Abstract
Background
Wide-ranging evidence on the occurrence of fluoroquinolone (FQ) resistance genetic determinants in African Salmonella strains is not available. The main objectives of this study were to assess the heterogeneity, estimate pooled proportions and describe the preponderance of FQ-resistance determinants in typhoidal and non-typhoidal Salmonella (NTS) isolates of Africa.
Methods
Genetic and phenotypic data on 6103 Salmonella isolates were considered. Meta- and frequency analyses were performed depending on the number of studies by category, number of isolates and risks of bias. A random effects model was used to assess heterogeneity and estimate pooled proportions. Relative and cumulative frequencies were calculated to describe the overall preponderance of FQ-resistance determinants in quinolone resistant isolates.
Results
The pooled proportion of gyrA mutants (Salmonella enterica serovar Typhi, Salmonella enterica serovar Typhimurium, and Salmonella enterica serovar Enteritidis) was estimated at 5.7% (95% Confidence interval (CI) = 2.6, 9.8; Tau squared (T2) = 0.1105), and was higher in S. Typhi than in S. Typhimurium (odds ratio (OR) = 3.3, 95%CI = 2, 5.7). The proportions of each of gyrB and parC mutants, and strains with Plasmid Mediated Quinolone Resistance genes (qnrA, qnrB and qnrS) were low (≤ 0.3%). Overall, 23 mutant serotypes were identified, and most strains had mutations at codons encoding Ser83 and Asp87 of gyrA (82%, 95%CI = 78, 86).
Conclusions
Mutations at gyrA appear to account for ciprofloxacin non-susceptibility in most clinical Salmonella strains in Africa. The estimates could be harnessed to develop a mismatch-amplification mutation-assay for the detection of FQ-resistant strains in Africa.
Introduction
Salmonella is one of the major causes of morbidity and mortality in Africa [1]. The serotypes the most frequently associated with invasive disease are S. Typhi, S. Typhimurium and S. Enteritidis [1, 2]. Incidence of invasive non-typhoidal Salmonella (iNTS) disease as high as 227 per 100,000 [range 152–341] [3] and a case fatality of 19% (276/1427) among low risk populations [4], higher S. Typhi blood stream infections in children than in adults (OR, 1.7; 95% CI = 1,2.7) [5], higher iNTS disease in high than in low malaria endemic settings (OR = 4.9 (1.6,14.9) [6], higher malaria-NTS co-infection in children than in adults [7] and a wide-spread occurrence of antimicrobial resistant strains [8,9] have been documented.
Increased resistance to chloramphenicol led to the increased use of ampicillin and cotrimoxazole [10], and the subsequent emergence of strains multiply resistant to these drugs led to the use of FQs [11]. However, FQ-resistance ensued shortly thereafter [11], and this has been linked to chromosomal mutations and plasmid-borne genes [12]. Infections with strains with high minimum inhibitory concentrations (MICs) have been associated with more treatment failures than those due to strains with low MICs (relative risk = 5.75; 95% CI = 1.8, to 18.7) [13]. Due to multiple mechanisms, recognition of FQ-resistance in Salmonella is complicated [14], and treatment failures have necessitated modifications of the break point levels of susceptibility/resistance [15, 16].
Selection pressure triggered by drugs, geographic and social transmission environments have been proposed as factors attributable to variation of gyrA mutations in Mycobacterium tuberculosis [17]. Correspondingly, the variations in the occurrence of FQ-resistant Salmonella among WHO regions [18] and within Africa [8,19] could be due to distinctions in the occurrence of genetic determinants across serotypes and differences in selection pressure across locations. However, wide-ranging comparative studies (typhoidal vs. NTS) or systematic reviews/meta-analyses on the occurrence of FQ-resistance genetic determinants across serotypes, and countries/regions in Africa are not available. The main objectives of this study were to assess the heterogeneity, estimate pooled proportions and describe the preponderance of genetic determinants that confer resistance to FQs in typhoidal and non-typhoidal Salmonella isolates of Africa.
Methods
The study was carried out according to the guidelines set-forth in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [20, 21] (S1 Checklist). The questions were on the heterogeneity and preponderance of genetic determinants of FQ-resistance in typhoidal and non-typhoidal Salmonella isolates of Africa. The study populations were salmonellae isolated from humans, animals and animal products. The study designs included prospective and retrospective studies, and case reports. Meta- and/or frequency analyses were performed by sampling populations and methodological features of the studies. The primary outcome of interest was the proportions of FQ-resistance genetic determinants. The secondary outcomes were the phenotypic proportions: multi-drug resistance (MDR), nalidixic acid non-susceptibility (Nalns), ciprofloxacin non-susceptibility (Cipns), MDR + Cipns, and non-MDR + Cipns). MDR was defined as resistance to chloramphenicol, ampicillin and cotrimoxazole. Nalidixic acid susceptibility was defined as minimum inhibitory concentration (MIC) ≤ 16μg/mL [22]. Ciprofloxacin susceptibility was defined as an MIC ≤ 0.06μg/mL [16, 22]. Intermediate susceptibility and resistance were combined as non-susceptible.
Study search
We searched published studies in PubMed, Google Scholar and African Journals Online (AJOL). A systematic search was done to avoid duplication of the subject: Salmonella AND Africa AND (‘systematic review’ OR ‘meta-analysis’). Multiple search strings (medical subject headings [MeSH] and text words [tiab]) were tried to identify primary studies. The search string that enabled to locate most studies was the following: Salmonella AND (quinolone OR fluoroquinolone) AND (Africa OR Algeria OR Angola OR Benin OR Botswana OR ‘Burkina Faso’ OR Burundi OR Cameroon OR ‘Cape Verde’ OR ‘Central African Republic’ OR Chad OR Comoros OR ‘Congo Republic’ OR ‘Côte d'Ivoire’ OR ‘Ivory Coast’ OR ‘Democratic Republic of the Congo’ OR Djibouti OR Egypt OR ‘Equatorial Guinea’ OR Eritrea OR Ethiopia OR Gabon OR Gambia OR Ghana OR Guinea OR ‘Guinea Bissau’ OR Kenya OR Lesotho OR Liberia OR Libya OR Madagascar OR Malawi OR Mali OR Mauritius OR Mauritania OR Morocco OR Mozambique OR Namibia OR Niger OR Nigeria OR ‘North Sudan’ OR Rwanda OR ‘Republic of Sudan’ OR ‘Sāo Tome and Principe’ OR Senegal OR Seychelles OR ‘Sierra Leone’ OR Somalia OR ‘South Africa’ OR ‘South Sudan’ OR Sudan OR Swaziland OR Tanzania OR Togo OR Tunisia OR Uganda OR ‘United Republic of Tanzania’ OR ‘Western Sahara’ OR ‘Saharawi Arab Democratic Republic’ OR Zambia OR Zimbabwe). Reference lists were scanned to make-out additional reports. The last search was done on January 21, 2018.
Inclusion and exclusion criteria
We excluded reviews, and studies with titles and/or abstracts unrelated to the primary outcomes of interest. Studies with the following characteristics were screened for eligibility: (i) published in English or French, (ii) reported genetic determinants of resistance to FQs, (iii) Salmonella isolated from humans, or domestic animals or animal products, and (iv) serotyped. Letters, correspondence and reports with unclear data were not included. An outbreak was defined as the occurrence of two or more cases of salmonellosis due to consumption of contaminated food/water from the same source [23]. Salmonellae isolated from samples collected from diverse locations, over a long period of time, and with different AMR and/or genomic patterns were considered as non-outbreak isolates.
Data extraction
The following data were considered and extracted: (i) study identifier: first author, year of publication, year of study, country, target population (humans, domestic animals and animal products), and sampling population (health-care/nosocomial, community, travel, animal species/products); (ii) methods: sample size, sampling method, sample, drug susceptibility test (DST), quinolones, break point level/interpretive standard, and gene detection (phenotype/genotype based); (iii) results: numbers of isolates (overall, typhoidal and NTS), numbers of isolates subjected to DST, numbers of MDR strains, numbers of nalidixic acid non-susceptible strains (Nalns), numbers of ciprofloxacin non-susceptible strains (Cipns), numbers of MDR + Cipns strains, non-MDR + Cipns, minimum inhibitory concentrations (MICs)/zone diameters (ZD), number of strains examined to detect mutations (gyrA, gyrB, parC and parE), numbers of mutants, mutation positions, substituted amino acids, number of strains examined to detect Plasmid Mediated Quinolone Resistance genes (PMQR) (qnrA, qnrB, qnrC, qnrD, qnrS, aac(6’)-Ib-cr, qepA, oqxA/B), and numbers of strains with PMQR genes.
Study-specific extraction processes (calculations, exclusions and assumptions) are provided in the table of characteristics of studies (S1 Table). Briefly, data on isolates other than human and domestic animal/animal product origins or collected before 2000 or duplicates were excluded. Authors were contacted to solicit online/supplementary information (S1 Table). Data extraction was done twice–overall and by subgroup (typhoidal and NTS). A further extraction was done by serotype. Multiple cross checks were performed to ensure extraction harmony and fix incompatibilities. The data was extracted by GT.
Data preprocessing
We categorized the data by region–Central, Northern, Eastern, Western and Southern Africa. Multi-country data were handled by country. Homogeneity based frequencies (group/serotype, country/region) were calculated to circumvent problems associated with study precision [24, 25]. Unreported sample size (Ns) is reported as not reported (nr), but approximated: Ns = n/p; where n = number of isolates, and p = pooled proportion. If MICs were provided, the number of non-susceptible strains classified as susceptible–due to high break point levels of the earlier standards–was adjusted according to the modified break point level [16]. In the absence of phenotype data, numbers of MDR and quinolone non-susceptible strains were extrapolated from genotype data (MDR = cat + blaTEM + dfrA-sul1, and qnrS/gyrA mutation positive = quinolone non-susceptible). To normalize data distribution, study level estimates were double arcsine (t) transformed, t = sin-1(√n/N+1) + sin-1(√n+1/N+1), Se (t) = √1/N+0.5, [26].
Assessment of quality and risk of bias
Study validity was established by the inclusion criteria [27]. The methodological quality of each study was assessed following the Joanna Briggs Institute (JBI) critical appraisal tool [28], and components [21] for which there is a practical evidence of bias: (i) target population (humans vs. animals/animal products), (ii) sampling population (humans [27]–health care vs. non-health care–community, adoptees, travelers; animals/animal products–species, farms, slaughterhouse, markets), (iii) sample size (n ≤ 384 vs. higher; n = Z2PQ/d2; where Pexp = 0.5), (iv) study design (prospective, retrospective, collections, cases), sampling method (consecutive/probability vs. non-probability), (iv) samples (blood vs. others), (v) phenotype detection (E-test/dilution (MIC) vs. disk diffusion (ZD)) [15,16]; ciprofloxacin susceptibility break point level/interpretive standard (Cips ≤ 0. 06μg/mL vs. higher) [15,16], and (vi) genotype detection (PCR based vs. Whole Genome Sequence based). In each sampling population, studies with the following characteristics were considered as low risk studies: sample size (> 384), prospective/consecutive sampling, and Cips ≤ 0.06μg/mL. The Begg and Mazmudar rank correlation test was used to assess bias across studies (small study effects).
Data analysis
Microsoft Office Excel 2007, Stata (Version 11.1, Stata Corp, College Station, Texas), and Epi InfoTM (version 3.5.1, Center for Disease Control, CDC, USA) were used in data analysis.
Estimation of heterogeneity
We measured inconsistency using Cochran’s test (Q), tau squared (T2) and Higgin’s indexes (H2 and I2) [29]. The 95% confidence bounds of I2 were calculated using the following formula: ln (H) +/-1.965 (Se (ln H)) [29]. If Q > k, Se was calculated by the following formula: Se (ln H) = 0.5 [(ln Q—ln k-1) / (√2Q-√2k-3)]. If Q < K, Se was calculated by the following formula: Se (ln H) = √(1/2k – 4) [(3(k − 2)2–1) / (3(k − 2)2)], [29]. I2 estimates less than 25% and greater than 75%, respectively, were considered as substantially low and high heterogeneity. The significance of a difference in subgroups’ estimates was examined using a one tailed X2 test [30]; X2 = P(X > x), where X is X2 random variable.
Estimation of pooled proportion
We used a random effects model to pool double arcsine estimates. We performed single study omitted influence analyses to examine the sensitivities of pooled estimates. A pooled estimate was considered as robust, if all single study omitted pooled estimates and 95% CIs lie within or closer to its 95% CIs. Pooled estimates were back transformed to proportions (p): p = 0.5 {1-sgn (cos t) [1- (N (sin t)2 + (sin t)2–1/ (N sin t))2]0.5}; where N = sample size [25]. The Yates corrected Chi square test was used to evaluate the significance of a difference between estimates. Alpha (α) was set at 0.05. Odds ratios (OR) were calculated to evaluate the magnitude of superiority or inferiority of estimates.
Proportion of gyrA mutant infection
The proportion of patients infected with gyrA mutants was derived from the pooled proportions of mutant strains, and the pooled proportion of patients with invasive salmonellosis. The proportions of typhoidal and iNTS disease were estimated using data of studies that isolated both typhoidal and non-typhoidal Salmonella. The isolates have been recovered from 47337 samples collected between 2007 and 2015 in nine countries (S1 Table).
Relative and cumulative frequencies
Relative and cumulative frequencies were calculated to describe the overall preponderance of mutated genes, PMQR genes, mutated positions, and substituted amino acids in quinolone resistant salmonellae. The proportion of a mutated or PMQR gene was calculated by dividing the number of strains in which the mutated or PMQR gene was found by the number of quinolone resistant strains examined to detect the genotype. The preponderance of a mutation position (‘hot spot’) was calculated by dividing the frequency of the mutation by the cumulative frequency of mutations within the QRDR. The occurrence of a specific mutation (position and amino acid) was calculated by dividing the frequency of the mutation (position and amino acid) by the cumulative frequency of mutations in the QRDR. The predominance of an amino acid substituted at a specific mutation position was calculated by dividing the frequency of the amino acid by the cumulative frequency of mutations at the specific position.
