Abstract
Reactive cognitive control refers to a complementary set of cognitive operations by which individuals monitor for and detect the presence of goal-interfering conflict (i.e., conflict monitoring/evaluation) and, subsequently, initiate attention-focusing and response selection processes to bolster goal-directed action in the face of such conflict (regulative control). The purpose of the current study was to characterize the nature of conflict adaptation in both components of this dynamic process across sequences of trials and, more broadly, across time as participants complete a cognitive control task. Fifty-two young adults completed a standard arrow flanker task while behavioral and event-related potential (ERP) data were recorded. Multilevel modeling of sequences of compatible and incompatible trials over time showed that whereas response time (RT) data demonstrated a typical conflict adaptation effect throughout the task, N2 and frontal slow wave (FSW) indices of conflict monitoring and regulative control, respectively, demonstrated significant conflict adaptation only during the early part of the task. Moreover, although differential change in N2 and FSW over time suggested that conflict monitoring and regulative control were dissociable, a reciprocal relation between them was maintained throughout the task and was not present in a component theoretically unrelated to conflict adaptation (visual attention-related N1). Findings are discussed in terms of compensatory processes that help to maintain goal-directed performance even as control-related neural responses become fatigued.
Humans’ ability to flexibly adjust attention and responses according to current goals, particularly overcoming prepotent or habitual responses, reflects a property of the information-processing system known as cognitive control (Alexander & Brown, 2010; Miller & Cohen, 2001; Shenhav, Cohen, & Botvinick, 2016). In particular, when a given context contains stimuli that have the potential to interfere with goal-directed action, cognitive control is needed to perform a number of complementary functions, including maintaining current goals in working memory, focusing attention on goal-relevant stimulus features so that goal-irrelevant features can be ignored, and biasing motor responding in favor of goal-appropriate responses (e.g., Botvinick, & Cohen, 2014; Braver, 2012; Jacoby, Jennings, & Hay, 1996; Meyer & Kieras, 1997).
The operation of these processes in the laboratory is commonly examined using speeded response time (RT) tasks in which visual targets are presented amid goal-challenging distracters. For example, in the Flanker task (Eriksen & Eriksen, 1974) participants must identify a target flanked on both sides by distracters associated either with the same response as the target (i.e., compatible or congruent trials) or an opposing response (i.e., incompatible or incongruent trials). The commonly observed compatibility effect (or congruency effect) reflects participants’ superior performance on compatible relative to incompatible trials, and is taken as evidence that response activation is influenced by flanker stimuli (see Coles, Bashore, Gratton, Eriksen, & Donchin, 1985; Coles, Gratton, & Donchin, 1988; Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988). That is, incompatible flankers elicit response conflict.
The malleability of cognitive control is evident in the fact that the compatibility effect varies as a function of context. Gratton, Coles, and Donchin (1992) first reported that the compatibility effect is smaller when the previous flanker trial was incompatible versus compatible, a finding that since has been replicated many times (for reviews see Clayson & Larson, 2013; Verguts & Notebaert, 2008). The dominant explanation for this phenomenon is that the experience of conflict—particularly when conflict is aversive (see Desender, Van Opstal, & Van den Bussche, 2014; Driesbach & Fischer, 2015; Inzlicht, Bartholow, & Hirsch, 2015; Steenbergen, Band, & Hommel, 2009)—elicits adjustments in cognitive control so that subsequent conflict can be better managed—a so-called conflict adaptation effect.1
This explanation accords with dominant theories emphasizing the dynamic interplay of conflict monitoring, attention control and response implementation. In particular, the conflict monitoring theory (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick & Cohen, 2014; Botvinick, Cohen, & Carter, 2004; Yeung, 2013) posits complementary processes responsible for adjusting control when performance is threatened by interference. Specifically, the theory posits an evaluative component that monitors performance for instances of conflict; when conflict is detected, a second, regulative component is responsible for increasing control to ensure that intended responses are implemented. Evidence from event-related potential (ERP) and functional magnetic brain imaging (fMRI) studies has associated the evaluative, conflict-monitoring process with activity in the medial prefrontal cortex (mPFC), particularly dorsal anterior cingulate cortex (dACC; Carter et al., 1999; Carter & van Veen, 2007; Sheth et al., 2012; Yeung, Botvinick, & Cohen, 2004), whereas the regulative control process has been associated with activity in dorsolateral areas of PFC (dlPFC) responsible for goal representation and response selection (Kerns et al., 2004; MacDonald, Cohen, Stenger, & Carter, 2000; West, Bailey, Tiernan, Boonsuk, & Gilbert, 2012). According to the dual mechanisms of control model (DMC; Braver, 2012), the interplay of these two components describes a reactive control process, whereby control fluctuates moment-to-moment in reaction to the presence of conflict.
Several studies have produced evidence consistent with the basic premise that the detection of conflict by dACC is associated with increased activation dlPFC (i.e., regulative control) shortly thereafter (e.g., Durston et al., 2003; Kerns et al., 2004, 2006). Other work has shown that whereas dACC is sensitive to the occurrence of conflict, dlPFC responds to information signaling upcoming conflict (MacDonald et al., 2000). Critically, however, no research to date has tested a reciprocal premise of the conflict monitoring theory that increased regulative control should reduce the extent to which evaluative conflict monitoring is engaged thereafter. To the extent that the experience of conflict instigates greater regulative control (Kerns et al., 2004), and following the logic outlined in the DMC that greater engagement of dlPFC should down-regulate mPFC reactivity to conflict “in the moment,” it stands to reason that regulative control in response to an instance of conflict should predict reduced conflict monitoring-related neural responses thereafter. Indirect evidence supporting this idea has come from studies demonstrating that mPFC responses on incompatible trials are reduced following incompatible relative to compatible trials (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Clayson & Larson, 2011, 2012; Forster, Carter, Cohen & Cho, 2011). But the specific premise that neural responses associated with regulative control predict reduced neural conflict-monitoring reactivity has never been tested. One goal of the current research was to test this basic premise, using ERP measures that previous research has associated with conflict monitoring and regulative aspects of control, thereby filling a critical gap in the literature pertaining to the dynamic nature of control variability associated with the reactive control process (Braver, 2012).
More generally, the current research aimed to model the extent to which behavioral and neural indices of conflict adaptation vary across time as participants engage in a typical laboratory control task. Just as control-related processes are hypothesized to fluctuate on a moment-to-moment basis (Botvinick et al., 2001; Braver, 2012) there are a number of reasons to hypothesize that the extent of this fluctuation also should vary over longer time periods. In particular, several theories predict (e.g., Botvinick, & Cohen, 2014; Shenhav, Cohen, & Botvinick, 2016; Robinson, Schmeichel, & Inzlicht, 2010) and empirical evidence suggests (Fischer, Dreisbach, & Goschke, 2008) that at least some aspects of control are resource-intensive and therefore susceptible to fatigue. For example, research has shown that both time-on-task (e.g., Barwick, Arnett, & Slobounov, 2012; Boksem, Meijman, & Lorist, 2006; Warm, Matthews, & Finomore, 2008) and previous mental effort (Hagger, 2010) result in fatigue and impaired performance on laboratory control tasks. Another possibility suggested by recent theories is that flagging motivation leads people to disengage from tasks that require control, which similarly can result in performance decrements (Braver et al., 2014; Francis & Inzlicht, 2016; Kool, McGuire, Rosen, & Botvinick, 2010; Kouneiher, Charron, & Koechlin, 2009).
To the extent that such effects reflect changes in activation of neural circuits associated with control, measures of such activation should show reductions as time-on-task increases. Some previous research provides support for this idea. For example, Boksem et al. (2006) found that ERP measures of conflict monitoring decreased from the first 20 min to the final 20 min of a very long (2 hr) cognitive control task, and that the amplitude reduction was evident only for high-conflict trials (also see Lorist, Boksem, & Ridderinkhof, 2005). To date, however, few studies have investigated whether behavioral and neural indices of conflict adaptation vary over the course of a cognitive control task (see Lorist & Jolij, 2012), nor, more specifically, have previous investigations tested the extent to which neural measures reflecting evaluative/conflict-monitoring versus regulative aspects of reactive cognitive control vary differentially over the course of task engagement. In other words, the dynamic interplay between these two, theoretically distinct aspects of conflict adaptation hypothesized in dominant neurocognitive theories of control (Botvinick & Cohen, 2014; Braver, 2012) has yet to be fully characterized.
The Current Study
The current study had three primary aims: (1) to characterize the nature of conflict adaptation effects in both evaluative/conflict-monitoring and regulative aspects of reactive cognitive control; (2) to model the nature of change in these processes over the course of a standard cognitive control task; and (3) to examine the extent to which conflict adaptation-related increases in regulative control predict subsequent change in evaluative, conflict-monitoring processes and behavioral performance, and whether these relationships change over the course of the task. The amplitude of the fronto-central N2, a transient negativity emerging 200–350 ms after stimulus onset and known to be generated in mPFC (van Veen & Carter, 2002), served as a neural index of conflict monitoring. Numerous studies have shown that N2 amplitude is larger on incompatible than compatible trials in conflict tasks (see Folstein & Van Petten, 2008; Larson, Clayson, & Clawson, 2014; van Veen & Carter, 2002), suggesting it reflects the evaluative/conflict monitoring function of control (Yeung et al., 2004). Moreover, neuroimaging and ERP data consistently show that indices of mPFC activation, including the N2, show conflict adaptation effects, being smaller for high-conflict stimuli when preceded by other high-conflict stimuli relative to low-conflict stimuli (e.g., Botvinick et al., 1999; Clayson & Larson, 2011, 2012; Forster et al., 2011; Larson, Kaufman, & Perlstein, 2009; Sheth et al., 2012).
