Abstract
Nucleobases are the carriers of the genetic information in RNA and DNA for all life on Earth. Their presence in meteorites clearly indicates that compounds of biological importance can form via non-biological processes in extraterrestrial environments. Recent experimental studies have shown that the pyrimidine-based nucleobases uracil and cytosine can be easily formed from the ultraviolet irradiation of pyrimidine in H2O-rich ice mixtures that simulate astrophysical processes. In contrast, thymine, which is found only in DNA, is more difficult to form under the same experimental conditions, as its formation usually requires a higher photon dose. Earlier quantum chemical studies confirmed that the reaction pathways were favorable provided that several H2O molecules surrounded the reactants. However, the present quantum chemical study shows that the formation of thymine is limited because of the inefficiency of the methylation of pyrimidine and its oxidized derivatives in an H2O ice, as supported by the laboratory studies. Our results constrain the formation of thymine in astrophysical environments and thus the inventory of organic molecules delivered to the early Earth, and have implications for the role of thymine and DNA in the origin of life.
I. Introduction
Nucleobases are the informational sub-units of ribonucleic and deoxyribonucleic acids (RNA and DNA) that carry the genetic information for all life on Earth. They play an essential role in the biomolecular processes in living organisms, and are believed to have played an important role in the emergence of life on Earth about 3.8 billion years ago. Nucleobases are nitrogen-bearing cyclic molecules, or N-heterocycles, structurally based on two compounds: pyrimidine (C4H4N2; for uracil, cytosine, and thymine) and purine (C5H4N4; for adenine and guanine).1 N-Heterocycles have not been observed in situ in astrophysical environments thus far despite an extensive search,2–4 but several of them have been detected in carbonaceous meteorites,5–7 with isotopic ratios consistent with an extraterrestrial origin.8 The only meteoritic pyrimidine-based compound reported to date is uracil,6 although the presence of cytosine, which may be converted into uracil during the extraction protocol,9,10 cannot be ruled out. In contrast, the non-detection of thymine in meteorites is not completely explained, although several possibilities have been suggested.11
Some or all of these meteoritic N-heterocycles must therefore have been formed in a pre-terrestrial environment. One plausible chemical pathway for their formation is via photo-processes of ices condensed on the surface of cold (10–100 K) interstellar or protostellar grains, known from astronomical observations to contain volatile species such as H2O, CH3OH, CO, CO2, CH4, and NH3,12,13 as well as larger molecules including polycyclic aromatic hydrocarbons (PAHs)14–16 and N-heterocycles. Other possible pathways include polymerization processes similar to what is believed to occur during PAH formation,17 but involving hydrogen cyanide (HCN) instead of acetylene (C2H2).18,19 Laboratory studies have shown that the ultraviolet (UV) photo-irradiation of mixtures of H2O, CH3OH, CO, CO2, CH4, and NH3 ices leads to the formation of a variety of complex organic molecules such as amino acids20–22 and other compounds of biological interest,23–25 most of which have been detected in meteorites.26–28 Similarly, the nucleobases uracil and cytosine are formed when pyrimidine is irradiated in ices containing H2O together with combinations of CH3OH, CH4, and/or NH3.1,29,30 Quantum chemical calculations have shown that the UV-induced oxidation of pyrimidine in the condensed phase favors the formation of uracil and its precursor 4(3H)-pyrimidone,31 and that the presence of multiple H2O molecules surrounding the reactants is essential for this process, i.e., the de-protonation reactions would not occur in the gas phase. Similar processes are believed to be true for the addition of NH2 groups, as OH and NH2 radicals are both nucleophiles and their energies of formation from H2O and NH3 are comparable.32,33 Radiation-induced alteration of nucleic acid bases and base pairs have also been studied previously using quantum chemical methods.34–37
In the present study, we performed quantum chemical computations to understand the pathways for the formation of thymine as well as other methyl-bearing pyrimidine derivatives and compare the results with UV photo-irradiation experiments of pyrimidine mixed in H2O+CH3OH and H2O+CH4 ices performed at 15–20 K under ultra-high vacuum (≤5×10−8 mbar).1,11,29,30 In those experiments, ices were subjected to two distinct photon doses: some of them were irradiated with the same photon dose as that used for the formation of uracil and cytosine under similar experimental conditions1,29 (“lower photon dose”, i.e., about ~0.25–0.35 photons per deposited molecule), while others were exposed to a photon dose which was about 3 times higher (“higher photon dose”, i.e., ~0.65–0.90 photons molecule−1).11,38
Guided by the detection—or non-detection—of compounds in the experimental studies, we performed quantum chemical calculations using density functional theory B3LYP/MP2//cc-pVTZ on the reactants, intermediates, and final products involved in the processes in order to determine the reaction pathways to the formation of thymine and explain why its formation is not as efficient as those of uracil and cytosine under similar conditions. Finally, we discuss the results from an astrophysical and astrobiological perspective to assess the possible role of thymine in the origin of life on Earth.
