INTRODUCTION
Among all the chronic autoimmune rheumatic disorders, Sjögren’s disease (SD) is among the most difficult to evaluate and manage. Clinicians are frequently challenged to differentiate symptoms related to disease activity from those that result from pre-existing damage. Additionally, the presence of multiple SD-related comorbidities, including anxiety, depression and fibromyalgia,1,2 may influence the severity of patient symptoms and further complicate the evaluation process. Furthermore, in the clinical setting, a thorough investigation of patient complaints will often reveal multiple potential causes for the same symptom.3
Presently, no cure or remittive agent for SD exists. Treatment goals remain (1) symptom palliation, (2) prevention of complications and, (3) for rheumatologists, proper selection of patients for immunosuppressive therapy. In SD the frequent occurrence of oral and ocular manifestations and complications also mandates a multidisciplinary approach to optimize care. Unfortunately, the paucity of well-designed, controlled studies in the SD medical and dental literature frequently leaves the clinician with little guidance. Therefore, the approach to treating SD in the United States has differed widely among various institutions and providers.
HIGH BURDEN OF ILLNESS
Several studies have documented that quality of life (QOL) is diminished in primary SD subjects compared with healthy controls1,4,5 and, in some cases, diminished to the degree seen in other subject groups, such as those with rheumatoid arthritis (RA) and/or fibromyalgia.5 One study found less overall end organ damage in primary SD compared with systemic lupus (SLE) but concluded that the degree of functional disability was the same for both disorders.6 Patients with SD may also incur increased health care costs7,8 and, not surprisingly, increased dental care costs.9 A study from England reported that annual health care costs in primary SD (£2188) were twice that of community controls (£949) and comparable to those of subjects with RA (£2693).8 Thus, the burden of illness in primary SD is quite substantial.
GUIDELINES DEVELOPMENT
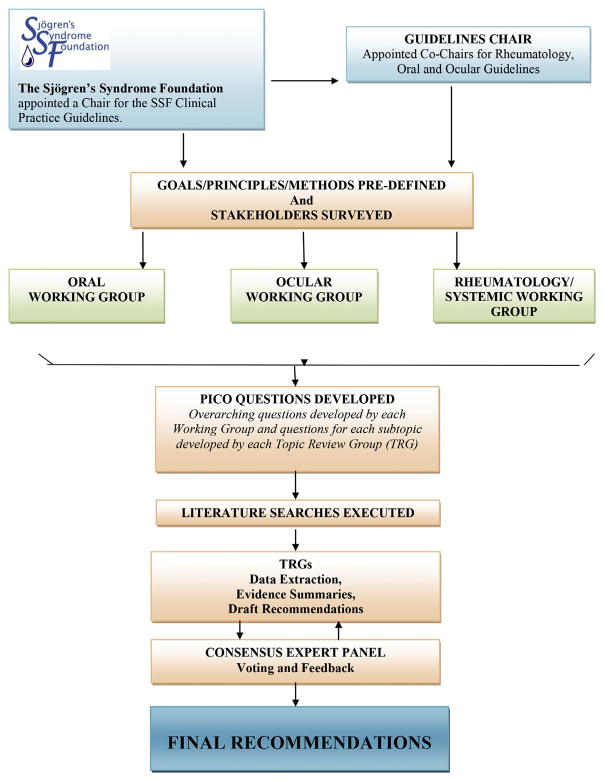
In 2010, the Sjögren’s Syndrome Foundation (SSF) enlisted the help of more than 200 professional volunteers nationwide to develop the first ever clinical practice guidelines (CPGs) for SD patients in the United States. The framework for this process is summarized in Fig. 1. The goals were to improve the quality and consistency of care and to ease the uncertainty of providers, patients, and insurers regarding coverage and reimbursement issues. All working groups followed a highly rigorous process with guidance from major professional organizations including the Institute of Medicine, American Dental Association, American Academy of Ophthalmology (AAO), and the American College of Rheumatology (ACR). The Appraisal of Guidelines for Research and Evaluation (AGREE) was used.10,11 Overreaching methodological principles included transparency, involvement of key stakeholders, and consistency of methods. All participants completed ACR conflict of interest forms.
Fig. 1.
The SSF clinical practice guidelines process.
DEFINING CLINICAL ISSUES
All key stakeholders, including patients and providers of various disciplines, from academia and the community, were surveyed to identify pertinent clinical issues. Topics were assigned to 1 of 3 working groups: Oral, Ocular, or Rheumatologic-Systemic; prioritized; and reformatted as PICO (population, intervention, comparison, and outcome) questions.12 Bias was reduced as much as possible by defining a priori all methodology elements, including protocol worksheets, data extraction tables, and literature search terms.
