Summary
Group 2 innate lymphoid cells (ILC2s) play crucial roles in type 2 immune responses associated with allergic and autoimmune diseases, viral and helminth infections and tissue homoeostasis. Experimental models show that in helminth infections ILC2s provide an early source of type 2 cytokines and therefore are essential for the induction of potentially protective type 2 responses. Much of our knowledge of ILC2s in helminth infections has come from experimental mouse models with very few studies analysing ILC2s in natural human infections. In attempts to harness knowledge from paradigms of the development of protective immunity in human helminth infections for vaccine development, the role of ILC2 cells could be pivotal. So far, potential vaccines against human helminth infections have failed to provide effective protection when evaluated in human studies. In addition to appropriate antigen selection, it is apparent that more detailed knowledge on mechanisms of induction and maintenance of protective immune responses is required. Therefore, there is need to understand how ILC2 cells induce type 2 responses and subsequently support the development of a protective immune response in the context of immunizations. Within this review, we summarize the current knowledge of the biology of ILC2s, discuss the importance of ILC2s in human helminth infections and explore how ILC2 responses could be boosted to efficiently induce protective immunity.
Keywords: Group 2 innate lymphoid cells, helminth infection, infection & treatment, innate lymphoid cells, schistosomiasis, TH2 immune responses, vaccination
1. INTRODUCTION
Group 2 innate lymphoid cells (ILC2s) were originally identified in experimental mouse models of helminth infections. Several studies published in 2010 utilized reporter mouse strains marking either interleukin (IL‐)131 or IL‐42 producing cells to identify a cell type, which did not express classical lineage markers of T, B, NK, myeloid or dendritic cells.2 These lineage‐negative innate lymphocytes produced classical T helper type 2 (TH2) cytokines in response to IL‐25 and IL‐33. In mice infected with the murine helminth parasite Nippostrongylus brasiliensis, these cells acted as an early source of IL‐13 and were essential for timely worm expulsion.1, 2 These innate cells, later designated as ILC2s,3 are now well characterized, and their importance in mediating pathology in asthmatic and allergic diseases as well as in viral infections has been described (reviewed in4, 5, 6, 7). Subsequently, further innate lymphoid cells were described mirroring the different adaptive CD4+ T cells; group 1 innate lymphoid cells (ILC1s) are the innate counterparts of TH1 CD4+ T cells, ILC2s are the counterpart of TH2 CD4+ T cells, and group 3 innate lymphoid cells (ILC3s) mirror TH17 and TH22 (reviewed in8). In contrast to T helper CD4+ T cells, and despite the fact that they are of lymphoid origin, ILCs do not express T‐cell receptors and lack any antigen specificity. The discovery of innate lymphoid cells has introduced a new immunological field and transformed our understanding of innate immune responses and the generation of the adaptive immune system.
Experimental studies have demonstrated that ILC2 cells are involved in tissue repair and homoeostasis9 (reviewed in10) which is an important consideration for tissue‐dwelling helminth. In addition, the involvement in parasite expulsion in intestinal helminths makes these cells important in immune protection against helminth infection and pathology. In one of only two studies of ILC2s in natural human helminth infection, we have shown that ILC2 cells are diminished in schistosome‐infected children and are restored to levels observed in children who are exposed to infection but remain uninfected following curative antihelminthic treatment.11
Within this review, we will discuss the current knowledge of the biology, function and regulation of ILC2s, their “potential” importance in human helminth infections and possibilities of utilizing ILC2 to boost protective immune response induced following treatment and vaccination. This knowledge could inform helminth control efforts as calls for helminth vaccine development escalate in the light of global mandates such as “Sustainable Development Goal 3” advocating for eradication or elimination of helminth infection.
2. THE BIOLOGY OF GROUP 2 INNATE LYMPHOID CELLS
In mice, ILC2s were originally identified as a type 2 cytokine expressing cell subset, which could not be classified by conventional lineage markers for T cells, B cells, NK cells, macrophages, dendritic cells, neutrophils, eosinophils, basophils or mast cells, but expressed the common leucocyte antigen (LCA) CD45 and their morphology resembled those of typical lymphocytes.1, 2, 12 Early studies identified the markers IL‐17 receptor B, in combination with IL‐17RA forming the IL‐25 receptor, the IL‐33 receptor (T1/ST2) with varying expression of the stem cell factor c‐kit (CD117).1, 2 These innate lymphoid‐like cells were given various names including nuocytes,1 innate helper type 2 cells (Ih2)2 or natural helper cells.13 They were enriched in mesentery13 and have been shown to express the common gamma chain (γc, CD132)‐associated receptors CD25 (IL‐2Rα) and CD127 (IL‐7Rα). IL‐7 be has been shown to play an essential role in the development and survival of ILC2s and ILC3s.14, 15, 16
Human ILC2s were initially described by Mjosberg et al.17 as being similar to murine ILC2s in lacking the expression of classical lineage defining markers, but being positive for the leucocyte marker CD45 and the IL‐7Rα (CD127). In addition, human ILC2s express the “chemokine receptor homologous molecule expressed on TH2 cells” (CRTH2=CD294),17 a marker well characterized for its expression on human CD4+ TH2 cells,18 the NK cell receptor NKR‐P1A (CD161)17 and ST219 (a member of the IL‐1 family receptors), which is part of the IL‐33 receptor complex.20 A combination of these markers is frequently used for identifying human ILC2s as Lin‐CD45+CD127+CRTH2+CD161+(ST2+)11, 17, 21, 22, 23 as we depict in the flow chart for analysing human ILC2 by flow cytometry (Figure 1).
Figure 1.
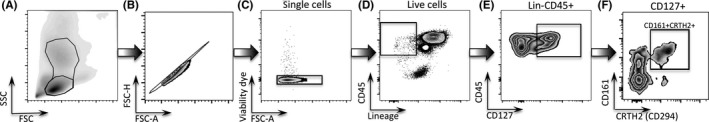
Identification of human ILC2s by flow cytometry as conducted in our studies. PBMCs were isolated from human peripheral blood and analysed by multifluorochrome‐based flow cytometry. PBMCs were gated on leucocytes (A), single cells (B) and live cells using a viability dye (C). Live single cells were gated on lineage negative (CD3, CD14, CD16, CD19, CD20, CD56, CD123, CD11c, αβTCR γδTCR), CD45+ (D), CD127+ (E) and CD161+CRTH2+ cells (F), which finally leads to the identification of lin‐CD45+CD127+CRTH2+CD161+ ILC2s
Apart from the IL‐7Rα, ILC2s express the IL‐2Rα (CD25)17 and both IL‐2 and IL‐7 are indispensable for the development, homoeostasis and activation of ILC2s.13, 24, 25 The IL‐7Rα chain forms a heterodimer with the “thymic stromal lymphopoietin” (TSLP) receptor26 a further characteristic marker of human ILC2s.25 TSLP is able to activate cytokine production by ILC2s, but works more efficiently in combination with IL‐2 and has synergistic effects with IL‐33.25 IL‐33 (or IL‐1F11) is a IL‐1 family member and acts via the IL‐33 receptor.20 Furthermore, IL‐25 activates cytokines production by ILC2s signalling via the IL‐25 receptor, a heterodimer of IL‐17RB and IL‐17RA. IL‐25, IL‐33 and TSLP can be seen as the classical ILC2 activating cytokines and often referred to as alarmins (alarm signals). Hematopoietic cells can produce alarmins, but the primary sources are nonhematopoietic cells. IL‐33 is primary produced by endothelial and epithelial cells,27, 28, 29 but can be released by macrophages30 or dendritic cells.31 In contrast, tuft cells, a subset of epithelial cells of the small intestine with previously more or less unknown function, were identified as a major source of IL‐25,32, 33, 34 which is required for ILC2 homoeostasis. The numbers of tuft cells increase significantly when exposed to intestinal parasites.