II. Theoretical Methods
The formation pathways of thymine from pyrimidine have been investigated using ab initio quantum chemical computations, employing methods that proved efficient for the study of the formation of uracil from the UV-induced oxidation of pyrimidine in pure H2O ice.31 In order to address the environmental effects of the water matrix certain rate-limiting steps of important reactions were explored by explicitly involving water molecules in the reaction pathway. Geometries of the reactants, intermediates, and products were optimized using density functional theory and perturbation theory methods. Becke’s three-parameter exchange functional B339 combined with the correlation functional of Lee, Yang, and Parr40 was employed in conjunction with Pople’s 6-31G(d,p) split valence basis set. Geometrical structures of all the molecules reported in this work were fully optimized and represent true minima, confirmed by subsequent frequency calculations. The absolute energies and energy differences reported here were obtained at the B3LYP/6-31G(d,p) optimized geometries using the second-order Møller-Plesset perturbation theory (MP2) based on a restricted Hartree-Fock reference function for closed shell species, and the second-order Z-averaged perturbation theory (ZAPT2)41,42 (which is also based on a restricted Hartree-Fock reference function) for the open shell species in conjunction with Dunning’s correlation consistent valence triple zeta basis sets (cc-pVTZ). The use of unrestricted MP2 (UMP2) for the open shell radicals, in some cases, showed a large spin contamination, which can lead to poor UMP2 results. ZAPT2, on the contrary, is based on symmetric spin orbitals,43 and leads to Sx eigenfunctions in the n-particle space. ZPAT2 has been shown to have much less spin contamination compared to UMP2 and other restricted open shell based perturbation theories, and a larger radius of convergence.42 All the computations were performed using the Q-CHEM 3.2 suite of ab initio quantum chemical codes.44
III. Results and discussion
A. Study of the formation mechanisms
Three gas-phase reaction mechanisms involving the OH and CH3 radicals, as well as cationic reactants are explored. Formation of thymine from pyrimidine requires two oxidations in positions 2 and 4 of the pyrimidine ring, and a methylation in position 5. Figure 1 illustrates the three pathways studied here for the formation of thymine via the successive addition of OH and CH3 groups to pyrimidine in different orders: double oxidation followed by methylation (Route 1); methylation followed by double oxidation (Route 2); and first oxidation, then methylation, and then second oxidation (Route 3).
Figure 1.
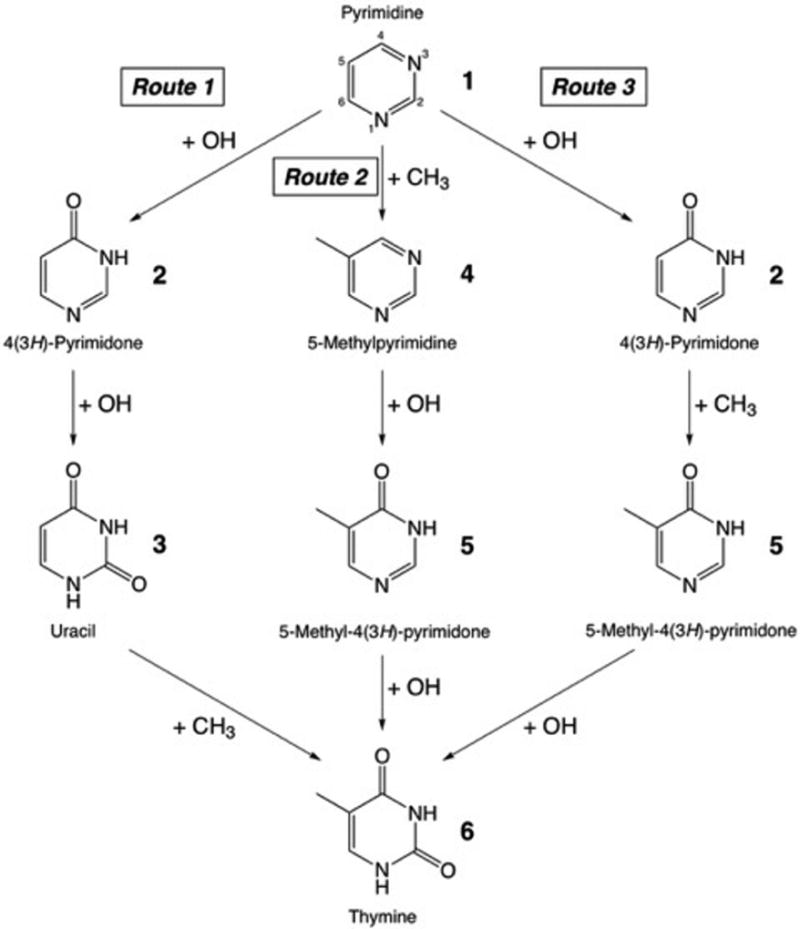
Diagram illustrating the three pathways from pyrimidine 1 to thymine 6 studied in this work with quantum chemical computations. The energetics and stability of the intermediate compounds given here for each step of each route (compounds 2–5) are discussed in Section II.
For each of these routes, we examined different types of mechanisms, involving reactions of OH and CH3 radicals with pyrimidine and its derivatives (including all the intermediate compounds) in their cationic forms, their uncharged radical forms, and their neutral forms. These reactions are followed by the loss of a hydrogen atom or a proton, which is assisted by neighboring H2O molecules. Each association reaction presented here takes place between a radical cation and a neutral radical with a non-zero dipole moment. Furthermore, the “hole” in the radical cation is always located in the π space of the aromatic ring, meaning that the attack by the neutral radical may occur on any atom in the ring without a barrier. In other words, a bond is formed, but no electron pair is broken during the association reaction. The initial nucleophilic addition of OH or CH3 is followed by the loss of a proton which, as we showed previously,31 is spontaneous in the presence of a polar proton acceptor such as H2O. Consequently, the distribution of products and intermediates in these reactions is driven by the overall thermodynamics. Similar to the UV-induced oxidation of pyrimidine in pure H2O ice under comparable conditions,31 the cationic mechanism, i.e., the association of the cations of pyrimidine and its derivatives with OH and CH3 radicals followed by the loss of a proton, appears to be the most efficient of all the routes and steps studied here. Consequently, in the following discussion we will focus on the reactions between cationic species and OH/CH3 radicals.
