Abstract
Purpose/Objective
Recently, we developed RSI, a clinically-validated molecular signature that estimates tumor radiosensitivity. Here, we test whether integrating RSI with molecular subtype refines the classification of local recurrence risk in breast cancer
Methods and Materials
RSI and molecular subtype were evaluated in 343 patients treated with breast-conserving therapy including whole-breast RT plus/minus tumor bed boost (dose range, 45 – 72 Gy). The follow up for patients without recurrence was 10 years. Clinical endpoint was local recurrence-free survival (LRFS).
Results
While RSI did not uniformly predict for local recurrence across the entire cohort, combining RSI and molecular subtype identifies a subpopulation with an increased risk of local recurrence: triple negative (TN) and radioresistant (TN-R) (Ref –TN-R, HR=0.37 (0.15, 0.92) p=0.02). TN patients that were RSI-Sensitive/Intermediate (RSI-S/Int) had similar LR rates as LUM patients (HR=0.86 (0.47, 1.57) p=0.63). On multivariate analysis (MVA) combined RSI-Molecular Subtype (p=0.004), along with age (p=0.001) were the most significant predictors of LR. In contrast, integrating RSI into the LUM subtype did not identify additional risk groups. We hypothesized that RT dose escalation was impacting radioresistance in the LUM subtype and serving as a confounder. Indeed, increased RT dose decreased LR only in the LUM-R subset (HR = 0.23 (0.05, 0.98), p=0.03). On MVA, RT dose was an independent variable only in the LUMA/B-RR subset (HR=0.025 (0.001, 0.946), p=0.046), along with age (p=0.008), T stage (p=0.004) and chemotherapy (p=0.008).
Conclusions
Combined molecular subtype-RSI identifies a novel molecular sub-population (Triple Negative and Radioresistant) with an increased risk of local recurrence after BCT. We propose RSI-molecular subtype may be useful in guiding RT-based decisions in breast cancer.
Introduction
Therapeutic benefit from radiotherapy (RT) plays an important role in the overall outcome of breast cancer patients. Classic prospective studies have demonstrated that post-lumpectomy RT to 50 Gy reduces local recurrences by 75% when compared to patients treated with lumpectomy alone (1). Furthermore, the Oxford meta-analysis has shown that RT provides a modest decrease in overall mortality from breast cancer at 15 years that is roughly equivalent to the survival benefit of polychemotherapy (2) (3). Therefore, local therapy benefit translates to an overall survival benefit.
Clinical data supports the presence of a gradient of RT dose response/benefit in breast cancer. The EORTC 22881-10882 boost vs. no boost trial (50 Gy vs. 66 Gy) showed reduction of absolute local recurrence in patients treated with BCT (10.2% vs. 6.2%), consistent with the existence of a radioresistant sub-population that benefit from additional RT dose (4). However, although remarkable progress has been made in defining the molecular variables that define outcome in breast cancer, the biologic variables that define RT treatment benefit remain elusive.
To address this, we developed a gene expression molecular signature to estimate cellular radiosensitivity (RSI) that has been independently clinically validated in RT-treated patients in multiple disease sites (5, 6). In breast cancer, RSI identifies a radiosensitive population (RSI-S) that has a decreased risk of distant metastasis after treatment with RT.
Importantly, we showed in two independent datasets (n=503) that RSI is RT-specific since it was not prognostic in patients treated with surgery only (i.e. mastectomy) (7). Furthermore, we demonstrated in subset analyses that the impact of RSI was affected by ER receptor status. Although, RSI-S patients had an improved DMFS (Distant Metastasis Free Survival), this effect was more pronounced in ER+ patients. Thus, we proposed that RSI may serve as a predictive biomarker of RT benefit in breast cancer and that the therapeutic impact of RT at current doses was larger in ER+ tumors. This hypothesis is consistent with results of the Oxford analysis that demonstrated that RT provides a larger proportional reduction in recurrence risk in ER+ tumors when compared with ER- tumors (3).
