Abstract
Objective
Diagnosis of obesity using traditional body mass index (BMI) using length may not be a reliable indicator of body composition in spina bifida (SB). We examine traditional and surrogate measures of adiposity in adults with SB, correlated with activity, metabolic disease, attitudes towards exercise and quality of life.
Design
Adult subjects with SB underwent obesity classification using BMI by length and arm span, abdominal girth and percent trunk fat (TF) on dual energy X-ray absorptiometry (DXA). Quality of life measures, activity level and metabolic laboratory values were also reviewed.
Results
Among eighteen subjects (6 male, 12 female), median age was 26.5 (range 19–37) years, with level of lesion 16.7% ≤L2, 61.1% L3-4, and 22.2% ≥L5, respectively. Median weight was 71.8 (IQR 62.4, 85.8) kg, similar between sexes (P = 0.66). With median length of 152.0 (IQR 141.8, 163.3) cm, median conventional BMI was 29.4 m/kg2, with 7 (43.8%) subjects with BMI >30. Median BMI by arm span was 30.2 m/kg2, abdominal girth of 105.5 cm, and TF 45.7%. More subjects were classified as obese using alternate measures, with 9 (56.3%) by arm span, 14 (82.4%) by abdominal girth and 15 (83.3%) by TF (P = 0.008). Reclassification of obesity from conventional BMI was significant when using TF (P = 0.03). No difference in quality of life measures, activity level and metabolic abnormalities was demonstrated between obese and non-obese subjects.
Conclusions
Conventional determination of obesity using BMI by length is an insensitive marker in adults with SB. Adults with SB are more often classified as obese using TF by DXA.
Keywords: Anthropometric measures, Body fat, Body mass index, Obesity, Spina bifida
Introduction
In the United States, there has been a dramatic increase in the prevalence of obesity over the past two decades, with the Center for Disease Control (CDC) reporting obesity in 2012 in 35% of adults and 17% of adolescents and children, and higher prevalence in people with physical impairments and immobility.1–3 Obesity, and specifically central adiposity,4–6 has been linked to the development of metabolic syndrome, an amalgam of symptoms predictive of morbidity and mortality due to cardiovascular disease, stroke, and diabetes mellitus.7–11 Furthermore, implications of obesity may also correlate with activity level, attitudes toward exercise and quality of life.
In the past 40 years, advances in understanding and care of spina bifida (SB) has resulted in significant improvements in life expectancy, with 78% reaching adulthood.12 With increasing life expectancy, risk of chronic medical problems such as cardiovascular disease, renal impairment, obesity and diabetes will become an important part of medical care for the growing adult SB population. People with SB are more commonly obese, with 35–37% prevalence reported in the literature, although some advocate that true prevalence of obesity may be even higher.13,14 Risk factors for obesity in this population include limited ambulation, sedentary lifestyle, decreased lean body mass and resting energy expenditure, and neuroendocrine abnormalities due to hydrocephalus.14 The importance of recognizing and addressing obesity in people with SB is clear, but the development of more accurate methods of obesity measurement remains a continual challenge. Conventional methods of classifying obesity using body mass index (BMI) based on height and weight in the SB population are limited by lower limb and trunk hypoplasia, vertebral anomalies, musculoskeletal deformities, and body fat redistribution may even further limit accuracy.15 Furthermore, conventional calculations of BMI does not specify body fat distribution despite the evidence that central obesity has the highest association with increased mortality.16,17
Conventional BMI calculations are known to underestimate obesity in patients with spinal cord injury (SCI), due to lower muscle mass in the lower extremities and torso and concomitant higher fat mass.18 Similarly, these changes are shared with adults with SB, reflecting a common pitfall of using traditional measures of BMI in these populations. DXA has been identified as one of the most accurate methods of measuring body composition in people with SCI.19–21 Studies of physical activity and body composition in people with SB have therefore employed differing methods of defining obesity, ranging from calculation of BMI using height measured from joint to joint,22–24 BMI using arm span,14 or dual energy X-ray absorptiometry (DXA) trunk fat percentage.18,25–26 To address the lack of consensus regarding obesity measurement standards in adults with SB, the primary goal of our study is to assess the accuracy of various anthropometric measures of determining adiposity and assess the sensitivity of each measure in characterizing obesity. As a secondary goal, we also investigate the correlation between obesity and activity level, attitudes toward exercise, and subsequent effects on quality of life and risk factors for metabolic syndrome.