Another stimulus-locked ERP component sensitive to the presence of conflict is the frontal slow wave (FSW). The FSW is a low-frequency, negative-going voltage deflection arising late in stimulus-locked ERP epochs (generally 600–1000 ms) over frontal and central scalp locations (West & Alain, 1999, 2000; West & Bailey, 2012) that is larger following high-conflict relative to low-conflict trials in cognitive control tasks (Bailey, West, & Anderson, 2010; Bartholow, Dickter, & Sestir, 2006; Curtin & Fairchild, 2003; West, Bailey, Tiernan, Boonsuk, & Gilbert, 2012), including the flanker (Bailey, Bartholow, Saults, & Lust, 2014). The FSW is thought to reflect adjustment-related activity in the lateral PFC (West & Bailey, 2012; West et al., 2012), in that FSW amplitude is correlated with behavioral indices of control adjustment (Bailey et al., 2014; Bartholow et al., 2006) and varies along with the amount of conflict in a block of trials (West & Bailey, 2012). Given these findings, coupled with other evidence demonstrating a role for dlPFC in regulative control (De Pisapia, & Braver, 2006.; Kerns et al., 2004; Kerns, 2006; MacDonald et al., 2000; Marklund & Persson, 2012), the amplitude of the FSW was used to represent the regulative component of reactive control.2
To examine change over time in control-related responses data from this study were analyzed using mixed-effects multilevel modeling (MLM). Recent reports have emphasized the advantages of MLM over traditional repeated-measures analysis of variance (rmANOVA) for psychophysiological data (Kristjansson, Kircher, & Webb, 2007; Tibon & Levy, 2015; Tremblay & Newman, 2015; Vossen et al., 2011). Of greatest relevance for the current research, MLM allows the modeling of data from individual trials over time (Tibon & Levy, 2015; Vossen et al., 2011). Virtually all theoretical models of cognitive control posit dynamic temporal relationships between multiple control-related processes—conflict adaptation is an example—but traditional approaches to neurophysiological signal quantification (e.g., signal averaging across all trials within conditions) and analysis (rmANOVA) are not well suited to modeling such dynamics, for several reasons. For example, the typical signal averaging technique assumes that each time-locked ERP consists of a signal (i.e., the neurocognitive process of interest) and random noise, and that the signal remains constant across all trials. This latter assumption is not valid in situations where signal change is anticipated (e.g., from fatigue or habituation; see Boksem et al., 2006; Kato, Endo, & Kizuka, 2009; Woestenburg, Verbaten, van Hees, & Slangen, 1983). Moreover, signal averaging necessarily ignores potentially interesting sources of variation related to any such changes, particularly in situations where the signal might vary over time in some conditions more than others.
MLM can handle large numbers of repeated measurements per person, which means data from individual trials can be modeled using “Trial” as a continuous predictor. This approach is arguably preferable to other techniques, such as binning of trials into sequential blocks, which still result in considerable loss of within-person variability (see Vossen et al., 2011). Modeling of individual trials is facilitated by the fact that MLM can account for multiple sources of variability, including that attributable to individual subjects, stimuli, electrodes, and so on, and can accommodate specification of error terms at each level of nesting (e.g., electrodes within subjects). Additionally, missing data need not result in listwise deletion of cases or imputation of missing values, as MLM is robust to imbalanced data structures across individual cases.
The ability to model data from individual trials allowed us to examine a number of issues related to this study’s aims. First, we examined whether conflict adaptation effects in neural measures of conflict monitoring and regulative control decrease with time-on-task. To the extent that conflict adaptation requires cognitive control resources that are susceptible to depletion over time due to fatigue or other influences (e.g., Boksem et al., 2006), it could be expected that conflict adaptation effects will be more evident earlier compared to later in the course of a task. This is consistent with the suggestion that N2-related conflict adaptation effects have been more apparent in studies with fewer trials (see West & Bailey, 2012).
Second, although a number of studies have shown support for the conflict monitoring theory’s prediction that mPFC responses to conflict positively predict subsequent dlPFC activation (e.g., Durston et al., 2003; Kerns et al., 2004, 2006), no existing studies have examined the complementary prediction that dlPFC activation in response to conflict should have a negative (i.e., down-regulating) effect on mPFC responses. Based on predictions derived from conflict-monitoring and DMC theories (Botvinick et al., 2001; Braver, 2012), we hypothesized that FSW amplitude elicited on a given trial would be inversely associated with N2 amplitude elicited on the next trial. We similarly predicted that the extent of regulative control elicited by a given stimulus would predict performance on the subsequent trial, such that previous-trial FSW amplitude would be positively associated with current-trial response time (RT) (i.e., larger FSW would predict faster RT).
Method
Participants
Individuals contributing data for the current report were among 142 young adults (ages 21–32 years) who participated in a study investigating the acute effects of alcohol and caffeine on cognitive control and its neural correlates (see Bailey et al., 2016). The current report includes data from participants who were randomly assigned to the control condition (consumed neither alcohol nor caffeine; n = 27) or a placebo condition (consumed a beverage they were told contained alcohol and caffeine but actually contained neither; n = 25) of that larger study. Participants were recruited from the Columbia, MO community using e-mail circulars, advertisements, and posted flyers. Eligible individuals completed lab sessions for which they were paid $12/hour.3
Materials
Flanker task
On each of 800 experimental trials (8 blocks of 100 trials) participants viewed arrow arrays and were to identify the direction of the central arrow (right or left) via button press. On compatible trials, the flanker (peripheral) and target arrows indicated the same response (i.e., →→→→→ or ←←←←←); on incompatible trials, the flankers and target indicated opposing responses (i.e., →→←→→ or ←←→←←). Compatible and incompatible arrays were presented pseudorandomly, with the constraints that they occurred with equal probability and left- and right-hand responses occurred equally often. Arrays were presented for 100 ms with an unlimited response window followed by a randomly-varying inter-trial interval (1100 or 1500 ms). Participants completed 100 practice trials prior to the experimental trials.
Electrophysiological recording
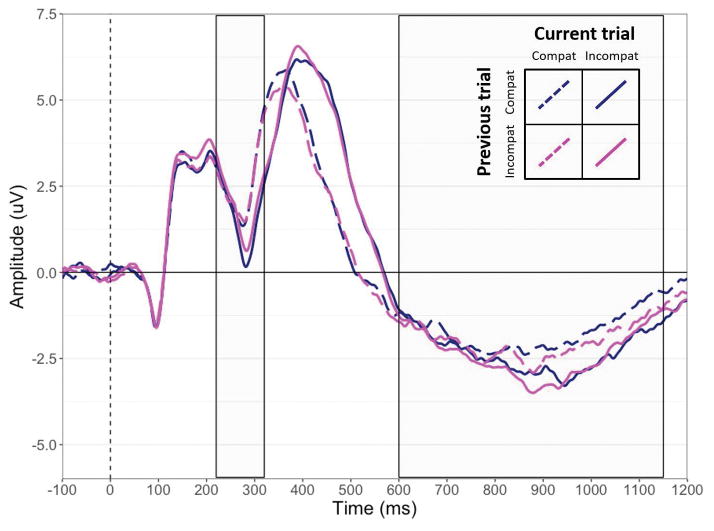
The electroencephalogram (EEG) was sampled at 1,000 Hz (filtered online at 0.05–40 Hz) with 32 tin electrodes placed in standard locations (American Encephalographic Society, 1994). Scalp electrodes were referenced online to the right mastoid; an average mastoid reference was derived offline. Impedance was kept below 5 kΩ. Blinks were removed from the EEG off-line using a regression-based procedure. EEG data were segmented into epochs of −100 to 1200 ms of post-stimulus activity and scanned for artifacts (trials containing voltage deflections of +/− 75 μV [< 3% of all trials] were discarded). Consistent with previous studies (see Folstein & Van Petten, 2008), visual inspection of the grand average waveforms (see Figure 1) indicated that the N2 emerged ~220–320 ms post-stimulus and was most prominent at frontal and central scalp sites (electrodes F3, Fz, F4, FC3, FCz, FC4, C3, Cz, and C4). Thus, the N2 was measured as the average amplitude 220–320 ms post-stimulus at those nine electrodes. The grand averages also show a more sustained negativity emerging around 600 ms post-stimulus and also most prominent at frontal and fronto-central scalp sites, consistent with the FSW observed in previous cognitive control tasks (e.g., Bailey et al., 2014; Bartholow et al., 2006; Curtin & Fairchild, 2003; West & Alain, 1999). Therefore, the FSW was quantified as the average amplitude 600–1150 ms post-stimulus at the same nine-electrode array used to quantify the N2.