ILC2s express a variety of additional receptors involved in the activation and homoeostasis. Expression of the IL‐4Rα (CD124) was shown in mice, and basophil‐derived IL‐4 can positively control ILC2s.35 As IL‐4 is secreted by ILC2s, IL‐4 could potentially act as an autocrine feedback mechanism for activation of ILC2s. However, the exact role of IL‐4 in controlling activation of human ILC2s is currently unknown. ILC2s are also the main source of IL‐9, another common γ chain (γc) cytokine,36, 37 with expression of IL‐9 receptor being essential for ILC2 activation, survival of activated ILC2s and finally for efficient helminth worm expulsion in mouse experimental models.36 IL‐9 released by lung resident ILC2s plays a central role in the epithelial response to murine N. brasiliensis infection by inducing IL‐5 and IL‐13 production.38 Gene expression analyses indicated that the IL‐9 receptor is expressed on murine ILC2s, and in humans, expression of this receptor has been shown on blood and lung ILC2s.21 The CRTH2 is a crucial marker for the identification of human ILC2s17 and for classical TH2 cells.18, 39 The agonist for CRTH2 is prostaglandin (PG)D2, a well‐characterized mediator of allergic asthma40 released by activated mast cells. PGD2 is crucial for chemotaxis of TH2 cells41 and drives accumulation of ILC2s in inflamed tissues.42
Murine ILC2s isolated from lymph nodes and the spleen, and to a less extent, ILC2s from the peritoneal or broncho‐alveolar lavage, express major histocompatibility complex class‐II (MHC‐II) molecules. They also express the co‐stimulatory molecules CD80 and CD86.43 Expression of MHC‐II in combination with co‐stimulatory molecules allows a direct interaction with CD4+ T cells and can drive CD4+ T‐cell expansion and activation and TH2 polarization and is important for efficient worm expulsion in murine infections of N. brasiliensis. Accordingly, it had been demonstrated that human ILC2s isolated from peripheral blood express high levels of HLR‐DR, CD80 and CD86.43
Similar to all other immune responses, the function of ILC2s needs counter‐regulation allowing control of their function. Type 1 and type 2 interferons can negatively regulate ILC2s,23, 44 and both types of interferons are long known to inhibit helminth‐driven TH2 responses. Additionally, ILC2s can be suppressed by IL‐2745 and express the inhibitory receptor killer cell lectin‐like receptor G1 (KLRG1). In human ILC2s, the ligand of KLRG1, E‐cadherin, inhibited expression of GATA3 and production of TH2‐cytokines.46 GATA3, the transcription factor essential for TH2 CD4+ T‐cell polarization and function, is crucial for ILC2 differentiation, maintenance and activation,24, 25 and also used as identifying marker to distinguish them from other ILC subsets. Furthermore, development, differentiation and function of ILC2s depend on RORα,16, 47 T‐cell factor‐1 (TCF‐1)48 and GFI1.49
ILC2s are now considered to play a central role in inducing type 2 immune responses in mice. Following activation, ILC2s secrete type 2 cytokines and activate various and complex immune responses, which are characteristic for type 2 responses including B‐cell activation and isotype switching to IgE, induction of eosinophilia, polarization of alternative activated macrophages and initiation of an adaptive TH2 T‐cell response including generation of TH2 memory CD4+ T cells as outlined and described in Figure 2.
Figure 2.
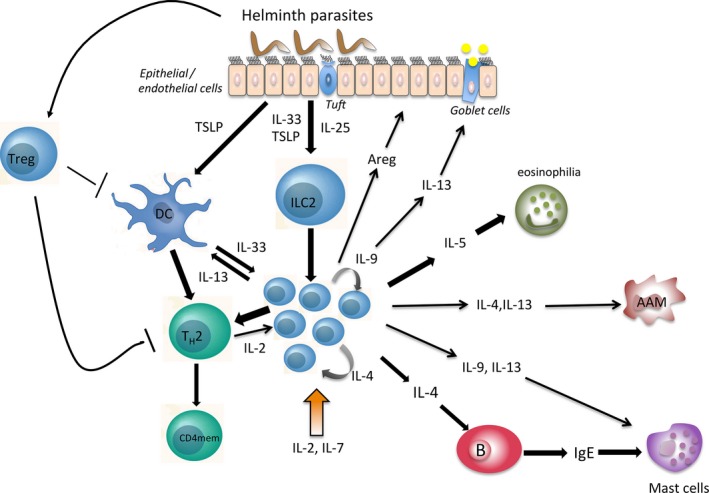
Helminth‐induced immune responses mediated by ILC2s. Helminth parasites trigger the secretion of alarmins by endothelial or epithelial cells (IL‐33, TSLP)27, 28, 29 or by tuft cells (IL‐25).32, 33, 34 Myeloid cells (dendritic cells (DC) or macrophages) can also release IL‐33 and thereby activate ILC2s.30, 31 ILC2 activation is maintained and multiplied by IL‐4 and IL‐9 (acting in an autocrine manner)36 and require IL‐2 and IL‐7 for homoeostasis and activation. ILC2s secrets type 2 cytokines upon activation. IL‐5 induces eosinophilia,139, 151 and IL‐4 triggers B cells and induces isotype switching to IgE. Furthermore, IL‐13 can activate mucus secretion by goblet cells,1, 16, 152 act on mast cells (potentially in conjunction with IL‐9152) and regulate DC migration.153 IL‐4 and IL‐13 can also induce alternative activated macrophages (AAM).154 ILC2s also secrete amphiregulin (Areg) important for tissue repair.9 Furthermore, ILC2s interact with TH2 CD4+ T cells (TH2), which induces TH2 immune response43, 155 and IL‐2 secreted by T cells could further sustain ILC2 responses and further affect generation of T‐cell memory,156 which is altered in chronic helminth infections.84 Helminth can induce regulatory T cells (Treg), which potentially can dampen the development of full protective immune response118
2.1. Common γc cytokine receptors
Common gamma chain (γc) (CD132) cytokine receptors play a central role in the development, homoeostasis and function of several immune cell lineages and are indispensable for the immune system itself. Therefore, it is not surprising that common γc cytokines and the corresponding receptors are also essential for the development of ILC2s. The IL‐7Rα chain, forming a heterodimer with the common γc (also known as common IL‐2 receptor gamma chain), was one of the first surface receptors identified as marker for ILCs, and the development of ILC2s was depending on the common γc and IL‐7.13 The IL‐2Rα is also a marker human ILC2s and provides an important co‐stimulatory signal for the activation of ILC2s.25
Using a reporter mouse strain, ILC2s, rather than CD4+ T cells, were also identified as main source of IL‐9 in a model of airway inflammation.37 More importantly, IL‐9 acts as feedback signal enhancing the cytokine production by ILC2s. The importance of IL‐9 as a feedback signal was subsequently confirmed in experimental infection with N. brasiliensis, in which IL‐9 receptor expressing ILC2 is important for restoring tissue damage caused by the lung stage of N. brasiliensis.36, 38 Hence, common γc receptors play a pivotal in the development, maintenance and activation of ILC2s.
In T cells, common γc signalling is mainly mediated by three pathways: the JAK‐STAT pathway, the mitogen‐activated protein kinases (MAPK)‐Erk pathway and the phosphoinositide 3‐kinase (PI3K) pathway. Binding of cytokines to its corresponding receptors leads to an activation of Janus kinases (JAKs), which are associated with the receptor (reviewed in50). JAK activation leads to a phosphorylation of tyrosine residues within the receptor chain causing binding, phosphorylation and dimerization of signal transducer and activator of transcription (STAT), which then translocate to the nucleus and starts specific transcription. There are several STAT molecules partially determining specific effects of cytokines. IL‐2, IL‐7 and IL‐9 mainly activate STAT5, whereas IL‐4 mainly induces the activation of STAT6. Interestingly, the TSLPR, which contains a IL‐7Rα chain, but no common γc, activates STAT5 in a JAK‐independent way.51 IL‐2 and TSLP efficiently induce STAT5 phosphorylation in human ILC2s, while IL‐33 elicits a moderate phosphorylation of STAT3.25
The JAK‐STAT signalling pathway is tightly regulated to control strength, duration and specificity of activation. Suppressor of cytokine signalling (SOCS) molecule comprises a family of eight members SOCS1‐SOCS7 and the cytokine‐inducible SH2 domain protein (CISH) of which four are shown to be important in T‐cell signalling (CISH, SOCS1‐SOCS3) (reviewed in52). The SOCS molecules including CISH have been shown to regulate STAT signalling and modulate T helper polarization.53, 54, 55 Both the MAPK‐Erk and the PI3K pathways play central roles in the development, homoeostasis and functions of several innate and adaptive immune cells. Both pathways contribute to T helper polarization56, 57, 58, 59 including differentiation of TH2 cells.60, 61 While the importance of common γc cytokine receptors for ILC2s is well described for mice, the precise signalling pathways controlling the development and function of human ILC2s remain to be investigated.
