Abstract
Aim:
We studied externally controlled anticancer effects of binding tumor growth inhibiting synthetic peptides to magnetoelectric nanoparticles (MENs) on treatment of glioblastomas.
Methods:
Hydrothermally synthesized 30-nm MENs had the core–shell composition of CoFe2O4@BaTiO3. Molecules of growth hormone-releasing hormone antagonist of the MIA class (MIA690) were chemically bound to MENs. In vitro experiments utilized human glioblastoma cells (U-87MG) and human brain microvascular endothelial cells.
Results:
The studies demonstrated externally controlled high-efficacy binding of MIA690 to MENs, targeted specificity to glioblastoma cells and on-demand release of the peptide by application of d.c. and a.c. magnetic fields, respectively.
Conclusion:
The results support the use of MENs as an effective drug delivery carrier for growth hormone-releasing hormone antagonists in the treatment of human glioblastomas.
Keywords: : cancer/oncology, gene/drug delivery, nanoparticles
Targeted cancer therapy remains a major challenge in the eradication of tumors. Drug delivery systems using nanoparticles offer a novel approach to specifically delivering drugs to cancer cells while sparing normal cells [1–4]. Glioblastoma multiforme (GBM) is the most frequent malignant primary brain tumor representing 32% of all brain tumor cases in adults, and 81% of malignant tumors [5]. With the current treatment approach including surgical resection and radiotherapy with concomitant chemotherapy, GBM still has a grim prognosis with a median overall survival of 15–18 months [6]. A major challenge in the treatment of GBM is to deliver therapeutics across the blood–brain barrier (BBB), a network of specialized brain endothelial cells with intercapillary distances of approximately 40 μm that tightly regulate ionic composition, prevent macromolecules and unwanted cells from entering the brain to protect the CNS from neurotoxins [7,8]. Moreover, it is believed that antitumor drugs cannot reach glioblastoma stem-like cells because they hide in a hypoxic microenvironment near BBB vasculature [9]. The use of targeted drug delivery with relatively small magnetically directed nanoparticles (<40 nm) has been demonstrated as an efficient way to cross BBB, target cancerous cells and permit an on-demand release of antitumor drugs [10].
Recently developed magnetoelectric nanoparticles (MENs) are a novel class of nanoparticles to enable targeted drug delivery across BBB and externally controlled release deep in the brain [11–14]. Due to the multiferroic physics of MENs, these nanoparticles offer properties that cannot be achieved by any other type of nanoparticles, such as traditional ferromagnetic nonsuperparamagnetic and superparamagnetic iron oxide nanoparticles and other magnetic or nonmagnetic metallic and polymer nanostructures. Like the traditional magnetic nanoparticles, MENs have a nonzero saturation magnetization and therefore can be navigated throughout the body by the application of magnetic field gradients, and localized using conventional image-guided magnetic approaches such as MRI and magnetic particle imaging. In addition, unlike the traditional nanoparticles, MENs display a quantum mechanically induced nonzero magnetoelectric (ME) effect; the ME effect allows a reciprocal control including a control of the nanoparticles’ electric dipole moments by a local magnetic field and a control of their magnetic moments by a local electric field, respectively. Because MENs’ electric dipole moment also defines drug carrier properties, mainly; the strength of the bond between the nanoparticle and the loaded drug, and the local electric field in MENs’ proximity, drug delivery and release also can be controlled via application of special sequences of d.c. and a.c. magnetic fields. Previously, through in vitro and in vivo experiments, the following properties have been demonstrated. First, MENs were shown to deliver antiretroviral therapy across BBB to eradicate HIV-1 virus hidden deep in the brain [15]. Second, drug-loaded MENs demonstrated a relatively high specificity to cancer cells by penetrating the cancer cell membrane, while sparing the surrounding healthy cells, and then releasing the drug intracellularly via application of d.c. and a.c. magnetic fields, respectively. The hypothesis of high-specificity targeted delivery has been verified through in vitro and in vivo studies on ovarian cancer in mice bearing SKOV-3 human ovarian carcinoma xenografts [11,12].
Hypothalamic growth hormone (GH)-releasing hormone (GHRH) regulates the synthesis and release of GH in the pituitary gland [16]. GHRH and its mRNA are expressed in many human cancers, suggesting that it may act as a tumor growth factor [17,18]. GHRH is specifically expressed in glioblastomas. GHRH antagonists have been studied as a treatment for this tumor type [19,20]. GHRH antagonists are a class of antitumorigenic peptides that block the release of insulin-like growth factor I (IGF-1), a tumor factor growth factor that plays an important role in the mechanism of malignant transformation, metastasis and tumorigenesis of various cancers, including GBM [21–23]. GHRH antagonists exert direct effects on GHRH receptors on tumor cells by reducing IGF-I and IGF-II in tumor tissue. GHRH antagonists also directly compete with autocrine/paracrine secretion of GHRH that is known to enhance cancer cell proliferation [24]. However, major challenges in treatment with GHRH antagonist include prolonging drug half-life and delivering drug across the BBB for localized drug release specifically to glioblastoma cells [25,26]. It is well established that the MIA class of GHRH antagonists exhibits high binding affinities to GHRH receptors and display anticancer properties [21,22,25–32]. MIA690 is a synthetic peptide that belongs to the class of MIA GHRH antagonists and has been recently developed as a promising treatment for glioblastomas [32,33].
Combining the effectiveness of GHRH antagonists and the MEN-based externally controlled targeted drug delivery may provide a promising therapy in treating GBM. However, open questions include whether MIA690 peptides can bind to MENs with sufficient affinity to penetrate human glioblastoma cell membranes and then be released on-demand from MENs into the intracellular space following a sequence of externally applied d.c. and a.c. magnetic fields according to the MENs’ ME physics. Furthermore, it is important to demonstrate that MENs can avoid uptake in nonmalignant cells of the brain vasculature when exposed to equivalent d.c. field gradients and thus minimize any collateral damage.
Therefore, the primary aims of this paper are to present an in vitro study in which MIA690 is efficiently bound to MENs as a drug delivery carrier, MIA690-loaded MENs can deliver GHRH to human glioblastoma cell membranes via application of a d.c. magnetic field and release of MIA690 through the application of an a.c. magnetic field without thermal damage. We demonstrate that the unique properties of MENs allow it to specifically target human glioblastoma cells, providing a potential nanotechnology solution to overcome challenges in the treatment of GBM.
Materials & methods
Materials
MENs were synthesized using chemicals purchased from Sigma–Aldrich (MO, USA). Cellular experiments in vitro utilized the human glioblastoma cell line (U-87MG) and human brain microvascular endothelial cells (HBMECs) obtained from the commercial provider American Tissue Culture Collection (VA, USA). Modified Eagle's medium (MEM), Dulbecco's Modified Eagle's medium (DMEM), fetal bovine serum (FBS) from Gibco (NY, USA) and penicillin-streptomycin (penstrep) were obtained from Science–Cell, Inc. (CA, USA). For fluorescence experiments, fluorescein isothiocyanate (FITC) amine reactive dye and 4′,6-Diamidino-2-Phenylindole (DAPI) were purchased from Thermo Fisher Scientific (MA, USA). All reagents met or exceeded ACS standards for procedures requiring stringent quality specifications.
