Abstract
Background: Canola is an agro-economically oilseed crop. Yield loss due to fungal disease of stem rot caused by Sclerotinia sclerotiorum is a serious problem in canola cultivation. Thaumatin-like proteins are large groups of the pathogenesis-related proteins which provide resistance to the fungal infection in response to invading pathogens and play a key role in plant defense system.
Objectives: Transformation of the rice tlp into canola via Agrobacterium-mediated transformation and evaluation of the antifungal activity of the expressed TLP in the transgenic events on the S. sclerotiorum growth was subject to investigation.
Materials and methods: The canola (R line Hyola308) was used for transformation experiment. The vector, pBITLPRA1, was used for the stable transformation. The PCR and southern blotting techniques were used to confirm transgene’s presence in the transgenic canola events. Antifungal activity of transgenic plants was evaluated by the radial diffusion and spore germination assays. T2 transgenic plants were evaluated by the intact leaf inoculation method in greenhouse assay.
Results: In this study, pBITLPRA1 construct containing tlp gene was introduced into canola and the transformed plants were verified by PCR. The glucanase activity of tlp gene in T0 generation was measured and transgenic plants with high activity were assessed by Southern blot analysis to confirm the copy number of the gene. Also, antifungal activity of the single copy T0 transgenic plants against Sclerotinia sclerotiorum was evaluated by radial diffusion and spore germination assays. In greenhouse assay, evaluation of T2 transgenic plants by the intact leaf inoculation method demonstrated that following the infection with S. sclerotiorum, there was a significant reduction in the lesion’s diameter in transgenic lines compared to the non-transgenic ones.
Conclusions: These results revealed that expression of TLP has an inhibitory effect against fungus compared to non-transgenic plants both in vitro and in vivo (i.e., greenhouse condition). These transgenic lines could be used as the additional sources of disease resistance for canola breeding program.
Keywords: Brassica napus, Oryza sativa, Plant transformation, Sclerotinia sclerotiorum, Thaumatin-like protein
1. Background
Canola (Brassica napus L.) is the most important source of oil seed in the world. The plant suffers from several diseases which result in the reduction of the yield and quality of production (1). Sclerotinia stem rot caused by Sclerotinia sclerotiorum is the second most damaging disease of the oilseed rape yield worldwide, that infects agro-economically crops such as sunflower (Helianthus annuus), soybean (Glycine max) and oilseed (Brassica napus) (2-3).
The use of chemical fungicides against plant pathogens is one of the primary strategies in the disease management programs. Generally, the method is expensive and labor intensive due to the environmental risks such as ecosystem pollution and production of biological toxin residues for human and livestock health (4-5).
Genetic manipulation of the plants conferring fungal resistance is one of the efficient methods in the prevention and management of the fungal diseases. Pathogenesis-related proteins (PR-proteins) which are well-known plant proteins provide resistance to fungal infection in the plants (6). The PR-proteins are able to inhibit the growth of pathogens and improve to plant’s pathogenic fungal resistance (7-8).
Thaumatin-like proteins (TLPs) share sequence similarity with thaumatin which is a sweet-tasting protein from Thaumatococcus daniellii (9-10). TLPs have the inhibitory effect on the phytopathogenic fungi through inhibition of spore germination and mycelium growth (11-13). It is believed that TLPs can change the permeability of the cell membrane to target fungi, glucan binding and glucanase activities as well as other biological functions (14-16). Regarding multifunctional activity against a broad spectrum of the phytopathogens, TLPs can be considered as the ideal candidates in plant transformation in order to produce resistant crops.
2. Objectives
In the present study, we transformed a synthetic construct harboring tlp gene from Oryza sativa in canola via Agrobacterium–mediated method. The Transgenic plants were evaluated in order to assess the enhanced plant resistance to the plant pathogenic fungus S. sclerotiorum under in vitro and green house conditions.
Materials and Methods
3.1. Plant Material, Fungi and Plasmids
The Hyola308 R line of rapeseed (B. napus L.) was used as a transgene recipient was kindly provided by the Oilseed and Development Company, Tehran, Iran. The strain of the Sclerotinia sclerotiorum was kindly provided by Dr. H. Afshari-Azad (Plant Pests and Diseases Research Institute, Agricultural Research and Education Organization, Tehran, Iran).
