Abstract
Objective
The impact of childhood differentiated thyroid carcinoma (DTC) on psychosocial development has not yet been studied. The aim of this study was to evaluate the achievement of psychosocial developmental milestones in long-term survivors of childhood DTC.
Design and methods
Survivors of childhood DTC diagnosed between 1970 and 2013 were included. Reasons for exclusion were age <18 or >35 years at follow-up, a follow-up period <5 years or diagnosis with DTC as a second malignant neoplasm. Survivors gathered peer controls of similar age and sex (n = 30). A comparison group non-affected with cancer (n = 508) and other childhood cancer survivors (CCS) were also used to compare psychosocial development. To assess the achievement of psychosocial milestones (social, autonomy and psychosexual development), the course of life questionnaire (CoLQ) was used.
Results
We included 39 survivors of childhood DTC (response rate 83.0%, mean age at diagnosis 15.6 years, and mean age at evaluation 26.1 years). CoLQ scores did not significantly differ between survivors of childhood DTC and the two non-affected groups. CoLQ scores of childhood DTC survivors were compared to scores of other CCS diagnosed at similar ages (n = 76). DTC survivors scored significantly higher on social development than other CCS, but scores were similar on autonomy and psychosexual developmental scales.
Conclusions
Survivors of childhood DTC showed similar development on social, autonomy, and psychosexual domains compared to non-affected individuals. Social development was slightly more favorable in DTC survivors than in other CCS, but was similar on autonomy and psychosexual domains.
Background
Childhood diagnosis of thyroid cancer is rare. Age-adjusted incidence rates of pediatric thyroid cancer are reported between 0.6 and 1.2 per 100 000 per year, but rates are increasing (1, 2, 3). Differentiated thyroid carcinoma (DTC) is the most frequently diagnosed histological subtype of this disease, accounting for over 90% of the thyroid cancers in children (1). The incidence of childhood DTC peaks during puberty and mainly affects girls (4, 5). The effect of DTC on the psychosocial development of children diagnosed with this disease has not been studied.
Childhood cancer arises in a period where several psychosocial developmental milestones are reached (6), also referred to as ‘the course of life’ (7). The psychosocial development from childhood toward adulthood involves increasing independence, growth of more symmetrical parent–adolescent relationships, maturation of personality and identity, and encountering and maintaining (first) psychosexual and social contacts (8). The diagnosis and treatment of childhood cancer may impair the psychosocial development as some childhood cancer survivors (CCS) have been shown to be hindered in their development. For example, CCS had fewer friends and tended to be older when they experienced their first sexual intimacy. However, data are inconclusive (7, 9, 10, 11, 12). In survivors of various types of childhood cancer the biological behavior of the cancer and the impact of treatment interfere differently in the course of life: survivors of brain tumors seem most severely affected (9, 10, 11, 13).
Unfortunately, despite the excellent cure rates, most DTC survivors remain patients for the rest of their lives. Treatment of DTC consists of a thyroidectomy, and in case of lymph node metastases, it is extended with a lymph node dissection in the neck. In the Netherlands, about a third of the survivors of childhood DTC experience surgical complications: the most common complications are hypoparathyroidism and recurrent laryngeal nerve damage (5). Surgery is followed by one or more administrations of radioactive iodine (131-I). During the 131-I administrations, patients are withdrawn from thyroid hormone for several weeks; this causes deep hypothyroidism, involving changes in metabolism (14). Initial treatment usually takes from one to several years. Subsequently, lifelong thyroid hormone therapy is initiated. Especially for high-risk patients, relatively high levels of thyroid hormone are prescribed to induce subclinical hyperthyroidism, which decreases the risk of cancer recurrence (15). However, large fluctuations in thyroid hormone levels are known to have a great impact on well-being, behavior, and learning ability (16, 17). Childhood DTC has an excellent survival, with 5-year and 10-year survival rates >95% (4, 5). Furthermore, specific aspects of DTC survivorship include lifelong use of hormone medication and blood tests as well as the possibility of cancer recurrences even after decades of disease-free survival (18).
An explorative study was set up, ultimately to evaluate whether interventions for these survivors are necessary. Our aims were (I) to compare the developmental milestones reached by adult survivors of childhood DTC and by individuals non-affected with cancer; (II) to compare psychosocial development in adult survivors of childhood DTC and in survivors of other types of childhood cancer and (III) to assess whether the psychosocial development of survivors of childhood DTC is associated with medical characteristics.
Subjects and methods
This cross-sectional study was performed in the context of a Dutch nationwide study examining medical characteristics and long-term treatment effects (including a paper questionnaire) of childhood DTC from 2012 to 2014 (5). The Institutional Review Board of the University Medical Center Groningen approved the study on behalf of all participating institutions (ABR NL40572.042.12, file number 2012/183). This study was registered in the Netherlands Trial Registry (trial registration number 3448).