Multiple studies have defined several clinico-pathological features as predictors of local recurrence risk in breast cancer including stage, age, surgical margins, grade and others (8, 9). More recently, local recurrence risk has been associated with breast cancer molecular subtypes. Both loco-regional and distant failures are increased in triple negative (TN) and Her-2-enriched (not treated with trastuzumab) patients. Loco-regional failures for TNs have been reported to range between 10 – 30% whereas local failures for luminal patients are generally reported below 5% (10-13). An important distinction between RSI and these classic prognostic factors is that RSI was developed to estimate differences in radiophenotype. Thus, it is expected to provide information regarding the potential benefit of RT, i.e. a predictive biomarker, if these differences play a role in determining clinical outcome. In contrast, prognostic biomarkers are independent of treatment effect and are directly related to clinical outcome (14).
In this study we test whether RSI impacts the ability to define local recurrence risk in patients treated with BCT. We hypothesized that combining RSI with classic prognostic factors would enhance the ability to define local recurrence risk. Since molecular subtype has been shown to predict for local recurrence risk, we tested whether combining both RSI with breast cancer molecular subtype would further refine the ability to define local recurrence risk in breast cancer.
Material and Methods
Patients
This dataset was developed under an IRB-approved protocol and has been previously described. It includes 343 patients with breast cancer treated at four Dutch (Netherlands Cancer Institute, Radboud University Medical Center, Erasmus Medical Center and Ziekenyhuis Amstelland) and one French center (Institut Curie, Paris, France)(Supplementary figure 1) (15). The dataset was specifically designed to develop a molecular signature of local recurrence after BCT. Thus, this dataset overrepresented local-failures from what would be expected in a random population (LR = 34.7%).. Inclusion criteria included patients with invasive primary breast carcinoma diagnosed before age 50, premenopausal without prior history of malignancy. Patients were treated between January 1984 - November 2002 with breast-conserving surgery including either axillary dissection or sentinel node procedure and post-operative radiation to the breast +/- regional lymphatics. Median RT dose to the whole breast was 50 Gy (range 45-55) and 248/342 patients received a tumor bed boost (median dose 15 Gy, range 6-26). Details on chemotherapy and hormone therapy have been described previously (15).
The clinical endpoint was the development of an ipsilateral breast recurrence within the first 10 years after completion of primary treatment that showed clinical, histologic and/or genomic features consistent with the original tumor. Patients without local recurrence required a minimum follow up of 10 years.
Tissue Processing and Microarray
Details of tissue processing, RNA isolation and Q/A procedures have been previously described (15). RNA was hybridized to Illumina Human Whole Genome V3.0 arrays. Normalized data (Variance Stabilization Transformation and Robust Spline Normalization in the Lumi Bioconductor package as described by the authors of the dataset) was downloaded from GEO and used for this study.
RSI determination
This was performed as previously described. Probesets utilized for each gene were mapped to the Illumina WGv6 platform (Supplemental Table 1) as done in previous studies (5, 6). The mean expression for probesets for each gene was utilized. Briefly, each of the ten genes in the assay was ranked according to gene expression (from the highest (10) to the lowest expressed gene (1)). RSI was determined using the previously published ranked-based linear algorithm:
RSI=-0.0098009*AR + 0.0128283*cJun + 0.0254552*STAT1 - 0.0017589*PKC - 0.0038171*RelA + 0.1070213*cABL – 0.0002509*SUMO1 – 0.0092431*PAK2 - 0.0204469*HDAC1 – 0.0441683*IRF1
Genes are ranked on each patient individually and RSI is determined without reference to the overall cohort.
Definition of RSI Subtype
RSI was originally trained in 48 cancer cell lines as a continuous variable based on cellular radiosensitivity as determined by survival fraction at 2 Gy (SF2) (6). Thus RSI is inversely proportional to radiosensitivity (high RSI = radioresistance). We pre-defined the RSI-Radioresistant (RSI-R) subtype as the top 25th percentile of RSI scores. The RSI-radiosensitive (RSI-S) subtype was the lower 25th percentile as in previous studies (5, 7). Patients with RSI scores between the 25th – 75th percentile were deemed RSI-intermediate, which is first defined for this dataset (RSI-I).