3. LOCATION OF ILC2s AND THE IMPLICATION FOR HUMAN HELMINTH INFECTIONS
ILC2s have been identified in various tissues. Using reporter mice in experimental models of N. brasiliensis infection, ILC2s were identified in the spleen, liver, mesenteric lymph nodes, the intestine, fat‐associated lymphoid clusters1, 2, 13 and skin.62 In humans, ILC2s have been described in nasal polyps, tonsils, gastrointestinal tract, peripheral blood17, 25 and the lung.9, 17 ILC2 are also described in human skin46, 63 with their migration to the skin being associated with PGD2, the ligand for CRTH2,63 and the skin‐homing marker cutaneous lymphocyte antigen.64 Overall, mucosa‐associated tissues of the lung, intestine and skin are now widely accepted as the most important locations for ILC2s.
Helminths have complicated and diverse life histories, differing in their route and site of infection, migration within the human host, location of adult worms and exit of juveniles or eggs. This diversity in helminth biology results in heterogeneous acquired immune responses to helminth parasites reflected by fundamental differences in in vitro experiments and in immuno‐epidemiological studies (reviewed in65). These life history differences, together with differences in niches relative to the location of ILC2s, imply differences in the encounter between the parasite/parasite products and ILC2 cells. For instance, helminths such as Schistosoma spp. (a trematode), Strongyloides stercoralis or hookworms (Ancylostoma duodenale and Necator americanus; nematodes) are skin‐penetrating parasites, meaning that the infective stage and/or the tissue damage caused by the skin penetration can trigger ILC2s.
A percutaneous infection by Schistosoma mansoni larvae elicits a transient expression of TSLP and IL‐33.66 Although not directly shown, the release of the cytokines is likely to activate ILC2s. Larvae (L3) of vector‐transmitted filarial nematodes (Wuchereria bancrofti Brugia malayi, Loa Loa, Mansonella perstans) also need to penetrate the skin or the bite wound during the blood meal of the vector. Onchocerca volvulus, Mansonella streptocerca and L. Loa directly develop a cutaneous filariasis with adults residing in subcutaneous tissues. It remains to be investigated whether dermal ILC2s do indeed play a role in initiating antifilarial immune responses following skin penetration and in cutaneous filariasis.
Infection via the skin causes a certain degree of tissue damage67 and induces wound healing.66 ILC2s are crucial for cutaneous wound healing.68 These data suggest that ILC2s may have an additional function in wound healing of damaged tissue caused by skin‐penetrating parasites.
Several helminth species have evolved a critical lung stage, which can be either transient (Ascaris, Schistosoma, Strongyloides spp.) or more persistent (W. bancrofti, B. malayi, L. Loa) (reviewed in69). Lung stages of helminths can cause tissue damage in the lung and affect mucosal integrity. In experimental mouse models, the crucial role of ILC2, mediated by IL‐9, acting in autocrine manner, in tissue repair and lung homoeostasis, has been well documented.36, 37, 38 Activated macrophages are also involved in limiting tissue damage during lung migration, a process requiring IL‐4/IL‐13 signalling70; although in this former study the exact source of these cytokines was not determined, ILC2s should be considered as source of these cytokines. Mature adults of gastrointestinal helminths (hookworms, S. stercoralis) reside in different parts of the intestine and influence and/or damage the epithelial tissue resulting in a release of IL‐25, IL‐33 and TSLP, triggering ILC2s.71 As the intestinal tissue is a main compartment where ILC2s are located, it is likely that activated ILC2s play a major role in initiating the immune response in these helminth infections. It will be difficult to prove this role of intestinal ILC2s in a human infection, but mouse experimental studies strongly support this as reviewed in.72
Adult schistosomes reside in mesenteric (S. mansoni, S. japonicum) or in the perivesical venous plexus (Schistosoma haematobium)73 were they interact with the epithelium. Moreover, eggs released by the females need to penetrate the bladder wall (S. haematobium) or migrate to the intestine (S. mansoni), damaging epithelial tissue. As outlined above damaging the epithelium could trigger ILC2s, but so far, there are no studies investigating whether schistosomes induce ILC2 directly or indirectly through tissue damage.
ILCs derive from a common lymphoid precursor in bone marrow74 expressing the integrin α4β7, mediating migration to endothelial venues and mucosal tissues, and chemokine receptor CXCR6 mediating migration to the intestine.75 Additionally, a lineage‐specific precursor has been identified for ILC2s.24, 47 However, it has been suggested that ILC2s proliferate within tissues and are rarely replenished from the bone marrow.45 More committed progenitors were also identified in secondary lymphoid organs.76 Therefore, the contribution of circulating ILC2s from peripheral blood to tissue‐resident ILC2 pool needs to be studied in more detail to allow interpretation of immuno‐epidemiological data based on human blood, as theoretically, blood ILC2s may be important in blood residing pathogens including schistosomes.
Much of our knowledge about ILC2s in helminth infections derives from experimental infection with N. brasiliensis, a murine gastrointestinal parasite and most experimental infections cover days or a few weeks following initial infection. In human, however, adult worms can live for years, and in the case of Schistosoma spp. even decades.77 For people living in endemic areas, an infection does not occur solely at a single time point, but instead occurs more gradually with multiple infection events, resulting in hosts carrying different life stages of the parasites concurrently. Hence, most helminth parasite can cause a chronic long‐lasting disease, which cannot be recapitulated in the mouse experimental model. Hence, knowledge about the function and importance of ILC2 obtained from experimental models cannot necessarily extrapolated to natural human infections.
4. ILC2s IN HUMAN HELMINTH INFECTION—WHAT WE DO “NOT” KNOW
So far there are very few studies that have analysed ILC2s in natural human helminth infection, a fact, which is not surprising considering the history and biology of ILC2s outlined above. ILC2s constitute a small fraction of human blood leucocytes. In our studies in a Zimbabwean population, a mean of 0.031% (median 0.023%, range 0.003‐0.133, N=72) of live‐gated leucocytes was denoted as ILC2s, a proportion which is comparable to data published elsewhere17 and data in Caucasians (unpublished data). Of note, ILC2s are hardly detectable in peripheral blood of naïve mice.1, 2
In humans, proportions are slightly higher in the skin, ileum, lung and tonsils compared to peripheral blood and are increased in inflamed tissues such as inflamed nasal polyps and skin lesions of patients with atopic dermatitis.17, 46, 64, 78 Furthermore, proportions of ILC2s are increased in lung specimens of patients with severe forms of asthma. However, it remains contradictory whether the proportions of ILC2s are higher in lung specimens compared to blood of the same patient.79, 80 Such analyses of human tissues are informative but are beyond the scope of immuno‐epidemiological approaches such as the ones we have previously published.