Route 1
The formation of thymine 6 via Route 1 (Fig. 1) involves two successive oxidations of pyrimidine 1, followed by one methylation. Based on our previous experimental and theoretical work, we know that the oxidation of pyrimidine is an efficient process in an H2O ice matrix, and that 4(3H)-pyrimidone 2 and uracil 3 are the most energetically favorable singly and doubly oxidized pyrimidine derivatives, respectively.1,31 Therefore, it is reasonable to assume that 2 and 3 will also be the most abundant oxidized pyrimidines in any other H2O-rich ices. The energetics of such reactions have been shown earlier.31 The subsequent addition of a methyl group to the uracil cation is favorable from an energetic point of view only when intermediate species are surrounded by excess H2O, which plays the role of proton acceptor for the formation of the final products, as shown by the energies of the reactants and products (Fig. 2). Our calculations also predict that the formation of 6-methyluracil, an isomer of thymine, is lower in energy by 1.8 kcal mol−1, although the protonated intermediate of thymine 6 is lower in energy. Thymine was observed in some of the experimental samples, while 6-methyluracil was not observed in any of them.11 This suggests that the stability of these products under such experimental conditions (condensed phase) may be different from the gas phase. Methylation of uracil towards thymine, though favorable by about −85 kcal mol−1, is not as strong as its oxiation. The oxidation of pyrimidine was found to be favorable by about −135 kcal mol−1, and the oxidation of 4(3H)-pyrimidone towards the formation of uracil by about −105 kcal mol−1 in an H2O ice matrix. Furthermore, thymine synthesis requires an additional CH3 group addition compared with uracil or cytosine, which may explain why thymine could only be detected in experimental samples produced from the irradiation of H2O:CH4:pyrimidine ices with a higher UV dose.11 At the same time, high UV doses may also lead to the destruction of thymine and other products.
Figure 2.
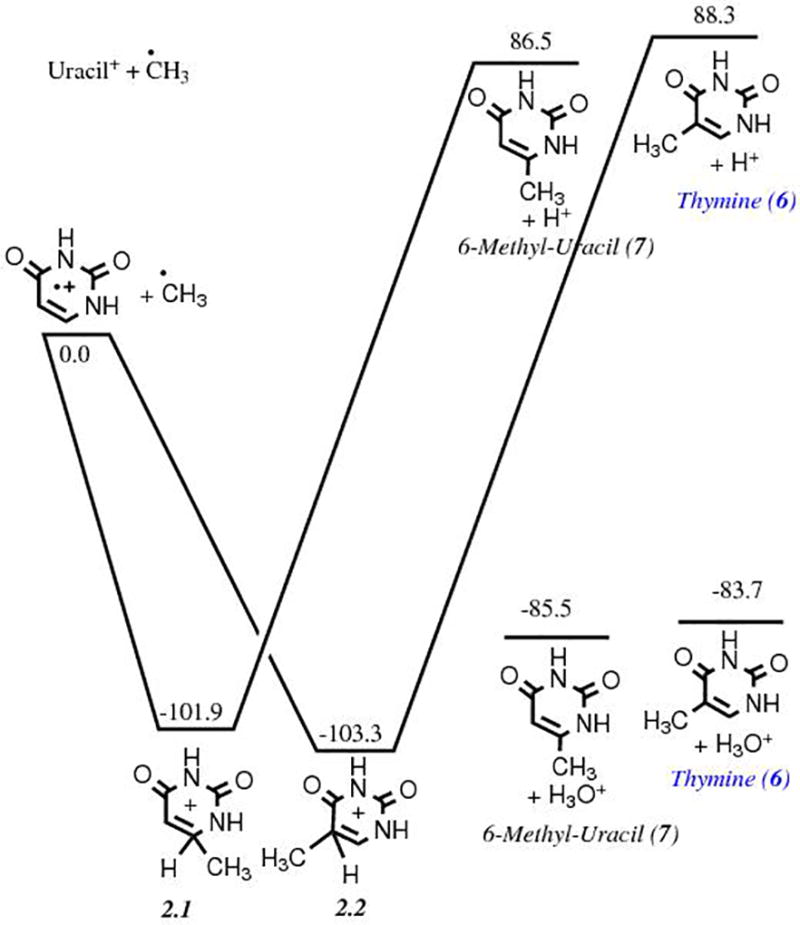
Energy diagram showing the energies (in kcal mol−1) and structures of the reactants and products corresponding to the third step of Route 1 (Fig. 1), i.e., the methylation of uracil 3 towards the formation of thymine 6. The formation of uracil from the double oxidation of pyrimidine has been studied in detail in a previous work.31
Route 2
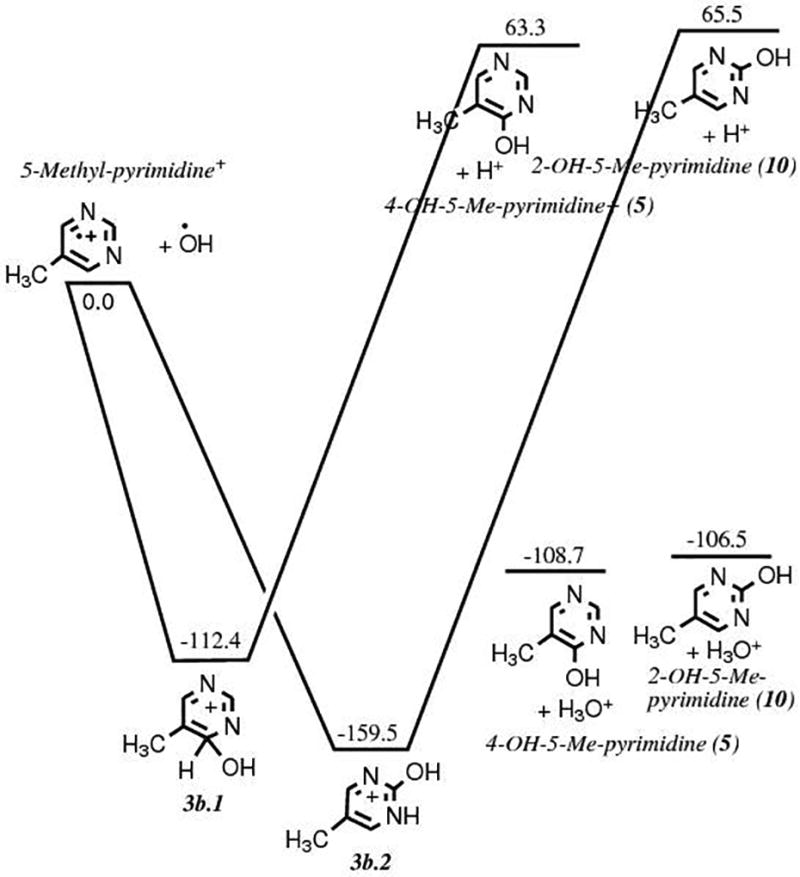