Methods
We performed an institutional review board approved cohort comparison of adult (≥18 years old) subjects with SB. Eighteen consecutive subjects were identified and enrolled in the clinic setting and asked to complete a standardized questionnaire at our institution (Rehabilitation Institute of Chicago, Chicago, IL, USA). Subject demographics including age at the time of study, sex, level of neurologic lesion, ambulatory status, history of ventriculo-peritoneal shunt and mobility were obtained from subject report and chart review of the electronic medical record. The questionnaire consisted of the Satisfaction with Life Scale (SWLS),27 the Functional Mobility Scale (FMS),28 an adaptation of the Barriers to Physical Activity and Disability Survey (B-PADS),29 the SF-12 Health Survey, with modification for those with spinal cord injury (SF-12v2)30 and a survey of approximate number of hours per week spent on specific activities (work, school sports and fitness, church, television or computer, social activities, and housekeeping). Overall quality of life was evaluated using the SF-12v2 questionnaire, consisting of Mental Health (MCS) and Physical Health Composite Scores (PCS).30 The average value was calculated for mental and physical domains on a scale of 0 to 100, with a lower score indicating a lower quality of life. These MCS and PCS values were compared to age-matched average scores in the United States.30 General satisfaction with life was evaluated using the SWLS, on a scale of 0 to 35, with a higher score indicating higher satisfaction.27 Ambulatory status and mobility were evaluated on subject interview as well as using the FMS, with higher score indicating greater independence in mobility and ambulation.28 The B-PADS was used to identify potential barriers to physical activity and exercise in subjects.29
Segmental height measurements (segment 1 (sitting height) is measured from head to hip; segment 2 is measured from spine to knee; segment 3 is measured from knee to sole) were taken to the nearest centimeter using a stadiometer. Total length was calculated by summation of the three segmental measurements. Weight of the subject was obtained to the nearest kilogram with a wheelchair balance scale. Arm span was measured from the tip of the right middle finger to the tip of the left middle finger, with arms fully extended in 90-degree abduction with a flexible tape to the nearest centimeter. Conventional BMI (BMI by height) was calculated from measured weight and total length. BMI calculations using arm span (kg/m2) were calculated as an alternative BMI determination using a measure of length that is typically unchanged between adults with SB and the general population. Abdominal circumference (cm) was measured at the level just above the uppermost lateral border of the ilium with no intervening clothing or gown, using a flexible tape to the nearest centimeter. Body composition measurement of percent trunk fat (TF) was obtained by DXA using Hologic QDR 4500A fan beam densitometer, using version 12.7.2 software (Hologic, Bedford, MA, USA). In this study, obesity was defined as percent TF by DXA of ≥30% in males and ≥35% in females, correlating to the 85th percentile31 a BMI value >30 kg/m2, or an abdominal circumference >102 cm in males and >88 cm in females.32 Overweight was defined as BMI value >25 kg/m2, or an abdominal circumference >94 cm in males or >80 cm in female.32 These reference values for adiposity and obesity are based on population-based studies of the general adult community.
The electronic medical record was used to review available laboratory results, vital signs and medication history, including lipid panel, fasting blood sugar, hemoglobin A1c (HbA1c), blood pressure measurements and medications pertaining to diabetes, hypertension or hyperlipidemia. Any medications to treat hyperlipidemia, diabetes, or hypertension were also recorded. Laboratory and blood pressure values were defined as abnormal based on the following criterion: 1) triglyceride ≥ 150 mg/dL; 2) total cholesterol ≥ 200 mg/dL; 3) high-density lipoprotein (HDL) ≤ 40 mg/dL; 4) low-density lipoprotein (LDL) > 190 mg/dL; 5) fasting blood sugar ≥ 126 mg/dL; 6) HbA1c ≥ 6.5%; 7) systolic or diastolic blood pressure ≥ 130/85.33 In cases in which multiple lab values or vital signs were available, all results were reviewed rather than averaging of measurements.
For statistical analysis, χ2 tests were used for categorical variables. Mann-Whitney U test was used for scale variables. Scores for each questionnaire are reported in all patients as well as by sex and obesity, as judged by DXA percent TF. The four measures of obesity (BMI by length, BMI by arm span, abdominal circumference, and DXA percent TF) were also compared. For all statistical analyses, P < 0.05 was considered statistically significant. Analysis was performed using SPSS®, version 22 (IBM Corp., Armonk, NY, USA).
Results
Of the 18 subjects included in the study, six (33.3%) were male and 12 (66.7%) were female. Mean age at the time of the study was 26.5 years (range 19–37). Level of lesion was most commonly L3–L4 (61.1%) and most subjects had a history of ventriculo-peritoneal shunt (94.4%). No statistically significant differences existed between the male and female subjects included in the study in these demographics (Table 1).
Table 1.
Characteristics of patients. Statistical significance was calculated using Mann-Whitney U test.
| All (n = 18) | Male (n = 6) | Female (n = 12) | P-value | |
|---|---|---|---|---|
| median (range) | ||||
| Age | 26.5 (19–37) | 26.5 (21–29) | 27.0 (19–37) | |
| Level of lesion | n (%) | 0.89 | ||
| Thoracic – L2 | 3 (16.7) | 2 (33.3) | 1 (8.3) | |
| L3–4 | 11 (61.1) | 2 (33.3) | 9 (75.0) | |
| L5 or lower | 4 (22.2) | 2 (33.3) | 2 (16.7) | |
| VP shunt | 17 (94.4) | 5 (83.3) | 12 (100) | 0.62 |
VP, ventriculo-peritoneal.