Synthesis & characterization of magnetoelectric nanoparticles
MENs were synthesized in our laboratory by conventional methods [34]. In the first step, CoFe2O4 core particles were prepared by the standard hydrothermal method. Thus, 0.58 g of Co(NO3)2•6H2O and 0.16 g of Fe(NO3)3•9H2O were dissolved in 150 ml of aqueous solution. An aqueous mixture of polyvinylpyrrolidone (0.2 g) and sodium borohydride (0.9 g) was then added and stirred at 70°C for 12 h. The precursor solution of BaTiO3 was prepared by mixing 174 mg of BaCo3 and 5 g citric acid with 240 μl titanium isopropoxide in ethanolic solution. CoFe2O4 cores were added to the BaTiO3 precursor solution and sonicated until fully dispersed (∼2 h). The mixture was heated at 90°C with continuous stirring overnight to form a milky opaque gel. The gel was placed in the KSL-1100x high-temperature muffle furnace from MTI Corporation (CA, USA) to calcine at 600°C for 5 h with controlled ramping temperatures to obtain core–shell of MENs of approximately 30 nm diameter. Size distribution of MENs was confirmed by atomic force microscope imaging to assess grain height using the Bruker Nanoscope IIIa Multimode (MA, USA) and TEM imaging using the FEI Talos F200X instrument (OR, USA).
Conjugation of growth hormone-releasing hormone antagonist peptide MIA690 to magnetoelectric nanoparticles
Five grams of 30 nm CoFe2O4-BaTiO3 core–shell MENs were resuspended in 1 ml sterile phosphate-buffered saline (PBS, pH 7.4) and sonicated for 1 min. To improve the biocompatibility of MENs, 0.1 ml of glycerol monooleate (GMO) was mixed with the MEN core–shell and rotated for 1 h. GMO-MENs were washed thrice with PBS, then N-(3-Dimethylaminopropyl)-N′-ethyl-carbodiimide hydrochloride (EDC) at 1 mg/ml concentration was added to the solution and incubated for 1 h by slow mechanical stirring. The GHRH antagonist, synthesized by R-Z Cai and AV Schally using solid-phase methods was conjugated to the functionalized GMO-MENs to produce a stock solution of MIA690-GMO-MENs that could be mixed with cell culture media to a final peptide concentration of 1 μM MIA690. The treatment with GHRH antagonists at the concentration of 1 μM has previously reduced cell viability and generated antitumor effects in GBM cell culture [26]. To determine the binding efficacy of MIA690 to GMO-MENs, stock solution was centrifuged at 4000 r.p.m. to separate unbound peptide from the pellet. The supernatant was resuspended in PBS and absorbance was measured spectrophotometrically and concentrations were plotted against a calibration curve of known MIA690 concentration.
Cell culture
U-87MG cells were grown in T-25 flasks seeded with 0.5 × 106 cells/flask and cultured with MEM media supplemented with 5% FBS and 1% penstrep. HBMECs were grown in DMEM media supplemented with 10% FBS and 1% penstrep. Cell cultures were incubated at 37°C with 5.0% CO2 in a humidified atmosphere. Media was replaced every 2–3 days until cells reached confluency. Confluent cells were detached with 0.25% trypsin/EDTA solution and reseeded.
Drug uptake experiments were performed at 80% confluency, and data were collected at 24 and 48 h post-treatment with MIA690-loaded MENs or MIA690 alone. Samples were read in duplicate and triplicate when necessary to obtain acceptable % CV values.
In vitro treatment
Cells were treated with MIA690 alone or MIA690-GMO-MENs in fresh media at a concentration of 1 μM of MIA690. Equivalent volumes of the vehicle solutions (‘naked’ GMO-MENs or PBS) were added to the media as controls. Each treatment group (MIA690, MIA690-GMO-MENs, GMO-MENs, PBS) was exposed to several magnetic field conditions to test the effect of a d.c. magnetic field gradient (reported in Oe) to induce penetration into U-87MG cells. Subsequently, selected treatment groups were exposed to an a.c. magnetic field (reported in Hz) to examine the on-demand release of MIA690 from the nanoparticle. The duration of exposure to a magnetic field was controlled to determine the optimal exposure time that would improve MIA690 uptake. We chose a combination of treatment groups based on prior MEN data [11,12,15]; U-87MG cells treated with MIA-GMO-MENs were exposed to the following magnetic fields in sequence: no field, 0 Oe + 0 Hz; 2 h 100 Oe + 0 Hz; 2 h 100 Oe + 50 Hz 30 min; 2 h 100 Oe + 50 Hz 2 h; 12 h 100 Oe + 50 Hz 30 min; 12 h 100 Oe + 50 Hz 2 h (Supplementary Figure 1).
Intracellular release of growth hormone-releasing hormone antagonist MIA690 from magnetoelectric nanoparticles in glioblastoma multiforme
To determine the amount of intracellular uptake and release of MIA690, the amount of free drug in the cell lysate was quantified spectrophometrically. It is accepted that cell lysate components represent the intracellular content of cells [35]. Following the treatment and exposure to magnetic fields, U-87MG cells were allowed to incubate further for 24 or 48 h. Then media was discarded and cells were washed with PBS and detached with 0.25% trypsin/EDTA solution. Cells were collected in a conical tube, pelleted by centrifugation and washed thrice with ice-cold PBS. Cells were resuspended in 1 ml DMSO and sonicated briefly to induce cell lysis. After 1 h of incubation at 37°C, the solution was centrifuged at 10,000 r.p.m. for 5 min to pellet cellular debris and MENs. The supernatant containing the cell lysate was collected to measure the intracellular concentration of MIA690.
Quantification of MIA690 uptake in cell lysate
An aliquot (20 μl) of cell lysate from each treatment group was diluted in buffer and measured spectrophometrically on scan mode to capture peak signatures of the peptide. A standard curve was generated by resuspending known concentrations of MIA690 and creating serial dilutions. Peak absorbance of MIA690 was captured at the maximum wavelength of 220 nm. MIA690 uptake was normalized to the protein content of cells lysate, which is representative of the number of cells in culture. Protein content was determined immediately after collection to avoid protein degradation with the Bradford method using Bio–Rad Protein Assay Kit at an absorbance of 595 nm according to Bio–Rad protocol (CA, USA). Final results were reported as % MIA690 per microgram protein. Spectrophometric measurements were performed by Cary 100 UV-Vis spectrophotometer (CA, USA).
