Abstract
The expression of extracellular matrix protein periostin (POSTN) was attenuated in Med1−/− mouse embryonic fibroblasts (MEFs), which exhibited a decreased capability to support hematopoietic progenitor cells (HPCs) in vitro. When bone marrow (BM) cells were cocultured with mitomycin C-treated Med1+/+ MEFs, or OP-9 or MS-5 BM stromal cells, in the presence of anti-POSTN antibody, the growth of BM cells and number of long-term culture-initiating cells (LTC-ICs) were attenuated. When BM cells were cocultured with Med1−/− MEFs in the presence of recombinant POSTN, the growth of BM cells and the number of LTC-ICs were restored. Moreover, antibody-mediated blockage of stromal cells-derived POSTN markedly reduced the growth and cobblestone formation, a leukemic stem cell feature, of stromal cell-dependent MB-1 myeloblastoma cells. POSTN was expressed both in BM cells and variably in different BM stromal cells. Expression in the latter cells was increased by physical interaction with hematopoietic cells. The receptor for POSTN, integrin αvβ3, was expressed abundantly in BM stromal cells. The addition of recombinant POSTN to BM stromal cells induced intracellular signaling downstream of integrin αvβ3. These results suggest that stromal cell POSTN supports both normal HPCs and leukemia-initiating cells in vitro, at least in part, indirectly by acting on stromal cells in an autocrine or paracrine manner.
Keywords: bone marrow stromal cells, hematopoietic niche, extracellular matrix protein, myeloblastoma cells, Mediator transcriptional coregulator complex
Introduction
The maintenance and differentiation of hematopoietic stem/progenitor cells (HSPCs) is strictly controlled by a bone marrow (BM) microenvironment called the hematopoietic niche, and thereby postnatal hematopoietic homeostasis is maintained. The mesenchymal stem cells (MSCs) and related stromal cells within the periarteriolar and perisinusoidal niches are now regarded as the major niche components. The molecular basis of communication between mesenchymal and hematopoietic cells, involving niche factors that restrain or expand HSPCs, has been extensively investigated [reviewed in 1–4]. Many of the major known niche factors, including CXC chemokine ligand 12 (CXCL12), stem cell factor (SCF) and transforming growth factor (TGF)-β among others, are produced by mesenchymal stem or stromal cells, while megakaryocytes, which constitute another niche, also provide CXCL4, TGF-β and fibroblast growth factor (FGF)1/2.
The Mediator, composed of about 31 subunits, is a master transcriptional coregulator complex that is essential for global transcription governed by RNA polymerase II [reviewed in 5]. Among the Mediator subunits, MED1 acts as a specific coactivator for activators that include nuclear receptors [reviewed in 6,7]. We previously reported that Med1−/− mouse embryonic fibroblasts (MEFs) have an attenuated ability to support hematopoietic progenitor cells (HPCs) relative wild-type MEFs, and that the attenuated expression of full-length osteopontin or FGF7 in Med1−/− MEFs is responsible for the observed phenotype. Thus, osteopontin and FGF7, produced by BM mesenchymal stromal cells, have been identified as important niche factors [8,9].
Periostin (POSTN), first identified in osteoblasts and later found to be expressed more widely, is the fasciclin family extracellular matrix (ECM) protein that is induced by TGF-β, together with its paralog BIGH3. It interacts with other ECM proteins such as fibronectin, type I collagen and tenascin-C, and constitutes the structural basis for tissues [10]. POSTN also induces intracellular signaling through its receptor, integrin αvβ3, and contributes to some pathological conditions such as scar formation after myocardial infarction, and, as a malignant niche factor, cancer cell migration, infiltration and survival [11].
Postn, Cxcl12 and Ccl9 are the direct targets of the transcriptional activator early B-cell factor (EBF) within mouse OP-9 BM stromal cells [12]. POSTN, produced by OP-9 cells, has recently been reported to be required for optimal B lymphopoiesis in vitro [13], suggesting that POSTN, as well as CXCL12, are B cell-specific niche factors within the BM microenvironment. Moreover, increased expression of POSTN within BM stromal cells might correlate with myelofibrosis, leading to the interesting hypothesis that POSTN might be a niche factor for clonal expansion in some form of chronic myeloproliferative diseases [reviewed in 14].
In this study we show that POSTN expression is attenuated in Med1−/− MEFs, and that POSTN produced by BM mesenchymal stromal cells is required for optimal support of both normal HPCs and leukemia-initiating cells in vitro. We propose that POSTN may be a bona fide niche factor for both normal and malignant hematopoiesis.
Methods
Cell culture
Stable lines of Med1+/+p53−/− and Med1−/−p53−/− MEFs in C57BL6 background are described elsewhere [8]. MEFs, mouse BM-derived MSCs obtained from GIBCO, and 293T cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal bovine serum (FBS). The OP-9 cells, distributed by RIKEN BRC through the National Bio-Resource of the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and MS-5 cells [15] were maintained as described [8]. The niche-dependent MB-1 myeloblastoma cells were maintained by coculturing with mitomycin C (MMC)-treated OP-9 cells as described [16,17].