Figure 1.
Grand average ERP waveforms averaged across nine electrodes (F3, Fz, F4, FC3, FCz, FC4, C3, Cz, and C4), plotted as a function of current and previous trial type (compatible, incompatible). The dotted vertical line indicates stimulus onset and the boxes indicate the time windows during which the mean amplitude of the N2 and FSW were computed. Compat = compatible trials; incompat = incompatible trials.
Additionally, we quantified the posterior N1, an early-latency component typically elicited in response to visual stimulation. The N1 is moderated by selective attention and thus has been most commonly enlisted to examine attentional processing (Mangun & Hillyard, 1991). As the N1 is theoretically not related to cognitive control, demonstrating that FSW does not significantly predict N1 on the subsequent trial would confirm the discriminant validity of the relationship between the FSW and N2 by implying that any such relationship is not the product of a general similarity in the EEG across trials. The N1 was quantified as the average amplitude 125–220 ms post-stimulus at electrodes P3, PZ, P4, O2, and O1.
Procedure
Upon arrival, participants provided informed consent and completed affidavits assessing compliance with pre-session protocols (e.g., abstention from alcohol and other drugs for the previous 24 hr). Participants then were escorted to an EEG acquisition suite where experimenters placed and tested the electrodes. Next, participants completed the practice trials followed by a break during which they consumed a tonic water beverage (according to the goals of the larger study). They then completed the experimental trials of the flanker task, after which they were debriefed and dismissed.
Data Analysis
Data from two participants were completely discarded due to experimenter errors during data collection (n = 1) and failure to demonstrate adequate performance in the flanker task (< 50% accuracy; n = 1). Equipment malfunctions resulted in the loss of 13 additional subjects’ EEG data. One additional subject’s ERP data was discarded due to a large number of artifacts in the data (only 23.3% usable trials). This resulted in a final sample of 50 individuals for RT analyses and 36 individuals for separate analyses of N2 and FSW amplitude. ERP data were unable to be merged with the behavioral data for three additional subjects, resulting in a sample of 33 individuals for analyses examining relationships between variables. Although this small sample size can adversely affect tests of group-level variances within a multilevel model, regression coefficients and their standard errors are estimated without bias, even with a sample size of 30 (Maas & Hox, 2005).
The R package ‘lme4’ (Bates et al., 2015) was used to fit multilevel models for data analysis. To determine the most appropriate random effects structure, we used the model-specification procedures described by Matuscheck et al. (2017), starting with a maximal model and then removing random slopes based on the magnitude of the correlations between random effects (.80 was used as the cutoff threshold). Satterthwaite approximations were used to estimate degrees of freedom and to obtain two-tailed p values; in situations where degrees of freedom were > 200, we report the results as z statistics. Data and code used for analysis can be found at https://github.com/hiv8r3/ConfDyn.
All models utilized single trial-level data rather than individual participant means. Separate MLMs were constructed to predict each outcome measure of interest (RT, N2, and FSW) using previous trial type (compatible, incompatible), current trial type (compatible, incompatible), and trial as predictors. The intercept and all slopes were allowed to vary by subject and, where appropriate (N2 and FSW models), the intercept was allowed to vary by electrode nested with subject. For all models, individual trials were included only if correct responses were made on both the trial in question (current trial) and the trial preceding it (previous trial). Additionally, to reduce skew and eliminate fast “guessing” responses, trials with RTs < 100 ms and > 2000 ms were excluded from the analyses.
Models examining associations among the outcomes were constructed using the disaggregation procedure described by Curran and Bauer (2011), which permits separation of variability attributable to differences between persons from the variability attributable to processes of interest that differ within persons. The amplitude of the predictor variable on each trial was centered on each participant’s mean. To simplify these models, amplitude was averaged over the nine electrodes at which it was measured (i.e., so that only a single mean was used for each subject). The mean of the predictor variable was also added as a level-2 predictor in each model. This resulted in two predictors, one representing the between-person variation in the predictor and the other the within-person variation in the predictor. The within-person estimate is more appropriate for examining cognitive control dynamics, since within-person relationships reflect differences across occasions (i.e., trials) rather than differences across individuals. Disaggregating these two sources of variability allows for a clearer inference that changes in the activation of one control-related process are associated with changes in another control-related process within or across trials (Fleesom, 2007). In each model, the intercept was allowed to vary by subject. To test whether the relationship between predictor and criterion variables changed over the course of the task, trial and its interaction with the within-subjects effect were added to each of the disaggregation models.
Results
Fixed effects derived from models in which RT, N2, and FSW were predicted from current trial type, previous trial type, trial, and all interactions are given in Table 1. In these models, trial was rescaled and centered (see the Supplementary Materials for more information on the logic behind the rescaling procedure). As a result, the effects of current trial type, previous trial type, and their interaction are estimated at the midpoint of the experiment in the main models. Significant Current × Previous × Trial interactions were probed with additional models in which the trial variable was centered around the first trial (representing trials toward the beginning of the experiment) and the last trial (representing trials toward the end of the experiment), respectively, following procedures outlined by Aiken and West (1991). The average number of trials included in the ERP analyses for each condition for each subject are given in Table 2. MLM is robust to unbalanced data, through a procedure called partial pooling (Gelman & Hill, 2007; Tibon & Levy, 2015), and therefore differing numbers of trials per condition are less problematic than in traditional rmANOVA.
Table 1.
Fixed Effects Estimated by Multilevel Models Examining Each Dependent Measure Separately.
| RT |
N2 |
FSW |
||||
|---|---|---|---|---|---|---|
| b | p | b | p | b | p | |
| Current | 29.06 (1.29) | .000 | −.39 (.08) | .000 | −.25 (.08) | .004 |
| Previous | .53 (.48) | .271 | .07 (.05) | .163 | −.07 (.06) | .288 |
| Current × Previous | −1.33 (.57) | .023 | .08 (.06) | .218 | .04 (.06) | .559 |
| Trial | −1.13 (.20) | .000 | −.05 (.01) | .000 | .33 (.01) | .000 |
| Trial × Current | −.62 (.20) | .001 | −.01 (.01) | .464 | .05 (.01) | .000 |
| Trial × Previous | −.06 (.20) | .745 | −.05 (.01) | .000 | −.04 (.01) | .000 |
| Trial × Current × Previous | .03 (.20) | .867 | .02 (.01) | .024 | −.04 (.01) | .000 |
Note. Unstandardized betas are presented; standard errors of estimates are in parentheses. Satterthwaite approximations were used to estimate degrees of freedom to calculate p-values. For analyses, the Trial variable was re-scaled and centered to range from −4 to 4. Changing the range of the Trial variable in this way increases the betas associated with the effect of Trial and its interactions ten-fold (to aid in their interpretation), but does not influence significance testing. In the model presented in this table, the effects of Current trial, Previous trial, and their interaction are interpreted at the midpoint of the task. Categorical variables were effect coded.
Table 2.
Average Number of Trials Included in ERP Analyses as a Function of Condition
| Previous trial | Current trial |
|
|---|---|---|
| Compatible | Incompatible | |
| N2 | ||
| Compatible | 172.5 | 158.0 |
| Incompatible | 160.6 | 144.5 |
|
| ||
| FSW | ||
| Compatible | 167.8 | 154.2 |
| Incompatible | 156.5 | 141.4 |
|
| ||
| Disaggregate Analyses | ||
| Compatible | 140.2 | 140.8 |
| Incompatible | 142.4 | 129.9 |
RT
As indicated in Table 1, the model predicting RT showed a significant main effect of current trial type, such that participants were faster to correctly identify targets on compatible trials (M = 382 ms; SD = 104 ms) than on incompatible trials (M = 430 ms; SD = 106 ms). This main effect was qualified by a small but significant Current × Previous trial type interaction. The means presented in Figure 2 (panels B–D) show a pattern generally consistent with conflict adaptation: the compatibility effect was smaller following incompatible versus compatible trials. Because trial is centered and included in the model, these effects are interpreted at the midpoint of the task.
Figure 2.