Imaging intracellular uptake & specificity of magnetoelectric nanoparticles in U-87MG cells
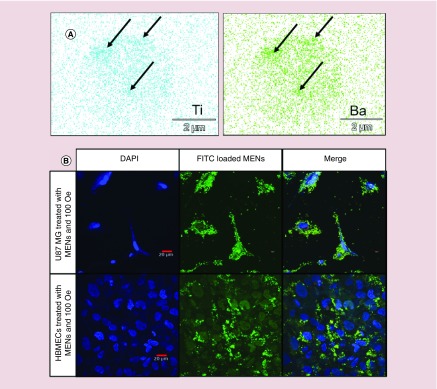
Cell lysate from each treatment group 24 h after treatment was collected for imaging with electron-dispersive spectroscopy (EDS) mode of scanning electron microscopy (SEM). EDS-SEM detects signature traces of Ba and Ti that are unique to MENs’ outer shell. The presence of Ba and Ti in cell lysate indicates efficient nanoelectroporation of U-87MG cells. One drop of lysate (∼10 μl) diluted in TE buffer was dried on a precleaned Si wafer and placed on copper tape mounting for imaging with a JEOL JIB-4500 multibeam system (focussed ion beam/SEM; MA, USA). The Thermo Scientific Noran system 7 performed EDS analysis. Intracellular uptake of GMO-MENs was observed by comparing the density of Ba and Ti signal in each image. ICP–MS confirmed the presence of Ba and Ti in the cell lysate.
For specificity data of MENs, malignant U-87MG cells and nonmalignant HBMECs were cultured as previously described and treated with FITC-loaded MENs (FITC MENs) and DAPI for nuclear staining. U-87MG cells and HBMECs were exposed to a magnetic field gradient of 100 Oe and intracellular uptake of MENs was quantified using a confocal fluorescent microscope Olympus Fluoview FS1200 (PA, USA).
Cell viability & magnetic nanoparticle hyperthermic effect
In vitro cell viability of U-87MG cells after treatment with MIA690 or MIA690-GMO-MENs and magnetic field exposure was determined using the trypan blue protocol [36]. Experimental conditions were identical to those of the treatment groups described previously. Reported results are relative to the control group treated with a PBS vehicle solution.
The magnetic fields used to induce nanoelectroporation may cause heat dissipation known to produce hyperthermic effects resulting in cell death. Infrared (IR) imaging captured by the FlIR-i3 camera (OR, USA) measured heat dissipation at each stage of treatment to ensure that cell culture temperatures remained within normal ranges. Temperatures were captured at the maximal magnetic field exposures.
Statistical analysis
Results are presented as mean ± standard deviation or percentages. Student's t-test and one-way ANOVA with post hoc analyses were conducted to assess significant differences between treatment groups. Linear regression models were used to assess relationships between variables of interest. Statistical analysis was performed using IBM SPSS software (IL, USA). P-values < 0.05 were considered statistically significant.
Results
Characterization of magnetoelectric nanoparticles
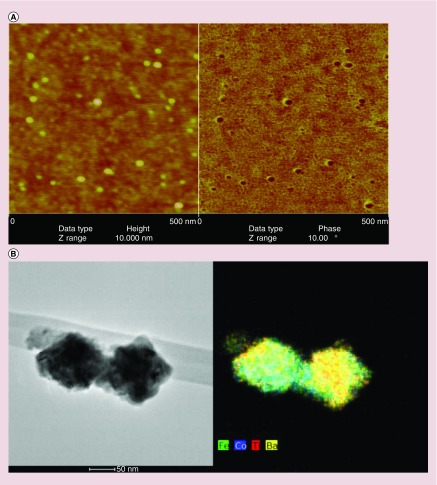
Atomic force microscope and TEM-EDS images of 30-nm MENs are shown in Figure 1A & B, respectively. X-ray diffraction confirmed the tetragonal crystal structure of BaTiO3 shell (space group P4 mm, a = 3.9940 Å, c = 4.0380 Å) and the cubic structure of CoFe2O4 cores (data not shown). Vibrating sample magnetometry results showed that the magnetic moment/coercivity of the CoFe2O4 core was approximately 1 emu/g and that of the core–shell particles was 40 emu/g.
Figure 1. . Atomic force microscope and transmission electron microscope images of core–shell structure of magnetoelectric nanoparticles.
(A) AFM images taken at 5.0 × 5.0 μm and a vertical scale of 0.10 μm. (B) Particle agglomeration occurs during preparation (drying) of colloidal stable fluids for TEM investigation. (C) EDS mapping of elemental composition of MENs. Scale bar is 50 nm.
AFM: Atomic force microscope; EDS: Electron-dispersive spectroscopy; MEN: Magnetoelectric nanoparticle.
Growth hormone-releasing hormone antagonist MIA690-loaded magnetoelectric nanoparticles
To ensure adequate biocompatibility, MENs were coated with GMO before conjugation with MIA690, as described in detail in ‘Materials & methods’ section. Using GMO ensured that the fields necessary for inducing local electroporation of the cell membrane and consequent release of the peptide into the cell were within adequate ranges of magnitudes (∼<100 Oe) and frequencies (∼<1000 Hz). The GMO functionalization approach has been previously described [37]. The effectiveness of the conjugation procedure was tested by creating a standard calibration curve from MIA690 stock solution and measuring the absorbance maxima of the unbound MIA690. The retained percentage of MIA690-GMO-MENs, P, was calculated using the following expression:
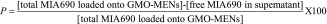 |
Results indicated that the functionalized GMO-MENs carrier could retain 72.7% of MIA690 (Figure 2).
Figure 2. . Bioconjugation of MIA690 to glycerol monooleate-magnetoelectric nanoparticles.
GMO-MENs can effectively bind MIA690 at room temperature in PBS solution (pH = 7.4).
GMO-MEN: Glycerol monooleate-magnetoelectric nanoparticle; PBS: Phosphate-buffered saline.
