Abstract
Mesenchymal stem cells (MSCs) can differentiate into chondroblasts, adipocytes, or cartilage under appropriate stimulation. Identifying a mechanism triggering the differentiation of MSCs into cartilage may help develop novel therapeutic approaches for treating heterotopic ossification, the pathological formation of lamellar bone in soft tissue outside the skeleton that can lead to debilitating immobility. Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor, stimulates growth hormone secretion, and has both orexigenic and adipogenic effects. This study sought to understand the potential involvement of the ERK1/2 signaling pathway in the ghrelin-induced growth of rat MSCs (rMSCs). We applied various concentrations of ghrelin to cultured rMSCs by observing the changes in the phosphorylation state of ERK1/2, p38, JNK as well as the type II collagen expression levels by western blot. The highest expression level for both type II collagen was obtained with 600 ng/mL ghrelin at 24 h. We found that the ghrelin-induced differentiation of rMSCs into cartilage was promoted primarily by the ERK1/2 pathway. Our study suggests that ghrelin induced differentiation of rMSCs into cartilage primarily through the ERK1/2 pathway.
Keywords: rMSCs, MAPK, Ghrelin, Cartilage differentiation, ERK1/2
Introduction
Bone marrow-derived mesenchymal cells (BMSCs) are among the best characterized of all stem cells studied so far, with great differentiation ability and being able to secrete factors beneficial for tissue repair (Zhu et al. 2013; Yin et al. 2016). Besides, they are of low immunogenicity and can exert potent immunosuppression (Zhou et al. 2016a, b). Moreover, MSCs have been widely reported to exert angiogenesis in in vitro and in vivo experiments (Zheng et al. 2016). Basic research on MSCs in certain fields has led to the initiation of clinical trials worldwide (Zhao et al. 2016b). To provide therapeutic benefits and further understand the mechanisms responsible for them, large numbers of cells are needed (Zhao et al. 2016a).
Ghrelin, a 28-amino-acid peptide identified as the first endogenous ligand for the growth hormone secretagogue receptor (GHS-R) (Xu et al. 2008; Wu et al. 2015), is found mainly in stomach and hypothalamus where it exercises biological activities such as regulating food intake and stimulating the release of growth hormone (GH) (Tena-Sempere 2005). It has been recently reported that ghrelin is also synthesized and secreted by cardiomyocytes and that ghrelin treatment inhibits cell death and apoptosis and promotes cell proliferation in cardiomyocytes (Tack and Corsetti 2015). In addition, several studies have shown that ghrelin is involved in regulating the differentiation of mesoderm-derived precursor cells including premyocyt, osteoblast, and preadipocyte either in vivo or in vitro (St-Onge et al. 2016; Shi et al. 2016).
Cellular signaling is essential for the cells’ ability to respond to the environment by integrating external cues to intracellular mediators and effectors. Activation of mitogen-activated protein kinases (MAPKs) constitutes a paradigm of intracellular signaling (Zhang et al. 2015, 2016). p38, a subgroup of the MAPKs, was initially identified as a transducer of the response to inflammatory and environmental stress conditions (Zhang et al. 2015, 2016; Xie et al. 2014). Phosphorylation of these threonine and tyrosine residues results in a conformational change that increases the accessibility of the active site and enhances catalysis (Urosevic et al. 2014). ERKs are activated in response to various cytokines and growth factors and mediate primarily mitogenic and anti-apoptotic signals (Smith et al. 2014).
Previously, we have found ghrelin is a growth factor important for the proliferation of mesenchymal stem cells, as well as essential to promote the differentiation of the mesenchymal stem cells to osteoblast cell. However, the effect of ghrelin on cartilage cells was not shown. In this study, ghrelin effects on the differentiation of the mesenchymal stem cells to cartilage cells was studied.
Materials and methods
Unless otherwise specified, all chemicals and reagents were purchased from Sigma Chemical Company (St. Louis, MO, USA). Antibodies to IgG, GAPDH, ALP, Runx2, Osterix, CD44, CD34, U0126 (an inhibitor of phospho-ERK1/2) ERK1/2, JNK, p90rsk, phospho-ERK1/2, phospho-JNK and phospho-p90rsk1(Ser380) were purchased from the Abcam Corporation Cambridge, MA, USA). ECL regent was purchased Pierce (Rockford, IL, USA). Alexa Fluor 488 goat anti-rabbit IgG (H + L) secondary antibody was purchased from Abcam Corporation.