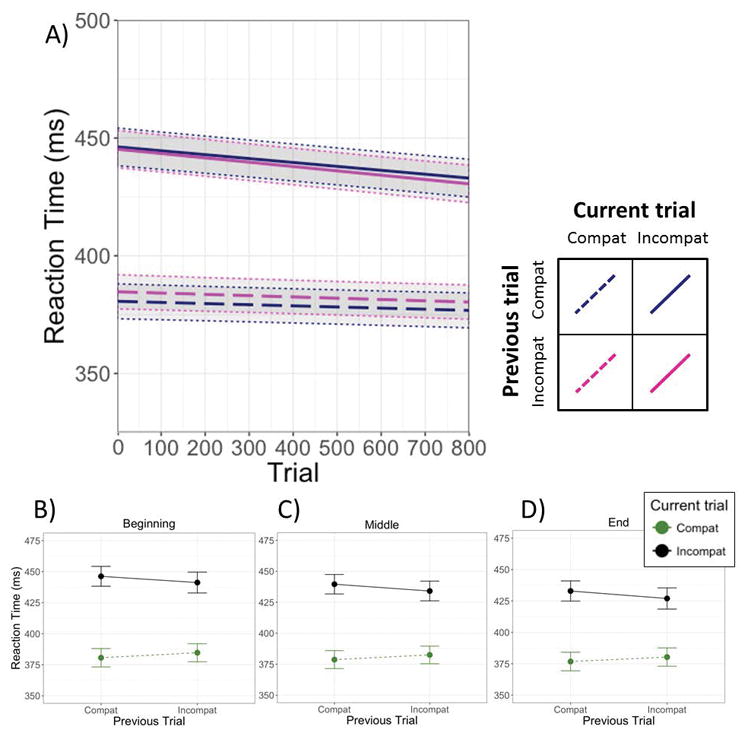
Panel A depicts the change in reaction times as a function of condition over the course of the experiment. The solid and dashed lines represent the simple slopes for the four sequential trial combinations of interest, estimated from the model corresponding to Table 1. Panels B, C, and D represent estimated (model-derived) means and standard errors for each condition at the beginning, middle, and end of the task, each estimated using a separate model with the Trial variable centered on the first trial, mean trial, and last trial, respectively.
The RT model also showed a main effect of trial (Table 1), indicating that RTs decreased over the course of the task. However, this effect was qualified by a significant Trial × Current trial interaction. Examination of the simple slopes (Figure 2A, Table 3) revealed that whereas RT for incompatible trials decreased over the course of the task, RT for compatible trials did not change. Neither the Trial × Previous trial type interaction nor the three-way Trial × Previous trial type × Current trial type interaction were significant. That is, the magnitude and form of the conflict adaptation effect in RT did not vary over the course of the task.4
Table 3.
Simple Slopes Describing the Change in Each Dependent Measure over the Course of the Experiment, Separately for Each Trial Condition.
| Previous trial | Current trial |
|
|---|---|---|
| Compatible | Incompatible | |
| RT | ||
| Compatible | −0.48 [−1.23, 0.26] | −1.66 [−2.44, −0.88] |
| Incompatible | −0.55 [−1.32, 0.23] | −1.85 [−2.66, −1.03] |
|
| ||
| N2 | ||
| Compatible | −0.03 [−0.06, 0.01] | 0.03 [−0.01, 0.06] |
| Incompatible | −0.09 [−0.13, −0.06] | −0.12 [−0.16, −0.08] |
|
| ||
| FSW | ||
| Compatible | 0.28 [0.24, 0.32] | 0.45 [0.41, 0.49] |
| Incompatible | 0.27 [0.23, 0.31] | 0.30 [0.26, 0.34] |
Note. The 95% confidence interval for each estimate is presented in brackets; intervals that do not cross zero are considered statistically significant (estimates in boldface).
N2 Amplitude
The model predicting N2 amplitude showed a significant main effect of current trial type (Table 1). As is evident in Figure 1, incompatible trials (M = 1.85 uV; SD = 9.79 uV) elicited larger (more negative) N2s than compatible trials (M = 2.65 uV; SD = 9.79 uV). The pattern of means in the N2 is suggestive of a conflict adaptation effect (Figure 1), in that incompatible trial N2s appeared larger when the previous trial was compatible versus incompatible, consistent with previous studies (e.g., Clayson & Larson, 2011; Forster et al., 2010). But neither the previous trial type main effect nor the Current × Previous trial type interaction were significant at the midpoint of the task.
However, the presence of a significant Previous × Current × Trial interaction suggests a more nuanced picture (see Table 1). This significant interaction was decomposed by fitting additional models with the trial variable centered around the beginning and the end of the task. These additional models revealed a significant conflict adaptation effect (i.e., a significant Current × Previous interaction) during the first part of the task, b = .158, t(60.3) = 2.24, p = .029, but not during the middle (b = .08, t[34.4] = 1.25, p = .218) or latter portion of the trials, b = −0.004, t(63.7) = −0.06, p =.95 (see panels B through D of Figure 3). In other words, conflict adaptation was more evident during approximately the first half of the trials and decreased over time.
Figure 3.
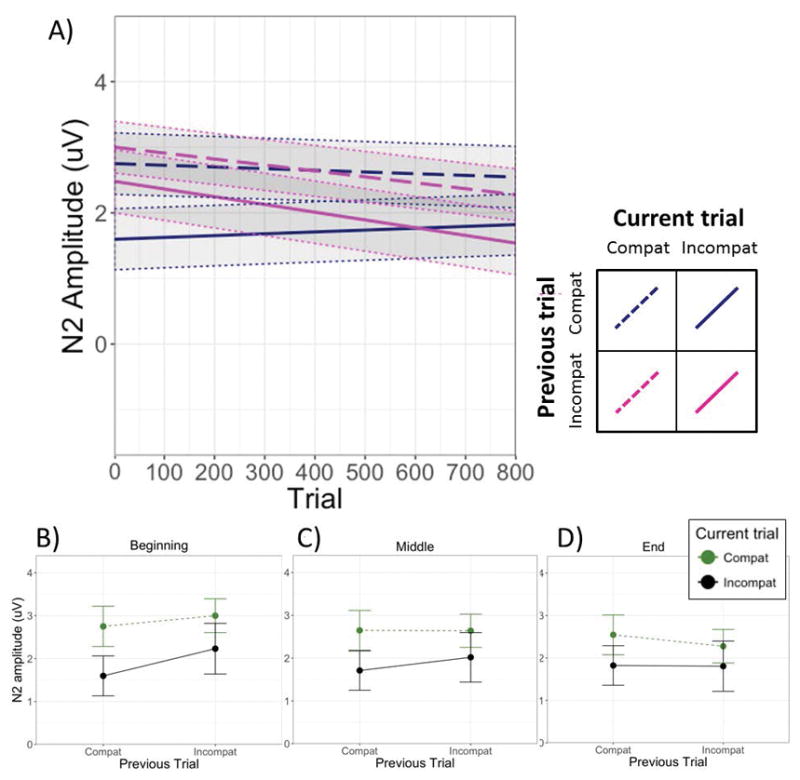
Panel A depicts the change in N2 amplitudes as a function of condition over the course of the experiment. The solid and dashed lines represent the simple slopes for the four sequential trial combinations of interest, estimated from the model corresponding to Table 1. Panels B, C, and D represent estimated (model-derived) means and standard errors for each condition at the beginning, middle, and end of the task, each estimated using a separate model with the Trial variable centered on the first trial, mean trial, and last trial, respectively.
Another way of interpreting these relationships is in terms of the difference in slopes representing the change in N2 amplitude over time in the four conditions of interest. Examination of the slopes and 95% confidence intervals in Table 3 indicates that whereas N2 amplitude elicited on trials following compatible trials remained relatively stable over the course of the task, the N2 elicited on trials following incompatible trials tended to get larger as the task progressed.
FSW Amplitude
A significant main effect of current trial type indicated that incompatible trials elicited larger (more negative) FSW amplitude (M = −2.31 uV; SD = 10.7 uV) than compatible trials (M = −1.82 uV; SD = 10.6 uV). Neither the main effect of previous trial type nor the interaction between current and previous trial type were significant at the midpoint of the task. As with the N2, however, the Previous × Current × Trial interaction was significant (see Table 1). Further exploration of the Previous × Current trial interaction at the beginning and end of the task using additional models indicated a significant conflict adaptation effect during the first part of the task, b = .18, t(68.2) = 2.49, p = .015, but not during the middle (b = .04, t[33.9] = 0.59, p = .56) or latter portion of the trials, b = −.11, t(73.0) = −1.47, p =.14 (see panels B through D of Figure 4).
Figure 4.
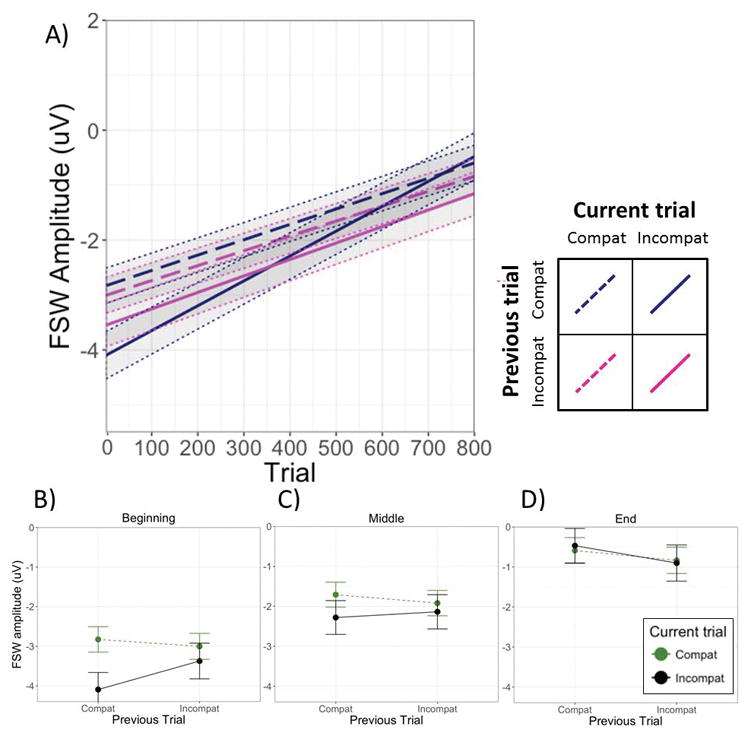
Panel A depicts the change in FSW amplitudes as a function of condition over the course of the experiment. The solid and dashed lines represent the simple slopes for the four sequential trial combinations of interest, estimated from the model corresponding to Table 1. Panels B, C, and D represent estimated (model-derived) means and standard errors for each condition at the beginning, middle, and end of the task, each estimated using a separate model with the Trial variable centered on the first trial, mean trial, and last trial, respectively.
