Abstract
Microphysiological systems and organs-on-chips promise to accelerate biomedical and pharmaceutical research by providing accurate in vitro replicas of human tissue. Aside from addressing the physiological accuracy of the model tissues, there is a pressing need for improving the throughput of these platforms. To do so, scalable data acquisition strategies must be introduced. To this end, we here present an instrumented 24-well plate platform for higher-throughput studies of engineered human stem cell-derived cardiac muscle tissues that recapitulate the laminar structure of the native ventricle. In each well of the platform, an embedded flexible strain gauge provides continuous and non-invasive readout of the contractile stress and beat rate of an engineered cardiac tissue. The sensors are based on micro-cracked titanium-gold thin films, which ensure that the sensors are highly compliant and robust. We demonstrate the value of the platform for toxicology and drug-testing purposes by performing 12 complete dose-response studies of cardiac and cardiotoxic drugs. Additionally, we showcase the ability to couple the cardiac tissues with endothelial barriers. In these studies, which mimic the passage of drugs through the blood vessels to the musculature of the heart, we regulate the temporal onset of cardiac drug responses by modulating endothelial barrier permeability in vitro.
Graphical abstract
Flexible sensors provide readout of the contractile stress and beat rate of up to 24 human stem cell-based cardiac tissues
Introduction
The staggering costs associated with drug development limit innovation and render cutting-edge therapies unattainable for countless patients. The limited efficiency of traditional preclinical models to identify toxic or ineffective compounds is a key part of this issue1, 2. Thus, retrospective analyses have found that merely ~50% of rodent studies are predictive of human toxicity3. The potential of organs-on-chips and microphysiological systems (MPS) is that in vitro engineered tissues based on human stem cells will provide more predictive preclinical drug assessments, at a lower cost. Since heart failure remains a leading cause of death and cardiotoxicity is one of the most prevalent causes of drug withdrawal4, tools for modeling the human cardiac musculature are of particular interest.
A central challenge for any microphysiological system is to faithfully recapitulate structural and functional hallmarks of the native tissue counterpart. In the mammalian heart, numerous sheets of highly aligned muscle cells wrap circumferentially around the heart chambers, following a spiraling angle from the epi- to the endo-cardium5, 6. Notably, each muscle layer merely spans approximately 4 cells in thickness, with each layer being spaced by the extensive connective tissue in the heart7. Muscular thin film (MTF) platforms mimic this laminar architecture in the form of engineered anisotropic 2D muscle tissues on soft material cantilever substrates8–10. Upon muscle tissue contraction, the cantilever bends proportionally to the stress generated by the tissue. By optically tracking cantilever motion in a microscope setup, changes in contractile stress can be quantified, enabling drug testing and disease modeling11, 12. However, the reliance on optical tracking as readout creates obstacles for scaling the assay to real-time and higher-throughput formats, as optical readouts are data-heavy, often require post-processing and are hardware intensive.
In this report, we present a multi-well cardiac MTF platform with integrated flexible strain gauges. The sensors are based on robust and compliant micro-cracked gold thin films, which can readily be deformed by the small stresses exerted by the engineered cardiac muscle tissue. The platform provides continuous electronic readout of the contractile stress and beat rate of up to 24 separate cardiac tissues. The electronic readout streamlines experimentation and data acquisition, which we demonstrate by performing dose-response studies of 12 cardiac drugs on human induced pluripotent stem cell-derived cardiomyocyte (hiPS-CM) tissues. Additionally, the open-well format of the platform allows us to easily couple the cardiac MTFs with endothelial barrier inserts, and study the temporal drug transport across the barriers. We take advantage of this to mimic the changes in cardiac drug transport across the vasculature endothelium that occurs during inflammation, by exposing the endothelial barriers to TNF-α13–16.
Results & Discussion
Device Principle & Engineered Cardiac Tissues
The central part of the device is a ~25µm thick, multilayer poly(dimethylsiloxane) (PDMS)-based cantilever that combines control of tissue structure with electronic readout of the contractile function; see Fig 1. On the surface of each cantilever is an engineered, cardiac thin film tissue, based on either hiPS-CMs or neonatal rat ventricular myocytes (NRVM). Upon tissue contraction and shortening, the underlying PDMS cantilever is bent and a flexible titanium-gold thin-film strain gauge, embedded in the cantilever, is strained. This generates an increase in resistance proportional to the tissue contractile stress; see Fig 1a-b. We recently introduced this general principle in a lower-throughput device that relied on highly specialized 3D-printing equipment for fabrication17, 18. In addition to significantly increasing the throughput, we here focused on designing a generally accessible platform, by basing device manufacture on more commonly available and scalable tools. To this end, we applied sequential spin-coating to fabricate the bulk of the stacked PDMS cantilever structure and used a CO2 laser cutter to define the cantilevers12. Spin coating provided a facile method for deposition of multiple PDMS layers, each in the µm range. Similarly, e-beam evaporation was applied to create titanium-gold thin-film strain gauges. The fabrication procedure is outlined in supplementary Fig. S1.
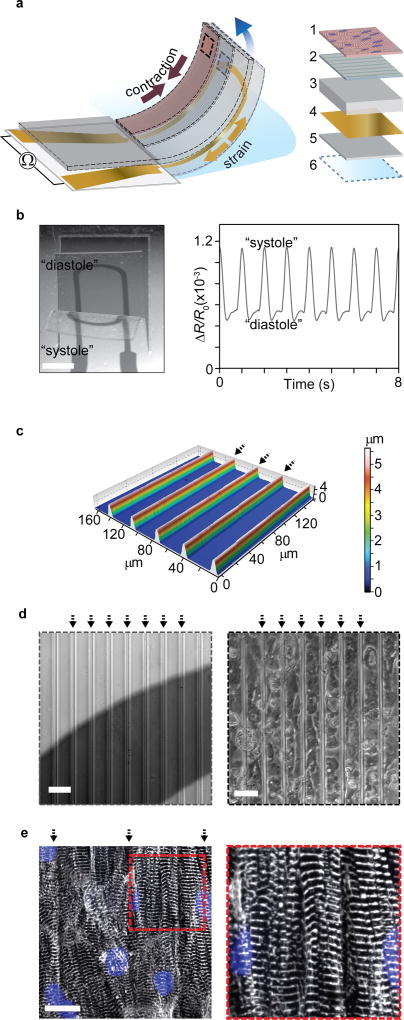
Figure 1. Engineered cardiac muscle tissue on cantilever with embedded flexible thin-film strain gauge.
(a) Principle sketch of device cantilever and constituent layers 1: Engineered cardiac muscle tissue 2: tissue-aligning micro-molded or micro-patterned layer 3: PDMS layer, 4: Ti-Au thin-film sensor layer, 5: bottom PDMS layer 6: PNIPAAM release layer. (b) Example microscope image of the deflecting cantilever and corresponding electrical readout, scale bar 2 mm. (c) Optical profilometer characterization of molded 500 kPa PDMS surface. Arrows indicate barrier peaks. (d) Left: Bright field optical micrograph of grooves on cantilevers applied for structural guidance of hiPS-CM tissues, strain gauge wire seen a dark area. Right: Bright field optical micrograph of hiPS-CM tissue on molded PDMS top layer, scale bars 40 µm. (e) Confocal microscopy of immunostained hiPS-CM tissue, blue: DAPI nuclei stain, white α-actinin. Scale bar 20 µm.