Isolation and culture of rMSCs
The rMSCs were obtained from a neonatal New Zealand white rabbit. The bone marrow was flushed with low glucose Dulbecco’s modified Eagle’s medium (DMEM) using a 1 mL syringe. Cells were harvested in culture dish, cultured in an incubator at 37 °C with 5% CO2. The animal protocol was approved by The Inner Mongolia Medical University Experimental Animal Management Committee.
Immunofluorescence
The rMSCs, at room temperature, were fixed in 3.7% paraformaldehyde for half an hour. Blocked with 1% bovine serum albumin in PBS with 10% goat serum overnight at 4 °C. The samples were then incubated with primary antibodies diluted in PBS. The primary antibody binding was detected with an Alexa Fluor 488 goat anti-rabbit IgG (H + L) secondary antibody. The fluorescence images were captured with a microscope.
Flow cytometric analysis
Cells were stained with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies against rat CD44, CD34. Cells were also stained with FITC- or PE-labeled isotype-matched immunoglobulins and used as negative controls. The stained cells were analyzed using a FACSCalibur cytometer and CellQuest software (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Western blotting
The protein, obtained from rMSCs, were separated using electrophoresis on 12% SDS-PAGE and transferred to immunoblot nitrocellulose membranes. Membranes were blocked and then incubated with primary antibodies overnight at 4 °C. The membranes were subsequently washed three times with PBS, incubated with peroxidase-conjugated secondary antibodies. Using ECL reagents to reveal the results (Pierce, Rockford, IL, USA).
Cartilage differentiation