BM cell culture and colony-forming assays
Mouse BM cells in α-modified Eagle’s medium with 20 % FBS were plated on a dish for several hours, and adherent cells (non-hematopoietic cells and macrophages) were eliminated. These BM cells (1 × 106) were cocultured with MMC-treated MEFs, or OP-9 or MS5 cells on 12-well plates, in MyeloCult M5300 (Stem Cell Technologies, Canada) in the absence or presence of recombinant (r) mouse (m) POSTN, or 0.5 µg/ml anti-mPOSTN rabbit polyclonal antibody (Ab) (H-300: sc-67233; Santa Cruz Biotechnology) or normal rabbit IgG (Medical & Biological Laboratories). In some experiments, transwell (12-well; Corning) was used to exclude the effects of physical interaction between BM cells and stromal cells.
For colony-forming assays, half of the medium was replaced with fresh medium every week for 4 weeks. Cells were trypsinized, harvested and cultured in complete methylcellulose medium (MethoCult M3434; Stem Cell Technologies) for all types of colonies at 37 °C for 14 days, and the colonies were counted [8,9].
Bromodeoxyuridine (BrdU) incorporation
DNA synthesis of cocultured hematopoietic cells in 24-well plates was measured by bromodeoxyuridine (BrdU) incorporation as described [8,9].
Quantitative RT-PCR
Total cellular RNA (0.5 µg), isolated with Isogen II (Toyobo), was reverse-transcribed with ReverTra Ace qPCR RT Master Mix with a gDNA Remover kit (Toyobo). Quantitative PCR (StepOnePlus Real-Time PCR system; Life Technologies) was performed for quantitation of various mouse and human mRNAs. Mouse or human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization of the results. The sequences of the primers used for amplification are available upon request.
Western blot analysis and ELISA
For western blot analysis, total cell lysates, separated by SDS-PAGE, were probed with polyclonal Abs against mouse focal adhesion kinase (FAK) (#3285; Cell Signaling), phospho-mFAK (#3283; Cell Signaling), mitogen-activated protein (MAP) kinase ERK1/ERK2 (Millipore), and phospho-MAP kinase ERK1/ERK2 (Tyr202/204) (#4370; Cell Signaling), and monoclonal Abs against mouse integrin αv/CD51 (NBP1-96739; Novus Biologicals), mouse integrin β3 (I19620; Transduction Laboratories), and β-actin (BioLegend). Chemiluminescence was detected by an ImageQuant LAS 4000mini (GE Healthcare).
For quantitation of mPOSTN protein, ELISA was performed by using Quantikine ELISA Mouse Periostin/OSF-2 Immunoassay kit (MOSF20; R&D Systems).
Statistical analysis
All numerical results are expressed as means ± SD. Data were evaluated using Student’s t test (for two groups) or one-way analysis of variance (ANOVA) (for more than two groups) for statistical comparisons. Two-way ANOVA was used for studies over prolonged time and, when significant, values for each time point were compared. P < 0.05 was considered significant. P < 0.05 and P < 0.01 were represented by * and **, respectively. Reproducibility of all data was confirmed by repeating the experiments more than twice.
Results
POSTN, downregulated in Med1−/− MEFs, mediates growth of BM cells in MEF-based coculture
MEFs have an early mesenchymal feature and are known to support HPCs in vitro [8]. We have previously reported that the Med1−/− MEFs have reduced ability to promote growth of BM cells and to support of HPCs in vitro, at least partly, through the reduced production of full-length osteopontin [8] and FGF7 [9]. Comparative microarray analysis of the Med1+/+p53−/− and Med1−/−p53−/− MEFs has revealed that Postn expression is also attenuated in Med1−/− MEFs (GEO accession number GSE22471) [8]. These findings and the proposed role for POSTN as a niche factor for rabbit and mouse B lymphopoiesis [13], prompted us to investigate whether POSTN also acts as a niche factor for other lineages of hematopoiesis.