3.2. General Procedures
To produce the construct pBITLPRA1 containing the tlp gene, the glucuronidase reporter gene (GUS) was removed by XbaI/ SacI restriction enzymes; the same restriction enzymes were used so that the tlp gene from the pJNG1 can be cloned into pBI121 plant expression vector. The pBITLPRA1 construct was confirmed by PCR assays using different combinations of the gene and vector specific primers and was also transferred into A. tumefaciens by freeze-thaw method (17). The A. tumefaciens harboring the pBITLPRA1 vector was used in the experiment.
3.3. Plant Transformation and Selection of Transformants
The process of plant transformation and selection of transformants was achieved according to Aghazadeh et al. (18).
3.4. Molecular Analyses of the Putative Transformants
Genomic DNA was extracted from the transgenic and non-transgenic plants as reported by Rogers et al. (19). Primary molecular confirmation of the putative transgenic plants (kanamycin-resistant plants) was done using PCR technique by OTLPF/OTLPR primers (Table 1). Also, southern blot analysis was carried out using a probe amplified by CaMV35SF/ CaMV35SR primers, producing a 631 bp fragment.
Table 1. Primers used in this study.
| Name of the primers | Primer sequences (5´-3´) |
| NOSR | GTGAAGCTTCCCGATCTAGTAACAT |
| OTLPF | GCTCTAGAATGACCAAAAAGACCAAAGCC |
| OTLPR | GGAGCTCTCATGGGCAGAAGA CGACC |
| Fe35S | CGGAATTCGCATGCCTGCAGGTCCCCAG |
| Renos | CCAGTGAATTCCCGATCTAGTAAC |
| CaMV35SF | GGACTAACTGCATCAAGAACACAG |
| CaMV35SR | GACGCACAATCCCACTATCCTTC |
| VirGF | ATGATTGTACATCCTTCACG |
| VirGR | TGCTGTTTTTATCAGTTGAG |
3.5. Glucanase Activity
β-1,3-glucanase activity was studied using laminarin as the substrate as per the protocol described by Kumar et al. (20).
3.6. Fungal Growth Inhibition Assay
Radial diffusion assay was used in order to test the antifungal activity of the transgenes (21) Also, as a quantitative method, spore germination assay was used to assess fungal growth inhibition (22).
3.7. Sytox Green Uptake
Sytox green staining experiment was achieved as described by Thevissen et al. (1999).
3.8. Generation of Homozygous Transgenic Plants
Generation of homozygous transgenic plants was done according to Moradyar et al. (22).
3.9. Greenhouse Antifungal Assay
Greenhouse antifungal assay was used in order to test the antifungal activity of the transgenes in T2 generation of the plants (22).
3.10. Statistical Analysis
Statistical comparisons were done using a completely randomized design with 3 replicates (SPSS, Chicago, IL). The Duncan’s multiple range tests were used for comparison of the means.
4. Results
4.1. Plasmid Construction and Plant Transformation
The pBITLPRA1construct was confirmed by using different combinations of the gene and vector specific primers in the PCR assays (Fig. 1A). Further analysis by DNA sequencing confirmed that open reading frame of the rice tlp gene in pBI121 locates between the CaMV35S promoter and nopaline synthase terminator (data not shown). The recombinant plasmid pBITLPRA1 was transferred into the Agrobacterium tumefaciens and then introduced into the R line Hyolla 308 of B. napus. PCR assay using OTLPF/NOSR primers verified the putative transgenic plants aiming to confirm the presence of tlp gene (Fig. 1B and Table 1). Consequently, 15 lines were identified to be positive for tlp gene. All transgenic plants exhibited a normal phenotype in terms of morphology and growth characteristics when compared to the non-transformed plants. Also, virG specific primers (Table 1) were used in the PCR for detecting Agrobacterium contamination in putative transgenic plants due to probable escape from kanamycin selection. The PCR assay detected an expected 1000 bp fragment in Agrobacterium DNA template, while with DNA from the transformed plants such fragment was not produced (data not shown).