The rMSCs were plated at a density of 5000 cells/cm2 and exposed to standard differentiation-inducing media for 21 days. The medium (5 μg/ml insulin, 5 μg/ml transferrin, 5 μg/ml selenous acid, 0.1 M dexamethasone, 0.17 mM ascorbic acid–2-phosphate, 1 mM sodium pyruvate, 0.35 mM proline, 1.25 mg/ml BSA, and 10 μg/ml transforming growth factor beta 3) was changed twice per week. Cartilage differentiation was achieved following standard in vitro protocols (Fromigué et al. 1998).
Statistical analysis
One-way analysis of variance (ANOVA) with GraphPad Prism version 5 software (GraphPad Software, La Jolla, CA, USA, www.graphpad.com/company/) was used for statistical analysis of the data. Data are presented as mean ± S.D.
Results
Identification of rMSCs
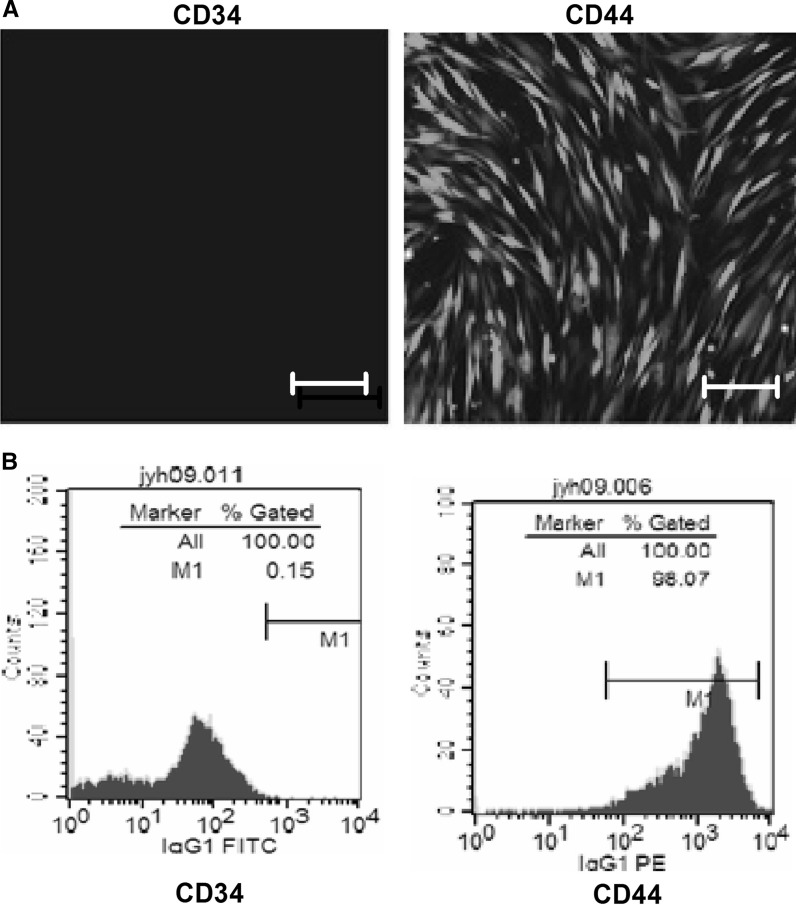
On day 10, cells reached 80% confluence. On day 13, the cells displayed a uniform spindle shape and reached 100% confluence. To further identify the rMSCs, the expression of CD34 and CD44 was examined by immunofluorescence. The cells were CD44 positive but CD34 negative (Fig. 1a). Flow cytometric analysis showed that the isolated cells strongly expressed the surface markers of mesenchymal stem cells, such as CD44, but almost no markers CD34 (Fig 1B).
Fig. 1.
Identification of rMSCs. a Immunofluorescence staining showing the expression of CD34 and CD44 in cultured rMSCs. Scale bar: 50 μm. b Flow cytometry showing the expression of CD34 and CD44 in cultured rMSCs
Optimal time for ghrelin-induced effects on the differentiation of rMSCs into cartilage
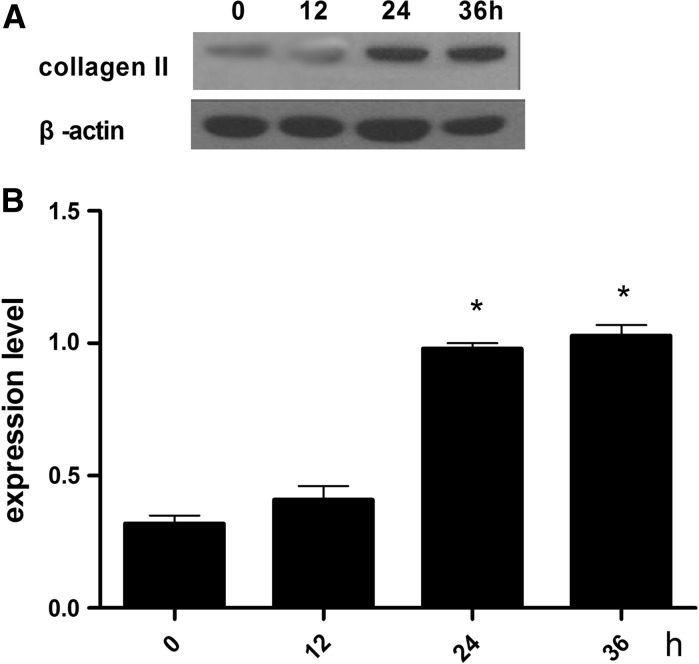
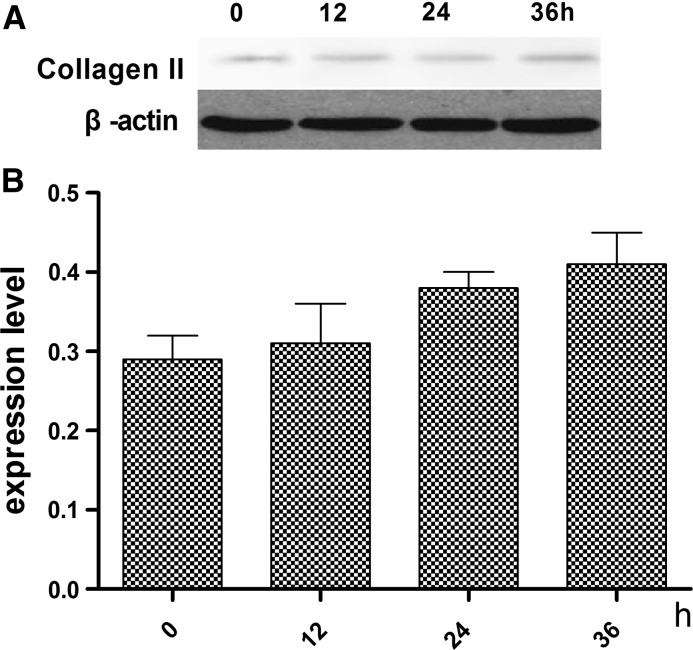
The optimal of ghrelin for the effects on the differentiation of rMSCs into cartilage was established. Figure 2 shows the expression levels of type II collagen and glycosaminoglycan at a ghrelin concentration of 600 ng/mL at 0, 12, 24, and 36 h. The highest expression level for both type II collagen was in presence of 600 ng/mL ghrelin at 24 h.