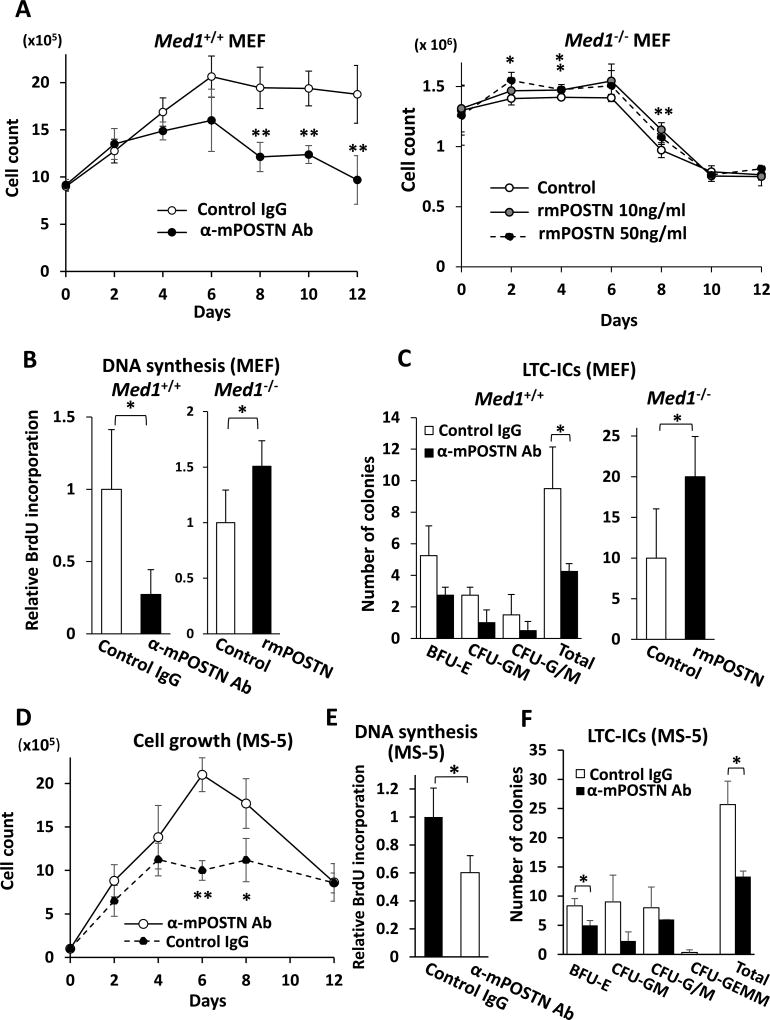
We first examined the effect of POSTN on mitogenicity of BM cells cocultured with MEFs. When normal BM cells were cocultured with MMC-treated Med1+/+ MEFs in the presence of anti-mPOSTN Ab, the number of BM cells was reduced compared to the control (Fig. 1A, left panel). These cells also showed reduced DNA synthesis (Fig. 1B, left panel), but the rate of cell death was comparable to that of the control as revealed by trypan blue staining. However, the number (Fig.1A, right panel) and DNA synthesis level (Fig. 1B, right panel) of BM cells increased when cocultured with Med1−/− MEFs in the presence of excess amount of exogenous rmPOSTN, whereas the rate of cell death was unchanged relative to that in the control. The significant effect of exogenous rmPOSTN in growth was reproducible three times, although marginal due probably to the residual POSTN present in the Med1−/− MEFs (see below) that was sufficient for a close to maximal effect. These results indicate that the extracellular POSTN in the MEF-based coculture system has a role in mitogenicity of BM cells.
Fig. 1. POSTN promotes BM cell growth and HPCs support on MEFs and MS5 BM stromal cells.
(A) The number of BM cells, when cocultured on Med1+/+ MEFs, decreased in the presence of anti-mPOSTN Ab (left panel) and, when cocultured on Med1−/− MEFs, slightly increased in the presence of rmPOSTN. α, anti.
(B) DNA synthesis of BM cells, measured by BrdU incorporation, on Med1+/+ MEFs decreased in the presence of anti-mPOSTN Ab (left panel) and, on Med1−/− MEFs, increased in the presence of 10 ng/ml rmPOSTN.
(C) HSPCs support was quantitated by LTC-ICs. On Med1+/+ MEFs, the number of colonies decreased in the presence of anti-mPOSTN Ab (left panel) and, on Med1−/− MEFs, total LTC-ICs increased in the presence of 10 ng/ml rmPOSTN (right panel).
(D, E) The number (D) and DNA synthesis (E) of BM cells, when cocultured with MS-5 cells, decreased in the presence of anti-mPOSTN Ab.
(F) The number of LTC-ICs, when BM cells were cocultured with MS-5 cell, decreased in the presence of anti-mPOSTN Ab.
N = 4 (A–F).
Periostin mediates support of HPCs in MEF-based coculture
We next analyzed the role of POSTN in supporting HPCs in MEF-based BM cells coculture. When BM cells were cocultured with Med1+/+ MEFs in the presence of anti-mPOSTN Ab, the number of LTC-ICs decreased, with variably lowered support of both erythroid and myelomonocytic colonies (Fig. 1C, left panel). The number of LTC-ICs increased (Fig. 1C, right panel) when cocultured with Med1−/− MEFs in the presence of rmPOSTN. These results suggest that the extracellular POSTN supports HPCs in the MEF-based coculture system.
POSTN mediates growth of BM cells and support of LTC-ICs in coculture with BM mesenchymal stromal cells
We attempted to confirm that the abovementioned MEF-based conclusion holds good in the coculture of BM cells and BM mesenchymal stromal cells. When BM cells were cocultured with MMC-treated MS-5 or OP-9 BM stromal cells in the presence of anti-mPOSTN Ab, the growth (Fig. 1D, supplementary Fig. E1A) and mitogenicity (Fig. 1E, supplementary Fig. E1B) of BM cells, as well as the number of LTC-ICs (Fig. 1F, supplementary Fig. E1C), declined. Reciprocally, when an excessive amount of rmPOSTN was added to the BM cell coculture with MS-5 cells, the number of BM cells and the level of DNA synthesis increased (supplementary Fig. E1D,E). These results clearly confirm the conclusion that POSTN mediates mitogenicity of BM cells and HPCs support.
Niche cell-derived POSTN supports niche-dependent MB-1 myeloblastoma cells
The MB-1 myeloblastoma cell line, originally established from a patient with myeloid crisis chronic myeloid leukemia, is a mesenchymal stromal cell-dependent cell line. These cells are unique in that they grow by forming cobblestone areas in the presence of niche cells but die of apoptosis when detached from stromal cells, thus possessing the characteristics of leukemic stem cells in vitro [16,17]. We used MB-1 cells to analyze the role of POSTN in supporting the myeloid leukemic stem/initiating cells.
