Abstract
Chronic HIV infection due to effective antiretroviral treatment has resulted in a broad range of clinical complications, including accelerated heart disease. Individuals with HIV infection have a 1.5 to 2 times higher incidence of cardiovascular diseases than their uninfected counterparts; however, the underlying mechanisms are poorly understood. To explore the link between HIV infection and cardiovascular diseases, we used postmortem human heart tissues obtained from HIV-infected and control uninfected individuals to examine connexin 43 (Cx43) expression and distribution and HIV-associated inflammation. Here, we demonstrate that Cx43 is dysregulated in the hearts of HIV-infected individuals. In all HIV heart samples analyzed, there were areas where Cx43 was overexpressed and found along the lateral membrane of the cardiomyocyte and in the intercalated disks. Areas of HIV tissue with anomalous Cx43 expression and localization also showed calcium overload, sarcofilamental atrophy, and accumulation of collagen. All these changes were independent of viral replication, CD4 counts, inflammation, and type of antiretroviral treatment. Overall, we propose that HIV infection increases Cx43 expression in heart, resulting in tissue damage that likely contributes to the high rates of cardiovascular disease in HIV-infected individuals.
HIV/AIDS represents a growing concern with an estimated 36 million cases worldwide and 1.2 million in the United States. The introduction of effective antiretroviral treatment (ART) has led to increased life span of HIV-infected (HIV+) individuals almost becoming a chronic disease. However, the prevalence of cardiovascular disease or cardiomyopathy in the HIV-infected population is 1.5 to 2 times higher than in the uninfected (HIV−) population1, 2, 3, 4 and may be due to chronic inflammation, hypertension, endothelial dysfunction, insulin resistance, toxicity of antiretroviral drugs, and dyslipidemia.5, 6, 7 HIV cardiomyopathy is characterized by loss of cardiomyocytes and their replacement by fibrous tissue and infiltration of immune cells.8, 9, 10 However, the pathogenesis of heart disease in HIV+ individuals remains largely unknown. We propose that the dysregulation of connexin 43 (Cx43) channels play a key role in the pathogenesis of HIV heart disease.
Gap junctions (GJs) allow the exchange of ions, metabolites, second messengers, and small RNAs and serve as low resistance bridges between coupled cells that enable action potential propagation in excitable tissues and coordination of metabolism.11 In the healthy adult heart, Cx43 is abundantly expressed in the myocardium and is mainly located in GJs at the intercalated disks. Alterations in Cx43 expression and/or subcellular distribution in the heart have been associated with several diseases, including hypertension, infarction, ischemia, hypertrophic cardiomyopathy, atrial fibrillation, and arrhythmias.12, 13, 14, 15, 16, 17 However, the effect of HIV on Cx43 expression and distribution in the heart has not been examined. Recently, we described that HIV-tat protein, the trans-activator of the virus, binds to the Cx43 promoter in human astrocytes and enhances Cx43 expression, thereby increasing gap junctional communication and likely the number of open hemichannels.18 It is unknown whether the same mechanism of Cx43 up-regulation occurs in cardiomyocytes. Here, we report that Cx43 is overexpressed and mis-localized in all postmortem heart tissue samples analyzed from HIV-infected individuals. These changes in Cx43 were independent of CD4 counts, HIV replication status, and ART. Furthermore, areas with dysregulated Cx43 expression and distribution also have signs of calcium overload, mitochondrial proliferation, muscle compromise, and fibrosis. Thus, we propose that overexpressed Cx43 is a main contributor to the increased heart disease observed in HIV-infected individuals.
Materials and Methods
Study Participants
Donors were recruited by National NeuroAIDS Tissue Consortium (NNTC) as part of a National Institute of Mental Health and National Institute of Neurological Disorders and Stroke program to collect, store, and distribute samples obtained from HIV+ and HIV− individuals. Uninfected and HIV-infected individuals were uniformly distributed among age and comorbidities (Table 1 and data not shown). The only uneven characteristic of our population was sex, most of our uninfected and HIV-infected samples were obtained from men. In addition, the population analyzed had a broad spectrum of CD4 counts, plasma viral load, ART, and length of HIV infection. The uninfected population analyzed was cognitively normal. In contrast, 49% of the HIV-infected population was neurocognitive normal (28 cases), 18% cognitive impaired (10 cases), 28% was cognitive impaired to other or uncertain cause (16 cases), and 5% was unable to reliably assign neurocognitive diagnostic (3 cases). These data indicated that even HIV-infected individuals had normal cognition, yet they may have significant alterations in heart disease as demonstrated below.
Table 1.
Patient Information
| Patient No. | HIV status | Age, years | Sex | ART current ARVs (2 years of death) | CD4 counts, cells/mm | Viral load, copies/mL | Time with HIV, years |
|---|---|---|---|---|---|---|---|
| 1 | + | 45 | M | 3TC, ABC, APV, CBV, D4T, DDC, DDI, DLV, EFV, HU, IDV, NFV, NVP, RTV, SQV, ZDV | 25 | 124,380 | 13 |
| 2 | + | 58 | M | 3TC, ABC, APV, DDI, IDV, NVP, ZDV | 87 | 3806 | 9 |
| 3 | + | 24 | M | NR | 141 | NR | <1 |
| 4 | + | 61 | M | 3TC, RTV, SQV, TFV | 69 | 40 | 28 |
| 5 | + | 23 | M | 3TC, DDI, NVP | 4 | 200 | 1 |
| 6 | + | 55 | M | 3TC | 3 | 240,337 | 7 |
| 7 | + | 58 | M | 3TC, ABC, EFV, KTA, RTV | 72 | 279 | <1 |
| 8 | + | 43 | M | ABC, KTA, NVP, RTV, TFV | 12 | 90,258 | 16 |
| 9 | + | 47 | M | 3TC, KTA, NFV, NVP, RTV, TFV, ZDV | 199 | NR | 17 |
| 10 | + | 52 | M | NR | NR | NR | 16 |
| 11 | + | 51 | M | FTC, TFV | 34 | 605,555 | 19 |
| 12 | + | 56 | M | ATV, EFV, RTV, TFV | 365 | 25,669 | 16 |
| 13 | + | 50 | M | ND | 253 | 400 | NR |
| 14 | + | 44 | M | Yes, NR | NR | NR | 23 |
| 15 | + | 52 | M | NR | 630 | 40 | 23 |
| 16 | + | 49 | M | 3TC, ABC, KTA, RTV, TFV, TZV, ZDV | 273 | 400 | 10 |
| 17 | + | 44 | M | NR | 78 | 3484 | 4 |
| 18 | + | 64 | M | DRV, EPZ, RTV | 299 | 40 | 18 |
| 19 | + | 39 | M | D4T, DDI, NFV | NR | 400 | 7 |
| 20 | + | 35 | M | ABC, APV, D4T | 40 | 250,000 | 13 |
| 21 | − | 69 | M | NA | NA | NA | NA |
| 22 | − | 38 | M | NA | NA | NA | NA |
| 23 | − | 51 | F | NA | NA | NA | NA |
| 24 | − | 54 | M | NA | NA | NA | NA |
| 25 | − | 66 | M | NA | NA | NA | NA |
| 26 | − | 74 | F | NA | NA | NA | NA |
| 27 | + | 42 | M | 3TC, D4T, FTV, SQV | 101 | 400 | 5 |
| 28 | + | 30 | M | APV, CBV, NVP | 115 | 63,909 | 9 |
| 29 | + | 49 | M | NR | 14 | 651,401 | <1 |
| 30 | + | 60 | M | NR | 24 | 61,223 | 10 |
| 31 | + | 47 | M | DRV, RTV, TRU | 61 | 48 | 20 |
| 32 | + | 60 | M | ATR | 398 | ND | 11 |
| 33 | + | 46 | M | 3TC, ATR, CBV, EFV, KTA, NFV, RTV | 22 | ND | 7 |
| 34 | + | 59 | M | 3TC, CBV, D4T, NVP, TRU | 158 | 40 | 13 |
