Abstract
There is a large unmet need for effective therapeutic approaches for glioma, the most malignant brain tumor. Clinical and preclinical studies have enormously expanded our knowledge about the molecular aspects of this deadly disease and its interaction with the host immune system. In this review we highlight the wide array of immunotherapeutic interventions that are currently being tested in glioma patients. Given the molecular heterogeneity, tumor immunoediting and the profound immunosuppression that characterize glioma, it has become clear that combinatorial approaches targeting multiple pathways tailored to the genetic signature of the tumor will be required in order to achieve optimal therapeutic efficacy.
Keywords: : cancer vaccines, checkpoint blockade, gene therapy, glioma, immunotherapy, passive immunotherapy
Glioma subtypes & molecular classification
Gliomas are the most common primary brain tumors with an estimated incidence of approximately 7 per 100,000 people in USA, representing 27% of all CNS tumors and 80% of malignant tumors [1]. Until 2016, the WHO classified gliomas based on histology and categorized them into three principal groups: astrocytoma, oligodendroglioma and oligoastrocytoma [2,3]. Gliomas are further separated based on their degree of anaplasia into WHO grades I-IV, of which WHO grade I is assigned to lesions with slow growth and better prognosis; and WHO grade IV is assigned to the most malignant tumors represented by high-grade gliomas (HGG) or glioblastomas [2–4].
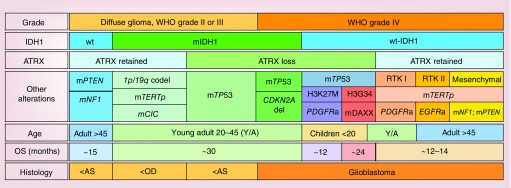
With the advancement of genomics, transcriptomics and epigenomics, together with the incorporation of high-throughput technology and histological methods in the analysis of the glioma specimens, several molecular markers have been identified, for example, TERT, PDGFR, PTEN, IDH, PI3K, ATRX, EGFR and H3 histone family member 3A (Figure 1) [5–7]. These markers are associated with specific tumor phenotypes and indicate the need to define new glioma subtypes [6–10]. With the introduction of molecular data to tumor classification, the WHO 2016 classification underwent a notable improvement from the classical histological classification [3,11]. One of the more significant criteria is the mutation status of IDH1. Mutation of IDH1 at arginine 132 (R132H) is present in around 80% of low-grade gliomas (LGG; WHO grade II) and anaplastic astrocytomas (WHO grade III), as well as in a subset of HGG (WHO grade IV) [12,13]. IDH1-R132H results in the production of the oncometabolite R-2-hydroxyglutarate, which can inhibit a variety of α-ketoglutarate-dependent dioxygenases, such as prolyl-4 hydroxylase, prolyl hydroxylase and the ten-eleven translocation family of DNA hydroxylases, which also function as histone demethylases [14,15]. 2-hydroxyglutarate also induces histone 3 hypermethylation, and is sufficient for formation of a glioma CpG island methylator phenotype thus causing a global hypermethylation phenotype in glioma cells [16,17]. Generally, patients with IDH1 mutation have better prognosis and better response to treatment [7,9,10,18]. Therefore, gliomas can be separated into two large groups: mutant IDH1 and wild-type IDH1 (wt-IDH1) (Figure 1). In turn, mutant IDH1 LGG can be further dissected into two subgroups according to 1p/19q or ATRX status, which are mutually exclusive (Figure 1) [3,9,10,12]. Mutant IDH1 with 1p/19q co-deletion is associated with oligodendroglioma phenotype in diffuse LGG [10,19]. In this subgroup, TERTp and CIC mutations are also present (Figure 1) [9,10,12]. Mutant IDH1 with ATRX loss and TP53 mutation is associated with astrocytoma and oligoastrocytoma phenotypes (Figure 1) [9,10,12,19]. This particular subtype of glioma can progress in malignancy to reach WHO IV grade [20]. For this reason, these molecular markers can also be found in the most aggressive forms of glioma [3]. On the other hand, gliomas harboring wt-IDH1 represent most of the WHO grade IV gliomas. Gliomas expressing wt-IDH1, with loss of TP53 and ATRX, and mutations in H3 histone family member 3A, including H3K27M and H3G34, are typically found in pediatric and young adult patients [19,21]. Gliomas with wt-IDH1 that have retained ATRX typically co-express TERTp mutations and alterations in regulators of the RTK-RAS-PI3K signaling cascade and are typically encountered in adult patients (Figure 1) [3,4,6,11]. RTK I is a molecular subgroup of glioblastomas that generally arises in young adults, characterized by TERTp mutation and PDGFRA amplification [4,11]. Glioblastoma can also be divided in primary and secondary. Primary glioblastomas are generated de novo and represent almost 90% of glioblastoma patients [3,22]. Secondary glioblastomas develop from diffuse lower grade glioma [22]. They also harbor different molecular alterations. For example, EGFR overexpression is prevalent in primary glioblastoma, but is rare in secondary [23]. In contrast, TP53 mutation is rare in primary glioblastoma; however, is a characteristic of secondary glioblastoma [23]. In addition, IDH1 mutation and ATRX inactivation are typically found in secondary glioblastoma together with TP53 mutation [3,22]. Therefore, primary and secondary glioblastoma correspond to a distinctive brain-tumor entities differing in origin and molecular characteristics.
Figure 1. . Overview of the major subtypes of glioma.
AS: Astrocytoma; OD: Oligodendroglioma; OS: Overall survival.
In summary, gliomas represent a heterogeneous group of brain tumors that can be classified according to histology, malignancy, age range and genetic/epigenetic alterations. The molecular features of these tumors are crucial for accurate diagnosis, and also for designing therapeutic strategies tailored to tumor subtypes. We hypothesized specific molecular alterations can impact glioma responses to therapies.
Glioma prognosis & treatment
Glioma treatment modalities include surgical resection, radiation therapy and/or chemotherapy. Treatment strategies are influenced by the recently revised 2016 WHO brain tumor classification guidelines [3]. Maximal safe surgical resection is the primary treatment strategy for LGG. The most common LGG in adults is oligodendroglioma, a grade II tumor by the 2016 WHO classification. Molecular and genetic characteristics, such as IDH mutation and codeletion of the 1p/19q chromosomal arms are becoming increasingly important for stratifying patients based on response to treatment. In most cases, the standard treatment for oligodendroglioma beyond surgery is radiotherapy followed by procarbazine, lomustine and vincristine chemotherapy [24]. For those patients with anaplastic astrocytomas, the standard of care (SOC) involves maximal safe resection or biopsy followed by involved-field radiotherapy to 60 Gy given in 1.8-2 Gy fractions [25]. Median survival time is doubled in patients receiving adjuvant radiotherapy versus surgery alone in randomized trials [26]. However, whole brain radiation therapy can significantly impact patient cognitive functions. First-line treatment also includes the use of chemotherapeutics with modest increases in 1- and 2-year survival times (58–63% and 31–37%, respectively) [27]. Glioma (WHO IV) carries the poorest prognosis, and surgical resection is a key element for initial management versus diagnostic biopsy. However, the benefit is mitigated if the patient is left with a neurologic deficit which significantly impairs daily function. Radiotherapy and adjuvant temozolomide (TMZ) have been the SOC for glioblastoma (GBM) patients based on the treatment protocol per Stupp et al. in a 2005 randomized controlled trial [28]. Patients receiving TMZ in addition to radiotherapy after surgery experienced a 2.5-month survival advantage compared with those receiving adjuvant radiotherapy alone [28]. Several important prognostic indicators exist, the most important of which include patient age and functional status, measured commonly on the Karnofsky Performance Scale and MGMT promoter methylation [29]. Current treatments for anaplastic oligodendroglioma and oligoastrocytoma, both WHO grade III tumors, represent a more rapidly evolving paradigm and insight into future glioma treatment effort. The molecular biology of these tumors and significance of well-recognized genetic aberrancies are leading to new treatment options and targeted therapies [25]. Evidence challenging the central dogma of the brain as an immune privileged organ and the success of immunotherapeutic approaches in other cancers has driven the investigation of several immune-based approaches in preclinical models and in numerous clinical trials [30–34].
Innate immune responses in glioma
Although the brain was originally considered an immune privileged organ, studies showed that due to disturbance of the blood–brain barrier integrity during inflammation or cancer and the presence of lymphatic outflow channels, the immune system is able to interact with cells within the CNS [30–32]. Immune cell infiltration has been demonstrated in patients with malignant glioma; however, endogenous immune responses fail to control the disease [35]. This is mainly due to glioma-mediated suppression of the infiltrating immune cells by mechanisms such as TGF-β secretion, release of LDH5 or expression of galectin-1 (gal-1) [36–38]. Immune surveillance by innate immune cells is the first line of defense against malignant cells. Natural killer (NK) cells are the main effector cells of the innate immune system mediating antitumor responses. Activation of NK cells is tightly regulated based on germline-encoded activating and inhibitory receptors [39,40]. Data from our laboratory showed that NK cells can mediate an antiglioma immune response which is suppressed by gal-1 expression by glioma cells [41]. Additional studies demonstrated that glioma-derived LDH5 and TGF-β abrogate the antiglioma activity of NK cells. LDH5 induces expression of activating receptor NKG2D ligands on myeloid cells, resulting in downregulation of NKG2D on NK cells and thereby decreased antiglioma reactivity [36]. Moreover, glioma cell-derived TGF-β has been shown to inhibit expression of activating NKG2D ligands MICA and ULBP2 on glioma cells, which facilitates downregulation of NKG2D receptor on immune cells, including NK cells and CD8+ T cells [37,42].
It was recently demonstrated that a myeloid cell population (Gr-1+CD11b+) is required for NK cell-mediated glioma eradication in the absence of immunosuppressive gal-1 [43]. Immunodepletion of Gr-1+ or Ly6C+ expressing cells in an orthotopic glioma mouse model resulted in abrogation of tumor rejection, demonstrating the importance of this myeloid cell population for the innate antiglioma immune response [43]. Additionally, studies demonstrated a role for TLR-mediated immune activation in the context of glioma-targeting immunotherapeutic approaches. TLRs are germline-encoded receptors, some of which are expressed on the cell surface while others are expressed in the endosomal compartment [44]. Natural TLR ligands are common pathogen-associated molecular patterns, such as viral ssRNA (TLR7) or dsRNA (TLR3) or bacterial components like lipopolysaccharide (LPS; TLR4) or CpG-containing dsDNA (TLR9) [44]. TLR activation can also be induced by recognition of endogenous damage-associated molecular patterns released upon cell death [45]. Once activated, TLR signaling results in production of proinflammatory cytokines and upregulation of co-stimulatory molecules, thereby mediating activation of the adaptive immune response. We demonstrated in vivo that release of the endogenous TLR2 ligand HMGB1 (high mobility group box 1) from dying glioma cells mediates tumor regression following combined immunotherapy/cytotoxic therapy [46]. In this therapeutic approach, adenoviral vectors encoding for herpes simplex type 1-thymidine kinase (TK) and FMS-like Tyrosine kinase 3 ligand (Flt3L) are delivered into the brain where glioma cell death is induced upon systemic ganciclovir treatment [47]. This in turn facilitates the release of tumor antigens and the endogenous TLR2 ligand HMGB1, which then activates dendritic cells (DCs) that were recruited into the brain by Flt3L. Activation of TLR signaling in recruited DCs induces CD8+ T cell-dependent glioma regression as well as antiglioma immunological memory [46]. In addition to TLR-induced immune activation by endogenous TLR ligands, administration of synthetic TLR ligands as single agents or as adjuvants in combination with peptide vaccines is under investigation. Studies in glioma mouse models showed that topical application of imiquimod (TLR7/8 ligand) can convert plasmacytoid dendritic cells (pDCs) into antiglioma effector cells [48]. In a different glioma mouse model, topical imiquimod administration exerted therapeutic effect by inducing glioma-reactive CD8+ T cells and reducing the number of CD4+Foxp3+ Treg cells [49]. Importantly, synthetic TLR agonists have entered clinical trials as adjuvant to amplify the therapeutic effect of peptide vaccines (NCT01920191, NCT02454634).