Participants
DTC survivors
For the nationwide study, all patients diagnosed with DTC at age ≤18 years between 1970 and 2013 treated in the Netherlands were included (5). Exclusion criteria were, as previously reported: follow-up <5 years after diagnosis, attained age <18 years, diagnosis of DTC as a second malignant neoplasm, lack of understanding of the Dutch language, and 131-I administration within three months before evaluation (19). For the current study, survivors aged >35 years during evaluation were also excluded because the questionnaire is validated only up to the age of 35 years (7).
Groups used for comparison
Comparison to individuals unaffected by cancer
DTC survivors were compared to a group of persons unaffected by cancer from the survivors’ environment (peer controls) and compared to an unaffected group reflecting the general population (comparison group).
Peer controls: DTC survivors were asked to find one or two peers of the same sex and age (range plus or minus 5 years from the survivor’s age at evaluation, minimum age 18 years). Controls who had a medical history of malignancy were excluded. Although we preferred peers (e.g. friends, colleagues), we allowed inclusion of family members, provided they met the in/exclusion criteria (19). For the current substudy, peer controls aged >35 years were also excluded.
All DTC survivors and peer controls gave written informed consent before participating in the study.
Comparison group: A comparison group, gathered by general practitioners in a previous study, was used (7). For this comparison, group persons aged 18–30 years, with no history of cancer and with the ability to understand Dutch questionnaires were included. This group consisted of 508 persons (consisting of 53% females and 47% males).
Comparison to survivors of other childhood cancers
Survivors of other childhood cancers: For this comparison, we used data of other CCS, gathered in a study by Stam and coworkers (7). These survivors were diagnosed with cancer at age <18 years, were at least 5 years in follow-up, aged 17–30 years during evaluation, and were able to understand Dutch questionnaires. The different cancer diagnoses were grouped as leukemia/lymphoma (e.g. acute lymphoblastic leukemia, (non-) Hodgkin lymphoma), solid tumors (e.g. rhabdomyosarcoma, osteosarcoma) and brain tumors (7). Three hundred and fifty-three CCS were eligible for comparison. Survivors of thyroid cancer (n = 3) were excluded from analyses since we only wanted to compare DTC survivors to other types of childhood cancer. Subsequently, 350 CCS of other types of cancer (hereafter referred to as CCS) were available for comparison.
Measures
Psychosocial development
Psychosocial development was assessed using the course of life questionnaire (CoLQ) (7), which measures achievement of developmental milestones on five domains: (1) social development, (2) autonomy development, (3) psychosexual development, (4) antisocial behavior, and (5) substance use and gambling. To restrict the length of the questionnaire, for the current study only domains 1–3 were evaluated. Possible scores for each item were scored 1 (milestone not (yet) achieved) or 2 (milestone achieved). Scores were added-up to form 3 scales: social development (12 items, range: 12–24), autonomy development (6 items, range: 6–12) and psychosexual development (4 items, range: 4–8). Higher scores indicate earlier achievement or achievement of more developmental milestones. The validity of the course of life scales is good. The internal consistency of the scales is satisfactory, except the autonomy scale, probably because the items refer to diverging aspects of autonomy (7). The Cronbach’s alphas in the populations under study were moderate to good: (1) social development (range: 12–24): DTC survivors 0.70, peer controls 0.76, comparison group 0.71, CCS 0.75; (2) autonomy development (range: 6–12): DTC survivors 0.52, peer controls 0.49, comparison group 0.49, CCS 0.43; (3) psychosexual development (range: 4–8): DTC survivors 0.91, peer controls 0.75, comparison group 0.71, CCS 0.74.
Sociodemographic and medical data
Sociodemographics (marital status, educational level (low, medium and high) and employment status) and data regarding disease, treatment, and follow-up characteristics were retrieved from medical records and a self-administered questionnaire.
Statistical methods
Data are presented as median (interquartile range) unless otherwise specified. Characteristics and scores on the CoLQ (on scale level and item level) of survivors of childhood DTC were compared separately with the characteristics or scores of the peer controls, the comparison group and the CCS (total, and divided by type of diagnosis). Associations between medical characteristics and scores on developmental domains were evaluated.
χ 2 tests or Fisher’s exact tests (if >20% of the cells had an expected count of <5) were used for categorical variables. Mann–Whitney U and Kruskal–Wallis tests were performed for non-normally distributed continuous or ordinal variables. Spearman’s rank correlation coefficient was used to correlate two non-normally distributed continuous and/or ordinal variables. The tests performed are described in the table legends. Missing or unknown values were excluded from statistical testing (pairwise deletion). To compensate for multiple testing, we considered differences to be statistically significant at P < 0.01. All tests were two-sided. IBM SPSS Statistics for Windows, version 23 (IBM) was used for statistical analyses.