| 35 | + | 60 | M | EPZ, KTA | 497 | 48 | 6 |
| 36 | + | 54 | M | 3TC, ABC, ATR, ATV, CBV, DRV, EPZ, KTA, NFV, RTV, TRU | 384 | 40 | 12 |
| 37 | + | 71 | M | 3TC, ATV, KTA, RTV, TRU, ZDV | 403 | 40 | 16 |
| 38 | + | 61 | M | NR | 315 | NR | 20 |
| 39 | + | 39 | M | DRV, KTA, RTV, TRU | 112 | 40 | 15 |
| 40 | + | 31 | M | NR | NR | 16,032 | 2 |
| 41 | + | 34 | M | NR | NR | 54,148 | 6 |
| 42 | + | 64 | M | 3TC, D4T | 96 | 2064 | 12 |
| 43 | + | 50 | M | TZV | 256 | 400 | 15 |
| 44 | + | 40 | M | NR | NR | NR | 5 |
| 45 | + | 39 | M | 3TC, ABC, D4T, EFV, IDV, KTA, NVP | 13 | 75,000 | 14 |
| 46 | + | 59 | M | 3TC, DDC, DLV, EFV, TFV, ZDV | 32 | 400 | 12 |
| 47 | + | 37 | M | NR | NR | 30,769 | 9 |
| 48 | + | 43 | M | NR | NR | NR | NR |
| 49 | + | 29 | F | NR | NR | NR | 10 |
| 50 | + | 37 | M | NR | 24 | NR | 5 |
| 51 | + | 35 | M | ATV, FTC, RTV, TFV | NR | NR | 11 |
| 52 | + | 52 | M | 3TC, D4T, NVP | 41 | 400 | 7 |
| 53 | + | 34 | M | ATV, RTV, TRU | 24 | 322 | 6 |
| 54 | + | 58 | M | NR | 5 | 505,903 | 23 |
| 55 | + | 52 | M | RTV, SQV, TRU | 317 | 500 | 10 |
| 56 | + | 57 | M | KTA, TRU | 518 | 50 | 17 |
| 57 | − | 83 | M | NA | NA | NA | NA |
| 58 | + | 25 | M | NR | NR | NR | NR |
| 59 | + | 46 | M | NR | NR | NR | NR |
| 60 | + | 51 | M | NR | NR | NR | 15 |
| 61 | − | 53 | F | NA | NA | NA | NA |
| 62 | + | 70 | M | EPZ, TMC | NR | <50 | NR |
| 63 | − | 58 | M | NA | NA | NA | NA |
| 64 | − | 43 | M | NA | NA | NA | NA |
| 65 | + | 59 | M | NR | 32 | 83,804 | <1 |
| 66 | + | 54 | F | EFV, TRU | 156 | 50 | NR |
| 67 | − | 63 | M | NA | NA | NA | NA |
| 68 | + | 46 | M | NR | 61 | 50 | 17 |
| 69 | − | 42 | F | NA | NA | NA | NA |
F, female; M, male; 3TC, lamivudine (Epivir); ABC, abacavir (Ziagen); APV, amprenavir (Agenerase); ART, antiretroviral treatment; ARV, antiretroviral; ATR, efavirenz 600 mg + emtricitabine 200 mg + tenofivir DF 300 mg (Atripla); ATV, atazanavir (Reyataz); CBV, zidovudine + lamivudine (Combivir); D4T, stavudine (Zerit); DDC, zalcitabine (Hivid); DDI, didanosine (Videx); DLV, delaviridine (Rescriptor); DRV, TMC-114, darunavir (Prezista); EFV, efavirenz (Sustiva); EPZ, ziagen + epivir (Epzicom); FTC, emtricitabine (Coviracil; Emtriva); FTV, SQV2, saquinavir-sgc (Fortovase); HU, hydroxyurea; IDV, indinavir (Crixivan); KTA, LPV/RTV, lopinavir/ritonavir (Kaletra); NA, not applicable; ND, not determined; NFV, nelfinavir (Viracept); NR, not recorded; NVP, nevirapine (Viramune); RTV, ritonavir (Norvir); SQV, saquinavir (Invirase); TFV, tenofivir DF (Viread); TMC, TMC-125, etravirine (Intelence); TRU, emtricitabine + tenofovir (Truvada); TZV, AZT + 3 TC + abacavir; zidovudine + lamivudine + abacavir (Trizivir); ZDV, zidovudine (alias AZT; Retrovir).
Immunofluorescence and Confocal Microscopy
Tissue sections were incubated in blocking solution for 2 hours at room temperature and then in diluted primary antibody (anti-pan Cx43: dilution 1:2000, Sigma C6219; Sigma-Aldrich, St. Louis, MO; or anti–N-cadherin: dilution 1:250, BD 61092; Becton Dickinson, Franklin Lakes, NJ) overnight at 4°C. Tissues were washed several times with phosphate-buffered saline at room temperature and incubated with the appropriate secondary antibody for at least 2 hours at room temperature, followed by another wash in phosphate-buffered saline. Tissues were examined using an A1 Nikon confocal microscope (Nikon, Tokyo, Japan). Antibody specificity was confirmed by replacing the primary antibody with a nonspecific myeloma protein of the same isotype or nonimmune serum as previously described.19, 20 The expression level and distribution of the target protein were analyzed using the three-dimensional reconstructions and deconvolution, followed by the generation of regions of interest. With the use of the corresponding regions of interest the numbers of positive pixels for the whole deconvoluted three-dimensional construction were calculated. The expression measurement was determined by quantifying the intensity of positive pixels for Cx43 and lipofuscin. To compare control and HIV conditions, a similar number of cells (300 to 800 cells) and total area were analyzed. This analysis avoids usual problems of this kind of experiment, such as changes in cell number or excessive inflammation. Details of the procedure, including deparaffinization, antigen retrieval, blocking, immunohistochemical staining with primary and secondary antibodies, and specificity testing, were previously described.19, 21
RNA Isolation and Quantitative RT-PCR
Total RNA was isolated from 10-mm3 pieces of ventricular heart using RNeasy plus universal tissue mini kit from Qiagen (Hilden, Germany) according to the manufacturer's instructions and quantified using a Nanodrop (Thermo Scientific, Wilmington, DE). Synthesis of cDNA was performed using iScript cDNA synthesis kit according to the manufacturer's instructions (Bio-Rad, Hercules, CA). Relative mRNA expression of Cx43 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was determined using Sybr green kits (Life Technologies, Carlsbad, CA). Results are represented as a relative expression of Cx43 normalized to that of GAPDH as a housekeeping gene. The ΔCt value was determined by subtracting the average Ct of the housekeeping gene from the average Ct of the Cx43 gene (official name GJA1). Expression of Cx43 mRNA was compared between the uninfected and HIV-infected individuals and analyzed by paired two-tailed t-test.
Western Blot Analysis
Small ventricular heart pieces were homogenized in RIPA buffer (Cell Signaling, Beverly, MA) containing protease inhibitors (Cell Signaling, Danvers, MA), and 40 μg of protein was electrophoresed on a 10% polyacrylamide gel (Bio-Rad) and transferred to nitrocellulose membranes. Membranes were probed with rabbit antibodies to connexin43 (Sigma-Aldrich) and GAPDH (Santa Cruz Technologies, Carlsbad, CA). Densitometric analysis was performed using ImageJ software version 1.51j8 (NIH, Bethesda, MD).
Von Kossa Staining
Human heart tissue frozen sections were fixed in cold ethanol for 15 to 20 minutes. After rinsing, the fixed tissues were incubated with 5% silver nitrate solution (ab150687; Abcam, Cambridge, UK) and cross-linked under UV light for 30 minutes and then rinsed twice with distilled water. The tissue sections were incubated with (5%) sodium thiosulfate solution for 5 minutes, rinsed twice with distilled water, incubated with Nuclear Fast Red solution for 5 minutes, and rinsed three times with distilled water to remove excess stain. Serial sections were used to identify areas with compromised Cx43 expression.
Electron Microscopy
Fresh postmortem samples were fixed with 2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 mol/L sodium cacodylate buffer, postfixed in 1% osmium tetroxide, followed by 2% uranyl acetate, dehydrated through a graded series of ethanol, and embedded in LX112 resin (LADD Research Industries, Burlington, VT). Ultrathin sections were cut on a Reichert Ultracut UCT, stained with uranyl acetate, followed by lead citrate, and viewed on a JEOL 1200EX transmission electron microscope at 80 kv.