Molecular Subtypes
Tumors were grouped into molecular subtypes according to their gene expression profile, i.e. luminal A (LUMA, ER+ and/or PR+, HER2-, AURKA-), luminal B (LUMB)(ER+ and/or PR+, HER2-, AURKA+), luminalHER (LUMHER)(ER+ and/or PR+, HER2+), Triple-negative (TN)(ER-, PR-, HER2-) and HER2+ (Her-2-enriched)(ER-, PR-, HER2+) based on the expression level of genes ESR1, PR, ERBB2 and AURKA. For this analysis we included the smaller subset of LUM-HER within the LUMB as previously suggested (16). The expression thresholds were optimized from tumors with known IHC data. The microarray-derived ER, PR, and HER2 statuses were determined according to the optimal sensitivity-specificity cutoff and then applied to all tumors without IHC data.
Statistics
Sociodemographic and clinico-pathological characteristics by RSI subtype (RSI-S, RSI-I, and RSI-R), and by dose level (<=66Gy, >66Gy), were compared using the exact Pearson chi-square test for categorical variables, and exact Wilcoxon rank sum test for continuous variables, both using the Monte Carlo estimation. LR was estimated using the Kaplan–Meier method and the log-rank test was used to identify differences by RSI group and dose level. The association between RSI group, dose level, and LR was assessed with multivariable Cox proportional hazards regression, adjusting for potential confounders. All analyses were conducted with SAS (version 9.3) and tests were two-sided with a significance level of 0.05.
Results
Distribution of Clinical Variables and RSI subtype
Supplementary Table 2 shows the distribution of clinico-pathological variables across each RSI subtype. There were no differences in distribution for the majority of clinical variables analyzed including T stage, nodal status, margins, grade, presence of DCIS, chemotherapy, and hormonal therapy. Interestingly, the RSI-S subtype was more prevalent in tumor with high mitotic index (p=0.003) and high proliferation (p=0.035). Similarly, the RSI-R subtype was more prevalent in less proliferative tumors. There were also differences in the distribution of RSI subtype and the biological subtypes. The RSI-S subtype was more frequent in TN patients (p=0.0004).
In the original report of this dataset, patients receiving RT doses above 66 Gy had a trend toward lower local recurrence risk (15). Thus, we compared the distribution of clinico-pathological variables between patients that received high and standard radiation dose (>66 Gy vs. ≤ 66 Gy) (supplementary table 3). As expected patients on the high dose group had a higher prevalence of positive surgical margins (p<0.0001), lymph node positive disease (p=0.032) and chemotherapy use (p=0.014). In addition, patients on the high dose group had a higher proportion of ER+ disease (p=0.028). There were no other differences identified between high and standard dose group in any of the variables studied including RSI subtype (supplementary table 3).
Integrating RSI and Molecular Subtypes in Breast Cancer
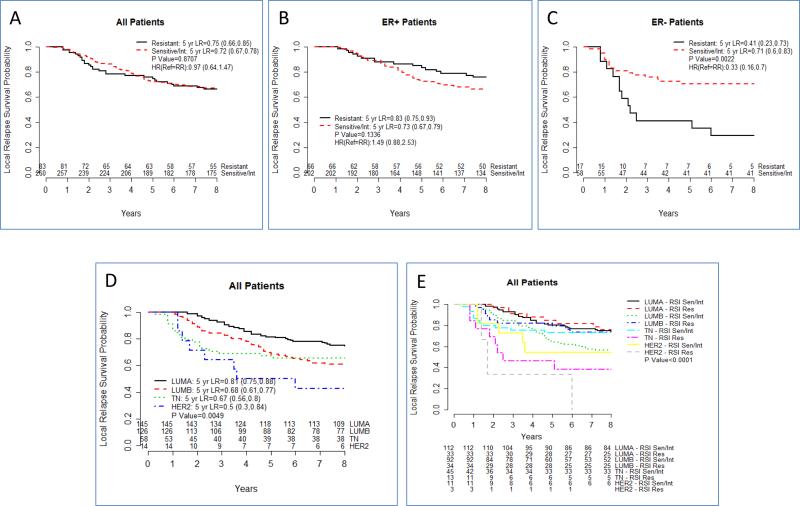
We initially tested RSI as an overall predictor of local recurrence. As shown in figure 1A, RSI did not identify differences in outcome between RSI-S/I and RSI-R patients. However and similar to prior studies, when we evaluate RSI by ER-receptor status, significant local recurrence risk differences are observed (Figure 1B and C). In ER-negative patients, RSI-R patients have a significantly higher risk of failure when compared with RSI-S or Int patients (Ref: RSI-R, HR=0.33, (0.16, 0.7), p=0.002). On MVA, RSI is a significant predictor of local recurrence risk in ER-negative patients (table 1, p=0.0048). In contrast no differences were identified by RSI in ER-positive patients.