Fig. 2.
Expression levels of type II collagen in cartilage induced by standard differentiation-inducing medium supplemebted with 600 ng/mL ghrelin. a Expression of type II collagen assessed by western blot. b Contrast gray value of type II collagen based on the western blot, presented as mean ± SD (n = 5). *Significantly different from the 0 h group (P < 0.05). Contrast gray value of type II collagen = gray value of type II collagen/gray value of β-actin
Ghrelin promotes the differentiation of rMSCs into cartilage via the ERK1/2 pathway
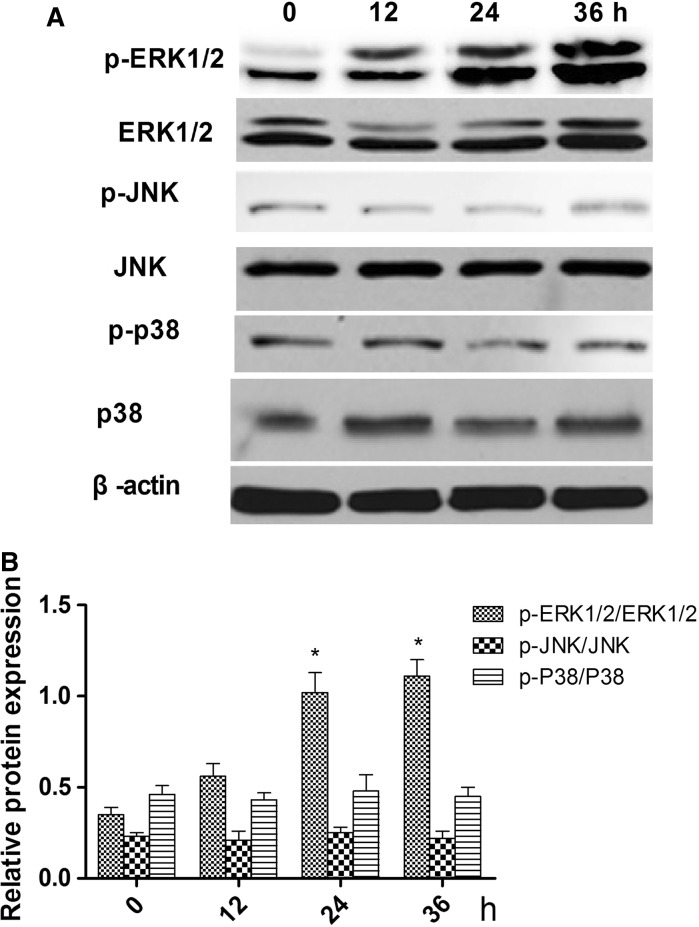
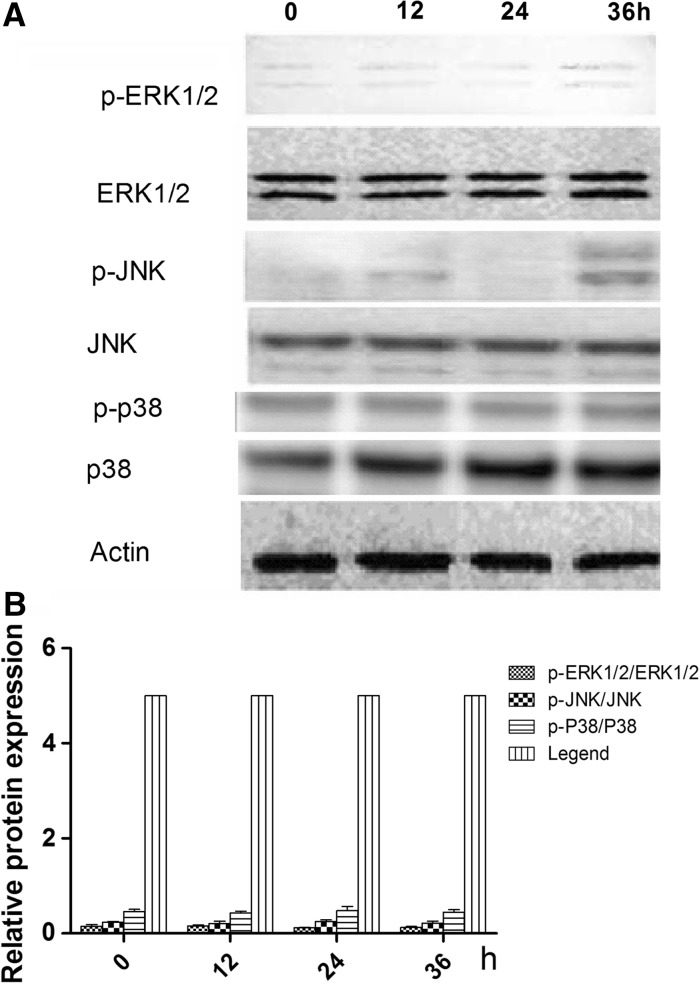
To identify the signaling pathway through which ghrelin acts to promote the differentiation of rMSCs into cartilage, cells were treated with 600 ng/mL of ghrelin for 0, 12, 24 and 36 h, and the phosphorylation levels of ERK1/2, JNK, p38 were examined on 0, 12, 24, and 36 h (Fig. 3a). Figure 3b shows that the ratios of phosphorylated to non-phosphorylated ERK1/2 were higher on 24 and 36 h than on 0 and 12 h.
Fig. 3.