Statistical Analysis
Statistical analyses were performed using Origin Lab software version 7.2 (OriginLab Corporation, Northampton, MA). Analysis of variance was used to compare the different groups.
Results
Description of Our Cohort of Uninfected and HIV-Infected Individuals
Our studies were focused on the regulation of cardiac Cx43 in HIV-infected individuals using postmortem human ventricular cardiac tissue obtained from 69 individuals (12 uninfected and 57 HIV-infected) as described in Table 1.
Our uninfected population was eight men and four women with a mean age of 57.8 ± 13.7 years. Our HIV-infected population was 55 men (96.5%) and two women (3.5%) with a mean age of 47.7 ± 11.5 years and mean duration of HIV infection of 11.5 ± 6.7 years, 6.89 ± 1.57 × 104 HIV RNA copies/mL, and 157.3 ± 164.61 CD4 cells/mm3 (Table 1). At the time of death, most of the HIV+ individuals were on ART that included protease inhibitors (Table 1), which have been reported to have intrinsic endothelial toxicity.22, 23 Most of the individuals whose cardiac tissue was analyzed had been followed by the National NeuroAIDS Tissue Consortium (Table 1).
HIV-Infected Individuals Have Increased Expression and Mislocalization of Cx43
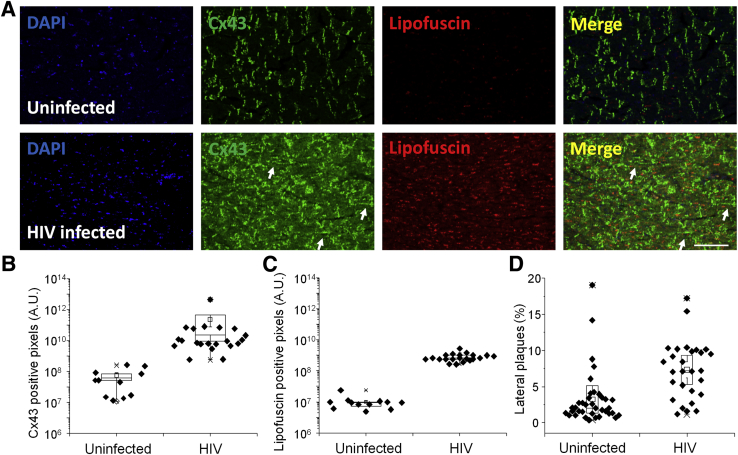
Ventricular tissue sections were stained for Cx43 to analyze expression and distribution by confocal microscopy. Normally, Cx43 mainly localizes at contacts between myocytes at the intercalated disks.16, 24, 25, 26, 27 Cx43 containing GJs form a low resistance bridge between cardiomyocytes that mediate action potential propagation and metabolic communication along the muscle fiber. Changes in expression and localization of GJs underlie several forms of heart disease.8, 12, 14, 16, 26, 28, 29, 30, 31, 32, 33, 34, 35 Our data confirmed that Cx43 in the heart from uninfected individuals was mainly localized at the intercalated disks as expected (n = 12) (Figure 1A). However, in all HIV-infected individuals analyzed, irrespective of HIV replication status, CD4 counts, and antiretroviral used, Cx43 staining was increased compared with uninfected individuals (n = 57; P = 0.001; HIV compared with uninfected samples) (Figure 1, A and B). Furthermore, the increased Cx43 in hearts of HIV-infected individuals was distributed predominantly along the lateral margins of the muscle fibers instead of at the intercalated disks (P = 0.05) (Figure 1, A and D). Lateralization of Cx43 is associated with several heart diseases such as ischemia, reperfusion injury, and arrhythmias.8, 12, 14, 16, 26, 28, 29, 30, 31, 32, 33, 34, 35 Interestingly, increased expression of Cx43, its lateralization, and lipofuscin accumulation was present in all HIV-infected individuals analyzed (P = 0.0003) (Figure 1, A and C), suggesting chronic heart damage. Lipofuscin accumulation is also associated with accelerated aging (Figure 1, A and C). Thus, we propose that increased Cx43 expression and lateralization of the protein in HIV-infected individual results in damage to the heart.
Figure 1.
Expression of connexin 43 (Cx43) in human hearts is up-regulated in HIV-infected individuals compared with heart tissues obtained from uninfected individuals. Postmortem ventricular heart tissue was obtained at the time of autopsy from uninfected and HIV-infected individuals (HIV+) as described in Table 1. Because of the limited numbers of uninfected hearts, two to three distinct areas were quantified. A: Immunohistochemistry was performed using paraffin-embedded and fresh tissues and analyzed by confocal microscopy. Representative tissue staining for nuclei (DAPI), Cx43 (fluorescein isothiocyanate), and lipofuscin (red) in uninfected and HIV-infected individuals. Arrows indicate the Cx43 lateralization in the tissues. Cx43 was up-regulated at the intercalated disks and in the lateral membrane of the cardiomyocytes in all individuals analyzed. B: Quantification of Cx43-positive pixels in human ventricular tissue sections from uninfected and HIV-infected individuals. C: Quantification of the total number of lipofuscin-positive pixels was obtained in uninfected and HIV-infected individuals. D: Quantification of Cx43 containing lateral plaques in cardiac ventricular tissue sections obtained from uninfected and HIV-infected individuals. Data are expressed as means ± SD. n = 69 samples (A); n = 3 tissue sections per individual (B and D); n = 2 tissue sections per individual (C). Scale bar = 200 μm. A.U., arbitrary unit.
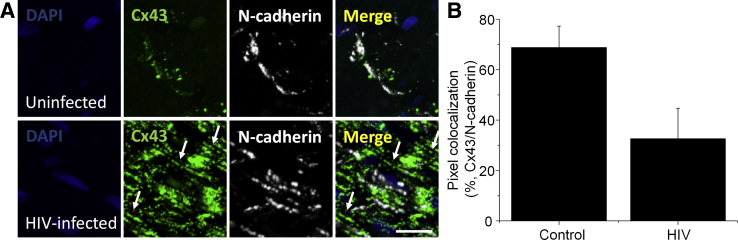
To confirm that HIV infection induced the lateralization of Cx43 in cardiomyocytes, we stained for Cx43 and N-cadherin to better define the three-dimensional structure of the intercalated disk as previously reported.36 In uninfected conditions 68.8% ± 8.5% of Cx43 colocalized with N-cadherin at the intercalated disks (Figure 2). In the HIV-infected condition, only 32.65% ± 12.02% of Cx43 colocalized with N-cadherin, supporting the hypothesis that Cx43 was mis-localized into lateral membranes of the cardiomyocytes (P = 0.0007 versus uninfected tissues) (Figure 2).
Figure 2.
Lateralized connexin 43 (Cx43) does not colocalize with N-cadherin in heart tissues obtained from HIV-infected individuals. Postmortem ventricular heart tissue was used. Immunohistochemistry was performed using paraffin-embedded and fresh tissues and analyzed by confocal microscopy and three-dimensional reconstruction to examine colocalization of Cx43 and N-cadherin. A: Representative tissue staining for nuclei (DAPI), Cx43 (fluorescein isothiocyanate), and N-cadherin (cyanine 5) in uninfected and HIV-infected tissues. Arrows indicate lateralized Cx43 that also lacks staining for N-cadherin. In heart tissues obtained from uninfected individuals, most Cx43 localizes at the intercalated disk. In contrast, in heart tissues obtained from HIV-infected individuals, most Cx43 does not colocalize with N-cadherin and mainly localizes at the lateral membrane of the cardiomyocyte. B: Quantification of Cx43-positive pixels that colocalize with N-cadherin–positive pixels in HIV− (control) and HIV+ conditions. Data are expressed as means ± SD. Scale bar = 38 μm.