Figure 1.
Integrating RSI subtype with breast molecular phenotypes refines the genomic classification of breast cancer. (A) Local recurrece risk for RSI S/Int vs RSI-R in all (B) ER-positive and (C) ER-negative patients. (D) Local recurrence risk as a function of breast mlecular subtype. (E) Local recurrence risk combining breast molecular subtype and RSI
Table 1.
Multivariable Analysis for ER Negative Patients
| Variable | Level Compared to Ref | Hazard Ratio | P Value |
|---|---|---|---|
| RSI | Sensitive/Int | 0.284 (0.119, 0.681) | 0.0048 |
| age | 0.955 (0.894, 1.02) | 0.1712 | |
| rttotaldose | >66Gy | 1.381 (0.378, 5.048) | 0.6253 |
| pT | pT2-3 | 1.498 (0.59, 3.8) | 0.3951 |
| surgicalmargin | marg+ | 0.505 (0.027, 9.358) | 0.6462 |
| pN | pN+ | 0.743 (0.189, 2.915) | 0.6701 |
| ct | ChT | 1.96 (0.55, 6.983) | 0.299 |
| ht | HT | 2.689 (0.172, 42.114) | 0.481 |
| subtypebyge | TN | 0.447 (0.151, 1.324) | 0.1463 |
Next, we evaluated the impact of breast molecular subtype (Figure 1D). As shown, molecular subtype is an important predictor of outcome in these patients (p=0.0049). However, combining RSI and molecular subtype further refines the classification of local recurrence risk, specifically in the TN and Her-2 enriched subtypes (Figure 1E and Table 2). In the TN subtype, RSI-R patients had a larger risk than RSI-S/Int that was statistically significant (Ref-RSI-R, HR=0.37 (0.15, 0.92), p=0.02). Interestingly, TN-S /Int had similar local recurrence rates as luminal patients (HR=0.86 (0.0.47, 1.57) p=0.63). On MVA combined RSI-Molecular Subtype was the best predictor of local recurrence in the dataset along with age (HR=0.94, p=0.001) (table 3).
Table 2.
Univariate analysis of RSI in each molecular subtype
| Variable | Level Compared to Ref | Hazard Ratio | P Value |
|---|---|---|---|
| LUMA (n=145) | Sensitive/Intermediate | 1.13 (0.52, 2.47) | 0.7553 |
| LUMB (n=126) | Sensitive/Intermediate | 1.73 (0.87, 3.45) | 0.1127 |
| TN (n=58) | Sensitive/Intermediate | 0.37 (0.15, 0.92) | 0.0248 |
| Her-2-enriched (n=14) | Sensitive/Intermediate | 0.33 (0.08, 1.42) | 0.108 |
Table 3.
