Abstract
Background and Purpose
Although preclinical studies have shown inflammation to mediate perihematomal edema (PHE) after intracerebral hemorrhage (ICH), clinical data are lacking. Leukocyte count, often used to gauge serum inflammation, has been correlated with poor outcome, but its relationship with PHE remains unknown. Our aim was to test the hypothesis that leukocyte count is associated with PHE growth.
Methods
We included patients with ICH admitted to a tertiary-care stroke center between 2011-2015. The primary outcome was absolute PHE growth over 24 hours, calculated using semi-automated planimetry. Linear regression models were constructed to study the relationship between absolute and differential leukocyte counts (monocyte count and neutrophil-lymphocyte ratio [NLR]), and 24-hour PHE growth.
Results
A total of 153 patients were included. Median hematoma and PHE volumes at baseline were 14.4 (interquartile range [IQR], 6.3-36.3) and 14.0 (IQR, 5.9-27.8), respectively. In linear regression analysis adjusted for demographics and ICH characteristics, absolute leukocyte count was not associated with PHE growth (beta, 0.07, standard error [SE], 0.15, p=0.09). In secondary analyses, NLR was correlated with PHE growth (beta, 0.22; SE, 0.08; p=0.005).
Conclusions
Higher NLR is independently associated with PHE growth. This suggests that PHE growth can be predicted using differential leukocyte counts on admission.
Keywords: Intracerebral hemorrhage, inflammation, leukocyte, neurotrophils, brain edema
Perihematomal edema (PHE) is considered a radiological surrogate for secondary injury and inflammation, and is independently associated with mortality and poor functional outcomes in ICH patients.1 In clinical studies, peripheral blood leukocyte count has been used as a marker for central nervous system inflammation. Absolute and differential leukocyte counts (such as monocyte count and neutrophil-lymphocyte ratio [NLR]) have been correlated with hematoma expansion and mortality after ICH.2, 3 Although PHE is believed to be orchestrated by inflammatory pathways, a similar association between PHE and leukocyte count has yet to be elucidated in clinical studies. With ongoing clinical trials in ICH targeting secondary injury4, it is important to identify patients at risk for PHE growth early on. Therefore, we aimed to assess the relationship between serum leukocyte counts and PHE growth.
Methods
We performed a retrospective cohort study of ICH patients from the Cornell AcutE Stroke Academic Registry (CAESAR) from January 2011 to December 2015. We included all ICH patients over the age of 18 years, who had non-contrast computed tomography scans (CT) performed on admission and at 24 hours. We excluded patients with secondary causes of ICH (i.e., trauma, aneurysms, tumors, arteriovenous malformations), surgical hematoma evacuation, and conditions with associated leukocytosis such as infection (in the first week) and hematological malignancies. The study was approved by the institutional review board at Weill Cornell Medicine.
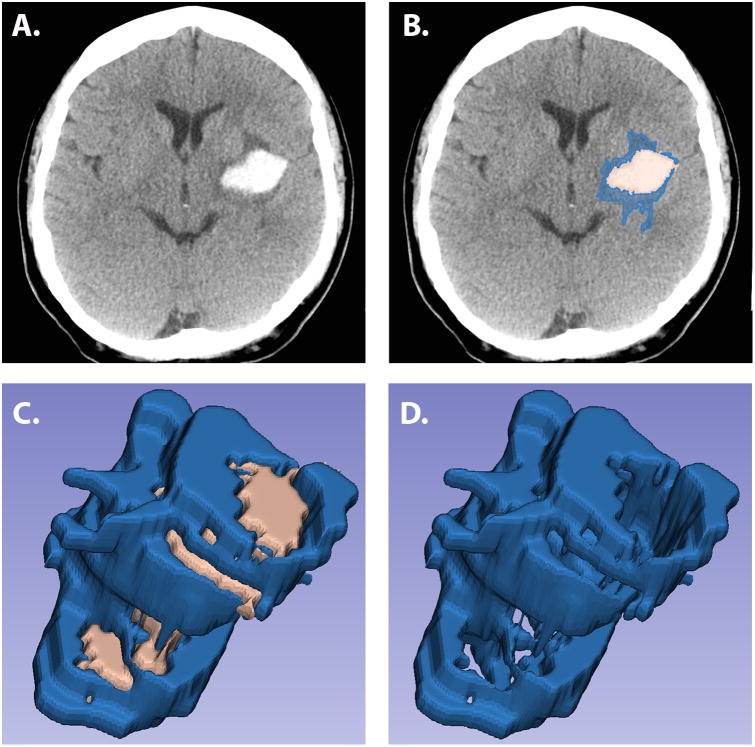
We measured hematoma and PHE volumes on the baseline scan and the scan closest in time to 24 hours after baseline. A single investigator (A.M.G) measured hematoma and PHE volumes using semi-automated planimetry with 3D Slicer5, 6 (Figure1) detailed in the Supplemental Material. This method of PHE determination has excellent inter-rater reliability.7 Additionally, the inter-rater intraclass correlation coefficients were 0.82 (0.75-0.89) between two independent readers (A.M.G and S.B.M) suggesting excellent agreement. Hematoma expansion was defined as an increase in the absolute baseline hematoma volume by either 33% or > 6 milliliters (mL) over 24 hours.8 Our predictor variables were absolute leukocyte count, NLR and monocyte count. The primary outcome measure was PHE growth, defined as the difference in the absolute PHE volumes on CT between the 24 hours and baseline studies as used in prior studies.9
Figure 1.
