Abstract
Background
Suicide is a heterogeneous phenomenon, and thus defining more homogeneous subgroups may help in understanding its underlying biology and ultimately in its prevention. Suicidal ideation is far more common than suicidal behavior and predicts future suicide attempts. Hypothalamic–pituitary–adrenal (HPA)-axis reactivity has been implicated in individuals with suicidal ideation but findings are mixed with some studies showing increased and others demonstrating decreased reactivity. This suggests that dysregulation of HPA-axis is related to a specific character of suicidal ideation. We hypothesized that individuals with brief suicidal ideation are more stress responsive than those with longer/continuous ideation.
Methods
Thirty-five individuals with major depressive disorder (MDD) and 23 healthy volunteers (HVs), aged 18–65 years, underwent the Trier Social Stress Test (TSST). Salivary cortisol was measured at 6 time-points before and during TSST. Total severity and duration of current suicidal ideation were assessed using the Beck Scale for Suicidal Ideation (SSI). Brief suicidal ideators (N=18), longer/continuous ideators (N=17) and HVs were compared regarding cortisol response, baseline cortisol and total output.
Results
Participants with brief suicidal ideation had greater cortisol response compared to those with longer/continuous ideation and HVs, even after controlling for relevant covariates. However, total SSI score was not associated with cortisol response. Baseline cortisol and total output were not related to overall severity or duration of suicidal ideation.
Limitations
The cross-sectional design and modest sample limit generalizability of the results.
Conclusions
Hyper-responsiveness of HPA-axis to social stress is associated with brief suicidal ideation, possibly defining a pathway for exploring the biological subtyping of suicidal individuals.
Keywords: Trier Social Stress Test, hypothalamic–pituitary–adrenal axis, cortisol, suicidal ideation, major depressive disorder
1. INTRODUCTION
Suicide is a worldwide problem resulting in the death of over 800,000 people every year (WHO, 2016). Suicidal behavior is a heterogeneous phenomenon with complex underlying biology and risk factors (Chaudhury et al., 2016; Mann and Currier, 2007; van Heeringen and Mann, 2014). Defining more homogeneous subgroups may improve the understanding of its underlying biology and ultimately aid in prediction and prevention of future suicide.
Suicidal ideation is the first step on a path toward dying by suicide (Nock et al., 2008). Despite the importance of suicidal ideation in predicting future suicide attempts (Fawcett et al., 1987; Kessler et al., 1999; Oquendo et al., 2004), little empirical research has been conducted on its dimensions/patterns. Suicidal thoughts can range from transient thoughts that life is not worth living (Nock and Banaji, 2007) to persistent rumination about death (Oquendo et al., 2003), or even manifest as an intense delusional preoccupation with self-destruction (Goldney et al., 1989). Importantly, some data suggest that individuals with brief, fleeting suicidal ideation have a comparable risk for future suicide attempts to those with persistent ideation (Wilcox et al., 2010). Community (Reinherz et al., 2006) as well as clinical (Pfeffer et al., 1993) cohort studies found that adolescents with suicidal thoughts, even if mild or of an apparently low severity, are more likely to have serious suicidal behavior in adulthood. Witte et al. (2005) found that fluctuation of suicidal ideation over time is a potent predictor for previous suicide attempts. Hall et al. (1999) reported that over two thirds of individuals who made serious suicide attempts requiring hospitalization had only brief suicidal ideation with no specific plan prior to their attempt. The biological correlates of these brief, fluctuating thoughts of killing oneself are largely unknown.
Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis has been suggested to increase the risk for suicidal behavior (Mann, 2003; Turecki, 2005; van Heeringen and Mann, 2014). Although some studies report that HPA axis hyperactivity, as assessed by non-suppression on the dexamethasone suppression test (DST), predicts completed suicide (Coryell and Schlesser, 2001; Mann et al., 2006; Yerevanian et al., 2004), and to a lesser extent, future suicide attempts (Jokinen et al., 2008; Jokinen and Nordstrom, 2009), others do not (Black et al., 2002; Fountoulakis et al., 2004; Maes et al., 1989; Pitchot et al., 2008). A few DST studies have examined the association of HPA axis activity with suicidal ideation, and, again, their results are mixed. Both HPA axis hyper-responsivity (López-Ibor et al., 1985) as well as hypo-responsivity (Pfennig et al., 2005) have been found in relation to suicidal thoughts. In an effort to understand these mixed results, Lindqvist et al. (2008) examined the relationship between suicide intent and HPA axis reactivity using the DST and reported that subjects with low suicide intent had greater cortisol response.