The architecture of the tissues was engineered to recapitulate the laminar structure and anisotropy of native cardiac musculature, following principles described in our previous reports8, 10, 18. To develop hiPS-CM tissues, we used microgrooves molded in 500 kPa PDMS. Groove barriers were 5µm tall and 4µm wide and were coated with Fibronectin (FN) prior to cell seeding; see Fig 1c-d. Culturing hiPS-CMs on the molded substrates gave rise to tissues with highly aligned sarcomeres; see Fig 1e. Correspondingly, the hiPS-CM tissues displayed a sarcomeric organizational order parameter (OOP)19 of 0.31; see supplementary Fig S2. For NRVM-based tissues, we used micro-contact printing to apply FN line patterns and generate anisotropic engineered tissues, as previously described8, 12. The OOP for NRVM tissues was 0.40; see supplementary Fig. S2. Both classes of tissues further displayed electrical connectivity through gap junctions; see supplementary Fig S3 and S4. The engineered tissues thus replicate essential structural hallmarks of the musculature of the heart.
Micro-Cracked Titanium-Gold Thin Films as Sensor Material
To achieve reliable device fabrication and readout, it was essential to limit the overall thickness of each layer of the cantilever, and of the titanium-gold strain gauge sensors in particular. Metal thin films can be deposited consistently in the nanometer range using E-beam evaporation, providing a robust route to sensor fabrication. Yet, beyond limiting the sensor thickness, we found that it was crucial to control the thin-film micro-structure. The Ti-Au thin films were deposited on thin ~5 µm PDMS substrates, which constitute the bottom part of the final cantilevers. We initially observed that macroscopic cracks occurred frequently and spontaneously when applying Ti adhesion layers of thicknesses 5 nm and greater on these µm thin PDMS substrates; see Fig 2a. Such macroscopic cracks commonly led to discontinuities and device failure. We subsequently noted that these macroscopic cracks did not occur when reducing the Ti adhesion layer thickness to 3 nm. SEM imaging revealed that this corresponded to a transition in the micro-structure of the Ti-Au thin-films from micro-buckled to micro-cracked20, 21; see Fig 2a. In accordance with previous findings, the micro-cracked thin films were highly stretchable, allowing strains in excess of 10% without failure20, 22; see Fig 2b. We attribute the absence of macroscopic cracks in the micro-cracked thin films to their ability to withstand the large strains that may arise at edges and impurities. In addition to overcoming macroscopic failures, the micro-cracked thin films gave rise to reliable signals with negligible dependency on rate in the 0.1% strain regime that was found to be relevant for the final device; see Fig 2c. Also, no notable changes in baseline resistance were detected during week-long tissue culture. Hence, the micro-cracked Ti-Au thin films provide a robust basis for the instrumented MTF cantilevers.
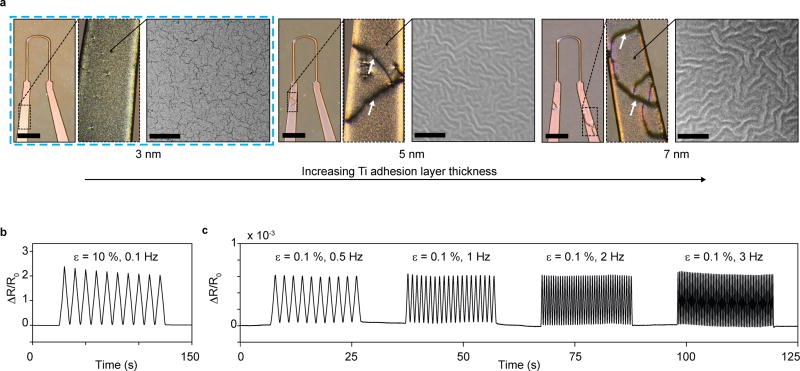
Figure 2. Titanium-Gold thin-film micro-structure, appearance and mechanical robustness.
(a) Optical micrographs (left, scale bars 2 mm) and SEM images (right, scale bars 5 µm) of 20 nm Au thin films deposited using 3, 5 or 7 nm Ti adhesion layers, on 5 µm thick PDMS substrates. For 5 nm and 7 nm Ti adhesion layers, buckled micro-structures were observed. These micro-buckled films frequently displayed macroscopic failure cracks in device formulations, deposited through shadow masks. For all thin films applied in devices, 1 nm Ti was added onto Au surface to ensure adhesion to subsequent PDMS layers. Thus, in final devices 3 nm Ti, 20 nm Au, and 1 nm Ti was deposited sequentially. These films yielded robust and reproducible devices. (b-c) Uniaxial strain tests of 3-20-1 nm Ti-Au-Ti thin films deposited on 5 µm thick PDMS substrates. (b) Cyclic 10 % strain did not lead to failure in thin-film conductivity. (c) Cyclic strain in the 0.1 % regime relevant to the final devices, displayed negligible dependency on strain rate, in the frequency range of tissue contractions.
The thickness of the titanium adhesion layer has previously been reported to have a pronounced effect on the micro-structure of titanium-gold thin films deposited on PDMS substrates21. In previous reports, adhesion layers thicknesses of up to 6 nm, as compared to the 3 nm observed here, were reported to give rise to micro-cracked structures21. Surprisingly, we found that the threshold below which micro-cracking occurs depends on PDMS substrate thickness. When increasing the PDMS substrate thickness above the 5 µm used in our device, we observed an increasing tendency towards developing micro-cracks and a decreasing tendency towards micro-buckles. For instance, 5 nm Ti adhesion layers gave rise to micro-cracked structures on ~1 mm thick PDMS substrates, while buckles formed on PDMS substrates of 60 µm and less; see supplementary Figs S5–7.
It is not clear how the PDMS substrate thickness might affect the thin-film micro-structure. It is possible that our observations may be a result of artifacts or indirect effects such as minor changes in surface properties21. Previously, the two types of micro-structure have been attributed to whether the Au layer grows on a continuous or discontinuous Ti layer 21. Buckled thin films were attributed to growth on continuous adhesion layers and micro-cracked thin films were attributed to thinner adhesion layers with a lower degree of PDMS coverage21. We propose that the degree of coverage of the Ti adhesion layer may be affected by the PDMS substrate thickness, potentially as part of a lateral edge effect.
Sensor Characterization & Device Readout
To enable conversion of the thin-film strain gauge readout to the contractile stresses generated by the engineered cardiac muscle tissues, we conducted electromechanical and optical characterization of the device components. Initially, we applied a series of uniaxial strain tests to determine the relationship between sensor wire strain and resistance. By comparing these tests with the readouts obtained in the MTF experiments, we found the sensor strains occurring upon tissue contraction to be in the order of 0.1 %. Our uniaxial strain tests indicate that in this regime, the micro-cracked thin films display an approximately linear strain-resistance relationship with an apparent gauge factor of 0.6, see Fig 3a-b and supplementary Figs S8 and S9. This low gauge factor arises from a significant part of the in-plane strain being relaxed in the form of out-of-plane displacements and rotations of the micro-cracked thin film20. However, these modes of relaxation are essential to the operation of the device since they make the thin-film gauges more compliant.