Figure 1.
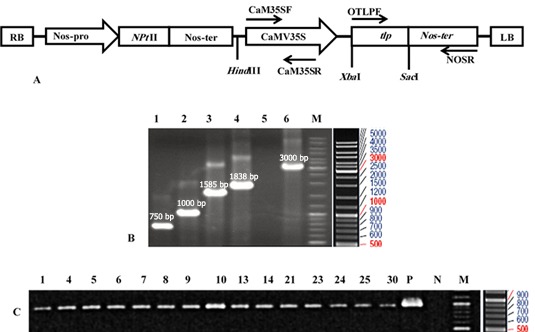
The schematic representation of the T-DNA region of pBITLPRA1 construct (A), PCR of the tlp gene in pBITLPRA1 vector, and the expected band size (B) as well as putative transgenic canola plants (C). A: pBITLPRA1 construct with the expected restriction pattern; RB: right border, Nos-ter: nopaline synthase terminator, NptII: neomycin phosphotransferase II, Nos-P: nopaline synthase promoter, and tlp: Rice tlp, LB: Left border. B: Lane1: OTLPF/OTLPR primers, Lane2: OTLPF/Renos primers, Lane3: Fe35s/OTLPR primers, Lane4: Fe35S/Renos primers, Lane5: negative control and Lane6: positive control (pBIGUS+), M: DNA ladder mix, C: PCR analysis of tlp gene in the putative transgenic canola plants (1–15transformants) by using OTLPF/Renos primers, band size 1000 bp, P: Positive control (Plasmid pBIRATLPRA1), N: Negative control (wild-type), and M: DNA ladder mix
4.2. β-1,3-Glucanase Activity Assay
To check whether rice TLP protein exhibits glucanase activity, total protein extracted from the leaves was incubated with laminarin. Detectable absorbance at 595nm showed that TLP protein bears glucanse activity, and the range of activity was detected from 1.72 ± 0.18 to 7.91 ± 0.24 U.µg-1. Among the 15 lines, five transgenic lines from the T0 generation (plants 9, 21, 24, 25 and 30) showed the highest glucanase activity when compared to non-transgenic plant; hence they were selected for further analysis (Fig. 2).
Figure 2.
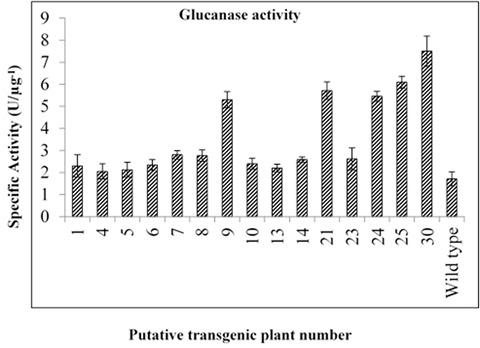
Comparison of glucanase activity in all transformants harboring tlp gene; each value represents the mean (± standard error) of the three independent experimental replications. The production of 1 µmol of the colorful product per hour was considered as one unit of activity (U) p< 0.01.
4.3.Southern Blot Analysis
DNA of the 5 putative transgenic lines showing high glucanase activity was used for Southern blot analysis. The results indicated that three PCR-positive transgenic lines (21, 25 and 30) had a single integrated tlp gene, and lines 9 and 24 had two copies in their genome (Fig. 3). The non-transformed canola did not show the corresponding band. The single copy lines were selected for further analysis.
Figure 3.