Together, these studies demonstrate that glioma can be infiltrated and recognized by cells of the innate as well as adaptive immune system; however, in most of the cases these immune interactions are effectively suppressed by glioma tumor cells. Therefore, counteracting glioma-mediated immune suppression is a prerequisite for the development of new and more effective immunotherapies for this devastating disease.
Immunosuppression in glioma
Glioma-mediated immunosuppression depends on the local production of cytokines and chemokines and the recruitment of regulatory, immunosupressive cells (Figure 2) [50]. TGF-β and IL-10 are central to maintaining the immunosuppressive glioma microenvironment. These cytokines are not only secreted by tumor-infiltrating immune cells, but also by glioma cells themselves [50]. IL-10 inhibits the activation and effector functions of DCs, macrophages and T cells, modulates the growth and differentiation of immune cells, and limits the expression of MHC class II in monocytes [50,51]. IL-10 has also been shown to upregulate PD-L1 in circulating monocytes and tumor-associated macrophages in glioma [52]. Interestingly, IL-10 is not always a mediator of immunosuppression and also exerts proinflammatory and antitumor effects. It has been found that T cells’ inhibition of glioma growth relies on high levels of IL-10 [53]. Furthermore, genetic ablation of IL-10 facilitates tumor growth and metastasis development in mouse models of colon cancer, an effect that was associated with the expansion of myeloid-derived suppressor cells (MDSCs) and Tregs [54]. In addition, IL-10 has been shown to stimulate macrophages to produce antiangiogenic cytokines and to promote antitumor NK-cell responses [50].
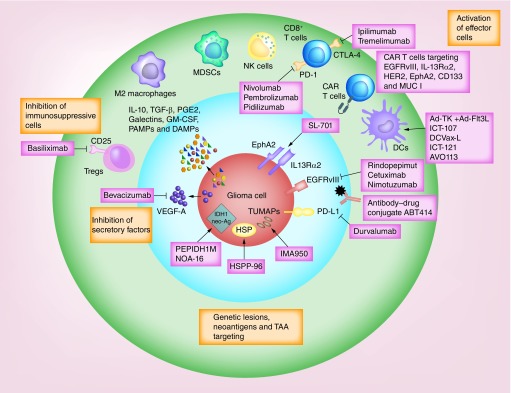
Figure 2. . Immunotherapy strategies in clinical trials.
Glioma evades the immune system through a variety of ways. Antiglioma immunotherapeutic strategies are targeting T-cell exhaustion, immunosuppressive cells and angiogenesis and testing peptide or dendritic cell vaccines-based approaches to enhance the antitumor adaptive immune response or the activity of effector cells using chimeric antigen receptor T cells.
TGF-β is a pleiotropic cytokine involved in many biological functions, including the blockade of T-cell activation and proliferation, as well as the induction of Tregs [55,56]. It was first isolated from patients with glioma and is considered an important immunosuppressive factor [57–59]. TGF-β expression levels correlate with higher tumor grades and worse prognosis [60]. Nevertheless, this cytokine plays a complex role in glioma. In the early stages of tumor growth, TGF-β acts as a tumor suppressor [61]. At later stages, however, glioma cells stop responding to these TGF-β-mediated inhibitory signals and instead TGF-β enhances tumor progression through different mechanisms such as increasing angiogenesis and promoting the expansion of Tregs [62].
Glioma secrete other immunosuppressive factors such as CSF-1, VEGF, PGE2, NO, Arg I, IDO and Gal-1 [63]. VEGF does not only induce angiogenesis, but also inhibits the functional maturation of DCs [64]. PGE2 downregulates the production of Th1 cytokines (IL-2, IFN-γ and TNF-α) and upregulates Th2 cytokines (IL-10, IL-6, IL-4) [65,66]. Both TGF-β and PGE2 can act synergistically to induce a regulatory phenotype in DCs [67]. Studies performed in mice harboring subcutaneous glioma have shown that systemic immunosuppression associated with growing gliomas is partly mediated by the overproduction of NO in splenocytes [68]. Inhibition of inducible NO synthase, using either mercaptoethylguanidine or l-N(6)-(1-Iminoethyl)-l-lysine (l-NIL) could boost IFN-γ-based immunotherapy approach in rats with intracranial tumors [69,70]. Arg I has been proposed as a mechanism of monocyte-mediated inhibition of T-cell function in glioma patients and researchers have found an expanded population of circulating degranulated neutrophils, which is associated with elevated levels of serum Arg I and decreased T-cell CD3ζ expression in the peripheral blood of glioma patients [71]. IDO expression is higher in HGG than in LGG and correlates with worse overall survival rates [72]. IDO expression has been shown to increase the recruitment of immunosuppressive Tregs, promoting tumor escape [73]. Another immunosuppressive ligand expressed by glioma cells, Gal-1, enhances tumor cell migration, induces T-cell apoptosis and inhibition of T-cell proliferation, expansion and accumulation of Tregs and protumoral DCs, and its expression was found to correlate with the grade of astrocytic tumors and with a dismal prognosis [38,74,75].
Soluble factors that bind to pattern recognition receptors, such as TLRs on microglia and activate them, have also been involved in the maintenance of an immunosuppressive microenvironment in glioma [76,77]. Although glioma-derived chemokines (i.e., CSF-1 or CCL2) attract and activate microglia, locally produced TGF-β and PGE2 induces an anti-inflammatory phenotype in these cells, reducing their antigen-presenting activity and facilitating the infiltration of immunosuppressive cells such as Th2 cells and Tregs [78–81]. Glioma-derived CCL22 and CCL2 also recruit Tregs that express CCR4 into the tumor microenvironment [82,83]. Thus, blockade of these chemokines could improve antitumor immunity.
Tumor progression depends on the accumulation of genetic and epigenetic alterations in cancer cells resulting in a complex and heterogeneous cellular composition at the site of tumor growth [84,85]. This heterogeneous environment contains an immunosuppressive network of immune cells such as MDSCs, tumor-associated macrophages/microglia (TAMs) and Tregs [86]. Of particular interest are the MDSCs which promote tumor growth by variety of mechanisms including inhibition of T-cell proliferation and effector functions, suppression of NK and NKT activity, recruitment of Tregs, secretion of immune suppressive cytokines and upregulation of checkpoint receptor ligands such as PD-L1 [87,88]. MDSCs can also promote tumor angiogenesis via the secretion of VEGF as well as matrix metallopeptidase 9 [89,90].
In mice, MDSCs are characterized by the dual expression of CD11b and Gr-1 surface markers. They are further distinguished into polymorphonuclear (PMN-MDSCs) or monocytic (M-MDSCs). The two subtypes differ not only in the surface makers (i.e., CD11b+, Ly6G+, Ly6Clow for the PMN-MDSCs, and CD11b+, Ly6G-, Ly6Chigh for the M-MDSCs) but also in the main mechanism involved in immunosuppression. The generation of Arg I and NO is the main suppressive mechanism of M-MDSC, whereas the PMN-MDSC suppresses CD8+ T-cell responses mainly by producing reactive oxygen species [91–93]. There are multiple strategies for targeting MDSCs in glioma [94,95]. MDSCs depletion and/or blockade of their inhibitory mechanisms seem to be the most effective method. We have recently shown that depletion of immune-suppressive MDSCs in glioma-bearing mice markedly enhances the efficacy of immunostimulatory/cytotoxic gene therapy [95]. Another possible strategy for targeting MDSCs is by promoting their differentiation into mature cells. Using an in vitro cell differentiation model, two groups showed that the macrophage migration inhibitory factor can be targeted to enhance DC differentiation from MDSCs [96,97]. TAMs are another dominant immune cell type in glioma [98,99]. Glioma can activate M2 polarized macrophages, which secrete IL-10 and TGF-β, and inhibit T-cell proliferation [100]. The exact mechanism for such effect is still not clear, although the CSF-1 receptor could be a possible mediator [101]. Gabrusiewicz et al. showed that in glioma patients, TAMs resembled the phenotype of nonpolarized M0 macrophages with partially immune-suppressive phenotype [98]. More recently, TAM recruitment has been connected to NF1 gene expression in glioma and the resistance to radiotherapy in some glioma patients may be associated with the presence of M2 macrophages [102].
In addition to MDSCs and TAMs, glioma cells promote the accumulation of Tregs [103]. Tregs restrain antitumor immune response through numerous mechanisms [102]. They can mediate inhibition of functional T cells through the interaction of CTLA-4 (on Tregs) with CD80/86 (on T cells) [104], by the activation of the perforin/granzyme B pathway to cause target cell death [105], by suppressing the release of IL-2 and IFN-γ [106,107], or by expression of TGF-β [108] which can also inhibit NK cells activity [109]. Many reports have illustrated the correlation between blocking the activity of Tregs with more effective antiglioma effector response and improved survival [103,104]. This can be done by a variety of mechanisms such as blocking CTLA-4 [110], inhibiting the signaling pathways that lead to FoxP3 activation (i.e., STAT3) [111–113], or by targeting CD25 [114]. The use of chemotherapeutic alkylating agents (such as temozolomide and cyclophosphamide) has also been reported to inhibit Treg activity [113,115,116].
In summary, glioma cells secrete numerous chemokines, cytokines and growth factors that promote infiltration and expansion of MDSCs, microglia, macrophages and Tregs that directly enhance the invasion of glioma cells, dampen antitumor immune response and accelerate tumor progression. Such immunosuppressive networks are crucial targets for the development of effective immunotherapies.
T-cell exhaustion in glioma
Acquisition of effector functions by naive T cells in response to acute infections is accompanied by robust proliferation, transcriptional, metabolic and epigenetic reprogramming [117–119]. Memory T cells arise from a small subset of these activated T cells upon the resolution of infection, while the majority of the T cells die [117]. However, during chronic infections or inflammation, such as in cancer, an altered state of exhaustion is generated in T cells. Hallmarks of exhausted T cells include loss of effector functions, expression of various inhibitory receptors, metabolic and transcriptional derangements [117,120]. Typically as exhaustion develops, the ability of the T cells to release IL-2 and undergo proliferation is lost, followed by the failure to produce IFN-γ, TNFα and undergo degranulation which results in the release of cytolytic granules from the T cells and is essential for granzyme-dependent killing [117,120]. There is also an increase in the amount and diversity of inhibitory receptors expressed by the T cells. Some of the common inhibitory receptors associated with T-cell exhaustion are CTLA-4, PD-1, T-cell immunoreceptor with immunoglobulin and ITIM domains, LAG-3, 2B4, B and T lymphocyte attenuator and TIM3 [117,118,121,122]. It has been shown that patients with primary intracranial tumors have impaired cell-mediated immunity, with the majority of patients failing to respond to common recall skin test antigens and to neoantigens in vivo [123–125]. T-cell receptor (TCR)-mediated signaling in peripheral blood lymphocytes was also shown to be defective in T cells obtained from patients with primary brain tumors [126]. In addition, T cells from these patients showed a marked reduction in tyrosine phosphorylation in response to mitogens. Reduction in CD4 and CD8 T cells has been reported in the tumor and circulation of GBM patients [127]. Using immunohistochemistry and flow cytometry, PD-L1 expression was analyzed in 94 GBM patients. A total of 60.6% of GBM patients had tumors with at least 1% or more PD-L1-positive cells and 5.32% had 50% or greater PD-L1-positive cells. Higher PD-L1 expression was also observed to be correlated with a worse outcome [128]. Interestingly, since tumors with high mutational burdens may be more immunogenic, immune checkpoint inhibition using nivolumab was tested in two siblings with recurrent multifocal biallelic mismatch repair deficiency GBM with clinically significant responses [129].