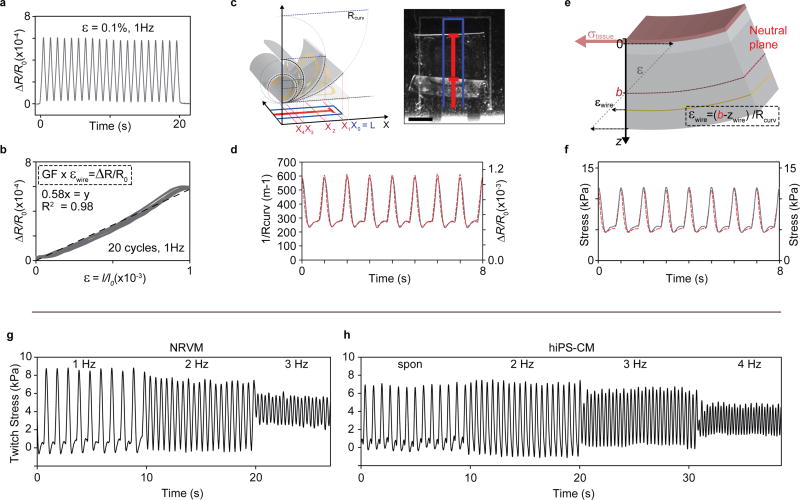
Figure 3. Thin-film sensor calibration and device mechanical model.
(a) Relative resistance change of 3-20-1 nm Ti-Au-Ti thin films deposited on 5 µm PDMS substrates, upon cyclic 0.1 % uniaxial strain at 1 Hz. (b) Relative resistance change vs. strain for cyclic 0.1 % uniaxial strain at 1 Hz. Linear fit (dashed line) indicate a gauge factor (GF) of app. 0.58. (c) optical tracking of cantilever deflection, scale bar 1 mm. (d) Concurrent optical tracking of cantilever curvature (left axis, dashed red line) and electrical readout of relative resistance change (right axis, solid grey line) from cantilever with NRVM tissue. (e) Principle sketch of mechanical model based on Stoney’s equation, applied to convert electrical and optical readout to stress generated by the engineered muscle tissues. By taking advantage of the concurrent optical and electrical readout, the placement of cantilever neutral axis b could be determined, see supplementary information (f) Stress values obtained applying mechanical model to convert optical (left axis, dashed red line) and electrical (right axis, solid grey line) readouts displayed in (d) to tissue stress values. (g) Force-frequency of twitch stress of NRVM-based tissues, paced at 1, 2, 3 Hz at day 4 after seeding on devices (h) Force-frequency of twitch stress of hiPS-CM tissues, spontaneously contracting and paced at 2, 3, 4 Hz at day 6 after seeding.
Subsequently, we applied optical tracking to determine the relationship between the curvature of the cantilever and the resistance signal, generally finding a satisfactory correspondence between electrical and optical signal, see Fig 3c-d and supplementary Figs S10-S14. Importantly, the sensor readout showed a high degree of repeatability across multiple cantilevers from independent fabrication runs; see supplementary Figs S11-S14. This indicates that sensor fabrication is consistent and that the sensors are durable, even to week-long exposure to tissue culture environment and repeated cyclic stretching caused by spontaneous tissue contractions. Yet, we did observe minor discrepancies between optical tracking and sensor readout. At times, a sharp decrease in resistance prior to the increase associated with tissue contraction was detected. We attribute this to a brief uniaxial compression of the gauge, before the tissue contraction overcomes the viscous and inertial forces that hinder out of plane movement of the cantilever18. Importantly, these brief artifacts did not influence the final tissue twitch stress values.
Finally, to fully describe the multilayer cantilever, we applied a modified version of Stoney’s equation; see Fig 3e and supplementary information. Applying this model we estimated the effective Young’s modulus of micro-cracked thin film to be ~0.8 GPa and thus significantly lower than the modulus of bulk gold. Most importantly, tissue twitch stress values of 5–10 kPa were obtained for NRVM MTFs, in very good agreement with previous findings10, 18; see Fig 3f and supplementary Fig S13. We further evaluated the force frequency relationship of the engineered tissues, finding that both hiPS and NRVM MTFs displayed negative force-frequency relationships, indicative of some degree of immaturity23; see Fig 3g-h. Noting that, also for the higher frequencies, we observed a good correspondence between sensor readout and an optical tracking; see supplementary Fig S12.
Instrumented 24-Well Platform
The integrated electrical readouts facilitate scaling up the number of assays that can be run in parallel. Our platform allows the contractile stress and beat rate of up to 24 biological replicates to be continuously evaluated, see Fig 4 and supplementary Fig S15–17. The wells of the device were made from machined polycarbonate. This limits the total volume of PDMS per well to the cantilever itself, (<0.5 µl volume) thereby reducing concerns related to absorption of hydrophobic drugs24. Using a custom recording setup and electrical pacing inserts, the devices facilitate running multiple parallel drug dose-response studies with replicates and references, inside an incubator; see Fig 4 and supplementary Fig S15–17.
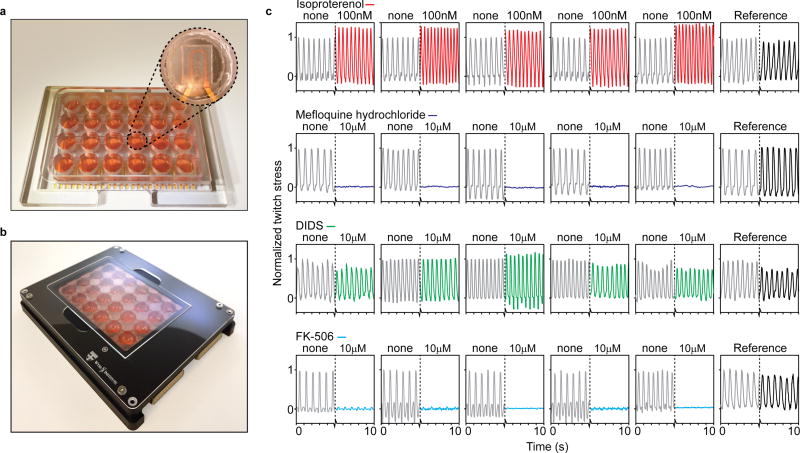
Figure 4. Instrumented 24-well platform enables multiple parallel experiments.
(a) Example 24-well device, with polycarbonate multi-well housing. Insert: Example cantilever in well. (b) 24-well device in recording holder applied for data acquisition inside incubator environments. (c) 24 example readouts of hiPS-CM tissues applied in drug dose-response experiments, with parallel replicates and references. From top: 100 nM Isoproterenol increased beat rate and spontaneous contractile stress, 10 µM Mefloquine hydrochloride completely disrupted contraction, 10 µM Disodium 4,4'-diisothiocyanatostilbene-2,2'-disulfonate (DIDS) did not cause any notably effects, 10 µM FK-506 markedly decreased contractile stress, but not beat rate.