Anthropometric measures of obesity
Results of anthropometric measurements are shown in Table 2. Median weight of patients was 71.8 kg (IQR 62.4, 85.8). Arm span was overall similar to total length (153.8 cm and 155.0 cm, respectively), but arm span measurements were significantly longer in males than in females (171.0 cm versus 148.0 cm, P = 0.005), while total length was similar between sexes. Median BMI calculated using total length was 29.4 kg/m2 (IQR 26.1, 36.7). Median BMI calculated using arm span was 30.2 kg/m2 (IQR 22.8, 34.9). While BMI by arm span tended to be lower in males (22.8 kg/m2) than in females (34.9 kg/m2) due to the significant difference in arm span between sexes, the difference in BMI between sexes was not statistically significant (P = 0.15) despite being clinically significant in terms of classification of obesity. Median abdominal girth was 105.5 cm (IQR 93.0, 111.0), without significant difference between sexes. In contrast, median DXA trunk fat % was 45.7% (IQR 36.5, 50.0), with significantly higher fat % in females than males (47.5% versus 31.0% respectively, P = 0.001).
Table 2.
Anthropometric measures. Total length was calculated as the sum of segments 1 through 3 (as described in the methods of the manuscript). Statistical significance was calculated using Mann-Whitney U test.
| All (n = 18) | Male (n = 6) | Female (n = 12) | P (95% CI) | |
|---|---|---|---|---|
| median (IQR) | ||||
| Weight (kg) | 71.8 (62.4, 85.8) | 68.7 (58.6, 83.0) | 71.8 (63.7, 86.8) | 0.66 |
| Arm span (cm) | 153.8 (147.8, 163.3) | 171.0 (162.0, 178.0) | 148.0 (147.0, 153.75) | 0.005 |
| Total length - right (cm) | 155.0 (137.8, 165.5) | 159.0 (146.0, 169.0) | 153.0 (137.5, 165.0) | 0.58 |
| Segment 1 - trunk (cm) | 69.0 (67.0, 77.3) | 69.0 (69.0, 70.0) | 69.0 (66.0, 77.5) | |
| Segment 2R - spine to knee (cm) | 43.0 (38.5, 48.6) | 44.0 (40.0, 48.5) | 42.0 (37.0, 48.5) | |
| Segment 3R - knee to heel (cm) | 39.0 (35.0, 42.5) | 45.0 (37.0, 48.0) | 38.0 (34.5, 40.8) | |
| BMI (kg/m2) | ||||
| by total length (right) | 29.4 (26.1, 36.7) | 26.5 (25.3, 34.3) | 29.4 (26.5, 38.1) | 0.58 |
| by arm span | 30.2 (22.8, 37.4) | 22.8 (21.5, 29.6) | 34.9 (29.7, 27.5) | 0.15 |
| Abdominal girth (cm) | 105.5 (93.0, 111.0) | 98.0 (81.8, 109.8) | 106.0 (101.5, 110.5) | 0.46 |
| DXA fat % | 45.7 (36.5, 50.0) | 31.0 (27.9, 37.3) | 47.5 (45.6, 51.6) | 0.001 |
BMI, body mass index (kg/m2); DXA, dual X-ray absorptiometry.
By conventional BMI (calculated using height) cutoffs for obesity, seven (43.8%) subjects were obese, which was subsequently used as a baseline to compare alternate measures of obesity (Table 3). However, when BMI was calculated using arm span, two additional subjects were reclassified as obese for a total of nine (56.3%) patients. The difference in obesity classification between BMI using height versus arm span was not significantly different (P = 0.63). As shown in Figure 1 and Table 3, a significant increase in obesity classification using abdominal circumference and DXA trunk fat % (P = 0.06 and P = 0.03, respectively). By measure of abdominal girth, 14 (82.4%) subjects were obese, while 15 (83.3%) were obese by DXA trunk fat %. Missing data points for anthropometric data, as indicated in Table 3, were due to either encounter time limitations or subject preference.
Table 3.
Obesity classification using different anthropometric measures. Obesity was defined as BMI (by height or arm span) > 30 kg/m2, abdominal girth > 102 cm in males or > 88 cm in females, or by DXA trunk fat % ≥ 30% in males or ≥ 35% in females. Overweight was defined as BMI > 25 kg/m2 or abdominal girth > 94 cm in males or > 80 cm in females. No accepted overweight standard value for DXA trunk fat percent is available. Statistical significance relative to conventional BMI (by height) was calculated using McNemar test.
| Normal | Overweight | Obese | Reclassified as Obese (compared to BMI by height) | P-value | |
|---|---|---|---|---|---|
| x (%) | |||||
| BMI by height (n = 16) | 3 (18.8) | 6 (37.5) | 7 (43.8) | n/a | n/a |
| BMI by arm span (n = 16) | 5 (31.3) | 2 (12.5) | 9 (56.3) | 2 (12.5) | 0.63 |
| Abdominal girth (n = 17) | 3 (17.6) | 0 (0) | 14 (82.4) | 5 (29.4) | 0.06 |
| DXA trunk fat % (n = 18) | 3 (16.7) | n/a | 15 (83.3) | 6 (33.3) | 0.03 |
BMI, body mass index (kg/m2); DXA, dual X-ray absorptiometry.
Figure 1.