Results
Search and selection of studies
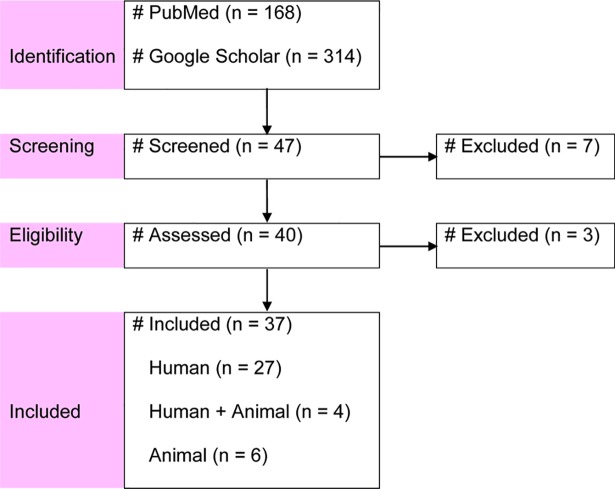
Fig 1 presents a flow diagram of the search and selection of studies. One systematic review on FQ-resistant enteric bacteria–with 10 reports on Salmonella [8]–was identified, but the heterogeneity and predominance of the resistance determinants have not been described. Other systematic reviews/meta-analyses that reported heterogeneity estimates, and genotypic and phenotypic proportions were not found. In total, 47 reports were screened, and 37 were included [31–67].
Fig 1. Flow diagram of search and selection of studies.
Characteristics of included studies
The characteristics of included studies are given in S1 Table. The studies were prospective and retrospective and included surveillance–case series [31–38, 50, 63], laboratory-based [42, 51,59]–and cross-sectional [40,52,58,62,66] studies, case reports [48,49,53,64], studies on hospital and laboratory collections [41,43,46,47,65, 67], multi-country collections [39,44,45,54–56], cross-sectional and/or country-based collections [57, 60, 61]. All human isolates but some from adopted children were recovered from patients who sought medical attention for reasons of fever and gastroenteritis or other systemic infections. One-hundred and thirty-eight isolates were isolated from symptomatic or asymptomatic adopted children [44–46, 50, 65]. Other isolates were from patients hospitalized due to travel-related infections [54–56, 65] and cases [48, 49, 53, 64]. Patient characteristics that included pre-sampling antimicrobial use, infection onset and comorbidities have not been reported in most studies. Of the isolates of animal/animal product origins, nine were from diarrheic calves [58] and others were recovered from a variety of samples and animal species–cattle, swine and poultry (S2 Table).
Overall, data on 6103 isolates recovered from humans (n = 5137) [31–56, 63–67], and animals/animal products (n = 966) [40, 52, 55–62] were considered. The salmonellae have been isolated from more than 100,000 samples. The numbers of typhoidal and NTS isolates, respectively, were 2003 and 4100. Most human isolates of community setting origins were from SSA (99.2%, 5040/5082)–Western (n = 1983), Central (n = 1673), Southern (n = 788) and Eastern Africa (n = 596). Most isolates of animal/animal product origins (59.3%, 573/966) were from Northern Africa. Nalidixic acid and/or ciprofloxacin were the test drugs, except in three studies that as well tested isolates’ susceptibility to norfloxacin [58], ofloxacin [59, 66] and enrofloxacin [66]. Resistance gene detection was phenotype–PCR/sequence based [31–37, 40–42, 44–47, 49–66] and whole genome sequence (WGS) based [38, 39, 43, 48, 67].
Quality assessment and risk appraisal
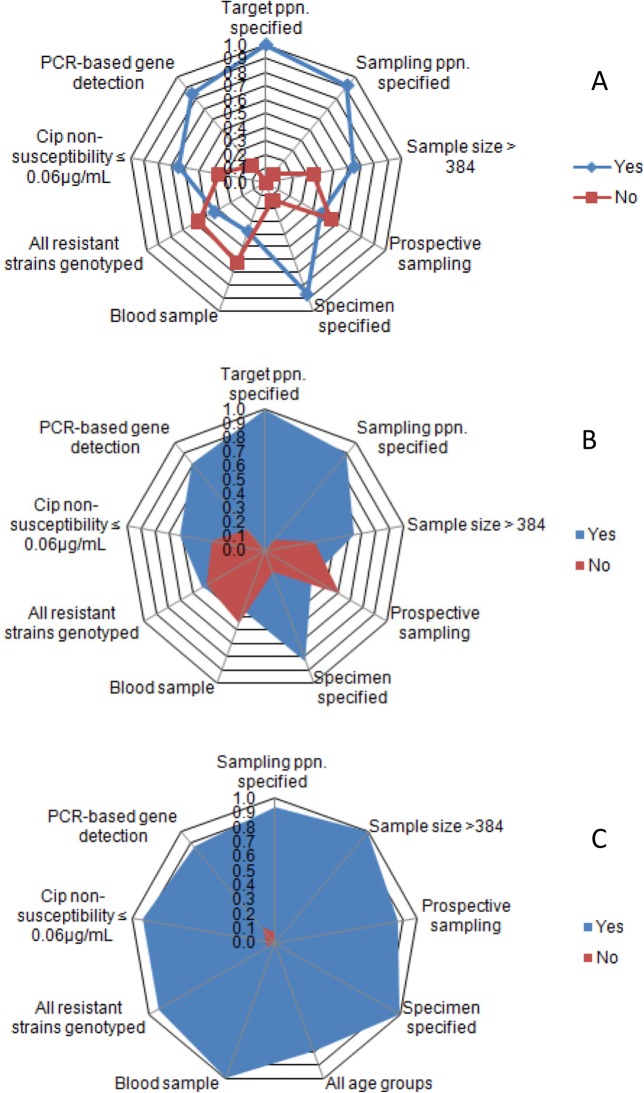
The methodological qualities and risks of bias of included studies are given in S2 Table and S3 Table. Fig 2 presents the proportion of studies by methods. Sampling population, sample size (n > 384), sample type, prospective/consecutive sampling, and Cips cutoff level at ≤ 0.06μg/mL were each reported in 45.9% or more of the studies. Of the studies on isolates of human origins, 25.8% were relatively homogeneous with respect to the sampling population and methods (community, sample size > 384, prospective sampling, blood sample, Cips cutoff level at ≤ 0.06μg/mL, and gene detection), [31–38]. The studies on animals and animal products were few and generally heterogeneous. Accordingly, meta- and frequency analyses were performed depending on the number of studies by category, number of isolates, and risks of bias/heterogeneity.
Fig 2. Radar plots of proportions of studies by methods.
All studies (A), All human studies (B), Studies used in meta-analysis (C).
Meta-analyses were performed to assess the heterogeneity and estimate pooled phenotypic and genotypic proportions of serotypes commonly associated with invasive disease in humans (S. Typhi, S. Typhimurium, and S. Enteritidis). For this purpose, we used data of studies that employed relatively uniform methods [31–39, 63] (S4 Table). Established standards to assess PCR associated bias [68], and to infer drug susceptibility on genotype based tests [69] are not available. However, the correlation between phenotype and genotype is strongly positive [70], and the margin of equivalence between phenotype and genotype based tests was considered insignificant. The minimum sample size and number of isolates included in the meta-analyses were 626 and 10, respectively, and as all isolates were recovered from fairly large samples [71], random fluctuations of uncertain significance were considered unimportant. The Begg and Mazmudar rank correlation test did not suggest across study bias/small study effects (Kendall’s score = 5; P > 0.05).
The number of studies on isolates (n > 10) recovered from each of animal and animal products by species, adoptees and of traveler origins were less than five; a disk (5μg) diffusion test does not reliably indicate Cipns [16], and higher breakpoint levels (> 0.06μg/mL) underestimate the proportion of Cipns strains. In addition, prior to 2011 a standardized International definition of MDR was not available [72], and MDR has not been uniformly defined in several studies other than those included in the meta-analyses. Accordingly, data from animals/animal products, adoptees, travelers and from studies with small sample sizes and/or methods that could likely introduce bias/heterogeneity [40–62, 64– 67], (S3 Table) were used to estimate relative and cumulative frequencies of mutations, PMQR genes, substituted amino acids, and mutant serotypes.
Heterogeneity of phenotypic proportions
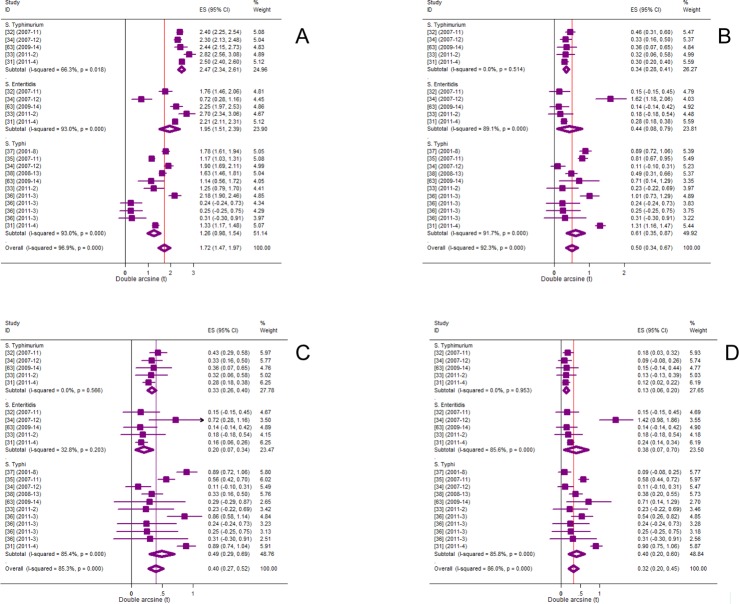
Fig 3 presents forest plots of proportions of MDR, and ciprofloxacin non-susceptible strains. The residuals of the heterogeneity estimates for each of MDR (P = 8E-94) and Cipns (P = 1E-22) invasive strains (S. Typhi, S. Typhimurium and S. Enteritidis) were highly significant and imply across-group differences. T2 estimates for MDR and Cipns strains, respectively, were 0.3171 and 0.1234, and those of MDR-Cipns, and nMDR-Cipns were 0.0595 and 0.0628, respectively (Table 1).
Fig 3. Forest plots of phenotypic proportions.
MDR (A), Cipns (B), MDR-Cipns (C), nMDR-Cipns (D).
Table 1. Heterogeneity of phenotypic proportions.
| I2 (95%CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Phenotype | Serotype | N | K | Q | T2 | I2 | L | U |
| MDR | S. Typhimurium | 800 | 5 | 12 | 0.0144 | 66 | 12 | 87 |
| S. Enteritidis | 530 | 5 | 57 | 0.2249 | 93 | 87 | 96 | |
| S. Typhi | 836 | 11 | 143 | 0.1882 | 93 | 89 | 95 | |
| Overall | 2166 | 21 | 640 | 0.3171 | 97 | 96 | 98 | |
| Cipns | S. Typhimurium | 800 | 5 | 3 | 0.0000 | 0 | 0 | 75 |
| S. Enteritidis | 530 | 5 | 37 | 0.1393 | 89 | 77 | 95 | |
| S. Typhi | 836 | 11 | 120 | 0.1558 | 92 | 87 | 95 | |
| Overall | 2166 | 21 | 261 | 0.1234 | 92 | 90 | 94 | |
| MDR-Cipns | S. Typhimurium | 800 | 5 | 3 | 0 | 0 | 0 | 72 |
| S. Enteritidis | 530 | 5 | 6 | 0.0083 | 33 | 0 | 75 | |
| S. Typhi | 836 | 11 | 68 | 0.0828 | 85 | 76 | 91 | |
| Overall | 2166 | 21 | 136 | 0.0595 | 85 | 79 | 90 | |
| nMDR-Cipns | S. Typhimurium | 800 | 5 | 1 | 0 | 0 | 0 | 0 |
| S. Enteritidis | 530 | 5 | 28 | 0.1011 | 86 | 68 | 94 | |
| S. Typhi | 836 | 11 | 70 | 0.0855 | 86 | 76 | 92 | |
| Overall | 2166 | 21 | 143 | 0.0628 | 86 | 80 | 90 | |
| Inv-salm | iNTS | 47337 | 11 | 1196 | 0.031 | 99 | 99 | 99 |
| Typhoidal | 47337 | 11 | 308 | 0.0078 | 97 | 96 | 98 | |
| Overall | 47337 | 11 | 1548 | 0.0178 | 99 | 98 | 99 | |
Cipns, ciprofloxacin non-susceptible; Inv-salm, invasive salmonellosis; K, number of studies; L, lower limit; MDR, multi-drug resistant; MDR-Cipns, multi-drug resistant + ciprofloxacin non-susceptible; N, number of isolates; nMDR-Cipns, non-multidrug resistant + ciprofloxacin non-susceptible; Q, Cochran’s-test; T2, tau squared; U, upper limit.