Thus approaches targeting T-cell exhaustion could provide clinical benefit in glioma. CTLA-4 and PD-1 have been identified as the two major inhibitory receptors/checkpoints involved in T-cell exhaustion and monoclonal antibodies targeting CTLA-4 (ipilimumab and tremelimumab) and PD-1 (nivolumab and pembrolizumab) have been tested for safety and efficacy in clinical trials for melanoma [34,130,131], non-small-cell lung cancer [132,133] and renal cell carcinoma [134]. Based on the impressive clinical benefit observed in the treatment of melanoma by the use of checkpoint inhibitors a number of preclinical and clinical studies are investigating their use in glioma.
Immunotherapeutic approaches in clinical trials
Various preclinical studies have demonstrated the success of immunotherapy-based approaches in animal models and many Phase I and II clinical trials have shown immunotherapy to be safe and in some cases improve progression-free survival (PFS) and overall survival (OS) [135–139]. Below, we provide an overview of the immunological approaches which yielded promising results in the preclinical setting and are currently being tested in the clinic (Figure 2).
Targeting immune checkpoints
Preclinical studies using murine models with orthotopic-transplanted gliomas have shown great benefit with checkpoint inhibitors used individually or with other immunotherapeutic strategies [95,110,140,141]. Administration of CTLA-4 blocking antibodies improved the survival of animals bearing intracranial SMA-560 tumors and in combination with IL-12 also caused tumor regression in GL261 tumor models [110,140]. Tumor eradication in both studies was accompanied by reduction of Tregs in the tumor microenvironment and an increase in effector CD8 T cells. Promising results from clinical trials for metastatic melanoma using ipilimumab in combination with gp100 vaccine or dacarbazine and the US FDA approval for ipilimumab's use in malignant melanoma have fueled research into its use for the treatment of other cancers [34]. Currently two clinical trials are assessing the use of anti-CTLA-4 antibodies (ipilimumab and tremelimumab) in the treatment of recurrent glioma (NCT02794883 and NCT02017717). The NCT02794883 trial evaluates the combination of tremelimumab with durvalumab, a PD-L1 blocking antibody and the NCT02017717 evaluates the combination of ipilimumab with nivolumab, a PD-1 blocking antibody. Preclinical studies investigating the PD-1 checkpoint blockade in glioma have shown antitumor efficacy [142]. Combination of PD-1 blocking antibodies with radiotherapy enhanced the survival of GL261 glioma-bearing mice [142,143]. PD-1 blocking antibodies, pembrolizumab and nivolumab were approved by the FDA for use in metastatic melanoma in 2014 and for non-small-cell lung cancer in 2015. Multiple clinical trials are investigating the efficacy of anti-PD-1 and anti-PD-L1 antibodies in malignant glioma. In addition to NCT02794883 and NCT02017717, two Phase I/II studies will test the use of pembrolizumab with or without bevacizumab (NCT02337491) or pembrolizumab in combination with laser ablation (NCT02311582) in patients with recurrent glioma. NCT01952769 will evaluate the use of pidilizumab (humanized anti-PD-1 monoclonal antibody) against diffuse intrinsic pontine glioma and recurrent glioma and NCT02336165 is testing MEDI4736 (anti-PD-L1 monoclonal antibody) in combination with radiotherapy and bevacizumab. As shown in Table 1, several other clinical trials are also testing the use of immune checkpoint inhibitors in malignant glioma.
Table 1. . Current clinical trials evaluating checkpoint inhibition.
| NCT# | Phase | Study title | Current status | Inclusion diagnosis | Intervention | Target |
|---|---|---|---|---|---|---|
| NCT02017717 | Phase III | A Study of the Effectiveness and Safety of Nivolumab Compared to Bevacizumab and of Nivolumab With or Without Ipilimumab in Glioblastoma Patients (CheckMate 143) | Recruiting | Recurrent glioblastoma | Nivolumab alone vs bevacizumab or nivolumab alone or with ipilimumab | PD-1, CTLA-4 |
| NCT02327078 | Phase I/II with glioblastoma cohort in Phase II | A Study of the Safety, Tolerability, and Efficacy of Epacadostat Administered in Combination With Nivolumab in Select Advanced Cancers (ECHO-204) | Recruiting | Recurrent glioblastoma | Nivolumab + epacadostat | PD-1, IDO1 |
| NCT02311582 | Phase I/II | MK-3475 in Combination With MRI-guided Laser Ablation in Recurrent Malignant Gliomas | Recruiting | Recurrent malignant gliomas | MK-3475 in combination with mri-guided laser ablation | PD-1 |
| NCT02313272 | Phase I | Hypofractionated Stereotactic Irradiation (HFSRT) With Pembrolizumab and Bevacizumab for Recurrent High Grade Gliomas | Recruiting | Recurrent high grade gliomas | Pembrolizumab with radiation therapy and bevacizumab | PD-1 + VEGF |
| NCT02335918 | Phase I/II with Phase II only for glioblastoma | A Dose Escalation and Cohort Expansion Study of Anti-CD27 (Varlilumab) and Anti-PD-1 (Nivolumab) in Advanced Refractory Solid Tumors | Recruiting | Glioblastoma | Combination of varlilumab and nivolumab | CD27 + PD-1 |
| NCT02337686 | Phase II | Pharmacodynamic Study of Pembrolizumab in Patients With Recurrent Glioblastoma | Recruiting | Recurrent glioblastoma | Pembrolizumab | PD-1 |
| NCT02550249 | Phase II | Neoadjuvant Nivolumab in Glioblastoma (Neo-nivo) | Recruiting | Primary and recurrent Glioblastoma | Nivolumab | PD-1 |
| NCT02529072 | Phase I | Nivolumab With DC Vaccines for Recurrent Brain Tumors (AVERT) | Recruiting | Recurrent grade iii and grade iv brain tumors | Nivolumab with DC vaccines | PD-1 |
| NCT02526017 | Phase Ia/Ib | Study of FPA008 in Combination With Nivolumab in Patients With Selected Advanced Cancers (FPA008-003) | Recruiting | Malignant glioma | FPA008 in combination with nivolumab | CSF-1R + PD-1 |
| NCT02617589 | Phase III | An Investigational Immuno-therapy Study of Nivolumab Compared to Temozolomide, Each Given With Radiation Therapy, for Newly-diagnosed Patients With Glioblastoma (GBM, a Malignant Brain Cancer) (CheckMate 498) | Recruiting | Newly diagnosed adults with unmethylated MGMT glioblastoma | Nivolumab + radiation vs temozolomide + radiation | PD-1 |
| NCT02648633 | Phase I | Stereotactic Radiosurgery With Nivolumab and Valproate in Patients With Recurrent Glioblastoma | Recruiting | Recurrent glioblastoma | Stereotactic radiosurgery with nivolumab and concurrent valproate | PD-1 |
| NCT02658279 | Proof-of-concept, pilot study | Pembrolizumab (MK-3475) in Patients With Recurrent Malignant Glioma With a Hypermutator Phenotype | Recruiting | Recurrent malignant glioma with a hypermutator phenotype | Pembrolizumab (MK-3475) | PD-1 |
| NCT02658981 | Phase I | Anti-LAG-3 or Urelumab Alone and in Combination With Nivolumab in Treating Patients With Recurrent Glioblastoma | Recruiting | Recurrent GBM | Anti-LAG-3 or urelumab alone and in combination with nivolumab | LAG-3 + CD137 + PD-1 |
| NCT02667587 | Phase III | An Investigational Immuno-therapy Study of Temozolomide Plus Radiation Therapy With Nivolumab or Placebo, for Newly Diagnosed Patients With Glioblastoma (GBM, a Malignant Brain Cancer) (CheckMate548) | Recruiting | MGMT-methylated glioblastoma | Temozolomide + radiation therapy with nivolumab or placebo | PD-1 |
| NCT02798406 | Phase II | Combination Adenovirus + Pembrolizumab to Trigger Immune Virus Effects (CAPTIVE) | Recruiting | Recurrent glioblastoma or gliosarcoma | DNX-2401 + Pembrolizumab | PD-1 |
| NCT02829723 | Phase I/II | Phase I/II Study of BLZ945 Single Agent or BLZ945 in Combination With PDR001 in Advanced Solid Tumors | Recruiting | Glioblastoma | BLZ945 single agent or BLZ945 in combination with PDR001 | CSF1R + PD-1 |
| NCT02852655 | Pilot | A Pilot Surgical Trial To Evaluate Early Immunologic Pharmacodynamic Parameters For The PD-1 Checkpoint Inhibitor, Pembrolizumab (MK-3475), In Patients With Surgically Accessible Recurrent/Progressive Glioblastoma | Recruiting | Recurrent/progressive glioblastoma | Pembrolizumab (MK-3475) | PD-1 |
| NCT02336165 | Phase II | Phase II Study of MEDI4736 in Patients With Glioblastoma | Not recruiting | Unmethylayed MGMT GBM and recurrent GBM | MEDI4736 alone or with radiotherapy or with bevacizumab | PD-L1 |
| NCT02794883 | Phase II | Tremelimumab and Durvalumab in Combination or Alone in Treating Patients With Recurrent Malignant Glioma | Recruiting | Recurrent malignant glioma | Tremelimumab and durvalumab (MEDI4736) alone and in combination | CTLA-4 + PD-L1 |
| NCT02937844 | Phase I | Pilot Study of Autologous Chimeric Switch Receptor Modified T Cells in Recurrent Glioblastoma Multiforme | Recruiting | Glioblastoma multiforme | Anti-PD-L1 CSR T cells + cyclophosphamide + fludarabine | PD-L1 |
| NCT02866747 | Phase I/II | A Study Evaluating the Association of Hypofractionated Stereotactic Radiation Therapy and Durvalumab for Patients With Recurrent Glioblastoma (STERIMGLI) | Recruiting | Recurrent glioblastoma | Durvalumab + radiation therapy | PD-L1 |
| NCT02968940 | Phase II | Avelumab With Hypofractionated Radiation Therapy in Adults With IDH Mutant Glioblastoma | Recruiting | Transformed IDH mutant glioblastoma | Avelumab + hypofractionated radiation therapy | PD-L1 |
DC: Dendritic cell; GBM: Glioblastoma.
Preclinical studies are investigating the potential of other checkpoints such as TIM-3, IDO, LAG-3 and adenosine A2a receptor as therapeutic targets in glioma [144]. Combination of anti-PD-1, anti-TIM-3 and focal radiation resulted in regression of murine gliomas and combination of IDO, CTLA-4 and PD-L1 blockade induced long-term survival in 100% of the glioma-bearing animals [145,146].