Another way of interpreting the significant Previous × Current × Trial interaction is in terms of the difference in slopes representing the change in FSW amplitude over time in the four conditions of interest. As evident by the slope values given in Table 3 (and depicted in Figure 4), FSW amplitude decreased relatively dramatically over the course of the task in all conditions, but more so in the previous compatible, current incompatible condition than in any of the others. Inspection of the 95% confidence intervals in each condition shows that whereas the slopes in each of the other conditions did not differ significantly (i.e., confidence intervals overlapped), the slope in the previous compatible, current incompatible condition differed from each of the others.
Associations among Control-related Processes and Performance
Conflict monitoring predicting regulative control
Conflict monitoring theory predicts that detection of conflict increases the implementation of regulative control (see Kerns et al., 2004). Although not directly related to the aims of the current study, we tested the magnitude of this association in the current data by predicting FSW amplitude from N2 amplitude elicited within the same trial. Consistent with theory, the within-subject effect of N2 was strongly associated with the FSW within the same trial, b = .59, z = 89.4, p < .001. Moreover, when trial was added as a predictor, this association was not moderated by trial, b = −.002 (z < 1, p = .538), indicating that the size of this association remained relatively constant throughout the task.
Regulative control predicting conflict monitoring
The prediction that the extent of regulative control (FSW) elicited on one trial would down-regulate conflict monitoring (N2) on the next trial was tested using a model in which previous-trial FSW amplitude was used to predict current-trial N2 amplitude. The model results indicated a significant between-subjects effect, b = −0.11, z = −2.0, p = .043, suggesting that individuals with larger previous-trial FSW also tend to experience smaller current-trial N2 reactivity. More importantly, a significant within-subjects effect, b = −0.05, z = −6.7, p < .001, indicated that a larger FSW on one trial predicted a smaller N2 on the following trial regardless of the magnitude of an individual’s FSW relative to others in the sample. When trial was added as a predictor, there was no interaction between the within-subject effect and trial, b = 0.00, z = .37, p = .711, suggesting that the sequential relationship between the FSW and N2 did not change over the course of the task.
We also examined whether this relationship varied according to trial type by estimating an additional model with previous trial FSW amplitude, previous trial type, and current trial type predicting current-trial N2 amplitude. This model produced a significant interaction between FSW amplitude, current trial type, and previous trial type, b = 0.10, z = 3.57, p < .001. To understand this interaction, we examined the effect of previous-trial FSW on current trial N2 separately for the four trial sequence conditions. There was a significant within-subjects effect of FSW in cI (previous compatible, current incompatible) trials, b = −0.05, z = −4.0, p < .001, and iC (previous incompatible, current compatible) trials, b = −0.08, z = −5.9, p < .001. However, this relationship did not hold for iI trials, b = −0.02, z = −1.4, p = .165, or cC trials, b = −0.02, z = −1.8, p = .065.
Regulative control predicting RT
Conflict-monitoring theory also predicts that enhanced regulative control should improve performance (e.g., by limiting the influence of subsequent conflict). The disaggregation model examining the relationship between FSW and RT showed a nonsignificant between-subjects effect (b = 0.26, z = .44, p = .663) but a significant within-subjects effect, b = 0.21, z = 3.0, p = .002, indicating that larger (more negative) FSW amplitude on one trial predicted better performance (faster RTs) on the subsequent trial. When trial was added as a predictor, the interaction between the within-subject effect and trial was also nonsignificant, b = 0.06, z = 1.9, p = .056suggesting the relationship between previous-trial FSW and current-trial RT was maintained throughout the task.
Regulative control predicting visual-attentional processing
To confirm that these relationships are indicative of links between theoretical constructs rather than simply the result of common method variance, we additionally examined the relationship between previous-trial FSW and current-trial N1. The disaggregation model examining the relationship between FSW and N1 on the following trial showed a nonsignificant between-subjects effect (b = −0.07, z = −1.4, p = .149), as well as a nonsignificant within-subjects effect (b = −.001, z = −.19, p = .846). Additionally, when trial was added as a predictor, the interaction between the within-subjects effect and trial was also nonsignificant, b = −.001, z = −2.6, p = .796.
Discussion
Dominant theories of cognitive control (Botvinick et al., 2001; Botvinick & Cohen, 2014; Braver, 2012) posit multiple components of control responsible for ensuring acceptable levels of performance when goal-directed action is threatened by distracting or goal-conflicting information. Moreover, these theories posit dynamic, reciprocal associations between the components of control rooted in mPFC (conflict monitoring) and lateral PFC (regulative control) as they react to varying levels of conflict. Although such control dynamics have generated intense interest among researchers (see Clayson & Larson, 2011; Forster et al., 2010; Larson et al., 2009; Verguts & Notebaert, 2008), there is a dearth of evidence concerning how the control components respond to each other and how their sensitivity to changing demands varies over the course of a challenging task.
Several aspects of the current data help to address this gap in understanding. First, although conflict adaptation (Gratton et al., 1992) was present in behavioral performance throughout the task, neurophysiological correlates of conflict monitoring (N2) and regulative control (FSW) showed a more time-limited adaptation effect. Specifically, both the N2 and FSW showed patterns indicative of conflict adaptation during early but not later trials (see Figures 3–4). Whereas previous studies have reported decreased compatibility effects (current trial compatibility) in conflict-monitoring-related neural responses from the first to the last block of a lengthy control task (Boksem et al., 2006; Kato et al., 2009), little previous work has examined how the conflict adaptation effect (i.e., Previous × Current trial compatibility) in such a measure varies over time (but see Lorist & Jolij, 2012). The fact that the N2 effect diminished as the task progressed is consistent with the observation that conflict adaptation effects in the N2 have been more apparent in studies with fewer trials (see West & Bailey, 2012).
Of greater relevance to testing the full scope of the conflict monitoring theory is whether fluctuation in the instigation of regulative control, which in theory reflects compensatory adjustments in attention and perceptual selection aimed at reducing the influence of subsequent conflict (see Berlyne, 1960; Botvinick et al., 2001), are associated with modulation of subsequent mPFC reactivity. The current results showed mixed evidence in support of this idea, in the form of a negative association between FSW elicited on a given trial and N2 amplitude elicited on the subsequent trial. To our knowledge, this study is the first in either the ERP or fMRI literatures to demonstrate the posited inverse association between a neural reflection of regulative control elicited on a given trial and a subsequent conflict-monitoring response. The strength of this relationship did not change over time, suggesting that the down-regulation of conflict reactivity following a regulatory control increase does not become less efficient or decoupled over time, even though the absolute magnitude of regulatory control responses might decrease. Importantly, however, this predicted association was evident for only some types of sequences (cI and iC trials) and not for others (iI and cC trials). The strongest form of conflict-monitoring theory would predict that, other factors being equal, the experience of conflict on a given trial would produce a large reactive control response (both conflict-monitoring [N2] and regulative [FSW]) on that trial, followed by a reduced conflict-monitoring response (i.e., N2 amplitude) to conflict experienced on the next trial. The lack of a significant association for iI trials in our data could reflect that regulative control is imperfect and does not consistently down-regulate the conflict-monitoring process when two high-conflict stimuli are encountered in a row. Additional research is needed to further test this idea and determine the extent to which the observed association on iC and cI trials in the current data is related to conflict adaptation or some other process.
An additional piece of evidence suggesting that the FSW→N2 association across trials was related in some way to conflict adaptation—and is not merely an artifact of a general similarity in the EEG across trials within the same brain—comes from the analysis showing that FSW amplitude on a given trial was unrelated to the amplitude of the N1 on the next trial. If any observed association across trials was due to psychological processes unrelated to conflict-related processing (e.g., visual attention) or even nonpsychological processes (e.g., volume conduction or signal drift), we would expect the FSW to correlate with an earlier-latency component on subsequent trials. This did not occur, providing some evidence of discriminant validity for the predicted association between regulative and evaluative reactive control across trials.