Results
I) Comparison to individuals unaffected with cancer
Sample characteristics
One hundred and five survivors were included in the nationwide study (of 169 eligible subjects, response rate 62.1%) (5). Of the 47 survivors eligible for the current study, 39 (83.0%) participated (Supplementary Fig. 1, see section on supplementary data given at the end of this article). The survivors gathered 59 peer controls, 30 of whom participated: 26 peer controls were excluded because of an age >35 years and 3 peer controls were excluded because they did not meet matching criteria.
Demographic characteristics appear in Table 1. Median age of the DTC survivors at evaluation was 26.2 years (range 18.8–35.7), compared to a median age of 25.8 years (range 19.4–34.4, P = 0.628) of peer controls and a median age of 23.8 years of the comparison group (range 18.0–31.0, P = 0.012). The group of DTC survivors had significantly more females than the comparison group (87% vs 53%, P < 0.001). DTC survivors reported significantly more frequently higher levels of education than the comparison group (P = 0.002). Other characteristics did not significantly differ between DTC survivors and the other two groups.
Table 1.
Characteristics of survivors of childhood DTC vs individuals non-affected with cancer.
| DTC survivors | Peer controls | P value | Comparison group | P value | |
|---|---|---|---|---|---|
| n | 39 | 30 | 508 | ||
| Age at evaluation (years) | 26.2 (18.8–35.7) | 25.8 (19.4–34.4) | 0.628† | 23.8 (18.0–31.0) | 0.012† |
| Sex, n (%) | 0.690‡ | <0.001*§ | |||
| Female | 34 (87) | 28 (93) | 269 (53) | ||
| Male | 5 (13) | 2 (7) | 239 (47) | ||
| Employment, n (%) | 0.576‡ | 1.000‡ | |||
| Employed and/or student | 38 (97) | 28 (93) | 486 (96) | ||
| Not employed and no student | 1 (3) | 2 (7) | 21 (4) | ||
| Missing | 0 (0) | 0 (0) | 1 (0) | ||
| Completed education, n (%) | 0.035‡ | 0.002*§ | |||
| Low level | 7 (18) | 0 (0) | 143 (28) | ||
| Medium level | 15 (39) | 12 (40) | 246 (48) | ||
| High level | 17 (44) | 18 (60) | 97 (19) | ||
| Missing | 0 (0) | 0 (0) | 22 (4) | ||
| Marital status‖, n (%) | 0.303§ | n.a. | |||
| Relationship | 24 (62) | 22 (73) | – | ||
| No relationship | 15 (38) | 8 (27) | – | ||
| Marital status‖, n (%) | n.a. | n.a. | |||
| Not married and not living together | – | – | 299 (59) | ||
| Married or living together | – | – | 192 (38) | ||
| Missing | – | – | 17 (3) |
†Mann–Whitney U test; ‡Fisher’s exact test; §Chi square test; ‖answer options regarding marital status of two different questionnaires were non-mergeable, therefore these are shown separately. Missing values were excluded for statistical testing (pairwise deletion). Continuous variables are presented as median (range). *Indicates a statistically significant difference (P < 0.01).
CCS, childhood cancer survivors; DTC, differentiated thyroid carcinoma; n.a., not applicable.
All DTC survivors and peer controls had the Dutch nationality. Ninety-seven percent of the comparison group had the Dutch nationality; this was not significantly different from the DTC survivors (P = 0.558).
Psychosocial development
Because scores on psychosocial development did not differ between males and females in all evaluated groups (data not shown), no correction for sex was performed.
Scale scores: Scores of the survivors of childhood DTC on all three developmental milestone scales (i.e. social development, autonomy development and psychosexual development) did not differ significantly from those of peer controls or of the comparison group (P = 0.592, P = 0.084, P = 0.841, P = 0.233, P = 0.241, and P = 0.556, respectively, Table 2).
Table 2.
Scores on psychosocial developmental domains: comparison of survivors of childhood DTC with individuals non-affected with cancer.
| DTC survivors | Peer controls | P value | Comparison group | P value | |
|---|---|---|---|---|---|
| n | 39 | 30 | 508 | ||
| Social development† | 22 (20.3, 23) | 22 (18, 23) | 0.592 | 21 (19, 23) | 0.233 |
| Autonomy development‡ | 9 (8, 10) | 10 (9, 11) | 0.084 | 9 (8, 11) | 0.241 |
| Psychosexual development§ | 8 (6.5, 8) | 8 (6, 8) | 0.841 | 8 (7, 8) | 0.556 |
Scores are shown as median (p25, p27). Comparisons between DTC survivors and other groups were performed using Mann–Whitney U tests. Higher scores indicate earlier achievement or achievement of more psychosocial developmental milestones; †scale ranges 12–24; ‡scale ranges 6–12; §scale ranges 4–8. Missing values were excluded from statistical testing (pairwise deletion).