Immune checkpoint blockade appears to be an exciting avenue to develop based on the preclinical studies. An important consideration, however, is the use of GL261 as a tumor model in a variety of these studies. Tumors generated by implantation of GL261 cells mimic many of the features of GBM including neovascularization, pseudopalisading necrosis, perivascular organization and angiogenesis [147]. These characteristics have therefore prompted the use of this model to test a variety of antitumor strategies. With a high mutational burden, however, GL261 tumors may potentially generate neoantigens leading to the development of a large T-cell repertoire and a high response rate to immunotherapies. Human GBM is highly immunosuppressive though and it is therefore important to validate the efficacy of immunotherapeutic approaches including checkpoint inhibition with other rodent models (Table 2) and with tumors containing the mutations commonly found in human GBM (Figure 1). This would also allow for the development of personalized immunotherapy strategies.
Table 2. . Rodent models for brain tumors.
| Induction | Species/strain | Source | Pathology | Applications | Ref. |
|---|---|---|---|---|---|
| Cell line inoculation | C57BL/6 | GL26 cells | GBM/ependymoblastoma | DC vaccines engineered to express IL-12. Treg depletion. Metronomic chemotherapy. Combined conditionally cytotoxic and immunostimulatory gene therapy |
[1–5] |
| GL26.1 cells | GBM/ependymoblastoma | Immunological checkpoint blockade. Antitumor DC vaccines + Treg blockade with anti-CD25 antibody. Peptide vaccinations + TGF-β neutralizing antibody | [6–10] | ||
| CT-2A cells | Anaplastic astrocytoma | Genetically modified T cells targeting EGFRvIII and IL13Rα2 | [11,12] | ||
| VM-Dk | SMA-560 cells | Anaplastic astrocytoma | Overexpression of soluble CD70 ligand. Inhibition of TGF-β signaling. EGFRvIII CAR-modified T-cell therapy. Antitumor DC vaccines |
[13–17] | |
| B6D2F1 | 4C8 cells | Oligodendroglioma, astrocytoma | Cationic liposome–DNA complexes. HSV vaccines encoding IL-12 | [18,19] | |
| Cell line inoculation | LEWIS | CNS-1 cells | GBM | Antitumor DC vaccines. TLR agonists. Metronomic chemotherapy. Combined conditionally cytotoxic and immunostimulatory gene therapy | [20–23] |
| FISHER 344 | F98 cells | GBM | Combined conditionally cytotoxic and immunostimulant gene therapy. Cellular vaccinations + GM-CSF. Upregulation of costimulatory molecules |
[5,24,25] | |
| RG2 cells | Anaplastic astrocytoma | Gene therapy-mediated delivery of chemokines and cytokines. Metronomic chemotherapy | [26,27] | ||
| 9L cells | Gliosarcoma | Gene therapy-mediated delivery of proinflammatory cytokines. Tumor vaccination + TGF-β inhibition | [5,28,29] | ||
| Genetic engineering | GFAP-Cre | Lentiviral-mediated knock down of NF1 and p53 | Mesenchymal GBM | [30] | |
| p53 KO | Lentiviral-mediated delivery of Ras and AKT | GBM | [31] | ||
| C57BL/6FVB/nBalb/c | Sleeping beauty transposon plasmids encoding for NRAS, AKT, SV40-LgT, EGFRvIII, shp53 | Grade III astrocytoma, GBM | [32,33] | ||
| p53, Arf or Ink4a-Arf KO Gtv-a mice | RCAS-mediated delivery of PDGF | GBM/oligodendroglioma | TAM targeting with a CSF-1R inhibitor | [34,35] | |
| Ntva-a mice | RCAS-mediated delivery of PDGF, Ras and AKT | GBM | [36] | ||
| Ink4a-Arf KO Gtv-a mice | RCAS-mediated delivery of Ras and AKT | GBM/gliosarcoma | [37] | ||
| Genetic engineering | Sprague Dawley | Retroviral-mediated delivery of PDGF | GBM | [38] | |
| Lentiviral-mediated delivery of PDGF, H-RAS, AKT | GBM | [39] | |||
DC: Dendritic cell; GBM: Glioblastoma; HSV: Herpes simplex virus; RCAS: Retroviral vectors derived from the SR-A strain of Rous sarcoma virus; TAM: Tumor-associated microglia.
Immunotherapy with peptide vaccines
Numerous glioma-associated antigens such as IL-13Rα2, HER2, EphA2, gp100 and AIM-2 are being targeted in glioma [148–150]. Additionally tumor-specific neoantigens such as EGFRvIII are being used to target tumor cells specifically [150,151]. EGFRvIII is expressed in about 20–30% of glioma patients [151]. Evaluation of a combination of EGFRvIII-specific peptide (PEP-3, rindopepimut) keyhole limpet hemocyanin conjugate vaccine plus GM-CSF with standard radiotherapy and chemotherapy in 18 patients expressing EGFRvIII showed a median survival of 26 months (Phase II multicenter study, ACTIVATe, NCT00643097) [152]. After adjustment for age and Karnofsky performance status, the OS of vaccinated patients was greater than that observed in a matched control group. Interestingly 82% of the tumors had lost EGFRvIII expression indicating treatment-induced immunoediting [152]. Immunoediting was also observed in a subsequent Phase II (ACT III, NCT00458601) trial using the combination of rindopepimut, GM-CSF and standard and dose-intensified TMZ with a decrease in EGFRvIII immunoreactivity in 67% of the patients and an OS of 21.8 months [138]. Phase III study of rindopepimut/GM-CSF with adjuvant TMZ in patients with newly diagnosed glioma (ACT IV, NCT01480479) has been discontinued as the median OS with rindopepimut was 20.4 months compared with 21.1 months in the control arm [153]. ReACT (NCT01498328) is a Phase II study of rindopepimut/GM-CSF plus bevacizumab in patients with relapsed EGFRvIII-positive glioma [154]. Interim analysis shows rindopepimut induces potent EGFRvIII-specific immune response and tumor regression, and appears to significantly prolong survival when administered with bevacizumab in patients with relapsed glioma [155].
To overcome the risk of immunoediting and disease recurrence following single peptide vaccinations, investigations testing peptide combinations are underway. Results from the NCT01130077 trial using the combination of EphA2, IL-13Rα2 and survivin with tetanus toxoid and Poly I:C in pediatric brain stem and HGG showed the development of peptide-specific immune responses and indications of immune cell infiltrates. Five out of twenty-six children showed pseudoprogression that was manageable, nineteen showed stable disease and two showed progressive disease [156]. The NCT02078648 study is testing the SL-701 vaccine (IL13Rα2, EphA2 and survivin) in combination with bevacizumab in patients with newly diagnosed glioma. A Phase I trial of IMA950 (consisting of peptides derived from the following proteins: brevican; chondroitin sulfate proteoglycan 4; fatty acid binding protein 7; insulin-like growth factor 2 mRNA binding protein 3; neuroligin 4, X-linked; neuronal cell adhesion molecule; protein tyrosine phosphatase, receptortype, Z polypeptide 1; tenascin C; Met proto-oncogene; baculoviral inhibitor of apoptosis repeat-containing 5; and HBV core antigen) in 45 patients with newly diagnosed glioma receiving maintenance TMZ showed 36 of 40 patients as single-peptide responders and 20 patients as multipeptide responders. However, the median OS was 15.3 months [157]. NCT01920191 tested the combination of intradermal IMA950 with intra muscular Poly-ICLC as an adjuvant in combination with TMZ in newly diagnosed HLA-A2 glioma patients. Preliminary results showed improvement in the median OS with two of the six patients showing induction of both peptide-specific CD4 and CD8 T cells [158].
NCT02149225 is a GAPVAC Phase I trial in newly diagnosed glioblastoma patients testing vaccines using both tumor-associated peptides and tumor-specific peptides, derived by expression profiling of tumors from individual patients. A Phase I clinical trial is testing the safety and efficacy of personalized neoantigen vaccines with radiotherapy for patients with MGMT unmethylated, newly diagnosed glioma (NCT02287428).
The genetic makeup of glioma seems to affect its response to immunotherapeutic strategies. Our lab has recently shown that tumors with ATRX loss have increased genetic instability [159]. Genome wide data analysis of human gliomas showed that ATRX mutation is associated with increased mutation rate at the single nucleotide variant level. Such tumors may therefore be more immunogenic. IDH1 and IDH2 have been found to be mutated in >80% of WHO grade II/III astrocytomas, oligodendrogliomas and oligoastrocytomas [9,12]. The study by Schumacher et al. has shown that immunotherapeutic approaches can target this neoantigen [160].
Currently NCT02193347 (RESIST) is testing the use of IDH1 peptide vaccine (PEPIDH1M) in combination with GM-CSF and tetanus toxoid in recurrent grade II glioma positive for IDH1-R132H in adults. NOA-16 (NCT02454634) is another Phase I study testing an IDH1 peptide vaccine in IDH1 mutant WHO grade III-IV tumors that also show ATRX loss without 1p/19q codeletion.
Certain HSP such as HSP70 and HSP90 have been shown to bind glioma antigens and induce innate and adaptive immune responses. Most trials using HSPs as vaccines have used the HSP–peptide complex 96. A Phase I trial (NCT00293423) using HSP vaccine in recurrent glioma showed tumor-specific responses [161]. A Phase II trial showed median PFS and OS as 19.1 and 42.6 weeks, respectively in patients given the vaccine postsurgical resection [135]. NCT01814813 is a Phase II study testing the combination of HSP–peptide complex 96 with bevacizumab postsurgical resection in patients with recurrent disease.
Immunotherapy with DC vaccines
Successful preclinical studies have prompted a number of clinical studies using DC vaccines. DCs can be loaded with peptides, tumor cell lysates, tumor-derived mRNA, viral antigens and cancer stem cells, all of which can be tailored to the individual makeup of a tumor. An autologous DC vaccine, ICT-107, consisting of six peptides (AIM-2, MAGE1, TRP-2, gp100, HER2 and IL-13Ra2) was tested in combination with radiochemotherapy in a Phase I trial in glioma patients [137]. An OS of 38.4 months was noted along with an increased production of IFNγ and TNFα in stimulated CD8 T cells, and a Phase III trial (NCT02546102) is currently recruiting patients to further investigate this treatment. In a Phase I/II trial of 22 patients with malignant glioma, administration of a type 1-polarized DCs pulsed with synthetic peptides (EphA2, IL-13Rα2, YKL-40 and gp100) and poly IC, 58% of the patients developed an immune response specific to at least one antigen, and IL-12 production by DCs was observed to positively correlate with PFS [162]. A Phase I study evaluated the safety and efficacy of an autologous tumor lysate-based DC vaccine. The median survival time for patients with recurrent glioma was determined to be 133 weeks with a significant increase in CD8 T cell activity [163]. An ongoing Phase III study (NCT00045968) is using an autologous DC vaccine (DCVax-L) prepared by loading the DCs with proteins from the patient's own tumor.