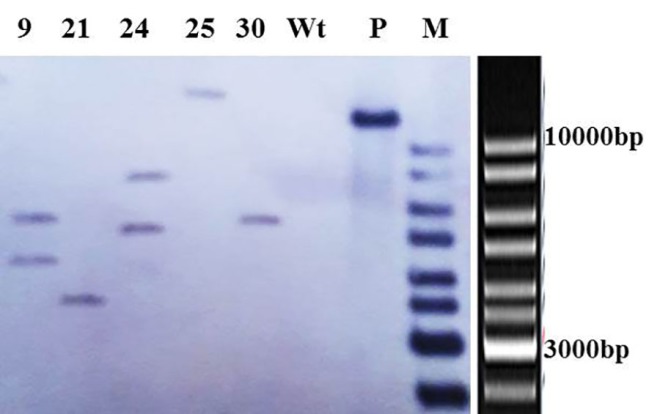
Southern blot analysis of the HindIII-digested genomic DNA from the representative pBITLPRA1-transformed plants (i.e., PCR-positive plants having the highest enzyme activity) in the T0 generation; the number of bands reflects the number of transgene’s insertions, Wt: non-transgenic plant, P: Positive control (linear pBITLPRA1 by HindIII) and MW: DNA ladder mix.
4.4. In vitro Antifungal Assays
Antifungal activity of the rice TLP protein was assessed using spore germination and radial diffusion assays. The single copy T0 transgenic plants having a high level of glucanase activity were selected for bioassays. The obtained results from spore germination and radial diffusion assays were showed that the crude extracted proteins from the single copy transgenic lines (21, 25 and 30) inhibited the fungal hyphal growth compared to the controls (Fig. 4A). In comparison to the wild type plants fungal growth inhibition for S. sclerotiorum was estimated from 49.98-51.9%, respectively (Fig. 4B).
Figure 4.
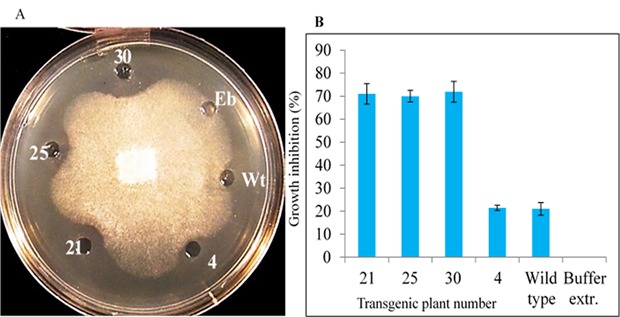
Radial diffusion and spore germination assays of the crude protein extracts of the transgenic canola against S. sclerotiorum. A and B: the number reflects the number of transgenic extracted protein (21, 25, 30) with the high glucanase activity and have only one copy of the gene, Wt: non-transgenic crude protein extract as a negative control, 4: event 4 crude protein extract as a control (lower glucanase activity), Eb: protein extraction buffer as a control; each value represents the mean (± standard error) of the three independent experiments.
4.5. Sytox Green Uptake
Analysis by fluorescence microscopy showed that the rice TLP protein is able to permeate into S. sclerotiorum hyphal membrane. The presence of the fluorescent dye is an indicator of the damaged cell membrane which increases the membrane permeability to sytox green. Fluorescence was observed in the treated hyphae of S. sclerotiorum, which demonstrates the antifungal activity thorough rice TLP protein by pore formation in the cell membrane (Fig. 5).
Figure 5.
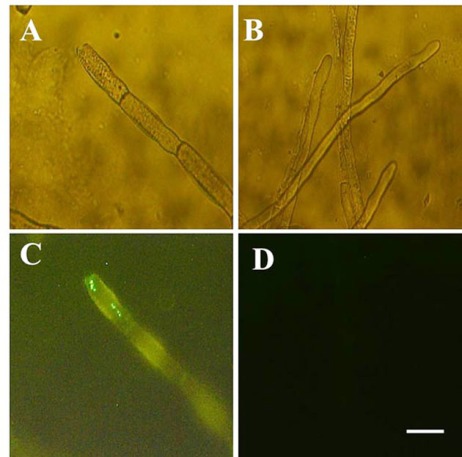
Fluorescence microscopy observation of the fungal mycelium in the presence of Sytox green; S. sclerotiorum mycelium was grown on PDB supplemented with 0.2 mM Sytox Green and 5 mM MgCl2 treated for 180 min in the crude protein from transgenic line 30 (A) or crude protein from the wild type (B). A, B are the fluorescence microscopic image, C and D are light microscopic images. Bar, 10 mm.