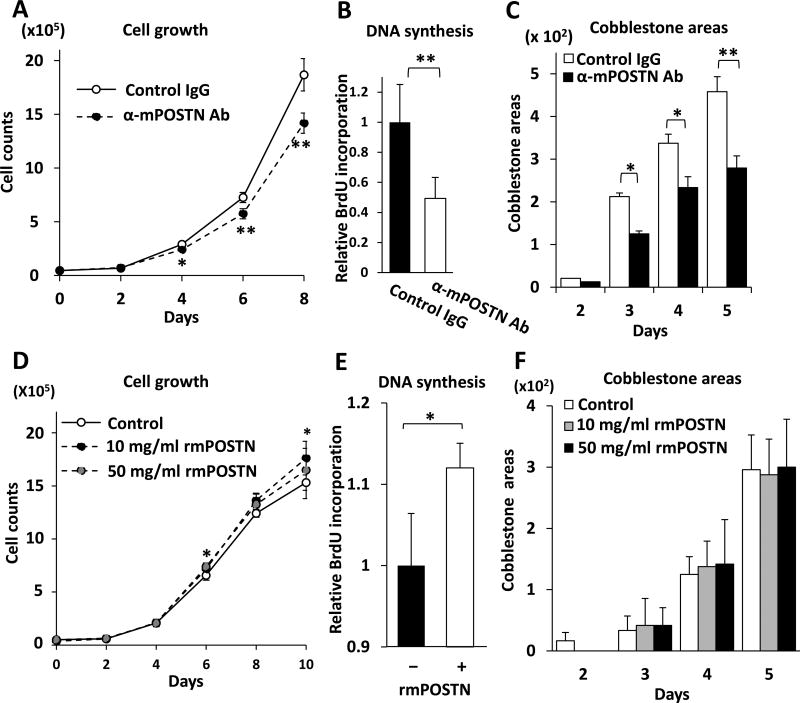
When MB-1 cells were cocultured with MMC-treated MS-5 or OP-9 BM stromal cells in the presence of anti-mPOSTN Ab, the number of MB-1 cells declined compared to the analysis with control IgG (Fig. 2A, supplementary Fig. E2A). Since most cells were viable with less than 1% trypan blue-positive cells in both cases, the reduction in the number of MB-1 cells is attributed to defective growth. Consistent with this hypothesis was the observation that anti-mPOSTN Ab also decreased the mitogenicity of MB-1 cells (Fig. 2B, supplementary Fig. E2B). The number of cobblestone areas was also reduced in the presence of anti-mPOSTN Ab (Fig. 2C, supplementary Fig. E2C). These effects are brought about by the POSTN produced by stromal cells, because MB-1 cells did not express any detectable level of POSTN mRNA despite the reference gene GAPDH being expressed abundantly (data not shown).
Fig. 2. MS-5 cell POSTN supports MB-1 niche-dependent myeloblastoma cells.
(A, D) The number of MB-1 cells cocultured with MS-5 cells decreased in the presence of anti-mPOSTN Ab (A), and increased in the presence of excess amount of exogenous rmPOSTN (D).
(B, E) Mitogenicity of MB-1 cells, cocultured with MS-5 cells, measured by BrdU incorporation, was attenuated in the presence of anti-mPOSTN Ab (B), and slightly increased in the presence of excess amount of exogenous rmPOSTN (E).
(C, F) The number of cobblestone areas per visual field formed by MB-1 cells cocultured with MS-5 cells was counted. The number decreased in the presence of anti-mPOSTN Ab (C), but was unchanged when excess amount of exogenous rmPOSTN was added (F).
N = 3 (A, E), 8 (B), or 4 (C, D, F).
The addition of exogenous rmPOSTN to the coculture with MS-5 cells slightly increased the growth (Fig. 2D) and DNA synthesis level (Fig. 2E) of MB-1 cells, but did not have an effect on the number of cobblestone areas (Fig. 2F). Therefore, rmPOSTN apparently increased the size of (i.e., the number of cells per) cobblestone areas. These data clearly suggest that BM stromal cell-derived POSTN is important for the optimal support/growth of stromal cell-dependent myeloblastic leukemia cells; it may constitute a leukemia-initiating cell niche.