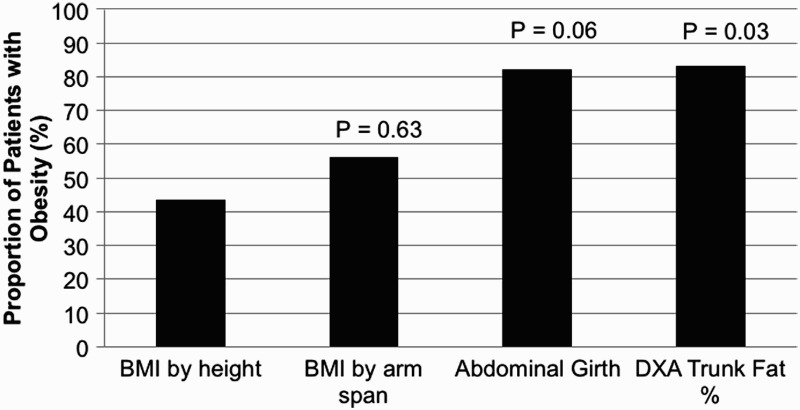
Reclassification of obesity using different anthropometric measures. Obesity was defined using BMI (by height or arm span), abdominal girth and DXA trunk fat percent as described in the manuscript. Classification by abdominal girth and trunk fat percentage resulted in a significantly increased rate of obesity. Statistical significance relative to conventional BMI (by height) was calculated using McNemar test. BMI, body mass index (kg/m2). DXA, dual X-ray absorptiometry.
Relationships between obesity on activity level and quality of life
Subjects were categorized as normal BMI versus obese using DXA trunk fat % standards and questionnaire elements were compared between the two groups (Table 4). No statistically significant difference in questionnaire elements between the two groups was demonstrated. Of note, in both the normal BMI and obese subject groups, SWLS scores were very similar, with average scores indicating an “average” satisfaction with life. Using the B-PADS questionnaire, the results suggest that most subjects (86.7% of obese patients and 100% of healthy subjects) want to join an exercise program, but a smaller portion (80% of obese subjects and 66.7% of healthy subjects) have ever exercised, with a smaller contingent ever having exercised regularly. Four (22.2%) subjects expressed concerns about exercising at a regular gym and five (27.8%) did not feel that a gym coordinator would know how to set up an exercise program to meet his or her needs. While all subjects report being instructed by a physician to exercise, only half recall being given specifics on where or how to do so. A minority of subjects identified family responsibilities or job as an obstacle for exercise, and most subjects identified parents, siblings, peers and role models as valuing exercise. Quality of life mental and physical health domains, as measured using the SF-12v2, tended to be lower in the obese subject group (46.5 ± 14.3 and 42.4 ± 10.3, respectively) compared to the normal BMI group (50.2 ± 14.6 and 50.3 ± 5.0, respectively), although this difference was not statistically significant (P = 0.74 and P = 0.2).
Table 4.
Survey findings, metabolic data and obesity classification using trunk fat percentage. Obesity was defined by dual X-ray absorptiometry trunk fat % ≥ 30% in males or ≥ 35% in females. No significant differences in survey scoers between healthy and obese patients. Statistical significance was calculated using Mann-Whitney U test.
| Normal (n = 3) | Obese (n = 15) | P-value | |
|---|---|---|---|
| Mean (SD) | |||
| Functional Mobility Scale (FMS) | 0.53 | ||
| Walking 5m | 3 (0) | 3 (1.6) | |
| Walking 50m | 3 (0) | 2.5 (1.9) | |
| Walking 500m | 3 (0) | 1.5 (1.4) | |
| Activity Level | 7.5 (0.7) | 5.6 (1.6) | 0.23 |
| Satisfaction with Life Scale (SWLS) | 20 (9.9) | 19.4 (7.8) | 0.17 |
| Barriers to Physcial Activity and Disability Surgery (B-PADS) | n (%) | ||
| Q1 want to join an exercise program? | 3 (100) | 13 (86.7) | 0.74 |
| Q2 have you ever exercised? | 2 (66.7) | 12 (80) | 0.74 |
| Q2a If yes, did you ever have health probs that caused you to stop exercising? | 0 (0) | 2 (13.3) | 0.77 |
| Q3 Ever injured from exercising? | 2 (66.7) | 1 (6.7) | 0.13 |
| Q4 Gone to fitness center, but was a negative experience | 0 (0) | 2 (13.3) | 0.74 |
| Q5 ever exercised regularly? | 2 (66.7) | 8 (53.3) | 0.74 |
| Q6 Know of a fitness center you could get to? | 3 (100) | 11 (73.3) | 0.5 |
| Q6a If yes, want to go? | 2 (66.7) | 10 (66.7) | 0.8 |
| Q7 willing to spend money to go? | 2 (66.7) | 11 (73.3) | 0.91 |
| Q8 concerns about exercising at YMCA? | 0 (0) | 4 (26.7) | 0.5 |
| Q9 Do you feel coordinator at YMCA would know how to set up an exercise program to meet your needs? | 1 (33.3) | 12 (80) | 0.25 |
| Q10 feel an exercise program could help you? | 3 (100) | 15 (100) | 1 |
| Q12 Doctor ever told you to exercise? | 2 (100) | 10 (100) | 1 |
| Q12a If yes, specifics? | 1 (50) | 5 (50) | 1 |
| Q13 satisfied with physical appearance, so don't need to exercise | 2 (100) | 1 (10) | 0.06 |
| Q14 family responsibilities prevent exercise | 0 (0) | 3 (30) | 0.61 |
| Q15 Job prevents from exercise | 0 (0) | 1 (10) | 1 |
| Q16 parents/siblings value exercise | 2 (100) | 8 (80) | 0.76 |
| Q17 Peers value exercising | 1 (50) | 6 (60) | 1 |
| Q18 Role models value exercising | 1 (50) | 7 (70) | 0.76 |
| Weekly activities (hrs/wk) | Mean (SD) | ||
| work | 19.5 (21.9) | 6.8 (10.0) | 0.17 |