TOPIC REVIEW AND THE DELPHI CONSENSUS PROCESS
Topic review groups (TRGs) of at least 2 to 3 providers were established for each clinical question to review the medical or dental literature, complete data extraction tables, and write an evidence summary. The TRG, as a whole, rated the strength of the evidence, developed a draft recommendation, and rated the strength of the recommendation based on a variation of grading of recommendations, assessment, development, and evaluation (GRADE).13 For the dry eye guidelines the AAO Preferred Practice Pattern guidelines for level of evidence were also followed.14 Any definition of primary SD (ie, SD without an associated connective tissue disorder) based on published classification criteria were accepted for guideline development. Data on patients with secondary SD were not used in this analysis.
A consensus expert panel (CEP) of pertinent specialists, providers from other disciplines, and stakeholders provided feedback and voted on each recommendation. A modified Delphi process was used with 75% agreement required for consensus. Revision of guidelines that failed to achieve consensus was permitted up to 3 rounds before the recommendation was discarded.
Guidelines for Oral Management
Rationale
Salivary dysfunction in SD can lead to serious and costly oral health complications. Study subjects with SD have significantly more dental caries, tooth extractions, and higher lifetime dental costs then do controls.15 SD patients who lose their dentition often have problems with denture wear and find that dental implants provide the only viable long-term alternative. Most patients in the United States lack sufficient dental insurance to cover these expenses and pay most costs out-of-pocket. It is, therefore, incumbent on every dentist and oral medicine specialist to consider the diagnosis of SD in patients with accelerated caries and initiate a management program for caries prophylaxis early in the disease course.
Recommendations
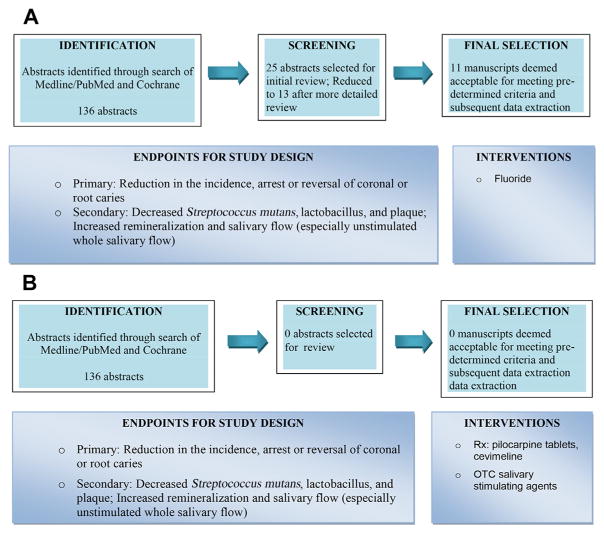
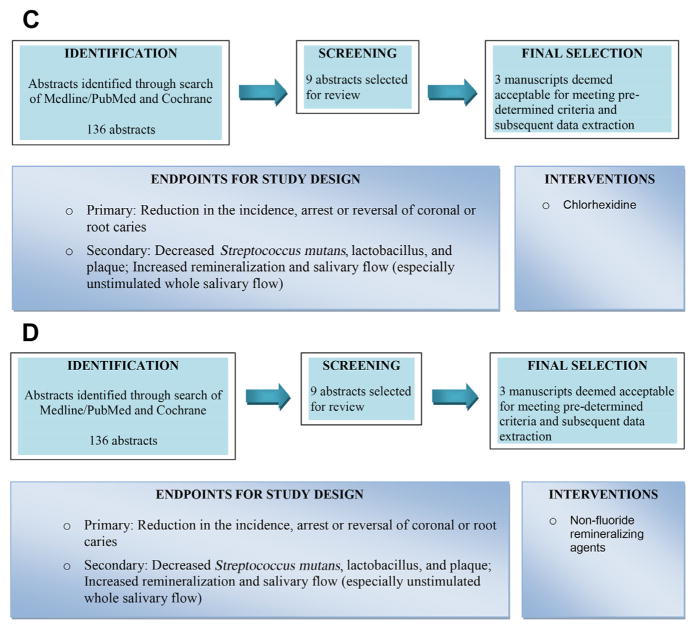
To develop CPGs for caries prophylaxis in SD, the Oral Working Group reviewed dental literature concerning the use of fluoride, salivary stimulation, antimicrobials, and remineralizing agents. Fig. 2 is a summary of this process. Further details, including findings from extensive literature reviews, protocol worksheets, data extraction tables, and summaries of dental evidence, have been previously reported.16 The clinical questions and oral guidelines for caries prophylaxis in SD are summarized in Box 1. The clinician is encouraged to consider all recommendations as potential therapies to be used either singly or in combination for the individual patient.