POSTN is expressed in various mesenchymal cells and BM cells
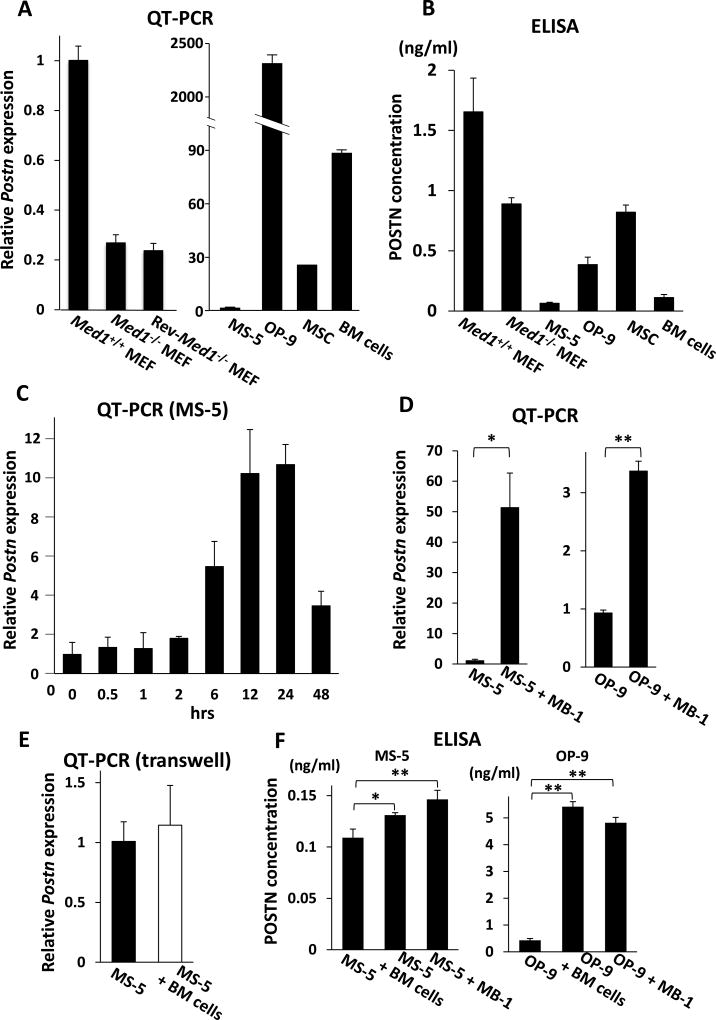
Consistent with the microarray data (above), the expression of Postn mRNA was much lower in Med1−/− MEFs. The expression level was not restored by introducing MED1 to Med1−/− MEFs (Rev-Med1−/− MEFs) (Fig. 3A, left panel), indicating that the effect of MED1 on Postn transcription in MEFs is indirect. The production of POSTN protein, both the extracellular secreted form (Fig. 3B) and the membrane-associated or intracellular form (supplementary Fig. E3A), was reduced by half in Med1−/− MEFs.
Fig. 3. POSTN expression in various mesenchymal cells and BM cells.
(A) Quantitative PCR. Postn mRNA was reduced in Med1−/− MEFs compared with Med1+/+ MEFs, and was not restored in Rev-Med1−/− MEFs (left panel). Postn was variably expressed in BM stromal cells and BM cells (right panel). The values are plotted as the fold increase versus the value in Med1+/+ MEFs (left panel) or MS-5 cells (right panel).
(B) ELISA of culture media. POSTN was secreted by various mesenchymal cells and BM cells.
(C–E) Quantitative PCR. MS-5 cell Postn mRNA was prominently induced when cocultured with BM cells (C). MS-5 cell or OP-9 cell Postn mRNA was induced when cocultured with MB-1 cells for 24 h (D). When cocultured for 24 h in transwell to inhibit direct contact between BM cells and MS-5 cells, Postn mRNA was not induced (E).
(F) ELISA of culture media. mPOSTN secretion was induced by physical interactions of MS-5 or OP-9 cells with BM cells or MB-1 cells.
N = 3 (A–F).
The BM mesenchymal stromal cells express Postn mRNA at different levels, ranging from low in MS-5 cells to high in OP-9 cells (Fig. 3A, right panel). Corresponding differences in the expression of both extracellular and cell-associated POSTN protein were also observed in these cells. BM cells also express a considerable amount of POSTN both at mRNA and protein levels (Fig. 3B, supplementary Fig. E3A).
Intriguingly and unexpectedly, Postn mRNA expression was robustly induced after MS-5 cells were cocultured with BM cells. The enhanced Postn transcription was not immediate but was first observed after 6 h of coculture. Therefore, the enhanced Postn expression was probably indirect. The expression level further increased and stayed high up to 24-h and then decreased (Fig. 3C). When OP-9 cells and BM cells were cocultured, Postn expression decreased slightly at first, but began to increase after 6-h and remained high for 24-h (supplementary Fig. E4A). Mouse Postn expression was also prominently enhanced after MS-5 or OP-9 cells were cocultured with MB-1 cells (Figure 3D). Since MB-1 cells are human, MS-5 or OP-9 stromal cells are the source of enhanced Postn mRNA. Postn expression was not enhanced when BM cells were cocultured but physically separated from MS-5 or OP-9 cells using transwell culture wells (Fig. 3E, supplementary Fig. E4B), which indicates that the enhanced Postn expression was dependent on physical contact of BM stromal cells with hematopoietic cells. Extracellularly secreted POSTN was also increased by the coculture (Fig. 3F), while the levels of stromal cell-associated POSTN were unchanged (supplementary Fig. E3B). These data suggest that POSTN in BM stromal cells is induced by the tactile interaction with hematopoietic cells.
POSTN receptor integrin αvβ3 is expressed on BM mesenchymal stromal cells
To gain insights into the mechanism of action of POSTN in the growth and support of normal and malignant hematopoietic cells, we analyzed the expression of POSTN receptor integrin αvβ3.