Cardiac Drug Dose-Response Studies
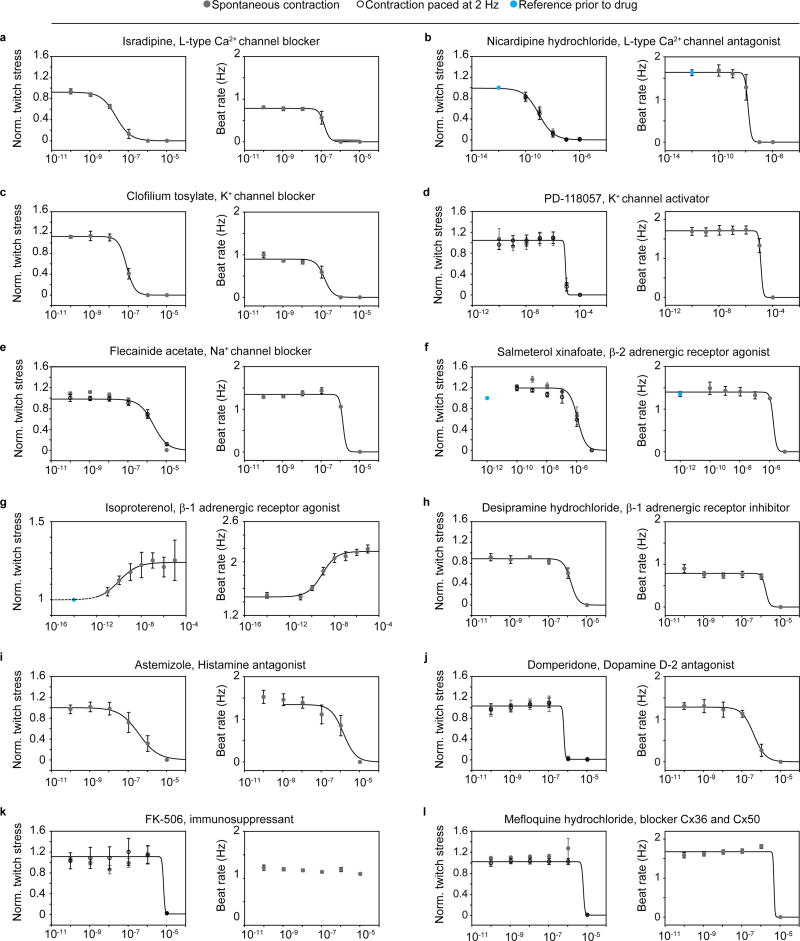
To demonstrate the value of the devices for toxicology and drug screening purposes, we performed sequential dose-response studies of 12 diverse drugs on engineered human cardiac muscle tissues, based on hiPS-CMs. The drugs were chosen to cover a range of cardiac functionalities, and their relative effects on spontaneous peak twitch stress as well as on spontaneous beat rate were studied; see Fig 5 and Table 1. For a subset of drugs the peak twitch stress for contractions paced at 2 Hz were also acquired.
Figure 5. Sequential drug dose-response studies on hiPS-CM-derived tissues.
Increasing drug doses were sequentially added, and tissue spontaneous beat rate and spontaneous twitch stress normalized to initial twitch stress, were recorded (grey circles). For a subset of the studies, the twitch stress while pacing electrically at 2 Hz (hollow circles) was also acquired. Error bars are S.E.M. Lines indicated 4-parameter logistic fits. EC/ IC50 values obtained from fits presented in Table 1. (a) Isradipine, N=5 (b) Nicardipine, N=4, (c) Clofilium tosylate, N=5 (d) PD-118057, N=5, (e) Flecanide acetate, N=5, (f) Salmeterol xinafoate, N=5, (g) Isoproterenol, N=6, (h) Desipramine hydrochloride N=4, (i) Astemizole N=5, (j) Domperidone N=5, (k) FK-506, N=5, (l) Mefloquine hydrochloride N=5.
Table 1. EC50 or IC50 values from sequential drug dose-response studies on hiPS-CM-derived tissues.
Values obtained from 4-parameter logistic fits to data presented in Fig 5. (spon): From spontaneous twitch stress (paced): From twitch stress while pacing electrically at 2 Hz.
| EC50 or IC50 | Twitch stress | Spontaneous beat frequency |
|---|---|---|
| Isradipine | 22.5 nM (spon.) | 147 nM |
| Nicardipine | 1.19 nM (spon.), 0.971nM (paced) | 15.2 nM |
| Clofilium | 81.6 nM (spon.) | 148 nM |
| PD-118057 | 8.8 µM (spon), 8.77 µM (paced) | 13.3 µM |
| Flecainide | 1.37 µM (spon), 2.12 µM (paced) | 1.37 µM |
| Salmeterol | 1.07 µM (spon), 1.08 µM (paced) | 1.79 µM |
| Isoproterenol | 0.107 nM (spon) | 0.844 nM |
| Desipramine | 1.49 µM (spon) | 1.75 µM |
| Astemizole | 0.361 µM (spon) | 1.44 µM |
| Domperidone | 0.731 µM (spon), 0.629 µM (paced) | 0.419 µM |
| FK-506 | 7.15 µM (spon), 7.00 µM (paced) | - |
| Mefloquine | 2.18 µM (spon), 6.48 µM (paced) | 1.44 µM |
Ca2+-channel antagonists are commonly used for treatment of hypertensive diseases25. We studied two types of Ca2+-channel blockers: Isradipine and Nicardipine; see Fig 5a-b. Both drugs decreased the twitch stress and spontaneous beat rate of the tissues, and both drugs affected twitch stress at 10-fold lower doses than beat rate. We obtained notably higher IC50 values for Israpidine than Nicardipine; see Table 1. This relative difference in potency between the drugs, as well as the range of IC50 values, are in accordance with previous studies on human papillary cardiac muscle strips 25.
Drugs targeting Na+ and K+ channels have classically been used for treatment of arrhythmias, but carry an inherent dose-dependent proarrhythmic risk 26, 27. At the same time, off-target interaction with hERG K+-channel is a most common concern in drug development, due to the risk of inducing ventricular arrhythmias, fibrillation and Torsades de Pointes (TdP)28. We tested hERG K+-channel blocker clofilium tosylate, Fig 5c, and the activator PD-118057, Fig 5d. For clofilium we observed a disruption of spontaneous contraction as indicated by a drop in beat rate and twitch stress with IC50 in the 100 nM range; see Table 1, matching the range previously found to induce TdP in isolated rabbit heart models in vitro 29. For PD-118057 we observed a disruption of spontaneous beat rate and twitch stress in the 10 µM range. Interestingly, previous in vitro electrophysiological evaluations of induced stem cell-derived cardiomyocytes carrying a long QT syndrome type 2 mutation found that a slightly lower dose decreased action potential (AP) duration and rescued early after-depolarisation events 30. We also perturbed Na+-channels through exposure to Flecainide acetate, observing a simultaneous drop in spontaneous beat rate and twitch stress in the single µM range; see Fig 5e and Table 1, illustrating the well-established risks associated with Flecainide 26, 31.