Non-contrast computed tomography scans showing a putaminal hemorrhage (Panel A) with demarcation of the differences in hypodensities (Panel B) and 3-dimensional reconstruction of hematoma and perihematomal volumes (Panel C) and 3-dimensional reconstruction of perihematomal edema only (Panel D).
Statistical Analysis
Continuous variables were represented as median (interquartile range [IQR]) since they were not normally distributed. We first performed univariable linear regression to identify variables associated with PHE growth, with the threshold of significance set at p<0.20 (Supplemental Table I). Natural logarithmic transformations were performed to minimize skewness of continuous variables when necessary. These covariates were then included in a multiple linear regression to assess the relationship of complete and differential leukocyte counts with PHE growth. The variance inflation factor was used to detect multicollinearity. Sensitivity analyses were performed based on 1) presence of seizures, which can cause leukocytosis, 2) external ventriculostomy drain (EVD) use since drainage of cerebrospinal fluid can potentially decrease PHE evolution, and 3) inpatient mortality. Statistical analyses were performed using Stata (version 14.0, College Station, TX). All analyses were two-tailed, and significance level was determined by p<0.05.
Results
A total of 153 patients with ICH were included. Median PHE growth over 24 hours was 3.1 mL (IQR, 5.4- 27.9) (Table1). We built three different linear regression models to study the absolute and differential components of the leukocyte count separately. Model covariates included age, baseline hematoma volume, intraventricular extension, EVD use, Glasgow Coma Scale, and times to baseline and 24-hour CT scans (Table 2). The absolute leukocyte count was not significantly correlated with PHE growth (beta, 0.07, standard error [SE], 0.15, p=0.09). There was a 22% increase in 24-hour PHE growth per unit increase in NLR (beta, 0.22; SE, 0.08; p=0.005). In sensitivity analyses stratified by presence of seizures in the first 24 hours, EVD placement and inpatient mortality, NLR remained significantly associated with PHE growth (Supplemental Table II).
Table 1. Baseline Demographic and Hematoma Characteristics of Patients with Intracerebral Hemorrhage.