Despite the existence of this predicted relationship, the current findings also provide evidence of the dissociation of the conflict-monitoring and regulative components of reactive control. Consideration of the patterns in the trial-level data of responses to incompatible trials is instructive here. The magnitude of responses on incompatible trials following compatible trials (i.e., cI trials) can be said to represent conflict reactivity, whereas responses on incompatible trials following incompatible trials (i.e., iI trials) represent adaptation to conflict. Although the conflict reactivity (cI) response in the N2 was robust and remained stable over the course of the task, the conflict reactivity response in the FSW showed a rather dramatic reduction over time. This dissociation suggests the possibility that regulative control reactivity to conflict is a resource-intensive process subject to fatigue. Similarly, the conflict adaptation response in the FSW also decreased over the course of the task but conflict adaptation in the N2 actually increased over time.
These differing patterns further suggest that although both mPFC (N2) and lateral PFC (FSW) are sensitive to conflict and show a decrease in the conflict adaptation effect (Previous trial × Current trial interaction) over time, they might do so for different reasons mainly related to responses on iI trials. Whereas the N2 on cI trials remained stable, the N2 on iI trials increased. In contrast, the dominant change in the regulative control response was that FSW amplitude on both cI and iI trials decreased, but the magnitude of this reduction was much larger for cI trials. Considered together, these patterns suggest a kind of compensatory process whereby control engagement shifts toward a greater reliance on the evaluative/conflict-monitoring component while the influence of the regulative component wanes over time. The DMC (Braver, 2012) similarly posits compensatory changes between reactive and proactive forms of control, particularly as the more resource-intensive proactive form becomes taxed. The current data suggest this same logic might apply to differing forms of reactive control, and that the regulative process might be more susceptible to depletion than is conflict monitoring.
Such compensation across these two modes of control could explain why, despite the apparent “disappearance” of conflict adaptation in both FSW and N2 as the task progressed, a behavioral conflict adaptation effect remained intact throughout the task. This kind of compensatory hypothesis was suggested by Boksem et al. (2006), who also observed a large reduction in the flanker compatibility effect in the N2 over time—and attributed this effect to fatigue (also see Beste, Kneiphof, & Woitalla, 2015)—but no reduction in the size of the effect in RT. As posited by Boksem and colleagues, “fatigue is more than an effort/reward imbalance and involves adaptive strategies to keep performance at an acceptable level under adverse internal circumstances” (p. 129). In the case of the current study, the “adverse internal circumstances” would appear to have been the sharp decline in the regulative control response over time, particularly on cI trials, and the adaptive strategy may have involved stronger reliance on conflict monitoring. In their study, Boksem et al. observed an overall RT slowing, which they attributed to participants taking longer to resolve conflict on incompatible trials as the conflict-monitoring response in N2 became exhausted. In contrast to those results, here we observed an overall decrease in RT across the task. It seems plausible that this could have resulted from the fact that the N2 response to conflict did not decrease and, instead, remained stable (cI trials) or increased (iI trials) over time.
Interpretation of the current findings should be considered in light of the study’s limitations. First, the sample was relatively modest in size compared to some in the conflict adaptation literature (e.g., Clawson, Clayson, Keith, Catron, & Larson, 2017; Clayson & Larson, 2011, 2012); the specific relationships among reactive control components investigated here should be tested in a larger sample. Nevertheless, the task involved a relatively large number of experimental trials (800). This is an important asset of the study given the mixed-model, trial-level approach to analyzing the data, which allowed for an internally reliable estimate of within-person effects. Given the focus of the current study on cognitive control dynamics, this aspect of the design is more important than between-person variability. Additionally, the participants were taking part in a study on the effects of alcohol and caffeine on cognition. Although none of the participants included in the present analyses actually ingested either substance, some of them (i.e., placebo group) were led to believe that they had, which could have influenced their performance motivation (e.g., Marczinski & Fillmore, 2005). However, statistical comparison of performance across the placebo and control groups showed no significant differences.
Second, the change in congruency sequence effects examined in this study has been framed primarily in terms of conflict adaptation. This account competes with alternative explanations grounded in learning and memory processes (for reviews see Schmidt, 2013; Weissman, Jiang, & Egner, 2014). In some previous reports, excluding sequences with stimulus or response repetitions has eliminated congruency sequence effects (Akçay & Hazeltine, 2007, 2011; Clayson & Larson, 2011; Funes, Lupiáñez, & Humphreys, 2010; Egner & Hirsch, 2005; Kunde & Wuhr, 2006; Notebaert & Verguts, 2007). Our own test of this hypothesis (see Supplementary Material) provided mixed support for both conflict adaptation and memory-based accounts. Full consideration of the implications of these varying accounts of congruency sequence effects and how they might account for changes across the course of the experiment is beyond the scope of this report.
Finally, although the estimation of change over time in cognitive control processes afforded by the trial-level MLM approach is a considerable strength of the study, effects associated with trial (i.e., time-on-task) should be interpreted with caution. We have suggested that changes over the course of the experiment might be due to fatigue, or the taxing of a cognitive resource. However, given the large number of trials used in the study, it is possible that practice resulted in alterations in the psychological mechanisms enlisted to perform the task and/or enabled the automatization of the conflict resolution process, thereby reducing the resource demands of conflict adaptation. It is therefore possible that the observed improvement in RT on incompatible trials is unrelated to the amplified conflict monitoring-related activity seen across the task as indexed by the bolstered N2. It is also possible that the diminished FSW seen as a function time-on-task is the result of a more efficient process being employed to perform the task, which countermands the need for increased control recruitment. Moreover, mental fatigue itself is a multi-faceted concept (Ackerman, 1990; Francis & Inzlicht, 2016; Kool & Botvinik, 2014; Kurzban, Duckworth, Kable, & Myers, 2013) with several disparate explanations. The current data are agnostic with respect to the processes that underlie behavioral and neural indices of cognitive fatigue, which range from resource depletion (Baumeister, Vohs, & Tice, 2007), emotion and motivation shifting (Francis & Inzlicht, 2016; van der Linden, 2011), and decision-theoretic choices (Kool & Botvinik, 2014). Furthermore, both time-on-task and the number of trials performed during a task can independently contribute to fatigue (Grinband et al., 2011). The design of the present study does not allow for the separation of these two potential sources of change.
In summary, the findings contribute to the understanding of reactive control processes theorized to underlie conflict adaptation effects. Both evaluative/conflict-monitoring (N2) and regulative control (FSW) demonstrated changes in conflict adaptation as the task progressed. Moreover, differential change in N2 and FSW over time suggest that conflict monitoring and regulative control were dissociable. Yet, the critical reciprocal relation between the two, whereby adaptation-related increases in regulative control on a given trial predict subsequent decreases in evaluative, conflict-monitoring processes was maintained throughout the task. Future research could explore how the distinction between reactive control dynamics as a function of adjacent trial sequences interfaces with the distinction between transient, stimulus-driven control (reactive control) versus sustained, internally-driven control (proactive control) (see Braver, 2012).
Supplementary Material
Acknowledgments
This research was supported by grant P60 AA011998 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Preparation of this article was supported by NIAAA grants R01 AA020970 and T32 AA013526, and by a University of Missouri Life Sciences Graduate Fellowship.
Footnotes
See the Supplementary Materials for brief discussion of alternative explanations of this effect, known more generally as the congruency sequence effect, along with analyses meant to address some of those alternative explanations.
Note that the conflict-related slow potential (i.e., conflict SP), frequently observed in ERP studies of the Stroop task (see Larson et al., 2009), also has been posited to reflect conflict adjustment or resolution (i.e., regulative control; see Larson et al., 2014). However, the conflict SP typically is not observed in flanker tasks. Future research should endeavor to determine the extent to which the FSW and conflict SP are related.
Eligibility criteria are detailed in Bailey et al. (2016).
Some previous research has indicated that the conflict adaptation effect (or congruency sequence effect) is eliminated when trial sequences with repeated features are eliminated (see Mayr et al., 2003). Thus, we examined the effects of both target repetitions (e.g. ≪>≪ followed by ≫≫>) and flanker repetitions (e.g., ≫≫ followed by ≫<≫) in our data; these analyses are presented in the Supplementary Materials. Summarizing, there was no evidence supporting the response priming view of the congruency sequence effect, and mixed support for both conflict-adaptation and feature integration accounts.
The authors declare no conflicts of interest related to the work described in this article.