Effects of 600 ng/ml ghrelin on mitogen-activated protein kinase (MAPK) activation in rBMSCs cells. a Expression of total and phosphorylated ERK1/2, JNKs, and p38 proteins. b Ratios of phosphorylated to non-phosphorylated ERK1/2, JNKs, and p38 based on the western blot, presented as mean ± SD (n = 5). *Significantly different from the 0 h group (P < 0.05). Contrast gray value of phosphorylation protein = gray value of phosphorylated protein/gray value of non-phoshporylated protein
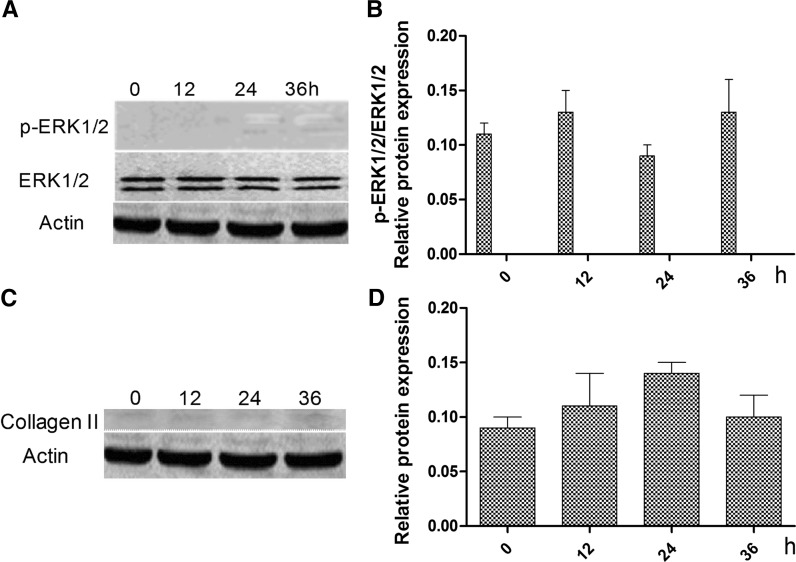
To verify that ghrelin promotes the differentiation of rMSCs into cartilage via the ERK1/2 pathway, the ERK1/2 signaling inhibitor U0126 (a specific inhibitor of ERK1/2 phosphorylation) was used to inhibit ERK1/2 phosphorylation activity. Following the addition of U0126, the cells were treated with ghrelin for 0, 12, 24, and 36 h. We found that the phosphorylation of ERK1/2 was not changed and was low (Fig. 4a, b). In addition, the expression levels of type II collagen were not changed and were very low (Fig. 4c, d).