Fig. 2.
(A) Review of fluoride use for caries prevention in SD. (B) Review of salivary stimulation for caries prevention in SD. OTC, over-the-counter. (C) Review of antimicrobials for caries prevention in SD. (D) Review of nonfluoride remineralizing agents for caries prevention in SD.
Box 1. Oral management guidelines for caries prophylaxis.
| Use of fluoride |
| Clinical questions |
|
|
|
| Recommendation |
|
|
| Topical fluoride should be used in SD patients with dry mouth. No information was available to answer the second question. Strength of recommendation: strong |
| Salivary stimulation |
| Clinical questions |
|
|
|
| Recommendation |
|
|
| While no studies to date link improved salivary function in SS patients to caries prevention, it is generally understood in the oral health community that increasing saliva may contribute to decreased caries incidence. Based on its expert opinion, the TRG recommends that SD patients with dry mouth increase saliva through gustatory, masticatory stimulation, and pharmaceutical agents; for example, sugar-free lozenges and/or chewing gum, xylitol, mannitol, and the prescription medications pilocarpine and cevimeline. Strength of recommendation: weak |
| Antimicrobials |
| Clinical questions |
|
|
|
| Recommendation |
|
|
| Chlorhexidine administered by varnish, gel, or rinse may be considered in SD patients with dry mouth and a high root caries rate. Strength of recommendation: weak |
| Nonfluoride remineralizing agents |
| Clinical questions |
|
|
|
| Recommendation |
|
|
| Nonfluoride remineralizing agents may be considered as an adjunct therapy in SD patients with dry mouth and a high root caries rate. Insufficient information was available to answer the second question. Strength of recommendation: moderate |
Guidelines for Ocular Management
Rationale
At least 2 prior surveys of SD patients conducted by the SSF have documented dry eye to be the single most troublesome symptom in SD.17,18 Additionally, dry eye is recognized as a debilitating symptom in the US Social Security Administration Disability Guidelines, which included SD as a specific listing for the first time in 2006. Dry eye can seriously compromise QOL19 and at least 1 study suggested that the impact of dry eye on QOL was comparable to that seen in patients with moderate to severe angina.20
Terminology
The development of ocular guidelines for the evaluation and management of dry eyes for SD used the definition of dry eye and other terminology reported in the 2007 International Dry Eye Workshop (DEWS).21 The DEWS report defined terms to characterize patient subsets, as well as clinical issues, and defined dry eye as, “a multi-factorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.”
Dry eye is classified into 2 categories: (1) aqueous-deficient dry eye related to decreased tear production and (2) evaporative dry eye most commonly caused by meibomian gland dysfunction (blepharitis). Both types of dry eye may occur in SD and often coexist in the same individual. Most patients are symptomatic and describe their discomfort as burning, stinging, foreign body sensation (grittiness), itching, or pain. Symptoms of visual disturbance may include fluctuation or blurring of vision, especially during reading or computer work, with transient improvement after blinking or the instillation of artificial tears. Interestingly, a recent study reported that as many as 40% of SD subjects with clear objective evidence of dry eyes had no symptoms, thus underscoring the necessity to thoroughly evaluate all SD patients for dry eye regardless of symptoms.22
Evaluation
The Ocular Working Group stressed the importance of comprehensive assessment of the SD patient to determine the cause and severity of dry eye before recommending treatment. This process involves the assessment of key ocular symptoms as described previously, as well as the examination of several objective parameters, including tear production, tear film stability, tear osmolarity, lid margin disease, and ocular surface damage. A summary of the diagnostic evaluation and recommended order of tests is included in Table 1.
Table 1.