References
- Ackerman D. A natural history of the senses. New York: Vintage Books; 1990. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. New York: Sage; 1991. doi.org/10.1037/10520-147. [Google Scholar]
- Alexander WH, Brown JW. Computational models of performance monitoring and cognitive control. Topics in cognitive science. 2010;2(4):658–677. doi: 10.1111/j.1756-8765.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K, Amlung M, Morris DH, Price MH, Von Gunten CD, McCarthy DM, Bartholow BD. Separate and joint effects of alcohol and caffeine on conflict monitoring and adaptation. Psychopharmacology. 2016;233:1245–1255. doi: 10.1007/s00213-016-4208-y. doi.org/10.1007/s0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K, Bartholow BD, Saults JS, Lust SA. Give me just a little more time: effects of alcohol on the failure and recovery of cognitive control. Journal of Abnormal Psychology. 2014;123(1):152. doi: 10.1037/a0035662. doi.org/10.1037/a0035662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K, West R, Anderson CA. A negative association between video game experience and proactive cognitive control. Psychophysiology. 2010;47(1):34–42. doi: 10.1111/j.1469-8986.2009.00925.x. doi.org/10.1111/j.1469-8986.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Dickter CL, Sestir MA. Stereotype activation and control of race bias: cognitive control of inhibition and its impairment by alcohol. Journal of Personality and Social Psychology. 2006;90(2):272–287. doi: 10.1037/0022-3514.90.2.272. doi.org/10.1037/0022-3514.90.2.272. [DOI] [PubMed] [Google Scholar]
- Barwick F, Arnett P, Slobounov S. EEG correlates of fatigue during administration of a neuropsychological test battery. Clinical Neurophysiology. 2012;123(2):278–284. doi: 10.1016/j.clinph.2011.06.027. doi.org/10.1016/j.clinph.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H. lme4: Linear mixed-effects models using Eigen and S4, 2014. R package version 1-1 2015 [Google Scholar]
- Baumeister RF, Vohs KD, Tice DM. The strength model of self-control. Current Directions in Psychological Science. 2007;16(6):351–355. doi.org/10.1111/j.1467-8721.2007.00534.x. [Google Scholar]
- Berlyne DE. Conflict, arousal and curiosity. New York: McGraw-Hill; 1960. doi.org/10.1037/11164-000. [Google Scholar]
- Beste C, Kneiphof J, Woitalla D. Effects of fatigue on cognitive control in neurosarcoidosis. European Neuropsychopharmacology. 2015;25(4):522–530. doi: 10.1016/j.euroneuro.2015.01.012. doi.org/10.1016/j.euroneuro.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biological psychology. 2006;72(2):123–132. doi: 10.1016/j.biopsycho.2005.08.007. doi.org/10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Bolger N, Laurenceau J-P. Intensive longitudinal methods: An introduction to diary and experience sampling research. New York: Guilford; 2013. [Google Scholar]
- Botvinick MM, Cohen JD. The computational and neural basis of cognitive control: charted territory and new frontiers. Cognitive Science. 2014;38(6):1249–1285. doi: 10.1111/cogs.12126. doi.org/10.1111/cogs.12126. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological review. 2001;108(3):624. doi: 10.1037/0033-295x.108.3.624. doi.org/10.1037/0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. doi.org/10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. doi.org/10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Krug MK, Chiew KS, Kool W, Westbrook JA. Mechanisms of motivation–cognition interaction: challenges and opportunities. Cognitive, Affective, & Behavioral Neuroscience. 2014;14(2):443–472. doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. doi.org/10.3758/CABN.7.4.367. [DOI] [PubMed] [Google Scholar]
- Clawson A, Clayson PE, Keith CM, Catron C, Larson MJ. Conflict and performance monitoring throughout the lifespan: An event-related potential (ERP) and temporospatial component analysis. Biological Psychology. 2017;124:87–99. doi: 10.1016/j.biopsycho.2017.01.012. doi.org/10.1016/j.biopsycho.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Clayson PE, Larson MJ. Conflict adaptation and sequential trial effects: Support for the conflict monitoring theory. Neuropsychologia. 2011;49:1953–1961. doi: 10.1016/j.neuropsychologia.2011.03.023. doi.org/10.1016/j.neuropsychologia.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Clayson PE, Larson MJ. Effects of repetition priming on electrophysiological and behavioral indices of conflict adaptation and cognitive control. Psychophysiology. 2011;48(12):1621–1630. doi: 10.1111/j.1469-8986.2011.01265.x. doi.org/10.1111/j.1469-8986.2011.01265.x. [DOI] [PubMed] [Google Scholar]
- Clayson PE, Larson MJ. Cognitive performance and electrophysiological indices of cognitive control: A validation study of conflict adaptation. Psychophysiology. 2012;49(5):627–637. doi: 10.1111/j.1469-8986.2011.01345.x. doi.org/10.1111/j.1469-8986.2011.01345.x. [DOI] [PubMed] [Google Scholar]
- Coles MG, Gratton G, Donchin E. Detecting early communication: Using measures of movement-related potentials to illuminate human information processing. Biological psychology. 1988;26(1):69–89. doi: 10.1016/0301-0511(88)90014-2. doi.org/10.1016/0301-0511(88)90014-2. [DOI] [PubMed] [Google Scholar]
- Coles MG, Gratton G, Bashore TR, Eriksen CW, Donchin E. A psychophysiological investigation of the continuous flow model of human information processing. Journal of Experimental Psychology: Human Perception and Performance. 1985;11(5):529–553. doi: 10.1037//0096-1523.11.5.529. doi.org/10.1037/0096-1523.11.5.529. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annual review of psychology. 2011;62:583–619. doi: 10.1146/annurev.psych.093008.100356. doi.org/10.1146/annurev.psych.093008.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: implications for regulation of behavior during response conflict. Journal of abnormal psychology. 2003;112(3):424–436. doi: 10.1037/0021-843x.112.3.424. doi.org/10.1037/0021-843X.112.3.424. [DOI] [PubMed] [Google Scholar]
- De Pisapia N, Braver TS. A model of dual control mechanisms through anterior cingulate and prefrontal cortex interactions. Neurocomputing. 2006;69(10):1322–1326. doi.org/10.1016/j.neucom.2005.12.100. [Google Scholar]
- Desender K, Van Opstal F, Van den Bussche E. Feeling the conflict: The crucial role of conflict experience in adaptation. Psychological Science. 2014;25(3):675–683. doi: 10.1177/0956797613511468. doi.org/10.1177/0956797613511468. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Fischer R. Conflicts as aversive signals for control adaptation. Current Directions in Psychological Science. 2015;24(4):255–260. doi.org/10.1177/0963721415569569. [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage. 2003;20:2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. doi.org/10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychological methods. 2007;12(2):121–138. doi: 10.1037/1082-989X.12.2.121. doi.org/10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Attention, Perception, & Psychophysics. 1974;16(1):143–149. doi.org/10.3758/BF03203267. [Google Scholar]
- Fischer R, Dreisbach G, Goschke T. Context-sensitive adjustments of cognitive control: conflict-adaptation effects are modulated by processing demands of the ongoing task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(3):712–718. doi: 10.1037/0278-7393.34.3.712. doi.org/10.1037/0278-7393.34.3.712. [DOI] [PubMed] [Google Scholar]
- Fleeson W. Studying personality processes: Explaining change in between-persons longitudinal and within-person multilevel models. In: Robins RW, Fraley RC, Krueger RF, editors. Handbook of research methods in personality psychology. New York, NY, US: Guilford Press; 2007. pp. 523–542. [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. doi.org/10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster SE, Carter CS, Cohen JD, Cho RY. Parametric manipulation of the conflict signal and control-state adaptation. Journal of Cognitive Neuroscience. 2010;23(4):923–935. doi: 10.1162/jocn.2010.21458. doi.org/10.1162/jocn.2010.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis Z, Inzlicht M. Proximate and ultimate causes of ego depletion. In: Hirt E, editor. Self-Regulation and Ego Control. New York: Elsevier; 2016. pp. 373–398. doi.org/10.1016/B978-0-12-801850-7.00018-4. [Google Scholar]
- Fritz J, Dreisbach G. Conflicts as aversive signals: Conflict priming increases negative judgments for neutral stimuli. Cognitive, Affective, & Behavioral Neuroscience. 2013;13(2):311–317. doi: 10.3758/s13415-012-0147-1. doi.org/10.3758/s13415-012-0147-1. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121(4):480. doi: 10.1037//0096-3445.121.4.480. doi.org/10.1037/0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Sirevaag EJ, Eriksen CW, Donchin E. Pre-and poststimulus activation of response channels: a psychophysiological analysis. Journal of Experimental Psychology: Human perception and performance. 1988;14(3):331–344. doi: 10.1037//0096-1523.14.3.331. doi.org/10.1037/0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011;57(2):303–311. doi: 10.1016/j.neuroimage.2010.12.027. doi.org/10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NL. Ego depletion and the strength model of self-control: a meta-analysis. Psychological Bulletin. 2010;136:495–525. doi: 10.1037/a0019486. doi.org/10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hommel B, Proctor RW, Vu KPL. A feature-integration account of sequential effects in the Simon task. Psychological Research. 2004;68:1–17. doi: 10.1007/s00426-003-0132-y. doi.org/10.1007/s00426-003-0132-y. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsh JB. Emotional foundations of cognitive control. Trends in Cognitive Sciences. 2015;19(3):126–132. doi: 10.1016/j.tics.2015.01.004. doi.org/10.1016/j.tics.