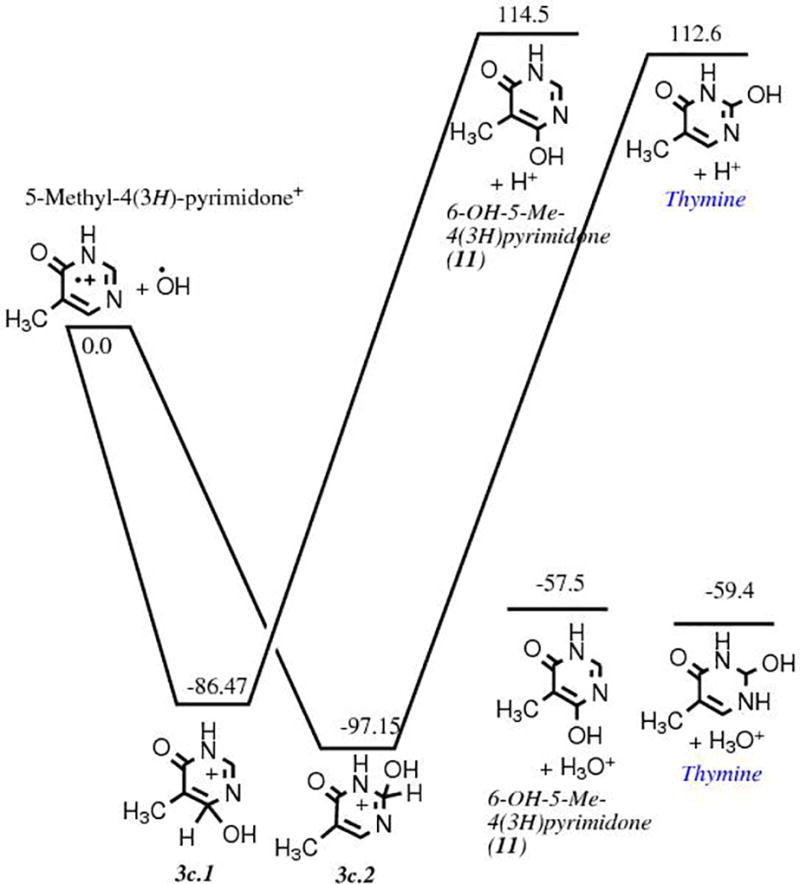
This route involves the methylation of pyrimidine 1 as a first step. Our quantum chemical computations show that such a reaction in a pure methane ice matrix via a cationic mechanism is not favorable (Fig. 3a). However, it becomes favorable in a polar, protic ice matrix such as H2O. The calculations do not show any clear preference for the position of the methyl group addition to the pyrimidic ring, as the energies of the three possible isomers fall within a 2.2 kcal mol−1 range. However, 4-methylpyrimidine 8 is statistically more likely to form than the two other isomers, including the precursor of thymine 5-methylpyrimidine 4, because it can be formed from the methylation of the carbon atoms in positions 4 and 6 of the pyrimidine ring. Experimentally, only small quantities of 4- and 5-methylpyrimidines were detected in samples produced after irradiation of pyrimidine in pure CH4 ices and H2O+CH4 ice mixtures with the higher UV photon dose,11 which confirms that methylation of pyrimidine is not an efficient process. Once it is formed, however, the oxidation of 4 in an H2O ice is energetically favorable and efficient (Fig. 3b). However, it should be noted that experiments in which 5-methylpyrimidine 4 in H2O ice was irradiated with UV photons resulted not only in the formation of thymine 6 and its precursors, but also in oxidized products in which the CH3 group has been cleaved from 4 (e.g. 4(3H)-pyrimidone 2 and uracil 3).11 The unusual stability of intermediate 3b.2 as compared to intermediate 3b.1 (Fig. 3b) is due to the migration of a proton from a carbon atom to a nitrogen atom. The formation of 5-methyl-4(3H)-pyrimidone 5 was found to be slightly favored over its isomer 5-methyl-2(1H)-pyrimidone 10 (Fig. 3b), but it does not affect the formation of thymine since the oxidation of either intermediate leads to thymine. Finally, the formation of thymine 6 via the oxidation of 5 (third and last step of Route 2) was found to be slightly favored over that of its isomer 4,6-dihydroxy-5-methylpyrimidine 11 by 2.0 kcal mol−1 after the oxidation of 5 (Fig. 3c), which corresponds to the third and last step of Route 2. Thus, while thymine can in principle form via this route, it is not expected to be as efficient as Route 1, in part due to the fact that it is not easy to add a methyl group to the pyrimidine and due to the possibility of cleaving of the added methyl group by subsequent irradiation or oxidation of the added methyl group.
Figure 3.