Evaluation of dry eye
| Observation or Test | What is Examined | Tools | Sign of Dry Eye |
|---|---|---|---|
| 1. Direct Observation | Tear function, tear stability and ocular surface | Corneal light reflex biomicroscope (additional instruments are available in the research setting) | Tear film instability Ocular surface irregularity |
| Meibomian gland disease | Biomicroscope | Presence of foamy debris | |
|
| |||
| 2. Osmolarity | Tear composition: levels of inflammatory mediators in tear film and conjunctiva | Osmometer (mostly limited to research settings but units are increasingly available for clinical practice) | Elevated osmolarity of the tear film |
|
| |||
| 3. Fluorescein Tear Break-Up Time | Tear film stability | Fluorescein dye Slit-lamp |
Rapid tear film breakup (<10 s) |
|
| |||
| 4. Corneal Staining | Ocular surface evaluation | Fluorescein Rose bengal or lissamine green dye |
Staining observed of mucus strands, filaments, and unprotected areas of the epithelium Staining patterns can designate severity of dry eye |
|
| |||
| 5. Schirmer 1 Test or Phenol Red Thread Test | Tear secretion rate | Schirmer tear test strip Small thread impregnated with phenol red dye A fluorophotometer is more sensitive than either of these but is usually not available in the clinical setting |
Schirmer 1: <5–7 mm of wetting after 5 min Phenol red thread test: <10 mm of wetting after 15 s |
Recommendations
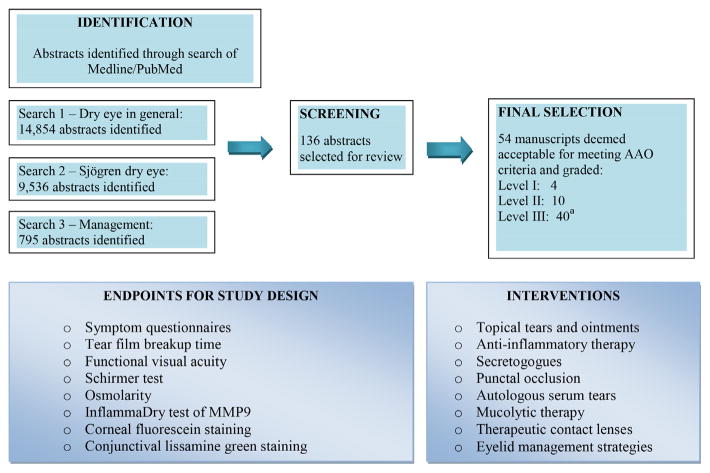
To develop SD-specific ocular CPGs, the dry eye literature was reviewed according to preselected criteria as summarized in Fig. 3. Studies on non-SD dry eye disease also guided management whenever considered essential. The CPGs for dry eye management in SD are outlined in Table 2 and organized by type of dry eye disease (aqueous deficient vs meibomian gland dysfunction) and level of severity. The latter is determined mainly by the presence or absence of ocular surface staining and the staining pattern. Conjunctival staining usually occurs before corneal staining and medial staining often occurs before temporal conjunctival staining. Early corneal staining is most often observed in the inferonasal cornea with central staining occurring later. A classic pattern of interpalpebral staining across the medial conjunctiva, cornea, and temporal conjunctiva, or the presence of ocular filaments, indicates advanced dry eye disease. If the results of treatment of the SD patient at a given level of severity are insufficient, the eye care provider is encouraged to follow recommendations for the next level of severity.
Fig. 3.
Review of treatments for dry eye. a Best evidence.
Table 2.
Guidelines for management of dry eye based on cause and severity
| Diagnosis | Treatment | Severity Level 1a | Severity Level 2 | Severity Level 3 | Severity Level 4 | Evidenceb | Recommendationc | |
|---|---|---|---|---|---|---|---|
| Dry eye disease: aqueous deficiency without meibomian gland disease | Education and environment or diet modification | — | — | — | Good | Strong | |
| Elimination of offending systemic medication | Good | Strong | |||||
| Artificial tears, gels, ointments | Good | Strong | |||||
| — | Omega 3 essential fatty acid supplement | — | — | Moderate | Moderate | ||
| Anti-inflammatory therapy: cyclosporine | Good | Moderate | |||||
| Anti-inflammatory therapy: pulse steroids | Good | Moderate | |||||
| Punctal plugs | Good | Moderate | |||||
| Secretagogues | Good | Moderate | |||||
| Moisture chamber spectacles | Good | Moderate | |||||
| — | — | Topical autologous serum | — | Good | Moderate | ||
| Contact lenses | Good | Moderate | |||||
| Permanent punctal occlusion | Good | Moderate | |||||
| — | — | — | Systemic anti- inflammatory medication | Moderate | Weak | ||
| Eyelid surgery | Good | Moderate | |||||
| Dry eye disease: aqueous deficiency with meibomian gland disease | Education and environment or diet modification | — | — | — | Good | Strong | |
| Elimination of offending systemic medication | Good | Strong | |||||
| Artificial tears with lipid component | Good | Strong | |||||
| Eyelid therapy: warm compress, massage | Good | Strong | |||||
| — | Omega 3 essential fatty acid supplement | — | — | Moderate | Moderate | ||
| Anti-inflammatory therapy: cyclosporine | Good | Moderate | |||||
| Anti-inflammatory therapy: topical steroids | Good | Moderate | |||||
| Topical azithromycin | Good | Moderate | |||||
| Liposomal spray | Good | Moderate | |||||
| Possible oral doxycycline | Good | Moderate | |||||
| Expression of meibomian glands | Good | Moderate | |||||
| Punctal plugs | Good | Moderate | |||||
| Secretagogues | Good | Moderate | |||||
| Moisture chamber spectacles | Good | Moderate | |||||
| — | — | Topical autologous serum | — | Good | Moderate | ||
| Contact lenses | Good | Moderate | |||||
| Permanent punctal occlusion | Good | Moderate | |||||
| LipiFlow pulsed thermal compression | Insufficient | Weak | |||||
| Probing of meibomian gland | Insufficient | Weak | |||||
| — | — | — | Systemic anti- inflammatory medication | Moderate | Weak | ||
| Eyelid surgery | Good | Moderate | |||||
Assumes use of the International DEWS severity scale.