| Demographic Characteristics | ICH cases N= 153 (%) |
|---|---|
| Age (years)1 | 66.0 (54.0-78.0) |
|
| |
| Sex | |
| Male | 92 (60.1) |
| Female | 61 (39.9) |
|
| |
| Race | |
| Caucasian | 124 (81.0) |
| African American | 18 (11.8) |
| Hispanic | 1 (0.7) |
| Others | 10 (6.5) |
|
| |
| Seizures within 24 hours | 12 (7.8) |
|
| |
| Diabetes mellitus | 26 (17.0) |
|
| |
| Hypertension | 97 (63.4) |
|
| |
| Hyperlipidemia | 50 (32.7) |
|
| |
| Atrial fibrillation | 14 (9.2) |
|
| |
| Anticoagulant medication | 12 (7.8) |
|
| |
| Antiplatelet medication | 49 (32.0) |
|
| |
| Antipyretic medication | 38 (24.8) |
|
| |
| ICH Characteristics | |
|
| |
| Admission GCS, median (IQR) | 13 (11-15) |
|
| |
| GCS <9 | 35 (22.9) |
|
| |
| Hematoma volume at baseline (mL)1 | 14.4 (6.6-36.0) |
|
| |
| Hematoma volume at 24 hours (mL)1 | 15.1 (6.1-30.7) |
|
| |
| Hematoma expansion | 25 (16.3) |
|
| |
| PHE at baseline (mL)1 | 14.0 (5.9-27.8) |
|
| |
| PHE growth in 24 hours (mL)1 | 3.1 (5.4-27.9) |
|
| |
| Location of ICH | |
| Lobar | 65 (42.5) |
| Basal Ganglia | 76 (49.7) |
| Infratentorial | 12 (7.8) |
|
| |
| Intraventricular extension | 56 (36.6) |
|
| |
| External ventricular drain (EVD) | 34 (22.2) |
|
| |
| Time to baseline CT scan (hours)1 | 5.0 (1.5-13.3) |
|
| |
| Time to 24-hour CT scan (hours)1 | 25.0 (18.8-38.5) |
|
| |
| Time to baselinetotal leukocyte count (hours)1 | 4.7 (1.6-12.6) |
|
| |
| Admission systolic blood pressure (mm Hg)1 | 160 (136- 186) |
|
| |
| Admission diastolic blood pressure (mm Hg)1 | 88 (75- 100) |
|
| |
| Admission INR1 | 1.1 (1.0-1.2) |
|
| |
| Hemoglobin A1C (gram/deciliter)1 | 5.6 (5.3- 6.7) |
|
| |
| Total leukocyte count (cells/mm3)1 | 9,000 (6,600-12,100) |
|
| |
| Neutrophil count (cells/mm3)1 | 6,607 (4,782-9,920) |
|
| |
| Lymphocyte count (cells/mm3)1 | 1,219 (756-1,536) |
|
| |
| Monocyte count (cells/mm3)1 | 602 (402-829) |
|
| |
| Neutrophil to lymphocyte ratio1 | 5.4 (3.4-11.4) |
Abbreviations: CT, Computerized Tomography; EVD, external ventricular drain; GCS, Glasgow Coma Scale; ICH, Intracerebral Hemorrhage; IQR, Interquartile Range; mL, Milliliters; PHE, Perihematomal Edema.
indicates values represented as median (interquartile range)
Table 2. Multivariable Linear Regression Analyses of Perihematomal Edema Growth After Intracerebral Hemorrhage.
| Covariates | PHE growth | |
|---|---|---|
| Beta (SE) | P value | |
| Model 1 | ||
| Absolute leukocyte count (per 1,000 cells/mm3) | 0.07 (0.15) | 0.09 |
| Model 2 | ||
| Neutrophil to Lymphocyte Ratio (per unit) | 0.22 (0.08) | 0.005 |
| Model 3 | ||
| Monocyte count (per 1,000 cells/mm3) | -0.11 (1.67) | 0.95 |
Each model was adjusted for baseline hematoma volume, hematoma expansion, intraventricular extension, external ventricular drain placement, Glasgow Coma Scale, and times to baseline and 24-hour computed tomorgraphy scans.
Abbreviations: Abbreviations: PHE, Perihematomal Edema; SE, Standard Error
Discussion
In this retrospective cohort study at a tertiary-care stroke center, we found baseline NLR to be independently correlated with 24-hour PHE growth after ICH. The inflammatory response following ICH is well-known to result in peripheral leukocytosis.10 ICH leads to microglial activation within hours which in turn secrete cytokines and chemokines that promote neutrophil and monocyte infiltration within 12 hours.11 Additionally, recent evidence also suggests that astrocytes shed extracellular vesicles which regulate peripheral leukocyte response in response to brain inflammation.12 Since inflammation is mainly responsible for PHE evolution4, we believe peripheral leukocyte count may aid in stratifying the risk of PHE growth at the time of presentation.