A Phase I/II study (NCT00846456) was conducted using DCs loaded with mRNA amplified from the cancer stem cells isolated from the patient's tumor. No severe side effects were observed and encouragingly the PFS in treated patients was 2.9-fold longer than the matched controls [139]. An ongoing Phase I trial (NCT02049489) in recurrent glioma is testing the safety of ICT-121, a DC vaccine targeting CD133, the antigen expressed on glioma stem cells [164]. With the identification of cytomegalovirus (CMV) and its gene products in glioma, preclinical studies have utilized this feature to develop targeted immunotherapy. NCT00626483 is a study evaluating the combination of anti-CD25 antibody, basiliximab with autologous DCs loaded with CMV pp65-lysosomal-associated membrane protein mRNA. Another study (NCT00639639) showed that preconditioning with tetanus/diphtheria toxoid prior to vaccination with pp65 RNA-pulsed DCs improves DC migration and survival [136]. ELEVATE is a Phase II randomized study (NCT02366728) currently recruiting patients with newly diagnosed glioma, is investigating preconditioning with tetanus toxoid or basiliximab prior to a CMV-targeted DC vaccine.
Immunological checkpoint blockade could improve the efficacy of antitumor DC vaccines in glioma patients, as it has been shown in preclinical studies [95,145,146,165]. AVERT, a Phase I clinical trial (NCT02529072) is evaluating the combination of CMV-targeted DC vaccine with PD-1-blocking antibody, nivolumab in patients with recurrent HGG. Another Phase II study combining the SOC with the DC vaccine, AVO113 and bevacizumab showed an increase in the median OS compared with the vaccine alone or bevacizumab alone group [166].
Immunotherapy with antibodies
This form of immunotherapy relies on targeting antigens uniquely expressed on glioma cells or molecules that are overexpressed by tumor cells. Several Phase II trials have tested the efficacy of anti-VEGF therapies because gliomas are highly vascularized tumors that express high amounts of VEGF. Most commonly used anti-VEGF antibody is bevacizumab that has been tested either alone (NCT00345163) or in combination with irinotecan (NCT00345163), etoposide (NCT00612430) or with concurrent radiotherapy (NCT00595322) [155,167–170]. The RTOG0825 and the AVAglio studies are prospective Phase III studies that tested the efficacy of TMZ-based radiochemotherapy with bevacizumab. No significant benefit in PFS or OS was seen in the RTOG 0825 study, while the AVAglio study showed an improvement of 4.4 months in the PFS with no change in the OS in the bevacizumab arm [171,172].
Monoclonal antibody therapy has been used to target EGFR using cetuximab. Combination of cetuximab with bevacizumab/irinotecan was not superior to bevacizumab/irinotecan alone [173]. A Phase I study (NCT01238237) showed that intra-arterial cerebral infusion of cetuximab and/or bevacizumab was safe for the treatment of recurrent gliomas in adults, and a Phase I/II trial is now evaluating the safety and efficacy of intra-arterial cetuximab and bevacizumab for the treatment of relapsed/refractory glioma in patients <22 years (NCT01884740). A Phase II study tested the combination of nimotuzumab (anti-EGFR monoclonal antibody) with concomitant radiation and vinorelbine in childhood diffuse pontine glioma [174].
A Phase III open label trial (NCT00753246) showed no significant benefit in OS by the addition of nimotuzumab to standard therapy for newly diagnosed glioma [175]. ABT414 is an antibody–drug conjugate that delivers the cytotoxic microtubule inhibitor, monomethyl auristatin F to cells with active EGFR or EGFRvIII [176]. A Phase I study (NCT02573324) tested the use of ABT414 alone or in combination with chemotherapy or chemotherapy and radiation and showed responses in 4 out of 12 patients [177].
Immunotherapy with adoptive T-cell transfer & chimeric antigen receptor T cells
Adoptive T cell transfer (ACT) involves the ex vivo production of autologous tumor reactive T cells that are directly transferred back to the patients. Initial studies using ACT for glioma involved the ex vivo expansion of T cells induced by culturing with tumor cells or the isolation of T cells from the draining lymph nodes (dLNs) following immunization with irradiated tumor cells and GM-CSF [178]. In a Phase I study in patients with recurrent glioma and CMV-positive serology, 4 out of 10 patients who received at least three T-cell infusions showed PFS at the time of data compilation and a median OS of 403 days, when infused with ex vivo expanded CMV-specific autologous T cells (Australia New Zealand Clinical Trial Registry; ACTRN12609000338268). While the therapy was deemed to be safe, no correlation was observed between the phenotype and functionality of T cells with PFS and further investigations are warranted [179].
Chimeric antigen receptor (CAR) T cells consist of the antigen-binding region of a monoclonal antibody fused with the signal transduction domain of CD3ζ or FcϵR1γ, and thus combine the specificity and avidity of a monoclonal antibody to the signaling pathways for T-cell effector functions [180]. Preclinical studies have used CAR T cells to target EGFRvIII, IL-13Rα2, HER2 and EphA2. The approach has also been shown to be safe with minimal side effects in a first-in-human pilot safety and feasibility trial (NCT00730613) targeting IL-13Rα2 in recurrent glioma [181]. Two out of three patients also developed transient antiglioma responses. Based on these findings, the IL13Rα2-targeted CAR T cells were further modified to incorporate 4-1BB (CD137) costimulation and a mutated IgG4-Fc linker. Central memory T cells were lentivirally transduced to produce these IL13BBζ CAR T cells. Early findings from one patient who received intracavity and intraventricular infusions showed clinical regression that was sustained for 7.5 months post the initiation of the CAR T-cell therapy. Disease recurrence was, however, observed at new locations after the last cycle, possibly due to the decreased expression of IL13Rα2 may be responsible. Additionally accumulation and expansion of the CAR T cells in the CSF in later cycles and over the 7-day infusion cycle were limited. The results from this patient have prompted the expansion of the Phase I study to evaluate intraventricular administration in a larger cohort of patients [181].
Ongoing clinical trials are evaluating the safety and efficacy of CAR T cells against EGFRvIII, IL-13Rα2, HER2, EphA2, CD133 and MUC I in malignant glioma. A Phase I study (NCT02209376) evaluated the feasibility and safety of manufacturing and administering CAR T cells redirected to EGFRvIII (CART-EGFRvIII) to patients with EGFRvIII-expressing recurrent GBM. No evidence of off-target toxicity or cytokine release syndrome was observed. The data showed evidence of CART-EGFRvIII trafficking to the brain tumor, proliferation of the CAR T cells and antitumor activity; however, robust compensatory immunosuppressive mechanisms including upregulation of IDO1 and PD-L1 and recruitment of Tregs were observed to develop [182].
A Phase I trial is also investigating the use of CMV-specific cytotoxic T lymphocytes expressing a CAR targeting HER2 in patients with glioma (NCT01109095). Infusion of HER2-specific CAR-modified CMV-specific T cells was shown to be well tolerated with no dose-limiting side effects. The study also showed clinical benefit in 8 out of 17 patients (partial response in one and stable disease in seven patients) thus warranting further trials [183].
Numerous investigations are looking into enhancing the specificity and antitumor activity of CAR T cells including the generation of tandem CARs, balanced-signal CAR, dual-receptor circuit CARs and CARs containing chimeric switch receptors [184–188]. The extracellular domain in chimeric switch receptor consists of PD-1 and the intracellular domain is stimulatory so that upon binding PD-L1 a stimulatory rather than inhibitory signal is generated [189]. Efforts are also being made to manage the toxicities associated with the administration of CAR T cells [190].
Oncolytic viral therapy
Viruses hijack host cells’ replication, eventually leading to host cell death and infection of the surrounding healthy cells. Some studies have shown how viruses can be targeted specifically to tumor cells [191,192]. In addition to causing cell death, virus infection also leads to the activation of innate and adaptive immune responses and therefore become attractive immunotherapeutic agents [193]. Two variants of HSV-1 containing mutations in ICP34.5 and ribonucleotide reductase (RNR) have been shown to be safe in Phase I trials and are currently in Phase II testing [194,195]. Other variants of HSV-1, such as M032 and rQNestin34.5 are in preclinical testing. ONYX-015 is an E1B mutant adenovirus that was shown be safe in Phase I study [196]. Another variant AdDelta24-RGD is currently in preclinical and clinical development [197–200]. Reovirus selectively infects cells with activated Ras pathways and when tested in Phase I study with recurrent glioma demonstrated safety and antiglioma activity [201]. An attenuated poliovirus PVS-RIPO showed efficacy in preclinical testing and is currently being tested in Phase I study (NCT01491893) [202,203]. Interim analysis from this study using historical controls seems to confer a survival advantage to the patients infused with PVS-RIPO [204]. H-1 is a parvovirus variant, that was shown to be oncolytic in rat and human GBM cell lines and is in a Phase I/IIa study for recurrent glioma (NCT01301430) [205]. NCT00390299 is a Phase I trial testing the measles virus variant, MV-CEA, in patients with recurrent glioma. Since the receptor for measles virus is expressed on healthy brain tissue and T cells, further work is ongoing to enhance the selectivity and safety of this virus [206,207]. Avian Newcastle disease virus has been tested in a Phase I/II trial (NCT01174537), with no serious side effects and a complete response in one patient [208]. To further enhance the therapeutic efficacy of the oncolytic virus and to reverse tumor-induced immunosuppression, NCT02798406 (CAPTIVE/KEYNOTE-192) is a Phase II trial of a conditionally replicative adenovirus, DNX-2401, in combination with anti-PD-1 monoclonal antibody, pembrolizumab in patients of recurrent glioma or gliosarcoma.
Immunostimulatory gene therapy
The aim of immune stimulatorygene therapy is to modulate the tumor environment such that a robust and effective antitumor immune response can be generated. In a Phase I trial, combination of suicide gene therapy using HSV-TK and IL-2 resulted in minimal side effects and partial response in 2 out of 12 patients [209]. IL-4 has been tested in Phase I study of IL-4-HSV-TK gene-modified autologous tumor to elicit an immune response [210]. A Phase I study conducted in Japan in patients with malignant glioma showed minimal toxicity and a 50% reduction in tumor size in two out of five patients that were given liposomal-mediated delivery of IFNβ [211]. A second Phase I trial that directly delivered Ad-hIFNβ into the tumor cavity and the surrounding area demonstrated safety and tumor cell apoptosis [212]. Adeno-associated viral vectors have been also developed to locally deliver adeno-associated virus (AAV)-IFN-β and tested in combination with chemotherapy. Since DNA replication is required for the synthesis of the second strand of DNA in order to activate the transcription of single-stranded AAV vector, chemotherapy was administered after viral gene therapy, improving the median survival of murine GBM models when compared with single treatments [213]. AAV vectors have been also employed to deliver IL-12 in rodent GBM models [214]. Our team has developed a conditionally cytotoxic immune stimulatory gene therapy mediated through the delivery of adenoviruses encoding HSV1-TK and Flt3L. This therapeutic approach results in tumor regression, long-term survival and a robust memory T-cell response in numerous preclinical glioma models. Importantly, concomitant treatment with temozolomide enhances the efficacy of this gene therapeutic approach in murine models of brain cancer [215]. This strategy is currently under investigation in a Phase I clinical trial in GBM patients (NCT01811992) [47,216–218].
The combination of immune gene therapeutic strategies with the blockade of immunosuppressive mechanisms could improve their efficacy in GBM patients. We have recently found that antibody-mediated blockade of immunological checkpoints and depletion of MDSCs, which constitute 40% of tumor-infiltrating immune cells, enhances the antitumor immune response induced by TK/Flt3L gene therapy in GBM mouse models [95]. Armed oncolytic HSV (oHSV G47Δ), which encodes for IL-12 has been shown to exhibit robust antitumor effects in murine models of GBM when combined with anti-CTLA-4, anti-PD-1 antibodies [219].