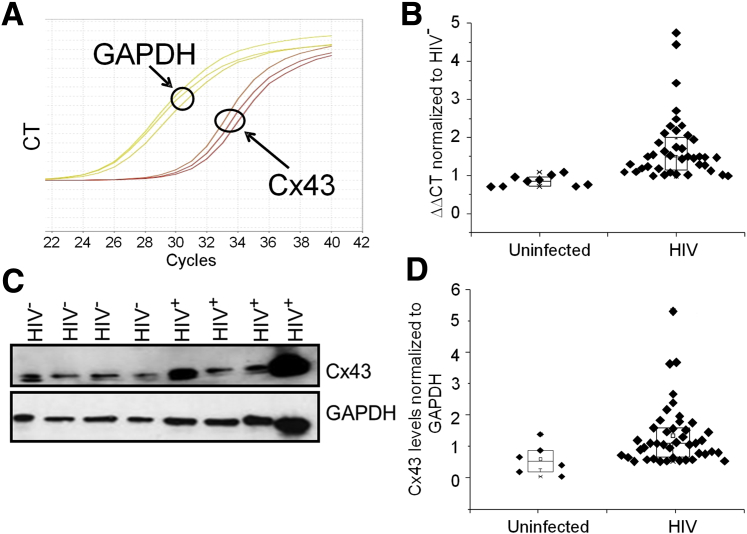
To further demonstrate that HIV infection increased the expression of Cx43 in heart tissues, we performed quantitative RT-PCR and Western blot analysis of cardiac tissue obtained from HIV patients as described in Table 1. Both Cx43 mRNA (n = 57; P = 0.03) (Figure 3, A and B) and protein (n = 12; P = 0.05) (Figure 3, C and D) were increased compared with heart tissue obtained from uninfected individuals. Our data confirmed the confocal data that Cx43 mRNA and protein increased in response to HIV infection in all cases analyzed. Further analysis of the HIV population described in Table 1 did not find any correlation between increased Cx43 (mRNA and protein) with type of antiretroviral treatment, CD4 counts, or viral load. The only feature that correlated with increased expression and localization of Cx43 was HIV infection.
Figure 3.
HIV infection increases expression of connexin 43 (Cx43) mRNA and protein in human ventricular heart tissue. A: Representative quantitative RT-PCR (RT-qPCR) amplifications for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Cx43. B: Quantification of the RT-qPCR experiments for tissues from uninfected and HIV-infected hearts. HIV infection (independent of antiretroviral treatment, CD4 counts, replication, and associated inflammation) increases Cx43 expression (mRNA and protein). Each point represents one individual. C: Representative Western blot analysis of Cx43 and GAPDH protein expression in HIV− and HIV+ hearts. GAPDH was used as a loading control; mouse astrocytes were used as a positive control for Cx43 and its phosphorylation (data not shown). D: Quantification of the Western blots for heart tissues obtained from uninfected and HIV-infected individuals. Each point represents one individual. Data are expressed as means ± SD. CT, cycle threshold.
Calcium Is Dysregulated in Ventricular Areas with Compromised Cx43 Expression and Localization
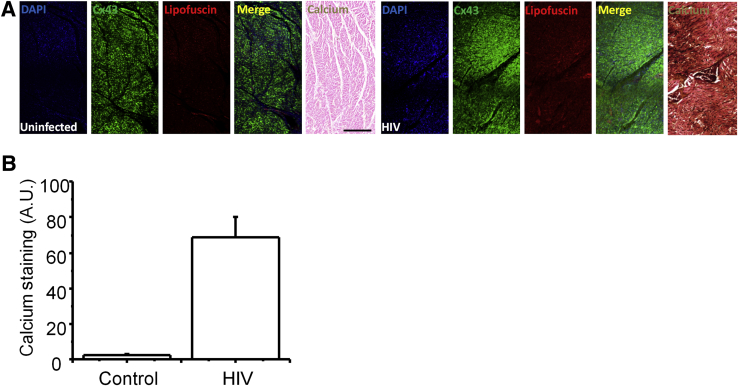
To examine whether changes in Cx43 localization and expression observed in heart tissue from HIV-infected individuals altered cardiomyocyte function, calcium metabolism was examined using Von Kossa staining.37, 38, 39 To perform these studies, serial tissue sections containing areas with normal and compromised Cx43 expression and localization were selected. Calcium normally diffuses through GJs31, 40, 41, 42 and is sequestered into the mitochondria and endoplasmic reticulum or is extruded from the cell.43, 44, 45, 46 In sections from uninfected individuals, Von Kossa staining was minimal, suggesting a normal calcium metabolism and diffusion (Figure 4A). In contrast, in tissue sections from HIV+ individuals different degrees of pathologic calcium accumulation were observed (Figure 4A). Calcium overload was particularly evident in areas with overexpressed and lateralized Cx43 and pronounced lipofuscin staining (Figure 4A). Quantification of the calcium staining indicated that HIV-infected tissues had an increased calcium staining compared with control uninfected tissues (n = 16; P = 0.005 versus uninfected samples) (Figure 4B).
Figure 4.
Areas with higher connexin 43 (Cx43) expression and mis-localization show calcium overload. Von Kossa staining to detect calcium deposits in heart sections from uninfected and HIV-infected individuals was performed. All these sections correspond to serial sections in which Cx43 was up-regulated or mis-localized. Sections stained for DAPI, Cx43, and lipofuscin (autofluorescence), and the merge, correspond to the same tissue section. The adjacent tissue section was stained for Von Kossa (labeled calcium). A: Staining for tissue sections obtained from uninfected individuals (uninfected) showing normal Cx43 distribution and low accumulation of lipofuscin with minimal calcium deposits. Staining for tissue sections obtained from HIV-infected individuals (HIV+) showing increased expression of Cx43 in several areas of the tissue and increased accumulation of lipofuscin also shows increased accumulation of calcium deposits in vast areas of the tissue (different degrees of brown-back staining). B: Quantification of calcium staining (dark staining) indicates an increase of 60% in Von Kossa staining. Data are expressed as means ± SD. n = 29 different individuals (A and B). Scale bar = 200 μm. A.U., arbitrary unit.
Ventricular Regions with Altered Cx43 Expression and Localization Show Muscle Compromise and Mitochondrial Proliferation Compared with Areas without Cx43 Dysregulation
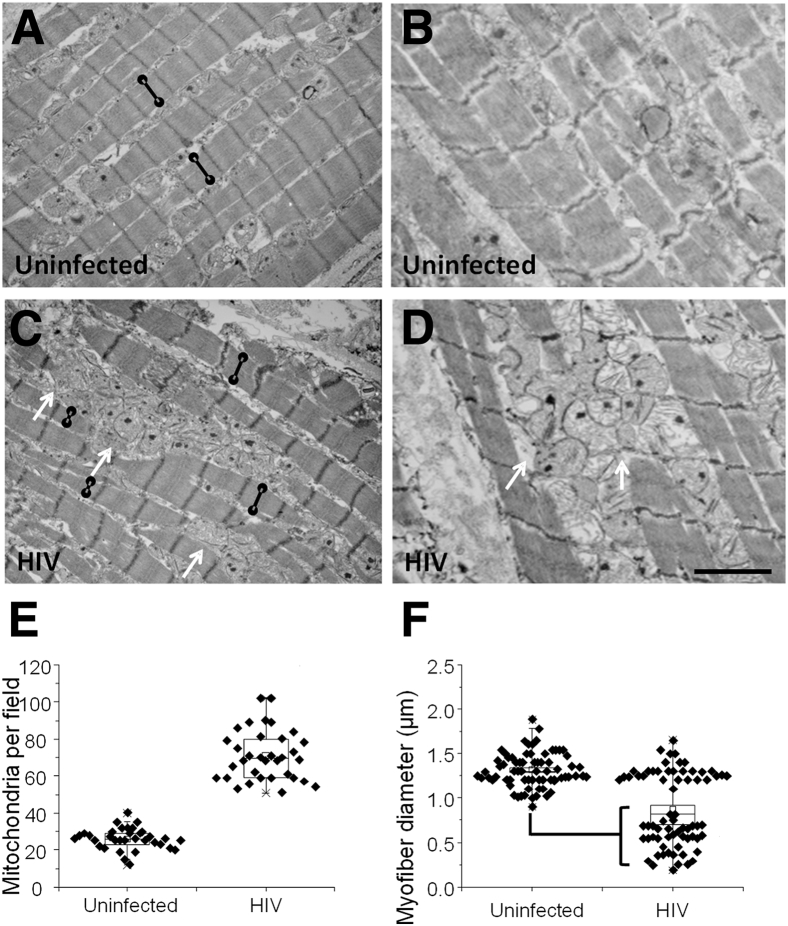
Transmission electron microscopy was used to further evaluate the damage in the heart tissue. Two representative examples each of heart tissues obtained from an uninfected and an HIV-infected individual are shown in Figure 5, A and B. In all HIV+ tissues analyzed, there was an increase in the number of mitochondria (P = 0.00003) (Figure 5, C–E), especially in areas with compromised Cx43 expression and localization. In addition, significant decrease in myofibril width in areas with compromised mitochondria (Figure 5, C, D, and F) was detected. Quantification of the myofibril diameter clearly indicated that HIV-infected individuals have two different populations of fibers: myofibrils of normal diameter and myofibrils of about half that diameter compared with uninfected tissues (P = 0.01) (Figure 5F). No significant differences were observed if the two populations of myofibers were quantified compared with the diameter of uninfected myofibers. These compromised myofibrils were associated with the proliferation and altered distribution of mitochondria (P < 0.001 versus uninfected conditions, n = 39).