CCS, childhood cancer survivors; DTC, differentiated thyroid carcinoma.
Item scores: Item scores regarding social development, autonomy development and psychosexual development during or after secondary school did not differ between survivors of childhood DTC and the peer controls or the comparison group (Supplementary Table 1a, b and c).
II) Comparison to survivors of other types of childhood cancer
Sample characteristics
Age at diagnosis was significantly different between survivors of childhood DTC and other CCS (15.6 vs 6.3 years old, respectively, P < 0.001); the large majority (35 out of 39, 90%) of survivors of childhood DTC were diagnosed during secondary school. We therefore chose to focus primarily on survivors (DTC and other CCS) diagnosed at age ≥12 years (the age at which Dutch children generally start secondary school).
Thirty-five of the DTC survivors were diagnosed at age ≥12 years (Supplementary Fig. 1). Of the 350 CCS non-affected with thyroid cancer 76 were diagnosed at age ≥12 years.
As shown in Table 3, the median age of these 35 DTC survivors at diagnosis was 15.8 years (range 12.0–18.7), and the median age at diagnosis of CCS was 14.0 years (range 12.0–17.0, P < 0.001). The group of DTC survivors included significantly more females than the CCS (91% vs 55%, P < 0.001). Median follow-up period for DTC survivors was 10.7 years (range 5.0–23.3), and for the CCS, it was 12.0 years (range 6.2–18.1, P = 0.088). DTC survivors and CCS did not differ significantly on other characteristics. Ninety-seven percent of the CCS group were Dutch; this was not significantly different from the DTC survivors (P = 1.000).
Table 3.
Characteristics of survivors of childhood DTC vs survivors of other childhood cancers (both diagnosed age ≥12 years old).
| DTC survivors | Other CCS | P value | |
|---|---|---|---|
| n | 35 | 76 | |
| At diagnosis | |||
| Age (years) | 15.8 (12.0–18.7) | 14.0 (12.0–17.0) | <0.001*† |
| Sex, n (%) | <0.001*§ | ||
| Female | 32 (91) | 42 (55) | |
| Male | 3 (9) | 34 (45) | |
| At follow-up | |||
| Age at evaluation (years) | 26.3 (18.8–35.7) | 26.2 (18.9–31.1) | 0.741† |
| Follow-up period (years) | 10.7 (5.0–23.3) | 12.0 (6.2–18.1) | 0.088† |
| Employment, n (%) | 1.000‡ | ||
| Employed and/or student | 34 (97) | 72 (95) | |
| Not employed and no student | 1 (3) | 3 (4) | |
| Missing | 0 (0) | 1 (1) | |
| Completed education, n (%) | 0.237§ | ||
| Low level | 6 (17) | 15 (20) | |
| Medium level | 13 (37) | 36 (47) | |
| High level | 16 (46) | 21 (28) | |
| Missing | 0 (0) | 4 (5) | |
| Marital status‖, n (%) | n.a. | ||
| Relationship | 23 (66) | – | |
| No relationship | 12 (34) | – | |
| Not married and not living together | – | 31 (55) | |
| Married or living together | – | 31 (41) | |
| Missing | – | 3 (4) |
†Mann–Whitney U test; ‡Fisher’s exact test; §Chi square test; ‖answer options regarding marital status of two different questionnaires were non-mergeable, therefore these are shown separately. Missing values were excluded for statistical testing (pairwise deletion). Continuous variables are presented as median (range). *Indicates a statistically significant difference (P < 0.01).
CCS, childhood cancer survivors; DTC, differentiated thyroid carcinoma; n.a., not applicable.
Psychosocial development
Scale scores: DTC survivors scored higher on social development than did CCS (both diagnosed at ≥12 years, 22 (21, 23) vs 21 (19, 22) out of 24, respectively, P = 0.005). DTC survivors also scored higher on social development compared to survivors of childhood leukemia diagnosed at age ≥12 years (22 (21, 23) vs 20 (18, 22) respectively, P = 0.001), but their scores were not significantly different from those of survivors of solid tumors or brain tumors. Scale scores of CCS and subgroups of CCS, compared to DTC survivors, did not differ on autonomy development and psychosexual development ( Table 4).
Table 4.