Given the central role of adrenergic receptors in regulating cardiac rate and contractile strength, we studied the effect of a β-2 agonist: Salmeterol xinafoate and a β-1 agonist: Isoproterenol; see Fig 5f-g and Table 1. For Salmeterol xinafoate, we observed a minor increase in twitch stress at low doses, in agreement with the canonical effects of β-2 agonists. At higher doses, we observed a cardiotoxic effect marked by stress and beat rate reduction in the µM range. A cardiotoxic effect at higher doses has previously been observed for hiPS-CM in vitro32. For the β-1 agonist Isoproterenol we observed well-described increases in spontaneous beat rate and spontaneous twitch stress with EC50 in the 0.1–1 nM range, comparable, while slightly lower than our previous studies on hiPS-CM based tissues 18, and studies by other groups of in vitro engineered cardiac stem cell-derived tissues33.
We additionally tested several non-cardiac drugs with severe cardiotoxic side effects. The antidepressant desipramine has been associated with fatal arrhythmias in several children34. For this drug, we observed an arrest of spontaneous contraction in the single µM range; see Fig 5h and Table 1. Astemizole is a histamine antagonist which was withdrawn due to hERG blockade side effects 35. For Astemizole we found that the spontaneous beating and stress were disrupted in the single µM range; see Fig 5i and Table 1. For the dopamine receptor agonist Domperidone, which has previously been found to induce arrhythmia and sudden cardiac death side effects 36, we observed a concerted disruption in tissue beat rate and contractile stress in the 0.5 µM range; See Fig 5j and Table 1. FK-506 is an immunosuppressant used in organ transplants to prevent transplant rejection, with calcineurin-related hypertension and hypertrophy cardiac side effects 37, 38. For this drug, we observed a diminished contractile stress in the 10 µM range, with no apparent influence on beat rate; see Fig 5k and Table 1. Finally, for the malarial drug Mefloquine, a range of cardiac side effects including arrhythmia and inotropic side effects have been reported39, 40. For this drug, we observed an abrupt drop in both contractile stress and rate at µM doses; see Fig 5l and Table 1. Collectively, the drug dose-response studies demonstrate the ability of the platform to detect a range of cardiac effects and side-effects on human stem cell-derived tissues, in a higher-throughput manner.
Cardiac Drug Exposure through Endothelial Barrier Inserts
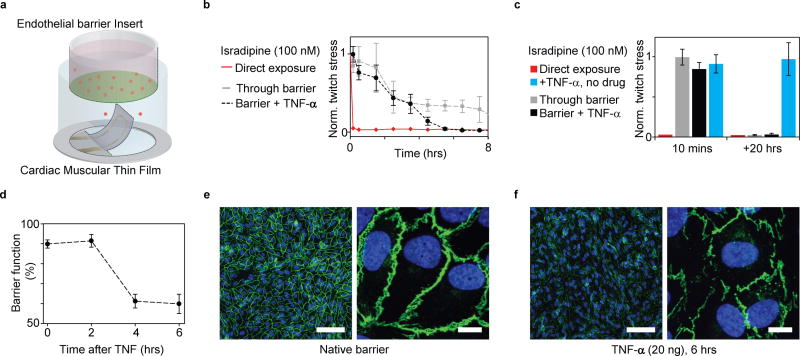
The standard open-well format of our instrumented cardiac platform makes it compatible with a range of common laboratory tools such as Transwell® inserts, which can support endothelial tissue barriers; see Fig 6a. The endothelium of the vasculature plays a critical role in regulating molecular transport to the heart musculature13, 15. Changes in endothelium permeability such as those occurring during inflammation can thus have a pronounced influence on the transport of cardiac drugs13, 14. To mimic such events, we studied the transport and subsequent cardiac effect of the Ca2+ channel blocker Isradipine, across a vascular endothelial barrier with and without co-exposure of TNF-α16.
Figure 6. Coupling endothelial barriers with cardiac MTFs to study drug transport.
(a) Schematic illustration of experiments. Endothelial barrier tissues in Transwell® inserts are introduced into the wells of the instrumented cardiac 24-well MTF platform. A cardiac drug (Isradipine) is introduced in the volume shielded by the endothelial barrier. Drug transport across barrier and resultant cardiac effects are recorded in real-time through embedded sensors. (b) Decrease in normalized contractile twitch stress over time. Direct exposure of Isradipine (100 nM) immediately halts contraction (red). Exposure of Isradipine (100 nM) through endothelial barrier inserts (grey line), leads to delayed drug effect, where contraction is still observed after > 7 h exposure. (N=3). Compromising endothelial barrier function by co exposure with TNF-α significantly accelerates drug diffusion, leading to a full effect of the drug after ~5 h. (c) After 20 h full effect of Isradipine was observed for all samples. TNF-α did not directly introduce changes in twitch stress of cardiac tissues. (d) Endothelial tissue barrier function decreases upon exposure to TNF-α (20 ng/ml). Barrier function was evaluated as percent of fluorescent marker (400 Da) contained inside barrier during 20 mins diffusion into reservoir without tracer (e) Confocal microscopy of immunostained intact endothelial barrier. Blue: DAPI nuclei stain, Green: VE-Cadherin, Scale bars left: 100 µm, right: 10 µm (f) Confocal microscopy of immunostained endothelial barrier after exposure to 20 ng/ml TNF-α for 6 h. Blue: DAPI nuclei stain, Green: VE-Cadherin, Scale bars left: 100 µm, right: 10 µm.
We added a high Isradipine dose, 100 nM, to the insert containing media and a confluent endothelial monolayer, and studied the passive transport across the barrier by evaluating the contractile stress of the cardiac MTFs below. As expected, the barrier significantly delayed the cardiac response to the drug, see Fig 6b-c. While direct exposure of the drug immediately disrupts the contractile function of the cardiac tissue, contractile function remains only partially affected after 8 h and fully disrupted after 20 h, when protected by the endothelial barrier. Notably, this temporal profile can be altered by disrupting the endothelial barrier using TNF-α, a potent regulator of vascular permeability in vivo16. TNF-α accelerated the passive transport of Isradipine across the endothelial tissue as evident by a complete disruption of contractile function being observed after 5.5 h; see Fig 6b. TNF-α alone did not decrease the contractile stress; see Fig 6c. We noted that the effect of TNF-α on endothelial barrier function was evident only after 4 hours of co-exposure. As control, we confirmed this response time by studying the effect of TNF-α isolated endothelial barriers; see Fig. 6d-f and supplementary Fig S18. Collectively, these studies demonstrate how the complexity of the assay can be increased to include aspects of drug transport, delivery and co-administration as well as paracrine signaling effects 41.