Nevertheless, there have been some advances in studying human ILC2 cells in the context of natural human infections. Nutman and colleagues analysed ILCs defined as lin‐CD45+CD127+CD117+ (c‐kit), comprising both ILC2s and ILC3s, in peripheral blood of filarial infected adults (L. Loa, W. bancrofti, O. volvulus).81 The frequency of c‐kit+ ILCs and IL‐13 producing cells among c‐kit+ ILCs was increased in filarial infected individuals. The proportion of c‐kit ILCs correlated with IL‐17‐producing CD4+ T cells and ex vivo stimulation of enriched ILCs released IL‐5 and IL‐13, but also IL‐10, IL‐17 and IFNγ.81
In a different study, we evaluated ILC2s in context of natural infection with S. haematobium in Zimbabwean children.11 Schistosome‐infected children aged 6‐13 years (as diagnosed by parasite egg excretion) had a significantly lower frequency of ILC2s in the peripheral blood compared to same‐age schistosome‐uninfected children (Figure 3A). In contrast, older infected children (aged 14‐18 years) had comparable levels of ILC2s to uninfected children.11 Proportions of ILC2s recovered following curative antihelminthic treatment (Figure 3B). Of note is the difference in these age groups; children are exposed to schistosome infection very young and therefore acquire infection at a young age.82 By the time they reach adolescence, they will have experienced several re‐infection events. Thus, it is possible that the ILC2 dynamics are reflecting differences occurring upon first vs repeated infection events. Older egg‐positive children had levels of ILC2s comparable to same‐age egg‐negative children. These older egg‐positive children show a schistosome‐specific antibody profile, which indicates a history of previous infection and also associated with the development of protective immunity, beginning to reduce re‐infection levels.83, 84, 85 This finding may indicate that ILC2s play a more pronounced role in the initiation of early immune response at a stage when effective TH2 responses are triggered and a full CD4+ T‐cell‐mediated TH2 response has not yet developed. In this context, it would be interesting to perform long‐term follow‐up studies, which analyse whether early changes in the proportion or phenotype of ILC2 are predictive for the development of, or the nature of a protective immune response. The study analysing ILCs in filarial infections mentioned above81 found increased proportions of ILCs in infected adults, which may indicate a complete contrasting function of ILCs in adults. For instance, ILC2s could play a role in diminishing tissue damage and promote epithelial healing to limit pathology or alternatively contribute to pathology. Differences between the two studies could reflect differences in the biology of trematodes and filarial nematodes. For instance, filarial parasites frequently harbour Wolbachia spp. endosymbionts,86 which could trigger TLR responses and potentially modify the response elicited by various ILC subsets including ILC2s and ILC3s. Whereas the precise pattern of TLR expression on ILC2s has not yet been specified, LTi‐like group 3 ILCs have been shown to express TLRs.87 Of note, the study by Boyd et al. analysed CD127+CD117+ ILCs comprising both ILC2s and ILC3s.81 To analyse the contribution of Wolbachia endosymbionts to ILC‐mediated immune responses, interventional studies with doxycycline, which targets Wolbachia spp. in filarial infections could be utilized.88, 89, 90 Furthermore, differences in the life cycle and age dynamics in different types of helminth infections could contribute to differences in ILC2s. Therefore, additional observational and interventional studies are required to decipher the precise role of ILC2s in various helminth infections and in the context of the complex dynamics of human helminth infections, which cannot completely be mimicked by experimental models. The mechanism responsible for differences in the frequency of ILC2 in peripheral blood in these human helminth studies remains unknown. One possibility is that one or all of proliferation, survival and homoeostasis of ILC2 is altered during helminth infection. Common γc cytokine signalling (as outlined above) could be altered during helminth infections. Chronic down‐modulation of IL‐7Rα on memory T cells has been shown for chronic viral infections,91, 92 a mechanism potentially affecting ILC2s in chronic parasite infections. However, expression of the IL‐7Rα chain on the surface of ILC2 was not altered during schistosome infections.11 Regulation of IL‐7 signalling is much more complex and could depend on the availability of IL‐7 and levels of soluble IL‐7Rα (generated by alternative splicing93), which has been shown to inhibit IL‐7 uptake94 or function as IL‐7 reservoir.95 Aberrant levels of plasma IL‐7 and soluble IL‐7R were recently shown in the context of human tuberculosis.96 In addition, modulation of downstream signalling in particular of the JAK‐STAT pathway including modulation of SOCS may influence homoeostasis and responsiveness of human ILC2s. Modulation of this signalling pathway in T cells has been shown for various infectious diseases including tuberculosis.97 Whether and to which degree common γc signalling is modulated in ILC2s in particular during helminth infection remains elusive. Furthermore, modulation of signalling via the IL‐9R and TSLPR could be regulated and may provide molecular targets for chemoprophylaxis or therapy.
Figure 3.
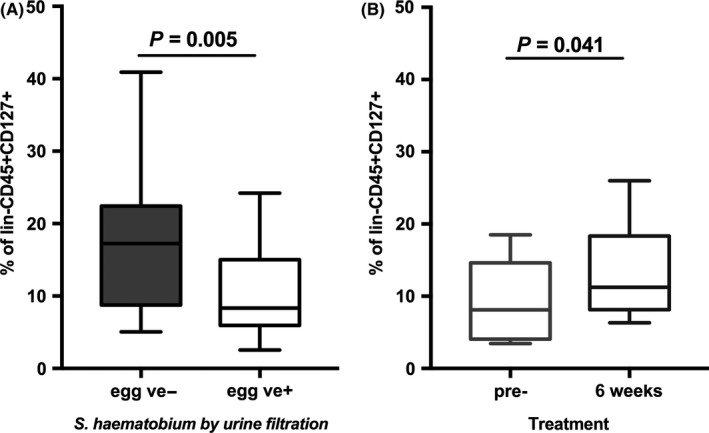
Proportions of ILC2s are diminished in schistosome‐infected children and restored by curative treatment. (A) Proportions of blood CD127+CD294+CD161+ ILC2s were compared between Schistosoma haematobium egg‐positive (+ve) children and S. haematobium egg‐negative (−ve) children (N=24 per group, age 6‐13 y). (B) Proportions of ILC2s of 12 individuals (aged 6‐13 y) were compared pre‐ vs 6 wk post‐treatment. Individuals were egg positive pretreatment and had cleared S. haematobium infections after treatment with the antihelminthic drug praziquantel. Figures are reproduced from data published in11
The impact of nutrition, particularly micronutrients on the immune system, is well established in experimental models. Micronutrient deficiency is widespread in helminth‐endemic areas with vitamin A deficiency being one of the most common. Interestingly, work in experimental studies indicates that vitamin A deficiency is characterized by an increase in ILC2 cells and increased production of IL‐13 by these cells to maintain mucosal barrier immunity to helminth infection under malnutrition.98 In addition, recent work has also highlighted that ILC2 cells predominantly depend on fatty acid (FA) metabolism during helminth infection.99 The vast majority of the world's malnourished people live in developing countries, where 13.5% of the population is undernourished100 and areas of malnutrition largely overlap with helminth‐endemic areas. Therefore, it is important to understand the development and function of ILC2 cells in populations exposed to helminth infection. There are many potential sources of heterogeneity, not least the gut microbiome structure. Mouse experimental studies have demonstrated that infection with the helminth Trichuris muris significantly altered the host gut microbiome structure, reducing the diversity and abundance of the Bacteroidetes, Prevotella and Parabacteroidetes.101 This dysbiosis was associated with a significant reduction in amounts of Vitamin D derivatives and a reduction in the breakdown of dietary plant‐derived carbohydrates involved in amino acid synthesis, with an associated reduction in the weight of the infected animals. We have demonstrated that the gut microbiome structure in children infected with schistosomes differed significantly from that of uninfected children from the same community.102 Although the development of ILC2 cells does not seem dependent on the gut microbiome, their function is dependent on the colonization of the gut by commensals (reviewed in103). However, the precise mechanisms of how/which signals from the gut microbes interact with the ILC2 to facilitate their maturation and function remains unknown. Answers to these questions will only come from studies conducted in context, in the relevant human populations.