Field-controlled uptake & on-demand release of MIA690 in U-87MG cells
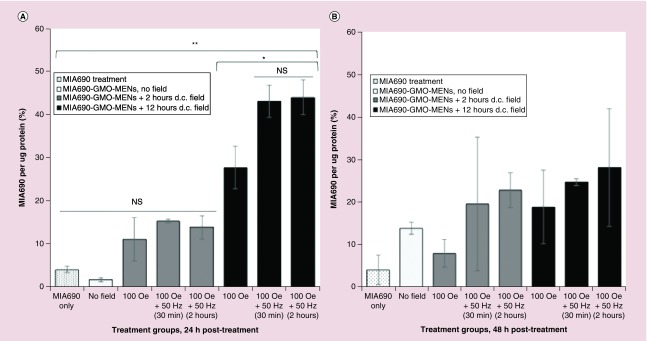
Several control and experimental U-87MG cell treatments were utilized to compare the effects of drug delivery treatment versus treatment alone (outlined in Materials & methods section). Briefly, cells were treated with PBS vehicle solution; GMO-MEN vehicle solution; MIA690 treatment only; and GMO-MENs + MIA690 treatment. Each treatment group was exposed to magnetic fields categorized as ‘before on-demand release’ (d.c. fields only) or ‘after on-demand release’ (d.c. field then a.c. field). Duration of magnetic field exposure was introduced as a third variable to investigate the effect of time on intracellular drug release (i.e., 2 or 12 h d.c. exposure + 30 min or 2 h a.c. exposure). Data were collected at two separate time points (24 and 48 h after field application) in replicates of at least n = 2. Free MIA690 drug in cell lysate was measured spectrophometrically against a standard calibration curve of MIA690, and the results were normalized to the protein content in the cell lysate. The absorbance maxima of MIA690 in cell lysate was 220 nm, which corresponds to the peptide bonds of MIA690. All necessary steps were taken in absorbance measurements (viz. blank lysates) to minimize the interference from cell components with MIA690 and normalized by the Bradford method. It should be noted that MIA690 needs to be released from the MENs to be bioactive and thus act on cell receptors. The time of release is dependent on the durations of the d.c. and a.c. field application, as shown in Figure 3. The increase in the incubation time with MENs complicates the estimation of membrane-bound GHRH because a fraction of MENs (with unreleased GHRH) may enter the cell (at 12 h). Therefore, we have reported the measured GHRH levels in the cell lysate. The Bradford method to detect proteins was used to normalize drug content in lysate relative to the number of cells in the sample. The Bradford method is reported to detect peptides >3000 kDa, which is beyond the limit of MIA690 (MW = 3934 Da). To ensure the peptide MIA690 did not significantly contribute to absorbances obtained by the Bradford method, we measured the maximum concentration of the peptide treatment (1 μM MIA690) using the Bradford method. Results indicated absorbances of MIA690 were negligible compared with the average protein content of cells indicating that MIA690 is of interest but does not contribute to protein values (Supplementary Figure 2). Results from intracellular measurements suggest that MIA690 uptake increased significantly only in cells treated with MIA690-loaded MENs and exposed to 100 Oe for 12 h in lysate collected 24 h post-treatment. Drug uptake increased by a factor of 6.9 for MIA690 carried by field-controlled MENs exposed to an extended d.c. field compared with the drug administered alone, and this factor increased to 11 with on-demand release using an a.c. magnetic field for 2 h (Figure 3A). Notably, cells exposed to brief d.c. fields (∼2 h) did not significantly increase intracellular release of MIA690 regardless of a.c. field exposure.
Figure 3. . Growth hormone-releasing hormone antagonist (MIA690) uptake relative to protein content in cell lysate across treatment groups.
(A) Intracellular levels of MIA690 increased significantly (p < 0.01) when U-87MG cells were treated with MIA690 bound to GMO-MENs and exposed to a prolonged d.c. magetic field (12 h) compared with MIA690 treatment alone. Release of MIA690 was further increased with the application of an a.c. field (p < 0.05). Intracellular levels of MIA690 did not differ significantly when drug was free in media or delivered bound to MEN carrier and not exposed to a magnetic field or a short duration of magnetic field (∼2 h d.c. field). (B) Drug uptake 48 h post-treatment did not show significant differences between groups when measured in cell lysate. Reported values were normalized relative to control treatments of PBS or PBS + MENs only vehicle solutions.
*p < 0.05; **p < 0.01.
GMO-MEN: Glycerol monooleate-magnetoelectric nanoparticle; MEN: Magnetoelectric nanoparticle; NS: No significance; PBS: Phosphate-buffered saline.
In cell lysate measurements 48 h post-treatment, uptake of MIA690 is increased by a factor of at least 4.6 in cells treated with MIA690-loaded MENs and exposed to 100 Oe for 12 h with and without the application of an a.c. field (Figure 3B); however, these results did not reach statistical significance.
U-87MG cell viability decreases with MIA690-loaded magnetoelectric nanoparticle delivery
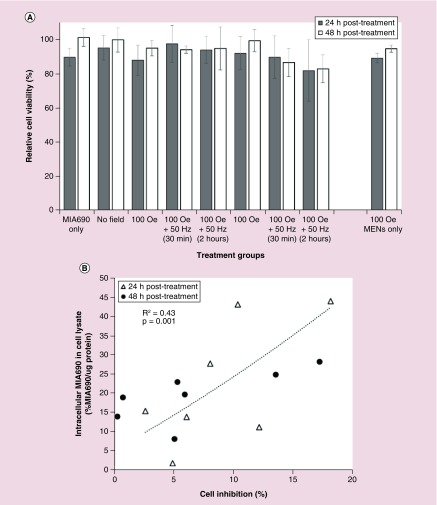
A key mechanism of action of the GHRH antagonist MIA690 is to inhibit cancer cell proliferation [32]. We investigated the effect on cell inhibition when MIA690 was delivered to U-87MG cells via field-controlled MENs as drug delivery vehicles. U-87MG cells were treated with MIA690 drug only, and MIA690 bound to GMO-MENs as a drug delivery vehicle. Vehicle controls included PBS only and GMO-MENs only under a magnetic field of 100 Oe. Viability was measured by taking an aliquot from the sample of cells and staining it with trypan blue, a dye that will distinguish between viable cells and dead cells in triplicate. Results show a decrease in cell viability at 24 h post-treatment when MIA690 was delivered through GMO-MENs and exposed to a field of 100 Oe d.c. for 12 h and 50 Hz for 2 h, however the results were not statistically significant compared with traditional treatment of MIA690 alone (-7.8%, p = 0.285). Cell death was enhanced at 48 h in cells treated with MIA690 bound to GMO-MENs and exposed to 100 Oe for 12 h then 50 Hz for 2 h compared with MIA690 treatment alone (-18.3%, p = 0.03) (Figure 4A). The relationship between % intracellular MIA690 levels and % cell inhibition is plotted in Figure 4B. There is a significant relationship between intracellular MIA690 levels and % cell inhibition, indicating that increased intracellular MIA690 is significantly associated with cell growth inhibition (R2 = 0.43, p = 0.001).
Figure 4. . Intracellular MIA690 release affects relative cell viability across treatment groups.
(A) Significant decrease in cell viability occurred in cells treated with MIA690-GMO-MENs for 12 h 100 Oe + 50 Hz for 2 h, compared with MIA treatment alone (-18.3%, p = 0.030). The results are mean ± standard error of mean of three independent measurements. (B) Intracellular MIA690 is significantly associated with cell growth inhibition in a linear regression model using data collected at 24 and 48 h post-treatment (R2 = 0.43, p = 0.001).
*p < 0.05 compared with ‘MIA690 only’ treatment group.
GMO: Glycerol monooleate; MEN: Magnetoelectric nanoparticle.
The effect of a low a.c. magnetic field on heat dissipation & cell viability
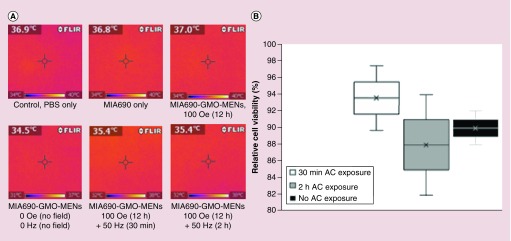
As expected, relatively low magnetic fields used to couple with MENs did not produce significant heating across treatment groups. The a.c. frequency used in this study is in the near-d.c. range and thus barely induces hysteresis-triggered thermal dissipation. FlIR-i3 IR imaging measured heat dissipation at each stage of field exposure to magnetic field. There were no significant differences between flask temperatures of control and GMO-MEN-treated flasks, regardless of the length of time or magentic field exposure (d.c. or a.c.). Cell viability did not differ significantly between GMO-MEN-treated cells with or without exposure to a.c. magnetic fields (Figure 5).