General Discussion
An inherent dilemma for MPS and organs-on-chips aimed at pharmaceutical applications is how to balance high biological content versus high experimental throughput. This tradeoff is reflected in multifarious approaches that have been taken in studying human stem cell-derived cardiomyocytes and engineered cardiac tissues. These range from construction of thicker 3D constructs33, 42–45, some even with embedded vasculatures46, to 2D single cell and cell-pair islands47. The extremities each hold value and drawbacks. For instance, increasing size and complexity of model tissue can simultaneously increase the amount of cells required and challenges associated with fabrication44, 48, 49. Similarly, 2D cell islands offer closely defined geometry, mechanical and chemical cellular environment, at the expense of tissue ensemble effects. Whether following a predominantly holistic or reductionist approach, a central challenge is capturing essential structural and functional hallmarks of the native counterpart in question. MTFs are inherently 2D with cell-cell connections being confined to a single plane, and do not support the incorporation of vasculature. Accordingly, studying effects such as drug diffusion through the tissue will require the use of a thicker model tissue. Yet, MTFs replicate the laminar structure, electrical connectivity and anisotropy of the heart musculature. Further, the soft cantilever substrates allow the tissue to shorten, similarly to in the native heart. In this report, we studied the responses of cardiac MTFs based on human induced stem cells, to 12 diverse cardiac drugs. Our findings largely match prior in vivo, ex vivo and engineered tissue findings, demonstrating the relevance of human cardiac MTFs for drug screening and toxicology applications.
Beyond considering the biological complexity of the model tissues, data acquisition considerations are of critical and often overlooked importance when aiming to scale MPS to the large numbers required for drug screening and toxicology applications. Integration of appropriate sensors represents an appealing route to scaling up the number of assays that can be run in parallel. However, sensor integration increases fabrication complexity, in particular since structure and function of tissue models should not be disrupted. Consequently, instrumented MPS that take advantage of electronic readouts to provide higher throughputs assays have only recently started to emerge18, 50. The 2D structure of MTF cardiac model tissues facilitates integration of strain gauges sensors while only minimally increasing fabrication complexity.
The micro-cracked titanium-gold thin films offer a number of benefits as sensor material for this application. In addition to a repeatable and scalable fabrication procedure, the sensors display a high degree of robustness, with no significant changes being observed during culture, and with similar sensitivity across independent cantilevers. The stretchability of the micro-cracked thin films additionally reduced the bending stiffness of the cantilevers; ensuring tissues were not hindered in contractile shortening. Yet, the micro-cracks also represent a draw-back as they are the central cause behind the relatively low gauge factor of the sensors. Alternative sensor materials such as soft composites based on elastomers with conducting particle additives are interesting alternatives, due to their low stiffness and high sensitivities18, 51.
Conclusions
In this report, we addressed key challenges associated with scaling cardiac microphysiological systems to the high throughputs required for preclinical and toxicological research. We demonstrated how incorporation of flexible thin-film sensors allowed fabrication of a multi-well platform with continuous readout of the contractile stress and beat rate of up to 24 human stem cell-derived engineered cardiac tissues. The platform significantly simplifies drug tests, which we demonstrated by performing full dose-response studies of 12 cardiac and cardiotoxic drugs. Further, the open-well design of the platforms ensured compatibility with a range of standard laboratory tools, to enable coupled studies of cardiac drug transport across human vascular endothelial barriers inserts. The platform fabrication relies on generally accessible, scalable techniques, and on robust micro-cracked Ti-Au thin films for sensing. The methodology presented in this report is thus generally applicable and well-suited for cardiac drug screening and disease modelling purposes. It therefore provides a path towards high-throughput, high-quality preclinical research, based on human stem-cell derived cardiac tissues.
Materials & Methods
Device fabrication
24-well devices were fabricated on 127.9 mm×85.8 mm×1.0 mm glass substrates, following a bottom-up procedure. First, silver contact pads on the device edge were made by stencil printing of AG-510 from Conductive Compounds, annealing at 190 °C for 30 mins. Subsequently, Poly(N-isopropylacrylamide) (PNIPAAM) release layer islands were made using a pressure sensitive pen to dispense 2 w/v% solution in isopropanol or by masked spin coating of a 10w/v% PNIPAAM solution in 1-Butanol. Subsequently, PDMS (Sylgard 184 Dow-Corning) layers were made through stepwise spin-coating and curing overnight at 65 °C. For bottom PDMS either 4000 rpm or 5000 rpm (5 mins) were used; equivalent to a 5.2 or 3.3 µm thick layer, respectively. Ti-Au-Ti (3 nm, 20 nm, 1 nm) sensor wires were added using a custom stainless steel shadow mask (NewCut) and an e-beam evaporator (Denton), starting deposition at 0.9–1×10−6 Torr. To ensure localization of the resistance to the cantilever loop, 100 nm Au, 1 nm Ti was added to the non-cantilever wire parts. Following evaporation, a middle PDMS layer was added by spin-coating at 2000 rpm or 1500 rpm (5 mins); equivalent to a 11.9 or 16.2 µm thick layer, respectively. Following curing, thin films were annealed to release pre-stress by heating to 190 °C for 30 mins. Lastly, a softer PDMS coating was added by spincoating a 2:1 mix of Sylgard 527 and Sylgard 184 (Dow-Corning) at 2500 rpm (5 mins); equivalent to a 6.2 µm thick coating. For hiPS-CM devices, the soft PDMS layer was micro-molded (30 µm×4 µm×5 µm, gap width×barrier width×barrier height) using fluorinated (Tridecafluoro-1, 1, 2, 2-Tetrahydrooctyl)-1-Trichlorosilane, United Chem) PDMS stamps. Cantilevers were defined using a CO2 laser-cutter (Epilog). Milled polycarbonate wells were attached to the device by casting a thin PDMS gasket onto the well part and attaching it to PDMS-covered glass substrate using plasma treatment.
Device cell seeding and tissue culture
Human iPS-CM tissues: Fully assembled devices with micro-molded cantilevers were sterilized using UV-Ozone. Subsequently, wells were incubated with a 50 µg/ml solution of human Fibronectin (FN, BD biosciences) in PBS, for at least 1 h. After removal of the FN solution, hiPS-CMs (Cor4U, Axiogenesis, Germany) were seeded at ~200 k/cm2 in designated Commercial Cor4U media (Axiogenesis, Germany). Cor4U media was changed every 2 days.
Neonatal Rat Ventricular Myocyte tissues: Non-molded devices were sterilized using UV-Ozone. FN line patterns (15 µm×4 µm) were micro-contact printed onto cantilevers using previously established procedures12. Primary NRVMs were seeded at a density of ~140 k/cm2 in 10 % FBS in media 199 (Lonza). Cell media was replaced every 2 days, applying 2 % FBS in media 199 (Lonza). NRVMs were obtained from newborn Sprague Dawley rats (n ≥ 10 litters, per harvest) following procedures approved by the Harvard University Animal Care and Use Committee; the Institutional Animal Care and Use Committee (IACUC). The procedures follow "US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training", the Guide for the Care and Use of Laboratory Animals 8th Ed., the Animal Welfare Act/Regulations, as well as other federal, state and city laws and regulations.