Conclusion
The extensive molecular characterization of gliomas, coupled with the 2016 WHO histological classification, have been instrumental in improving our understanding of glioma progression and the response to therapeutics. Also, the genetic lesions encountered within the glioma cells, play a critical role in reprogramming the immune TME. This has opened the horizon for scientists to investigate novel glioma treatment strategies. Work in the field has led to the conclusion that there is a need for combinatorial treatments, in order to elicit higher efficacy and better outcomes in the clinic. In particular, immunotherapies offer very promising approaches for prolonging patient survival; in several ongoing clinical trials immunotherapies have shown evidence of significant anti-tumor outcomes, i.e., circulating specific anti-glioma T cells and higher infiltration of activated immune cells into the TME.
Future perspective
Glioma is a devastating disease and despite many years of research the prognosis remains dismal. Significant progress has been made in developing immunotherapeutic regimens and these may soon be included in the SOC. Several challenges, however, need to be overcome, the chief among which is the intratumor heterogeneity [220].
Given the enormous increase in availability of gene expression, epigenetic and molecular pathway analysis, a personalized therapeutic approach tailored to the tumor would be ideal. A second point to consider is the standardization of diagnostic, therapeutic response and efficacy criteria for clinical trials, making it easier to interpret results and compare outcomes across different clinical trials. The immunotherapy response assessment in neuro-oncology criteria is being established in this regard [221]. Repeat tissue sampling is extremely challenging for CNS tumors and the assessment of therapeutic efficacy is further complicated by the associated edema and pseudoprogression. Efforts are also being made to identify unique biomarkers to serve as inclusion criterion or that can be of prognostic value to predict the response to a particular therapy using tumor-derived DNA from the cerebrospinal fluid [222]. It is also apparent that given the tumor heterogeneity and immunoediting resulting from treatment, a single approach will not be sufficient and successful treatment will require the combination of multipronged therapies such as those combining multiple checkpoint inhibitors with radiation, the combination of checkpoint inhibitors with IDO inhibitors or the combination of checkpoint inhibitors with immune stimulatory gene therapy or with vaccination strategies [95,145,146]. Ongoing clinical trials are testing combinatorial approaches to achieve broad and durable clinical efficacy. Another important factor to consider for the integration of immunotherapy with the current SOC, is the effect of radiation and TMZ on cells of the immune system. Hyperfractionated radiation has been found to correlate with CD4 T-cell depletion [223]. TMZ also causes lymphopenia and it is therefore critical to evaluate the novel immunotherapeutic approaches in the context of SOC [224,225]. Of note, our lab has shown that TMZ administration does not affect the therapeutic efficacy of the TK/Flt3L immunotherapy approach currently in a Phase I study [215].
Executive summary.
Recent molecular characterization of several glioma subtypes, raises the possibility of tailoring treatments to specific genetic lesions encountered in these tumors. This will give rise to precision medicine-based therapies for glioma patients.
The presence of the blood–brain barrier hampers the efficacy of chemotherapies for brain tumors. Immunotherapies, which rely on the migration of activated, tumor antigen-specific cytotoxic T cells, could yield efficacious therapeutic options, as activated immune cells can migrate across the blood–brain barrier.
The use of oncolytic therapeutic approaches, which induce the release of tumor-derived ligands capable of stimulating the immune system of the host, provides an exciting therapeutic modality, triggering immunogenic tumor cell death.
Improvements in genetically engineered chimeric antigen receptor T cells, in order to improve their survival, tumor penetration and in vivo expansion, may provide an attractive therapeutic modality.
Combination therapies, including standard of care together with immunotherapies provide improved efficacy.
Adding immune checkpoint blockade to immunotherapies, would provide another layer of enhancement of therapeutic efficacy.
A coordinated, multi-institutional approach would be required to analyze the results from multicentric Phase I clinical trials, which would enable to move these exciting novel therapies in a timelier fashion into the clinical arena in order to benefit glioma patients.
Supplementary Material
Footnotes
Supplementary data
Supplementary information includes one table Supplementary Table. To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/imt-2017-0122
Financial & competing interests disclosure
This work was supported by the NIH/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants R37NS094804, R01NS074387 and R21NS091555, to M G Castro; NIH/NINDS Grants R01NS076991, R01NS082311 and R01NS096756 to P R Lowenstein; NIH/NCI U01CA224160-01 and NIH/NIBIB R01EB022563 to M G Castro and P R Lowenstein; D Shah and M G Castro are supported in part by NIH/NCI T32CA009676; University of Michigan M-Cubed and the Center for RNA Biomedicine to M G Castro; the University of Michigan Medical School Department of Neurosurgery and the Comprehensive Cancer Center; Leah's Happy Hearts, Chad Tough Foundation, and the Phase One Foundation; Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET PIP 114-201101-00353, Cooperación Internacional CONICET-NIH, doctoral fellowship to AS Asad) and Agencia Nacional de Promoción Científica y Tecnológica (PICT-2013-0310 and PICT-2015-3309) to M Candolfi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro. Oncol. 2015;17(Suppl. 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta. Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta. Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]; • Significant advance in the classification of CNS tumors after taking into consideration the molecular patterns encountered.
- 4.Reifenberger G, Wirsching HG, Knobbe-Thomsen CB, Weller M. Advances in the molecular genetics of gliomas – implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017;14(7):434–452. doi: 10.1038/nrclinonc.2016.204. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig K, Kornblum HI. Molecular markers in glioma. J. Neurooncol. 2017 doi: 10.1007/s11060-017-2379-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan CW, Verhaak RG, Mckenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N. Brat DJ, Verhaak RG, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 2015;372(26):2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masui K, Mischel PS, Reifenberger G. Molecular classification of gliomas. Handb. Clin. Neurol. 2016;134:97–120. doi: 10.1016/B978-0-12-802997-8.00006-2. [DOI] [PubMed] [Google Scholar]
- 12.Bai H, Harmanci AS, Erson-Omay EZ, et al. Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat. Genet. 2016;48(1):59–66. doi: 10.1038/ng.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karsy M, Guan J, Cohen AL, Jensen RL, Colman H. New molecular considerations for glioma: IDH, ATRX, BRAF, TERT, H3 K27M. Curr. Neurol. Neurosci. Rep. 2017;17(2):19. doi: 10.1007/s11910-017-0722-5. [DOI] [PubMed] [Google Scholar]
- 17.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venteicher AS, Tirosh I, Hebert C, et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017 doi: 10.1126/science.aai8478. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaichana KL, Mcgirt MJ, Laterra J, Olivi A, Quinones-Hinojosa A. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J. Neurosurg. 2010;112(1):10–17. doi: 10.3171/2008.10.JNS08608. [DOI] [PubMed] [Google Scholar]
- 21.Bjerke L, Mackay A, Nandhabalan M, et al. Histone H3.3. mutations drive pediatric glioblastoma through upregulation of MYCN. Cancer Discov. 2013;3(5):512–519. doi: 10.1158/2159-8290.CD-12-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013;19(4):764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6(3):217–223. doi: 10.1111/j.1750-3639.1996.tb00848.x. discussion 223–214. [DOI] [PubMed] [Google Scholar]
- 24.Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N. Engl. J. Med. 2016;374(14):1344–1355. doi: 10.1056/NEJMoa1500925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weller M, Van Den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):E395–E403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 26.Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N. Engl. J. Med. 1980;303(23):1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- 27.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 28.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 29.O'toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J. Med. 1991;155(4):384–387. [PMC free article] [PubMed] [Google Scholar]
- 30.Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J. Leukoc. Biol. 2006;80(4):797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 31.Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2(4):269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 32.Davies DC. Blood–brain barrier breakdown in septic encephalopathy and brain tumours. J. Anat. 2002;200(6):639–646. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 34.Hodi FS, O'day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J. Neurosurg. 2011;115(3):505–511. doi: 10.3171/2011.4.JNS101172. [DOI] [PubMed] [Google Scholar]
- 36.Crane CA, Austgen K, Haberthur K, et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc. Natl Acad. Sci. USA. 2014;111(35):12823–12828. doi: 10.1073/pnas.1413933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisele G, Wischhusen J, Mittelbronn M, et al. TGF-β and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129(Pt 9):2416–2425. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 38.Verschuere T, De Vleeschouwer S, Lefranc F, Kiss R, Van Gool SW. Galectin-1 and immunotherapy for brain cancer. Expert Rev. Neurother. 2011;11(4):533–543. doi: 10.1586/ern.11.40. [DOI] [PubMed] [Google Scholar]
- 39.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer. 2016;16(1):7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 40.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nature Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker GJ, Chockley P, Yadav VN, et al. Natural killer cells eradicate galectin-1-deficient glioma in the absence of adaptive immunity. Cancer Res. 2014;74(18):5079–5090. doi: 10.1158/0008-5472.CAN-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friese MA, Wischhusen J, Wick W, et al. RNA interference targeting transforming growth factor-β enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo . Cancer Res. 2004;64(20):7596–7603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 43.Baker GJ, Chockley P, Zamler D, Castro MG, Lowenstein PR. Natural killer cells require monocytic Gr-1(+)/CD11b(+) myeloid cells to eradicate orthotopically engrafted glioma cells. Oncoimmunology. 2016;5(6):e1163461. doi: 10.1080/2162402X.2016.1163461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 45.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunological Rev. 2012;249(1):158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtin JF, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65(16):7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drobits B, Holcmann M, Amberg N, et al. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J. Clin. Invest. 2012;122(2):575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong Z, Ohlfest JR. Topical imiquimod has therapeutic and immunomodulatory effects against intracranial tumors. J. Immunother. 2011;34(3):264–269. doi: 10.1097/CJI.0b013e318209eed4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perng P, Lim M. Immunosuppressive mechanisms of malignant gliomas: parallels at non-CNS sites. Front. Oncol. 2015;5:153. doi: 10.3389/fonc.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore KW, De Waal Malefyt R, Coffman RL, O'garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 52.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 2013;19(12):3165–3175. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Vleeschouwer S, Spencer Lopes I, Ceuppens JL, Van Gool SW. Persistent IL-10 production is required for glioma growth suppressive activity by Th1-directed effector cells after stimulation with tumor lysate-loaded dendritic cells. J. Neurooncol. 2007;84(2):131–140. doi: 10.1007/s11060-007-9362-y. [DOI] [PubMed] [Google Scholar]
- 54.Tanikawa T, Wilke CM, Kryczek I, et al. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012;72(2):420–429. doi: 10.1158/0008-5472.CAN-10-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fontana A, Bodmer S, Frei K, Malipiero U, Siepl C. Expression of TGF-β 2 in human glioblastoma: a role in resistance to immune rejection? Ciba Found. Symp. 1991;157:232–238. doi: 10.1002/9780470514061.ch15. discussion 238–241. [DOI] [PubMed] [Google Scholar]
- 57.Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J. Exp. Med. 1972;136(6):1631–1647. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zagzag D, Salnikow K, Chiriboga L, et al. Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab. Invest. 2005;85(3):328–341. doi: 10.1038/labinvest.3700233. [DOI] [PubMed] [Google Scholar]
- 59.Bodmer S, Strommer K, Frei K, et al. Immunosuppression and transforming growth factor-β in glioblastoma. Preferential production of transforming growth factor-β 2. J. Immunol. 1989;143(10):3222–3229. [PubMed] [Google Scholar]