To date, both experimental and human studies have mainly focused on infection. However, there is also another aspect of human helminthiases in which the immune response plays a central role, that is immunopathology. Eggs are mainly responsible for the pathology‐associated schistosomiasis, and egg‐induced immunopathology can occur in the chronic form of the disease. Eggs laid by adults worms, which reside in the vesical plexus of the bladder or mesenteric veins of the liver, can be carried to portal venules in the liver and to the bladder or genital tract where eggs become trapped and eventually form granulomas and can induce immune‐mediated fibrosis. The degree of pathology depends on the balance of type 1, type 2 and type 17 immune responses (reviewed in104, 105). Severe forms of pathology are associated with TH1/TH17, whereas mild pathology is associated with a combination of regulatory and TH2 response. However, the type 2 cytokine IL‐13 also contributes to hepatic fibrosis.106 In mouse models, it has been shown that ILC2s are a likely source for IL‐13 in hepatic fibrosis.107 Hepatic IL‐33 triggered the expansion and activation of liver‐resident ILC2s, which produced IL‐13 and mediated fibrosis.107, 108 In human intestinal schistosomiasis, the majority of patients develop a less severe form of the disease, but about 5%‐10% suffer from hepatosplenic schistosomiasis with progressive fibrosis.109 To what extent, hepatic ILC2s contribute to the development of severe forms of schistosomiasis remains to be investigated. Furthermore, the impact of environmental enteropathy, which affects gut permeability, exacerbated by helminth infections has yet to be investigated.110
5. THE POTENTIAL IMPACT FOR TREATMENT STRATEGIES AND SUCCESSFUL VACCINATION
The development of successful vaccinations against human parasitic infections and in particular against helminth infections has proven challenging. Although there are some promising vaccine candidates for instance, a vaccine against hookworms,111 currently there is no licenced vaccine against helminth infections for use in human. The reasons for lack of progress in human helminth vaccinology are manifold. Most helminths have complex life cycles with intermediates hosts and reservoirs and several life cycle stages even within the human host leading to highly variable and complex antigen pattern. Helminths typically induce a type 2 response, which is potentially protective. However, work over the last decade has shown that helminths have evolved immune evasion mechanisms allowing the establishment of long‐lasting infections and modulation of pathology (reviewed in112, 113, 114, 115). To do so, helminths utilize immunosuppressive and immunoevasive mechanisms, mediated through various mechanisms. For instance, the importance of regulatory T cells has been shown for filarial116, 117 and schistosome infections118, 119 and excretory‐secretory products released by helminth parasites can directly induce regulatory T cells.120 In the cases of schistosomiasis suppression of immune responses induced by worms can delay the development of protective immunity.121 Mechanisms of how the host eventually manages to express a resistance phenotype have been a subject of our research, leading to the description of the threshold hypothesis122; that is, the host needs to experience a threshold of antigens to mount an effective immune response and that these antigens become available following worm death. We and others have also demonstrated the requirement of the ratio of regulatory vs effector cellular immune to favour effector responses for expression of resistance.118 However, the precise mechanism of the induction of a protective response remains elusive. The description of the ILC2 cells bridging the innate and adaptive immune system could potentially shed light to this aspect of schistosome immunobiology.
Therefore, apart from the search of new vaccine candidates, new strategies to trigger and boost the development of effective immune responses should be investigated. ILC2s are of major importance for the induction of effective type 2 immune responses (Figure 2) and are in particularly crucial in early immune response and hence are a promising target to boost responses. Here, it is interesting to note that in an experimental mouse model excretory‐secretory products of Heligmosomoides polygyrus inhibited the production of TH2 cytokines by ILC2s through the blockade of IL‐33,123 indirectly indicating the importance for dampening ILC2 responses for parasite survival. Overcoming such inhibition and efficient triggering response mediated by ILC2s could be an important step in triggering protective responses against helminth infections. However, our knowledge of the role of ILC2 biology in helminth infections is still very limited to allow a predication if modulation of ILC2s can improve immune response and thereby improve vaccine efficacy.
For schistosomiasis, protective immune responses can build up over time under constant exposure124 and repeated treatment can boost specific immune responses.125 Therefore, “Infection and treatment” (I&T) strategies are a potential alternative to induce protective immune responses,126 which so far has proven to be the most efficient method to induce protection. The efficacy of this approach has been recently shown for human malaria infections.127 Understanding the dynamics of ILC2 involvement in inducing protective immune responses might better inform targeting of treatment. For example, we are currently testing the potential for inducing protective immune responses in schistosomiasis following treatment of the first very infection event. Human immunology and mouse experimental studies of helminths and Plasmodium infections suggest that the number of antiparasite treatments required to induce protective immune responses can be reduced by treating people following first infection.128, 129, 130, 131, 132
6. MODULATING ILC2 RESPONSES
Common gamma γc cytokines and their receptors are crucial for homoeostasis and activation of ILC2s and therefore are potential targets to boost ILC2 responses thereby potentially increase the effectiveness of vaccinations or I&T approaches. IL‐2 therapy has a long history in antitumour therapy,133 and therapies with low‐dose IL‐2 are currently tested in autoimmune disease such as hepatitis C virus‐related vasculitis134 and type 1 diabetes.135, 136 Early on, it has been recognized that IL‐2 therapy can lead to increased plasma levels of IL‐5 and eosinophila137, 138 an effect that, at least in mouse models, is caused by an activation of ILC2s.139 Side effects of low doses of IL‐2 are considered to be relatively safe, but in the context of autoimmune diseases are used to expand regulatory T cells (reviewed in140), which may contradict attempts to trigger a protective response in helminth infections. However, with detailed investigations of treatment regimes regarding the dose and duration of the IL‐2 therapy might help to tackle this problem. For instance, regulatory T cells may expand only after a few weeks of IL‐2 therapy, whereas ILC2 activation may occur quicker in particular if incorporated in I&T approaches or if applied with vaccinations.
IL‐7, another common gamma γc cytokine, is also considered for use in cancer141, 142, 143 and chronic viral infections,144 highlighting the potential in immunotherapies. This is particularly important in the carinogenic trematodes, S. haematobium, Opisthorchis viverrini and Clonorchis sinensis where one of the pathological manifestations of these infections is cancer in different organs (bladder, bile duct and liver)145 for which we currently do not have any therapeutic interventions beyond surgery. As IL‐7 is crucial for the development and homoeostasis of human ILC2s, its potential to increase responses mediated by ILC2s in vaccination and/or I&T protocols should be investigated.
Apart from the direct use of cytokines in immunotherapies, molecules crucial for the downstream signalling induced by these cytokine could be targeted. Interestingly, the effects by IL‐7 in the study on chronic viral infections were partially mediated by repression of SOCS3.144 Hence, targeting the JAK/STAT or the MAPK/Erk pathway including SOCS inhibitors may have the potential to increase ILC2 activation, but also TH2 responses in general.53, 146
The main trigger of the ILC2 activity are the alarmins IL‐25, IL‐33 and TSLP, but their potential as activators in immunotherapy has not been investigated in detail. However, blocking alarmins has been considered for treating allergic diseases,147, 148, 149 but has not really gone beyond experimental testing with only initial studies in human.150 ILC2 targeting alarmins could be also used in combination with common γc cytokines. Overall, specific modulation of ILC2 activity to improve vaccine or I&T‐induced protective immune responses is an exciting idea. Precise treatment strategies need to be carefully approved to avoid induction of regulatory T cells or to avoid the induction of allergic immune responses. Before attempting ILC2 targeting strategies to build up protective immune responses, much more work needs to be done on dissecting mechanisms and signalling pathways in ILC2s.
7. CONCLUSIONS
Within a few years of their discovery, ILCs have revolutionized immunology research, added a new layer of complexity to the immune system as a whole and transformed our understanding of how immune responses are initiated and maintained. ILCs have been shown to be important in allergic disorders, autoimmune diseases, viral infections and even in tumour immunology. Experimental mouse models of helminth infections have led to increased understanding of the ILC2 biology and provided mechanistic details of the crucial role of ILC2s in inducing TH2 responses. Human studies testing the hypotheses from these mouse models lag behind, creating a knowledge gap. However, the limited studies of ILC2s in the context of natural human infections have already started to yield interesting results on the nature and function of ILC2s. Given the complexity and diversity of human helminth infections, much more work needs to be done to obtain a complete figure about the role of ILC2 and the underlying immunological pathways and mechanisms of their function in human helminth infections. While the study of the role and function of human ILC is still in its infancy, rapid incorporation of the knowledge of these cells in our paradigm of the nature and development of protective immunity is essential for helminth vaccinology and optimal treatment strategies.
CONFLICT OF INTEREST
The authors have declared that no competing interests exist.