Our study has some noteworthy limitations. First, the generalizability of our results may be limited by selection biases, small sample size, and the retrospective nature of this analysis. Second, exclusion of patients who underwent surgical evacuation limits our results to only medically managed ICH patients. Third, we assessed PHE only up to the first 24 hours while it is known that PHE evolves well beyond this time frame.13 Finally, we also did not have information on body temperature and osmotherapy, both being known predictors of PHE growth.4
In conclusion, our study demonstrates that baseline NLR is significantly associated with PHE growth in the first 24 hours, suggesting a potential role of inflammatory pathways in the evolution of PHE. While these inflammatory pathways may be potential therapeutic targets, larger prospective studies using additional time points of PHE assessment are required to confirm the role of leukocytes in PHE growth.
Supplementary Material
Acknowledgments
Sources of Funding: A. Gusdon: National Natural Science Foundation of China grant 81550110260.
B. Navi: NIH K23NS091395; Florence Gould Endowment for Stroke Discovery.
C. Iadecola: NIH grants R37NS089323, R01NS034179, R01NS037853, R01NS095441, and R01NS100447.
A. Gupta: KL2TR000458 from the NIH/NCATS.
H. Kamel: NIH grants K23NS082367, R01NS097443; Michael Goldberg Stroke Research Fund.
S. Murthy: American Academy of Neurology/American Brain Foundation; Leon Levy Neuroscience Foundation.
Footnotes
Disclosures: None
References
- 1.Murthy SB, Moradiya Y, Dawson J, Lees KR, Hanley DF, Ziai WC, et al. Perihematomal edema and functional outcomes in intracerebral hemorrhage: Influence of hematoma volume and location. Stroke. 2015;46:3088–3092. doi: 10.1161/STROKEAHA.115.010054. [DOI] [PubMed] [Google Scholar]
- 2.Walsh KB, Sekar P, Langefeld CD, Moomaw CJ, Elkind MS, Boehme AK, et al. Monocyte count and 30-day case fatality in intracerebral hemorrhage. Stroke. 2015;46:2302–2304. doi: 10.1161/STROKEAHA.115.009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. 2016;47:1654–1657. doi: 10.1161/STROKEAHA.116.013627. [DOI] [PubMed] [Google Scholar]
- 4.Urday S, Kimberly WT, Beslow LA, Vortmeyer AO, Selim MH, Rosand J, et al. Targeting secondary injury in intracerebral haemorrhage--perihaematomal oedema. Nat Rev Neurol. 2015;11:111–122. doi: 10.1038/nrneurol.2014.264. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalo Dominguez M, Hernandez C, Ruisoto P, Juanes JA, Prats A, Hernandez T. Morphological and volumetric assessment of cerebral ventricular system with 3d slicer software. Journal of medical systems. 2016;40:154. doi: 10.1007/s10916-016-0510-9. [DOI] [PubMed] [Google Scholar]
- 6.3D Slicer. [Accessed on September 15, 2016]; http://www.Slicer.Org.
- 7.Urday S, Beslow LA, Goldstein DW, Vashkevich A, Ayres AM, Battey TW, et al. Measurement of perihematomal edema in intracerebral hemorrhage. Stroke. 2015;46:1116–1119. doi: 10.1161/STROKEAHA.114.007565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer SA, Brun NC, Broderick J, Davis S, Diringer MN, Skolnick BE, et al. Safety and feasibility of recombinant factor viia for acute intracerebral hemorrhage. Stroke. 2005;36:74–79. doi: 10.1161/01.STR.0000149628.80251.b8. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Arima H, Wu G, Heeley E, Delcourt C, Zhou J, et al. Prognostic significance of perihematomal edema in acute intracerebral hemorrhage: Pooled analysis from the intensive blood pressure reduction in acute cerebral hemorrhage trial studies. Stroke. 2015;46:1009–1013. doi: 10.1161/STROKEAHA.114.007154. [DOI] [PubMed] [Google Scholar]
- 10.Askenase MH, Sansing LH. Stages of the inflammatory response in pathology and tissue repair after intracerebral hemorrhage. Semin Neurol. 2016;36:288–297. doi: 10.1055/s-0036-1582132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickens AM, Tovar YRLB, Yoo SW, Trout AL, Bae M, Kanmogne M, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10:eaai7696. doi: 10.1126/scisignal.aai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatasubramanian C, Mlynash M, Finley-Caulfield A, Eyngorn I, Kalimuthu R, Snider RW, et al. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke. 2011;42:73–80. doi: 10.1161/STROKEAHA.110.590646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.