- 60.Zhang J, Yang W, Zhao D, et al. Correlation between TSP-1, TGF-β and PPAR-γ expression levels and glioma microvascular density. Oncol. Lett. 2014;7(1):95–100. doi: 10.3892/ol.2013.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Platten M, Wick W, Weller M. Malignant glioma biology: role for TGF-β in growth, motility, angiogenesis, and immune escape. Microsc. Res. Tech. 2001;52(4):401–410. doi: 10.1002/1097-0029(20010215)52:4<401::AID-JEMT1025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 62.Vega EA, Graner MW, Sampson JH. Combating immunosuppression in glioma. Future Oncol. 2008;4(3):433–442. doi: 10.2217/14796694.4.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kduom E, Weller M, Heimberger A. Immunosuppressive mechanisms in glioblastoma. Neuro-Oncology. 2015;17(suppl_7):vii9–vii14. doi: 10.1093/neuonc/nov151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2(10):1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 65.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55(1):115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J. Immunol. 2004;173(7):4352–4359. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- 67.Razavi SM, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G. Immune evasion strategies of glioblaγstoma. Front Surg. 2016;3:11. doi: 10.3389/fsurg.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hegardt P, Widegren B, Sjogren HO. Nitric-oxide-dependent systemic immunosuppression in animals with progressively growing malignant gliomas. Cell Immunol. 2000;200(2):116–127. doi: 10.1006/cimm.2000.1625. [DOI] [PubMed] [Google Scholar]
- 69.Badn W, Visse E, Darabi A, Smith KE, Salford LG, Siesjo P. Postimmunization with IFN-γ-secreting glioma cells combined with the inducible nitric oxide synthase inhibitor mercaptoethylguanidine prolongs survival of rats with intracerebral tumors. J. Immunol. 2007;179(6):4231–4238. doi: 10.4049/jimmunol.179.6.4231. [DOI] [PubMed] [Google Scholar]
- 70.Badn W, Hegardt P, Fellert MA, et al. Inhibition of inducible nitric oxide synthase enhances anti-tumour immune responses in rats immunized with IFN-γ-secreting glioma cells. Scand. J. Immunol. 2007;65(3):289–297. doi: 10.1111/j.1365-3083.2007.01901.x. [DOI] [PubMed] [Google Scholar]
- 71.Sippel TR, White J, Nag K, et al. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin. Cancer Res. 2011;17(22):6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- 72.Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. 2013;72(6):1031–1038. doi: 10.1227/NEU.0b013e31828cf945. discussion 1038–1039. [DOI] [PubMed] [Google Scholar]
- 73.Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res. 2012;18(22):6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rubinstein N, Alvarez M, Zwirner NW, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; a potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5(3):241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 75.Camby I, Belot N, Lefranc F, et al. Galectin-1 modulates human glioblastoma cell migration into the brain through modifications to the actin cytoskeleton and levels of expression of small GTPases. J. Neuropathol. Exp. Neurol. 2002;61(7):585–596. doi: 10.1093/jnen/61.7.585. [DOI] [PubMed] [Google Scholar]
- 76.Sato Y, Goto Y, Narita N, Hoon DS. Cancer cells expressing Toll-like receptors and the tumor microenvironment. Cancer Microenviron. 2009;2(Suppl. 1):205–214. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galvao RP, Zong H. Inflammation and gliomagenesis: bi-directional communication at early and late stages of tumor progression. Curr. Pathobiol. Rep. 2013;1(1):19–28. doi: 10.1007/s40139-012-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakayamada S, Takahashi H, Kanno Y, O'shea JJ. Helper T cell diversity and plasticity. Curr. Opin. Immunol. 2012;24(3):297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009;4(4):399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2012;60(3):502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 81.O'keefe GM, Nguyen VT, Benveniste EN. Class II transactivator and class II MHC gene expression in microglia: modulation by the cytokines TGF-β, IL-4, IL-13 and IL-10. Eur. J. Immunol. 1999;29(4):1275–1285. doi: 10.1002/(SICI)1521-4141(199904)29:04<1275::AID-IMMU1275>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 82.Jacobs JF, Idema AJ, Bol KF, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J. Neuroimmunol. 2010;225(1-2):195–199. doi: 10.1016/j.jneuroim.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 83.Jordan JT, Sun W, Hussain SF, Deangulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol. Immunother. 2008;57(1):123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mack SC, Hubert CG, Miller TE, Taylor MD, Rich JN. An epigenetic gateway to brain tumor cell identity. Nat. Neurosci. 2016;19(1):10–19. doi: 10.1038/nn.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lichtenstein AV. Cancer: evolutionary, genetic and epigenetic aspects. Clin. Epigenetics. 2010;1(3–4):85–100. doi: 10.1007/s13148-010-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mirghorbani M, Van Gool S, Rezaei N. Myeloid-derived suppressor cells in glioma. Expert Rev. Neurother. 2013;13(12):1395–1406. doi: 10.1586/14737175.2013.857603. [DOI] [PubMed] [Google Scholar]
- 88.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang L, Debusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 90.Kusmartsev S, Eruslanov E, Kubler H, et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 2008;181(1):346–353. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- 91.Dolcetti L, Peranzoni E, Ugel S, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 2010;40(1):22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 92.Raychaudhuri B, Rayman P, Ireland J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro. Oncol. 2011;13(6):591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 2004;172(2):989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 94.Wesolowski R, Markowitz J, Carson WE., 3rd Myeloid derived suppressor cells – a new therapeutic target in the treatment of cancer. J. Immunother. Cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamran N, Kadiyala P, Saxena M, et al. Immunosuppressive myeloid cells’ blockade in the glioma microenvironment enhances the efficacy of immune-stimulatory gene therapy. Mol. Ther. 2017;25(1):232–248. doi: 10.1016/j.ymthe.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar R, De Mooij T, Peterson TE, et al. Modulating glioma-mediated myeloid-derived suppressor cell development with sulforaphane. PloS ONE. 2017;12(6):e0179012. doi: 10.1371/journal.pone.0179012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yaddanapudi K, Rendon BE, Lamont G, et al. MIF is necessary for late-stage melanoma patient MDSC immune suppression and differentiation. Cancer Immunol. Res. 2016;4(2):101–112. doi: 10.1158/2326-6066.CIR-15-0070-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gabrusiewicz K, Rodriguez B, Wei J, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight. 2016;1(2) doi: 10.1172/jci.insight.85841. pii: e85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J. Pathol. 2008;216(1):15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 100.Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-oncology. 2010;12(11):1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pyonteck, Akkari, Schuhmacher, Bowman, Sevenich, Quail CSF-1R inhibition alters macrophage polarizaation and blocks glioma progression. Nat. Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56.e46. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-oncology. 2006;8(3):234–243. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Characterizes Tregs within the tumor of glioma patients and showed the presence of Tregs in the tumor and increased frequency in the blood as compared with controls.
- 104.Von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005;6(4):338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 105.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21(4):589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 106.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001;193(11):1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188(2):287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 2001;194(5):629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghiringhelli F, Menard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-β-dependent manner. J. Exp. Med. 2005;202(8):1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 2007;13(7):2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 111.Kong LY, Wei J, Sharma AK, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol. Immunother. 2009;58(7):1023–1032. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heimberger AB, Kong LY, Abou-Ghazal M, et al. The role of Tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin. Neurosurg. 2009;56:98–106. [PubMed] [Google Scholar]
- 113.Jordan JT, Sun W, Hussain SF, Deangulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol. Immunother. 2008;57(1):123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin. Cancer Res. 2006;12(14 Pt 1):4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 115.Taieb J, Chaput N, Schartz N, et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J. Immunol. 2006;176(5):2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 116.Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J. Clin. Oncol. 2004;22(4):610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 117.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Catakovic K, Klieser E, Neureiter D, Geisberger R. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun. Signal. 2017;15(1):1. doi: 10.1186/s12964-016-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sen DR, Kaminski J, Barnitz RA, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354(6316):1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mckinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523(7562):612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mirzaei R, Sarkar S, Yong VW. T cell exhaustion in glioblastoma: intricacies of immune checkpoints. Trends Immunol. 2017;38(2):104–115. doi: 10.1016/j.it.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 122.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J. Exp. Med. 1972;136(6):1631–1647. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elliott LH, Brooks WH, Roszman TL. Cytokinetic basis for the impaired activation of lymphocytes from patients with primary intracranial tumors. J. Immunol. 1984;132(3):1208–1215. [PubMed] [Google Scholar]
- 125.Brooks WH, Roszman TL, Rogers AS. Impairment of rosette-forming T lymphocytes in patients with primary intracranial tumors. Cancer. 1976;37(4):1869–1873. doi: 10.1002/1097-0142(197604)37:4<1869::aid-cncr2820370435>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 126.Morford LA, Elliott LH, Carlson SL, Brooks WH, Roszman TL. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J. Immunol. 1997;159(9):4415–4425. [PubMed] [Google Scholar]
- 127.Kmiecik J, Poli A, Brons NH, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 2013;264(1-2):71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 128.Nduom EK, Wei J, Yaghi NK, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro. Oncol. 2016;18(2):195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J. Clin. Oncol. 2016;34(19):2206–2211. doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- 130.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 131.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, Phase II trial. Lancet Oncol. 2012;13(5):459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 132.Lim SH, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Pembrolizumab for the treatment of non-small cell lung cancer. Expert Opin. Biol. Ther. 2016;16(3):397–406. doi: 10.1517/14712598.2016.1145652. [DOI] [PubMed] [Google Scholar]
- 133.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Escudier B, Sharma P, Mcdermott DF, et al. CheckMate 025 randomized Phase III study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur. Urol. 2017 doi: 10.1016/j.eururo.2017.02.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 135.Bloch O, Crane CA, Fuks Y, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a Phase II, single-arm trial. Neuro. Oncol. 2014;16(2):274–279. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Shows that preconditioning of the vaccination site with tetanus toxoid enhanced the efficacy of dendritic cell vaccination. More than 50% of the patients survived longer than 40 months.
- 137.Phuphanich S, Wheeler CJ, Rudnick JD, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. 2013;62(1):125–135. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schuster J, Lai RK, Recht LD, et al. A Phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro. Oncol. 2015;17(6):854–861. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vik-Mo EO, Nyakas M, Mikkelsen BV, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol. Immunother. 2013;62(9):1499–1509. doi: 10.1007/s00262-013-1453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vom Berg J, Vrohlings M, Haller S, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J. Exp. Med. 2013;210(13):2803–2811. doi: 10.1084/jem.20130678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT., Jr Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J. Immunother. 2012;35(5):385–389. doi: 10.1097/CJI.0b013e3182562d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013;86(2):343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang BY, Zhan YP, Zong WJ, et al. The PD-1/B7-H1 pathway modulates the natural killer cells versus mouse glioma stem cells. PloS one. 2015;10(8):e0134715. doi: 10.1371/journal.pone.0134715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luksik AS, Maxwell R, Garzon-Muvdi T, Lim M. The role of immune checkpoint inhibition in the treatment of brain tumors. Neurotherapeutics. 2017 doi: 10.1007/s13311-017-0513-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim JE, Patel MA, Mangraviti A, et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin. Cancer Res. 2017;23(1):124–136. doi: 10.1158/1078-0432.CCR-15-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014;20(20):5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates the role of checkpoint inhibition in gliomas and the importance of targeting multiple pathways for successful treatment.