ACKNOWLEDGEMENTS
The current work of NN is supported by the German‐African Cooperation Initiative of the “Deutsche Forschungsgemeinschaft” (Grant No: JA 1479/5‐1). FM is supported by the Wellcome Trust, Thrasher Research Fund and OAK Foundation. We thank Daniel R Neill (University of Liverpool, UK) and Padraic Fallon (Trinity College Dublin, Ireland) for the invitation to contribute to this Special Issue of Parasite Immunology.
Nausch N, Mutapi F. Group 2 ILCs: A way of enhancing immune protection against human helminths? Parasite Immunol. 2018;40:e12450 https://doi.org/10.1111/pim.12450
REFERENCES
- 1. Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature. 2010;464:1367‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL‐13‐expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489‐11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spits H, Artis D, Colonna M, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145‐149. [DOI] [PubMed] [Google Scholar]
- 4. Peebles RS Jr. At the bedside: the emergence of group 2 innate lymphoid cells in human disease. J Leukoc Biol. 2015;97:469‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doherty TA. At the bench: understanding group 2 innate lymphoid cells in disease. J Leukoc Biol. 2015;97:455‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Licona‐Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536‐542. [DOI] [PubMed] [Google Scholar]
- 7. Halim TY. Group 2 innate lymphoid cells in disease. Int Immunol. 2016;28:13‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eberl G, Di Santo JP, Vivier E. The brave new world of innate lymphoid cells. Nat Immunol. 2015;16:1‐5. [DOI] [PubMed] [Google Scholar]
- 9. Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765‐774. [DOI] [PubMed] [Google Scholar]
- 11. Nausch N, Appleby LJ, Sparks AM, Midzi N, Mduluza T, Mutapi F. Group 2 innate lymphoid cell proportions are diminished in young helminth infected children and restored by curative anti‐helminthic treatment. PLoS Negl Trop Dis. 2015;9:e0003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)‐25‐dependent cell population that provides IL‐4, IL‐5, and IL‐13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue‐associated c‐Kit(+)Sca‐1(+) lymphoid cells. Nature. 2010;463:540‐544. [DOI] [PubMed] [Google Scholar]
- 14. Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647‐675. [DOI] [PubMed] [Google Scholar]
- 16. Wong SH, Walker JA, Jolin HE, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mjosberg JM, Trifari S, Crellin NK, et al. Human IL‐25‐ and IL‐33‐responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055‐1062. [DOI] [PubMed] [Google Scholar]
- 18. Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30:2972‐2979. [DOI] [PubMed] [Google Scholar]
- 19. Brestoff JR, Kim BS, Saenz SA, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL‐1 receptor accessory protein and ST2 comprise the IL‐33 receptor complex. J Immunol. 2007;179:2551‐2555. [DOI] [PubMed] [Google Scholar]
- 21. Bal SM, Bernink JH, Nagasawa M, et al. IL‐1beta, IL‐4 and IL‐12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17:636‐645. [DOI] [PubMed] [Google Scholar]
- 22. Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duerr CU, McCarthy CD, Mindt BC, et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol. 2016;17:65‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoyler T, Klose CS, Souabni A, et al. The transcription factor GATA‐3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mjosberg J, Bernink J, Golebski K, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649‐659. [DOI] [PubMed] [Google Scholar]
- 26. He R, Geha RS. Thymic stromal lymphopoietin. Ann N Y Acad Sci. 2010;1183:13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baekkevold ES, Roussigne M, Yamanaka T, et al. Molecular characterization of NF‐HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carriere V, Roussel L, Ortega N, et al. IL‐33, the IL‐1‐like cytokine ligand for ST2 receptor, is a chromatin‐associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moussion C, Ortega N, Girard JP. The IL‐1‐like cytokine IL‐33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joshi AD, Oak SR, Hartigan AJ, et al. Interleukin‐33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yanagawa Y, Suzuki M, Matsumoto M, Togashi H. Prostaglandin E(2) enhances IL‐33 production by dendritic cells. Immunol Lett. 2011;141:55‐60. [DOI] [PubMed] [Google Scholar]
- 32. Gerbe F, Sidot E, Smyth DJ, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Howitt MR, Lavoie S, Michaud M, et al. Tuft cells, taste‐chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. von Moltke J, Ji M, Liang HE, Locksley RM. Tuft‐cell‐derived IL‐25 regulates an intestinal ILC2‐epithelial response circuit. Nature. 2016;529:221‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Motomura Y, Morita H, Moro K, et al. Basophil‐derived interleukin‐4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758‐771. [DOI] [PubMed] [Google Scholar]
- 36. Turner JE, Morrison PJ, Wilhelm C, et al. IL‐9‐mediated survival of type 2 innate lymphoid cells promotes damage control in helminth‐induced lung inflammation. J Exp Med. 2013;210:2951‐2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilhelm C, Hirota K, Stieglitz B, et al. An IL‐9 fate reporter demonstrates the induction of an innate IL‐9 response in lung inflammation. Nat Immunol. 2011;12:1071‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang HE, Locksley RM. Group 2 innate lymphoid cells utilize the IRF4‐IL‐9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol. 2016;9:275‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagata K, Tanaka K, Ogawa K, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278‐1286. [PubMed] [Google Scholar]
- 40. Matsuoka T, Hirata M, Tanaka H, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013‐2017. [DOI] [PubMed] [Google Scholar]
- 41. Hirai H, Tanaka K, Yoshie O, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven‐transmembrane receptor CRTH2. J Exp Med. 2001;193:255‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wojno ED, Monticelli LA, Tran SV, et al. The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015;8:1313‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oliphant CJ, Hwang YY, Walker JA, et al. MHCII‐mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Molofsky AB, Van Gool F, Liang HE, et al. Interleukin‐33 and interferon‐gamma counter‐regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity. 2015;43:161‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moro K, Kabata H, Tanabe M, et al. Interferon and IL‐27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol. 2016;17:76‐86. [DOI] [PubMed] [Google Scholar]
- 46. Salimi M, Barlow JL, Saunders SP, et al. A role for IL‐25 and IL‐33‐driven type‐2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939‐2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic‐acid‐receptor‐related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463‐474. [DOI] [PubMed] [Google Scholar]
- 48. Mielke LA, Groom JR, Rankin LC, et al. TCF‐1 controls ILC2 and NKp46+RORgammat+ innate lymphocyte differentiation and protection in intestinal inflammation. J Immunol. 2013;191:4383‐4391. [DOI] [PubMed] [Google Scholar]
- 49. Spooner CJ, Lesch J, Yan D, et al. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nat Immunol. 2013;14:1229‐1236. [DOI] [PubMed] [Google Scholar]
- 50. Shuai K, Liu B. Regulation of JAK‐STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900‐911. [DOI] [PubMed] [Google Scholar]
- 51. Liu YJ, Soumelis V, Watanabe N, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193‐219. [DOI] [PubMed] [Google Scholar]
- 52. Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503‐529. [DOI] [PubMed] [Google Scholar]
- 53. Yang XO, Zhang H, Kim BS, et al. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol. 2013;14:732‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kleinsteuber K, Heesch K, Schattling S, et al. SOCS3 promotes interleukin‐17 expression of human T cells. Blood. 2012;120:4374‐4382. [DOI] [PubMed] [Google Scholar]
- 55. Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 2009;30:592‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haylock‐Jacobs S, Comerford I, Bunting M, et al. PI3Kdelta drives the pathogenesis of experimental autoimmune encephalomyelitis by inhibiting effector T cell apoptosis and promoting Th17 differentiation. J Autoimmun. 2011;36:278‐287. [DOI] [PubMed] [Google Scholar]
- 57. Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3‐kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122‐5128. [DOI] [PubMed] [Google Scholar]