Figure 5.
Electron microscopic analysis of hearts obtained from HIV-infected individuals shows that areas with compromised connexin 43 (Cx43) localization and calcium metabolism have extensive mitochondrial proliferation and decreased myofibril width. Areas with normal and increased Cx43 expression were identified and subjected to electron microscopy analysis. A and B: Representative sections from an HIV− heart. Black lines with circles indicate myofibril width. C and D: Representative sections from an HIV+ heart showing mitochondrial proliferation (arrows) and a decrease in myofibril width (black lines with circles). E: Quantification of mitochondrial numbers in heart tissue showing a significant increase in HIV+ tissues. F: Quantification of myofibril width in heart tissues obtained from uninfected and HIV-infected individuals. There are no statistically significant differences between uninfected and HIV-infected individuals in myofibril width. However, analysis of tissues obtained from HIV-infected individuals indicates the presence of two distinct populations of myofibrils, one with normal width and a compromised one that is significantly different that that uninfected individual. Data are expressed as means ± SD. n = 5 grids per patient from five different individuals were analyzed (F). P = 0.01, HIV small myofibers compared with uninfected conditions (F). Scale bar = 1.9 μm.
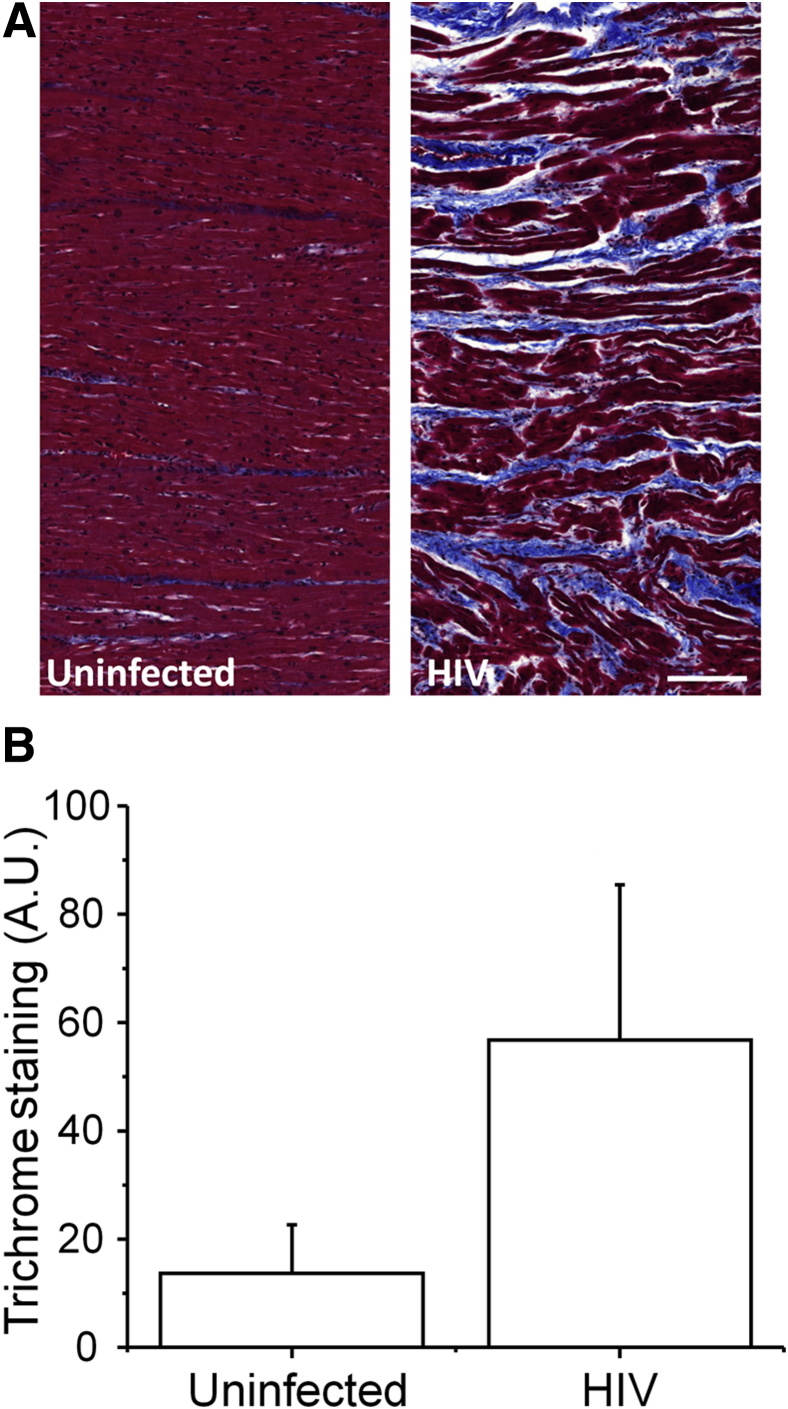
To further examine the damage in heart tissue obtained from HIV-infected individuals, trichrome stain for detection of collagen fibers and fibrin and periodic acid-Schiff for detection of polysaccharides was performed. Heart tissues obtained from uninfected cells have minimal trichrome staining, especially in blood vessels (Figure 6A). In contrast, HIV+ heart tissue showed increased muscle disorganization as described above and increased accumulation of collagen or fibrin between the muscle fibers (Figure 6A). Quantification of the trichrome staining indicated a significant increase in accumulation of collagen or fibrin between cardiomyocytes in areas with compromised Cx43 expression and distribution (P = 0.001 versus uninfected samples) (Figure 6B). No significant changes in periodic acid-Schiff staining were detected between uninfected and HIV-infected tissue sections (data not shown). In summary, significant damage was observed in areas with compromised Cx43 expression and localization.
Figure 6.
HIV infection increases the accumulation of collagen/fibrin in areas with increased expression and mis-localized connexin 43 (Cx43). Trichrome staining (blue) was performed to detect accumulation of collagen and fibrin in serial sections with areas already identified to have increased and mis-localized Cx43 expression. A: Trichrome staining of tissue sections obtained from uninfected and HIV-infected individuals. B: Quantification of trichrome staining from individuals indicates that HIV infection increases tissue scaring. n = 10 uninfected individuals (A and B); n = 19 HIV-infected individuals (A and B). Scale bar = 200 μm. A.U., arbitrary unit. Data are expressed as means ± SD.
Discussion
Our data demonstrate that HIV increases Cx43 expression at the intercalated disk and at the lateral membrane of the cardiomyocytes. We found that ventricular areas with Cx43 overexpression and mis-localization also have high calcium levels, increased fibrous tissue, and the proliferation of mitochondria. Thus, we propose that HIV-mediated up-regulation of Cx43 contributes to the onset and progression of heart disease in HIV+ individuals.