As a pharmacologic manipulation, the DST may not accurately depict functioning of the HPA axis in response to social and environmental stressors (McGirr et al., 2011), such as those produced by staged-situation laboratory stressors (e.g., (Giletta et al., 2015; Keilp et al., 2016; McGirr et al., 2010; Melhem et al., 2016; Wilson et al., 2016), like the Trier Social Stress Test (TSST) (Kirschbaum et al., 1993). Some TSST studies have found lower pre-task baseline cortisol (Keilp et al., 2016; Melhem et al., 2016) and blunted total cortisol output (Melhem et al., 2016; O’Connor et al., 2017a) in suicide attempters, but no differences in cortisol response to stress. O’Connor et al. (2017a), in turn, found that lower cortisol reactivity predicted elevated suicidal thoughts at 1-month follow up in suicide attempters, but not in ideators who did not attempt suicide. However, Giletta et al. (2015) reported that both hyper-responsivity as well as hypo-responsivity (at a trend level) were associated with suicidal ideation. A next logical step is to examine the relationship between cortisol response to social stress and the character of suicidal ideation, particularly its duration.
In the current study, we examined the relationship between HPA axis reactivity using the TSST and current suicidal ideation in individuals with major depressive disorder (MDD). Stress-responsive individuals likely have increased emotional reactivity and are proposed to develop sudden, transient suicidal thoughts following stressful life events (Bernanke et al., 2017). Thus, we hypothesized that those with brief, fleeting suicidal ideation would show greater HPA axis reactivity, as indicated by elevated levels of cortisol response rather than baseline or total cortisol output (Pruessner et al., 2003), compared to those with longer or continuous periods of ideation.
2. METHODS
2.1. Participants
Participants were recruited through the Molecular Imaging and Neuropathology Division (MIND) Clinic at Columbia University (New York, NY, SA). This study was approved by the New York State Psychiatric Institute Institutional Review Board and all participants gave their written informed consent. Thirty-five participants who met the DSM-IV (American Psychiatric Association, 1994) criteria for MDD and have current suicidal ideation, and 23 healthy volunteers (HVs) were included. Depressed participants were classified as brief suicidal ideators (N = 18) or longer/continuous ideators (N = 17), using the time dimension item on the Beck Scale for Suicidal Ideation (SSI) (Beck et al., 1979) (see below for details). Inclusion criteria for depressed participants were assessed through clinical interview and included: 1) 18–65 years of age; 2) DSM-IV diagnosis of MDD; and 4) capacity to provide informed consent. Exclusion criteria included: 1) unstable medical condition; 2) current alcohol or substance use disorder (past diagnosis allowed if in remission for ≥6 months); 3) bipolar disorder or schizophrenia (comorbid anxiety disorders were not excluded); 5) marked cognitive impairment that would interfere with participation. Criteria for HVs were similar except for the required absence of psychiatric history (specific phobia was permitted) or family history of a mood or psychotic disorder or suicidal behavior in a first-degree relative.
2.2. Clinical assessment
Lifetime Axis I DSM-IV diagnoses were assessed using the Structured Clinical Interview for DSM-IV (SCID I) (First et al., 1995). Self- and clinician rated current severity of depressive symptoms was evaluated with the Beck Depression Inventory (BDI) (Beck et al., 1961) and the Hamilton Depression Rating Scale-17 item (HDRS) (Hamilton, 1960), respectively. Hopelessness was assessed with the Beck Hopelessness Scale (Beck et al., 1974).
Suicide history was assessed with the Columbia Suicide History Form (Oquendo et al., 2003). Suicidal ideation was evaluated with the Beck Scale for Suicidal Ideation (Beck et al., 1979), a 19-item scale that assesses thoughts, feelings, and plans regarding suicide. Item 6 on this scale assesses the time dimension of suicidal ideation. The duration of suicidal ideation on this item is classified into 3 categories: 1) brief, fleeting periods, 2) longer periods, and 3) continuous (chronic) or almost continuous. Longer and continuous ideators were then added together into a single group.
2.3. Trier Social Stress Test (TSST)
The TSST is a well-established procedure used to study psychological and physiological indices of stress response (Kirschbaum et al., 1993). The procedure involves administering a 5-minute personal introduction speech followed by 5 minutes of a speeded mental arithmetic task, in the presence of a test administrator and two observers who serve as the confederates. The administrator and observers respond neutrally to the participant and provide stern feedback for computation errors. The test was performed at the same time for all participants in order to control for diurnal variation in cortisol levels: mid-afternoon at 2.30 p.m., when cortisol levels are declining to improve the signal to baseline ratio (Kudielka et al., 2004). Saliva samples were obtained at 6 time points: −15, and −5 min before the procedure began, and then at 4 intervals of time from the start of the task: 15, 25, 35, and 45 min. Subjective mood was evaluated using the Profile of Mood States (POMS; (McNair et al., 1992) at −20, 10, and 40 min.