Heterogeneity of genotypic proportions
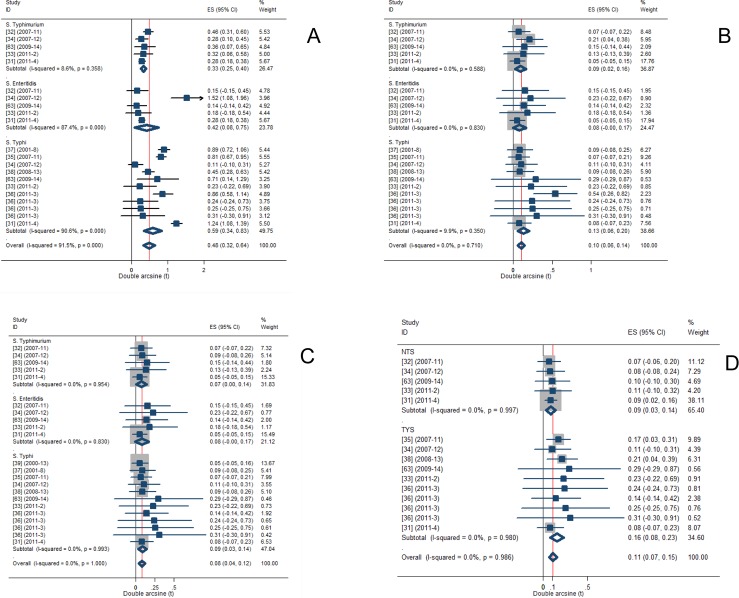
Fig 4 presents forest plots of proportions of mutants, and strains with PMQR genes. The residual of the heterogeneity estimates of gyrA mutant typhoidal and non-typhi Salmonella was significant (P = 5E-21). The estimate was higher in S. Typhi (T2 = 0.137) than in NTS (T2 = 0.0259), and in S. Enteritidis than in S. Typhimurium (Table 2). The heterogeneity estimates for each of gyrB and parC mutants, and strains with PMQR genes (qnrA, qnrB, and qnrC) did not differ between typhoidal and NTS subgroups (P > 0.05).
Fig 4. Forest plots of genotypic proportions.
gyrA (A), gyrB (B), parC (C), qnr (A,B,S) (D).
Table 2. Heterogeneity of genotypic proportions.
| I2 (95%CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Serotype | N | K | Q | T2 | I2 | L | U |
| gyrA | S. Typhimurium | 800 | 5 | 4 | 0.0007 | 9 | 0 | 81 |
| S. Enteritidis | 830 | 5 | 35 | 0.1182 | 87 | 73 | 94 | |
| S. Typhi | 836 | 11 | 107 | 0.137 | 91 | 85 | 94 | |
| Overall | 2166 | 21 | 242 | 0.1105 | 92 | 88 | 94 | |
| gyrB | S. Typhimurium | 800 | 5 | 3 | 0 | 0 | 0 | 71 |
| S. Enteritidis | 530 | 5 | 1 | 0 | 0 | 0 | 44 | |
| S. Typhi | 836 | 11 | 11 | 0.0016 | 10 | 0 | 50 | |
| Overall | 2166 | 21 | 16 | 0 | 0 | 0 | 34 | |
| parC | S. Typhimurium | 800 | 5 | 1 | 0 | 0 | 0 | 0 |
| S. Enteritidis | 530 | 5 | 1 | 0 | 0 | 0 | 44 | |
| S. Typhi | 1180 | 12 | 3 | 0 | 0 | 0 | 0 | |
| Overall | 2510 | 22 | 5 | 0 | 0 | 0 | 0 | |
| qnr(A,B,S) | NTS | 1330 | 5 | 0 | 0 | 0 | 0 | 0 |
| TYS | 700 | 10 | 3 | 0 | 0 | 0 | 0 | |
| Overall | 2030 | 15 | 5 | 0 | 0 | 0 | 0 | |
K, number of studies; L, lower limit; N, number of isolates; Q, Cochran’s-test
T2, tau squared; U, upper limit.
Pooled phenotypic proportions
Pooled proportions of MDR and Cipns strains are given in Table 3. For each analysis, the single study omitted estimates including the 95%CIs lie within or closer to the 95% confidence bounds of the respective pooled estimate. The proportion of MDR S. Typhimurium was higher than the proportion of MDR S. Typhi (OR = 15.6, 95%CI = 11.8, 20.5). Conversely, the proportion of Cipns S. Typhi was higher than the proportion of Cipns S. Typhimurium (OR = 3.3, 95%CI = 2, 5.5). The proportions of MDR-Cipns and nMDR-Cipns isolates, respectively, were estimated at 3.8% (95%CI = 1.8, 6.5) and 2.7% (95%CI = 0.9, 5.3) (Table 3). Of 785 isolates tested with both ciprofloxacin and nalidixic acid [32, 33, 35, 37,63–65], 70(8.9%) were non-susceptible to both drugs.
Table 3. Pooled phenotypic proportions.
| P (95%CI) | |||||||
|---|---|---|---|---|---|---|---|
| Phenotype | Serotype | N | K | P | L | U | OR (95%CI) |
| MDR | S. Typhimurium | 800 | 5 | 89.3 | 84.8 | 93.1 | Tp vs.Ty; 15.6 (11.8,20.5) |
| S. Enteritidis | 530 | 5 | 68.5 | 47.1 | 86.6 | ||
| S. Typhi | 836 | 11 | 34.8 | 22.3 | 48.5 | ||
| Overall | 2166 | 21 | 57.4 | 44.9 | 69.5 | ||
| Cipns | S. Typhimurium | 800 | 5 | 2.9 | 1.8 | 4.1 | Ty vs. Tp; 3.3 (2,5.5) |
| S. Enteritidis | 530 | 5 | 4.6 | 0.1 | 14.8 | ||
| S. Typhi | 836 | 11 | 8.9 | 3 | 17.6 | ||
| Overall | 2166 | 21 | 6.2 | 2.8 | 10.7 | ||
| MDR-Cipns | S. Typhimurium | 800 | 5 | 2.6 | 1.6 | 3.9 | |
| S. Enteritidis | 530 | 5 | 1 | 0 | 2.8 | ||
| S. Typhi | 836 | 11 | 5.9 | 2.1 | 11.4 | ||
| Overall | 2166 | 21 | 3.8 | 1.8 | 6.5 | ||
| nMDR-Cipns | S. Typhimurium | 800 | 5 | 0.4 | 0 | 1 | |
| S. Enteritidis | 530 | 5 | 5.2 | 0 | 17.9 | ||
| S. Typhi | 836 | 11 | 3.5 | 0.7 | 8.4 | ||
| Overall | 2166 | 21 | 2.7 | 0.9 | 5.3 | ||
| Inv-salm | iNTS | 47337 | 11 | 1.9 | 0.7 | 3.6 | NTS vs. TyS; 1.1(0.98,1.2) |
| Typhoidal | 47337 | 11 | 1.7 | 1.1 | 2.5 | ||
| Overall | 47337 | 11 | 1.8 | 1.1 | 2.6 | ||
Cipns; ciprofloxacin non-susceptible; Inv-salm, invasive salmonellosis; K, number of studies; L, lower limit; MDR, multi-drug resistant; MDR-Cipns; multi-drug resistant + ciprofloxacin non-susceptible; N, number of isolates; nMDR-Cipns; non-multidrug resistant + ciprofloxacin non-susceptible; NTS, non-typhoidal Salmonella; OR, odds ratio; P, pooled proportion; Q, Cochran’s-test; T2, tau squared; Tp, S. Typhimurium; Ty, S. Typhi; TyS, typhoidal Salmonella; U, upper limit.
Pooled genotypic proportions
The pooled proportions of mutants and strains with PMQR genes are given in Table 4. All single study omitted estimates including the 95%CIs lie within or closer to the 95% confidence bounds of the respective means. Mutations at gyrA, gyrB and parC but parE have been identified (Table 4). The overall pooled proportion of gyrA mutants was estimated at 5.7% (95%CI = 2.6, 9.8). The proportion of gyrA mutants was higher in typhoidal than in non-typhi Salmonella (OR = 2.9, 95%CI = 1.9, 4.4), and in S. Typhi than in S. Typhimurium (OR = 3.3, 95%CI = 2, 5.7). The proportions of gyrB and parC mutants, and strains with PMQR genes (qnrA, qnrB and qnrS), respectively, were estimated at 0.2% (95%CI = 0.1, 05), 0.1% (95%CI = 0, 3), and 0.3% (95%CI = 0.1, 0.6).
Table 4. Pooled genotypic proportions.
| P (95%CI) | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | Serotype | N | K | P | L | U | OR (95%CI) |
| gyrA | S Typhimurium | 800 | 5 | 2.6 | 1.6 | 4 | |
| S. Enteritidis | 530 | 5 | 4.2 | 0.1 | 13.2 | ||
| S. Typhi | 836 | 11 | 8.3 | 2.8 | 16.2 | Ty vs. Tp; 3.3 (2,5.7) | |
| Overall | 2166 | 21 | 5.7 | 2.6 | 9.8 | ||
| gyrB | S. Typhimurium | 800 | 5 | 0.2 | 0 | 0.6 | |
| S. Enteritidis | 530 | 5 | 0.1 | 0 | 0.6 | ||
| S. Typhi | 836 | 11 | 0.4 | 0 | 1 | ||
| Overall | 2166 | 21 | 0.2 | 0.1 | 0.5 | ||
| parC | S Typhimurium | 800 | 5 | 0.1 | 0 | 0.5 | |
| S. Enteritidis | 530 | 5 | 0.1 | 0 | 0.6 | ||
| S. Typhi | 1180 | 21 | 0.2 | 0 | 0.5 | ||
| Overall | 2510 | 21 | 0.1 | 0 | 0.3 | ||
| qnr(A,B,S) | iNTS | 1330 | 5 | 0.1 | 0 | 0.4 | |
| S. Typhi | 700 | 10 | 0.5 | 0.1 | 1.2 | ||
| Overall | 2030 | 15 | 0.3 | 0.1 | 0.6 | ||
Cipns, ciprofloxacin non-susceptible; K, number of studies; L, lower; N, number of isolates; OR, odds ratio; P, pooled proportion; Tp, S. Typhimurium; Ty, S. Typhi; U, upper limit.
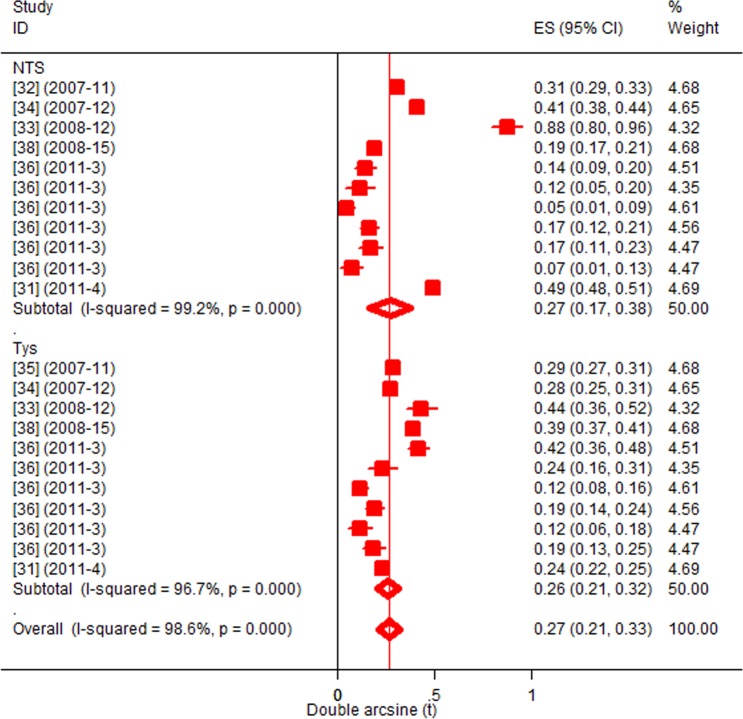
Pooled proportion of gyrA mutant invasive infections
Fig 5 presents the occurrence of invasive Salmonella (typhoidal and non-typhi) disease in SSA. The estimates were based on data from nine countries [31–36, 38]. The overall pooled proportion was estimated at 1.8% (95%CI = 1.1, 2.6). The proportions of iNTS disease (1.9%, 95%CI = 0.7, 3.6) and typhoidal infections (1.7%, 95%CI = 1.1, 2.5) did not differ (P > 0.05). The pooled proportion of infections with gyrA mutants was estimated at 0.1% (95%CI = 0.03, 0.26).
Fig 5. Forest plot of proportions of invasive Salmonellosis.
Preponderance of gene mutations
Relative and cumulative frequencies of gene mutations in quinolone resistant Salmonellae are given in Table 5. Mutations at gyrA were the most frequent (82%, 95%CI = 78, 86). Of these, 92.8% (256) were recovered from human patients who sought medical attention and three asymptomatic adopted children. Mutations at gyrB and parC, respectively, were identified in 2.9% (95%CI = 1.5, 5.6) and 26.6% (95%CI = 21.9, 31.8) of the isolates tested for each genotype. No parE mutation has been reported.
Table 5. Relative and cumulative frequencies of gene mutations.
| P (95%CI)‡ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Host | Group | Setting | N | P | L | U | References |
| gyrA | Human | NTS | Cm, Ad | 94 | 77.7 | 68.2 | 84.9 | [31–34,45–49,51–54,63] |
| Human | TyS | Cm | 162 | 84 | 77.5 | 88.8 | [31,35–38,41–43,63,64] | |
| Human | NTS | Tr | 47 | 100 | 92.4 | 100 | [54–56] | |
| Animal | NTS | A/P | 33 | 60.6 | 43.7 | 75.3 | [52, 55,56,60] | |
| Overall | 336 | 82.1 | 77.7 | 85.9 | [31–38, 41–43, 45–49, 51–56,63,64] | |||
| gyrB | Human | NTS | Cm, Ad | 62 | 4.8 | 1.7 | 13.3 | [31–34,46,48,49,51,52,54,63] |
| Human | TyS | Cm | 137 | 2.2 | 0.7 | 6.2 | [31,35,36,38,41–43,63,64] | |
| Human | NTS | Tr | 47 | 0 | 0 | 7.6 | [54–56] | |
| Animal | NTS | A/P | 32 | 6.3 | 1.7 | 20.1 | [52,55,56] | |
| Overall | 278 | 2.9 | 1.5 | 5.6 | [31–36,38, 41–43, 46,48,49, 51–56,63,64] | |||
| parC | Human | NTS | Cm, Ad | 63 | 22.2 | 13.7 | 33.9 | [31–34,46,48,49,51–54,63] |
| Human | TyS | Cm, | 162 | 4.9 | 2.5 | 9.4 | [31,35–38,41–43,63,64] | |
| Human | NTS | Tr | 47 | 87.2 | 74.8 | 94 | [54–56] | |
| Animal | NTS | A/P | 33 | 54.5 | 38 | 70.2 | [52,55,56,60] | |
| Overall | 305 | 26.6 | 21.9 | 31.8 | [31–36,38,41–43,46,48,49,51–56,60,63,64] | |||
| parE | Human | NTS | Cm, Ad | 30 | 0 | 0 | 11.4 | [34,48,49,51,52,54] |
| Human | TyS | Cm | 68 | 0 | 0 | 5.3 | [36,37,38,41–43] | |
| Human | NTS | Tr | 47 | 0 | 0 | 7.6 | [54–56] | |
| Animal | NTS | A/P | 32 | 0 | 0 | 10.7 | [52,55,56] | |
| Overall | 177 | 0 | 0 | 2.1 | [34,36–38,41–43,48,49,51,52,54–56] | |||
Ad, adoptees; A/P, animals/animal products; Cm, community; P, proportion; L, lower limit; N, number; NTS, non-typhoidal Salmonella; OR, odds ratio; Tr, travel-associated; TyS, typhoidal Salmonella; U, upper limit.