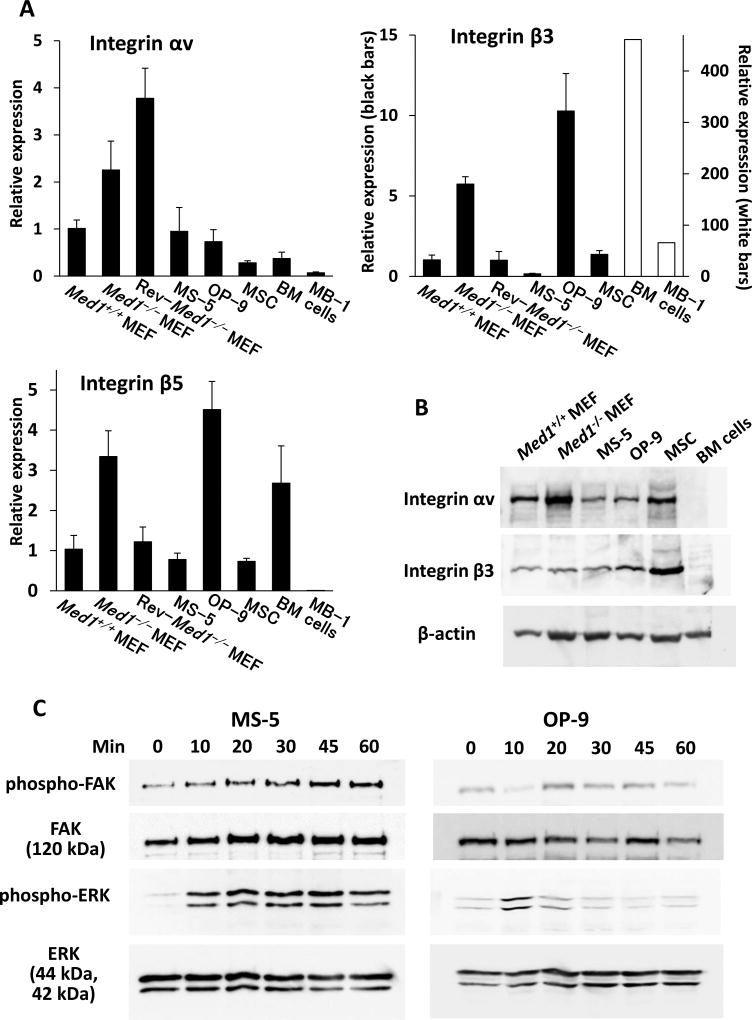
Integrin αv, β3, and β5 mRNAs were differentially expressed in BM mesenchymal cells and hematopoietic cells. β3 mRNA was especially prominent in both BM cells and MB-1 cells (Fig. 4A). Western blot analysis showed that αv and β3 were expressed on various mesenchymal cells but, in our hands, these expression levels were below the detection level on BM cells (Fig. 4B). These data suggest that integrin αvβ3 is abundant on BM mesenchymal stromal cells but not on BM cells. However, it does not exclude the possibility that functional integrin αvβ3 might be enriched on HSPCs, as suggested previously [13,18], and MB-1 cells might express a small amount of αvβ3 protein.
Fig. 4. mPOSTN induces phosphorylation of BM stromal cell FAK and MAP kinases through integrin αvβ3.
(A) Quantitative PCR. αv, β3, and β5 transcription in various mesenchymal cells and hematopoietic cells. N = 4.
(B) Western blot. Integrin αvβ3 was expressed strongly in various mesenchymal cells but was undetectable in BM cells.
(C) The phosphorylation of FAK and MAP kinases in MS-5 or OP-9 cells was induced after rmPOSTN addition.
POSTN induces intracellular signaling in BM mesenchymal stromal cells
Given that POSTN expression was induced in BM stromal cells upon physical interaction with BM cells, that integrin αvβ3 was richly expressed on these cells, and that, in the case of MB-1 cells, POSTN was not expressed by hematopoietic cells, we hypothesized that BM stromal cell POSTN might act on stromal cells in an autocrine or paracrine manner through their expression of integrin αvβ3. We explored this possibility by testing whether treatment of these cells with exogenous POSTN elicited intracellular signaling.
MS-5 cells express a relatively small amount of endogenous POSTN (above). When an excess amount of exogenous POSTN was added to MS-5 cells after 24-h serum starvation, FAK (the immediate target of integrin αvβ3) was slightly, and MAP kinases ERK1/ERK2 (the intermediate hub of various intracellular signals) was more robustly, phosphorylated as early as 10 min, and the phosphorylation was sustained for over 60 min. OP-9 cells, which express much more endogenous POSTN, also showed increased phosphorylation of these kinases but at a relatively lower level compared to MS-5 cells (Fig. 4C). These results clearly indicate that POSTN effectively activates integrin αvβ3 and subsequent intracellular signaling in BM stromal cells.
Discussion
We propose that, in our in vitro niche model, POSTN is a niche factor both for normal hematopoiesis and for support of myeloblastic leukemia-initiating cells. While both BM cells and BM stromal cells produce POSTN, the role of stromal cells that robustly induce Postn expression upon physical interaction with hematopoietic cells is highlighted. POSTN appears to support HPCs and myeloblastic leukemia-initiating cells, at least in part, indirectly by POSTN- and integrin αvβ3-activated BM stromal cells.