2.4. Salivary Samples Collection and Assay
Saliva was collected via the Sarstedt Salivette Synthetic Swab saliva collection system (Catalogue # 51.1534.500 Sarstedt, Newton, NC 28658, USA). Samples were stored at −30 °C until assayed for cortisol by radioimmunoassay. Primary antibodies raised against cortisol-3-O-carboxymethyloxime-BSA and iodine labeled cortisol were purchased from MP Biomedicals. Cortisol standards were purchased from Sigma Chemical, anti-rabbit globulin serum in conjunction with polyethylene glycol was used for separation of the bound and free fractions. All samples and standards were analyzed in duplicate. The intra and inter-assay coefficient of variation was 4.7% at 1 ng/ml and 7.4% at 0.25 ng/ml, respectively.
2.5. Statistical analysis
All analyses were performed in IBM SPSS Statistics (SPSS Inc., Chicago, Illinois, USA, Version 23.0). In preliminary analyses, all quantitative variables, by diagnostic group, were checked for outliers. Clinical and demographic variables were compared among the study groups using one-way analysis of variance (ANOVA) (and brief ideators and longer/continuous ideators groups compared with planned contrasts using two sample two-tailed t-tests) for continuous variables, and chi-square tests of independence and Fisher’s Exact test for categorical variables. Salivary cortisol data were skewed, and were log-transformed. The primary outcome was cortisol response to stress assessed by measuring area under the curve (AUCI) with respect to the participant’s log-transformed baseline cortisol values (Pruessner et al., 2003). The association between cortisol response and total SSI scores was examined using the Spearman’s correlation coefficient. The difference in cortisol response between the three groups was firstly assessed using ANOVA. When outliers were present in the cortisol response (AUCI) (defined as being outside the box-and-whisker plot), analyses were repeated after removing the outlier to check their influence on the result. Post hoc Turkey’s test was then performed to contrast the brief to longer/continuous ideators as well as to HVs. Additionally, demographic and clinical variables were examined for their effects on cortisol response values. These included age, sex, body mass index (BMI), current smoking status, current medication status, history of physical or sexual abuse and past history of substance abuse or dependence. The effect of contraceptives use on cortisol response was specifically examined due to their ability to suppress HPA axis activity (Kirschbaum et al., 1999) by reducing the production of endogenous ovarian steroids that potentiate the HPA axis activity (Roca et al., 2003). A univariate analysis of covariance (ANCOVA) was then performed to compare the cortisol response between the study groups while controlling for variables with a significant association with cortisol response or duration of suicidal ideation. A Chi-square goodness of fit test was performed to compare the quadratic and linear association between the cortisol response (AUCI) and the chronicity of suicidal ideation in two logistic regression models.
We also analyzed the baseline (pre-task) cortisol levels as well as the total cortisol output, computed using AUC with respect to ground (AUCG) (see (Pruessner et al., 2003)) and examined their association with SSI scores and duration of suicidal ideation. One participant with longer/continuous ideation did not have any post-baseline cortisol data and was included only in analyses comparing baseline cortisol.
The number of participants with history of suicide attempts (N = 10) in the current study was too few to examine the association between suicidal behavior and the cortisol values.
3. RESULTS
Participants’ (N = 58) demographic and clinical characteristics are shown in Table 1. Depressed brief ideators (N = 18), depressed longer/continuous ideators (N = 17) and healthy volunteers (N = 23) did not differ in terms of age, sex, race, ethnicity, marital status, employment status, smoking status and years of education. The longer/continuous suicidal ideators were more severely depressed than brief ideators as indicated by HDRS (t(33) = 2.347, p = 0.025), but the two groups showed no statistically significant differences regarding BDI or Beck Hopelessness scale scores, history of physical or sexual abuse and past history of substance abuse or dependence.
Table (1).