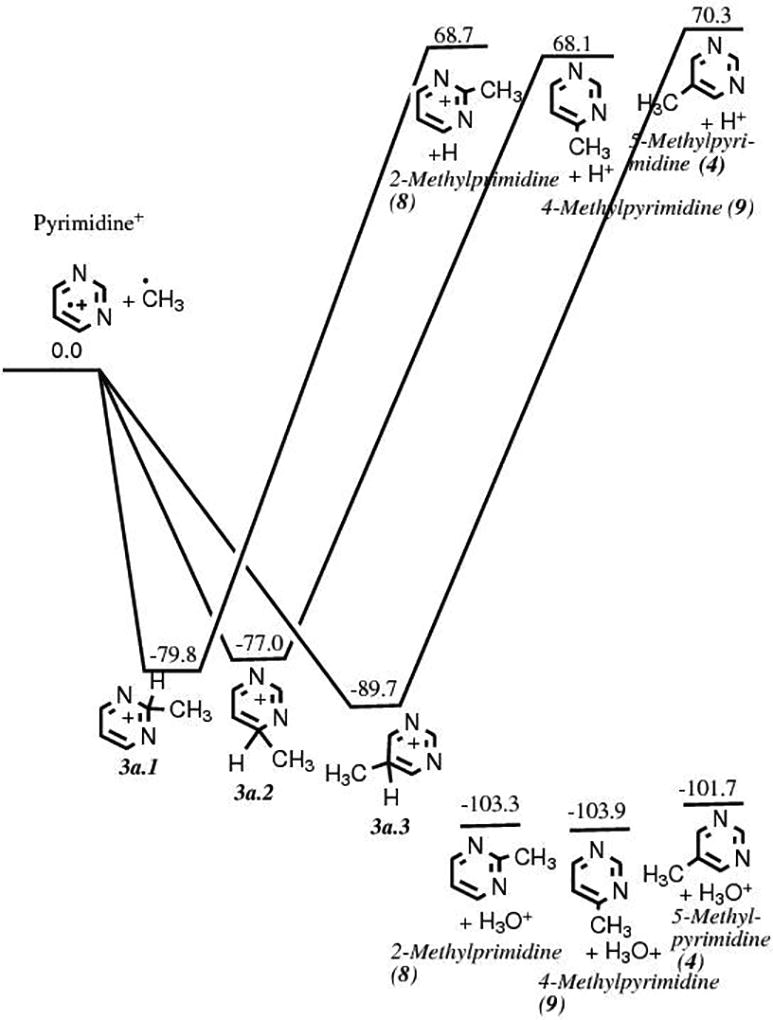
a. Energy diagram showing the energies (in kcal mol−1) and structures of the reactants and products for the methylation of pyrimidine via an ionic mechanism, corresponding to the first step of the formation mechanism illustrated in Route 2 (Fig. 1).
b. Energy diagram showing the energies (in kcal mol−1) and structures of the reactants and products for the oxidation of 5-methylpyrimidine 4 via an ionic mechanism, corresponding to the second step of the formation mechanism illustrated in Route 2 (Fig. 1).
c. Energy diagram showing the energies (in kcal mol−1) and structures of the reactants for the oxidation of 5-methyl-4(3H)-pyrimidone 5, corresponding to the third step of the formation mechanism illustrated in Route 2 (Fig. 1).
Route 3
Alternatively, methylation can also take place on a singly oxidized intermediate, most probably 4(3H)-pyrimidone 2.1,31 The formation of 2 from the oxidation of pyrimidine was studied and published earlier.31 Computations indicate that the formation of 5-methyl-4(3H)-pyrimidone 5 from 2, which is the desired product toward the formation of thymine via the cationic mechanism, is slightly less favored than its isomers 2-methyl-4(3H)-pyrimidone 12 and 6-methyl-4(3H)-pyrimidone 13 by 1.0 and 0.5 kcal mol−1, respectively (Fig. 4). Although all three products might be formed, experimentally none of them were detected in the laboratory studies.11 Finally, as for Route 2, the oxidation of cationic 5 slightly favors the formation of thymine 6 over its isomer 11 with the same energy difference (Fig. 3c). While theoretically possible, the formation of thymine via Route 3 would be much less efficient compared to Route 1 or Route 2.
Figure 4.
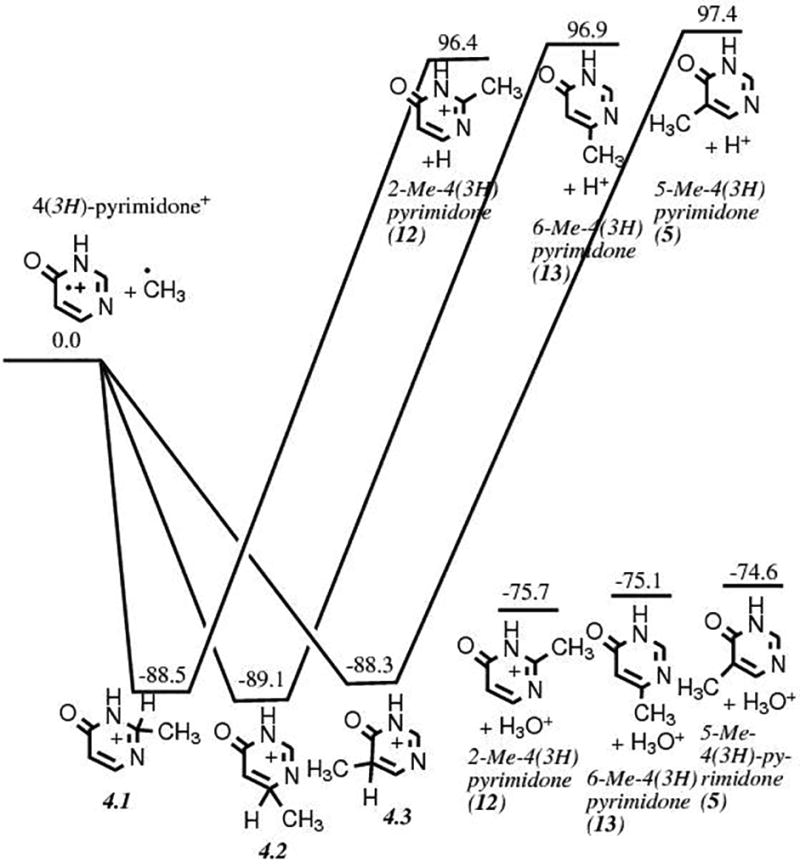
Energy diagram showing the energies (in kcal mol−1) and structures of the reactants for the methylation of 4(3H)-pyrimidone 2, corresponding to the second step of the formation mechanism illustrated in Route 3 (Fig. 1).