Fig. 4.
Effects of addition of U1206 in combination with 600 ng/mL of ghrelin on the expression of ERK1/2, p-ERK1/2 and type II collagen proteins. a Expression of total and phosphorylated ERK1/2 proteins. b Ratio of the contrast gray value of phosphorylation to non-phosphorylated ERK1/2 based on the western blot. c Expression of type II collagen. d Contrast gray value of type II collagen based on the western blot, presented as mean ± SD (n = 5). *Significantly different from the 0 h group (P < 0.05)
When this treatment was repeated with the addition of 10−9 mg/ml D-Lys3-GHRP-6, compared with treatment with ghrelin only, the phosphorylation of ERK1/2 was reduced (Fig. 5a). The phosphorylation status of the JNKs and p38 were not significantly modified (Fig. 5b), nor were the levels of type II collagen (Fig. 6).
Fig. 5.
Effects of ghrelin receptor inhibitor (D-Lys3-GHRP-6) in combination with 600 ng/mL of ghrelin on ghrelin-mediated MAPK activation in rMSCs cells. a Expression of total and phosphorylated ERK1/2, JNKs, and p38 proteins. b Contrast gray value of phosphorylation of ERK1/2, JNKs and p38 based on the western blot, presented as mean ± SD (n = 5). Ratios of phosphorylated to non-phosphorylated protein = gray value of phosphorylation protein/ gray value of protein. *Significantly different from the 0 h group (P < 0.05)
Fig. 6.
Expression of type II collagen by cartilage after treatment with the ghrelin receptor inhibitor (D-Lys3-GHRP-6) in combination with 600 ng/mL of ghrelin. a Expression of type II collagen by western blot. b Contrast gray value of type II collagen based on the western blot, presented as mean ± SD (n = 5). Contrast gray value of type II collagen = gray value of type II collagen/gray value of β-actin. *Significantly different from the 0 h group (P < 0.05)
This result suggests that ERK1/2 plays a key role in ghrelin’s ability to accelerate the growth of rMSCs. To test this, U0126 (a specific inhibitor of ERK1/2 phosphorylation) was used to silence ERK1/2 expression. In the presence of U0126, ghrelin (600 ng/ml) did not accelerate the expression levels of type II collagen.
Discussion
It may help to develop novel therapeutic approaches for treating heterotopic ossification, and to find trigger the differentiation of rMSCs into cartilage mechanisms (Kara et al. 2015). Here, the ghrelin-induced differentiation of rMSCs into cartilage was promoted primarily via the ERK1/2 pathway was shown, and when U0126 was added, the function of ghrelin inducing differentiation of rMSCs into cartilage was inhibited.
Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor (GHSR), is a 28-amino-acid peptide produced from a 117-amino acid preprohormone (Rambhojan et al. 2015). It has been recently reported that ghrelin is also synthesized and secreted by cardiomyocytes and that ghrelin treatment inhibits cell death and apoptosis and promotes cell proliferation in cardiomyocytes (Moon et al. 2009; Gao et al. 2013). Previously, we have found that ghrelin (600 ng/ml) can promote the growth of rMSCs and the rMSCs differentiation to osteoblast cell. Ghrelin/GHSR signaling pathway accelerates rMSC growth and promotes rMSC differentiation to osteoblasts mainly through an ERK-dependent pathway (Ye and Jiang 2015). In this study, we found that ghrelin can promote the rMSCs differentiation to cartilage, especially at 24 h. At that time, as the marker protein, the type II collagen was expressed at higher levels at 24 h than at 0 and 12 h.