Endothelial Barrier Culture
Prior to cell seeding, polycarbonate Transwell® inserts with 0.4 µm pores (Corning) were incubated with 50 µg/mL human fibronectin (FN, BD biosciences) in PBS for 2 h and inserts were rinsed twice with PBS. Human umbilical vein endothelial cells (ATCC) were seeded at a density of ~75 k/cm2 in commercial endothelial growth media and supplements containing 2 % FBS (Lonza). Media was changed daily and exchanged to reduced serum media containing 0.5 % FBS on day 3 and 4.
Data acquisition & stress calculation
Data were acquired using a custom polycarbonate connector and a Keithley Multichannel DMM 3706a. The 2-wire resistance recordings were generally obtained, at sampling rates ≥60 Hz.
To convert the resistance readout to stresses generated by the tissues, we applied a mechanical model based on a modified Stoney’s equation. After determining the mechanical properties of each layer in the cantilever, as well as the gauge factor of the Ti-Au thin film, this model provided linear conversion constants between relative changes in resistance and tissue contractile stress; see supplementary information for details. Custom MATLAB (MathWorks, Natick, MA) codes were applied for quantifying relative resistance changes as well as for converting to stress. For electrically paced samples, a median filter (5) was applied to filter noise from pacing.
Cumulative drug dose studies
Cumulative dosing of cardiac drugs was performed on engineered hiPS-CM tissues inside incubator at day 6–9 after seeding. 900 µl serum-free media was added to each cell well prior to drug dose experiments. A dilution series of the drugs in media was added in 9 µl doses. Samples were incubated with dose 10 mins prior to recording. For each dose, 30 s were recorded per channel. Isoproterenol stocks were kept at 4 °C prior to dosing. Pacing was applied using custom platinum wire electrodes. Each n in analysis denotes biological replicate of a tissue in separate isolated well with associated sensor and media. Drugs were obtained from Sigma-Aldrich (LO2219).
Endothelial barrier testing and disruption
Barrier function tests were performed using Oregon Green 488 carboxylic acid (~412 Da) and Alexa Fluor 555 succinimidyl ester (~1250 Da) as fluorescent tracers (ThermoFisher). Fluorescent media containing 1 µg/ml of each tracer was added to the top insert and incubated for 20 min. Samples of top and bottom media were collected and fluorescent intensity was measured using a Synergy HT plate reader (BioTek) to estimate barrier permeability. Barrier function was calculated as percent tracer contained in top reservoir. To disrupt barrier function, human tumor necrosis factor alpha (Sigma-Aldrich) was diluted in endothelial media to a concentration of 20 ng/mL and was added to inserts. Fluorescent intensity of top and bottom media was measured every 2 h to estimate barrier disruption over the course of 6 h.
Tissue immuno-staining and OOP structural analysis
All immunocytochemistry procedures were conducted at room temperature. Cardiac samples were fixed with 4 % PFA/PBS (v/v) solution for 10 mins and then permeabilized with 0.05 % Triton-X/PBS (v/v) solution for 10 mins. Subsequently, samples were incubated for 1 h with monoclonal sarcomeric α-actinin (clone EA-53; Sigma-Aldrich) and Cx43 (ab11370; abcam) primary antibodies, washed three times in PBS, and finally counterstained with Alexa Fluor 488-conjugated anti-mouse secondary antibody and Alexa Fluor 546-conjugated anti-rabbit secondary antibody, Alexa Fluor 633-conjugated Phalloidin and DAPI (Invitrogen). Endothelial barrier tissues were fixed using with 4 % PFA/PBS (v/v) for 10 mins and permeabilized using 0.05 % Triton-X/PBS (v/v) solution for 2 mins. Subsequently, samples were incubated with 5% Bovine Serum Albumin/PBS (w/v) for 30 mins and washed three times in 0.5 % BSA/PBS (w/v). Samples were incubated for 1 h with rabbit polyclonal antibody to VE-cadherin (Abcam, ab33168) and mouse monoclonal antibody to CD31 (PECAM) (Abcam, ab24590) and rinsed three times with 0.5% BSA/PBS. Samples were counterstained for 2 h with Alexa Flour 488-conjugated anti-rabbit secondary antibody, Alexa Fluor 546-conjugated anti-mouse secondary antibody, and DAPI (ThermoFisher) and rinsed three times with 0.5% BSA/PBS. Samples were mounted on coverslips using ProLong Gold Antifade (ThermoFisher). Samples were imaged using confocal microscopy (Olympus) and z-stacks were projected to acquire images of cardiac tissues and endothelial barriers. The alignment and spatial organization of α-actinin positive structures were evaluated using the OOP methodology, previously described 19. Briefly, for both NRVM and hiPS-CM tissues, z-stacks of this sarcomere stain was acquired for 5 independent FOVs (from at least 3 samples). Following z-projection, the angular orientation of all sarcomeres was calculated and the degree of similarity in their orientations was quantified in terms of their OOP using a custom MATLAB (MathWorks, Natick, MA) code19.
Supplementary Material
Acknowledgments
This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF award no. 1541959. CNS is part of Harvard University. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UH3TR000522; U. S. Army Research Laboratory and the U. S. Army Research Office under Contract No. W911NF-12-2-0036; and the Harvard University Materials Research Science and Engineering Center (MRSEC), NSF Award number DMR-1420570. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Office, Army Research Laboratory, the U.S. Government or the National Institutes of Health. The U.S. Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation hereon. Authors thanks K. Hudson and M. Rosnach for assistance with photography, as well as J. Ferrier and A. Cho for their assistance with 3D rendering and shadow-mask fabrications. Authors additionally thank F.S. Pasqualini, S.P. Sheehy, J.A. Goss, T.A. Busbee and T. Biering-Sorensen for helpful discussions.
Footnotes
The authors declare no completing financial interests.