4.6. Generation of Homozygous Transgenic Plants
To produce homozygous line, five T1 plants were grown from each of the single-copy transgenic events (21, 25 and 30). The T1 plants were self-fertilized, then, T2 seeds were harvested from individual plants, under greenhouse conditions. To select homozygous events, segregation analysis was performed by kanamycin resistance on the MS medium. The obtained results indicated that T2 progenies of the hemizygous T1 plants segregated at a 3:1 ratio (kanamycin resistance: sensitive), that seeds of homozygous T1 plants were fully germinated on selective medium and were completely green. As a result, T2 progenies of event 21 (60 seeds) showed 3:1 segregation for resistance to kanamycin that was hemizygous, but seeds of events 25 and 30 were fully germinated on a selective medium which was homozygous. The homozygous events (25 and 30) were selected for greenhouse antifungal assay.
4.7. Greenhouse Antifungal Assay
The results of intact plant leaves’ antifungal assay have estimated the lesion diameter for transgenic plants 30 and 25 from 15.24 ± 0.22 to 16.39 ± 0.17 mm, respectively, while the lesion diameter for non-transgenic plants was estimated 32 ± 0.19 mm (Fig. 6). These results have indicated the significant differences in the severity of the disease between transgenic lines and non-transgenic plant.
Figure 6.

Greenhouse evaluation of the homozygous T2 transgenic lines with S. sclerotiorum in order to assess the level of resistance. Wild type (as a control) and T2 transgenic plants (25 and 30) were inoculated with S. sclerotiorum and pictures were taken 72 hour post-inoculation (hpi).
5. Discussion
Canola is an agro-economically oilseed crop in the world. However, phytopathogenic fungi can cause damage in several organs, which reduce the growth and seed yield of canola. Genetic manipulation of the plants is one of the most efficient methods for management of the fungal diseases. TLPs as a class of PR-5 family, have been isolated and characterized from different plants such as corn (16), rye(23), Camellia sinensis (11) and have shown to enhance the resistance of the plans to the fungal infection or stress conditions (6-7, 16).
In the current study, radial diffusion and spore germination assays by the crude protein extracted from transgenic plants have shown to exhibit growth inhibition of the S. sclerotiorum fungus. All the three transgenic lines (21, 25, and 30) were able to inhibit the fungal growth of the S. sclerotiorum. In agreement with our finding, Zamani et al. (24) have reported that transgenic canola harboring rye tlp gene could inhibit the progress of the S. sclerotiorum fungal growth (24). The same findings have also reported that overexpression of tlp gene demonstrates an inhibitory effect on phytopathogenic fungi through inhibition of the spore germination and mycelial growth (11, 25, 26).
PR-5 family members have various biological functions such as changing the permeability of the target fungal cell membrane by mean of transmembrane pore formation (14) and glucanase activity (16, 20, 27). In the present study, rice tlp gene has shown to have glucanase activity. The same finding has also been demonstrated in different reports (16, 20, 28).
The results of sytox green uptake have also revealed that the rice TLP protein is able to change the permeability of the S. sclerotiorum hyphal membrane. Fluorescence was observed in the treated hyphae of S. sclerotiorum, which demonstrates the antifungal activity of the rice TLP protein through altering plasma membrane permeability. Similar results were obtained from membrane permeability of Alternaria sp. spores by PI staining in which SindOLP-treated spores have indicated a distinct fluorescence compared to the control sample (14).
Greenhouse antifungal assay on the leaves of the intact plants (i.e., T2 generation) showed a 50 percent reduction in the lesion’s diameter in transgenic lines towards S. sclerotiorum. Velazhahan and Muthukrishnan (2003) have also reported that T2 homozygous transgenic tobacco plants harboring tlp gene showed limited lesions by the phythopathogenic fungus (29).
In the present study, we have shown that expressing a gene encoding TLP in the canola can efficiently enhance the resistance to S. sclerotiorum, along with as inhibiting the symptoms of the disease. These transgenic lines could be used as the additional sources of disease resistance for the canola breeding program.
Acknowledgments
The present work has been supported by the National Institute of Genetic Engineering and Biotechnology (Project No. 407-M).