Multivariate Analysis combining RSI and Molecular Subtype in Breast Cancer
| Variable | Level Compared to Ref | Hazard Ratio | P Value |
|---|---|---|---|
| subtypebyge_RSI | HER2, Resistant | 3.048 (0.776, 11.971) | 0.1103 |
| Ref: TN, Resistant | HER2, Sensitive/Int | 0.608 (0.169, 2.179) | 0.4447 |
| LUMA, Resistant | 0.232 (0.079, 0.683) | 0.008 | |
| LUMA, Sensitive/Int | 0.308 (0.132, 0.717) | 0.0063 | |
| LUMB, Resistant | 0.334 (0.119, 0.936) | 0.0369 | |
| LUMB, Sensitive/Int | 0.494 (0.214, 1.139) | 0.0979 | |
| TN, Sensitive/Int | 0.239 (0.088, 0.648) | 0.0049 | |
| age | 0.941 (0.907, 0.977) | 0.0014 | |
| rttotaldose | >66Gy | 0.686 (0.409, 1.15) | 0.1531 |
| pT | pT2-3 | 1.083 (0.711, 1.651) | 0.7094 |
| surgicalmargin | marg+ | 1.517 (0.771, 2.988) | 0.2277 |
| pN | pN+ | 1.048 (0.522, 2.103) | 0.8956 |
| ct | ChT | 0.738 (0.376, 1.449) | 0.3773 |
| ht | HT | 2.064 (1.099, 3.878) | 0.0243 |
• Only 9% of all patients received HT
Intrinsic Radiosensitivity May Be Influenced by Biological Context
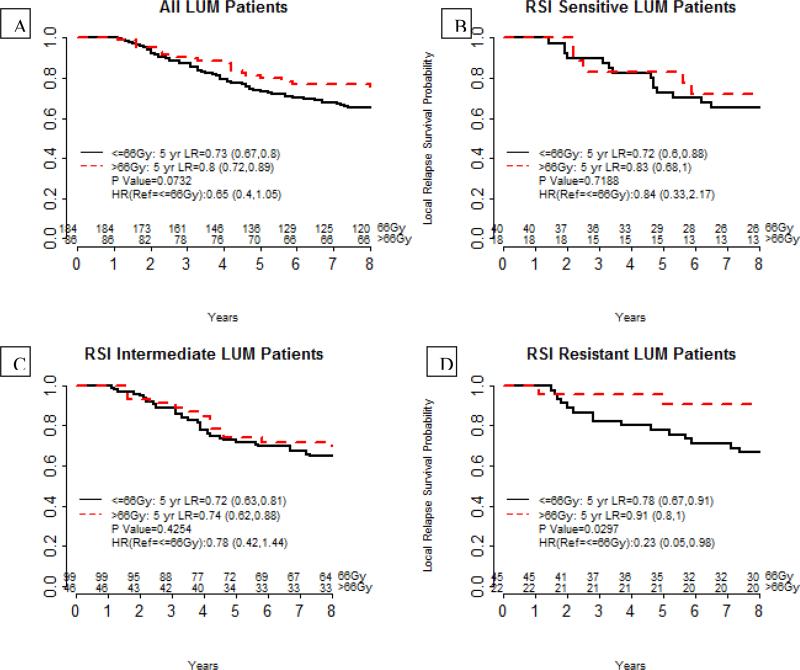
RSI did not identify any differences in local recurrence risk in ER-positive tumors. One possible explanation is that as suggested by the Oxford analysis, RT exerts a larger therapeutic impact in this population. Since our hypothesis is that RSI is a predictive biomarker, it is possible that RT dose could be serving as a confounder. Thus we analyzed the impact of RT dose in our dataset. In figure 2, we show the impact of RT dose dichotomized at 66 Gy. Indeed, increased RT dose (≤ 66 Gy vs. > 66Gy), resulted in an overall trend to lower LR only in ER-positive patients (LUM) (HR=0.65 (0.4, 1.05), p=0.07). In contrast, RT dose escalation did not impact local recurrence risk in ER-negative patients (HR=1.11 (0.45, 2.75), p=0.81, data not shown). However, when accounting for RSI, increased RT dose decreased LR only in the LUM RSI-R subset (HR = 0.23 (0.05, 0.98), p=0.03) but not in the RSI-S/Int patients (HR=0.78 (0.42, 1.44), p=0.42, HR=0.84 (0.33, 2.17), p=0.72).
Figure 2.