‡Score confidence interval.
Preponderance of mutated codons
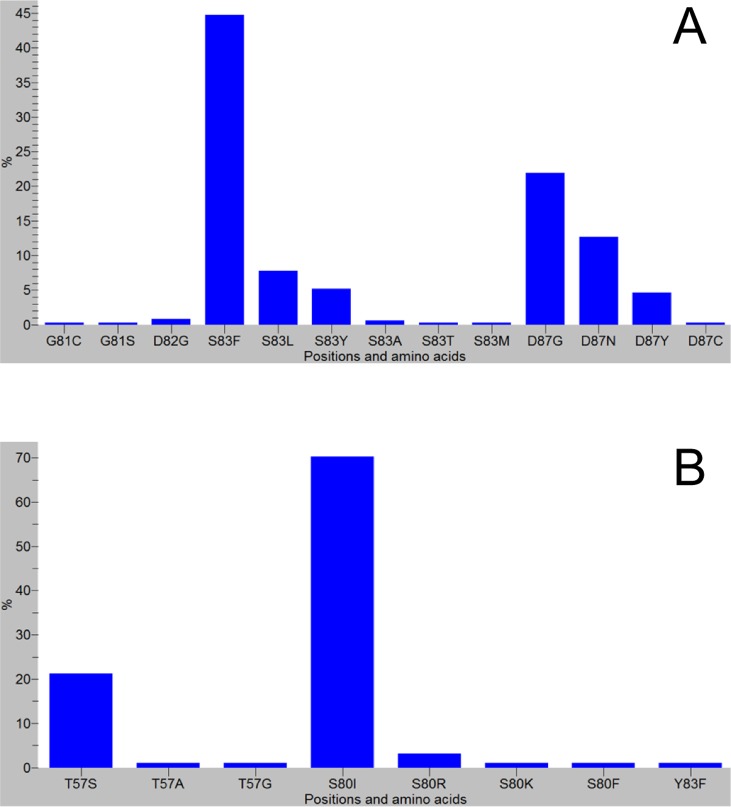
The most frequently mutated codons in the QRDR of gyrA were codons for Ser83 (60%, 204/346) and Asp87 (39.6%, 137/346) (Fig 6). Other gyrA codons included codons encoding Gly81, Asp82, Ala119 [42] and Glu133 [35, 36, 63]. At parC, mutations most frequently occurred at codons encoding Ser80 followed by Thr57, and most have been identified in association with gyrA mutations (Ser83 or Asp87).
Fig 6. Mutated codons and substituted amino acids.
gyrA, (A); parC, (B).
Preponderance of substituted amino acids
The amino acids the most frequently substituted at GyrA were phenylalanine (155/204) and leucine (27/204) at Ser83, and glycine (76/137) and asparagine (44/137) at Asp87 (Table 6). The amino acids the most frequently substituted at positions Ser80 and Thr57 of ParC, respectively, were isoleucine (66/71) and serine (20/22).
Table 6. Frequencies of substituted amino acids.
| Substituted amino acids | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme | Position | np | Phe | Leu | Tyr | Thr | Gly | Asn | Cys | Asp | Ile | Ser | Arg | Lys | Ala | Met | His | References |
| GyrA | Ser83 | 204 | 155 | 27 | 18 | 1 | - | - | - | - | - | - | - | - | 2 | 1 | - | ‡R |
| (ns = 276) | Asp87 | 137 | - | - | 16 | - | 76 | 44 | 1 | - | - | - | - | - | - | - | - | †R |
| Asp82 | 3 | - | - | - | - | 3 | - | - | - | - | - | - | - | - | - | - | [42] | |
| Gly81 | 2 | - | - | - | - | - | - | 1 | - | - | 1 | - | - | - | - | - | [33, 42] | |
| GyrB | Ser464 | 3 | 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [36] |
| (ns = 8) | Ser463 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | [52] |
| Glu466 | 2 | - | - | - | - | - | - | - | 2 | - | - | - | - | - | - | - | [34,49] | |
| Val423 | 2 | - | - | - | - | 2 | - | - | - | - | - | - | - | - | - | - | [52] | |
| Asp459 | 2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 | [52] | |
| ParC | Ser80 | 71 | 1 | - | - | - | - | - | - | - | 66 | - | 3 | 1 | - | - | - | [42,49,52–56] |
| (ns = 81) | Thr57 | 22 | - | - | - | - | 1 | - | - | - | - | 20 | - | - | 1 | - | - | [36,42,46,52,60] |
| Tyr83 | 1 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [52] | |
Ala, alanine; Arg, arginine; Asn, asparagine; Asp, aspartic acid; Cys, cystine; Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys, lysine; Met, methionine; np, number of mutations by position; ns, number of strains; Phe, phenylalanine; R, references; Ser, serine; Thr, threonine; Tyr, tyrosine.
Preponderance of PMQR genes
Of the PMQR genes, qnrA, qnrB, qnrS, aac(6′)-Ib-cr and qepA but qnrC (0/9 4) [31, 40, 62, 65], qnrD (0/203) [31, 38, 40, 43, 48, 49, 51, 52, 54–56, 62, 65] and oqxA/B (0/85) [31,55] have been identified (Table 7). The proportions of strains with qnrA, qnrB, qnrS, and aac(6′)-Ib-cr genes, respectively, were 1.5% (95%CI = 0.8, 2.9), 5.9% (95%CI = 4.2, 8.3), 3.1% (95%CI = 1.9, 5.1), and 4.2% (95%CI = 2.6, 6.6). Most isolates with PMQR genes were NTS.
Table 7. Frequencies of strains with PMQR genes.
| P (95%CI)‡ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Host | Group | Setting | N | P | L | U | References |
| qnrA | Human | NTS | Cm, Ad | 105 | 5.7 | 2.6 | 11.9 | [31–34,44–52,54,63,65,66] |
| Human | TyS | Cm | 159 | 0 | 0 | 2.4 | [31,35–38,42,43,63,64] | |
| Human | NTS | Tr | 48 | 0 | 0 | 7.4 | [54–56,65] | |
| Animal | NTS | A/P | 223 | 0.9 | 0.2 | 3.2 | [40,52,55–62] | |
| Overall | 535 | 1.5 | 0.8 | 2.9 | [31–38,40,42–52,54–56,63–66] | |||
| qnrB | Human | NTS | Cm, Ad | 76 | 19.7 | 12.3 | 30 | [31–34,45,46,48–52,54,63,65,66] |
| Human | TyS | Cm | 159 | 0 | 0 | 2.4 | [31,35–38,42,43,63,64] | |
| Human | NTS | Tr | 48 | 2.1 | 0.4 | 10.9 | [54–56,65] | |
| Animal | NTS | A/P | 223 | 6.3 | 3.8 | 10.3 | [40,52,55–62] | |
| Overall | 506 | 5.9 | 4.2 | 8.3 | [31–38,40,42–52,54–56,63–66] | |||
| qnrS | Human | NTS | Cm, Ad | 76 | 0 | 0 | 4.8 | [31–34,45,46,48–52,54,63,65,66] |
| Human | TyS | Cm | 134 | 1.5 | 0.4 | 5.3 | [31,35,36,38,42,43,63,64] | |
| Human | NTS | Tr | 48 | 0 | 0 | 7.4 | [54–56,65] | |
| Animal | NTS | A/P | 223 | 5.8 | 3.4 | 9.7 | [40,52,55–62] | |
| Overall | 481 | 3.1 | 1.9 | 5.1 | [31–38,40,42–52,54–56,63–66] | |||
| aac(6′)-Ib-cr | Human | NTS | Cm, Ad | 45 | 17.8 | 9.3 | 31.3 | [31,45,48–52,54,65] |
| Human | TyS | Cm | 95 | 0 | 0 | 3.9 | [31,37,38,43] | |
| Human | NTS | Tr | 48 | 0 | 0 | 7.4 | [54–56,65] | |
| Animal | NTS | A/P | 219 | 4.1 | 2.2 | 7.6 | [52,55–59,61,62] | |
| Overall | 407 | 4.2 | 2.6 | 6.6 | [31,37,38,43,45,48–52,54–59,61,62,65] | |||
| qepA | Human | NTS | Cm, Ad | 49 | 0 | 0 | 7.3 | [31,34,48,49,51,52,54,65] |
| Human | TyS | Cm | 82 | 0 | 0 | 4.5 | [31,36,38,43] | |
| Human | NTS | Tr | 48 | 0 | 0 | 7.4 | [54–56,65] | |
| Animal | NTS | A/P | 168 | 0.6 | 0.1 | 3.3 | [52,55,56,59,61] | |
| Overall | 347 | 0.3 | 0.1 | 1.6 | [31,34,36,38,43,48,49,51,52,54–56,61,65] | |||
Ad, adoptees; A/P, animals/animal products; Cm, community setting; L, lower limit; N, number; P, proportion; Tr, travel associated; U, upper limit.
‡Score confidence interval.
Mutant serotypes
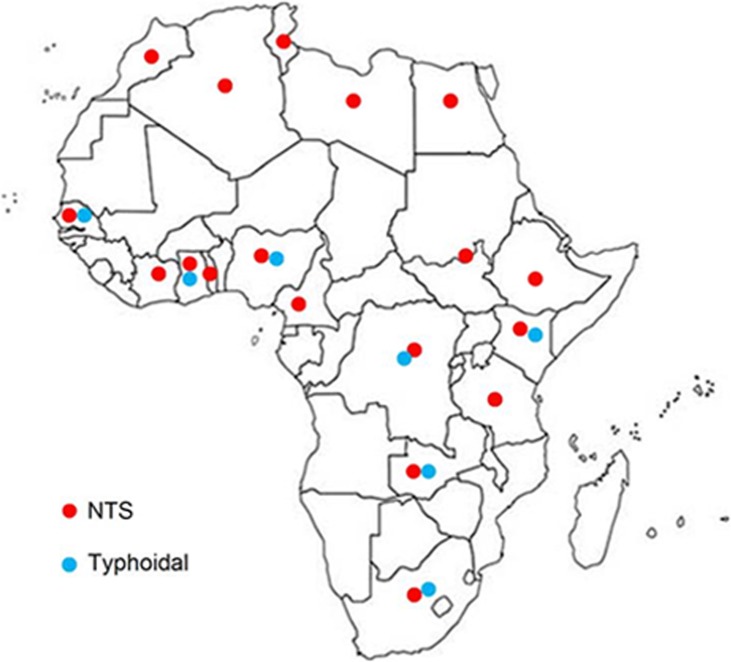
A distribution map of reported gyrA mutant typhoidal and non-typhoidal Salmonella is given in Fig 7. Twenty-three mutant serotypes have been identified (Table 8). S. Typhi was the most frequently reported (139/287) followed by S. Kentucky (69/287), S. Typhimurium (34/287) and S. Enteritidis (16/287). The numbers of mutations differed between typhoidal and NTS subgroups. Most double (9/10), triple (56/59) and quadruple (10/11) mutants were NTS. Most triple (47/63) and quadruple (10/63) mutants were S. Kentucky strains [52–56].
Fig 7. Distribution of reported gyrA mutants.
Table 8. Reported occurrence of mutant serotypes.
| Regions | Source | Serotype | N | gyrA | gyrB | parC | References |
|---|---|---|---|---|---|---|---|
| ECWN | Human | S. Typhi | 139 | 135 | 3 | 7 | [31, 35–38,42,43,63,64] |
| EWN | H/A | S. Kentucky | 69 | 69 | - | 63 | [52–56] |
| CWS | Human | S. Typhimurium | 34 | 32 | 2 | - | [31–34,47,49,63] |
| CW | Human | S. Enteritidis | 16 | 16 | - | - | [31,34] |
| Southern | Human | S. Isangi | 7 | 7 | - | - | [47] |
| Eastern | Human | S. Concord | 4 | 3 | - | 4 | [46] |
| Southern | Human | S. Senftenberg | 2 | 2 | - | 2 | [48] |
| Eastern | Animal | S. Livingstone | 2 | 2 | - | - | [52] |
| Southern | Human | S. Kissi | 1 | 1 | - | - | [47] |
| Southern | Human | S. Kivu | 1 | 1 | - | - | [47] |
| Western | Human | S. Paratyphi A | 1 | 1 | - | 1 | [36] |
| Southern | Human | S. Reading | 1 | 1 | - | - | [47] |
| Central | Human | Salmonella 4,5 | 1 | 1 | - | - | [32] |
| Southern | Human | Untypable | 1 | 1 | - | - | [47] |
| Eastern | Human | V:ROUGH-O;-:- | 1 | - | 1 | - | [52] |
| Eastern | Animal | I:6;7,14:-:I,w | 1 | 1 | - | - | [52] |
| Eastern | Animal | S. Agona | 1 | - | - | 1 | [52] |
| Eastern | Animal | S. Braenderup | 1 | - | 1 | 1 | [52] |
| Northern | Animal | S. Hadar | 1 | 1 | - | 1 | [60] |
| Eastern | Animal | S. Haifa | 1 | 1 | - | - | [52] |
| Eastern | Animal | S. Miami | 1 | - | - | 1 | [52] |
| Eastern | Animal | S. Mikawasima | 1 | - | 1 | - | [52] |
| Eastern | Animal | S. Virchow | 1 | 1 | - | - | [52] |
CW, Central and Western; CWS, Central, Western and Southern; ECWN, Eastern, Central, Western and Northern; EWN, Eastern, Western and Northern.