- 58. Soond DR, Bjorgo E, Moltu K, et al. PI3K p110delta regulates T‐cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115:2203‐2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Agrawal A, Dillon S, Denning TL, Pulendran B. ERK1‐/‐ mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2006;176:5788‐5796. [DOI] [PubMed] [Google Scholar]
- 60. Nashed BF, Zhang T, Al‐Alwan M, et al. Role of the phosphoinositide 3‐kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol. 2007;37:416‐424. [DOI] [PubMed] [Google Scholar]
- 61. Dillon S, Agrawal A, Van Dyke T, et al. A Toll‐like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal‐regulated kinase mitogen‐activated protein kinase and c‐Fos in dendritic cells. J Immunol. 2004;172:4733‐4743. [DOI] [PubMed] [Google Scholar]
- 62. Roediger B, Kyle R, Yip KH, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xue L, Salimi M, Panse I, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor‐homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Teunissen MB, Munneke JM, Bernink JH, et al. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J Invest Dermatol. 2014;134:2351‐2360. [DOI] [PubMed] [Google Scholar]
- 65. Bourke CD, Maizels RM, Mutapi F. Acquired immune heterogeneity and its sources in human helminth infection. Parasitology. 2011;138:139‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bourke CD, Prendergast CT, Sanin DE, Oulton TE, Hall RJ, Mountford AP. Epidermal keratinocytes initiate wound healing and pro‐inflammatory immune responses following percutaneous schistosome infection. Int J Parasitol. 2015;45:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McKerrow JH, Jones P, Sage H, Pino‐Heiss S. Proteinases from invasive larvae of the trematode parasite Schistosoma mansoni degrade connective‐tissue and basement‐membrane macromolecules. Biochem J. 1985;231:47‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rak GD, Osborne LC, Siracusa MC, et al. IL‐33‐dependent group 2 innate lymphoid cells promote cutaneous wound healing. J Invest Dermatol. 2016;136:487‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Craig JM, Scott AL. Helminths in the lungs. Parasite Immunol. 2014;36:463‐474. [DOI] [PubMed] [Google Scholar]
- 70. Chen F, Liu Z, Wu W, et al. An essential role for TH2‐type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Patel N, Wu W, Mishra PK, et al. A2B adenosine receptor induces protective antihelminth type 2 immune responses. Cell Host Microbe. 2014;15:339‐350. [DOI] [PubMed] [Google Scholar]
- 72. Filbey K, Bouchery T, Le Gros G. The role of ILC2 in hookworm infection. Parasite Immunol. 2017;00:e12429 https://doi.org/10.1111/pim.12429. PubMed PMID: 28369954. [DOI] [PubMed] [Google Scholar]
- 73. Secor WE, Colley DG, eds. Schistosomiasis, 1st edn Boston: Springer US; 2005. [Google Scholar]
- 74. Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: natural helper cells derive from lymphoid progenitors. J Immunol. 2011;187:5505‐5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Satoh‐Takayama N, Serafini N, Verrier T, et al. The chemokine receptor CXCR6 controls the functional topography of interleukin‐22 producing intestinal innate lymphoid cells. Immunity. 2014;41:776‐788. [DOI] [PubMed] [Google Scholar]
- 76. Scoville SD, Mundy‐Bosse BL, Zhang MH, et al. A progenitor cell expressing transcription factor RORgammat generates all human innate lymphoid cell subsets. Immunity. 2016;44:1140‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wilkins HA. The epidemiology of schistosome infections in man In: Rollinson D, Simpson AJG, eds. The Biology of Schistosomes: From Genes to Latrines. London, UK: Academic Press; 1987:379‐397. [Google Scholar]
- 78. Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL‐33‐independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75‐86 e8. [DOI] [PubMed] [Google Scholar]
- 80. Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2016;137:624‐626 e6. [DOI] [PubMed] [Google Scholar]
- 81. Boyd A, Ribeiro JM, Nutman TB. Human CD117 (cKit)+ innate lymphoid cells have a discrete transcriptional profile at homeostasis and are expanded during filarial infection. PLoS ONE. 2014;9:e108649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Woolhouse ME, Mutapi F, Ndhlovu PD, Chandiwana SK, Hagan P. Exposure, infection and immune responses to Schistosoma haematobium in young children. Parasitology. 2000;120(Pt 1):37‐44. [DOI] [PubMed] [Google Scholar]
- 83. Milner T, Reilly L, Nausch N, et al. Circulating cytokine levels and antibody responses to human Schistosoma haematobium: IL‐5 and IL‐10 levels depend upon age and infection status. Parasite Immunol. 2010;32:710‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nausch N, Bourke CD, Appleby LJ, et al. Proportions of CD4+ memory T cells are altered in individuals chronically infected with Schistosoma haematobium . Sci Rep. 2012;2:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rujeni N, Nausch N, Bourke CD, et al. Atopy is inversely related to schistosome infection intensity: a comparative study in Zimbabwean villages with distinct levels of Schistosoma haematobium infection. Int Arch Allergy Immunol. 2012;158:288‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hoerauf A, Volkmann L, Hamelmann C, et al. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet. 2000;355:1242‐1243. [DOI] [PubMed] [Google Scholar]
- 87. Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL‐22+ cells and LTi‐like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207:281‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Coulibaly YI, Dembele B, Diallo AA, et al. A randomized trial of doxycycline for Mansonella perstans infection. N Engl J Med. 2009;361:1448‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hoerauf A, Specht S, Buttner M, et al. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo‐controlled study. Med Microbiol Immunol. 2008;197:295‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double‐blind, randomised placebo‐controlled trial. Lancet. 2005;365:2116‐2121. [DOI] [PubMed] [Google Scholar]
- 91. Boutboul F, Puthier D, Appay V, et al. Modulation of interleukin‐7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005;19:1981‐1986. [DOI] [PubMed] [Google Scholar]
- 92. Koesters SA, Alimonti JB, Wachihi C, et al. IL‐7Ralpha expression on CD4+ T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur J Immunol. 2006;36:336‐344. [DOI] [PubMed] [Google Scholar]
- 93. Rane L, Vudattu N, Bourcier K, et al. Alternative splicing of interleukin‐7 (IL‐7) and interleukin‐7 receptor alpha (IL‐7Ralpha) in peripheral blood from patients with multiple sclerosis (MS). J Neuroimmunol. 2010;222:82‐86. [DOI] [PubMed] [Google Scholar]
- 94. Crawley AM, Faucher S, Angel JB. Soluble IL‐7R alpha (sCD127) inhibits IL‐7 activity and is increased in HIV infection. J Immunol. 2010;184:4679‐4687. [DOI] [PubMed] [Google Scholar]
- 95. Lundstrom W, Highfill S, Walsh ST, et al. Soluble IL7Ralpha potentiates IL‐7 bioactivity and promotes autoimmunity. Proc Natl Acad Sci USA. 2013;110:E1761‐E1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lundtoft C, Afum‐Adjei Awuah A, Rimpler J, et al. Aberrant plasma IL‐7 and soluble IL‐7 receptor levels indicate impaired T‐cell response to IL‐7 in human tuberculosis. PLoS Pathog. 2017;13:e1006425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jacobsen M, Repsilber D, Kleinsteuber K, et al. Suppressor of cytokine signaling‐3 is affected in T‐cells from tuberculosisTB patients. Clin Microbiol Infect. 2011;17:1323‐1331. [DOI] [PubMed] [Google Scholar]
- 98. Spencer SP, Wilhelm C, Yang Q, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wilhelm C, Harrison OJ, Schmitt V, et al. Critical role of fatty acid metabolism in ILC2‐mediated barrier protection during malnutrition and helminth infection. J Exp Med. 2016;213:1409‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. FAO, IFAD, WFP . The State of Food Insecurity in the World 2015: Meeting the 2015 international hunger targets: taking stock of uneven progress. Rome: FAO; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Houlden A, Hayes KS, Bancroft AJ, et al. Chronic Trichuris muris infection in C57BL/6 mice causes significant changes in host microbiota and metabolome: effects reversed by pathogen clearance. PLoS ONE. 2015;10:e0125945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kay GL, Millard A, Sergeant MJ, et al. Differences in the faecal microbiome in Schistosoma haematobium infected children vs. uninfected children. PLoS Negl Trop Dis. 2015;9:e0003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65‐74. [DOI] [PubMed] [Google Scholar]