Figure 5. . The effect of a.c. magnetic fields on heat dissipation of magnetoelectric nanoparticles and cell viability.
(A) Heat images displaying surface temperatures of cell cultures treated with MENs compared with controls. No significant differences in temperatures were observed between groups. (B) Relative cell viability between groups exposed to varying durations of a.c. magnetic fields. Exposure to a.c. magnetic fields had no significant effects on cell viability.
MEN: Magnetoelectric nanoparticle.
Magnetoelectric nanoparticles specifically penetrate U-87MG cells
The intracellular uptake of MENs was imaged across treatment groups using EDS-SEM. The EDS-SEM provides an atomic-level elemental composition of the material and identifies atomic signatures of GMO-MENs. Because MENs are composed of a CoFe2O4 core with a BaTiO3 shell, the atomic signatures of Ba and Ti indicate the presence of MENs in the cell lysate. The samples showing the highest traces of Ba and Ti were those exposed to 100 Oe for 12 h compared with controls, indicating that MENs loaded with MIA690 effectively penetrate U-87MG cells with the application of a d.c. magnetic field (Figure 6A). ICP–MS was performed to quantify amounts of Ba and Ti in the cell lysate. Results showed that following d.c. treatment for 12 h, the average Ba concentration was 18.7 ± 2.7 μg/l and Ti concentration was 98.6 ± 2.5 μg/l, confirming the presence of MENs in cell lysate (data not shown).
Figure 6. . Magnetoelectric nanoparticle uptake in malignant glioblastoma cells using a relatively weak d.c. magnetic field.
(A) EDS-SEM images of signature trace elements Ti and Ba in the cell lysate of U-87MG cells treated with MIA-GMO-MENs exposed to 100 Oe d.c. magnetic field. The arrows indicate where the MENs are most concentrated in the sample. (B) Confocal images showing the specific interaction of MENs with malignant glioblastoma cells. FITC-MENs are specifically associated with U-87MG cells. Uptake is not evident in nonmalignant HBMECs.
EDS: Electron-dispersive spectroscopy; FITC: Fluorescein isothiocyanate; GMO: Glycerol monooleate; HBMEC: Human brain microvascular endothelial cell; MEN: Magnetoelectric nanoparticle; SEM: Scanning electron microscopy.
Specificity of MENs to target cancer cells was investigated by treating healthy cells (HBMECs) and cancer cells (U-87MGs) with fluorescently labeled MENs. FITC-loaded MENs were redispersed in cell media and exposed to a d.c. magnetic field (∼100 Oe) hypothesized to produce electroporation in cancer cells while sparing healthy cells. Results of confocal fluorescent images indicate that FITC-MENs are highly localized in the cytoplasm of the malignant U-87MG cells and no definite association is found with the nonmalignant HMBECs (Figure 6B).
Discussion
Current treatment modalities for malignant glioblastomas including surgery, radiotherapy, chemotherapy or their combinations are being applied with limited success [38–40]. GHRH antagonists are being developed to improve the outcome of chemotherapy and the survival of brain cancer patients and improve chemotherapy outcomes [16,21,41]. The beneficial effects of GHRH antagonist in experimental treatment models include suppression of the pituitary-hepatic IGF axis, and direct inhibition of the autocrine/paracrine activity of GHRH. However, delivery of GHRH antagonists to target sites in the brain is limited by their short half-life in circulation and relatively inefficient delivery to glioblastoma. Therefore, an approach for tightly binding GHRH antagonists to a carrier particle to avoid degradation, directing the drug-loaded conjugate across the BBB and controlling the release of the drug may provide improved treatment outcomes.
The use of MENs as drug delivery vehicles for cancer therapies is being studied both in vitro and in vivo [12,37]. Previously, we have shown that MENs as drug carriers can be loaded with a GHRH antagonist, administrated intravenously and then navigated through BBB via application of a d.c. magnetic field gradient. In general, MRI or magnetic particle imaging can be used as an imaging modality to provide an image guided delivery of MENs directly to tumor sites using d.c. magnetic field gradient. Recently, it has been shown that MENs can be localized to tumor sites due to a physical mechanism which acts independently of the enhanced permeability and retention effect [42]. According to this mechanism, drug-loaded and charged MENs are attracted to the tumor cells due to their more conductive membrane surface and the resulting stronger Coulomb force compared with that for the normal cells. When in close proximity to the tumor cell membrane, MENs induce local electroporation of the cell membrane, the effect known as the nanoelectroporation, which further pulls the nanoparticles inside the cells. The specificity exists because the nanoelectroporation threshold field for the malignant cells is significantly lower than that for the surrounding nonmalignant cells [43,44]. Upon entering into the cytosol, an on-demand release of the peptide into the intracellular microenvironment is achieved by application of an a.c. magnetic field. As described in detail in previous publications, the physical mechanism of the release relies on the strong a.c. field dependence of the bond affinity between the nanoparticle and the drug due to the ME effect [15].
The present data support the concept that MENs can effectively bind to the GHRH antagonist MIA690 at physiological pH and can deliver MIA690 more efficiently to malignant glioblastoma cells than the free drug alone in vitro. Furthermore, the data confirm that MENs exposed to a controlled magnetic field show specificity to malignant glioblastoma cells while sparing nonmalignant cells.
Conjugation of MIA690 to GMO-MENs using an EDC-linker resulted in 72% of MIA690 loaded onto the GMO-MEN carrier at physiological pH. This result is consistent with the binding capacity of chemotherapeutic drugs to MENs previously reported [11]. An important challenge in the administration of GHRH antagonists is their short half-life [25]. Various nanocarriers are emerging as delivery systems to improve half-life of therapeutic peptides [45]. The binding force between MIA690 and MENs at physiological pH indicates that GHRH antagonists may avoid early degradation when loaded onto MEN carriers in order to reach target sites. Thompson et al. have shown that iron oxide nanoparticles can be dragged across an in vitro BBB through the application of a magnetic field gradient. Furthermore, recent studies have shown that manganese ferric oxide particles coated with serum albumin can cross in vivo BBB in mice through adsorptive transcytosis and through the gap junctions. Since MENs possess similar properties, we can expect their transport across BBB through similar mechanisms with an added advantage of no thermal damage associated with conventional iron oxide nanoparticles. It is worth noting that MEN-based delivery and release of peptides across BBB is independent of the biochemical microenvironment; in contrast, it is mostly determined by externally controlled d.c. and a.c. magnetic fields. Thus, a logical approach to MEN-based drug delivery is adjusting magnetic field gradients in sequence to penetrate the BBB with a stronger magnetic field gradient, and subsequently target glioblastoma with a weaker magnetic field [46,47].