Ciprofloxacin MICs and genetic determinants
Two levels of resistance (≤ 1μg/mL and ≥ 4μg/mL) were identified (Table 9). The highest MICs (4μg/mL to > 32 μg/mL) have been recorded in triple mutant S. Senftenberg [48], and S. Kentucky [53–56]. In other serotypes but three NTS with PMQR genes but no mutations [51], the MICs were ≤ 1μg/mL. In quadruple mutant S. Kentucky (gyrA-Ser83Phe and Asp87Gly, and parC-Ser80Ile and Thr57Ser), zone diameter (ZD) decreases of two to four-fold (8-14mm) were recorded [52].
Table 9. Ciprofloxacin MICs and genetic determinants.
| Serotype | N | gyrA | gyrB | parC | NM | PMQR | MIC (μg/mL) | References |
|---|---|---|---|---|---|---|---|---|
| S. Typhi | 48 | 0 | 0 | 0 | 0 | nd | 0.25–0.38 | [41] |
| NTS | 6 | 0 | 0 | 0 | 0 | + | 0.08–2 | [51] |
| S. Typhi | 126 | 123 | 3 | 0 | 1 | +/- | >0.06–1 | [31,35–37,43,63,64] |
| S. Typhi | 7 | 6 | 0 | 7 | 1–4 | - | 0.125–0.5 | [42] |
| S. Enteritidis | 16 | 16 | 0 | 0 | 1 | +/- | >0.06–0.5 | [31,34] |
| S. Kentucky | 6 | 6 | 0 | 0 | 1 | - | 0.125–0.5 | [56] |
| S. Kentucky | 53 | 53 | 0 | 53 | 3 | - | 4- >32 | [53–56] |
| S. Senftenberg | 2 | 2 | 0 | 2 | 3 | + | >4 | [48] |
| S. Senftenberg | 1 | 0 | 0 | 0 | 0 | + | ≥0.12 | [65] |
| S. Typhimurium | 21 | 20 | 1 | 0 | 1 | +/- | >0.06–0.5 | [31–34, 49,63] |
| S. Concord | 4 | 3 | 0 | 4 | 1,2 | +/- | 0.5–1 | [46] |
| S. Concord | 3 | 0 | 0 | 0 | 0 | + | 0.12–0.5 | [65] |
Discussion
Here, we describe the heterogeneity and preponderance of FQ-resistance genetic determinants in Salmonella isolates of Africa. Most invasive infections have been due to S. Typhimurium, S. Enteritidis and S. Typhi. This is consistent with reports of previous reviews/meta-analyses on the occurrence of salmonellosis in SSA [3, 4, 27]. The heterogeneity estimates demonstrate variability in the occurrence of MDR, CIpns, and gyrA mutants, but parallel occurrence of each of gyrB and parC mutants, and strains with PMQR genes in both typhoidal and non-typhi Salmonella.
The substantial inconsistency estimates imply potential variations in the distributions of MDR and FQ-resistant invasive strains across locations. For instance, the S. Typhi H58 lineage is frequently associated with MDR and FQ-resistance and reportedly predominate in Eastern and Southern Africa, but is rare in other regions [39]. By the same token, despite MDR non-typhi Salmonella occurs extensively [73], most iNTS diseases have been associated with two S. Typhimurium ST313 lineages–I and II [74]. These lineages emerged with MDR acquisition, and a chloramphenicol driven clonal replacement of lineage I by lineage II, and dispersal of the latter from the Central to other regions have been postulated [75,76]. Similarly, two S. Enteritidis clades (West African and Central/East African) resistant to one or more antimicrobial classes (1–4) have been implicated in iNTS disease [77]. Nevertheless, the considerable differences in the occurrence of iNTS disease (e.g. Central –1186/24370, Eastern- 9/4407), (Fig 4) suggest variability in the distribution of these lineages (S. Typhimurium and S. Enteritidis) across regions and countries, and locations within a country. Similarly, a systematic review [4], and a recent multi-country based study [78] have reported variability in the occurrence of iNTS disease and/or typhoidal infection in SSA.
The variability in drug resistance might essentially have been due to differences in the pattern and frequency of use of antimicrobials across locations and time. In resource-poor settings, antimicrobial self-medication is widespread and has been linked with inappropriate use [79]. Elsewhere in Asia, the convergence of poor public health system, non-prescription antimicrobial use, and rising income has been implicated as a factor that created favorable conditions for the selection and spread of drug resistant pathogens [80]. Antimicrobial use has also been associated with persisting AMR (up to a year) at the individual level [81], and resistant bacteria are common in communities with frequent non-prescription use [82]. In areas where typhoid fever is endemic, most patients self-treat [10], and non-prescription antimicrobial use as high as 100% has been documented in Eastern and Western Africa [82]. The extent of the problem could, however, differ across countries and within a country (urban/rural) depending on drug availability. For instance, in Kenya the FQs have been used since the last quarter of the 1990s, and of the S. Typhi isolated in 2000–2, 47% (48/102) were ciprofloxacin non-susceptible [41]. Likewise, in Nigeria the use of FQs was associated with an increase in the occurrence of FQ-resistant Escherichia coli between 2005 and 2009 [83]. By contrast, among NTS recovered from human samples in 2013/4 in Ethiopia, the occurrence of ciprofloxacin non-susceptible strains but two S. Kentucky and one V:ROUGH-O;-:- was low [52]. In general, the gyrA region is under a strong positive selection pressure, and mutations have reportedly occurred independently on multiple occasions [39]. Although FQ-resistant S. Typhi H58 emerged in Asia and disseminated globally–including Eastern and Southern Africa [39]–invasive S. Typhimurium [75, 76] and S. Enteritidis [77] originated in Africa, and are common causes of disease in Africa. Taken together, the AMR profiles (MDR-Cipns and nMDR-Cipns) of both typhoidal and iNTS isolates point towards a more than likely locally induced non-quinolone and quinolone based selection of FQ-resistant strains.
Most ciprofloxacin non-susceptible (Cipns) strains were nalidixic acid non-susceptible (Nalns). However, there have been reports of strains with the NalsCipns phenotype [36, 41, 43, 47, 51] with most being from Kenya [41]. This atypical phenotype has also been identified in S. Typhi [84, 85] and NTS [14] elsewhere. In India, Nals and Nalns phenotypes, respectively, were reported to have predictive values of 95% for Cips, and 36% for Cipns in S. Typhi [86]. Although reports on the distribution of strains with the NalsCipns phenotype are scarce, such strains could occur in several countries in Africa.
Mutations at Ser83 and Asp87 of gyrA were the most frequently identified, and appear to account for FQ-non-susceptibility in most strains. The occurrence of Glu133Gly mutation concomitantly with gyrA (Ser83 and Asp87) [35, 36, 63] and gyrB mutations [36], and in Nals and/or Cips [34, 35] strains implies a no association with FQ-resistance. In resistant isolates, mutations frequently occur between positions 67 and 106 [87], and in a sequence analysis of GenBank data, while 99.1% (212/214) of the NTS sequences carried the Glu133 residue, 96% (97/101) of the S. Typhi did not have it [88]. Furthermore, compared to gyrA mutations the occurrence of gyrB and parC mutations were low, and the relative contributions of these mutations to FQ-non-susceptibility in strains commonly implicated in invasive salmonellosis may be low. Among a global collection of S. Typhi, the gyrB mutant proportion was 2.2% (42/1832), and parC coding changes were detected in 1.6% (29/1832) with almost all (28/29) belonging to the H58 lineage [39]. The overall data demonstrate gyrA mutation as the foremost determinant of FQ-resistance, and Ser83 and Asp87 as the major mutation ‘hot spots’.
The estimates of the occurrence of salmonellosis in SSA [3, 4, 89, 90] differ, and underestimation of the incidence of iNTS disease but overestimation of that of typhoid fever was proposed [91]. Accordingly, to put the proportion of gyrA mutant infections in perspective, we estimated the proportion of invasive salmonellosis. As a result, the proportions of patients infected with iNTS and typhoidal strains were comparable, and the prevalence of infection with gyrA mutants was 0.1% (Table 3). The proportion of iNTS disease in SSA (1.9%) was in accord with the estimate of Reddy et al. [27] (~2%, 960/(58296–10230))–authors’ calculations after excluding data from North Africa where 99% of the isolates were S. Typhi). Nevertheless, as these estimates were not derived from population-based data, the true prevalence and incidence of invasive salmonellosis and thus infections with gyrA mutants could be higher. Furthermore, the estimates denote likely higher incidence rates than were estimated for iNTS disease ((227(152–341)/100000) [3], 0 to 237 (178–316) per 100000 person years [78]) and typhoidal infections ((10-100/100000) [89], (0 to 383 (274–535) per 100000 person years [78]). The differences in the estimated occurrence of the disease (prevalence/incidence) might have originated from differences in study periods, countries, locations, and numbers of study participants.
Ciprofloxacin was the test FQ, and data on other FQs is not available. However, the varied amino-acid substitutions at mutation positions suggest potential cross-resistance to other FQs. Lower binding of Ser83→Phe/Tyr to nalidixic acid, and Asp87→Gly/Tyr to ciprofloxacin [92], Ser83→Phe/Leu substitution to ciprofloxacin resistance but not to levofloxacin [93], Ser83→Tyr/Ile substitution with resistance to both ciprofloxacin and levofloxacin [93], and Ser83Phe + Asp87Val of gyrA + parC mutations with resistance to ciprofloxacin, ofloxacin, levofloxacin and gatifloxacin [94] have been reported. In Escherichia coli, similarities in drug structures and genotypes were proposed as factors accounting for parallel ciprofloxacin and norfloxacin MICs, and levofloxacin and gatifloxacin MICs [95].
The occurrence of PMQR genes was generally low, and comparable between isolates of human and animal origins. Reports show regional differences in the occurrence of PMQR genes. For instance, in the USA PMQR genes were reported to have been rare in Salmonella [96]. In contrast, among Salmonella collected from 13 countries in Europe, PMQR genes were detected in 59% (288/485) with qnrB being predominant (28.5%, 138/485) followed by qnrS (26%, 125/485) and qnrD (4.5%, 22/485) [97]. In a global analysis of reports on 20,960 enteric bacterial isolates, the prevalence of qnrA, qnrB, qnrS, and aac (6’)-Ib-cr was estimated at 1.5%, 4.6%, 2.4%, and 10.8%, respectively [68]. In Asia, higher occurrence of PMQR genes in enteric bacteria of human (65%, 414/642) [98] and animal origins (57%, 282/495) [99] have been reported. In general, PMQR genes reportedly provide low-level resistance that does not exceed the clinical breakpoint level [100], and the wide MIC ranges have been attributed to differences in plasmid copy number and gene expression [101].
Of the 23 mutant serotypes, triple and quadruple mutations have commonly been detected in S. Kentucky. This serotype has been identified from various samples collected from several countries in Africa and elsewhere [52–56, 102]. Whilst most S. Kentucky isolated in the earlier years were triple mutants [54–56], strains isolated from humans and domestic animals in 2013/4 in Ethiopia (n = 10) [52] were quadruple mutants. The data from Ethiopia is suggestive of the proclivity of S. Kentucky strains to mutate, and the distribution of the serotype across a range of hosts, countries and regions [52–56] implies its evolutionary success similar to S. Typhi H58 that reportedly has disseminated globally [39]. Furthermore, there have been reports of strains (ST198-X1) with genes that confer resistance to the cephalosporins (CMY-2, CTX-M-15 and VEB), carbapenemes (OXA-48 and VIM-2) and azithromycin (mphA) [53, 54]. Accordingly, the mutability, occurrence in various animal hosts and resistance to antimicrobials critically important for human health are reminiscent of the danger S. Kentucky strains might pose in the near future. A recent risk assessment study on Antibiotic Pan Drug Resistance (PDR) in the UK has estimated 284,000 cases of PDR Gram-negative bacteraemia leading to 79,000 deaths in a span of 20 years [103].
The ciprofloxacin MICs and associated genetic determinants depict a complex association between FQ-resistance phenotype and genotype, and what each determinant accounts for is indistinct (Table 9). This complexity might be due to effect modifying or confounding factors and lurking variables. In all reports included in this study, efflux and influx associated genetic mechanisms have not been addressed. A regression analysis could not be performed because of the limited number of studies and co-variable differences among studies. However, the frequent co-occurrence of target alterations and efflux activation [104], and the crucial role of the efflux system for the development of high-level resistance to FQs [101] have been reported. In Salmonella, AcrAB is the major multidrug efflux pump and RamA is a positive regulator of the acrAB transcription [105]. Nevertheless, the role of the efflux system in high-level quinolone resistant serotypes/strains has not been consistently described. For instance, a South African study on S. Typhi mutants has reported a 16- to 32- fold decrease in nalidixic acid MIC, and a 2 to 8- fold decrease in ciprofloxacin MIC in the presence of an efflux pump inhibitor [42]. By contrast, a study on S. Typhi and S. Paratyphi A strains of diverse origins, and with varying levels of resistance to nalidixic acid (16–1024 μg/mL) and ciprofloxacin (0.125–8 μg/mL) has reported the absence of mutations associated with the AcrAB efflux system genes [106]. Furthermore, although ramR mutations were associated with ciprofloxacin resistance in S. Typhimurium [107,108], in S. Kentucky, these mutations were recorded in only three of the 27 strains examined [109]. Accordingly, the AcrAB efflux system associated gene mutations do not appear to provide a general explanation for the wide MIC range recorded in S. Kentucky strains (4 to >32μg/mL) [53–56]. Overall, the data suggest potential interactions of the determinants of resistance (antagonistic, additive or synergistic) or mechanisms that have not yet been described.