Integration of RSI and molecular subtypes identifies a small sub-population that benefits from RT- dose escalation. (A)Local recurrence rates for all luminal patients are shown comparing patients treated 66 Gy or less (black) or more than 66 Gy (red). (B-D) Recurrence rates in luminal patients by RSI subtype. RT dose has no overall impact on local recurrence of luminal patients that are RSI-S (A) or RSI-Int (B). However, higher RT dose reduces the risk of local recurrence for RSI-R patients (D)(p=0.02)
Finally, we analyzed all luminal patients and found that dose effect was most pronounced in the luminal A/B RSI-R subset (Ref= (≤ 66 Gy, HR=0.13 (0.02,1.01), p=0.02) but not in the Lum-Her, RSI-R group (HR- 0.71 (0.07, 6.83), data not shown). On MVA, RT dose was an independent predictive variable only in the luminal A/B-RR subset (HR=0.025 (0.001, 0.946), p=0.046), along with age (p=0.008), T stage (p=0.004) and chemotherapy (p=0.008).
Discussion
The development of molecular predictors of local recurrence after breast conservation is central to the development of personalized treatment approaches in breast cancer. Here, we show that RSI, a previously developed and validated molecular signature of tumor radiosensitivity, may refine the classification of local recurrence risk in ER negative breast cancer patients treated with BCT.
RSI did not uniformly predict for local recurrence but only in a subset of patients. It uncovered differences in local recurrence in ER-negative patients but not in the ER-positive subset. Thus RSI may not reflect radioresistance for the whole breast cancer cohort. However, when RSI was integrated to the breast molecular subtypes, it found differences in outcomes between RSI-S/Int and RSI-R in both TN and Her-2-enriched subtypes, although it did not reach statistical significance in the latter perhaps due to a small sample size (n=14). In contrast, RSI-S/Int subsets within the TN subytpe had similar local recurrence risk as the luminal patients. These observations are similar to a prior published analysis, where we demonstrated that RSI is influenced by ER receptor status when predicting DM risk. One possible explanation for these findings is that the molecular determinants of radioresistance are different between disease sites and/or molecular subtypes. However we have validated RSI as a predictor of clinical outcome in RT-treated patients in multiple other disease sites (rectal, esophagus, pancreas, lung, prostate, head and neck and GBM) (5, 7, 17-20). Another possibility is that intrinsic radioresistance is influenced by biological context (i.e microenvironment or proliferation). For example expression of HIF-1α target genes is increased in TN patients, suggesting that these patients may have a more hypoxic microenvironment (21-23).
Local recurrence for TN and Her-2 subtype (not treated with trastuzumab) has been reported between 15%-30%, suggesting that RT alone may be insufficient in a subset of TN patients (i.e. TN-RSI-R)(10-13). In contrast, local recurrence for luminal patients in modern cohorts is around 5%. Indeed, some authors have suggested that TN patients are more radioresistant based on their higher local recurrence rate after BCT (11, 24)}(13). Our data supports that combining RSI and molecular subtype more accurately defines these sub-populations.
In a previous study we proposed that RSI subtype serves as a predictive biomarker of RT benefit (7). Unlike prognostic biomarkers which predict clinical outcome independent of treatment, predictive biomarkers are treatment specific and thus are critical for therapeutic decision-making (14). Previously, we showed that RSI was RT-specific as it only predicted DMFS in breast cancer patients that received RT (7). Although additional data is required, the current analyses provide further evidence that RSI may be a predictive biomarker of RT benefit. In addition they are suggestive that RSI may have clinical utility in informing RT-based treatment decisions in breast cancer.
There is clinical data that suggest the existence of a clinical gradient for RT benefit in breast cancer: 1. Systemic and local-regional benefit at 50 Gy, 2. Local-regional benefit at 50 Gy, 3. Local-regional benefit at 66 Gy, 4. No benefit at current doses. In this study we have shown that in combination with molecular subtype, RSI identifies patients least likely to benefit from RT (ER-negative/RSI-R, group 4 above) and patients more likely to benefit from RT dose escalation (LUM-RSI-R, group 3 above). Finally, in previous analyses we had shown that RSI, identified a sub-population with a decrease risk of systemic metastases after treatment with RT (group 1). Thus we propose that RSI provides the first opportunity to define clinical groups from the RT benefit perspective.