As a primary aim, we investigated the efficacy of MENs as a drug delivery system for the GHRH antagonist MIA690 to glioblastoma cells. We used U-87MG human glioblastoma malignant cells, which express the primary functional receptor responsible for mediating the effects of GHRH and GHRH antagonists in tumors [19,26]. Our results indicate that MENs loaded with MIA690 provided greater intracellular uptake compared with MIA690 treatment alone. Within a 24-h period, the percentage of MEN-mediated drug penetration was 6.9-times greater after a 12-h d.c. field exposure than after MIA690 treatment alone (p < 0.01). Penetration mediated by MENs was further enhanced with the subsequent application of an a.c. magnetic field, which increased an intracellular MIA690 uptake by a factor of 1.6 compared with 12-h d.c. treatment alone and 11-times compared with traditional free MIA690 treatment (p < 0.05 and p < 0.01, respectively). Our results show a significant relationship between MIA690 uptake and cell inhibition, and although the intracellular levels of MIA690 were not significant in cell lysate collected 48 h post-treatment, it may be a consequence of efflux of the GHRH antagonist, which is able to bind with high affinity to the GHRH membrane receptor and lead to an increase in cell death at 48 h. Taken together, the effect of MIA690 uptake at 24 h may be associated with a decline in U-87MG cell proliferation at 48 h post-treatment. To our knowledge, this is the first paper to demonstrate an intracellular drug delivery of a GHRH antagonist to glioblastoma cells and its effectiveness in enhancing inhibition of glioblastoma cells in vitro compared with traditional MIA690 treatment. Intracellular delivery of GHRH antagonists offers a novel modality to the existing GHRH treatments. Currently, the mechanism action of GHRH antagonists involves binding to external pituitary receptors to reduce secretion of GH that inhibits the release of IGF-I associated with tumorigenic effects in vivo, as well as its binding to GHRH receptors of local autocrine/paracrine tumor cells followed by internalization and increased tumorigenicity both in vivo and in vitro [17,24,48]. We demonstrated that MIA690-GMO-MENs wirelessly coupled to externally controlled magnetic fields increased intracellular levels of GHRH antagonist MIA690 in U-87MG glioblastoma cells. Furthermore, cell inhibition was significantly associated with the uptake of MIA690 (Figure 4B). These results imply that MEN-mediated uptake of MIA690 provides a potential intracellular GHRH antagonist pool to compete with endogenous tumorigenic GHRH.
Conventional magnetic nanoparticles exploit a.c. fields often at relatively high frequencies (∼>100 kHz) to heat tumor sites causing hyperthermic cell death [49,50]. Such high-frequency hypothermia-based therapies are prone to side effects because of the difficulty to control thermal effects. Alternatively, based on their new physical high-specificity targeting mechanism, MENs exhibit high-efficacy ME coupling that enables responsiveness to substantially lower frequency a.c. fields [11]. To ensure that MENs exposed to a.c. fields indeed did not contribute to cell death due to unintended hyperthermia, temperatures were measured on the surface of U-87MG cells across treatment groups using an IR camera (Figure 5A). As expected at such low frequencies and consistent with our previous studies, only negligible heat dissipation was observed [11]. We further investigated the effect of time of exposure to a low-frequency (50 Hz) a.c. field coupled to MENs on U-87MG cell viability. The results showed that even the longest (2 h) exposure barely affected cell viability (Figure 5B). It is important to note that a relatively new technology utilizing external a.c. electric fields is being developed to target gliomas. The technology known as tumor-treating field (TTF) interrupts cell growth of rapidly dividing tumor cells during stages of mitosis, leading to blebbing and resulting in cell death [51,52].
However, the TTF approach works in a relatively high-frequency range (100–500 kHz) and displays a relatively low specificity [53,54]. For comparison, MEN-based drug delivery utilizes a.c. frequencies in the near d.c. range, which are three orders of magnitude smaller than those in the TTF frequency range. Therefore, it is likely that the antitumorigenic effects in this study are due an intracellular drug uptake and not due to a direct disruption of mitosis. Taken together, these data indicate that MEN-mediated drug delivery may increase intracellular levels of MIA690 at 24 h and is associated with lower U-87MG cell viability at 48 h post-treatment. Highest MEN-mediated drug penetration was achieved when MIA-690-GMO-MENs were exposed to 100 Oe for 12 h + 50 Hz for 2 h. It is worth mentioning that future research into MEN-targeted drug delivery in combination with localizing TTF therapy to treat gliomas is warranted.
Conclusion
Overall, this study reports major improvements in therapy of human glioblastoma with a GHRH antagonist. Taking advantage of the unique properties of MENs, these results suggest that GMO-MENs effectively bind MIA690, provide controlled intracellular drug delivery and specifically target human glioblastoma cells.
Future perspective
MENs promise to solve the important challenge in the treatment of gliomas through the penetration of the intact BBB for directed therapeutic delivery [10,55,56]. Previously, it has been shown that drug-loaded MENs can penetrate the BBB and release the drug deep in the brain on demand. The current data indicate that MENs specifically penetrate human glioblastoma cells while sparing nonmalignant HBMECs. The implications of high-specificity MEN drug delivery to human glioblastoma cells are twofold: the potential to improve intracellular drug uptake in human glioblastomas, and avoiding intracellular accumulation in HBMECs. Certain nanoparticles may experience diminished transport across the BBB due to intracellular accumulation of large aggregates within the endothelial cells [57]. Conversely, MENs may overcome this type of accumulation in endothelial cells using a relatively weak d.c. magnetic field gradient to ‘pull’ them across the BBB into parenchymal tissues. As a final remark, it is noteworthy that, in general, this nanotechnology could be applied to treat not only brain tumors but also neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease.
Summary points.
Background
When used for targeted drug delivery, magnetoelectric nanoparticles (MENs) allow to control local intrinsic electric fields by externally applied magnetic fields.
Antagonists of growth hormone-releasing hormone (GHRH) are a class of synthetic peptides that inhibit tumor growth by blocking autocrine/paracrine GHRH and IGF-I activity by binding to the surface GHRH receptors.
The goal of this in vitro study is to study externally controlled anticancer effects of binding GHRH antagonist of the MIA class (MIA690) to MENs in glioblastomas.
Methods
Hydrothermally synthesized 30-nm MENs had the core–shell composition of CoFe2O4@BaTiO3.
Cellular experiments in vitro utilized the human glioblastoma cell line U-87MG and human brain microvascular endothelial cells.
The cells under study were treated with MIA690 alone or MIA690-MENs in fresh media under different field conditions to understand the cellular penetration and release mechanisms through atomic force microscopy, confocal microscopy and photoabsorption studies.
Results
We report high-efficacy binding of MIA690 to MEN-targeted on-demand release and specificity to human glioblastoma cells achieved by application of a specially tailored command sequence of d.c. and a.c. magnetic fields.
Intracellular drug uptake was 11-times greater in cells treated with MIA690-loaded MENs coupled with external d.c. and a.c. magnetic fields compared with treatment with MIA690 alone (p < 0.01).
We describe a significant relationship between intracellular MIA690 levels and decrease in cell viability (R2 = 0.43, p = 0.001) using MEN-based field-controlled delivery.