Limitations, bias and strength of evidence
We have adhered to the PRISMA statement for reporting systematic reviews and meta-analyses [21]. However, as is the case in systematic reviews/meta-analyses, this study has limitations. First, the number of studies was limited; data from North Africa was scarce, and a region-based analysis was not done. Second, although we retrieved all identified reports, the search might not have yielded a complete list, and communication with authors is not always productive [27,110]. As the proportions of gyrB, and parC mutants, and strains with PMQR genes were generally low, a missed study/data may not appreciably alter the respective pooled estimates. Nevertheless, we performed a post hoc sensitivity analysis to evaluate the potential effect of a missing study/data on the pooled proportion of gyrA mutant strains using the highest estimate as a proxy. As a result, the margin of equivalence was small (0.9%); the proxy-based estimate lies within the 95% confidence bounds of the estimate, and the estimates with and without a substitute did not significantly differ (Yates corrected X2 = 1.52, P = 0.217). Third, as ciprofloxacin was the FQ used to assess the phenotype and describe the genotypes, the genetic basis of potential cross-resistance to other FQs was not explored. Fourth, data on drug efflux and influx associated mechanisms are not available, and establishing a clear-cut relationship between genetic determinants and ciprofloxacin MICs is difficult.
We have considered studies with different designs [111], and established study validity by using the inclusion and exclusion criteria [27]. Several quality assessment tools (QATs)–checklists and scales–have been developed [111,112,113] for use in prevalence studies. Some of these tools have been recommended to assess the qualities of studies on infectious diseases [113,114]. However, the items and the weighting of the items are variable and inconsistent [112]. In addition, as critical components differ across domains and topics [21], we did not find a QAT matching the components addressed in this study. For this reason, a qualitative summary of the component quality items is presented (S2 Table and S3 Table). To supplement the summary, a post hoc sensitivity analysis on unmeasured confounding on the odds ratio of gyrA mutation in S. Typhi and NTS was performed. We considered a gyrA mutant prevalence of less than 10%; we approximated the RR from the OR estimate [115] and estimated the minimum bias factor and confounding strength as described by Mathur and VanderWeele (https://mmathur.shinyapps.io/meta_gui_2/), [116]. The input parameters and the output are given in S1 Fig, and the potential impact of bias is discussed here below. Our assessment is simplistic and intended to not run the risks of understating/overstating the bias and the evidence. Overall, other than the limitations described under the section on limitations (limited country representation and country level data), we presume that selection, reporting and confounding bias may not have a significant impact on the evidence on the preponderance of FQ-resistance determinants and the pooled estimates. Our assumptions are based on the following premises.
First, a cross-sectional sample of the patient population is the ideal sampling population to study low prevalence/rare conditions [117], and under such a setting 'random sampling' in its strictest sense is unethical, impractical and has not been employed. Recruitment and samplings were syndrome based (fever, gasteroeneritis and systemic infections). Most isolates have been recovered from samples collected in surveillance and cross-sectional studies, and the rest were isolated from samples collected in different populations, locations and over time. Assuming a crude infection prevalence of 1.8%, and a ciprofloxacin non-susceptible strain proportion of 6.2% (Table 3), the recovery rate of a non-susceptible strain is approximately one in 1000 samples. Furthermore, with the exception of isolates that failed to grow or lost, and serotype/phenotype based selections in a few cases–perhaps due to logistical reasons and study objectives–most isolates have been characterized. This study has captured data on serotypes most frequently implicated in invasive salmonellosis–widely reported (S. Typhi, S. Typhimurium, and S. Enteritidis) [1, 3, 4, 10, 28] and of local/regional importance (S. Concord, S. Isangi, S. Bovismorbificans, S. Stanleyville and S. Dublin) [1,10]. As these isolates were recovered from spatially and temporally divergent samples, it is reasonable to assume that they are close representatives of the predominant strains circulating in Africa. Consequently, the risk due to non-representation of the major serotypes and distortion of the estimates appear minimal.
Second, most of the data were from articles published in high-quality journals. However, determination of reporting bias is difficult [118,119,120], and the tools commonly employed to detect publication bias are not rigorous [120]. In this study we have captured data from studies with large and small sample sizes. Despite missed generic and phenotype/genotype information in some studies (S1 Table), genotype data was secured from most studies and for at least 94% of the isolates included herein. Systematic differences between unreported and reported data [118, 120] were not observed, and in each analysis only studies with complete outcomes [118] were considered.
Third, salmonellosis is principally a food-related infection largely of community setting origin [18]. Although there have been sporadic reports of health-care related Salmonella outbreaks [121], health-care associated infections in Africa [122] have been linked with Gram-negative bacilli other than Salmonella. Moreover, in a review of surveillance data of 33188 paediatric admissions in Kenya (2002–2009), Gram-negative bacilli other than Salmonella were the major causes of hospital-acquired bacteraemeia [123]. In this study, the difference between the pooled proportions of typhoidal and iNTS infections of community setting origin was marginal (Table 3). However, factors that may influence the occurrence of Salmonella and thus the phenotypic/genotypic proportions–e.g. malaria and HIV status–[5–7] have not been reported in most studies used to estimate pooled proportions. Nevertheless, the sensitivity analysis on unmeasured confounding on the occurrence of gyrA mutation in typhoidal and non-typhoidal Salmonella suggests the relative robustness of the pooled estimates to unmeasured confounding (S1 Fig).
Strength of evidence is related with the qualities of individual studies, and the size, reliability and robustness of the combined data [124]. Although the GRADE methodology [125] has been recommended to rate the evidence in systematic reviews addressing infectious diseases [113,126], the applicability of the criteria in prevalence studies is unclear [113,126]. Instead, we grouped studies as low risk studies to estimate pooled proportions, and low risk studies to calculate frequencies (S3 Table). To put the evidence in viewpoint, we considered the variance estimates/credibility intervals and the agreement between the meta- and frequency analyses (Tables 1–5). Accordingly, we judged the preponderance of genotypes (gyrA mutation predominance) as a first order (‘high quality’) evidence, and the pooled estimates as second rank (‘moderate quality’) evidence–subject to change depending on data accretion and over time.
Implications and significance
FQ-resistant Salmonella is in the high tier of the global pathogen priority list for research and development of new antimicrobials [127]. The synopsis may be of importance to health-care providers and researchers in the domain. First, the summary indicates the likely distribution of FQ-resistant serotypes across regions and the range of resistance to FQs. Accordingly, given the socio-economic and agro-ecologic similarities of most countries in SSA, the data could be extrapolated to countries where sufficient data is not available. Second, it shows the landscape of research on FQ-resistance determinants in Salmonella isolates of Africa and the gaps to address in further research undertakings. Third, as the contribution of each FQ-resistance genetic determinant is indistinct, a global meta-analysis or large-scale testing of diverse collections of FQ-resistant isolates to known genetic determinants may provide explanation on the relative contributions of each determinant and offer clues as to whether additional mechanisms are operating or not. Fourth, the occurrence of gyrA mutations in a significant proportion of FQ-non-susceptible strains implies the potential development and utility of a mismatch-amplification mutation-assay [128] to rapidly detect FQ-resistant invasive strains. Fifth, the unevenness of estimates on the incidence of typhoidal and iNTS disease necessitates an update of the estimates using current data.
Conclusions
Mutations at gyrA appear to account for ciprofloxacin resistance in most clinical strains. Compared to iNTS, estimates of gyrA mutant typhoidal Salmonella are heterogeneous. The composite data could be harnessed to develop a molecular assay that enables a rapid detection of FQ-resistant invasive strains. Further studies are needed to describe (i) the relative contributions of genetic determinants to FQ-resistance, (ii) the diversities of determinants, and the serotypes particularly in countries lacking adequate data, and (iii) on the incidence of typhoidal and iNTS disease.
Supporting information
(DOC)
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Alemayehu Godana and Workineh Korma for retrieving full-text articles. We thank Dr. Simon Le Hello (Institute Pasteur, France), Dr. Annelies Post and co-authors (Institute of Tropical Medicine, Belgium), Dr. Veerle Lejon (Institute of Tropical Medicine, Belgium), Dr. Tadesse Eguale (Aklilu Lemma Institute of PathoBiology, Ethiopia), and Dr. Hassan M Al-Emran (Bernhard Nocht Institute for Tropical Medicine, and German Center for Infection Research, partner site Hamburg-Borstel-Lübeck, Hamburg, Germany) for having provided us with additional information.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kariuki S, Gordon MA, Feasey N, Parry CM. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine. 2015; 33 Suppl 3: C21–C9. doi: 10.1016/j.vaccine.2015.03.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feasey NA, Masesa C, Jassi C, Faragher EB, Mallewa J, Mallewa M, et al. Three epidemics of invasive multidrug-resistant Salmonella bloodstream infection in Blantyre, Malawi, 1998–2014. Clin Infect Dis. 2015; 61Suppl 4:S363–71. doi: 10.1093/cid/civ691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global Burden of Invasive nontyphoidal Salmonella disease, 2010. Emerg Infect Dis. 2015; 21: 941–949. http://dx.doi.org/10.3201/eid2106.140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uche IV, MacLennan CA, Saul A. A Systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014). PLoS Negl Trop Dis. 2017; 11: e0005118 doi: 10.1371/journal.pntd.0005118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols C, Espinoza LMC, von Kalckreuth V, Aaby P, El Tayeb MA, Ali M, et al. Bloodstream infections and frequency of pretreatment associated with age and hospitalization status in sub-Saharan Africa. Clin Infect Dis. 2015; 61Suppl 4:S372–9. doi: 10.1093/cid/civ730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SE, Pak GD, Aaby P, Adu-Sarkodie Y, Ali M, Aseffa A, et al. The relationship between invasive nontyphoidal Salmonella disease, other bacterial bloodstream infections, and malaria in sub-Saharan Africa. Clin Infect Dis. 2016; 62Suppl1:S23–31. doi: 10.1093/cid/civ893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takem EN, Roca A, Cunnington A. The association between malaria and nontyphoid Salmonella bacteraemia in children in sub-Saharan Africa: a literature review. Malar J. 2014; 13:400 doi: 10.1186/1475-2875-13-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chattaway MA, Aboderin AO, Fashae K, Okoro CK, Opintan JA, Okeke IN. Fluoroquinolone resistant enteric bacteria in sub Saharan Africa: clones, implications and research needs. Front Microbiol. 2016; 7: 558 doi: 10.3389/fmicb.2016.00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leopold SJ, van Leth F, Tarekegn H, Schultsz C. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: a systematic review. Antimicrob Agents Chemother. 2014; 69: 2337–2353. doi: 10.1093/jac/dku176 [DOI] [PubMed] [Google Scholar]
- 10.Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 2015; 28: 901–937. doi: 10.1128/CMR.00002-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO). The diagnosis, treatment and prevention of typhoid fever. WHO/V&B/03.07. Geneva: WHO; 2003; Available from: http://apps.who.int/iris/handle/10665/68122.
- 12.Ruppé É, Woerther PL, Barbier F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann Intensive Care. 2015; 5: 61 doi: 10.1186/s13613-015-0061-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falagas ME, Tansarli GS, Rafailidis PI, Kapaskelis A, Vardakasa KZ. Impact of antibiotic MIC on infection outcome in patients with susceptible Gram-Negative bacteria: a systematic review and meta-Analysis. Antimicrob Agents Chemother. 2012; 56: 4214–4222. doi: 10.1128/AAC.00663-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphries RM, Fang FC, Aarestrup FM, Hindler JA. In vitro susceptibility testing of fluoroquinolone activity against Salmonella: recent changes to CLSI standards. Clin Infect Dis. 2012; 55:1107–1113. doi: 10.1093/cid/cis600 [DOI] [PubMed] [Google Scholar]
- 15.Clinical Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. CLSI, supplement M100S. Wayne, PA: Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- 16.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. EUCAST Version 7.1. 2017; Available from: http://www.eucast.org/clinical_breakpoints/.
- 17.Avalos E, Catanzaro D, Catanzaro A, Ganiats T, Brodine S, Alcaraz J, et al. Frequency and geographic distribution of gyrA and gyrB mutations associated with fluoroquinolone resistance in clinical Mycobacterium tuberculosis isolates: a systematic review. PLoS ONE. 2015; 10: e0120470 doi: 10.1371/journal.pone.0120470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO). Antimicrobial resistance: global report on surveillance. WHO. 2014; Available from: http://www.who.int/iris/handle/10665/112642.
- 19.Vlieghe E, Phoba MF, Tamfun JJM, Jacobs J. Antibiotic resistance among bacterial pathogens in Central Africa: a review of the published literature between 1955 and 2008. Int J Antimicrob Agents. 2009; 34: 295–303. doi: 10.1016/j.ijantimicag.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009; 6: e1000097 doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, John P A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339: b2700 doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. European Centre for Disease Prevention and Control. EFSA Journal. 2016;14:4380 doi: 10.2903/j.efsa.2016.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization (WHO). Foodborne disease outbreaks: Guidelines for investigation and control. 2008; Available from: www.who.int/ffodsafety/en/.