Despite the clinical importance of HIV heart disease the cause and contributing factors are poorly understood. Several laboratories using different models have demonstrated that HIV infection in combination with systemic inflammation and ART cytotoxicity results in cardiac pathologic processes.47, 48, 49 Furthermore, in the past couple of years, there is increasing clinical and experimental evidence that HIV infects cardiomyocytes in vitro and in vivo.50, 51, 52 Furthermore, it was reported that gp120 interaction with CXCR4 on the surface of cardiomyocytes triggers caspase-dependent mitochondrial activation and apoptosis.53 In addition to the potential direct effect of HIV in cardiomyocytes, several indirect mechanisms are also responsible for HIV heart damage, including an increase in proinflammatory cytokines, nitric oxide, and inducible nitric oxide synthase, which are released mainly by macrophages.54, 55, 56 In conclusion, several laboratories agree that the damage is multifactorial. Here, we propose that dysregulation of Cx43 also plays a role in HIV-associated cardiotoxicity.
Cx43 is an essential protein in heart development and function.12 In the normal heart, Cx43 is localized to intercalated disks, and little is found in the lateral membranes. Increased lateralization is often associated with reduced conduction velocity and increased in fibrosis.12, 14, 16, 25, 30, 31, 57 Normally, under inflammatory and infectious conditions Cx43 expression and GJ communication is reduced or shut down.11, 58 However, our data on astrocytes in the context of HIV infection indicate that the HIV protein, tat, the transactivator of the virus, binds to the Cx43 promoter, increasing Cx43 expression and gap junctional coupling to enable the spread of toxic intracellular second messengers among coupled cells, resulting in bystander apoptosis and inflammation.18, 58 HIV-tat, like other viral proteins, is secreted into the extracellular space even in the presence of effective ART59, 60 and may enter and affect cells that do not express HIV-tat. Our data indicate that in all of the heart tissues analyzed from HIV-infected individuals Cx43 was increased in abundance and was anomalously distributed along the lateral membranes. The lateral localization of Cx43 protein and the separation between the different muscle fibers suggest that Cx43 hemichannels may be an additional source of cellular compromise and inflammation as described in other systems.58, 61, 62 Opening of Cx43 hemichannels would increase Ca2+ influx into cardiomyocytes and allow Na+ influx, and K+ efflux and loss of ATP and other metabolites into the extracellular space. In agreement with the suggestion of increased hemichannel opening, our data indicate that all HIV-infected individuals have increased circulation of intracellular molecules transported by the opening of hemichannels. Furthermore, HIV infection of astrocytes and cell lines susceptible to HIV infection also results in the opening of Cx43-containing hemichannels.18, 58, 63 Thus, further studies are required to examine the role of Cx43 hemichannels in HIV-associated heart disease.
The increased accumulation of lipofuscin indicates accelerated aging processes in the HIV-infected population as described in other HIV-related pathologic conditions.64 Lipofuscin is a brownish pigment generated by the breakdown and absorption of damaged blood cells or lipids and is associated with aging. Thus, higher amounts of lipofuscin are representative of accelerated aging and/or alterations in lipid metabolism. Studies in mice indicate that aging or senescence normally decreases Cx43 expression and function in the heart,65 a condition different from the increase observed in our study. Furthermore, studies using SIV-infected monkeys indicate a critical role of CCR5 in gp120-mediated inflammation, recruitment of monocytes/macrophages into the cardiac tissue, and compromise of cardiac muscle and function.66, 67, 68, 69 Thus, we propose that an additional mechanism of heart disease in the HIV-infected population is altered in Cx43 expression, distribution, and function. Several groups also demonstrated the presence of Cx43-containing channels on the surface of the mitochondria,12, 70, 71 but the role of this mitochondrial Cx43 in cardiomyopathy is still under investigation. Our data indicate that both astrocytes and macrophages have compromised and enlarged mitochondria, resulting in significant changes in metabolism and bystander killing mechanisms mediated by connexin-containing channels, GJs, and hemichannels. Future studies will address these intriguing possibilities.
Conclusions
We consistently detected increased Cx43 mRNA and protein, altered localization of the protein, calcium accumulation, and deposition of collagen/fibrin in areas with Cx43 overexpression. All of these effects were independent of age, ART (including protease inhibitors), CD4 counts, viral load, and years living with HIV. Our studies in the brain and heart tissue indicate that HIV uses or targets Cx43 to spread toxicity or inflammation. In the heart, it is not known whether Cx43 up-regulation by HIV occurs during early infection or is a result of chronic damage by HIV and ART. Our data suggest that Cx43 channels are a main target of HIV infection in the body, including the brain and the heart. The targeting of Cx43 in all HIV-infected individuals provides a common mechanism of damage and a potential target to reduce or prevent heart disease in the HIV-infected population.
Acknowledgments
We thank the National NeuroAIDS Tissue Consortium (NNTC) for providing all samples, Dr. Susan Morgello for reading the manuscript, and Dr. Karen Maass (New York University) for providing the N-cadherin antibody.
Footnotes
Supported by the NIH grants MH096625 and NS105584 (E.A.E.) and Public Health Research Institute funding (E.A.E.). The National NeuroAIDS Tissue Consortium is made possible through funding from the National Institute of Mental Health and National Institute of Neurological Disorders and Stroke by the Manhattan HIV Brain Bank grant U24MH100931, Texas NeuroAIDS Research Center grant U24MH100930, National Neurological AIDS Bank grant U24MH100929, California NeuroAIDS Tissue Network grant U24MH100928, and Data Coordinating Center grant U24MH100925.
Disclosures: None declared.
References
- 1.Currier J.S., Taylor A., Boyd F., Dezii C.M., Kawabata H., Burtcel B., Maa J.F., Hodder S. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Triant V.A., Grinspoon S.K. Vascular dysfunction and cardiovascular complications. Curr Opin HIV AIDS. 2007;2:299–304. doi: 10.1097/COH.0b013e3281e7e831. [DOI] [PubMed] [Google Scholar]
- 3.Triant V.A., Lee H., Hadigan C., Grinspoon S.K. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obel N., Thomsen H.F., Kronborg G., Larsen C.S., Hildebrandt P.R., Sorensen H.T., Gerstoft J. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 5.Choy P.C., Siow Y.L., Mymin D., O K. Lipids and atherosclerosis. Biochem Cell Biol. 2004;82:212–224. doi: 10.1139/o03-085. [DOI] [PubMed] [Google Scholar]
- 6.Hui X., Lam K.S., Vanhoutte P.M., Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165:574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinik A.I., Wing R.R. The good, the bad, and the ugly in diabetic diets. Endocrinol Metab Clin North Am. 1992;21:237–279. [PubMed] [Google Scholar]
- 8.Tribulova N., Egan Benova T., Szeiffova Bacova B., Viczenczova C., Barancik M. New aspects of pathogenesis of atrial fibrillation: remodelling of intercalated discs. J Physiol Pharmacol. 2015;66:625–634. [PubMed] [Google Scholar]
- 9.Singh P., Hemal A., Agarwal S., Kumar D. Cardiac manifestations in HIV infected children. Indian J Pediatr. 2015;82:230–234. doi: 10.1007/s12098-014-1481-9. [DOI] [PubMed] [Google Scholar]