MENs exposed to a relatively low d.c. field (∼100 Oe) showed high-specificity uptake in human glioblastoma cells while sparing nonmalignant brain microvascular endothelial cells.
Conclusion
The results support the use of MENs as an effective drug delivery carrier for GHRH antagonists in the treatment of human glioblastomas.
Supplementary Material
Footnotes
Financial & competing interests disclosure
The authors acknowledge partial financial support from National Science Foundation (NSF) awards # ECCS-1408063, ECCS-0939514 and IIP-1237818, National Institute of Health (NIH) DA # R01DA034547–01, and Neuroscience Centers of Florida Foundation (NSCFF). The authors have no other relevant affiliations of financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: focus on cancer. Int. J. Nanomed. 2014;9:467–483. doi: 10.2147/IJN.S36654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy Chowdhury M, Schumann C, Bhakta Guha D, Guha G. Cancer nanotheranostics: strategies, promises and impediments. Biomed. Pharmacother. 2016;84:291–304. doi: 10.1016/j.biopha.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Liu R, Qin S, Yu R, Fu Y. Current status and future directions of nanoparticulate strategy for cancer immunotherapy. Curr. Drug Metab. 2016;17(8):755–762. doi: 10.2174/1389200217666160714095722. [DOI] [PubMed] [Google Scholar]
- 4.Biffi S, Voltan R, Rampazzo E, Prodi L, Zauli G, Secchiero P. Applications of nanoparticles in cancer medicine and beyond: optical and multimodal in vivo imaging, tissue targeting and drug delivery. Expert Opin. Drug Deliv. 2015;12(12):1837–1849. doi: 10.1517/17425247.2015.1071791. [DOI] [PubMed] [Google Scholar]
- 5.Ostrom Q, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the USA in 2008–2012. Neuro-oncology. 2015;17(Suppl. 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu R, Shimizu F, Hovinga K, et al. Molecular and clinical effects of notch inhibition in glioma patients: a Phase 0/I trial. Clin. Cancer Res. 2016;22(19):4786–4796. doi: 10.1158/1078-0432.CCR-16-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood–brain barrier: an engineering perspective. Front. Neuroeng. 2013;6(7):7–29. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Tellingen O, Yetkin Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat. Rev. Neurosci. 2011;12(9):495–508. doi: 10.1038/nrn3060. [DOI] [PubMed] [Google Scholar]
- 10.Mahmoudi K, Hadjipanayis C. The application of magnetic nanoparticles for the treatment of brain tumors. Front. Chem. 2014;2:109–109. doi: 10.3389/fchem.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guduru R, Liang P, Runowicz C, Nair M, Atluri V, Khizroev S. Magnetoelectric nanoparticles to enable field-controlled high-specificity drug delivery to eradicate ovarian cancer cells. Sci. Rep. 2013;3:2953–2953. doi: 10.1038/srep02953. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Shows magnetoelectric nanoparticles’ (MENs) ability to specifically penetrate cancer cells and deliver the cancer drug paclitaxel.
- 12.Rodzinski A, Guduru R, Liang P, et al. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci. Rep. 2016;6:20867–20867. doi: 10.1038/srep20867. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses the underlying physics that enable MENs to specifically penetrate cancer cells and release drugs on-demand intracellularly using externally controlled magnetic fields.
- 13.Kaushik A, Jayant R, Sagar V, Nair M. The potential of magnetoelectric nanocarriers for drug delivery. Expert Opin. Drug Deliv. 2014;11(10):1635–1646. doi: 10.1517/17425247.2014.933803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guduru R, Liang P, Hong J, et al. Magnetoelectric ‘spin’ on stimulating the brain. Nanomedicine. 2015;10(13):2051–2061. doi: 10.2217/nnm.15.52. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Contains data that show MENs are able to penetrate the brain in a mouse model.
- 15.Nair M, Guduru R, Liang P, Hong J, Sagar V, Khizroev S. Externally controlled on-demand release of anti-HIV drug using magnetoelectric nanoparticles as carriers. Nat. Comm. 2013;4:1707–1707. doi: 10.1038/ncomms2717. [DOI] [PubMed] [Google Scholar]; • Describes the bioconjugation of drugs to MENs and the controlled release of drugs using external magnetic fields.
- 16.Schally A, Varga J, Engel J. Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat. Clin. Pract. Endocrinol. Metab. 2008;4(1):33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 17.Kiaris H, Schally AV, Varga JL. Antagonists of growth hormone-releasing hormone inhibit the growth of U-87MG human glioblastoma in nude mice. Neoplasia. 2000;2(3):242–250. doi: 10.1038/sj.neo.7900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schally A, Varga J. Antagonists of growth hormone-releasing hormone in oncology. Comb. Chem. High Throughput Screen. 2006;9(3):163–170. doi: 10.2174/138620706776055449. [DOI] [PubMed] [Google Scholar]
- 19.Havt A, Schally A, Halmos G, et al. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc. Natl. Acad. Sci. USA. 2005;102(48):17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Codrici E, Enciu A, Popescu I, Mihai S, Tanase C. Glioma stem cells and their microenvironments: providers of challenging therapeutic targets. Stem Cells Int. 2016:5728438–5728438. doi: 10.1155/2016/5728438. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schally AV, Varga JL. Antagonistic analogs of growth hormone-releasing hormone: new potential antitumor agents. Trends Endocrinol. Metab. 1999;10(10):383–391. doi: 10.1016/s1043-2760(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 22.Rekasi Z, Czompoly T, Schally A, et al. Antagonist of growth hormone-releasing hormone induces apoptosis in LNCaP human prostate cancer cells through a Ca2-dependent pathway. Proc. Natl. Acad. Sci. USA. 2005;102(9):3435–3440. doi: 10.1073/pnas.0410006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schally AV, Szepeshazi K, Nagy A, Comaru Schally AM, Halmos G. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell. Mol. Life Sci. 2004;61(9):1042–1068. doi: 10.1007/s00018-004-3434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiaris H, Schally AV, Varga JL, Groot K, Armatis P. Growth hormone-releasing hormone: an autocrine growth factor for small cell lung carcinoma. Proc. Natl. Acad. Sci. USA. 1999;96(26):14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaeger L, Banks W, Varga J, Schally A. Antagonists of growth hormone-releasing hormone cross the blood–brain barrier: a potential applicability to treatment of brain tumors. Proc. Natl. Acad. Sci. USA. 2005;102(35):12495–12500. doi: 10.1073/pnas.0504163102. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Shows the antitumor effect and success of growth hormone-releasing hormone (GHRH) antagonists in traversing the blood–brain barrier.
- 26.Pozsgai E, Schally A, Zarandi M, Varga J, Vidaurre I, Bellyei S. The effect of GHRH antagonists on human glioblastomas and their mechanism of action. Int. J. Cancer. 2010;127(10):2313–2322. doi: 10.1002/ijc.25259. [DOI] [PubMed] [Google Scholar]; •• Describes the antitumor effects of GHRH antagonists on glioblastoma cells.