| school | 27.5 (10.6) | 5.75 (13.3) | 0.17 |
| sports/fitness | 4.5 (0.7) | 1.7 (2.2) | 0.17 |
| church/religious | 6 (8.5) | 1.0 (1.5) | 0.57 |
| TV/computer | 11 (5.7) | 32.9 (29.2) | 0.38 |
| social activities/friends | 9 (8.5) | 1 (1.4) | 0.23 |
| housekeeping | 3.4 (3.0) | 9.0 (14.6) | 0.17 |
| SF-12 v2 | Mean (SD) | ||
| MCS | 50.2 (14.6) | 46.5 (14.3) | 0.74 |
| PCS | 50.3 (5.0) | 42.4 (10.3) | 0.2 |
| Metabolic Data | Mean (SD) | ||
| Triglyceride (mg/dL) | 116.5 (2.1) | 127.2 (59.0) | |
| Total Cholesterol (mg/dL) | 168 (18.4) | 185.6 (21.1) | |
| HDL (mg/dL) | 42 (0) | 36.9 (10.8) | |
| LDL (mg/dL) | 102.5 (17.7) | 114.9 (16.7) | |
| Fasting Blood Glucose (mg/dL) | 104 (37.6) | 87.2 (14.9) | 0.22 |
| Hemoglobin A1c (%) | 6.4 (1.9) | 5.4 (0.3) | |
| Systolic Blood Pressure | 130.3 (7.5) | 125.8 (19.3) | 0.7 |
| Statin medication | none | none | |
| Diabetes medication | none | none | |
| Hypertensive medication | 1 patient | none |
MCS, Mental Health Composite Score; PCS, Physical Health Composite Score; HDL, high density lioprotein; LDL, low density lipoprotein.
Correlations between obesity and risk factors for metabolic syndrome
Laboratory values were available for 13 (72.2%) subjects and are outlined in Table 4. In general, the obese group tended to have more adverse findings than the normal BMI group with respect to triglyceride, total cholesterol, HDL and LDL levels, while the healthy group had more adverse features in fasting blood glucose, HbA1c and blood pressure measurements, with one subject with normal BMI on two antihypertensive medications. Although trend towards adverse lab values was demonstrated in both groups for specific metabolic lab measurements, most subjects remained within the range of normal reference values. Of note, within the normal weight cohort, one subject had significantly abnormal lab values (fasting blood glucose 147 mg/dL, HbA1c 7.8%), which may have skewed the group average.
Discussion
Population-based studies have reported a 59% higher rate of obesity and 88% higher rate of physical inactivity in SCI compared to the general population,34 but there is currently no accepted standard to identify obesity in individuals with SB. Although BMI (calculated using patient height) is used in the general population to identify individuals at elevated risk for obesity-related illness, it may not be a reliable indicator of body composition in adults with physical disabilities, with some suggesting lowering standard cutoffs for overweight and obese BMI in this population.35 While alternative measures of obesity have been suggested in the SB population, the accepted standard method of measurement remains controversial.
Conventional BMI calculations using height may be confounded in the SB population due to inability to use standard stadiometer or joint contractures.36,37 Use of arm span in the calculation of BMI in adults with SB has been reported,13 but others have suggested inaccuracies due to decreased torso length.36 Traditional anthropometric indices may be further limited in utility in adults with SCI or SB as these individuals tend to have lower lean tissue mass and higher fat mass,38 suggesting that measures that focus on body composition, such as bioelectrical impedance analysis (BIA), abdominal circumference or DXA, may be more accurate.39,40
In this study, we compared conventional measurements of BMI using height to alternate methods of anthropometric measurement in adults with SB, a previously infrequently studied population of growing proportions and importance. By conventional BMI using height, 43.8% of subjects were obese. However, when using alternative measures, increase in obesity rate in the same population was demonstrated. When BMI was calculated using arm span, 12.5% of subjects were reclassified to obese. A significant increase in subjects classified as obese was seen when subjects were assessed using anthropometric measures that focused on body composition and fat distribution. Abdominal girth re-categorized 29.4% of subjects initially deemed of normal BMI by conventional measures by height to obese, for a total of 82.4% obese. Trunk fat percentage by DXA similarly categorized 83.3% of subjects as obese. In other words, by using measurements that have been shown to better assess true body composition in the SCI population, obesity was identified almost two-fold as frequently, suggesting that a large portion of subjects thought to be of normal BMI by conventional measures may actually be obese.