References
- 1.Dong X, Ji R, Guo X, Foster SJ, Chen H, Dong C. et al. Expressing a gene encoding wheat oxalate oxidase enhances resistance to Sclerotinia sclerotiorum in oilseed rape (Brassica napus) Planta. 2008;228:331–40. doi: 10.1007/s00425-008-0740-2. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava M, Gupta SK, Saxena AP, Shittu LAJ, Gupta SK. A review of occurrence of fungal pathogens on significant brassicaceous vegetable crops and their control measures. Asian J Agric Sci. 2011;3(2):70–79. [Google Scholar]
- 3.Zhao J, Peltier A, Meng J, Osborn T, Grau C. Evaluation of Sclerotinia stem rot resistance in oilseed Brassica napus using a petiole inoculation technique under greenhouse conditions. Plant Disease. 2004;88(9):1033–9. doi: 10.1094/PDIS.2004.88.9.1033. [DOI] [PubMed] [Google Scholar]
- 4.Yadav SK. Pesticide Applications-Treat to Ecosystems. J Hum Ecol. 2010;32(1):37–45. [Google Scholar]
- 5.Abhilash PC, Singh N. Pesticide use and application: An Indian scenario. J Hazard Mater. 2009;165:1–12. doi: 10.1016/j.jhazmat.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 6.Chhikara S, Chaudhury D, Dhankher OP, Jaiwal PK. Combined expression of a barley class II chitinase and type I ribosome inactivating protein in transgenic Brassica juncea provides protection against Alternaria brassicae. Plant Cell, Tissue and Organ Cult. 2012;108(1):83–9. doi: 10.1007/s11240-011-0015-7. [DOI] [Google Scholar]
- 7.Chen W, Punja Z. Transgenic herbicide-and disease-tolerant carrot (Daucus carota L) plants obtained through Agrobacterium-mediated transformation. Plant Cell Rep. 2002;20(10):929–35. doi: 10.1007/s00299-001-0419-7. [DOI] [Google Scholar]
- 8.Esfahani K, Motallebi M, Zamani MR, Hashemi Sohi H, Jourabchi E. Transformation of Potato (Solanum tuberosum cv Savalan) by Chitinase and β-1, 3-Glucanase Genes of Myco-Parasitic Fungi Towards Improving Resistance to Rhizoctonia solani AG-3. Iran J Biotech. 2010;8(2):73–81. [Google Scholar]
- 9.Velazhahan R, Datta SK, Muthukrishnan S. The PR-5 family: thaumatin-like proteins Pathogenesis-related proteins in plants. Kluwer Academic CRC Presss, Dordrecht. 1999:303–345. [Google Scholar]
- 10.Wel H, Loeve K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. Eur J Biochem. 1972;31(2):221–5. doi: 10.1111/j.1432-1033.1972.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 11.Acharya K, Pal AK, Gulati A, Kumar S, Singh AK, Ahuja PS. Overexpression of Camellia sinensis thaumatin-like protein, CsTLP in potato confers enhanced resistance to Macrophomina phaseolina and Phytophthora infestans infection. Mol Biotechnol. 2013;54(2):609–22. doi: 10.1007/s12033-012-9603-y. [DOI] [PubMed] [Google Scholar]
- 12.Anžlovar S, Dermastia M. The comparative analysis of osmotins and osmotin-like PR-5 proteins. Plant Biol. 2003;5(2):116–24. doi: 10.1055/s-2003-40723. [DOI] [Google Scholar]
- 13.Van Loon L, Van Strien E. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol. 1999;55(2):85–97. doi: 10.1006/pmpp.1999.0213. [DOI] [Google Scholar]
- 14.Chowdhury S, Basu A, Kundu S. Cloning, Characterization, and Bacterial Over-Expression of an Osmotin-like Protein Gene from Solanum nigrum L with Antifungal Activity Against Three Necrotrophic Fungi. Mol Biotechnol. 2015;57(4):371–81. doi: 10.1007/s12033-014-9831-4. [DOI] [PubMed] [Google Scholar]
- 15.Liu J-J, Sturrock R, Ekramoddoullah AK. The superfamily of thaumatin-like proteins: its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010;29(5):419–36. doi: 10.1007/s00299-010-0826-8. [DOI] [PubMed] [Google Scholar]
- 16.Liu J-J, Zamani A, Ekramoddoullah AK. Expression profiling of a complex thaumatin-like protein family in western white pine. Planta. 2010;231(3):637–51. doi: 10.1007/s00425-009-1068-2. [DOI] [PubMed] [Google Scholar]
- 17. Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual . 2001. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York; 2001.