Scores on psychosocial developmental domains: comparison of survivors of childhood DTC with survivors of other childhood cancers (both diagnosed age ≥12 years old).
| DTC survivors | Childhood cancer survivors (CCS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total CCS | P value | Leukemia/lymphoma | P value | Solid tumors | P value | Brain tumors | P value | ||
| n | 35 | 76 | 45 | 23 | 8 | ||||
| Social development† | 22 (21, 23) | 21 (19, 22) | 0.005 | 20 (18, 22) | 0.001 | 22 (19, 23) | 0.164 | 21.5 (20.3, 22.8) | 0.391 |
| Autonomy development‡ | 9 (8, 10) | 9 (8, 10) | 0.688 | 10 (8, 10) | 0.753 | 9 (8, 10.5) | 0.677 | 9 (7.5, 11) | 0.890 |
| Psychosexual development§ | 8 (7, 8) | 7 (6, 8) | 0.020 | 7 (6, 8) | 0.040 | 7 (6, 8) | 0.083 | 5.5 (4.3, 8) | 0.097 |
Scores are shown as median (p25, p27). Comparisons between DTC survivors and other groups were performed using Mann–Whitney U tests. Higher scores indicate earlier achievement or achievement of more psychosocial developmental milestones; †scale ranges 12–24; ‡scale ranges 6–12; §scale ranges 4–8. Missing values were excluded from statistical testing (pairwise deletion). P values in bold indicate a significant value (P < 0.01).
CCS, childhood cancer survivors; DTC, differentiated thyroid carcinoma.
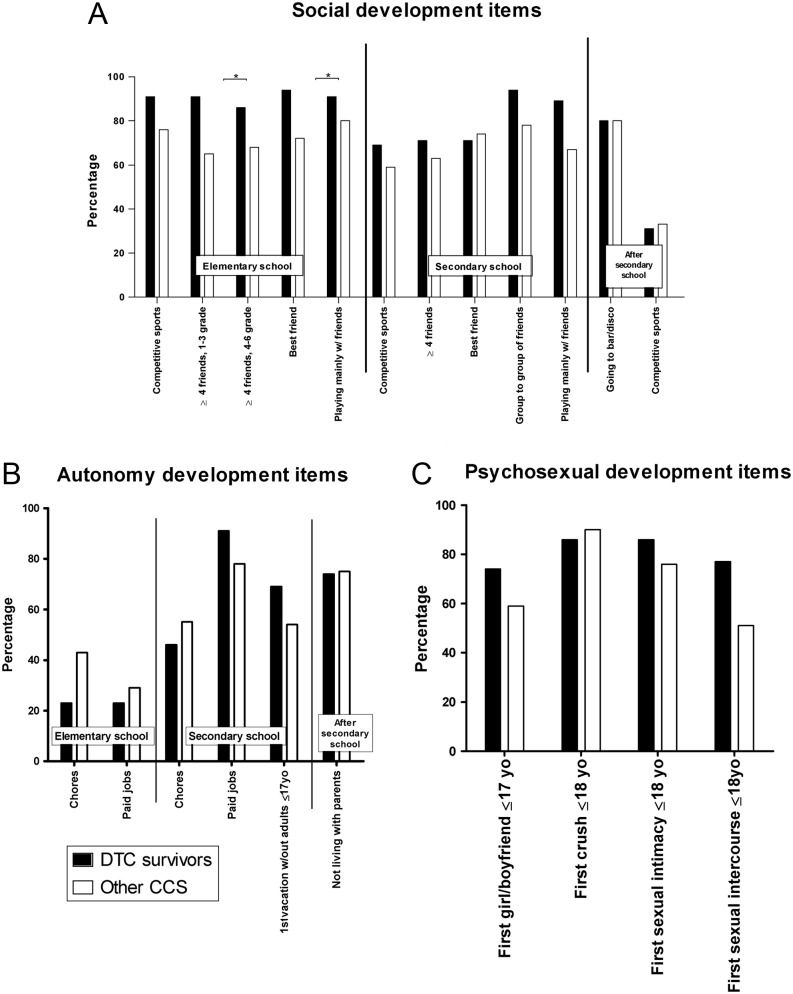
Item scores: Given the age at diagnosis of DTC survivors, only items that apply to the period during or after secondary school are discussed. Item scores regarding social development, autonomy development, and psychosexual development during or after secondary school did not differ between survivors of DTC and CCS diagnosed at ≥12 years old (Fig. 1 and Supplementary Table 2a, b and c).
Figure 1.
Scores of survivors of childhood DTC (n = 35) and childhood cancer survivors (CCS, n = 76) of other types of cancer, both diagnosed at age ≥12 years old, on individual items of the CoLQ. Scores indicate the percentage of the group that has reached the developmental milestone. Dark bars: DTC survivors, light bars: other CCS. An asterisk (*) indicates a P value <0.01.
Characteristics and psychosocial development scale scores of DTC survivors and CCS diagnosed at all ages are shown in Supplementary Tables 3 and 4.
Possible determinants of psychosocial development
Disease- treatment and, follow-up characteristics were not significantly associated with scores on domains of psychosocial development (Supplementary Table 5).