Although we consider that the major source of POSTN in coculture is BM stromal cells, particularly in the case of coculture with leukemia-initiating cells, the expression by BM cells is not negligible and might be responsible for an accessory but significant contribution. The total POSTN produced by both BM stromal cells and hematopoietic cells may together constitute a functional hematopoietic niche. Experiments using Postn−/− mouse-derived hematopoietic cells and/or CRISPR-CAS9 system-mediated knockout of Postn in BM stromal cells could answer this question in future.
The mechanism of POSTN action on BM stromal cells raises a question regarding the identity of putative niche molecules produced in these cells upon stimulation by POSTN. In one study, CXCL12 and IL-7 were found to be downregulated in Postn-knockdown OP-9 cells [13]. However, B lymphopoiesis was not restored by the addition of exogenous CXCL12 and IL-7, indicating that as yet unidentified niche molecules might be involved.
We propose that the mechanism of POSTN action is mainly, or at least partly, indirect because the expression of integrin αvβ3 on BM cells at the protein level is low compared to the level in BM stromal cells. However, αv and β3 are expressed at the mRNA level, and HSPCs (and MB-1 cells) may express integrin αvβ3 at an increased level [13]. In fact, HSPCs are reportedly supported by thrombopoietin through thrombopoietin receptor c-Mpl-associated integrin αvβ3 [19]. If HSPCs express functional integrin αvβ3, it is also reasonable to infer a direct effect of POSTN and integrin αvβ3 interaction on HSPCs and possibly leukemia-initiating cells.
Apart from a role in supporting hematopoietic cells, POSTN also constitutes a major ECM component. POSTN also enhances bone formation at the periosteum [20, reviewed in 14]. These effects of POSTN might add to the stability of the BM microenvironment, and might also facilitate the function of other niche factors.
Postn knockout mice are reportedly not defective in normal hematopoiesis. This suggests an in vivo redundancy made up of a complex network of a number of niche molecules and their interactions with their respective receptors on both hematopoietic cells and stromal cells, and of the mechanical and chemical elements of the microenvironment. A simple and defined in vitro system as ours is effective in reducing the complexity and enhancing our understanding. Now that POSTN is shown to be a niche factor in vitro, it is interesting to know if these knockout mice really have no altered phenotype in hematopoiesis. Studying in detail the stress hematopoiesis and leukemogenesis or crossing these mice with other mutant mice might lead to some interesting discoveries and would be worthwhile future research efforts.
Supplementary Material
(A, B, C) The number (A) and DNA synthesis measured by BrdU incorporation (B) of BM cells, and LTC-ICs (C), when cocultured on OP-9 cells, decreased in the presence of anti-mPOSTN Ab. α, anti.
(D, E) The number (D) and DNA synthesis (E) of BM cells, when cocultured with MS-5 cells, increased in the presence of rmPOSTN. 10 ng/ml POSTN was added in (E).
N = 4 (A, B, D, E), or 3 (C). *, P < 0.05; **, P < 0.01.
(A, B) The number (A) and DNA synthesis (B) of MB-1 cells cocultured with OP-9 cells decreased in the presence of anti-mPOSTN Ab.
(C) The number of cobblestone areas per visual field, formed by MB-1 cells cocultured with OP-9 cells, decreased in the presence of anti-mPOSTN Ab.
N = 3 (A), 8 (B), or 4 (C). *, P < 0.05; **, P < 0.01.
POSTN concentrations of whole cell extracts, obtained by sonicating cells in a buffer (100 mM KCl, 0.05 % NP-40, 10 mM Tris-HCl pH 7.9 (at 4 °C), 0.25 mM EDTA, and 10 % glycerol) and normalized by protein concentrations (420 µg/ml (A) and 284 µg/ml (B)) were measured by ELISA. The values were subtracted from the ELISA value of human 293T cell extract as the background.
(A) Various cells had variable levels of cell-associated POSTN.
(B) MS-5 or PO-9 cell-associated POSTN was not induced by physical attachment with BM cells.
N = 3.
(A, B) Quantitative PCR. OP-9 cell Postn mRNA was initially downregulated and then induced when cocultured with BM cells (A). When cocultured for 24 h in transwells to inhibit direct contact between BM cells and OP-9 cells, Postn mRNA was not induced (B).
N = 3.
Acknowledgments
We thank Katsuhiko Ito for MS-5 cells, Minato Nakazawa, Yoshimi Takai and members in our laboratories and Takai’s laboratory for helpful discussion, and Yoshimi Takai for sharing laboratory materials. This study was supported by grants from the MEXT (to M.I.).