B. The role of methylation
Our results suggest that methylation is the limiting step in the chemical pathways studied here towards the formation of thymine from pyrimidine in irradiated H2O-rich ices. Although methylation was experimentally shown to be more efficient when ices are subjected to a higher UV photon dose, such a high UV dose can also lead to the photo-destruction of methyl-bearing pyrimidine derivatives.11 Methylation is therefore significantly less efficient than oxidation, which takes place rapidly and efficiently for any photon dose (low or high).1,29
Consequently, the main reason why thymine does not efficiently form via the UV irradiation of pyrimidine in H2O+CH3OH and H2O+CH4 ices may be because methylation cannot compete with oxidation when reactions take place in an H2O ice matrix. Indeed, the presence of OH radicals in the matrix, which outnumber CH3 radicals, will oxidize pyrimidine and its derivatives.
C. The role of water
The presence of H2O in the ices has thus two contradictory effects for methylation: (i) it catalyzes the reactions by playing the role of a proton acceptor to assist the formation of final, stable products, including methyl-bearing pyrimidine derivatives, but (ii) at the same time it releases OH radicals into the matrix which oxidize pyrimidine and its derivatives, and inhibits methylation.
Further, while explicitly exploring the pathway leading to thymine from uracil, we experienced the role the water matrix plays in this process. The first step in Fig. 2 is a barrierless addition of a radical cation and a neutral radical. The second step is a loss of a proton assisted by water molecule(s). We showed in figure 2 from purely thermodynamic arguments that the reaction is energetically favorable only in presence of H2O molecules. But the question remains of the kinetic viability of such a proton abstraction process. By explicitly involving one and three water molecules in this process we find the following. When only one water molecule is included the ensuing complex between 2.2 (Fig. 2) and H2O [2.2-H2O] is a minimum in which the water is loosely attached with the leaving hydrogen atom, and is lower in energy compared to the combined energy of separated 2.2 and H2O. The forward reaction (leading to H3O+ plus thymine) sees an energy barrier of 19.6 kcal mol−1 above the combined energy of separated 2.2 and H2O. However, when three water molecules are arranged in a similar fashion as in H2O trimer around the proton,45 the energy barrier for proton transfer becomes a submerged barrier, i.e., the proton is transferred from 2.2 to the associated H2O trimer via a submerged barrier of −0.33 kcal mol−1, and the products thymine and protonated H2O trimer [H2O]3H+ are lower in energy compared to the combined energy of 2.2 and H2O trimer [H2O]3. The forward reaction, therefore, becomes kinetically favorable in the presence of at least three water molecules in this case, and with more water molecules the submerged barrier may disappear entirely.
In light of the results described in above, we propose that the most dominant pathway for the formation of thymine from pyrimidine in H2O+CH3OH and H2O+CH4 ices is Route 1 (Fig. 1), i.e., the double oxidation of pyrimidine to form uracil first, via the formation of 4(3H)-pyrimidone, followed by the methylation of uracil. Although this last reaction is the limiting step in this formation pathway, methylation of uracil is energetically allowed, and experiments showed that thymine can form in detectable amounts when the reactants are subjected to a sufficient photon dose.11
D. Astrophysical and astrobiological implications
In cold astrophysical environments such as dense molecular clouds, pyrimidine may condense on the surface of interstellar grains, embedded in a complex ice matrix consisting of H2O (dominant component) as well as other species including CH3OH, CO, CO2, CH4, NH3, and larger organic compounds.12–15 In addition, pyrimidine might be made in situ in the ices via radiation-induced substitution of carbon atoms in benzene with nitrogen from N-bearing ices such as NH3.16 In light of our previous experimental data11 combined with the present theoretical study, we suggest that thymine forms with a significantly smaller yield on cold grains than the two other pyrimidine-based nucleobases uracil and cytosine under the same physical and chemical conditions. This may explain why uracil has been detected in primitive meteorites, but thymine has not.5–7 The production yield of thymine may, however, increase in environments with a high UV radiation field. Such higher radiation doses, with up to 50 photons per molecule, are predicted by models in protostellar disks, where significant amounts of organic compounds are expected to be produced through the irradiation of mixed molecular ices (models suggest that ices in the protosolar disk may have received doses as high as 50 photons per molecule).46 Nonetheless, the lack of detection of thymine in meteorites suggests that the production of thymine in space is significantly less efficient compared to other pyrimidine-based compounds, simply because the reactions leading to its formation are not strongly favored in H2O ice-rich environments.
The absence of thymine in meteorites implies that it was only a minority compound in the inventory of organics that were delivered to the primitive Earth.47,48 The extraterrestrial delivery of such a uracil-rich, thymine-poor inventory may have influenced the initial emergence of an RNA world,49–51 in which RNA-based macromolecules (which require uracil but not thymine) mediated the function of self-replication and preceded the emergence of DNA (which requires thymine, but not uracil).