- 18.Aghazadeh R, Zamani MR, Motallebi M, Moradyar M, Moghadassi Jahromi Z. Co-transformation of canola by chimeric chitinase and tlp genes towards improving resistace to Sclerotinia sclerotiorum. World J Microbiol Biotechnol. 2016;32:144–155. doi: 10.1007/s11274-016-2104-6. [DOI] [PubMed] [Google Scholar]
- 19.Rogers SO, Bendich AJ. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol. 1985;5(2):69–76. doi: 10.1007/BF00020088. [DOI] [PubMed] [Google Scholar]
- 20.Kumar HGA, Hegde VL, Shetty SM, Venkatesh YP. Characterization and gene cloning of an acidic thaumatin-like protein (TLP 1), an allergen from sapodilla fruit (Manilkara zapota) Allergol Int. 2013;62(4):447–62. doi: 10.2332/allergolint.12-OA-0522. [DOI] [PubMed] [Google Scholar]
- 21.Brogue K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S. Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science. 1991;254(5035):1194–7. doi: 10.1126/science.254.5035.1194. [DOI] [PubMed] [Google Scholar]
- 22.Moradyar M, Motallebi M, Zamani MR, Aghazadeh R. Pathogen-induced expression of chimeric chitinase gene containing synthetic promoter confers antifungal resistance in transgenic canola. In Vitro Cell Dev Biol Plant. 2016;52(2):119–129. doi: 10.1007/s11627-016-9751-z. [DOI] [Google Scholar]
- 23.Thevissen K, Terras FRG, Broekaert WF. Permeabilization of Fungal Membranes by Plant Defensins Inhibits Fungal Growth. Appl Environ Microbiol. 1999;65(12):5451–5458. doi: 10.1128/aem.65.12.5451-5458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamani A, Motallebi M, Jonoubi P, Ghafarian-Nia NS, Zamani MR. Heterologous expression of the Secale cereal thaumatin-like protein in transgenic canola plants enhances resistance to stem rot disease. Iran J Biotechnol. 2012;10(2):87–95. [Google Scholar]
- 25.Menu-Bouaouiche L, Vriet C, Peumans WJ, Barre A, Van Damme EJM, Rougé P. A molecular basis for the endo-b1,3-glucanase activity of the thaumatin-like proteins from edible fruits. Biochimie. 2003;85:123–3. doi: 10.1016/S0300-9084(03)00058-0. [DOI] [PubMed] [Google Scholar]
- 26.Singh NK, Kumar KRR, Kumar D, Shukla P, Kirti P. Characterization of a pathogen induced thaumatin-like protein gene AdTLP from Arachis diogoi, a wild peanut. PloS one. 2013;8(12):e83963. doi: 10.1371/journal.pone.0083963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grenier J, Potvin C, Asselin A. Some fungi express β-1, 3-glucanases similar to thaumatin-like proteins. Mycologia. 2000:841–8. doi: 10.2307/3761579. [DOI] [Google Scholar]
- 28.Sakamoto Y, Watanabe H, Nagai M, Nakade K, Takahashi M, Sato T. Lentinula edodes tlg1 encodes a thaumatin-like protein that is involved in lentinan degradation and fruiting body senescence. Plant Physiol. 2006;141(2):793–801. doi: 10.1104/pp.106.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velazhahan R, Muthukrishnan S. Transgenic tobacco plants constitutively overexpressing a rice thaumatin-like protein (PR-5) show enhanced resistance to Alternaria alternata. Biologia Plantarum. 2003;47(3):347–54. doi: 10.1023/B:BIOP.0000023876.55053.5e. [DOI] [Google Scholar]