Discussion
The current study shows similar achievement of psychosocial developmental milestones in long-term survivors of childhood DTC as compared to non-affected groups. A slightly better social development in DTC survivors was observed compared to other CCS; differences with leukemia survivors were the most pronounced. Medical characteristics were not associated with a better or worse psychosocial development. Altogether, this indicates that even though the diagnosis of DTC during childhood is a life-altering event, the disease does not seem to have consequences related to altered psychosocial development. Current results align with the overall normal QoL reported in a previous study describing the same cohort (19).
Current results imply that survivors of childhood DTC may be less restricted in their psychosocial development than other CCS; previous studies report hampered psychosocial development in other CCS compared to a non-affected comparison group of similar sex and age (7). This indicates that the degree to which a child is hindered in his or her development depends on the type of cancer (13). For instance, survivors of brain tumors and CCS treated with neurotoxic treatment (i.e. cranial irradiation, intrathecal chemotherapy and specific intravenous chemotherapies) were found to be most vulnerable to impairment of psychosexual and social development as compared to other CCS (10, 11, 13, 20). However, this finding was not confirmed in the current study. This could be due to the fact that results in the current study were based on brain tumor survivors diagnosed during secondary school: brain tumor survivors diagnosed at younger ages were not represented while younger age at diagnosis of a brain tumor is associated with more developmental impairment (21).
Current results allow for conclusions regarding the majority of survivors of childhood DTC that was diagnosed during secondary school. Diagnosis of childhood DTC before secondary school occurs less frequently, therefore, concise conclusions cannot be made.
Factors that could interfere with psychosocial development (e.g. sex, age at diagnosis, age at follow-up, or follow-up duration Supplementary Table 5) were evaluated, but surprisingly showed no significant associations with psychosocial development. It may be that other, not investigated, factors may have played a role.
A first possible explanation for the normal psychosocial development of survivors of childhood DTC could lie in the excellent prognosis of the disease. In addition, the indolent course of DTC allows for flexibility in treatment, thus accommodating school schedules. In general, attending school benefits social development. The more favorable social development in DTC survivors compared to other CCS emphasizes this explanation. However, this statement remains speculative since we did not study school attendance. DTC treatment modalities differ from those of other types of cancer, using chemotherapeutics administered over longer periods of time. Another explanation could be the age upon diagnosis of most children with DTC. Relatively older upon diagnosis, these children as well as their parents have already experienced considerable developmental progression. For example, the foundations for social interactions (friendly as well as romantic) have already been formed. Lastly, fluctuations in thyroid hormone levels during periods of thyroid hormone withdrawal or long-term treatment effects of DTC, such as a weakened voice or the need for medication monitoring, may have less impact on psychosocial development than the aforementioned neurotoxicity and other physical or mental sequelae involved in other childhood cancer treatments (9, 10, 12, 22). It is common practice to substitute DTC survivors with levothyroxine after initial treatment and only use triiodothyronine in preparation for treatment with 131-I (23). As a result, current results suggest that neurological development of childhood DTC survivors is not affected by this approach to treatment.
Strengths and limitations
There is a great lack of knowledge regarding the long-term impact of childhood thyroid cancer on psychosocial domains (23). A strength of the current study is that it is the first to evaluate psychosocial development in a, though relatively small, unique cohort of survivors of childhood DTC. Using various groups for comparison allowed psychosocial development in these survivors to be placed in different perspectives. However, when interpreting the results, one must keep in mind the limitations of the study. One cannot use the results of this cross-sectional design in long-term survivors to elaborate about psychosocial development in the first 5 years after diagnosis, but eventual long-term results are promising. Not all predictors of psychosocial development were evaluated; for instance, we did not include the dependency of autonomy development on parenting behavior. Moreover, since most DTC survivors were diagnosed during secondary school, the items regarding elementary school were less relevant for the current population. However, the CoLQ is only validated containing all items (7).
Clinical implications
Current results may be reassuring for children newly diagnosed with DTC, and for their families and caregivers, regarding the possible psychosocial impact of their disease. However, the results do not imply that physicians need not monitor problems with psychosocial development in these survivors. This study presents data of a group of survivors, but individual differences should not be overlooked. Presenting patients with thyroid cancer as a good cancer makes them feel that physicians are downplaying their cancer experiences (24).
In conclusion, the current study aimed to evaluate the achievement of psychosocial developmental milestones in survivors of childhood DTC and found no delay in autonomy, social, and psychosexual domains after diagnosis compared to individuals non-affected with cancer. It did find a slightly more favorable development in DTC survivors compared to other CCS. However, before drawing definite conclusions, current findings need to be confirmed in subsequent studies.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by Stichting Kinderen Kankervrij (the Netherlands, Foundation Children Cancer-Free, Project 81). C M R is supported by the Dutch Cancer Society.