- 27.Fahrenholtz C, Rick F, Garcia M, et al. Preclinical efficacy of growth hormone-releasing hormone antagonists for androgen-dependent and castration-resistant human prostate cancer. Proc. Natl. Acad. Sci. USA. 2014;111(3):1084–1089. doi: 10.1073/pnas.1323102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Köster F, Jin L, Shen Y, et al. Effects of an antagonistic analog of growth hormone-releasing hormone on endometriosis in a mouse model and in vitro . Reprod. Sci. 2017;24(11):1503-–1511. doi: 10.1177/1933719117691140. [DOI] [PubMed] [Google Scholar]
- 29.Perez R, Schally A, Vidaurre I, Rincon R, Block N, Rick F. Antagonists of growth hormone-releasing hormone suppress in vivo tumor growth and gene expression in triple negative breast cancers. Oncotarget. 2012;3(9):988–997. doi: 10.18632/oncotarget.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schally A, Perez R, Block N, Rick F. Potentiating effects of GHRH analogs on the response to chemotherapy. Cell Cycle. 2015;14(5):699–704. doi: 10.1080/15384101.2015.1010893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szalontay L, Schally A, Popovics P, et al. Novel GHRH antagonists suppress the growth of human malignant melanoma by restoring nuclear p27 function. Cell Cycle. 2014;13(17):2790–2797. doi: 10.4161/15384101.2015.945879. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Explains the action of novel GHRH antagonists such as those used in the current manuscript.
- 32.Zarandi M, Cai R, Kovacs M, et al. Synthesis and structure-activity studies on novel analogs of human growth hormone releasing hormone (GHRH) with enhanced inhibitory activities on tumor growth. Peptides. 2017;89:60–70. doi: 10.1016/j.peptides.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Chu W, Law K, Chan S, et al. Antagonists of growth hormone-releasing hormone receptor induce apoptosis specifically in retinoblastoma cells. Proc. Natl. Acad. Sci. USA. 2016;113(50):14396–14401. doi: 10.1073/pnas.1617427113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corral Flores V, Bueno Baqués D, Ziolo RF. Synthesis and characterization of novel CoFe2O4–BaTiO3 multiferroic core–shell-type nanostructures. Acta Mater. 2010;58(3):764–769. [Google Scholar]; • Basic reference for the synthesis of MEN core–shell nanostructures.
- 35.Acton A. Scholarly Editions; Atlanta, GA, USA: 2013. Erythroid cells – advances in research and application. [Google Scholar]
- 36.Strober W, Coligan JE. Trypan blue exclusion test of cell viability. Curr. Proton. Immunol. 2001 doi: 10.1002/0471142735.ima03bs21. A3.B.3. [DOI] [PubMed] [Google Scholar]
- 37.Guduru R, Khizroev S. Magnetic field-controlled release of paclitaxel drug from functionalized magnetoelectric nanoparticles. Part. Part. Syst. Character. 2014;31(5):605–611. [Google Scholar]
- 38.Prados MD, Levin V. Biology and treatment of malignant glioma. Semin. Oncol. 2000;27(3 Suppl. 6):1–10. [PubMed] [Google Scholar]
- 39.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. Cancer J. Clin. 2002;52(1):23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 40.Kanashiro C, Schally A, Nagy A, Halmos G. Inhibition of experimental U-118MG glioblastoma by targeted cytotoxic analogs of bombesin and somatostatin is associated with a suppression of angiogenic and antiapoptotic mechanisms. Int. J. Oncol. 2005;27(1):169–174. [PubMed] [Google Scholar]
- 41.Barabutis N, Schally AV. Knocking down gene expression for growth hormone-releasing hormone inhibits proliferation of human cancer cell lines. Br. J. Cancer. 2008;98(11):1790–1796. doi: 10.1038/sj.bjc.6604386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stimphil E, Nagasetti A, Guduru R, et al. Physics considerations in targeted anticancer drug delivery by magnetoelectric nanoparticles. Appl. Phys. Rev. 2017;4(2):021101. [Google Scholar]
- 43.Ivey J, Latouche E, Sano M, Rossmeisl J, Davalos R, Verbridge S. Targeted cellular ablation based on the morphology of malignant cells. Sci. Rep. 2015;5:17157–17157. doi: 10.1038/srep17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pakhomova O, Gianulis E, Pakhomov AG. Springer; 2016. Different cell sensitivity to pulsed electric field; pp. 1–17. [Google Scholar]
- 45.Patel A, Cholkar K, Mitra A. Recent developments in protein and peptide parenteral delivery approaches. Ther. Deliv. 2014;5(3):337–365. doi: 10.4155/tde.14.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomsen LB, Linemann T, Pondman KM, et al. Uptake and transport of superparamagnetic iron oxide nanoparticles through human brain capillary endothelial cells. Chem. Neurosci. 2013;4(10):1352–1360. doi: 10.1021/cn400093z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yim YS, Choi JS, Kim GT, et al. A facile approach for the delivery of inorganic nanoparticles into the brain by passing through the blood–brain barrier (BBB) Chem. Commun. (Camb.) 2012;48:61–63. doi: 10.1039/c1cc15113d. [DOI] [PubMed] [Google Scholar]
- 48.Kahán Z, Arencibia JM, Csernus VJ, et al. Expression of growth hormone-releasing hormone (GHRH) messenger ribonucleic acid and the presence of biologically active GHRH in human breast, endometrial and ovarian cancers. J. Clin. Endocrinol. Metab. 1999;84(2):582–589. doi: 10.1210/jcem.84.2.5487. [DOI] [PubMed] [Google Scholar]
- 49.Bañobre López M, Teijeiro A, Rivas J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013;18(6):397–400. doi: 10.1016/j.rpor.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaur P, Aliru M, Chadha A, Asea A, Krishnan S. Hyperthermia using nanoparticles – promises and pitfalls. Int. J. Hyperther. 2016;32(1):76–88. doi: 10.3109/02656736.2015.1120889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanson K, Lok E, Wong E. An overview of alternating electric fields therapy (NovoTTF therapy) for the treatment of malignant glioma. Current Neurol. Neurosci. Rep. 2016;16(1):8–8. doi: 10.1007/s11910-015-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giladi M, Schneiderman R, Voloshin T, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci. Rep. 2015;5:18046–18046. doi: 10.1038/srep18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirson E, Dbalý V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA. 2007;104(24):10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies A, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Ann. NY Acad. Sci. 2013;1291:86–95. doi: 10.1111/nyas.12112. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez A, Tatter S, Debinski W. Neurosurgical techniques for disruption of the blood–brain barrier for glioblastoma treatment. Pharmaceutics. 2015;7(3):175–187. doi: 10.3390/pharmaceutics7030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eichler A, Chung E, Kodack D, Loeffler J, Fukumura D, Jain R. The biology of brain metastases – translation to new therapies. Nat. Rev. Clin. Oncol. 2011;8(6):344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu C, Zhao Y, Wong H, Cai J, Peng L, Tian X. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014;9:2241–2257. doi: 10.2147/IJN.S61288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.