In the general population, obesity and increased risk for metabolic syndrome have been associated with morbidity and mortality related to cardiovascular disease and diabetes.7–11 SCI literature has suggested waist circumference is more strongly correlated with hyperlipidemia than BMI, and may be a more clinically relevant measure of adiposity.37 Furthermore, waist circumference and hyperlipidemia have been shown in multiple studies in adults with SCI to be associated with a higher Framingham 30-year CVD risk score, earlier development of cardiovascular disease.41–44 Similar research investigating cardiovascular disease in adults with SB is lacking, although one study identified risk factors for cardiovascular disease in 42% of young people with SB, with an association with physical activity45 while another found a 32.4% prevalence of metabolic syndrome in adolescents with SB.46 Identifying adults with SB at higher risk for metabolic syndrome, cardiovascular disease and diabetes would facilitate early prevention and intervention, and should be a priority to clinicians caring for a growing adult population.
Our results show no statistically significant difference in laboratory metabolic values between obese and normal BMI adults with SB, although the small cohort of people examined likely limits ability to detect statistical significance. Of interest, despite 20% of adults with laboratory values suggesting hyperlipidemia, none were prescribed medication for hyper-lipidemia. Similarly, 56% of adults had documented blood pressures >130/85 mm Hg, but only one was prescribed antihypertensive medication.
Adults with SB, regardless of obesity, tended to report an average satisfaction with life (SWLS questionnaire) and mental and physical quality of life (SF-12v2 questionnaire) scores comparable to average scores in the United States. While no statistically significant difference in any questionnaire elements between the two groups was demonstrated, several findings are of importance regarding overall patterns and attitudes towards exercise. Using the B-PADS questionnaire, most adults with SB (88.9%) expressed a desire to join an exercise program, which was discordant with the proportion actually reporting a history of ever exercising in their lifetime (77.8%) and an even smaller portion exercising regularly (55.6%). One-fourth of adults surveyed expressed concerns about exercising at a regular gym due to their physical limitations, and did not feel that a gym coordinator would be able to help set up an exercise program for them. While 100% of adults with SB had been instructed by a physician in the past to exercise, unfortunately, only half report being given specifics as to how to do so, indicating as clinicians caring for this population, significant improvements in education may help facilitate exercise. While a minority of subjects identified family or work responsibilities as a restriction for exercise, it appears that the most common reasons cited as an obstacle for exercise are monetary concerns and lack of knowledge of appropriate gyms and exercises to meet their needs.
Despite the advantages of this study in a relatively infrequently studied population, there are several limitations that deserve mention. Firstly, the small number of adults with SB included limits the generalizability of results and ability to detect statistically significant differences. Secondly, while we demonstrate an important finding of high rate of reclassification of adults with SB as obese, metabolic and clinical correlation is limited by the snapshot of lab values and vital signs. Ideally, long-term follow-up to determine lifetime risk for diabetes, hypertension, hyperlipidemia and cardiovascular morbidity and mortality would be assessed relative to earlier anthropometric measurements of obesity. Finally, with any small cohort study of subjects, selection bias for inclusion and subject mix not representative of the overall adult SB population from a tertiary referral institution may be a confounder.
Conclusion
Currently no standard measure of adiposity and obesity in adults with SB exists, with current literature suggesting that conventional measures of BMI are flawed in this population. In comparing conventional measurements of BMI using height to alternate methods of anthropometric measurement in adults with SB, BMI measurements using height may underestimate true incidence of obesity in this population by almost half. In this study, abdominal circumference and DXA trunk fat percentage were most sensitive in determining obesity.
Activity level, quality of life and attitudes toward exercise were similar regardless of obesity. While exercise is accepted as important by both clinicians and adults with SB, actual execution of exercise may be limited by adequate instruction and accessibility to appropriate facilities. Earlier incorporation of community based physical activity may help change the frequency of exercise behavior in adults with disabilities as they age, and contribute to reduced risk of obesity and potentially of metabolic syndrome. Clinicians should discuss these issues early and often, and provide national and local resources for adaptive sports and recreation.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest None
Ethics approval None.