Author contribution statement
Marloes Nies: conceptualization, methodology, validation, software, formal analysis, investigation, data curation resources, writing – original draft, writing – review and editing, visualization. Bernadette L Dekker: methodology, validation, resources, writing – original draft, writing – review and editing. Esther Sulkers: conceptualization, resources, writing – original draft, writing – review and editing, supervision. Gea A Huizinga: conceptualization, methodology, validation, resources, writing – original draft, writing – review and editing, supervision. Mariëlle S Klein Hesselink: conceptualization, methodology, validation, investigation, data curation, writing – review and editing. Heleen Maurice-Stam: software, investigation, resources, writing – review and editing. Martha A Grootenhuis: investigation, resources, writing – review and editing. Adrienne H Brouwers: writing – review and editing. Johannes G M Burgerhof: software, formal analysis, writing – review and editing. Eveline W C M van Dam: resources, writing – review and editing. Bas Havekes: resources, writing – review and editing. Marry M van den Heuvel-Eibrink: resources, writing – review and editing. Eleonora P M Corssmit: resources, writing – review and editing. Leontien C M Kremer: writing – review and editing. Romana T Netea-Maier: resources, writing – review and editing. Heleen J H van der Pal: resources, writing – review and editing. Robin P Peeters: resources, writing – review and editing. John T M Plukker: writing – review and editing. Cécile M Ronckers: conceptualization, writing – review and editing. Hanneke M van Santen: conceptualization, resources, writing – review and editing. Anouk N A van der Horst-Schrivers: resources writing – original draft, writing – review and editing, supervision. Wim J E Tissing: conceptualization, methodology, validation, resources writing – original draft, writing – review and editing, supervision, project administration, funding acquisition. Gianni Bocca: conceptualization, methodology, validation, resources writing – original draft, writing – review and editing, supervision, project administration, funding acquisition. Thera P Links: conceptualization, methodology, validation, resources, data curation, writing – original draft, writing – review and editing, supervision, project administration, funding acquisition.
Acknowledgements
The authors are grateful to their colleagues in the Netherlands for referring patients for this study. They thank the registration teams of the Comprehensive Cancer Centers for the collection of data for the Netherlands Cancer Registry, as well as the scientific staff of the Netherlands Cancer Registry.
References
- 1.Dermody S, Walls A, Harley EH., Jr. Pediatric thyroid cancer: an update from the SEER database 2007–2012. International Journal of Pediatric Otorhinolaryngology 2016. 89 121–126. ( 10.1016/j.ijporl.2016.08.005) [DOI] [PubMed] [Google Scholar]
- 2.Steliarova-Foucher E, Stiller CA, Pukkala E, Lacour B, Plesko I, Parkin DM. Thyroid cancer incidence and survival among European children and adolescents (1978–1997): report from the Automated Childhood Cancer Information System project. European Journal of Cancer 2006. 42 2150–2169. ( 10.1016/j.ejca.2006.06.001) [DOI] [PubMed] [Google Scholar]
- 3.Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009. Pediatrics 2014. 134 e945–e955. ( 10.1542/peds.2013-3926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. Journal of Surgical Research 2009. 156 167–172. ( 10.1016/j.jss.2009.03.098) [DOI] [PubMed] [Google Scholar]
- 5.Klein Hesselink MS, Nies M, Bocca G, Brouwers AH, Burgerhof JG, van Dam EW, Havekes B, van den Heuvel-Eibrink MM, Corssmit EP, Kremer LC. et al Pediatric differentiated thyroid carcinoma in the Netherlands: a Nationwide Follow-Up study. Journal of Clinical Endocrinology and Metabolism 2016. 101 2031–2039. ( 10.1210/jc.2015-3290) [DOI] [PubMed] [Google Scholar]
- 6.Hay WJ, Levin M, Deterding R, Abzug M. Current Diagnosis and Treatment Pediatrics, 23rd ed. New York, NY: McGraw-Hill, 2016. [Google Scholar]
- 7.Stam H, Grootenhuis MA, Last BF. The course of life of survivors of childhood cancer. Psycho-Oncology 2005. 14 227–238. ( 10.1002/pon.839) [DOI] [PubMed] [Google Scholar]
- 8.Meeus W. Adolescent psychosocial development: a review of longitudinal models and research. Developmental Psychology 2016. 52 1969–1993. ( 10.1037/dev0000243) [DOI] [PubMed] [Google Scholar]