- 24.Tang C, Cui L, Xu Y, Xie L, Sun P, Liu C, et al. The incidence and drug resistance of Clostridium difficile infection in Mainland China: a systematic review and meta-analysis. Sci Rep. 2016; 6:37865 doi: 10.1038/srep37865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huong VTL, Ha N, Huy NT, Horby P, Nghia HDT, Thiem VD, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis. 2014; 20: 1105–1114. doi: 10.3201/eid2007.131594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013; 67:974–978. doi: 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 27.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010; 10: 417–432. doi: 10.1016/S1473-3099(10)70072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Joanna Briggs Institute. Reviewer’s manual Adelaide: The Joanna Briggs Institute; 2014. Available from: http://joannabriggs.org/research/critical-appraisal-tools-html. [Google Scholar]
- 29.Higgins JP, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 30.Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie et al. Meta-analysis of data from animal studies: A practical guide. J Neuroscience Methods. 2014: 221: 92–102. [DOI] [PubMed] [Google Scholar]
- 31.Kalonji LM, Post A, Phoba MF, Falay D, Ngbonda D, Muyembe JJ, et al. Invasive Salmonella infections at multiple surveillance sites in the Democratic Republic of the Congo, 2011–2014. Clin Infect Dis. 2015; 61:346–353. [DOI] [PubMed] [Google Scholar]
- 32.Lunguya O, Lejon V, Phoba MF, Bertrand S, Vanhoof R, Glupczynski Y, et al. Antimicrobial resistance in invasive non-typhoid Salmonella from the Democratic Republic of the Congo: emergence of decreased fluoroquinolone susceptibility and extended-spectrum beta lactamases. PLoS Negl Trop Dis. 2013; 7:e2103 doi: 10.1371/journal.pntd.0002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phoba MF, De Boeck H, Ifeka BB, Dawili J, Lunguya O, Vanhoof R, et al. Epidemic increase in Salmonella bloodstream infection in children, Bwamanda, the Democratic Republic of Congo. Eur J Clin Microbiol Infect Dis. 2014; 33: 79–87. doi: 10.1007/s10096-013-1931-8 [DOI] [PubMed] [Google Scholar]
- 34.Eibach D, Al-Emran HM, Dekker DM, Krumkamp R, Adu-Sarkodie Y, Espinoza LMC, et al. The emergence of reduced ciprofloxacin susceptibility in Salmonella enterica causing bloodstream infections in rural Ghana. Clin Infect Dis. 2016; 62 Suppl 1: S32–6. doi: 10.1093/cid/civ757 [DOI] [PubMed] [Google Scholar]
- 35.Lunguya O, Lejon V, Phoba MF, Bertrand S, Vanhoof R, Verhaegen J, et al. Salmonella Typhi in the Democratic Republic of the Congo: Fluoroquinolone decreased susceptibility on the rise. PLoS Negl Trop Dis. 2012; 6: e1921 doi: 10.1371/journal.pntd.0001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Emran HM, Eibach D, Krumkamp R, Ali M, Baker S, Biggs HM, et al. A multicountry molecular analysis of Salmonella enterica Serovar Typhi with reduced susceptibility to ciprofloxacin in Sub-Saharan Africa. Clin Infect Dis. 2016; 62 Suppl 1: S42–6. doi: 10.1093/cid/civ788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kariuki S, Revathi G, Kiiru J, Mengo DM, Mwituria J, Muyodi J. Typhoid in Kenya Is associated with a dominant multidrug-resistant Salmonella enterica Serovar Typhi haplotype that is also widespread in southeast Asia. J Clin Microbiol. 2010; 48:2171–2176. doi: 10.1128/JCM.01983-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.International Typhoid Consortium, Wong VK, Holt KE, Okoro C, Baker S, Pickard DJ, et al. Molecular surveillance identifies multiple transmissions of typhoid in West Africa. PLoS Negl Trop Dis. 2016; 10: e0004781 doi: 10.1371/journal.pntd.0004781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong VK, Baker S, Pickard DJ, Parkhill J, Page AJ, Feasey NA, et al. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet. 2015; 47: 632–639. doi: 10.1038/ng.3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raufu I, Bortolaia V, Svendsen CA, Ameh JA, Ambali AG, Aarestrup FM, et al. The first attempt of an active integrated laboratory-based Salmonella surveillance programme in the North-Eastern Region of Nigeria. J Appl Microbiol. 2013: 115:1059–1067. doi: 10.1111/jam.12304 [DOI] [PubMed] [Google Scholar]
- 41.Kariuki S, Revathi G, Muyodi J, Mwituria J, Munyalo A, Mirza S, et al. Characterization of multidrug-resistant typhoid outbreaks in Kenya. J Clin Microbiol. 2004; 42:1477–1482. doi: 10.1128/JCM.42.4.1477-1482.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith AM, Govender N, Keddy KH, GERMS-SA. Quinolone-resistant Salmonella Typhi in South Africa, 2003–2007. Epidemiol Infect. 2010; 138:86–90. doi: 10.1017/S0950268809990331 [DOI] [PubMed] [Google Scholar]
- 43.Fabre L, Delauné A, Espié E, Nygard K, Pardos M, Polomack L, et al. , Chromosomal integration of the extended-spectrum beta-lactamase gene blaCTX-M-15 in Salmonella enterica serotype Concord isolates from internationally adopted children. Antimicrob Agents Chemother. 2009; 53:1808–1816. doi: 10.1128/AAC.00451-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendriksen RS, Mikoleit M, Kornschober C, Rickert RL, Duyne SV, Kjelsø C, et al. Emergence of multidrug-resistant Salmonella Concord infections in Europe and the United States in children adopted from Ethiopia, 2003–2007. Pediatr Infect Dis J. 2009; 28:814–818. doi: 10.1097/INF.0b013e3181a3aeac [DOI] [PubMed] [Google Scholar]
- 45.Vanhoof R, Gillis P, Stévart O, Boland C, Vandenberg O, Fux F, et al. Transmission of multiple resistant Salmonella Concord from internationally adopted children to their adoptive families and social environment: proposition of guidelines. Eur J Clin Microbiol Infect Dis. 2012; 31:491–497. doi: 10.1007/s10096-011-1336-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hendriksen RS, Leekitcharoenphon P, Lukjancenko O, Lukwesa-Musyani C, Tambatamba B, Mwaba J, et al. Genomic signature of multidrug-resistant Salmonella enterica Serovar Typhi isolates related to a massive outbreak in Zambia between 2010 and 2012. J Clin Microbiol. 2015; 53: 262–272. doi: 10.1128/JCM.02026-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Govinden U, Mocktar C, Moodley P, Sturm AW, Essack SY. Detection of mutations in the gyrA of clinical Salmonella spp. Afr J Biotechnol. 2009; 8: 3911–3914. [Google Scholar]
- 48.Hendriksen RS, Joensen KG, Lukwesa-Musyani C, Kalondaa A, Leekitcharoenphon P, Nakazwe R, et al. Extremely drug-resistant Salmonella enterica serovar Senftenberg infections in patients in Zambia. J Clin Microbiol. 2013; 51: 284 doi: 10.1128/JCM.02227-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Emran H, Heisig A, Dekker D, Adu-Sarkodie Y, Espinoza LMC, Panzner U, et al. Detection of a novel gyrB mutation associated with fluoroquinolone-non susceptible Salmonella enterica serovar Typhimurium isolated from a bloodstream infection in Ghana. Clin Infect Dis. 2016; 62 Suppl 1:S47–9. doi: 10.1093/cid/civ790 [DOI] [PubMed] [Google Scholar]
- 50.Boisramé-Gastrin S, Tandé D, Münck MR, Gouriou S, Nordmann P, Naas T. Salmonella carriage in adopted children from Mali: 2001–08. J Antimicrob Chemother. 2011; 66: 2271–2276. doi: 10.1093/jac/dkr307 [DOI] [PubMed] [Google Scholar]
- 51.Harrois D, Breurec S, Seck A, Delauné A, Le Hello S, de la Gándara MP, et al. Prevalence and characterization of extended-spectrum β-lactamase-producing clinical Salmonella enterica isolates in Dakar, Senegal, from 1999 to 2009. Clin Microbiol Infect. 2014; 20:109–116. doi: 10.1111/1469-0691.12534 [DOI] [PubMed] [Google Scholar]
- 52.Eguale T, Birungi J, Asrat D, Njahira MN, Njuguna J, Gebreyes WA, et al. Genetic markers associated with resistance to beta-lactam and quinolone antimicrobials in non-typhoidal Salmonella isolates from humans and animals in central Ethiopia. Antimicrob Resist Infect Control. 2017; 6: doi: 10.1186/s13756-017-0171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seiffert SN, Perreten V, Johannes S, Droz S, Bodmer T, Endimiania A. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of Sequence Type 198 in a patient transferred from Libya to Switzerland. Antimicrob Agents Chemother. 2014; 58: 2446–2449. doi: 10.1128/AAC.02417-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Hello S, Harrois D, Bouchrif B, Sontag L, Elhani D, Guibert V, et al. Highly drug-resistant Salmonella enterica serotype Kentucky ST198-X1: a microbiological study. Lancet Infect Dis. 2013; 13: 672–679. doi: 10.1016/S1473-3099(13)70124-5 [DOI] [PubMed] [Google Scholar]
- 55.Le Hello S, Bekhit A, Granier SA, Barua H, Beutlich J, Zajac M˛ et al. The global establishment of a highly fluoroquinolone resistant Salmonella enterica serotype Kentucky ST198 strain. Front Microbiol. 2013; 4: doi: 10.3389/fmicb.2013.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Hello S, Hendriksen RS, Doublet B, Fisher I, Nielsen EM, Whichard JM, et al. International spread of an epidemic population of Salmonella enterica Serotype Kentucky ST198 resistant to ciprofloxacin. J Infect Dis. 2011; 204: 675–684. doi: 10.1093/infdis/jir409 [DOI] [PubMed] [Google Scholar]
- 57.Ahmed AM, Shimamoto T, Shimamoto T. Characterization of integrons and resistance genes in multidrug-resistant Salmonella enterica isolated from meat and dairy products in Egypt. Int J Food Microbiol. 2014; 189: 39–44. doi: 10.1016/j.ijfoodmicro.2014.07.031 [DOI] [PubMed] [Google Scholar]
- 58.Ahmed AM, Younis EE, Ishida Y, Shimamoto T. Genetic basis of multidrug resistance in Salmonella enterica serovars Enteritidis and Typhimurium isolated from diarrheic calves in Egypt. Acta Trop. 2009; 111:144–149. doi: 10.1016/j.actatropica.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 59.Al-Gallas N, Abbassi MS, Gharbi B, Manai M, Fayala MNB, Bichihi, et al. Occurrence of plasmid-mediated quinolone resistance determinants and rmtB gene in Salmonella enterica Serovar Enteritidis and Typhimurium isolated from food-animal products in Tunisia. Foodborne Pathog Dis. 2013; 10: doi: 10.1089/fpd.2012.1466 [DOI] [PubMed] [Google Scholar]
- 60.Murgia M, Bouchrif B, Timinouni M, Al-Qahtani A, Al-Ahdal MN, Cappuccinelli P, et al. Antibiotic resistance determinants and genetic analysis of Salmonella enterica isolated from food in Morocco. Int J Food Microbiol. 2015; 215: 31–39. doi: 10.1016/j.ijfoodmicro.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 61.Soufi L, Sáenz Y, de Toro M, Abbassi MS, Rojo-Bezares B, Vinué L, et al. Phenotypic and genotypic characterization of Salmonella enterica recovered from poultry meat in Tunisia and identification of new genetic traits. Vectorborn Zoonot Dis. 2012; 12: doi: 10.1089/vbz.2011.0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fashae K, Hendriksen RS. Diversity and antimicrobial susceptibility of Salmonella enterica serovars isolated from pig farms in Ibadan, Nigeria. Folia Microbiol (Praha). 2014; 59: 69–77. doi: 10.1007/s12223-013-0270-6 [DOI] [PubMed] [Google Scholar]
- 63.Falay D, Kuijpers LMF, Phoba MF, De Boeck H, Lunguya O, Vakaniaki E, et al. Microbiological, clinical and molecular findings of non-typhoidal Salmonella bloodstream infections associated with malaria, Oriental Province, Democratic Republic of the Congo. BMC Infectious Dis. 2016; 16: doi: 10.1186/s12879-016-1604-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phoba MF, Barbé B, Lunguya O, Masendu L, Lulengwa D, Dougan G, et al. Salmonella enterica serovar Typhi Producing CTX-M-15 Extended Spectrum β-Lactamase in the Democratic Republic of the Congo. Clin Infect Dis. 2017; 65:1229–1231. doi: 10.1093/cid/cix342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veldman K, Dierikx C, van Essen-Zandbergen A, van Pelt W, Mevius D. Characterization of multidrug-resistant, qnrB2-positive and extended-spectrum- β-lactamase-producing Salmonella Concord and Salmonella Senftenberg isolates. J Antimicrob Chemother. 2010; 65: 872–875. doi: 10.1093/jac/dkq049 [DOI] [PubMed] [Google Scholar]
- 66.Tabo D, Granier SA, Diguimbaye CD, Marault M, Brisabois A, Mama B, et al. Are Salmonella-Induced gastroenteritis neglected in developing countries? feedback from microbiological investigations in N’Djamena Hospitals, Chad. PLoS ONE. 2015; 10: e0136153 doi: 10.1371/journal.pone.0136153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bronowski C, Fookes MC, Gilderthorp R, Ashelford KE, Harris SR, Phiri A, et al. Genomic characterisation of invasive non-typhoidal Salmonella enterica Subspecies enterica Serovar Bovismorbificans isolates from Malawi. PLoS Negl Trop Dis. 2013; 7: e2557 doi: 10.1371/journal.pntd.0002557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-Mediated Quinolone Resistance: a multifaceted threat. Clin Microbiol Rev. 2009; 664–689. doi: 10.1128/CMR.00016-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellington MJ, Ekelund O, Aarestrup FM, Canton R, Doumith M, Giske C, et al. The role of whole genome sequencing in antimicrobial susceptibility testing of bacteria: report from the EUCAST Subcommittee. Clin Microbiol Infect. 2017; 23: 2e22 doi: 10.1016/j.cmi.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 70.Tyson GH, Zhao S, Li C, Ayers S, Sabo JL, Lam C, et al. Establishing genotypic cutoff values to measure antimicrobial resistance in Salmonella. Antimicrob Agents Chemother. 2017; 61: pii: e02140-16. doi: 10.1128/AAC.02140-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cornaglia G, Hryniewicz W, Jarlier V, Kahlmeter G, Mittermayer H, Stratchounski L, et al. European recommendations for antimicrobial resistance surveillance. Clin Microbiol Infect. 2004; 10: 349–383. doi: 10.1111/j.1198-743X.2004.00887.x [DOI] [PubMed] [Google Scholar]