Declarations The authors have no sources of funding or disclosures to report.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM.. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311(8):806–14. doi: 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Physical activity among adults with a disability—united states, 2005. MMWR Morb Mortal Wkly Rep 2007;56(39):1021–4. [PubMed] [Google Scholar]
- 3.State-specific prevalence of obesity among adults with disabilities -- eight states and the District of Columbia, 1998–1999. MMWR Morb Mortal Wkly Rep 2002;51(36):805–8. [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH.. Prevalence of the metabolic syndrome among US adults: findings from the third national health and nutrition examination survey. JAMA 2002;287(3):356–9. doi: 10.1001/jama.287.3.356 [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. . Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 6.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS.. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol 2013;62(10):921–5. doi: 10.1016/j.jacc.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kershaw EE, Flier JS.. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89(6):2548–56. doi: 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Parker JL, Lugus JJ, Walsh K.. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11(2):85–97. doi: 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prospective Studies C, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. . Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundstrom J, Riserus U, Byberg L, Zethelius B, Lithell H, Lind L.. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ 2006;332(7546):878–82. doi: 10.1136/bmj.38766.624097.1F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juonala M, Jarvisalo MJ, Maki-Torkko N, Kahonen M, Viikari JS, Raitakari OT.. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the cardiovascular risk in young finns study. Circulation 2005;112(10):1486–93. doi: 10.1161/CIRCULATIONAHA.104.502161 [DOI] [PubMed] [Google Scholar]
- 12.Wong LY, Paulozzi LJ.. Survival of infants with spina bifida: a population study, 1979–94. Paediatric Perinat Epidemiol 2001;15(4):374–8. doi: 10.1046/j.1365-3016.2001.00371.x [DOI] [PubMed] [Google Scholar]
- 13.Dosa NP, Foley JT, Eckrich M, Woodall-Ruff D, Liptak GS.. Obesity across the lifespan among persons with spina bifida. Disabil Rehabil 2009;31(11):914–20. doi: 10.1080/09638280802356476 [DOI] [PubMed] [Google Scholar]
- 14.van den Berg-Emons HJ, Bussmann JB, Meyerink HJ, Roebroeck ME, Stam HJ.. Body fat, fitness and level of everyday physical activity in adolescents and young adults with meningomyelocele. J Rehabil Med 2003;35(6):271–5. doi: 10.1080/16501970310012400 [DOI] [PubMed] [Google Scholar]
- 15.Shepherd K, Hickstein R, Shepherd R.. Neurogenic faecal incontinence in children with spina bifida: rectosphincteric responses and evaluation of a physiological rationale for management, including biofeedback conditioning. Aust Paediatr J 1983;19(2):97–9. [DOI] [PubMed] [Google Scholar]
- 16.Coutinho T, Goel K, Correa de Sa D, Carter RE, Hodge DO, Kragelund C, et al. . Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of "normal weight central obesity". J Am Coll Cardiol 2013;61(5):553–60. doi: 10.1016/j.jacc.2012.10.035 [DOI] [PubMed] [Google Scholar]
- 17.Carmienke S, Freitag MH, Pischon T, Schlattmann P, Fankhaenel T, Goebel H, et al. . General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr 2013;67(6):573–85. doi: 10.1038/ejcn.2013.61 [DOI] [PubMed] [Google Scholar]
- 18.McDonald CM, Abresch-Meyer AL, Nelson MD, Widman LM.. Body mass index and body composition measures by dual x-ray absorptiometry in patients aged 10 to 21 years with spinal cord injury. J Spinal Cord Med 2007;30 Suppl 1:S97–104. doi: 10.1080/10790268.2007.11754612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazess RB, Barden HS, Bisek JP, Hanson J.. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 1990;51(6):1106–12. [DOI] [PubMed] [Google Scholar]
- 20.Mazess RB, Barden HS, Hanson JA.. Body composition by dual-photon absorptiometry and dual-energy x-ray absorptiometry. Basic Lide Sci 1990;55:427–32. [DOI] [PubMed] [Google Scholar]
- 21.Chow YW, Inman C, Pollintine P, Sharp CA, Haddaway MJ, el Masry W, et al. . Ultrasound bone densitometry and dual energy X-ray absorptiometry in patients with spinal cord injury: a cross-sectional study. Spinal Cord 1996;34(12):736–41. doi: 10.1038/sc.1996.134 [DOI] [PubMed] [Google Scholar]
- 22.Buffart LM, van den Berg-Emons RJ, van Wijlen-Hempel MS, Stam HJ, Roebroeck ME.. Health-related physical fitness of adolescents and young adults with myelomeningocele. Eur J Appl Physiol 2008;103(2):181–8. doi: 10.1007/s00421-008-0684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruinings AL, van den Berg-Emons HJ, Buffart LM, van der Heijden-Maessen HC, Roebroeck ME, Stam HJ.. Energy cost and physical strain of daily activities in adolescents and young adults with myelomeningocele. Dev Med Child Neurol 2007;49(9):672–7. doi: 10.1111/j.1469-8749.2007.00672.x [DOI] [PubMed] [Google Scholar]
- 24.Buffart LM, Roebroeck ME, Rol M, Stam HJ, van den Berg-Emons RJ, Transition Research Group South-West N Triad of physical activity, aerobic fitness and obesity in adolescents and young adults with myelomeningocele. J Rehab Med 2008;40(1):70–5. doi: 10.2340/16501977-0135 [DOI] [PubMed] [Google Scholar]