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Endo H, Kizuka T. Mental fatigue and impaired response processes: event-related brain potentials in a Go/NoGo task. International Journal of Psychophysiology. 2009;72(2):204–211. doi: 10.1016/j.ijpsycho.2008.12.008. doi.org/10.1016/j.ijpsycho.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006;33(1):399–405. doi: 10.1016/j.neuroimage.2006.06.012. doi.org/10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. doi.org/10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kool W, Botvinick M. A labor/leisure tradeoff in cognitive control. Journal of Experimental Psychology: General. 2014;143(1):131–141. doi: 10.1037/a0031048. doi.org/10.1037/a0031048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool W, McGuire JT, Rosen ZB, Botvinick MM. Decision making and the avoidance of cognitive demand. Journal of Experimental Psychology: General. 2010;139(4):665–682. doi: 10.1037/a0020198. doi.org/10.1037/a0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nature neuroscience. 2009;12(7):939–945. doi: 10.1038/nn.2321. doi.org/10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures research designs in psychophysiology: An introduction to growth curve modeling. Psychophysiology. 2007;44(5):728–736. doi: 10.1111/j.1469-8986.2007.00544.x. doi.org/10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Kurzban R, Duckworth A, Kable JW, Myers J. An opportunity cost model of subjective effort and task performance. Behavioral and Brain Sciences. 2013;36(06):661–679. doi: 10.1017/S0140525X12003196. doi.org/10.1017/S0140525X12003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, Clayson PE, Clawson A. Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. International Journal of Psychophysiology. 2014;93(3):283–297. doi: 10.1016/j.ijpsycho.2014.06.007. doi.org/10.1016/j.ijpsycho.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kaufman DA, Perlstein WM. Neural time course of conflict adaptation effects on the Stroop task. Neuropsychologia. 2009;47(3):663–670. doi: 10.1016/j.neuropsychologia.2008.11.013. doi.org/10.1016/j.neuropsychologia.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Jolij J. Trial history effects in stroop task performance are independent of top-down control. PloS one. 2012;7(6):e39802. doi: 10.1371/journal.pone.0039802. doi.org/10.1371/journal.pone.0039802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorist MM, Boksem MA, Ridderinkhof KR. Impaired cognitive control and reduced cingulate activity during mental fatigue. Cognitive Brain Research. 2005;24(2):199–205. doi: 10.1016/j.cogbrainres.2005.01.018. doi.org/10.1016/j.cogbrainres.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Maas CJ, Hox JJ. Sufficient sample sizes for multilevel modeling. Methodology. 2005;1(3):86–92. doi.org/10.1027/1614-2241.1.3.86. [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. doi.org/10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Compensating for alcohol-induced impairment of control: effects on inhibition and activation of behavior. Psychopharmacology. 2005;181(2):337–346. doi: 10.1007/s00213-005-2269-4. doi.org/10.1007/s00213-005-2269-4. [DOI] [PubMed] [Google Scholar]
- Marklund P, Persson J. Context-dependent switching between proactive and reactive working memory control mechanisms in the right inferior frontal gyrus. Neuroimage. 2012;63(3):1552–1560. doi: 10.1016/j.neuroimage.2012.08.016. doi.org/10.1016/j.neuroimage.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Matuschek H, Kliegl R, Vasishth S, Baayen H, Bates D. Balancing Type I error and power in linear mixed models. Journal of Memory and Language. 2017;94:305–315. doi.org/10.1016/j.jml.2017.01.001. [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nature Neuroscience. 2003;6:450–452. doi: 10.1038/nn1051. doi.org/10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part 2. Accounts of psychological refractory-period phenomena. Psychological Review. 1997;104(4):749–791. doi: 10.1037/0033-295x.104.1.3. doi.org/10.1037/0033-295X.104.4.749. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. doi.org/10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Newman LA, Creer DJ, McGaughy JA. Cognitive control and the anterior cingulate cortex: how conflicting stimuli affect attentional control in the rat. Journal of Physiology-Paris. 2015;109:95–103. doi: 10.1016/j.jphysparis.2014.06.004. doi.org/10.1016/j.jphysparis.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Stins JF, Posthuma D, Polderman TJ, Boomsma DI, de Geus EJ. Accounting for sequential trial effects in the flanker task: Conflict adaptation or associative priming? Memory & Cognition. 2006;34(6):1260–1272. doi: 10.3758/bf03193270. doi.org/10.3758/BF03193270. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR. Micro-and macro-adjustments of task set: activation and suppression in conflict tasks. Psychological Research. 2002;66(4):312–323. doi: 10.1007/s00426-002-0104-7. doi.org/10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Schmeichel BJ, Inzlicht M. A cognitive control perspective of self-control strength and its depletion. Social and Personality Psychology Compass. 2010;4(3):189–200. doi.org/10.1111/j.1751-9004.2009.00244.x. [Google Scholar]
- Schmidt JR. Questioning conflict adaptation: proportion congruent and Gratton effects reconsidered. Psychonomic Bulletin & Review. 2013;20(4):615–630. doi: 10.3758/s13423-012-0373-0. doi.org/10.3758/s13423-012-0373-0. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience. 2016;19(10):1286–1291. doi: 10.1038/nn.4384. doi.org/10.1038/nn.4384. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, … Eskandar EN. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488(7410):218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen HV, Band GP, Hommel B. Reward counteracts conflict adaptation: Evidence for a role of affect in executive control. Psychological Science. 2009;20(12):1473–1477. doi: 10.1111/j.1467-9280.2009.02470.x. doi.org/10.1111/j.1467-9280.2009.02470.x. [DOI] [PubMed] [Google Scholar]
- Tibon R, Levy DA. Striking a balance: analyzing unbalanced event-related potential data. Frontiers in psychology. 2015;6 doi: 10.3389/fpsyg.2015.00555. doi.org/10.3389/fpsyg.2015.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay A, Newman AJ. Modeling nonlinear relationships in ERP data using mixed-effects regression with R examples. Psychophysiology. 2015;52(1):124–139. doi: 10.1111/psyp.12299. doi.org/10.1111/psyp.12299. [DOI] [PubMed] [Google Scholar]
- van der Linden D. The urge to stop: The cognitive and biological nature of acute mental fatigue. Cognitive fatigue: Multidisciplinary perspectives on current research and future applications. 2011:149–64. doi.org/10.1037/12343-007.
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology and Behavior. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Verguts T, Notebaert W. Hebbian learning of cognitive control: dealing with specific and nonspecific adaptation. Psychological Review. 2008;115(2):518–525. doi: 10.1037/0033-295X.115.2.518. doi.org/10.1037/0033-295X.115.2.518. [DOI] [PubMed] [Google Scholar]
- Verguts T, Notebaert W. Adaptation by binding: a learning account of cognitive control. Trends in Cognitive Sciences. 2009;13(6):252–257. doi: 10.1016/j.tics.2009.02.007. doi.org/10.1016/j.tics.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Vossen H, Van Breukelen G, Hermens H, Van Os J, Lousberg R. More potential in statistical analyses of event-related potentials: a mixed regression approach. International journal of methods in psychiatric research. 2011;20(3):e56–e68. doi: 10.1002/mpr.348. doi.org/10.1002/mpr.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warm JS, Matthews G, Finomore VS., Jr Vigilance, workload, and stress. Performance under stress. 2008:115–41. [Google Scholar]
- Weissman DH, Jiang J, Egner T. Determinants of congruency sequence effects without learning and memory confounds. Journal of Experimental Psychology: Human Perception and Performance. 2014;40(5):2022–2037. doi: 10.1037/a0037454. doi.org/10.1037/a0037454. [DOI] [PubMed] [Google Scholar]
- West R, Alain C. Event-related neural activity associated with the Stroop task. Cognitive Brain Research. 1999;8(2):157–164. doi: 10.1016/s0926-6410(99)00017-8. doi.org/10.1016/S0926-6410(99)00017-8. [DOI] [PubMed] [Google Scholar]
- West R, Alain C. Age-related decline in inhibitory control contributes to the increased Stroop effect observed in older adults. Psychophysiology. 2000;37(2):179–189. doi.org/10.1111/1469-8986.3720179. [PubMed] [Google Scholar]
- West R, Bailey K. ERP correlates of dual mechanisms of control in the counting Stroop task. Psychophysiology. 2012;49(10):1309–1318. doi: 10.1111/j.1469-8986.2012.01464.x. doi.org/10.1111/j.1469-8986.2012.01464.x. [DOI] [PubMed] [Google Scholar]
- West R, Bailey K, Tiernan BN, Boonsuk W, Gilbert S. The temporal dynamics of medial and lateral frontal neural activity related to proactive cognitive control. Neuropsychologia. 2012;50(14):3450–3460. doi: 10.1016/j.neuropsychologia.2012.10.011. doi.org/10.1016/j.neuropsychologia.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Woestenburg JC, Verbaten MN, Van Hees HH, Slangen JL. Single trial ERP estimation in the frequency domain using orthogonal polynomial trend analysis (OPTA): Estimation of individual habituation. Biological Psychology. 1983;17(2):173–191. doi: 10.1016/0301-0511(83)90018-2. doi.org/10.1016/0301-0511(83)90018-2. [DOI] [PubMed] [Google Scholar]
- Yeung N. Conflict monitoring and cognitive control. In: Ochsner KN, Kosslyn S, editors. The Oxford Handbook of Cognitive Neuroscience: Volume 2: The Cutting Edges. New York: Oxford University Press; 2013. pp. 275–299. doi.org/10.1093/oxfordhb/9780199988709.013.0018. [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological review. 2004;111(4):931–959. doi: 10.1037/0033-295x.111.4.939. doi.org/10.1037/0033-295X.111.4.931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.