Evidence is graded as good, moderate, and insufficient.
Recommendations are strong, moderate, and weak.
A detailed description of therapeutic options and the evidence that supports these recommendations has been previously reported.23 Patient education regarding the nature of the problem, aggravating factors and treatment goals is essential to successful management. Strategies include use of topical tear substitutes, gels and ointments, anti-inflammatory therapies, secretagogues, punctal occlusion, autologous serum tears, mucolytic agents, therapeutic contact lenses, and management of eyelid disease.
Guidelines for Rheumatologic-Systemic Management
Rationale
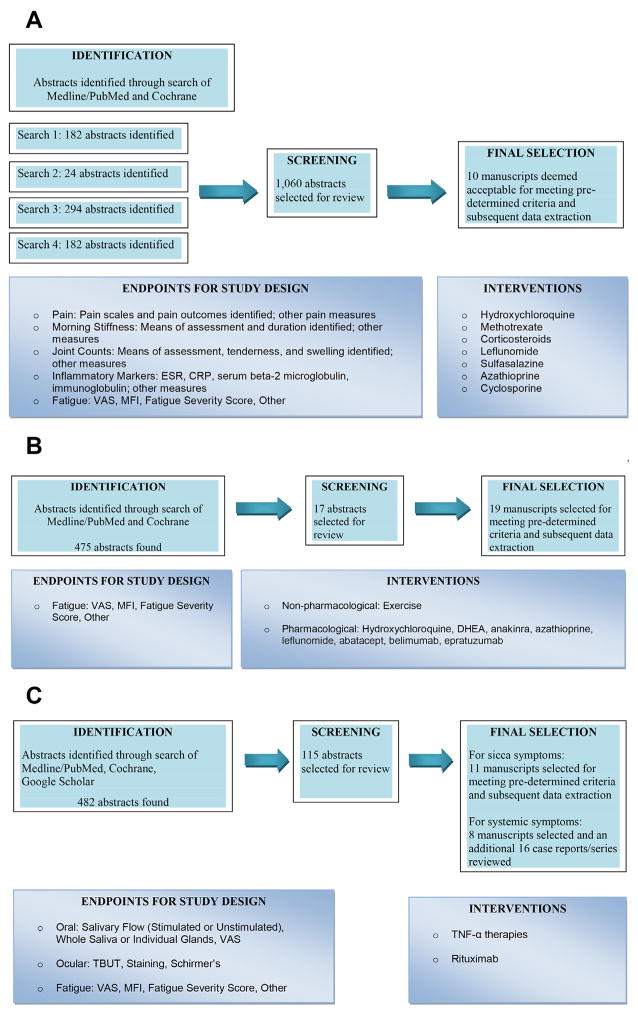
Morbidity in SD results not only from untreated sicca but also from internal organ involvement (Table 3) and an increased incidence of non-Hodgkin B cell lymphomas.24 The current treatment algorithms for serious organ manifestations of SD are frequently borrowed from management strategies used for closely related disorders such as SLE and RA. Initially, 97 potential topics for guideline development were identified by review of stakeholder surveys. After further discussion, the list was narrowed to 16 topics that were ranked by vote of the Rheumatologic-Systemic Working Group. Initial efforts were focused on the 3 most important topic areas: treatment of inflammatory musculoskeletal pain, management of fatigue, and the use of biological medications in SD. Study selection criteria and results of literature review for the first 3 topics are summarized in Fig. 4. Carsons and colleagues25 provide further details, including findings from extensive literature reviews, protocol worksheets, data extraction tables, evidence summaries, and discussion of the recommendations.
Table 3.