References
- 1.Schachter AD, Ramoni MF. Nature reviews Drug discovery. 2007;6:107–108. doi: 10.1038/nrd2246. [DOI] [PubMed] [Google Scholar]
- 2.DiMasi JA, Grabowski HG, Hansen RW. Journal of health economics. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W. Regulatory Toxicology and Pharmacology. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S. Circulation. 2013;127:e62–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckberg GD, Hoffman JI, Coghlan HC, Nanda NC. European Journal of Cardio-Thoracic Surgery. 2015;47:587–601. doi: 10.1093/ejcts/ezu278. [DOI] [PubMed] [Google Scholar]
- 6.Kocica MJ, Corno AF, Carreras-Costa F, Ballester-Rodes M, Moghbel MC, Cueva CN, Lackovic V, Kanjuh VI, Torrent-Guasp F. European Journal of Cardio-thoracic Surgery. 2006;29:S21–S40. doi: 10.1016/j.ejcts.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.LeGrice IJ, Smaill B, Chai L, Edgar S, Gavin J, Hunter PJ. American Journal of Physiology-Heart and Circulatory Physiology. 1995;269:H571–H582. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK. Science. 2007;317:1366–1370. doi: 10.1126/science.1146885. [DOI] [PubMed] [Google Scholar]
- 9.Grosberg A, Alford PW, McCain ML, Parker KK. Lab on a Chip. 2011;11:4165–4173. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCain ML, Agarwal A, Nesmith HW, Nesmith AP, Parker KK. Biomaterials. 2014;35:5462–5471. doi: 10.1016/j.biomaterials.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L. Nature medicine. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Lab on a Chip. 2013;13:3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Mariscal L, Nava P, Hernandez S. The Journal of membrane biology. 2005;207:55–68. doi: 10.1007/s00232-005-0807-y. [DOI] [PubMed] [Google Scholar]
- 14.Henry CB, Duling BR. American Journal of Physiology-Heart and Circulatory Physiology. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 15.Tirziu D, Giordano FJ, Simons M. Circulation. 2010;122:928–937. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royall JA, Berkow RL, Beckman JS, Cunningham MK, Matalon S, Freeman BA. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1989;257:L399–L410. doi: 10.1152/ajplung.1989.257.6.L399. [DOI] [PubMed] [Google Scholar]
- 17.Parker KK, Lind JU, Lewis JA, Vlassak JJ, Yuan H, Busbee TA, Perkins I, Chantre C. US Pat. App, 20170016875A1, WO 2015178980A3. 2017. [Google Scholar]
- 18.Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park S-J, Kotikian A, Nesmith AP, Campbell PH. Nature materials. 2017;16:303–308. doi: 10.1038/nmat4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasqualini FS, Sheehy SP, Agarwal A, Aratyn-Schaus Y, Parker KK. Stem cell reports. 2015;4:340–347. doi: 10.1016/j.stemcr.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacour SP, Chan D, Wagner S, Li T, Suo Z. Applied Physics Letters. 2006;88:204103. [Google Scholar]
- 21.Graudejus O, Görrn P, Wagner S. ACS applied materials & interfaces. 2010;2:1927–1933. doi: 10.1021/am1002537. [DOI] [PubMed] [Google Scholar]
- 22.Graz IM, Cotton DP, Lacour SP. Applied Physics Letters. 2009;94:071902. [Google Scholar]
- 23.Endoh M. European journal of pharmacology. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Toepke MW, Beebe DJ. Lab Chip. 2006;6:1484–1486. doi: 10.1039/b612140c. [DOI] [PubMed] [Google Scholar]
- 25.Schwinger RH, Böhm M, Erdmann E. Journal of cardiovascular pharmacology. 1990;15:892–899. doi: 10.1097/00005344-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. New England journal of medicine. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 27.Hohnloser SH. The American journal of cardiology. 1997;80:82G–89G. doi: 10.1016/s0002-9149(97)00717-0. [DOI] [PubMed] [Google Scholar]
- 28.Valentin J-P, Hammond T. Journal of pharmacological and toxicological methods. 2008;58:77–87. doi: 10.1016/j.vascn.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Eckardt L, Haverkamp W, Mertens H, Johna R, Clague JR, Borggrefe M, Breithardt G. Journal of cardiovascular pharmacology. 1998;32:425–434. doi: 10.1097/00005344-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Matsa E, Rajamohan D, Dick E, Young L, Mellor I, Staniforth A, Denning C. European heart journal. 2011;32:952–962. doi: 10.1093/eurheartj/ehr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borchard U, Boisten M. Journal of cardiovascular pharmacology. 1982;4:205–212. doi: 10.1097/00005344-198203000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Sirenko O, Cromwell EF, Crittenden C, Wignall JA, Wright FA, Rusyn I. Toxicology and applied pharmacology. 2013;273:500–507. doi: 10.1016/j.taap.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, Shadrin IY, Lam J, Xian H-Q, Snodgrass HR, Bursac N. Biomaterials. 2013;34:5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacher P, Kecskemeti V. Current pharmaceutical design. 2004;10:2463–2475. doi: 10.2174/1381612043383872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou ZF, Vorperian VR, Gong QM, Zhang ST, January CT. J. Cardiovasc. Electrophysiol. 1999;10:836–843. doi: 10.1111/j.1540-8167.1999.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 36.Johannes CB, Varas-Lorenzo C, McQuay LJ, Midkiff KD, Fife D. Pharmacoepidemiology and Drug Safety. 2010;19:881–888. doi: 10.1002/pds.2016. [DOI] [PubMed] [Google Scholar]
- 37.Atkison P, Joubert G, Barron A, Grant D, Paradis K, Seidman E, Wall W, Rosenberg H, Howard J, Williams S, Stiller C. Lancet. 1995;345:894–896. doi: 10.1016/s0140-6736(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 38.Miller LW. Am. J. Transplant. 2002;2:807–818. doi: 10.1034/j.1600-6143.2002.20902.x. [DOI] [PubMed] [Google Scholar]
- 39.Fonteyne W, Bauwens A, Jordaens L. Clin. Cardiol. 1996;19:967–968. doi: 10.1002/clc.4960191213. [DOI] [PubMed] [Google Scholar]
- 40.Coker SJ, Batey AJ, Lightbown ID, Diaz ME, Eisner DA. British Journal of Pharmacology. 2000;129:323–330. doi: 10.1038/sj.bjp.0703060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brutsaert DL. Physiological reviews. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 42.Godier-Furnémont AF, Tiburcy M, Wagner E, Dewenter M, Lämmle S, El-Armouche A, Lehnart SE, Vunjak-Novakovic G, Zimmermann W-H. Biomaterials. 2015;60:82–91. doi: 10.1016/j.biomaterials.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Biotechnology and bioengineering. 2000;68:106–114. [PubMed] [Google Scholar]
- 44.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. Tissue Engineering Part A. 2011;18:910–919. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thavandiran N, Dubois N, Mikryukov A, Massé S, Beca B, Simmons CA, Deshpande VS, McGarry JP, Chen CS, Nanthakumar K. Proceedings of the National Academy of Sciences. 2013;110:E4698–E4707. doi: 10.1073/pnas.1311120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L. Nature materials. 2016;15:669–678. doi: 10.1038/nmat4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aratyn-Schaus Y, Pasqualini FS, Yuan H, McCain ML, George J, Sheehy SP, Campbell PH, Parker KK. Journal of Cell Biology. 2016 doi: 10.1083/jcb.201508026. jcb. 201508026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huebsch N, Loskill P, Deveshwar N, Spencer CI, Judge LM, Mandegar MA, Fox CB, Mohamed TM, Ma Z, Mathur A. Scientific reports. 2016;6:24726. doi: 10.1038/srep24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP. Scientific reports. 2015;5:8883. doi: 10.1038/srep08883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odijk M, van der Meer A, Levner D, Kim H, van der Helm M, Segerink L, Frimat J, Hamilton G, Ingber D, van den Berg A. Lab on a Chip. 2015;15:745–752. doi: 10.1039/c4lc01219d. [DOI] [PubMed] [Google Scholar]
- 51.Boland CS, Khan U, Ryan G, Barwich S, Charifou R, Harvey A, Backes C, Li Z, Ferreira MS, Möbius ME. Science. 2016;354:1257–1260. doi: 10.1126/science.aag2879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.