There are several limitations to our study. First, RT dosing was not randomized. Therefore, it is possible that there are biases that we are unable to account for in our analysis. Second, RSI did not uniformly predict for local recurrence for the whole cohort. Third, this dataset was specifically designed to develop a molecular signature of local recurrence, so it may not be representative of the general breast cancer population and failures were overrepresented. Therefore, our analysis may be overestimating the absolute local recurrence risk for the novel populations identified. Fourth, these patients were mostly treated prior to the regular use of CT-based planning and thus this probably affected the accuracy of RT delivery. Finally, hormonal therapy was underutilized in this cohort as only 9% of patients received HT, while 78% of the population was ER+.
In conclusion we demonstrate that combining a novel molecular signature of radiosensitivity (RSI) with molecular subtypes further defines local recurrence risk in breast cancer. Although further confirmation is required, these data demonstrate that integrating a RSI into the classification of breast cancer is an important step towards the development of biology-based clinical decisions in breast cancer.
Supplementary Material
Summary.
We integrate a clinically-validated molecular measure of tumor radiosensitivity (RSI) with molecular phenotypes to define local recurrence risk in breast cancer. While RSI did not uniformly predict for local recurrence across the entire cohort, it identifies a sub-population of triple negative patients (RSI-determined radioresistant patients) with the highest risk of local recurrence. Combined RSI and molecular phenotype is the best predictor of local recurrence risk outperforming all other variables. Finally, we show that RSI identifies a radioresistant sub-population within the luminal phenotype that benefit from increased RT dose.
Table 4.
Multivariate analysis in the LUMA/B-RR patients
| Variable | Level Compared to Ref. | Variable | P Value |
|---|---|---|---|
| Age | 0.708 (0.549, 0.912) | 0.0075 | |
| RT dose (ref= <=66GY) | >66Gy | 0.025 (0.001, 0.946) | 0.0466 |
| T stage (ref=pT1) | pT2-3 | 40.066 (3.16, 508.082) | 0.0044 |
| Surgical margin ref=negative | positive | 0.087 (0.004, 1.781) | 0.1128 |
| N stage ref=negative | pN+ | 3.766 (0.445, 31.854) | 0.2235 |
| Chemotherapy (CT) ref=no CT | CT | 0.016 (0.001, 0.333) | 0.0076 |
| Hormonal therapy (HT)* Ref=no HT | HT | 123.725 (7.326, 2089.61) | 0.0008 |
| Luminal subtype ref=LUMA | LUMB | 0.509 (0.096, 2.702) | 0.4275 |
only 10 patients of 67 patients in the LUMA/B-RR subset received HT
Acknowledgments
Support: National Institutes of Health (R21CA101355/R21CA135620), US Army Medical Research and Materiel Command, National Functional Genomics Center (170220051) and Bankhead-Coley Foundation (09BB-22).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: JTR and SAE hold intellectual property related to the work presented in this article. They are also shareholders and officers of Cvergenx, Inc a molecular diagnostics company that holds the commercial rights to RSI
References
- 1.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002 Oct 17;347(16):1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 Dec 17;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011 Nov 12;378(9804):1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad WJ, Oei SB, Warlam-Rodenhuis CC, Pierart M, Collette L. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007 Aug 1;25(22):3259–65. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 5.Eschrich SA, Pramana J, Zhang H, Zhao H, Boulware D, Lee JH, Bloom G, Rocha-Lima C, Kelley S, Calvin DP, Yeatman TJ, Begg AC, Torres-Roca JF. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009 Oct 1;75(2):489–96. doi: 10.1016/j.ijrobp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eschrich S, Zhang H, Zhao H, Boulware D, Lee JH, Bloom G, Torres-Roca JF. Systems biology modeling of the radiation sensitivity network: a biomarker discovery platform. Int J Radiat Oncol Biol Phys. 2009 Oct 1;75(2):497–505. doi: 10.1016/j.ijrobp.