Demographic and Clinical Characteristics of Study Sample:
| Variable | Total Sample (N = 58) | Brief Ideators (N = 18) | Longer/Continuous Ideators (N = 17) | Healthy Volunteers (N = 23) | Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Three-group comparisona | Brief vs. Longer/Continuous Ideatorsb | |||||||||
| Demographic Characteristics | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | |
| Age (years) | 33.6 | 10.3 | 35.8 | 12.3 | 31.7 | 9.5 | 33.4 | 9.4 | 0.511 | 0.282 |
| Education (years) | 15.7 | 2.0 | 16.0 | 2.2 | 14.9 | 2.1 | 16.0 | 1.7 | 0.162 | 0.134 |
| Body Mass Index | 28.2 | 9.0 | 27.5 | 6.8 | 28.9 | 11.1 | 27.0 | 6.7 | 0.787 | 0.674 |
| N | % | N | % | N | % | N | % | p | p | |
| Sex; Female | 34 | 58.6 | 11 | 61.1 | 11 | 64.7 | 13 | 56.5 | 0.618 | 0.615 |
| Ethnicity; Hispanic | 16 | 27.6 | 4 | 22.2 | 6 | 35.3 | 6 | 26.1 | 0.673 | 0.471† |
| Race; White | 26 | 44.8 | 8 | 44.4 | 9 | 52.9 | 9 | 39.1 | 0.606 | 0.648 |
| Marital Status; Single | 40 | 70.0 | 12 | 66.7 | 11 | 64.7 | 17 | 73.9 | 0.985 | 0.937 |
| Employed | 33 | 56.9 | 12 | 66.7 | 8 | 47.1 | 13 | 56.5 | 0.375 | 0.163 |
| Smoking | 7 | 12.1 | 2 | 11.1 | 5 | 29.4 | 1 | 4.4 | 0.079† | 0.228† |
| Clinical Characteristics$ | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | p | ||||
| Beck Depression Inventory | 25.2 | 8.8 | 22.5 | 10.0 | 27.8 | 6.7 | 0.076 | |||
| Hamilton Depression Rating Scale | 18.9 | 5.2 | 17.0 | 5.4 | 20.9 | 4.3 | 0.025 | |||
| Beck Hopelessness Scale | 14.3 | 4.3 | 13.1 | 4.6 | 15.5 | 3.6 | 0.098 | |||
| N | % | N | % | N | % | p | ||||
| History of Physical or Sexual Abuse | 18 | 51.4 | 8 | 44.4 | 10 | 58.8 | 0.598 | |||
| History of Substance Abuse/Dependence | 9 | 25.7 | 6 | 33.3 | 3 | 17.6 | 0.856 | |||
| Medication Naïve | 10 | 28.6 | 5 | 27.8 | 5 | 29.4 | 0.824 | |||
One way ANOVA (df = 2,56) for continuous variables; chi-square analysis (df = 2) for categorical variables.
Independent two sample two-tailed t-test (df = 33) for continuous variables; chi-square analysis (df = 1) for categorical variables.
Fisher’s Exact test.
Statistics are calculated for MDD group only (N = 35).
Abbreviations: SD= standard deviation.
We examined the effect of demographic and clinical measures on our primary outcome variable (cortisol response; AUCI). Only smoking status had a significant effect on cortisol response. Smokers had significantly lower cortisol response compared to non-smokers (t(55) = 2.405, p = 0.020).
The current use of medications within the depressed group was not related to cortisol response (t(32) = 0.981, p = 0.334). However, participants were taking a wide variety of medications including antidepressants, benzodiazepines, oral contraceptive pills, antihistamines, anti-hypertensives, analgesics and multivitamin. We did not find statistically significant difference in cortisol response between women who are using contraceptive pills (N = 5) and those who do not (N = 30) (t(33) = 0.779, p = 0.442). No systematic effect could be ascribed to other categories of medications, and participants taking psychotropic medications (N = 4) showed no statistically significant difference in cortisol response compared to those taking other types of medication (N = 20) and unmedicated participants (N = 11) (Mann Whitney U test; p = 0.474).
Cortisol response (AUCI) was not related to age (r = −0.020, p = 0.883), or BMI (r = 0.079, p = 0.563). It was not statistically different between males and females (t(56) = 0.057, p = 0.954), and not affected by history of substance abuse/dependence (t(31) = 1.142, p = 0.264) or history of physical or sexual abuse (t(33) = 1.239, p = 0.225). Accordingly, we adjusted subsequent analyses only for smoking status as well as HDRS score, which showed difference between the brief and longer/continuous ideators.
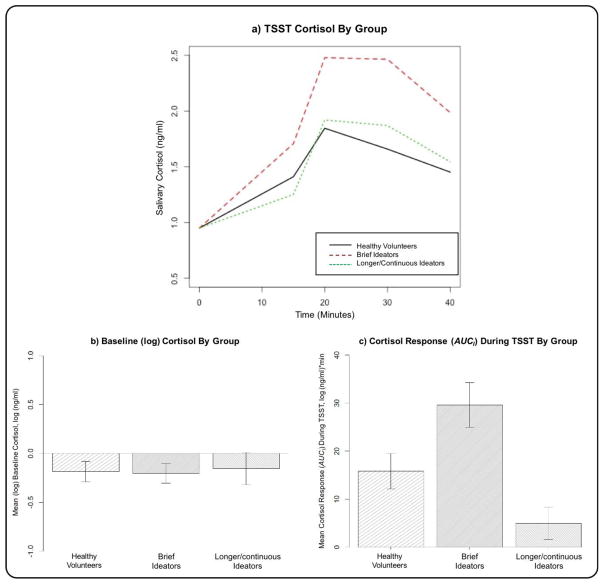
The three-group comparison of the brief ideators, longer/continuous ideators and HVs showed significant difference in cortisol response (AUCI) (F(2, 54) = 3.561, p = 0.035). This difference persisted after removing two outliers from the longer/continuous ideator group and four oultiers from the HVs (F(2, 52) = 8.147, p = 0.0008) (Figure 1a & 1c). Post hoc analysis revealed that brief ideators have greater cortisol response (AUCI) compared with longer/continuous ideators (p = 0.0006) and HVs (p = 0.038) but there were no difference between longer/continuous ideators and HVs (p = 0.166). There was no statistically significant quadratic association between the cortisol response (AUCI) and the chronicity of ideation (p = 0.724). The difference between brief and longer/continuous ideators remains significant after controlling for smoking status and HDRS scores (F(3, 30) = 4.258, p = 0.048).