References
- 1.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvi LM, Link DC. The hematopoietic stem cell niche in homeostasis and disease. Blood. 2015;126:2443–2451. doi: 10.1182/blood-2015-07-533588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik S, Roeder RG. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 2010;11:761–772. doi: 10.1038/nrg2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito M, Roeder RG. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol. Metab. 2001;12:127–134. doi: 10.1016/s1043-2760(00)00355-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Roeder RG. Mediator-dependent nuclear receptor function. Semin. Cell Dev. Biol. 2011;22:749–758. doi: 10.1016/j.semcdb.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumitomo A, Ishino R, Urahama N, Inoue K, Yonezawa K, Hasegawa N, Horie O, Matsuoka H, Kondo T, Roeder RG, Ito M. The transcriptional Mediator subunit MED1/TRAP220 in stromal cells is involved in hematopoietic stem/progenitor cell support through osteopontin expression. Mol. Cell. Biol. 2010;30:4818–4827. doi: 10.1128/MCB.01348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishino R, Minami K, Tanaka S, Nagai M, Matsui K, Hasegawa N, Roeder RG, Asano S, Ito M. FGF7 supports hematopoietic stem and progenitor cells and niche-dependent myeloblastoma cells via autocrine action on bone marrow stromal cells in vitro. Biochem. Biophys. Res. Commun. 2013;440:125–131. doi: 10.1016/j.bbrc.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 10.Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J. Biol. Chem. 2010;285:2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi Y, Kunita A, Iwata C, Komura D, Nishiyama T, Shimazu K, Takeshita K, Shibahara J, Kii I, Morishita Y, Yashiro M, Hirakawa K, Miyazono K, Kudo A, Fukayama M, Kashima TG. The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am. J. Pathol. 2014;184:859–870. doi: 10.1016/j.ajpath.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Lagergren A, Månsson R, Zetterblad J, Smith E, Basta B, Bryder D, Akerblad P, Sigvardsson M. The Cxcl12, Periostin and Ccl9 genes are direct targets for early B-cell factor in OP-9 stroma cells. J. Biol. Chem. 2007;282:14454–14462. doi: 10.1074/jbc.M610263200. [DOI] [PubMed] [Google Scholar]
- 13.Siewe BT, Kalis SL, Le PT, Witte PL, Choi S, Conway SJ, Druschitz L, Knight KL. In vitro requirement for periostin in B lymphopoiesis. Blood. 2011;117:3770–3779. doi: 10.1182/blood-2010-08-301119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klamer S, Voermans C. The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment. Cell Adh. Migr. 2014;8:563–577. doi: 10.4161/19336918.2014.968501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, Uchino H, Mori KJ. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp. Hematol. 1989;17:145–153. [PubMed] [Google Scholar]
- 16.Funayama K, Saito-Kurimoto Y, Ebihara Y, Shimane M, Nomura H, Tsuji K, Asano S. Adhesion-mediated self-renewal abilities of Ph+ blastoma cells. Biochem. Biophys. Res. Commun. 2010;396:193–198. doi: 10.1016/j.bbrc.2010.03.172. [DOI] [PubMed] [Google Scholar]
- 17.Funayama K, Shimane M, Nomura H, Asano S. An evidence for adhesion-mediated acquisition of acute myeloid leukemic stem cell-like immaturities. Biochem. Biophys. Res. Commun. 2010;392:271–276. doi: 10.1016/j.bbrc.2009.12.163. [DOI] [PubMed] [Google Scholar]
- 18.Umemoto T, Yamato M, Shiratsuchi Y, Terasawa M, Yang J, Nishida K, Kobayashi Y, Okano T. CD61 enriches long-term repopulating hematopoietic stem cells. Biochem. Biophys. Res. Commun. 2008;365:176–182. doi: 10.1016/j.bbrc.2007.10.168. [DOI] [PubMed] [Google Scholar]
- 19.Umemoto T, Yamato M, Ishihara J, Shiratsuchi Y, Utsumi M, Morita Y, Tsukui H, Terasawa M, Shibata T, Nishida K, Kobayashi Y, Petrich BG, Nakauchi H, Eto K, Okano T. Integrin-αvβ3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells. Blood. 2012;119:83–94. doi: 10.1182/blood-2011-02-335430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol. Cell. Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A, B, C) The number (A) and DNA synthesis measured by BrdU incorporation (B) of BM cells, and LTC-ICs (C), when cocultured on OP-9 cells, decreased in the presence of anti-mPOSTN Ab. α, anti.
(D, E) The number (D) and DNA synthesis (E) of BM cells, when cocultured with MS-5 cells, increased in the presence of rmPOSTN. 10 ng/ml POSTN was added in (E).
N = 4 (A, B, D, E), or 3 (C). *, P < 0.05; **, P < 0.01.
(A, B) The number (A) and DNA synthesis (B) of MB-1 cells cocultured with OP-9 cells decreased in the presence of anti-mPOSTN Ab.
(C) The number of cobblestone areas per visual field, formed by MB-1 cells cocultured with OP-9 cells, decreased in the presence of anti-mPOSTN Ab.
N = 3 (A), 8 (B), or 4 (C). *, P < 0.05; **, P < 0.01.
POSTN concentrations of whole cell extracts, obtained by sonicating cells in a buffer (100 mM KCl, 0.05 % NP-40, 10 mM Tris-HCl pH 7.9 (at 4 °C), 0.25 mM EDTA, and 10 % glycerol) and normalized by protein concentrations (420 µg/ml (A) and 284 µg/ml (B)) were measured by ELISA. The values were subtracted from the ELISA value of human 293T cell extract as the background.
(A) Various cells had variable levels of cell-associated POSTN.
(B) MS-5 or PO-9 cell-associated POSTN was not induced by physical attachment with BM cells.
N = 3.
(A, B) Quantitative PCR. OP-9 cell Postn mRNA was initially downregulated and then induced when cocultured with BM cells (A). When cocultured for 24 h in transwells to inhibit direct contact between BM cells and OP-9 cells, Postn mRNA was not induced (B).
N = 3.