- 10.Pham T.V., Torres M. Human immunodeficiency virus infection-related heart disease. Emerg Med Clin North Am. 2015;33:613–622. doi: 10.1016/j.emc.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Eugenin E.A., Basilio D., Saez J.C., Orellana J.A., Raine C.S., Bukauskas F., Bennett M.V., Berman J.W. The role of gap junction channels during physiologic and pathologic conditions of the human central nervous system. J Neuroimmune Pharmacol. 2012;7:499–518. doi: 10.1007/s11481-012-9352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michela P., Velia V., Aldo P., Ada P. Role of connexin 43 in cardiovascular diseases. Eur J Pharmacol. 2015;768:71–76. doi: 10.1016/j.ejphar.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Antanaviciute I., Ereminiene E., Vysockas V., Rackauskas M., Skipskis V., Rysevaite K., Treinys R., Benetis R., Jurevicius J., Skeberdis V.A. Exogenous connexin43-expressing autologous skeletal myoblasts ameliorate mechanical function and electrical activity of the rabbit heart after experimental infarction. Int J Exp Pathol. 2015;96:42–53. doi: 10.1111/iep.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtenbach S., Kurtenbach S., Zoidl G. Gap junction modulation and its implications for heart function. Front Physiol. 2014;5:82. doi: 10.3389/fphys.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan Z., Yaiw K.C., Wilhelmi V., Lam H., Rahbar A., Stragliotto G., Soderberg-Naucler C. Human cytomegalovirus immediate early proteins promote degradation of connexin 43 and disrupt gap junction communication: implications for a role in gliomagenesis. Carcinogenesis. 2014;35:145–154. doi: 10.1093/carcin/bgt292. [DOI] [PubMed] [Google Scholar]
- 16.Fontes M.S., van Veen T.A., de Bakker J.M., van Rijen H.V. Functional consequences of abnormal Cx43 expression in the heart. Biochim Biophys Acta. 2012;1818:2020–2029. doi: 10.1016/j.bbamem.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez J.P., Ramachandran J., Xie L.H., Contreras J.E., Fraidenraich D. Selective connexin43 inhibition prevents isoproterenol-induced arrhythmias and lethality in muscular dystrophy mice. Sci Rep. 2015;5:13490. doi: 10.1038/srep13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berman J.W., Carvallo L., Buckner C.M., Luers A., Prevedel L., Bennett M.V., Eugenin E.A. HIV-tat alters Connexin43 expression and trafficking in human astrocytes: role in NeuroAIDS. J Neuroinflammation. 2016;13:54. doi: 10.1186/s12974-016-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subbian S., Eugenin E., Kaplan G. Detection of Mycobacterium tuberculosis in latently infected lungs by immunohistochemistry and confocal microscopy. J Med Microbiol. 2014;63:1432–1435. doi: 10.1099/jmm.0.081091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rella C.E., Ruel N., Eugenin E.A. Development of imaging techniques to study the pathogenesis of biosafety level 2/3 infectious agents. Pathog Dis. 2014;72:167–173. doi: 10.1111/2049-632X.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marakalala M.J., Raju R.M., Sharma K., Zhang Y.J., Eugenin E.A., Prideaux B., Daudelin I.B., Chen P.Y., Booty M.G., Kim J.H., Eum S.Y., Via L.E., Behar S.M., Barry C.E., III, Mann M., Dartois V., Rubin E.J. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat Med. 2016;22:531–538. doi: 10.1038/nm.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thienemann F., Sliwa K., Rockstroh J.K. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J. 2013;34:3538–3546. doi: 10.1093/eurheartj/eht388. [DOI] [PubMed] [Google Scholar]
- 23.Reyskens K.M., Fisher T.L., Schisler J.C., O'Connor W.G., Rogers A.B., Willis M.S., Planesse C., Boyer F., Rondeau P., Bourdon E., Essop M.F. Cardio-metabolic effects of HIV protease inhibitors (lopinavir/ritonavir) PLoS One. 2013;8:e73347. doi: 10.1371/journal.pone.0073347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Kempen M.J., Fromaget C., Gros D., Moorman A.F., Lamers W.H. Spatial distribution of connexin43, the major cardiac gap junction protein, in the developing and adult rat heart. Circ Res. 1991;68:1638–1651. doi: 10.1161/01.res.68.6.1638. [DOI] [PubMed] [Google Scholar]
- 25.Fromaget C., el Aoumari A., Gros D. Distribution pattern of connexin 43, a gap junctional protein, during the differentiation of mouse heart myocytes. Differentiation. 1992;51:9–20. doi: 10.1111/j.1432-0436.1992.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 26.Severs N.J. Gap junction shape and orientation at the cardiac intercalated disk. Circ Res. 1989;65:1458–1462. doi: 10.1161/01.res.65.5.1458. [DOI] [PubMed] [Google Scholar]
- 27.Hoyt R.H., Cohen M.L., Saffitz J.E. Distribution and three-dimensional structure of intercellular junctions in canine myocardium. Circ Res. 1989;64:563–574. doi: 10.1161/01.res.64.3.563. [DOI] [PubMed] [Google Scholar]
- 28.Eckardt D., Theis M., Degen J., Ott T., van Rijen H.V., Kirchhoff S., Kim J.S., de Bakker J.M., Willecke K. Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J Mol Cell Cardiol. 2004;36:101–110. doi: 10.1016/j.yjmcc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Cobo M., Gingalewski C., Drujan D., De Maio A. Downregulation of connexin 43 gene expression in rat heart during inflammation. The role of tumour necrosis factor. Cytokine. 1999;11:216–224. doi: 10.1006/cyto.1998.0422. [DOI] [PubMed] [Google Scholar]
- 30.Hesketh G.G., Shah M.H., Halperin V.L., Cooke C.A., Akar F.G., Yen T.E., Kass D.A., Machamer C.E., Van Eyk J.E., Tomaselli G.F. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106:1153–1163. doi: 10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C., Meng Q., Yu X., Jing X., Xu P., Luo D. Regulatory effect of connexin 43 on basal Ca2+ signaling in rat ventricular myocytes. PLoS One. 2012;7:e36165. doi: 10.1371/journal.pone.0036165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oyamada M., Tsujii E., Tanaka H., Matsushita T., Takamatsu T. Abnormalities in gap junctions and Ca2+ dynamics in cardiomyocytes at the border zone of myocardial infarcts. Cell Commun Adhes. 2001;8:335–338. doi: 10.3109/15419060109080749. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Z., Zhang H., Lin N., Kang M., Zheng Y., Li C., Xu P., Wu Y., Luo D. Role of inositol-1,4,5-trisphosphate receptor in the regulation of calcium transients in neonatal rat ventricular myocytes. J Pharmacol Sci. 2014;126:37–46. [PubMed] [Google Scholar]
- 34.Delmar M., Makita N. Cardiac connexins, mutations and arrhythmias. Curr Opin Cardiol. 2012;27:236–241. doi: 10.1097/HCO.0b013e328352220e. [DOI] [PubMed] [Google Scholar]
- 35.Tribulova N., Knezl V., Okruhlicova L., Slezak J. Myocardial gap junctions: targets for novel approaches in the prevention of life-threatening cardiac arrhythmias. Physiol Res. 2008;57 Suppl 2:S1–S13. doi: 10.33549/physiolres.931546. [DOI] [PubMed] [Google Scholar]
- 36.Gutstein D.E., Liu F.Y., Meyers M.B., Choo A., Fishman G.I. The organization of adherens junctions and desmosomes at the cardiac intercalated disc is independent of gap junctions. J Cell Sci. 2003;116:875–885. doi: 10.1242/jcs.00258. [DOI] [PubMed] [Google Scholar]
- 37.Bills C.E., Eisenberg H., Pallante S.L. Complexes of organic acids with calcium phosphate: the Von Kossa stain as a clue to the composition of bone mineral. Johns Hopkins Med J. 1974;128:194–207. [PubMed] [Google Scholar]
- 38.Drut R. Calcified mitochondria in epithelial cells of the respiratory tract in upper respiratory thermal injury. Diagn Cytopathol. 1998;19:288–289. doi: 10.1002/(sici)1097-0339(199810)19:4<288::aid-dc12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Olbrich H.G., Herrmann G., Vandeplassche G., Michaelis H., Schneider M., Krause E., Kober G. Calcium overload in human giant cell myocarditis. J Clin Pathol. 1990;43:650–653. doi: 10.1136/jcp.43.8.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boengler K., Stahlhofen S., van de Sand A., Gres P., Ruiz-Meana M., Garcia-Dorado D., Heusch G., Schulz R. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol. 2009;104:141–147. doi: 10.1007/s00395-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 41.Lamont C., Luther P.W., Balke C.W., Wier W.G. Intercellular Ca2+ waves in rat heart muscle. J Physiol. 1998;512(Pt 3):669–676. doi: 10.1111/j.1469-7793.1998.669bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song D., Liu X., Liu R., Yang L., Zuo J., Liu W. Connexin 43 hemichannel regulates H9c2 cell proliferation by modulating intracellular ATP and [Ca2+] Acta Biochim Biophys Sin (Shanghai) 2010;42:472–482. doi: 10.1093/abbs/gmq047. [DOI] [PubMed] [Google Scholar]