Figure 1.
a) Cortisol values during and after the Trier Social Stress Test (TSST) in the healthy volunteers, brief suicidal ideators and longer/continuous ideators (the curves have been shifted to a common baseline value to illustrate cortisol response to TSST, regardless of baseline level). b) and c) show bar plots of the means and standard errors of pre-task baseline (log) cortisol, and cortisol response (AUCI) during the TSST, respectively, in the three groups.
No differences among the three groups in baseline cortisol (F(2, 55) = 2.235, p = 0.117, or F(2, 51) = 0.034, p = 0.967 after removing four outliers from the brief ideator group) (Figure 1b), or total cortisol output (AUCG) (F(2, 54) = 0.239, p = 0.788) were found.
Within the depressed suicidal ideators, total SSI scores were not correlated with cortisol response (AUCI) (Spearman’s r = −0.227, p = 0.196), baseline cortisol (Spearman’s r = 0.104, p = 0.553) or total cortisol output (AUCG) (Spearman’s r = −0.090, p = 0.611).
4. DISCUSSION
This is the first study to examine the relationship of HPA axis response to laboratory-induced social stress with duration of suicidal ideation in individuals with MDD. We found that depressed individuals with brief suicidal ideation have a greater cortisol response to a social stressor compared to those with longer and continuous ideation and healthy volunteers. This finding could not be explained by depression severity or smoking status, as it remained significant when these two factors were included as covariates. This finding represents a step toward delineating clinical characteristics of suicidal ideation associated with patterns of stress response.
Our finding has not been addressed in literature to date. Previous studies related overall severity of suicidal thoughts to the HPA axis reactivity as indicated by cortisol non-suppression in response to pharmacological challenges (López-Ibor et al., 1985; Maes et al., 1989; Pfennig et al., 2005) or cortisol response to laboratory-induced stresses (Giletta et al., 2015; O’Connor et al., 2017a), and their results are mixed. Giletta et al. (2015) demonstrated two subgroups of female adolescents that were more likely to have suicidal ideation at three-month follow-up after TSST: one with heightened and the other with blunted cortisol response to stress (at a trend level), suggesting a potential risk for developing suicidal ideation in individuals with either abnormally increased or decreased cortisol reactivity. Another prospective study using a similar task, the Maastricht Acute Stress Test, O’Connor et al. (2017a) found that lower cortisol reactivity predicted increased levels of suicide ideation at one-month follow-up in suicide attempters, but not in non-attempters. Although the findings of these two studies differ from our finding of no association between cortisol response and total SSI scores, the former are prospective while our study is cross-sectional. The results of studies that examined suicidal ideation-HPA axis reactivity using the DST also run in different directions. Pfennig et al. (2005) reported that suicidal ideation was associated with a lower adrenocorticotropic hormone (ACTH) and cortisol response in the combined dexamethasone/ACTH test. López-Ibor et al. (1985) reported elevated post-DST cortisol in relation to more severe suicidal thoughts in depressed suicidal inpatients. Maes et al. (1989) did not find such associations. These apparent inconsistencies may be explained if, as our results suggest, different patterns of cortisol response to social stress are related to a specific aspect of suicidal ideation, namely duration of ideation rather than the overall total score of suicidal ideation.
It should be noted that Heim et al. (2008b) demonstrated another stress-responsive subtype of depression where the combination of childhood abuse and depression is associated with greater stress reactivity (Heim et al., 2008a; Heim et al., 2000; Heim et al., 2004) compared with depression without history of early life adversity. However, these findings contrast to ours of no association between cortisol response to stress and history of physical or sexual abuse in the depressed group as well as with other studies where childhood trauma was associated with decreased rather than increased cortisol reactivity to stress in healthy adults (Carpenter et al., 2007; Carpenter et al., 2011; Lovallo, 2013) and in individuals with history of suicidal behavior or ideation (O’Connor et al., 2017b).