In this study, we found that the ghrelin-induced differentiation of rMSCs into cartilage was promoted primarily through the ERK1/2 pathway. The MAPKs are a super-family of serine/threonine kinases that include ERK, JNK, and p38 (Rodriguez-Pena et al. 2013). These kinases are involved primarily in the activation of nuclear transcription factors that control cell proliferation, differentiation, and apoptosis (Qiu et al. 2014). To verify the ERK1/2 function, the U1206 was used to inhibit the ERK1/2 phosphorylation activity. The type II collagen expression was reduced. Therefore, the function of rMSCs differentiation to cartilage was also inhibited. Further, the gherlin receptor (GHRS) was inhibited by D-Lys3-GHRP-6. We also found that type II collagen expressed was inhibited and the rMSCs differentiation to cartilage was also inhibited.
Overall, our results provide evidence that the ghrelin/GHSR signaling pathway promotes rMSC differentiation to cartilage mainly through an ERK-dependent pathway. In this study, we found that the ghrelin-induced differentiation of rMSCs into cartilage was promoted primarily bythe ERK1/2 pathway. Further studies are necessary before any clinical application is considered.
Acknowledgement
This work was supported by Inner Mongolia Natural Science Foundation (No. 2017BS0813).
References
- Fromigué O, Marie PJ, Lomri A. Bone morphogenetic protein-2 and transforming growth factor-β2 interact to modulate human bone marrow stromal cell proliferation and differentiation. J Cell Biochem. 1998;68:411–426. doi: 10.1002/(SICI)1097-4644(19980315)68:4<411::AID-JCB2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Gao M, Yang J, Wei R, Liu G, Zhang L, Wang H, Wang G, Gao H, Chen G, Hong T. Ghrelin induces cardiac lineage differentiation of human embryonic stem cells through ERK1/2 pathway. Int J Cardiol. 2013;167:2724–2733. doi: 10.1016/j.ijcard.2012.06.106. [DOI] [PubMed] [Google Scholar]
- Kara M, Ekiz T, Ozturk GT, Onat SS, Ozcakar L. Heterotopic ossification and peripheral nerve entrapment: ultrasound is a must-use imaging modality. Pain Med. 2015;16:1643–1644. doi: 10.1111/pme.12749. [DOI] [PubMed] [Google Scholar]
- Moon M, Kim S, Hwang L, Park S. Ghrelin regulates hippocampal neurogenesis in adult mice. Endocr J. 2009;56:525–531. doi: 10.1507/endocrj.K09E-089. [DOI] [PubMed] [Google Scholar]
- Qiu W, Chen N, Zhang Q, Zhuo L, Wang X, Wang D, Jin H. Resistin increases platelet P-selectin levels via p38 MAPK signal pathway. Diab Vasc Dis Res. 2014;11:121–124. doi: 10.1177/1479164113513912. [DOI] [PubMed] [Google Scholar]
- Rambhojan C, Bouaziz-Amar E, Larifla L, Deloumeaux J, Clepier J, Plumasseau J, Lacorte JM, Foucan L. Ghrelin, adipokines, metabolic factors in relation with weight status in school-children and results of a 1-year lifestyle intervention program. Nutr Metab (Lond) 2015;12:43. doi: 10.1186/s12986-015-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena JM, Diez-Muniz S, Bermejo C, Nombela C, Arroyo J. Activation of the yeast cell wall integrity MAPK pathway by zymolyase depends on protease and glucanase activities and requires the mucin-like protein Hkr1 but not Msb2. FEBS Lett. 2013;587:3675–3680. doi: 10.1016/j.febslet.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Shi L, Du X, Jiang H, Xie J. Ghrelin and neurodegenerative disorders—a review. Mol Neurobiol. 2016;54:1144–1155. doi: 10.1007/s12035-016-9729-1. [DOI] [PubMed] [Google Scholar]
- Smith MP, Sanchez-Laorden B, O'Brien K, Brunton H, Ferguson J, Young H, Dhomen N, Flaherty KT, Frederick DT, Cooper ZA, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFalpha. Cancer Discov. 2014;4:1214–1229. doi: 10.1158/2159-8290.CD-13-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge V, Watts A, Abizaid A. Ghrelin enhances cue-induced bar pressing for high fat food. Horm Behav. 2016;78:141–149. doi: 10.1016/j.yhbeh.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Tack J, Corsetti M. Ghrelin agonists as emerging prokinetic agents. Clin Gastroenterol Hepatol. 2015;13:2320–2322. doi: 10.1016/j.cgh.2015.08.032. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M. Ghrelin: novel regulator of gonadal function. J Endocrinol Invest. 2005;28:26–29. [PubMed] [Google Scholar]