- 9.Font-Gonzalez A, Feijen EL, Sieswerda E, van Dulmen-den Broeder E, Grootenhuis M, Maurice-Stam H, Caron H, Essink-Bot ML, van der Pal H, Geskus R. et al Social outcomes in adult survivors of childhood cancer compared to the general population: linkage of a cohort with population registers. Psycho-Oncology 2016. 25 933–941. ( 10.1002/pon.4040) [DOI] [PubMed] [Google Scholar]
- 10.Lehmann V, Tuinman MA, Keim MC, Winning AM, Olshefski RS, Bajwa RPS, Hagedoorn M, Gerhardt CA. Psychosexual development and satisfaction in long-term survivors of childhood cancer: neurotoxic treatment intensity as a risk indicator. Cancer 2017. 123 1869–1876. ( 10.1002/cncr.30513) [DOI] [PubMed] [Google Scholar]
- 11.Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B, Tersak JM, Ness KK. Social outcomes in the Childhood Cancer Survivor Study cohort. Journal of Clinical Oncology 2009. 27 2390–2395. ( 10.1200/JCO.2008.21.1458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frederick NN, Recklitis CJ, Blackmon JE, Bober S. Sexual dysfunction in young adult survivors of childhood cancer. Pediatric Blood and Cancer 2016. 63 1622–1628. ( 10.1002/pbc.26041) [DOI] [PubMed] [Google Scholar]
- 13.Maurice-Stam H, Grootenhuis MA, Caron HN, Last BF. Course of life of survivors of childhood cancer is related to quality of life in young adulthood. Journal of Psychosocial Oncology 2007. 25 43–58. ( 10.1300/J077v25n03_03) [DOI] [PubMed] [Google Scholar]
- 14.Maxon HR, 3rd, Smith HS. Radioiodine-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinology and Metabolism Clinics of North America 1990. 19 685–718. [PubMed] [Google Scholar]
- 15.Rivkees SA, Mazzaferri EL, Verburg FA, Reiners C, Luster M, Breuer CK, Dinauer CA, Udelsman R. The treatment of differentiated thyroid cancer in children: emphasis on surgical approach and radioactive iodine therapy. Endocrine Reviews 2011. 32 798–826. ( 10.1210/er.2011-0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder PR, Haugen BR, Pacini F, Reiners C, Schlumberger M, Sherman SI, Cooper DS, Schuff KG, Braverman LE, Skarulis MC. et al A comparison of short-term changes in health-related quality of life in thyroid carcinoma patients undergoing diagnostic evaluation with recombinant human thyrotropin compared with thyroid hormone withdrawal. Journal of Clinical Endocrinology and Metabolism 2006. 91 878–884. ( 10.1210/jc.2005-2064) [DOI] [PubMed] [Google Scholar]
- 17.Bauer AJ. Approach to the pediatric patient with Graves’ disease: when is definitive therapy warranted? Journal of Clinical Endocrinology and Metabolism 2011. 96 580–588. ( 10.1210/jc.2010-0898) [DOI] [PubMed] [Google Scholar]
- 18.Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. Journal of Clinical Endocrinology and Metabolism 2001. 86 1447–1463. ( 10.1210/jcem.86.4.7407) [DOI] [PubMed] [Google Scholar]
- 19.Nies M, Klein Hesselink MS, Huizinga GA, Sulkers E, Brouwers AH, Burgerhof JG, van Dam EW, Havekes B, van den Heuvel-Eibrink MM, Corssmit EP. et al Long-term quality of life in adult survivors of pediatric differentiated thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 2016. 102 1218–1226. ( 10.1210/jc.2016-2246) [DOI] [PubMed] [Google Scholar]
- 20.Howard AF, Tan de Bibiana J, Smillie K, Goddard K, Pritchard S, Olson R, Kazanjian A. Trajectories of social isolation in adult survivors of childhood cancer. Journal of Cancer Survivorship: Research and Practice 2014. 8 80–93. ( 10.1007/s11764-013-0321-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reimers TS, Ehrenfels S, Mortensen EL, Schmiegelow M, Sonderkaer S, Carstensen H, Schmiegelow K, Muller J. Cognitive deficits in long-term survivors of childhood brain tumors: Identification of predictive factors. Medical and Pediatric Oncology 2003. 40 26–34. ( 10.1002/mpo.10211) [DOI] [PubMed] [Google Scholar]
- 22.Wengenroth L, Rueegg CS, Michel G, Gianinazzi ME, Essig S, von der Weid NX, Grotzer M, Kuehni CE. & Swiss Paediatric Oncology Group SPOG. Concentration, working speed and memory: cognitive problems in young childhood cancer survivors and their siblings. Pediatric Blood and Cancer 2015. 62 875–882. ( 10.1002/pbc.25396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M. et al Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015. 25 716–759. ( 10.1089/thy.2014.0460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Easley J, Miedema B, Robinson L. It’s the ‘good’ cancer, so who cares? Perceived lack of support among young thyroid cancer survivors. Oncology Nursing Forum 2013. 40 596–600. ( 10.1188/13.ONF.596-600) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a