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eschrich SA, Fulp WJ, Pawitan Y, Foekens JA, Smid M, Martens JW, Echevarria M, Kamath V, Lee JH, Harris EE, Bergh J, Torres-Roca JF. Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res. 2012 Sep 15;18(18):5134–43. doi: 10.1158/1078-0432.CCR-12-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrieling C, Collette L, Fourquet A, Hoogenraad WJ, Horiot JC, Jager JJ, Bing Oei S, Peterse HL, Pierart M, Poortmans PM, Struikmans H, Van den Bogaert W, Bartelink H. Can patient-, treatment- and pathology-related characteristics explain the high local recurrence rate following breast-conserving therapy in young patients? Eur J Cancer. 2003 May;39(7):932–44. doi: 10.1016/s0959-8049(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 9.Harris JR, Lippman ME, Morrow M, Osborne CK. Diseases of the Breast. Lippincott Williams and Wilkings; 2010. [Google Scholar]
- 10.Abdulkarim BS, Cuartero J, Hanson J, Deschenes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011 Jul 20;29(21):2852–8. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010 Apr 1;28(10):1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 12.Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, Bellon JR, Wong JS, Smith BL, Harris JR. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011 Oct 10;29(29):3885–91. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012 Jun;133(3):831–41. doi: 10.1007/s10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- 14.Clark GM. Prognostic factors versus predictive factors: Examples from a clinical trial of erlotinib. Mol Oncol. 2008 Apr;1(4):406–12. doi: 10.1016/j.molonc.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Servant N, Bollet MA, Halfwerk H, Bleakley K, Kreike B, Jacob L, Sie D, Kerkhoven RM, Hupe P, Hadhri R, Fourquet A, Bartelink H, Barillot E, Sigal-Zafrani B, van de Vijver MJ. Search for a gene expression signature of breast cancer local recurrence in young women. Clin Cancer Res. 2012 Mar 15;18(6):1704–15. doi: 10.1158/1078-0432.CCR-11-1954. [DOI] [PubMed] [Google Scholar]
- 16.Eroles P, Bosch A, Perez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012 Oct;38(6):698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Caudell JJ, Eschrich SA, Torres-Roca JF, editors. ASTRO. San Francisco: Sep, 2014. Radiosensitivity Molecular Signature is Predictive of Overall Survival in Glioblastoma. 2014. [Google Scholar]
- 18.Creelan B, Eschrich S, Fulp W, Torres-Roca JF, editors. ASTRO. San Francisco: 2014. A Gene Expression Platform to Predict Benefit from Adjuvant External Beam Radiation in Resected Non-small Cell Lung Cancer. [Google Scholar]
- 19.Shridhar R, Strom T, Hoffe SE, Coppola D, Springett GM, Malafa MP, Eschrich S, Torres-Roca JF, editors. ASTRO. SAn Francisco: 2014. Radiosensensitivity Index Shows Promise for Predicting Outcomes with Adjuvant Radiation in Resectable Pancreatic Cancer Patients. [Google Scholar]
- 20.Torres-Roca JF, Erho N, Vergara I, Davicioni E, Jenkins RB, Den RB, Dicker A, Eschrich S, editors. ASTRO. San Francisco: Sep, 2014. A Molecular Signature of Radiosensitivity (RSI) is an RT-specific Biomarker in Prostate cancer. 2014. [Google Scholar]
- 21.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012 Oct 4;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu ZJ, Semenza GL, Zhang HF. Hypoxia-inducible factor 1 and breast cancer metastasis. Journal of Zhejiang University Science B. 2015 Jan;16(1):32–43. doi: 10.1631/jzus.B1400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999 Nov 15;59(22):5830–5. [PubMed] [Google Scholar]
- 24.Adkins FC, Gonzalez-Angulo AM, Lei X, Hernandez-Aya LF, Mittendorf EA, Litton JK, Wagner J, Hunt KK, Woodward WA, Meric-Bernstam F. Triple-negative breast cancer is not a contraindication for breast conservation. Ann Surg Oncol. 2011 Oct;18(11):3164–73. doi: 10.1245/s10434-011-1920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.