We did not find differences in baseline cortisol and total cortisol output (AUCG) among the study groups. However, our primary outcome measure, the cortisol response (AUCI; with respect to pre-task cortisol) better captures the reactivity of the HPA system compared to the total output measure (AUCG; with respect to the ground) because it controls for baseline cortisol levels (Pruessner et al., 2003). In fact, lower baseline cortisol levels were found in suicide attempters with mood disorders (Keilp et al., 2016), attempters who are offspring of mood disorder patients (Melhem et al., 2016) and individuals with family history of suicide deaths (McGirr et al., 2011). Melhem et al. (2016) also demonstrated blunted total cortisol output (AUCG), but not cortisol response (AUCI), in past suicide attempters compared to non-attempters. Interestingly, O’Connor et al. (2017a) found that participants who attempted suicide within the last 12 months appear to exhibit a clearly defined, blunted cortisol response to the laboratory stressor (AUCI), compared to those with a lifetime history of suicide attempt. Taken together, the baseline cortisol and total HPA hormonal output (AUCG) appear to be related to attempt status whereas the HPA axis reactivity, as indicated by cortisol response to stress (AUCI), is particularly affected by the time elapsed since suicide attempt, age of participants, and most importantly, the duration of suicidal ideation.
In summary, there appears to be evidence of two suicidal subgroups based on the patterns of suicidal ideation with differential cortisol response to stress. Brief suicidal ideators are more stress-responsive. They may have greater emotional reactivity, less cognitive control over their thoughts and emotions and thus are at high risk for impulsive suicidal behavior. On the other hand, persistent or chronic suicidal ideators are stress-non-responsive and tend to have more severe or chronic depression but good cognitive control over their thoughts allowing them to plan more carefully for their suicide attempts (Bernanke et al., 2017). Further research is needed to characterize the mechanisms of these associations and their inter-relationship with other risk factors for suicide.
4.1. Limitations
The modest sample size of the current study limits the generalizability of results. Replication of our findings with a larger sample size is a goal for future studies. Although the use of retrospective measures to assess suicidal ideation is well-validated, it may be helpful to test the variation of suicidal ideation over time and relate them to the pattern of stress response. We have not considered standardizing the phase of the menstrual cycle in relation to the time of the TSST, which has been shown to affect the HPA axis reactivity in healthy women (Roca et al., 2003). Finally, the cross-sectional design of the current study did not allow us to assess the potential of brief suicidal ideators for later conversion to chronic ideators.
4.2. Future Directions
Our results indicate a distinct suicidal endophenotype of brief suicidal ideation associated high biological reactivity to social stress. If these findings are replicated, future studies should further characterize this clinical subgroup with respect to additional characteristics such as cognitive style, association with impulsivity and aggression, and their neural correlates.
Highlights.
Suicide is a heterogenous phenomenon and thus defining more homogeneous subgroups may help in its prevention.
Hypothalamic–pituitary–adrenal (HPA) axis reactivity has been implicated in individuals with suicidal ideation.
This study shows that depressed brief suicidal ideators have greater cortisol response to a social stressor compared to those with longer periods or continuous ideation.
Acknowledgments
Role of funding source
This research was supported by grants from the National Institute of Mental Health: P50 MH090964 (PI: J. Mann), R01 MH61017 (PI: B. Stanley), R01 MH109326 (PI: B. Stanley & M. Oquendo), R01 MH062665 (PI: B. Stanley). Mina M. Rizk is supported by a scholarship from the Egyptian Cultural and Educational Bureau, Embassy of Egypt, Washington, D. C.
We thank the study participants and entire staff of the MIND Clinic for their time and effort.
Footnotes
Contributors
M.R. and B.S. conceptualized and wrote the manuscript. M.R. and H.G. performed the statistical analysis. M.R. and T.S. ran the TSST. J.K., M.E.S, M.A.O, J.J.M. and B.S. designed the study. All authors significantly edited and have approved the final manuscript.