Extraoral and extraglandular manifestations of Sjögren’s disease
| Area Affected | Symptoms |
|---|---|
| General | Fatigue, malaise, fevers |
| Ear, nose, and throat | Epistaxis, otitis media, conduction deafness, recurrent sinusitis |
| Gastrointestinal | Esophageal dysmotility, esophageal webs, reflux, atrophic gastritis, autoimmune pancreatitis, liver disease |
| Genitourinary | Vaginitis sicca, interstitial cystitis |
| Hematologic | Anemia, leukopenia, lymphopenia, cryoglobulinemia, lymphoma |
| Lungs | Xerotrachea, recurrent bronchitis or pneumonia, interstitial pneumonitis, pulmonary fibrosis, lung nodules, bronchiectasis, bronchiolitis obliterans with organizing pneumonia |
| Neurologic | Peripheral neuropathy, cranial neuropathy, autonomic neuropathy, central nervous system involvement |
| Renal | Interstitial nephritis, hyposthenuria, renal tubular acidosis (Types I, II), glomerulonephritis (rare) |
| Rheumatologic | Arthralgias, polyarthritis, myalgias, myositis, Raynaud’s phenomenon |
| Skin | Xeroderma, purpura, urticaria, vasculitis |
Fig. 4.
(A) Review of disease-modifying antirheumatic drug (DMARD) use for musculoskeletal pain in SD. (B) Review of treatments for fatigue in SD. (C) Review of biological medication use in SD.
Use of disease-modifying antirheumatic drugs for inflammatory musculoskeletal pain
Inflammatory arthralgias, myalgias and, in some cases, synovitis, can occur in SD and contribute to disease morbidity and patient disability. Guidelines for the use of disease-modifying antirheumatic drugs (DMARDS) for treatment of inflammatory musculoskeletal pain are represented in Box 2 and use a stepwise approach with hydroxychloroquine (HCQ) listed as first-line therapy. Although a recent randomized controlled study of HCQ in SD failed to meet the primary endpoint for pain,26 the moderate strength of the recommendation and 92% agreement of the CEP as guided by the modified Delphi process is based on the significant reported improvement of inflammatory markers and musculoskeletal pain in other studies,27–30 a moderate level of confidence that the guideline recommendation reflected best clinical practice and that sufficient evidence existed that potential benefits exceeded potential harms. In instances in which therapies were deemed equivalent with similar safety profiles, recommendations were grouped together to allow the physician final choice based on clinical experience and patient profile.
Box 2. Guidelines for disease-modifying antirheumatic drug use for musculoskeletal pain in Sjögren’s disease.
| DMARDs FOR INFLAMMATORY MSK PAIN |
Recommendations are provided with the following caveats and then listed in a step-by-step process:
|
| Recommendation 1: Hydroxychloroquine (HCQ) |
|
|
| A first-line of treatment of inflammatory musculoskeletal pain in primary SD should be HCQ. Strength of recommendation: moderate |
| Recommendation 2: Methotrexate (MTX) |
|
|
| If HCQ is not effective in the treatment of inflammatory musculoskeletal pain in primary SD, MTX alone may be considered. Strength of recommendation: moderate or |
| Recommendation 3: HCQ plus MTX |
|
|
| If either HCQ or MTX alone is not effective in the treatment of inflammatory musculoskeletal pain in primary SD, HCQ plus MTX may be considered. Strength of recommendation: moderate |
| Recommendation 4a: Short-term corticosteroids |
|
|
| If HCQ plus MTX is not effective in the treatment of inflammatory musculoskeletal pain in primary SD, short-term (1 month or less) corticosteroids of 15 mg or less a day may be considered. Strength of recommendation: strong |
| Recommendation 4b: Long-term corticosteroids |
|
|
| Long-term (more than 1 month) 15 mg or less a day corticosteroids may be useful in the management of inflammatory musculoskeletal pain in primary SD but efforts should be made to find a steroid-sparing agent as soon as possible. Strength of recommendation: moderate The following 3 (5, 6, and 7a and 7b) recommendations are numbered in order of the TRG’s preference and experience. However, the TRG is grouping these together to allow the physician to choose any of the following and in any order based on that physician’s experience and the individual patient. |
| Recommendation 5: Leflunomide |
|
|
| If HCQ and/or MTX or short-term (1 month or less) corticosteroids are not effective in the treatment of inflammatory musculoskeletal pain in primary SD, leflunomide may be considered. Strength of recommendation: weak |
| Recommendation 6: Sulfasalazine |
|
|
| If HCQ and/or MTX, corticosteroids, or leflunomide (Arava) are not effective in the treatment of inflammatory musculoskeletal pain in primary SD, sulfasalazine may be considered. Strength of recommendation: weak |
| Recommendation 7a: Azathioprine |
|
|
| If HCQ and/or MTX, corticosteroids, leflunomide, or sulfasalazine are not effective in the treatment of inflammatory musculoskeletal pain in primary SD, azathioprine may be considered. Strength of recommendation: weak |
| Recommendation 7b: Potential change in order |
|
|