IV. Conclusions
We have explored computationally the formation of thymine from the successive addition of OH and CH3 groups to pyrimidine when UV irradiated in mixed molecular ices via three different routes using quantum chemistry computations. Assuming a gas phase environment the reactions are found to be deficient in forming the desired products, but becomes feasible in a micro-solvated environment. We show that all the steps leading to the formation of thymine are energetically favorable when they take place in an H2O-rich environment such as an ice matrix. H2O plays the role of a catalyst by assisting the proton extraction towards the formation of the final, stable products. However, H2O ice has also a detrimental effect on the formation of methyl-bearing pyrimidine derivatives in general, and thymine in particular, as the presence of OH radicals will favor oxidation of pyrimidine and its derivatives, and inhibit their methylation. Our calculations were compared to recent experiments of UV irradiation of pyrimidine in ices containing H2O and a methyl group donor, either CH3OH or CH4. In these experiments, the formation of thymine was shown to be inefficient, occurring only when ices were subjected to a photon dose higher than the dose needed to form detectable amounts of uracil and cytosine under the same conditions. Although more photons are required to add the third group to pyrimidine and form thymine, a higher dose may also contribute more efficiently to the destruction of newly formed products, including thymine itself.
If the delivery of extraterrestrial materials were important for the origin of life on Earth, then the role of thymine may have been limited because of its low formation yield. More specifically, the detection of uracil and the non-detection of thymine in meteorites indicate that thymine may not have played a significant part in the initial inventory of organics delivered to the early Earth. Such an imbalance between uracil and thymine might have affected the inventory of prebiotic compounds that were delivered to the early Earth, and favored the emergence of an RNA world over a DNA world.
Acknowledgments
Part of this material is based upon work supported by the National Aeronautics and Space Administration through the NASA Astrobiology Institute under Cooperative Agreement Notice NNH13ZDA017C issued through the Science Mission Directorate. The NASA ‘Origins of Solar Systems’ program, the NASA consortium ‘Carbon in the galaxy’ grant, and the NASA Postdoctoral Programs are gratefully acknowledged. M.N., C.K.M., and S.A.S. would like to acknowledge Robert L. Walker (NASA Ames) for technical support.
References
- 1.Nuevo M, Milam SN, Sandford SA, Elsila JE, Dworkin JP. Astrobiology. 2009;9:683. doi: 10.1089/ast.2008.0324. [DOI] [PubMed] [Google Scholar]
- 2.Simon MN, Simon M. Astrophys. J. 1973;184:757. [Google Scholar]
- 3.Kuan Y-J, Charnley SB, Huang H-C, Tseng W-L, Kisiel Z. Monthly Not. R. Astron. Soc. 2003;345:650. [Google Scholar]
- 4.Charnley SB, Kuan Y-J, Huang H-C, Botta O, Butner HM, Cox N, Despois D, Ehrenfreund P, Kisiel Z, Lee Y-Y, Markwick AJ, Peeters Z, Rodgers SD. Adv. Space. Res. 2005;36:137. [Google Scholar]
- 5.Folsome CE, Lawless J, Romiez M, Ponnamperuma C. Nature. 1971;232:108. doi: 10.1038/232108a0. [DOI] [PubMed] [Google Scholar]
- 6.Stokes PG, Schwartz AW. Nature. 1979;282:709. [Google Scholar]
- 7.Callahan MP, Smith KE, Cleaves HJ, II, Ruzicka J, Stern JC, Glavin DP, House CH, Dworkin JP. Proc. Natl. Acad. Sci. USA. 2011;108:13995. doi: 10.1073/pnas.1106493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins Z, Botta O, Fogel ML, Sephton MA, Glavin DP, Watson JS, Dworkin JP, Schwartz AW, Ehrenfreund P. Earth Planet. Sci. Lett. 2008;270:130. [Google Scholar]
- 9.Shapiro R. Proc. Natl. Acad. Sci. USA. 1999;96:4396. doi: 10.1073/pnas.96.8.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson KE, Robertson MP, Levy M, Miller SL. Orig. Life Evol. Biosph. 2001;31:221. doi: 10.1023/a:1010652418557. [DOI] [PubMed] [Google Scholar]
- 11.Materese CK, Nuevo M, Bera PP, Lee TJ, Sandford SA. Astrobiology. 2013;13:948. doi: 10.1089/ast.2013.1044. [DOI] [PubMed] [Google Scholar]
- 12.Gibb EL, Whittet DCB, Boogert ECB, Tielens AGGM. Astrophys. J. Suppl. Ser. 2004;151:35. [Google Scholar]
- 13.Dartois E. Space Sci. Rev. 2005;119:293. [Google Scholar]
- 14.Sandford SA, Bernstein MP, Allamandola LJ. Astrophys J. 2004;607:346. doi: 10.1086/310376. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein MP, Sandford SA, Allamandola LJ. Astrophys J. Suppl. Ser. 2005;161:53. [Google Scholar]
- 16.Materese CK, Nuevo M, Sandford SA. Astrophys. J. 2015;800:116. doi: 10.1088/0004-637X/812/2/150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherchneff I, Barker JR, Tielens AGGM. Astrophys. J. 1992;401:516. [Google Scholar]