- 43.Bublitz M., Musgaard M., Poulsen H., Thogersen L., Olesen C., Schiott B., Morth J.P., Moller J.V., Nissen P. Ion pathways in the sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 2013;288:10759–10765. doi: 10.1074/jbc.R112.436550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decrock E., De Bock M., Wang N., Gadicherla A.K., Bol M., Delvaeye T., Vandenabeele P., Vinken M., Bultynck G., Krysko D.V., Leybaert L. IP3, a small molecule with a powerful message. Biochim Biophys Acta. 2013;1833:1772–1786. doi: 10.1016/j.bbamcr.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Dupont G., Combettes L. Fine tuning of cytosolic Ca (2+) oscillations. F1000Res. 2016:5. doi: 10.12688/f1000research.8438.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krebs J., Agellon L.B., Michalak M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: an integrated view of calcium signaling. Biochem Biophys Res Commun. 2015;460:114–121. doi: 10.1016/j.bbrc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Lambert C.T., Sandesara P.B., Hirsh B., Shaw L.J., Lewis W., Quyyumi A.A., Schinazi R.F., Post W.S., Sperling L. HIV, highly active antiretroviral therapy and the heart: a cellular to epidemiological review. HIV Med. 2016;17:411–424. doi: 10.1111/hiv.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ntusi N., O'Dwyer E., Dorrell L., Wainwright E., Piechnik S., Clutton G., Hancock G., Ferreira V., Cox P., Badri M., Karamitsos T., Emmanuel S., Clarke K., Neubauer S., Holloway C. HIV-1-related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging. 2016;9:e004430. doi: 10.1161/CIRCIMAGING.115.004430. [DOI] [PubMed] [Google Scholar]
- 49.Ntusi N.B., Taylor D., Naidoo N.G., Mendelson M. Progressive human immunodeficiency virus-associated vasculopathy: time to revise antiretroviral therapy guidelines? Cardiovasc J Afr. 2011;22:197–200. doi: 10.5830/CVJA-2010-048. [DOI] [PubMed] [Google Scholar]
- 50.Grody W.W., Cheng L., Lewis W. Infection of the heart by the human immunodeficiency virus. Am J Cardiol. 1990;66:203–206. doi: 10.1016/0002-9149(90)90589-s. [DOI] [PubMed] [Google Scholar]
- 51.Epstein J.E., Eichbaum Q.G., Lipshultz S.E. Cardiovascular manifestations of HIV infection. Compr Ther. 1996;22:485–491. [PubMed] [Google Scholar]
- 52.Rodriguez E.R., Nasim S., Hsia J., Sandin R.L., Ferreira A., Hilliard B.A., Ross A.M., Garrett C.T. Cardiac myocytes and dendritic cells harbor human immunodeficiency virus in infected patients with and without cardiac dysfunction: detection by multiplex, nested, polymerase chain reaction in individually microdissected cells from right ventricular endomyocardial biopsy tissue. Am J Cardiol. 1991;68:1511–1520. doi: 10.1016/0002-9149(91)90288-v. [DOI] [PubMed] [Google Scholar]
- 53.Lopes de Campos W.R., Chirwa N., London G., Rotherham L.S., Morris L., Mayosi B.M., Khati M. HIV-1 subtype C unproductively infects human cardiomyocytes in vitro and induces apoptosis mitigated by an anti-Gp120 aptamer. PLoS One. 2014;9:e110930. doi: 10.1371/journal.pone.0110930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monsuez J.J., Gallet B., Escaut L., Vayre F., Charniot J.C., Pulik M., Merad M., Minozzi C., Slama M., Weber S., Vittecoq D. Clinical outcome after coronary events in patients treated with HIV-protease inhibitors. Eur Heart J. 2000;21:2079–2080. doi: 10.1053/euhj.2000.2264. [DOI] [PubMed] [Google Scholar]
- 55.Monsuez J.J., Gallet B., Escaut L., Vayre F., Pulik M., Charniot J.C., Merad M., Slama M., Weber S., Vittecoq D. Cardiac side effects of anti-HIV agentsArch Mal Coeur Vaiss. 2000;93:835–840. French. [PubMed] [Google Scholar]
- 56.Monsuez J.J., Auperin I., Vittecoq D., Heshmati F. Pulmonary hypertension associated with thrombotic thrombocytopaenic purpura in AIDS patients. Eur Heart J. 1997;18:1036–1037. doi: 10.1093/oxfordjournals.eurheartj.a015367. [DOI] [PubMed] [Google Scholar]
- 57.Palatinus J.A., Rhett J.M., Gourdie R.G. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim Biophys Acta. 2012;1818:1831–1843. doi: 10.1016/j.bbamem.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eugenin E.A. Role of connexin/pannexin containing channels in infectious diseases. FEBS Lett. 2014;588:1389–1395. doi: 10.1016/j.febslet.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nath A., Steiner J. Synaptodendritic injury with HIV-Tat protein: what is the therapeutic target? Exp Neurol. 2014;251:112–114. doi: 10.1016/j.expneurol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bachani M., Sacktor N., McArthur J.C., Nath A., Rumbaugh J. Detection of anti-tat antibodies in CSF of individuals with HIV-associated neurocognitive disorders. J Neurovirol. 2013;19:82–88. doi: 10.1007/s13365-012-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malik S., Eugenin E.A. Mechanisms of HIV neuropathogenesis: role of cellular communication systems. Curr HIV Res. 2016;14:400–411. doi: 10.2174/1570162x14666160324124558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stehberg J., Moraga-Amaro R., Salazar C., Becerra A., Echeverria C., Orellana J.A., Bultynck G., Ponsaerts R., Leybaert L., Simon F., Saez J.C., Retamal M.A. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 2012;26:3649–3657. doi: 10.1096/fj.11-198416. [DOI] [PubMed] [Google Scholar]
- 63.Orellana J.A., Saez J.C., Bennett M.V., Berman J.W., Morgello S., Eugenin E.A. HIV increases the release of dickkopf-1 protein from human astrocytes by a Cx43 hemichannel-dependent mechanism. J Neurochem. 2014;128:752–763. doi: 10.1111/jnc.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calvo M., Martinez E. Update on metabolic issues in HIV patients. Curr Opin HIV AIDS. 2014;9:332–339. doi: 10.1097/COH.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 65.Bonda T.A., Szynaka B., Sokolowska M., Dziemidowicz M., Winnicka M.M., Chyczewski L., Kaminski K.A. Remodeling of the intercalated disc related to aging in the mouse heart. J Cardiol. 2016;68:261–268. doi: 10.1016/j.jjcc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Kelly K.M., Tocchetti C.G., Lyashkov A., Tarwater P.M., Bedja D., Graham D.R., Beck S.E., Metcalf Pate K.A., Queen S.E., Adams R.J., Paolocci N., Mankowski J.L. CCR5 inhibition prevents cardiac dysfunction in the SIV/macaque model of HIV. J Am Heart Assoc. 2014;3:e000874. doi: 10.1161/JAHA.114.000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelly K.M., Tarwater P.M., Karper J.M., Bedja D., Queen S.E., Tunin R.S., Adams R.J., Kass D.A., Mankowski J.L. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS. 2012;26:815–823. doi: 10.1097/QAD.0b013e3283518f01. [DOI] [PubMed] [Google Scholar]
- 68.Walker M., Caldwell R.W., Yoon Y., Nguyen T.T., Johnson J.A. deltaPKC interaction with the d subunit of F1Fo ATP synthase impairs energetics and exacerbates ischemia/reperfusion injury in isolated rat hearts. J Mol Cell Cardiol. 2015;89(Pt B):232–240. doi: 10.1016/j.yjmcc.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 69.Walker J.A., Beck G.A., Campbell J.H., Miller A.D., Burdo T.H., Williams K.C. Anti-alpha4 integrin antibody blocks monocyte/macrophage traffic to the heart and decreases cardiac pathology in a SIV infection model of AIDS. J Am Heart Assoc. 2015;4:e001932. doi: 10.1161/JAHA.115.001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gadicherla A.K., Wang N., Bulic M., Agullo-Pascual E., Lissoni A., De Smet M., Delmar M., Bultynck G., Krysko D.V., Camara A., Schluter K.D., Schulz R., Kwok W.M., Leybaert L. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res Cardiol. 2017;112:27. doi: 10.1007/s00395-017-0618-1. [DOI] [PubMed] [Google Scholar]
- 71.Baines C.P. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]