Trikalinos TA Evidence synthesis for diagnostic tests with partially ordered performance and no reference standard. Poster presented at: 38th Annual North American Meeting of the Society for Medical Decision Making; Vancouver, British Columbia, Canada;October 23-26, 2016. [Google Scholar]
- 21.United States Census Bureau. Table DP05 - ACS Demographic and Housing Estimates. https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed May 26, 2016.
- 22.Shea KM, Kammerer JS, Winston CA, Navin TR, Horsburgh CR. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am J Epidemiol. 2014;79(2):216-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Vital Statistics System. 2013 Mortality Tables. Vol. 6, No. 3. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_03.pdf. Updated April 11, 2017. Accessed December 14, 2016.
- 24.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44(11):990-997. [DOI] [PubMed] [Google Scholar]
- 25.Desale M, Bringardner P, Fitzgerald S, Page K, Shah M. Intensified case-finding for latent tuberculosis infection among the Baltimore City Hispanic population. J Immigr Minor Health. 2013;15(4):680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colson PW, Hirsch-Moverman Y, Bethel J, et al. ; Tuberculosis Epidemiologic Studies Consortium . Acceptance of treatment for latent tuberculosis infection: prospective cohort study in the United States and Canada. Int J Tuberc Lung Dis. 2013;17(4):473-479. [DOI] [PubMed] [Google Scholar]
- 27.Kopanoff DE, Snider DE Jr, Caras GJ. Isoniazid-related hepatitis: a US Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117(6):991-1001. [DOI] [PubMed] [Google Scholar]
- 28.Salgame P, Geadas C, Collins L, Jones-López E, Ellner JJ. Latent tuberculosis infection—revisiting and revising concepts. Tuberculosis (Edinb). 2015;95(4):373-384. [DOI] [PubMed] [Google Scholar]
- 29.Pérez A, Brown HS III, Restrepo BI. Association between tuberculosis and diabetes in the Mexican border and non-border regions of Texas. Am J Trop Med Hyg. 2006;74(4):604-611. [PMC free article] [PubMed] [Google Scholar]
- 30.Moran A, Harbour DV, Teeter LD, Musser JM, Graviss EA. Is alcohol use associated with cavitary disease in tuberculosis? Alcohol Clin Exp Res. 2007;31(1):33-38. [DOI] [PubMed] [Google Scholar]
- 31.Moonan PK, Ghosh S, Oeltmann JE, Kammerer JS, Cowan LS, Navin TR. Using genotyping and geospatial scanning to estimate recent Mycobacterium tuberculosis transmission, United States. Emerg Infect Dis. 2012;18(3):458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.United States Department of Health and Human Services Center for Medicare Services Physician fee schedule. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/PhysicianFeeSched. Accessed December 22, 2015.
- 33.United States Department of Health and Human Services Center for Medicare Services Clinical laboratory fee schedule. http://www.cms.gov/ClinicalLabFeesched/. Accessed December 22, 2015.
- 34.Truven Health Analytics. Micromedex 2.0. Drug Topics Red Book Online http://www.micromedexsolutions.com. Accessed January 13, 2017.
- 35.Agency for Healthcare Research and Quality Medical expenditure panel survey. http://meps.ahrq.gov/mepsweb/. Accessed July 15, 2013. [PubMed]
- 36.American Diabetes Association Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joyce AT, Iacoviello JM, Nag S, et al. End-stage renal disease–associated managed care costs among patients with and without diabetes. Diabetes Care. 2004;27(12):2829-2835. [DOI] [PubMed] [Google Scholar]
- 38.McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making. 2008;28(4):582-592. [DOI] [PubMed] [Google Scholar]
- 39.Guo N, Marra CA, Marra F, Moadebi S, Elwood RK, Fitzgerald JM. Health state utilities in latent and active tuberculosis. Value Health. 2008;11(7):1154-1161. [DOI] [PubMed] [Google Scholar]
- 40.Miramontes R, Hill AN, Yelk Woodruff RS, et al. Tuberculosis infection in the United States: prevalence estimates from the National Health and Nutrition Examination Survey, 2011-2012. PLoS One. 2015;10(11):e0140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am J Epidemiol. 1978;107(1):71-76. [DOI] [PubMed] [Google Scholar]
- 42.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis: a general review. Bibl Tuberc. 1970;26:28-106. [PubMed] [Google Scholar]
- 43.Centers for Medicare & Medicaid Services. Clinical laboratory fee schedule: details for title: 16CLAB. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Published December 2015. Accessed May 2016.
- 44.Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project National Inpatient Sample. Washington, DC: US Health and Human Services; 2015. [Google Scholar]
- 45.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 46.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. [DOI] [PubMed] [Google Scholar]
- 47.Miller TL, McNabb SJN, Hilsenrath P, Pasipanodya J, Weis SE Personal and societal health quality lost to tuberculosis. PLoS One.2009;4(4):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Meta-analysis