- 18.Ricca A, Bauschlicker CWJ, Bakes ELO. Icarus. 2001;154:516. [Google Scholar]
- 19.Hamid AM, Bera PP, Lee TJ, Aziz S, Al-Youbi A, El-Shall M. J. Phys. Chem. A. 2014;5:3392. doi: 10.1021/jz501648q. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein MP, Dworkin JP, Sandford SA, Cooper GW, Allamandola LJ. Nature. 2002;416:401. doi: 10.1038/416401a. [DOI] [PubMed] [Google Scholar]
- 21.Munoz Caro GM, Meierhenrich UJ, Schutte WA, Barbier B, Arcones Segovia A, Rosenbauer H, Thiemann WH-P, Brack A, Greenberg JM. Nature. 2002;416:403. doi: 10.1038/416403a. [DOI] [PubMed] [Google Scholar]
- 22.Nuevo M, Auger G, Blanot D, d'Hendecourt L. Orig. Life Evol. Biosph. 2008;38:37. doi: 10.1007/s11084-007-9117-y. [DOI] [PubMed] [Google Scholar]
- 23.Dworkin JP, Deamer DW, Sandford SA, Allamandola LJ. Proc. Natl. Acad. Sci. USA. 2001;98:815. doi: 10.1073/pnas.98.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuevo MB, H J, Meierhenrich UJ, d’Hendecourt L, Thiemann WH-P. Astrobiology. 2010;11:245. doi: 10.1089/ast.2009.0358. [DOI] [PubMed] [Google Scholar]
- 25.de Marcellus, Bertrand MP, Nuevo M, Westall F, d’Hendecourt L. Astrobiology. 2011;11:847. doi: 10.1089/ast.2011.0677. [DOI] [PubMed] [Google Scholar]
- 26.Cronin JR, Pizzarello S. Science. 1997;275 doi: 10.1126/science.275.5302.951. [DOI] [PubMed] [Google Scholar]
- 27.Cooper G, Kimmich N, Belisle W, Sarinana J, Brabham K, Garrel L. Naturew. 2001;414:879. doi: 10.1038/414879a. [DOI] [PubMed] [Google Scholar]
- 28.Sephton MA. Nat. Prod. Rep. 2002;19:292. doi: 10.1039/b103775g. [DOI] [PubMed] [Google Scholar]
- 29.Nuevo M, Milam SN, Sandford SA. Astrobiology. 2012;12:295. doi: 10.1089/ast.2011.0726. [DOI] [PubMed] [Google Scholar]
- 30.Nuevo M, Materese CK, Sandford SA. Astrophys. J. 2014;793:125. doi: 10.1088/0004-637X/812/2/150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bera PP, Nuevo M, Milam SN, Sandford SA, Lee TJ. J. Chem. Phys. 2010;133:104303. doi: 10.1063/1.3478524. [DOI] [PubMed] [Google Scholar]
- 32.Mordaunt DH, Dixon RN, Ashfold MNR. J. Chem. Phys. 1996;104:6472. [Google Scholar]
- 33.Woon D. Astrophys. J. 2002;571:177. [Google Scholar]
- 34.Bera PP, Schaefer HF. Proc. Natl. Acad. Sci. 2005;102:6698. doi: 10.1073/pnas.0408644102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bera PP, Schaefer HF. Trends and Perspectives in Modern Computational Science. Vol. 6. Brill Academic Publishers; 2006. p. 254. [Google Scholar]
- 36.Bera PP, Schaefer HF. AIP Conference Proceedings. Vol. 963. American Institute of Physics; Corfu(Greece): 2007. p. 10. [Google Scholar]
- 37.Lind MC, Bera PP, Richardson NA, Wheeler SE, Schaefer HF. Proc. Natl. Acad. Sci. 2006;103:7554. doi: 10.1073/pnas.0600654103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandford SA, Bera PP, Materese CK, Nuevo M, Lee TJ. Topics Curr. Chem. 2014;356:123. doi: 10.1007/128_2013_499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becke AD. J. Chem. Phys. 1993;98:5648. [Google Scholar]
- 40.Lee CT, Yang WT, Parr RG. Phys. Rev. B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 41.Lee TJ, Jayatilaka D. Chem. Phys. Lett. 1993;201:1. [Google Scholar]
- 42.Lee TJ, Rendell AP, Dyall KG, Jayatilaka D. J. Chem. Phys. 1994;100:7400. [Google Scholar]
- 43.Jayatilaka D, Lee TJ. Chem. Phys. Lett. 1992;199:211. [Google Scholar]
- 44.Shao Y, Molnar LF, Jung Y, Kussmann J, Ochsenfeld C, Brown ST, Gilbert ATB, Slipchenko LV, Levchenko SV, O'Neill DP, DiStasio RA, Lochan RC, Wang T, Beran GJO, Besley NA, Herbert JM, Lin CY, Van Voorhis T, Chien SH, Sodt A, Steele RP, Rassolov VA, Maslen PE, Korambath PP, Adamson RD, Austin B, Baker J, Byrd EFC, Dachsel H, Doerksen RJ, Dreuw A, Dunietz BD, Dutoi AD, Furlani TR, Gwaltney SR, Heyden A, Hirata S, Hsu CP, Kedziora G, Khalliulin RZ, Klunzinger P, Lee AM, Lee MS, Liang W, Lotan I, Nair N, Peters B, Proynov EI, Pieniazek PA, Rhee YM, Ritchie J, Rosta E, Sherrill CD, Simmonett AC, Subotnik JE, Woodcock HL, Zhang W, Bell AT, Chakraborty AK, Chipman DM, Keil FJ, Warshel A, Hehre WJ, Schaefer HF, Kong J, Krylov AI, Gill PMW, Head-Gordon M. Phys. Chem. Chem. Phys. 2006;8:3172. doi: 10.1039/b517914a. [DOI] [PubMed] [Google Scholar]
- 45.Hodges MP, Wales DJ. Chem. Phys. Lett. 2000;324:279. [Google Scholar]
- 46.Ciesla FJ, Sandford SA. Science. 2012;336:452. doi: 10.1126/science.1217291. [DOI] [PubMed] [Google Scholar]
- 47.Oro J. Nature. 1961;190:389. doi: 10.1038/190442a0. [DOI] [PubMed] [Google Scholar]
- 48.Chyba CF, Sagan C. Nature. 1992;355:125. doi: 10.1038/355125a0. [DOI] [PubMed] [Google Scholar]
- 49.Gilbert W. Naturew. 1986;319:618. [Google Scholar]
- 50.Joyce GF. Nature. 1989;338:217. doi: 10.1038/338217a0. [DOI] [PubMed] [Google Scholar]
- 51.Joyce GF. Nature. 2002;418:214. doi: 10.1038/418214a. [DOI] [PubMed] [Google Scholar]