| If major organ involvement occurs in the primary SD patient, azathioprine may be a better choice than leflunomide or sulfasalazine for the treatment of all complications, including inflammatory musculoskeletal pain. Strength of recommendation: moderate |
| Recommendation 8: Cyclosporine |
|
|
| If HCQ and/or MTX, corticosteroids, leflunomide, azathioprine, or sulfasalazine are not effective in the treatment of inflammatory musculoskeletal pain in primary SD, cyclosporine may be considered. Strength of recommendation: weak |
Methotrexate (MTX) was determined to be second-line therapy after HCQ based on some evidence for a true net effect30,31 and moderate confidence regarding a good safety profile. Although there is no reported evidence to support this guideline, combined therapy with HCQ and MTX was recommended as the third step if either drug alone was ineffective. This statement was based on the collective experience of the TRG-CEP and the knowledge that both therapies have been successfully combined to treat arthritis in closely related autoimmune rheumatic disorders (eg, RA, SLE). When adding MTX to HCQ, physicians may choose to lower the dose of HCQ as maintenance therapy.
Although no formal studies have reported efficacy on the short-term (≤1 month) use of corticosteroids (≤15 mg/day) for inflammatory musculoskeletal pain in SD, this practice is frequently followed in the United States and, therefore, listed as fourth-line therapy when the first 3 treatment approaches fail. There was a strong level of agreement among the CEP that this treatment approach reflects best clinical practice. Longer-term use of corticosteroids at similar doses was deemed equally efficacious but the strength of recommendation was lowered to moderate due to concern over potential side effects. Although this task can be quite challenging, the CEP recommended that every possible effort be made to find a steroid-sparing agent as soon as possible in glucocorticoid-responsive SD patients.
The algorithm concluded with grouping of leflunomide, sulfasalazine, and azathioprine together, followed by listing cyclosporine as a potential therapy for inflammatory musculoskeletal pain in SD. Evidence for these recommendations is scant32,33 and clinical experience with these medications in SD limited. One exception was emphasized. In situations when the SD patient has significant extraglandular involvement in association with inflammatory musculoskeletal pain, azathioprine would be preferred because of anecdotal evidence, case reports, and case series suggesting benefit for SD manifestations, including central nervous system disease, peripheral neuropathies, interstitial lung disease, and leukocytoclastic vasculitis.
Management of fatigue
Treatment of fatigue is among the greatest therapeutic challenges in the management of SD.34 In guidelines development, the TRG-CEP emphasized that causes of fatigue in SS are numerous3 and that proper therapy necessitates a thoughtful and comprehensive diagnostic approach. Guideline recommendations for fatigue are summarized in Box 3.
Box 3. Guidelines for treatment of fatigue in Sjögren’s disease.
| Fatigue |
| Recommendation 1: Exercise |
|
|
| Education about self-care measures should include advice about exercise to reduce fatigue in SD. Strength of recommendation: strong |
| Recommendation 2: Dehydroepiandrosterone (DHEA) |
|
|
| DHEA is not recommended for treatment of fatigue in SD. Strength of recommendation: strong |
| Recommendation 3: HCQ |
|
|
| HCQ may be considered in selected situations to treat fatigue in SD. Strength of recommendation: weak |
| Recommendation 4: Tumor necrosis factor (TNF)-α inhibitors |
|
|
| Neither etanercept nor infliximab is recommended for treatment of fatigue in SD. Strength of recommendation: strong For the following 10 therapeutic options addressed by the Fatigue TRG, there was insufficient evidence to issue a recommendation:
|
The only strongly recommended treatment of fatigue in SD was exercise, which provides the same benefit for SD patients35 that is seen in patients with RA, SLE, or multiple sclerosis. The panel also recommended that “hydroxychloroquine may be considered in selected situations to treat fatigue in Sjögren’s.” This approach is mainly based on uncontrolled studies as well as clinical experience and a favorable safety profile in both lupus and SD, given that evidence of benefit in placebo-controlled trials is lacking. Nevertheless, comments from the CEP during the first 2 voting rounds demonstrated strong support for keeping this option, especially in light of the perceived limitations of the controlled trials. When the draft recommendation was revised from “HCQ should not be used for fatigue” to the current recommendation listed previously, consensus agreement increased by 30% and enabled inclusion of this recommendation in the final guidelines. Currently, the CEP recommend against the use of dehydroepiandrosterone (DHEA)36,37 and tumor necrosis factor (TNF)-α inhibitors38,39 for fatigue, and found insufficient data and/or existing clinical experience to recommend use of anakinra, abatacept, belimumab, or epratuzumab for this indication.