- Urosevic J, Nebreda AR, Gomis RR. MAPK signaling control of colon cancer metastasis. Cell Cycle. 2014;13:2641–2642. doi: 10.4161/15384101.2014.946374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Fan X, Yu Y, Wang Y. Maternal serum ratio of ghrelin to obestatin decreased in preeclampsia. Pregnancy Hypertens. 2015;5:263–266. doi: 10.1016/j.preghy.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Xie Y, Peng Z, Shi M, Ji M, Guo H, Shi H. Metformin combined with p38 MAPK inhibitor improves cisplatin sensitivity in cisplatinresistant ovarian cancer. Mol Med Rep. 2014;10:2346–2350. doi: 10.3892/mmr.2014.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Li Y, An W, Zhang W. Ghrelin and cell differentiation. Acta Biochim Biophys Sin (Shanghai) 2008;40:841–847. doi: 10.1093/abbs/40.10.841. [DOI] [PubMed] [Google Scholar]
- Ye N, Jiang D. Ghrelin accelerates the growth and osteogenic differentiation of rabbit mesenchymal stem cells through the ERK1/2 pathway. BMC Biotechnol. 2015;15:51. doi: 10.1186/s12896-015-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q, Zhou YY, Wang P, Ma LI, Li P, Wang XG, She CH, Li WL. CD44 promotes the migration of bone marrow-derived mesenchymal stem cells toward glioma. Oncol Lett. 2016;11:2353–2358. doi: 10.3892/ol.2016.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Shi J, Mao SY, Xu YS, Zhang D, Feng LY, Zhang B, Yan YY, Wang SC, Pan JP, et al. Role of p38 MAPK in enhanced human cancer cells killing by the combination of aspirin and ABT-737. J Cell Mol Med. 2015;19:408–417. doi: 10.1111/jcmm.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lu M, Zhang Z, Tian X, Wang S, Lv D. Resveratrol reverses P-glycoprotein-mediated multidrug resistance of U2OS/ADR cells by suppressing the activation of the NF-kappaB and p38 MAPK signaling pathways. Oncol Lett. 2016;12:4147–4154. doi: 10.3892/ol.2016.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Hwang NS, Bichara DA, Saris DB, Malda J, Vacanti JP, Pomerantseva I, Sundback CA, Langer R, Anderson DG, Randolph MA. Chondrogenesis by bone marrow-derived mesenchymal stem cells grown in chondrocyte-conditioned medium for auricular reconstruction. J Tissue Eng Regen Med. 2016;11:121–125. doi: 10.1002/term.2171. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang X, Dong P, Xu Q, Ma Z, Mu Q, Sun X, Jiang Z. Bone marrow derived mesenchymal stem cells alleviated brain injury via down-regulation of interleukin-1β in focal cerebral ischemic rats. Am J Transl Res. 2016;8:1541–1550. [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Cai W, Zhou S, Xu L, Jiang C. Protective effect of bone marrow derived mesenchymal stem cells in lipopolysaccharide-induced acute lung injury mediated by claudin-4 in a rat model. Am J Transl Res. 2016;8:3769–3779. [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Carter BZ, Andreeff M. Bone-marrow-derived mesenchymal stem cells inhibit gastric aspiration lung injury and inflammation in rats. J Cell Mol Med. 2016;20:1706–1717. doi: 10.1111/jcmm.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HS, Carter BZ, Andreeff M. Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang. Cancer Biol Med. 2016;13:248–259. doi: 10.20892/j.issn.2095-3941.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Zhang T, Sun C, Yu A, Qi B, Cheng H. Bone marrow mesenchymal stem cells combined with calcium alginate gel modified by hTGF-beta1 for the construction of tissue-engineered cartilage in three-dimensional conditions. Exp Ther Med. 2013;5:95–101. doi: 10.3892/etm.2012.765. [DOI] [PMC free article] [PubMed] [Google Scholar]