- 147.Oh T, Fakurnejad S, Sayegh ET, et al. Immunocompetent murine models for the study of glioblastoma immunotherapy. J. Transl. Med. 2014;12:107. doi: 10.1186/1479-5876-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reardon DA, Wucherpfennig KW, Freeman G, et al. An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Rev. Vaccines. 2013;12(6):597–615. doi: 10.1586/erv.13.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weller M, Roth P, Preusser M, et al. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat. Rev. Neurol. 2017;13(6):363–374. doi: 10.1038/nrneurol.2017.64. [DOI] [PubMed] [Google Scholar]
- 150.Srinivasan VM, Ferguson SD, Lee S, Weathers SP, Kerrigan BCP, Heimberger AB. Tumor vaccines for malignant gliomas. Neurotherapeutics. 2017;14(2):345–357. doi: 10.1007/s13311-017-0522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heimberger AB, Suki D, Yang D, Shi W, Aldape K. The natural history of EGFR and EGFRvIII in glioblastoma patients. J. Transl. Med. 2005;3:38. doi: 10.1186/1479-5876-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weller M, Butowski N, Tran DD, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international Phase III trial. Lancet Oncol. 2017;18(10):1373–1385. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 154.Reardon DA, Vredenburgh JJ, Desjardins A, et al. REACT: a Phase II study of rindopepimut (CDX-110) plus bevacizumab (BV) in relapsed glioblastoma (GB) J. Clin. Oncol. 2012;30(15_suppl):TPS2103–TPS2103. [Google Scholar]
- 155.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 156.Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J. Clin. Oncol. 2014;32(19):2050–2058. doi: 10.1200/JCO.2013.54.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rampling R, Peoples S, Mulholland PJ, et al. A cancer research UK first time in human Phase I trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin. Cancer Res. 2016;22(19):4776–4785. doi: 10.1158/1078-0432.CCR-16-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Migliorini D, Dutoit V, Walker PR, Dietrich PY. 6PDPhase I/II study of IMA950 peptide vaccine with Poly-ICLC in combination with standard therapy in newly diagnosed A2 glioblastoma: preliminary results. Ann. Oncol. 2015;26(suppl_8):viii2–viii2. [Google Scholar]
- 159.Koschmann C, Calinescu AA, Nunez FJ, et al. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci. Transl. Med. 2016;8(328):328ra328. doi: 10.1126/scitranslmed.aac8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–327. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]; • Demonstrates that IDH1(R132H) contains an immunogenic epitope suitable for mutation-specific vaccination. Peptides encompassing the mutated region were presented on MHC II and induced mutation-specific CD4+ TH1 responses.
- 161.Crane CA, Han SJ, Ahn B, et al. Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin. Cancer Res. 2013;19(1):205–214. doi: 10.1158/1078-0432.CCR-11-3358. [DOI] [PubMed] [Google Scholar]
- 162.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {α}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64(14):4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 164.Brescia P, Ortensi B, Fornasari L, Levi D, Broggi G, Pelicci G. CD133 is essential for glioblastoma stem cell maintenance. Stem cells. 2013;31(5):857–869. doi: 10.1002/stem.1317. [DOI] [PubMed] [Google Scholar]
- 165.Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol. Res. 2016;4(2):124–135. doi: 10.1158/2326-6066.CIR-15-0151. [DOI] [PubMed] [Google Scholar]
- 166.Buchroithner J, Pichler J, Marosi C, et al. Vascular endothelia growth factor targeted therapy may improve the effect of dendritic cell-based cancer immune therapy. Int. J. Clin. Pharmacol. Ther. 2014;52(1):76–77. doi: 10.5414/CPXCES13EA02. [DOI] [PubMed] [Google Scholar]
- 167.Raizer JJ, Grimm S, Chamberlain MC, et al. A Phase II trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–5305. doi: 10.1002/cncr.25462. [DOI] [PubMed] [Google Scholar]
- 168.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 169.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 170.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(1):156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 172.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hasselbalch B, Lassen U, Hansen S, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a Phase II trial. Neuro. Oncol. 2010;12(5):508–516. doi: 10.1093/neuonc/nop063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Massimino M, Biassoni V, Miceli R, et al. Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J. Neurooncol. 2014;118(2):305–312. doi: 10.1007/s11060-014-1428-z. [DOI] [PubMed] [Google Scholar]
- 175.Westphal M, Heese O, Steinbach JP, et al. A randomised, open label Phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur. J. Cancer. 2015;51(4):522–532. doi: 10.1016/j.ejca.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 176.Phillips AC, Boghaert ER, Vaidya KS, et al. ABT-414, an antibody-drug conjugate targeting a tumor-selective EGFR epitope. Mol. Cancer Ther. 2016;15(4):661–669. doi: 10.1158/1535-7163.MCT-15-0901. [DOI] [PubMed] [Google Scholar]
- 177.Reardon DA, Lassman AB, Van Den Bent M, et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro-Oncology. 2017;19(7):965–975. doi: 10.1093/neuonc/now257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chandramohan V, Mitchell DA, Johnson LA, Sampson JH, Bigner DD. Antibody, T-cell and dendritic cell immunotherapy for malignant brain tumors. Future Oncol. 2013;9(7):977–990. doi: 10.2217/fon.13.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schuessler A, Smith C, Beagley L, et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74(13):3466–3476. doi: 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 180.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol. 2009;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brown CE, Badie B, Barish ME, et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015;21(18):4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.O'rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9(399) doi: 10.1126/scitranslmed.aaa0984. pii: eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First-in-human study demonstrates the safety and feasibility of intravenous delivery of EGFRvIII-directed chimeric antigen receptor T cells.
- 183.Ahmed N, Brawley V, Hegde M, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a Phase 1 dose-escalation trial. JAMA oncology. 2017;3(8):1094–1101. doi: 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Genssler S, Burger MC, Zhang C, et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology. 2016;5(4):e1119354. doi: 10.1080/2162402X.2015.1119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126(8):3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31(1):71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Roybal KT, Rupp LJ, Morsut L, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164(4):770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ankri C, Shamalov K, Horovitz-Fried M, Mauer S, Cohen CJ. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J. Immunol. 2013;191(8):4121–4129. doi: 10.4049/jimmunol.1203085. [DOI] [PubMed] [Google Scholar]
- 189.Liu X, Ranganathan R, Jiang S, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016;76(6):1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gardeck AM, Sheehan J, Low WC. Immune and viral therapies for malignant primary brain tumors. Expert Opin. Biol. Ther. 2017;17(4):457–474. doi: 10.1080/14712598.2017.1296132. [DOI] [PubMed] [Google Scholar]
- 192.Kroeger KM, Muhammad AK, Baker GJ, et al. Gene therapy and virotherapy: novel therapeutic approaches for brain tumors. Discov. Med. 2010;10(53):293–304. [PMC free article] [PubMed] [Google Scholar]
- 193.Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol. Res. 2014;2(4):295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 195.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a Phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 196.Ganly I, Kirn D, Eckhardt G, et al. A Phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000;6(3):798–806. [PubMed] [Google Scholar]
- 197.Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J. Natl Canc. Inst. 2003;95(9):652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 198.Jiang H, Gomez-Manzano C, Aoki H, et al. Examination of the therapeutic potential of δ-24-RGD in brain tumor stem cells: role of autophagic cell death. J. Natl Canc. Inst. 2007;99(18):1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 199.Jiang H, Clise-Dwyer K, Ruisaard KE, et al. δ-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PloS One. 2014;9(5):e97407. doi: 10.1371/journal.pone.0097407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Van Putten EH, Wembacher-Schroder E, Smits M, Dirven CM. Magnetic resonance imaging-based assessment of gadolinium-conjugated diethylenetriamine penta-acetic acid test-infusion in detecting dysfunction of convection-enhanced delivery catheters. World Neurosurg. 2016;89:272–279. doi: 10.1016/j.wneu.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 201.Forsyth P, Roldan G, George D, et al. A Phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16(3):627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 202.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc. Natl Acad. Sci. U S A. 1996;93(6):2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dobrikova EY, Goetz C, Walters RW, et al. Attenuation of neurovirulence, biodistribution, and shedding of a poliovirus:rhinovirus chimera after intrathalamic inoculation in Macaca fascicularis . J. Virol. 2012;86(5):2750–2759. doi: 10.1128/JVI.06427-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Desjardins A, Sampson JH, Peters KB, et al. Patient survival on the dose escalation phase of the Oncolytic Polio/Rhinovirus Recombinant (PVSRIPO) against WHO grade IV malignant glioma (MG) clinical trial compared to historical controls. J. Clin. Oncol. 2016;34(15_suppl):2061–2061. [Google Scholar]
- 205.Geletneky K, Kiprianova I, Ayache A, et al. Regression of advanced rat and human gliomas by local or systemic treatment with oncolytic parvovirus H-1 in rat models. Neuro. Oncol. 2010;12(8):804–814. doi: 10.1093/neuonc/noq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Allen C, Vongpunsawad S, Nakamura T, et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66(24):11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- 207.Allen C, Paraskevakou G, Iankov I, et al. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol. Ther. 2008;16(9):1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006;13(1):221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 209.Colombo F, Barzon L, Franchin E, et al. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther. 2005;12(10):835–848. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- 210.Okada H, Pollack IF, Lotze MT, et al. Gene therapy of malignant gliomas: a Phase I study of IL-4-HSV-TK gene-modified autologous tumor to elicit an immune response. Hum. Gene Ther. 2000;11(4):637–653. doi: 10.1089/10430340050015824. [DOI] [PubMed] [Google Scholar]
- 211.Yoshida J, Mizuno M, Fujii M, et al. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon β gene using cationic liposomes. Hum. Gene Ther. 2004;15(1):77–86. doi: 10.1089/10430340460732472. [DOI] [PubMed] [Google Scholar]
- 212.Chiocca EA, Smith KM, Mckinney B, et al. A Phase I trial of Ad.hIFN-β gene therapy for glioma. Mol Ther. 2008;16(3):618–626. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- 213.Guhasarkar D, Neiswender J, Su Q, Gao G, Sena-Esteves M. Intracranial AAV-IFN-β gene therapy eliminates invasive xenograft glioblastoma and improves survival in orthotopic syngeneic murine model. Mol. Oncol. 2017;11(2):180–193. doi: 10.1002/1878-0261.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chiu TL, Wang MJ, Su CC. The treatment of glioblastoma multiforme through activation of microglia and TRAIL induced by rAAV2-mediated IL-12 in a syngeneic rat model. J. Biomed. Sci. 2012;19:45. doi: 10.1186/1423-0127-19-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Candolfi M, Yagiz K, Wibowo M, et al. Temozolomide does not impair gene therapy-mediated antitumor immunity in syngeneic brain tumor models. Clin. Cancer Res. 2014;20(6):1555–1565. doi: 10.1158/1078-0432.CCR-13-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ghulam Muhammad AK, Candolfi M, King GD, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin. Cancer Res. 2009;15(19):6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Candolfi M, Curtin JF, Yagiz K, et al. B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia. 2011;13(10):947–960. doi: 10.1593/neo.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.King GD, Muhammad AK, Curtin JF, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro. Oncol. 2008;10(1):19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32(2):253–267. doi: 10.1016/j.ccell.2017.07.006. e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–542. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang Y, Springer S, Zhang M, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc. Natl Acad. Sci. USA. 2015;112(31):9704–9709. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin. Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huang J, Dewees TA, Badiyan SN, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int. J. Radiat. Oncol. Biol. Phys. 2015;92(5):1000–1007. doi: 10.1016/j.ijrobp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 225.Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. 2012;1(2):149–154. doi: 10.2217/cns.12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.