- 72.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18: 268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 73.Morpeth SC, Ramadhani HO, Crump JA. Invasive non-typhi Salmonella Disease in Africa. Clin Infect Dis. 2009: 49:606–611. doi: 10.1086/603553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kingsley RA, Msefula CL, Nicholas R. Thomson NR, Kariuki S, Holt KE, et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 2009; 19:2279–2287. doi: 10.1101/gr.091017.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, et al. Intra-continental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet. 2012; 44: 1215–1221. doi: 10.1038/ng.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okoro CK, Barquist L, Connor TR, Harris SR, Clare S, Stevens MP, et al. Signatures of Adaptation in human invasive Salmonella Typhimurium ST313 populations from Sub-Saharan Africa. PLoS Negl Trop Dis. 2015; 9: e0003611 doi: 10.1371/journal.pntd.0003611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feasey NA, Hadfield J, Keddy KH, Dallman TJ, Jacobs J, Deng X, et al. Distinct Salmonella Enteritidis lineages associated with enterocolitis in high-income settings and invasive disease in low-income settings. Nat Genet. 2016; 48: 1211–1217. doi: 10.1038/ng.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marks F, von Kalckreuth V, Aaby P, Adu-Sarkodie Y, El Tayeb MA, Ali M, et al. Incidence of invasive salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Glob Health. 2017; 5: e310–23. doi: 10.1016/S2214-109X(17)30022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ocan M, Obuku EA, Bwanga F, Akena D, Richard S, Jasper Ogwal-Okeng J, et al. Household antimicrobial self-medication: a systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health 2015; 15:742 doi: 10.1186/s12889-015-2109-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laxminarayan R, Chaudhury RR. Antibiotic resistance in India: drivers and opportunities for action. PLoS Med. 2016. 13: e1001974 doi: 10.1371/journal.pmed.1001974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096 doi: 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 82.Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011; 11: 692–701. doi: 10.1016/S1473-3099(11)70054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lamikanra A, Crowe JL, Lijek RS, Odetoyin BW, Wain J, Aboderin A, et al. Rapid evolution of fluoroquinolone resistant Escherichia coli in Nigeria is temporally associated with fluoroquinolone use. BMC Infect Dis. 2011; 11: doi: 10.1186/1471-2334-11-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cooke FJ, Wain J, Threlfall EJ. Fluoroquinolone resistance in Salmonella Typhi. BMJ. 2006; 333: 353–354. doi: 10.1136/bmj.333.7563.353-b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crump JA, Kretsinger K, Gay K. Clinical response and outcome of infection with Salmonella enterica serotype Typhi with decreased susceptibility to fluoroquinolones: a United States Foodnet multi-center retrospective cohort study. Antimicrob Agents Chemother. 2008; 52: 1278–1284. doi: 10.1128/AAC.01509-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ray P, Sharma J, Marak RSK, Garg RK. Predictive efficacy of nalidixic acid resistance as a marker of fluoroquinolone resistance in Salmonella enterica var Typhi. Indian J Med Res. 2006; 124: 105–108. [PubMed] [Google Scholar]
- 87.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother.1990; 34: 1271–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walther-Rasmussen J, Høiby N. Salmonella enterica serovar Typhi and S. Paratyphi A: need to expand the QRDR region? Epidemiol Infect. 2011; 139:1281–1283. doi: 10.1017/S0950268810002487 [DOI] [PubMed] [Google Scholar]
- 89.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004; 82: 346–353. [PMC free article] [PubMed] [Google Scholar]
- 90.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010; 50:882–889. doi: 10.1086/650733 [DOI] [PubMed] [Google Scholar]
- 91.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012; 379: 2489–2499. doi: 10.1016/S0140-6736(11)61752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vashist J. Vishvanath, Kapoor V, Kapil A, Yennamalli R, Subbarao N, et al. Interaction of nalidixic acid and ciprofloxacin with wild type and mutated quinolone resistant determining region of DNA gyrase A. Indian J Biochem Biophys. 2009; 49:147–153. [PubMed] [Google Scholar]
- 93.Fu Y, Zhang W, Wang H, Zhao S, Chen Y, Meng F, et al. Specific patterns of gyrA mutations determine the resistance difference to ciprofloxacin and levofloxacin in Klebsiella pneumoniae and Escherichia coli. BMC Infect Dis. 2013; 13: doi: 10.1186/1471-2334-13-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koirala KD, Thanh DP, Thapa SD, Arjyal A, Karkey A, Dongol S, et al. Highly resistant Salmonella enterica Serovar Typhi with a novel gyrA mutation raises questions about the long-term efficacy of older fluoroquinolones for treating typhoid Fever. Antimicrob Agents Chemother. 2012; 2761–2762. doi: 10.1128/AAC.06414-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boyd LB, Maynard MJ, Morgan-Linnell SK, Horton LB, Sucgang R, Hamill RJ. Relationships among ciprofloxacin, gatifloxacin, levofloxacin, and norfloxacin MICs for fluoroquinolone-resistant Escherichia coli clinical isolates. Antimicrob Agents Chemother. 2009; 53: 229–234. doi: 10.1128/AAC.00722-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frye JG, Jackson CR. Genetic mechanisms of antimicrobial resistance identified in Salmonella enterica, Escherichia coli, and Enteroccocus spp. isolated from US food animals. Front Microbiol. 2013; 4: doi: 10.3389/fmicb.2013.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Veldman K, Cavaco LM, Mevius D, Battisti A, Franco A, Botteldoorn N, et al. International collaborative study on the occurrence of plasmid-mediated quinolone resistance in Salmonella enterica and Escherichia coli isolated from animals, humans, food and the environment in 13 European countries. J Antimicrob Chemother. 2011; 66:1278–1286. doi: 10.1093/jac/dkr084 [DOI] [PubMed] [Google Scholar]
- 98.Yugendran T, Harish BN. High incidence of plasmid-mediated quinolone resistance genes among ciprofloxacin-resistant clinical isolates of Enterobacteriaceae at a tertiary care Hospital in Puducherry, India. Peer J. 2016; doi: 10.7717/peerj.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu BT, Liao XP, Yang SS, Wang XM, Li LL, Sun J, et al. Detection of mutations in the gyrA and parC genes in Escherichia coli isolates carrying plasmid-mediated quinolone resistance genes from diseased food-producing animals. J Medic Microbiol. 2012; 61: 1591–1599. doi: 10.1099/jmm.0.043307–0 [DOI] [PubMed] [Google Scholar]
- 100.Jacoby GA, Strahilevitz J, Hooper DC. Plasmid-mediated quinolone resistance. Microbiol Spectr. 2014; 2: doi: 10.1128/microbiolspec.PLAS-0006-2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Redgrave LS, Sutton SB, Webber MA, Piddock LJ. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014; 22: 438–445. doi: 10.1016/j.tim.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 102.Weill FX, Bertrand S, Guesnier F, Baucheron S, Cloeckaert A, Grimont PA. Ciprofloxacin-resistant Salmonella Kentucky in travelers. Emerg Infect Dis. 2006; 12:1611–1612. doi: 10.3201/eid1210.060589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carter D, Charlett A, Conti S, Robotham JV, Johnson AP, Livermore DM, et al. A risk assessment of antibiotic Pan-Drug-Resistance in the UK: Bayesian analysis of an expert elicitation study. Antibiotics. 2017; 6: 9 doi: 10.3390/antibiotics6010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacoby GA. Mechanisms of resistance to quinolones. Clin Infect Dis. 2005; 41: Suppl 2:S120–6. doi: 10.1086/428052 [DOI] [PubMed] [Google Scholar]
- 105.Li X-Z, Plésiat P, Nikaido H. The challenge of efflux mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev. 2015; 28: 337–418. doi: 10.1128/CMR.00117-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baucheron S, Monchaux I, Le Hello S, Weill FX, Cloeckaert A, et al. Lack of efflux mediated quinolone resistance in Salmonella enterica serovars Typhi and Paratyphi A. Front Microbiol. 2014; 5: doi: 10.3389/fmicb.2014.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abouzeed YM, Baucheron S, Cloeckaert A. ramR mutations involved in efflux-mediated multidrug resistance in Salmonella enterica Serovar Typhimurium. Antimicrob Agents Chemother. 2008; 52: 2428–4234. doi: 10.1128/AAC.00084-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun Y, Dai M, Hao H, Wang Y, Huang L, Almofti YA, et al. The role of ramA on the development of ciprofloxacin resistance in Salmonella enterica Serovar Typhimurium. PLoS ONE. 2011; 6: e23471 doi: 10.1371/journal.pone.0023471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baucheron S, Le Hello S, Doublet B, Giraud E, Weill FX, Cloeckaert A. ramR mutations affecting fluoroquinolone susceptibility in epidemic multidrug-resistant Salmonella enterica serovar Kentucky ST198. Front Microbiol. 2013; 4: doi: 10.3389/fmicb.2013.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 9: e1001300 doi: 10.1371/journal.pmed.1001300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harder T, Takla A, Rehfuess E, Sanchez-Vivar A, Matysiak-Klose D, Eckmanns T, et al. Evidence-based decision-making in infectious diseases epidemiology, prevention and control: matching research questions to study designs and quality appraisal tools. BMC Med Res Methodol. 2014; 14: doi: 10.1186/1471-2288-14-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007; 36: 666–676. doi: 10.1093/ije/dym018 [DOI] [PubMed] [Google Scholar]
- 113.Harder T, Takla A, Eckmanns T, Ellis S, Forland F, James R, et al. PRECEPT: an evidence assessment framework for infectious disease epidemiology, prevention and control. Euro Surveill. 2017; 22(40):pii = 16–00620. https://doi.org/10.2807/1560-7917.ES.2017.22.40.16-00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robert Koch Institute. PRECEPT project. Berlin: Robert Koch Institute; 2017. Available from: http://www.rki.de/EN/Content/Institute/DepartmentsUnits/InfDiseaseEpidem/Div33/.PRECEPT/PRECEPT_II_en.html. [Google Scholar]
- 115.VanderWeele TJ. On a square root transformation of the odds ratio for a common outcome. Epidemiology. 2017; 28: e58–e60. doi: 10.1097/EDE.0000000000000733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mathur MB, VanderWeele TJ. Sensitivity analysis for unmeasured confounding in meta-analyses. 2017; http://arXiv:1707.09076 [stat.ME]. [DOI] [PMC free article] [PubMed]
- 117.Mann CJ. Observational research methods. Research design II: cohort, cross-sectional, and case-control studies. Emerg Med J. 2003; 20: 54–60. doi: 10.1136/emj.20.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Higgins J, Green S. Cochrane handbook for systematic reviews and interventions. 2011; Available from: https://dhosth.files.wordpress.com/2011/12/cochrane-handbook-for-systematic-reviews-of-interventions.pdf.
- 119.Nakagawa S, Noble DWA, Senior AM., Lagisz M. Meta-evaluation of meta-analysis: ten appraisal questions for biologists. BMC Biology. 2017; 15: doi: 10.1186/s12915-017-0357-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J. et al. GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol. 2011; 64: 1277–1282. doi: 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 121.Smith AM, Mthanti MA, Haumann C, Tyalisi N, Boon GPG, Sooka A, et al. Nosocomial outbreak of Salmonella Typhimurium primarily affecting a pediatric ward, South Africa, 2012. J Clin Microbiol. 2014; 52: 627–631. doi: 10.1128/JCM.02422-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nejad SB, Allegranzi B, Syed SB, Ellis B, Pittet D. Health-care-associated infection in Africa: a systematic review. Bull World Health Organ. 2011; 89: 757–765. doi: 10.2471/BLT.11.088179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aiken AM, Mturi N, Njuguna P, Mohammed S, Berkley JA, Mwangi I, et al. Risk and causes of paediatric hospital-acquired bacteraemia in Kilifi District Hospital, Kenya: a prospective cohort study. Lancet. 2011; 378: 2021–2027. doi: 10.1016/S0140-6736(11)61622-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lohr K.N. Rating the strength of scientific evidence: relevance for quality improvement programs. Int J Qual Health Care. 2004; 16: 9–18. doi: 10.1093/intqhc/mzh005 [DOI] [PubMed] [Google Scholar]
- 125.GRADE Handbook: Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013. Available from: https://gdt.gradepro.org/app/handbook/handbook.html.
- 126.PRECEPT. Development of an evidence assessment framework for public health/ infectious disease prevention and control in Europe. 2015; Available from: https://www.rki.de/EN/Content/Institute/DepartmentsUnits/InfDiseaseEpidem/Div33/PRECEPT/PRECEPTFramework.pdf?blob=publi.
- 127.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2017; doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 128.Trespalacios AA, Rimbara E, Otero W, Reddy R, Graham DY. Improved allele-specific PCR assays for detection of clarithromycin and fluoroquinolone resistant Helicobacter pylori in gastric biopsies: identification of N87I mutation in GyrA. Diagn Microbiol Infect Dis. 2015; 8: 251–255. doi: 10.1016/j.diagmicrobio.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.