- 25.Widman LM, Abresch RT, Styne DM, McDonald CM.. Aerobic fitness and upper extremity strength in patients aged 11 to 21 years with spinal cord dysfunction as compared to ideal weight and overweight controls. J Spinal Cord Med 2007;30 Suppl 1:S88–96. doi: 10.1080/10790268.2007.11754611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ausili E, Focarelli B, Tabacco F, Fortunelli G, Caradonna P, Massimi L, et al. . Bone mineral density and body composition in a myelomeningocele children population: effects of walking ability and sport activity. Eur Rev Med Pharmacol Sci 2008;12(6):349–54. [PubMed] [Google Scholar]
- 27.Diener E, Emmons RA, Larsen RJ, Griffin S.. The Satisfaction With Life Scale. J Pers Assess 1985;49(1):71–5. doi: 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- 28.Graham HK, Harvey A, Rodda J, Nattrass GR, Pirpiris M.. The Functional Mobility Scale (FMS). J Pediatr Orthop 2004;24(5):514–20. doi: 10.1097/01241398-200409000-00011 [DOI] [PubMed] [Google Scholar]
- 29.Rimmer JH, Rubin SS, Braddock D.. Barriers to exercise in African American women with physical disabilities. Arch Phys Med Rehabil 2000;81(2):182–8. doi: 10.1016/S0003-9993(00)90138-2 [DOI] [PubMed] [Google Scholar]
- 30.Ware J, Kosinski M, DM T-B, Gandek B.. User's Manual for the SF-12v2® Health Survey With a Supplement Documenting SF-12® Health Survey. Lincoln, RI: QualityMetric Incorporated 2002. [Google Scholar]
- 31.Rodriguez G, Moreno LA, Blay MG, Blay VA, Garagorri JM, Sarria A, et al. . Body composition in adolescents: measurements and metabolic aspects. Int J Obes Relat Metab Disord 2004;28 Suppl 3:S54–8. doi: 10.1038/sj.ijo.0802805 [DOI] [PubMed] [Google Scholar]
- 32.Lean ME, Han TS, Morrison CE.. Waist circumference as a measure for indicating need for weight management. BMJ 1995;311(6998):158–61. doi: 10.1136/bmj.311.6998.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, et al. . 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: a report of the american college of cardiology/american heart association task force on practice guidelines. Circulation 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 34.Rimmer JH, Wang E, Yamaki K, Davis B.. Documenting Disparities in Obesity and Disability. Austin, TX: National Center for the Dissemination of Disability Research; 2009. [Google Scholar]
- 35.Weaver FM, Collins EG, Kurichi J, Miskevics S, Smith B, Rajan S, et al. . Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review. Am J Phys Med Rehabil 2007;86(1):22–9. doi: 10.1097/PHM.0b013e31802b8937 [DOI] [PubMed] [Google Scholar]
- 36.Rotenstein D, Adams M, Reigel DH.. Adult stature and anthropomorphic measurements of patients with myelomeningocele. Eur J Pediatr 1995;154(5):398–402. doi: 10.1007/BF02072114 [DOI] [PubMed] [Google Scholar]
- 37.Buchholz AC, Bugaresti JM.. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal cord 2005;43(9):513–8. doi: 10.1038/sj.sc.3101744 [DOI] [PubMed] [Google Scholar]
- 38.Rajan S, McNeely MJ, Warms C, Goldstein B.. Clinical assessment and management of obesity in individuals with spinal cord injury: a review. J Spinal Cord Med 2008;31(4):361–72. doi: 10.1080/10790268.2008.11760738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchholz AC, McGillivray CF, Pencharz PB.. The use of bioelectric impedance analysis to measure fluid compartments in subjects with chronic paraplegia. Arch Phys Med Rehabil 2003;84(6):854–61. doi: 10.1016/S0003-9993(02)04950-X [DOI] [PubMed] [Google Scholar]
- 40.Eriks-Hoogland I, Hilfiker R, Baumberger M, Balk S, Stucki G, Perret C.. Clinical assessment of obesity in persons with spinal cord injury: validity of waist circumference, body mass index, and anthropometric index. J Spinal Cord Med 2011;34(4):416–22. doi: 10.1179/2045772311Y.0000000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravensbergen HR, Lear SA, Claydon VE.. Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury. J Neurotrauma 2014;31(3):292–300. doi: 10.1089/neu.2013.3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauman WA, Spungen AM.. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am 2000;11(1):109–40. [PubMed] [Google Scholar]
- 43.Bauman WA, Spungen AM, Raza M, Rothstein J, Zhang RL, Zhong YG, et al. . Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med 1992;59(2):163–8. [PubMed] [Google Scholar]
- 44.Wilt TJ, Carlson KF, Goldish GD, MacDonald R, Niewoehner C, Rutks I, et al. . Carbohydrate and lipid disorders and relevant considerations in persons with spinal cord injury. Evid Rep Technol Assess (Full Rep) 2008;(163):1–95. [PMC free article] [PubMed] [Google Scholar]
- 45.Buffart LM, van den Berg-Emons RJ, Burdorf A, Janssen WG, Stam HJ, Roebroeck ME.. Cardiovascular disease risk factors and the relationships with physical activity, aerobic fitness, and body fat in adolescents and young adults with myelomeningocele. Arch Phys Med Rehabil 2008;89(11):2167–73. doi: 10.1016/j.apmr.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 46.Nelson MD, Widman LM, Abresch RT, Stanhope K, Havel PJ, Styne DM, et al Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med 2007;30 Suppl 1:S127–39. doi: 10.1080/10790268.2007.11754591 [DOI] [PMC free article] [PubMed] [Google Scholar]