Conflict of interest
Maria A. Oquendo, Barbara Stanley and J. John Mann receive royalties for commercial use of the Columbia Suicide Severity Rating Scale from the Research Foundation for Mental Hygiene. Dr. Oquendo’s family owns stock in Bristol Myers Squibb. Other authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. (DSM-IV) [Google Scholar]
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol. 1974;42:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Bernanke JA, Stanley BH, Oquendo MA. Toward fine-grained phenotyping of suicidal behavior: the role of suicidal subtypes. Mol Psychiatry. 2017;22:1080–1081. doi: 10.1038/mp.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DW, Monahan PO, Winokur G. The relationship between DST results and suicidal behavior. Annals of Clinical Psychiatry. 2002;14:83–88. doi: 10.1023/a:1016839404032. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2011;214:367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury SR, Singh T, Burke A, Stanley B, Mann JJ, Grunebaum M, Sublette ME, Oquendo MA. Clinical Correlates of Planned and Unplanned Suicide Attempts. J Nerv Ment Dis. 2016;204:806–811. doi: 10.1097/NMD.0000000000000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. Am J Psychiatry. 2001;158:748–753. doi: 10.1176/appi.ajp.158.5.748. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Scheftner W, Clark D, Hedeker D, Gibbons R, Coryell W. Clinical predictors of suicide in patients with major affective disorders: a controlled prospective study. Am J Psychiatry. 1987;144:35–40. doi: 10.1176/ajp.144.1.35. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0) Biometrics Research Dept., New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Fountoulakis KN, Iacovides A, Fotiou F, Nimatoudis J, Bascialla F, Ioannidou C, Kaprinis G, Bech P. Neurobiological and psychological correlates of suicidal attempts and thoughts of death in patients with major depression. Neuropsychobiology. 2004;49:42–52. doi: 10.1159/000075338. [DOI] [PubMed] [Google Scholar]
- Giletta M, Calhoun CD, Hastings PD, Rudolph KD, Nock MK, Prinstein MJ. Multi-Level Risk Factors for Suicidal Ideation Among at-Risk Adolescent Females: The Role of Hypothalamic-Pituitary-Adrenal Axis Responses to Stress. J Abnorm Child Psychol. 2015;43:807–820. doi: 10.1007/s10802-014-9897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldney RD, Winefield AH, Tiggemann M, Winefield HR, Smith S. Suicidal ideation in a young adult population. Acta Psychiatr Scand. 1989;79:481–489. doi: 10.1111/j.1600-0447.1989.tb10291.x. [DOI] [PubMed] [Google Scholar]
- Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics. 1999;40:18–27. doi: 10.1016/S0033-3182(99)71267-3. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008a;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008b;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Nordstrom AL, Nordstrom P. ROC analysis of dexamethasone suppression test threshold in suicide prediction after attempted suicide. J Affect Disord. 2008;106:145–152. doi: 10.1016/j.jad.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Nordstrom P. HPA axis hyperactivity and attempted suicide in young adult mood disorder inpatients. J Affect Disord. 2009;116:117–120. doi: 10.1016/j.jad.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Stanley BH, Beers SR, Melhem NM, Burke AK, Cooper TB, Oquendo MA, Brent DA, John Mann J. Further evidence of low baseline cortisol levels in suicide attempters. J Affect Disord. 2016;190:187–192. doi: 10.1016/j.jad.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Traskman-Bendz L, Vang F. Suicidal intent and the HPA-axis characteristics of suicide attempters with major depressive disorder and adjustment disorders. Arch Suicide Res. 2008;12:197–207. doi: 10.1080/13811110802100775. [DOI] [PubMed] [Google Scholar]
- López-Ibor JJJ, Saiz-Ruiz J, Perez De los Cobos JC. Biological correlations of suicide and aggressivity in major depressions (with melancholia): 5-hydroxyindoleacetic acid and cortisol in cerebral spinal fluid, dexamethasone suppression test and therapeutic response to 5-hydroxytryptophan. Neuropsychobiology. 1985;14:67–74. doi: 10.1159/000118207. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Early life adversity reduces stress reactivity and enhances impulsive behavior: implications for health behaviors. Int J Psychophysiol. 2013;90:8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Vandewoude M, Schotte C, Martin M, Blockx P, Scharpe S, Cosyns P. Hypothalamic-pituitary-adrenal and -thyroid axis dysfunctions and decrements in the availability of L-tryptophan as biological markers of suicidal ideation in major depressed females. Acta Psychiatr Scand. 1989;80:13–17. doi: 10.1111/j.1600-0447.1989.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier D. A review of prospective studies of biologic predictors of suicidal behavior in mood disorders. Arch Suicide Res. 2007;11:3–16. doi: 10.1080/13811110600993124. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier D, Stanley B, Oquendo MA, Amsel LV, Ellis SP. Can biological tests assist prediction of suicide in mood disorders? Int J Neuropsychopharmacol. 2006;9:465–474. doi: 10.1017/S1461145705005687. [DOI] [PubMed] [Google Scholar]