- 104. Larkin BM, Smith PM, Ponichtera HE, Shainheit MG, Rutitzky LI, Stadecker MJ. Induction and regulation of pathogenic Th17 cell responses in schistosomiasis. Semin Immunopathol. 2012;34:873‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL‐4 and IL‐13: IL‐13 is a profibrotic agent. J Immunol. 2000;164:2585‐2591. [DOI] [PubMed] [Google Scholar]
- 107. McHedlidze T, Waldner M, Zopf S, et al. Interleukin‐33‐dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Neumann K, Karimi K, Meiners J, et al. A proinflammatory role of type 2 innate lymphoid cells in murine immune‐mediated hepatitis. J Immunol. 2017;198:128‐137. [DOI] [PubMed] [Google Scholar]
- 109. Fallon PG. Immunopathology of schistosomiasis: a cautionary tale of mice and men. Immunol Today. 2000;21:29‐35. [DOI] [PubMed] [Google Scholar]
- 110. Lin A, Arnold BF, Afreen S, et al. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am J Trop Med Hyg. 2013;89:130‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hotez PJ, Diemert D, Bacon KM, et al. The human hookworm vaccine. Vaccine. 2013;31(Suppl 2):B227‐B232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25:585‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375‐388. [DOI] [PubMed] [Google Scholar]
- 114. Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733‐744. [DOI] [PubMed] [Google Scholar]
- 116. Wammes LJ, Hamid F, Wiria AE, et al. Regulatory T cells in human lymphatic filariasis: stronger functional activity in microfilaremics. PLoS Negl Trop Dis. 2012;6:e1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Metenou S, Dembele B, Konate S, et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184:5375‐5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nausch N, Midzi N, Mduluza T, Maizels RM, Mutapi F. Regulatory and activated T cells in human Schistosoma haematobium infections. PLoS ONE. 2011;6:e16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Watanabe K, Mwinzi PN, Black CL, et al. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am J Trop Med Hyg. 2007;77:676‐682. [PMC free article] [PubMed] [Google Scholar]
- 120. Grainger JR, Smith KA, Hewitson JP, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF‐beta pathway. J Exp Med. 2010;207:2331‐2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mitchell KM, Mutapi F, Woolhouse ME. The predicted impact of immunosuppression upon population age‐intensity profiles for schistosomiasis. Parasite Immunol. 2008;30:462‐470. [DOI] [PubMed] [Google Scholar]
- 122. Mitchell KM, Mutapi F, Savill NJ, Woolhouse ME. Explaining observed infection and antibody age‐profiles in populations with urogenital schistosomiasis. PLoS Comput Biol. 2011;7:e1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. McSorley HJ, Blair NF, Smith KA, McKenzie AN, Maizels RM. Blockade of IL‐33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7:1068‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Woolhouse ME, Taylor P, Matanhire D, Chandiwana SK. Acquired immunity and epidemiology of Schistosoma haematobium . Nature. 1991;351:757‐759. [DOI] [PubMed] [Google Scholar]
- 125. Mutapi F, Burchmore R, Mduluza T, et al. Praziquantel treatment of individuals exposed to Schistosoma haematobium enhances serological recognition of defined parasite antigens. J Infect Dis. 2005;192:1108‐1118. [DOI] [PubMed] [Google Scholar]
- 126. Mutapi F, Billingsley PF, Secor WE. Infection and treatment immunizations for successful parasite vaccines. Trends Parasitol. 2013;29:135‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mordmuller B, Surat G, Lagler H, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542:445‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. de Oliveira Fraga LA, Lamb EW, Moreno EC, et al. Rapid induction of IgE responses to a worm cysteine protease during murine pre‐patent schistosome infection. BMC Immunol. 2010;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. de Oliveira Fraga LA, Torrero MN, Tocheva AS, Mitre E, Davies SJ. Induction of type 2 responses by schistosome worms during prepatent infection. J Infect Dis. 2010;201:464‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468‐477. [DOI] [PubMed] [Google Scholar]
- 131. Roestenberg M, Teirlinck AC, McCall MB, et al. Long‐term protection against malaria after experimental sporozoite inoculation: an open‐label follow‐up study. Lancet. 2011;377:1770‐1776. [DOI] [PubMed] [Google Scholar]
- 132. van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475‐490. [DOI] [PubMed] [Google Scholar]
- 133. Rosenberg SA. IL‐2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451‐5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T‐cell responses to low‐dose interleukin‐2 in HCV‐induced vasculitis. N Engl J Med. 2011;365:2067‐2077. [DOI] [PubMed] [Google Scholar]
- 135. Rosenzwajg M, Churlaud G, Mallone R, et al. Low‐dose interleukin‐2 fosters a dose‐dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun. 2015;58:48‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Long SA, Rieck M, Sanda S, et al. Rapamycin/IL‐2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta‐cell function. Diabetes. 2012;61:2340‐2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. van Haelst PisaniC, Kovach JS, Kita H, et al. Administration of interleukin‐2 (IL‐2) results in increased plasma concentrations of IL‐5 and eosinophilia in patients with cancer. Blood. 1991;78:1538‐1544. [PubMed] [Google Scholar]
- 138. Cragun WC, Yamshchikov GV, Bissonette EA, et al. Low‐dose IL‐2 induces cytokine cascade, eosinophilia, and a transient Th2 shift in melanoma patients. Cancer Immunol Immunother. 2005;54:1095‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Van Gool F, Molofsky AB, Morar MM, et al. Interleukin‐5‐producing group 2 innate lymphoid cells control eosinophilia induced by interleukin‐2 therapy. Blood. 2014;124:3572‐3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Klatzmann D, Abbas AK. The promise of low‐dose interleukin‐2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15:283‐294. [DOI] [PubMed] [Google Scholar]
- 141. Pellegrini M, Calzascia T, Elford AR, et al. Adjuvant IL‐7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009;15:528‐536. [DOI] [PubMed] [Google Scholar]
- 142. Gao J, Zhao L, Wan YY, Zhu B. Mechanism of action of IL‐7 and its potential applications and limitations in cancer immunotherapy. Int J Mol Sci. 2015;16:10267‐10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. ElKassar N, Gress RE. An overview of IL‐7 biology and its use in immunotherapy. J Immunotoxicol. 2010;7:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pellegrini M, Calzascia T, Toe JG, et al. IL‐7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601‐613. [DOI] [PubMed] [Google Scholar]
- 145. Fried B, Reddy A, Mayer D. Helminths in human carcinogenesis. Cancer Lett. 2011;305:239‐249. [DOI] [PubMed] [Google Scholar]
- 146. Kim D, Kim SH, Cho SH, Shin K, Kim S. SOCS3 suppresses the expression of IL‐4 cytokine by inhibiting the phosphorylation of c‐Jun through the ERK signaling pathway in rat mast cell line RBL‐2H3. Mol Immunol. 2011;48:776‐781. [DOI] [PubMed] [Google Scholar]
- 147. Lei Y, Boinapally V, Zoltowska A, Adner M, Hellman L, Nilsson G. Vaccination against IL‐33 inhibits airway hyperresponsiveness and inflammation in a house dust mite model of asthma. PLoS ONE. 2015;10:e0133774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Knolle MD, Rana BM, McKenzie AN. IL‐25 as a potential therapeutic target in allergic asthma. Immunotherapy. 2015;7:607‐610. [DOI] [PubMed] [Google Scholar]
- 149. Han H, Xu W, Headley MB, et al. Thymic stromal lymphopoietin (TSLP)‐mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012;5:342‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gauvreau GM, O'Byrne PM, Boulet LP, et al. Effects of an anti‐TSLP antibody on allergen‐induced asthmatic responses. N Engl J Med. 2014;370:2102‐2110. [DOI] [PubMed] [Google Scholar]
- 151. Yasuda K, Muto T, Kawagoe T, et al. Contribution of IL‐33‐activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode‐infected mice. Proc Natl Acad Sci USA. 2012;109:3451‐3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL‐9‐deficient mice establish fundamental roles for IL‐9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573‐583. [DOI] [PubMed] [Google Scholar]
- 153. Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell‐mediated allergic lung inflammation. Immunity. 2014;40:425‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Molofsky AB, Nussbaum JC, Liang HE, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mirchandani AS, Besnard AG, Yip E, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442‐2448. [DOI] [PubMed] [Google Scholar]
- 156. Halim TY, Hwang YY, Scanlon ST, et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]