- McGirr A, Diaconu G, Berlim MT, Pruessner JC, Sable R, Cabot S, Turecki G. Dysregulation of the sympathetic nervous system, hypothalamic-pituitary-adrenal axis and executive function in individuals at risk for suicide. J Psychiatry Neurosci. 2010;35:399–408. doi: 10.1503/jpn.090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Diaconu G, Berlim MT, Turecki G. Personal and family history of suicidal behaviour is associated with lower peripheral cortisol in depressed outpatients. J Affect Disord. 2011;131:368–373. doi: 10.1016/j.jad.2010.10.050. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Manual for the profile of mood states revised. San Deigo, CA: Educational and Industrial Testing Service; 1992. [Google Scholar]
- Melhem NM, Keilp JG, Porta G, Oquendo MA, Burke A, Stanley B, Cooper TB, Mann JJ, Brent DA. Blunted HPA Axis Activity in Suicide Attempters Compared to those at High Risk for Suicidal Behavior. Neuropsychopharmacology. 2016;41:1447–1456. doi: 10.1038/npp.2015.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Banaji MR. Prediction of suicide ideation and attempts among adolescents using a brief performance-based test. Journal of consulting and clinical psychology. 2007;75:707. doi: 10.1037/0022-006X.75.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, Bruffaerts R, Chiu WT, de Girolamo G, Gluzman S, de Graaf R, Gureje O, Haro JM, Huang Y, Karam E, Kessler RC, Lepine JP, Levinson D, Medina-Mora ME, Ono Y, Posada-Villa J, Williams D. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192:98–105. doi: 10.1192/bjp.bp.107.040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DB, Green JA, Ferguson E, O’Carroll RE, O’Connor RC. Cortisol reactivity and suicidal behavior: Investigating the role of hypothalamic-pituitary-adrenal axis responses to stress in suicide attempters and ideators. Psychoneuroendocrinology. 2017a;75:183–191. doi: 10.1016/j.psyneuen.2016.10.019. [DOI] [PubMed] [Google Scholar]
- O’Connor DB, Green JA, Ferguson E, O’Carroll RE, O’Connor RC. Effects of childhood trauma on cortisol levels in suicide attempters and ideators. Psychoneuroendocrinology. 2017b;88:9–16. doi: 10.1016/j.psyneuen.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am J Psychiatry. 2004;161:1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: utility and limitations of research instruments. In: First MB, editor. Standardized evaluation in clinical practice. 1. American Psychiatric Publishing; Washington DC: 2003. pp. 103–130. [Google Scholar]
- Pfeffer CR, Klerman GL, Hurt SW, Kakuma T, Peskin JR, Siefker CA. Suicidal children grow up: rates and psychosocial risk factors for suicide attempts during follow-up. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32:106–113. doi: 10.1097/00004583-199301000-00016. [DOI] [PubMed] [Google Scholar]
- Pfennig A, Kunzel HE, Kern N, Ising M, Majer M, Fuchs B, Ernst G, Holsboer F, Binder EB. Hypothalamus-pituitary-adrenal system regulation and suicidal behavior in depression. Biol Psychiatry. 2005;57:336–342. doi: 10.1016/j.biopsych.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Pitchot W, Scantamburlo G, Pinto E, Hansenne M, Reggers J, Ansseau M, Legros JJ. Vasopressin-neurophysin and DST in major depression: relationship with suicidal behavior. J Psychiatr Res. 2008;42:684–688. doi: 10.1016/j.jpsychires.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Reinherz HZ, Tanner JL, Berger SR, Beardslee WR, Fitzmaurice GM. Adolescent suicidal ideation as predictive of psychopathology, suicidal behavior, and compromised functioning at age 30. American Journal of Psychiatry. 2006;163:1226–1232. doi: 10.1176/ajp.2006.163.7.1226. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. The Journal of Clinical Endocrinology & Metabolism. 2003;88:3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Turecki G. Dissecting the suicide phenotype: the role of impulsive-aggressive behaviours. J Psychiatry Neurosci. 2005;30:398–408. [PMC free article] [PubMed] [Google Scholar]
- van Heeringen K, Mann JJ. The neurobiology of suicide. Lancet Psychiatry. 2014;1:63–72. doi: 10.1016/S2215-0366(14)70220-2. [DOI] [PubMed] [Google Scholar]
- WHO. Monitoring Health For The SDGs. 2016. [Google Scholar]
- Wilcox HC, Arria AM, Caldeira KM, Vincent KB, Pinchevsky GM, O’Grady KE. Prevalence and predictors of persistent suicide ideation, plans, and attempts during college. Journal of affective disorders. 2010;127:287–294. doi: 10.1016/j.jad.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ST, Chesin M, Fertuck E, Keilp J, Brodsky B, Mann JJ, Sonmez CC, Benjamin-Phillips C, Stanley B. Heart rate variability and suicidal behavior. Psychiatry Res. 2016;240:241–247. doi: 10.1016/j.psychres.2016.04.033. [DOI] [PubMed] [Google Scholar]
- Witte TK, Fitzpatrick KK, Joiner TE, Schmidt NB. Variability in suicidal ideation: A better predictor of suicide attempts than intensity or duration of ideation? Journal of Affective Disorders. 2005;88:131–136. doi: 10.1016/j.jad.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Yerevanian BI, Feusner JD, Koek RJ, Mintz J. The dexamethasone suppression test as a predictor of suicidal behavior in unipolar depression. J Affect Disord. 2004;83:103–108. doi: 10.1016/j.jad.2004.08.009. [DOI] [PubMed] [Google Scholar]