Use of biologics in Sjögren’s disease
Recently, the study of biological therapies as potential remittive agents for SD has generated tremendous interest in the SD community. CPGs for use of biologics in SD are summarized in Box 4. The CEP recommended against the use of TNF-α inhibitors in SD, based on findings from 2 earlier studies,38,39 but emphasized this recommendation does not preclude the use of these agents in SD patients if needed for other indications (eg, overlapping manifestations with RA). The committee concluded that, among the various biologics studied to date, some evidence exists to justify the use of rituximab for sicca manifestations in selected patients with SD who otherwise fail more conservative and less costly measures. Although a recent, randomized, placebo-controlled trial of rituximab in SD failed to meet primary endpoints that included sicca symptoms,40 an analysis of secondary outcome measures41 and a smaller randomized, placebo-controlled trial42 provide evidence to support this recommendation. Rituximab was also recommended for SD patients with serious organ manifestations who fail more conservative and less costly therapies. This was based on results of a nonrandomized comparator trial43 and other large studies that described outcomes for systemic or internal organ manifestations in SD patients.44–47 Although not common, significant toxicity can be seen with rituximab as seen with other biologics. Patients with SD require careful monitoring for side effects as outlined in recommendation 6.
Box 4. Guidelines for use of biological medications in Sjögren’s disease.
| Biological Therapies |
| Recommendation 1: TNF-α inhibitors |
|
|
| TNF-α inhibitors should not be used to treat sicca symptoms in patients with primary SD. Strength of recommendation: strong |
| Recommendation 2: TNF-α inhibitor cautions |
|
|
If TNF-α inhibition therapy is used for RA or other related overlap conditions in SD patients, health care providers should consider and monitor for the following:
|
| Recommendation 3: Rituximab for keratoconjunctivitis sicca (KCS) |
|
|
| Rituximab may be considered as a therapeutic option for KCS in patients with primary SD and for whom conventional therapies, including topical moisturizers, secretagogues, anti-inflammatories, immunomodulators, and punctual occlusion, have proven insufficient. Strength of recommendation: weak |
| Recommendation 4: Rituximab for xerostomia |
|
|
| Rituximab may be considered as a therapeutic option for xerostomia in patients with primary SD with some evidence of residual salivary production, significant evidence of oral damage as determined by the clinician, and for whom conventional therapies, including topical moisturizers and secretagogues, have proven insufficient. Strength of recommendation: weak |
| Recommendation 5: Rituximab for systemic symptoms |
|
|
Rituximab may be considered as a therapeutic option for adults with primary SD and any or all of the following systemic manifestations:
|
| Recommendation 6: Rituximab cautions |
|
|
|
DISCUSSION AND FUTURE DIRECTIONS
SD remains a highly prevalent chronic autoimmune rheumatic disease with many un-met clinical needs. The process of CPG development has helped define the goals for future therapeutic studies. Of paramount importance is the need to develop SD-specific outcome measures that encompass the spectrum of organ system involvement and are sensitive to clinically meaningful change. Better staging to identify patients with early disease, and the discovery of novel biomarkers and/or genetic profiling to define specific patient subsets should facilitate better patient selection for targeted therapies. The design of future studies (eg, rituximab) should include evaluation time points and dosing regimens relevant to patients with SD rather than those with related disorders such as RA.
The working groups further recommended future clinical trials to (1) identify the most efficacious oral DMARD for inflammatory musculoskeletal pain; (2) expand studies of anti-B cell, anticytokine therapy (eg, BAFF, interleukin-6, interferon), inhibition of T-cell stimulation, and Janus kinase inhibitors for SD patients with early sicca and/or serious extraglandular manifestations; and (3) develop a multimodality approach for the management of SD-related fatigue, including pharmacologic and nonpharmacologic therapies.
Further research on the pathophysiology of dry eye as addressed in the recent second International DEWS will suggest new therapeutic targets for SD, including focused anti-inflammatory therapy (eg, topical anticytokines, integrin-directed therapy) and research into nanotechnology as applied to drug delivery for dry eye. Finally, further work in dentistry is needed to optimize the use of fluoride (eg, preparation, application, dosing regimen) and other adjunctive measures previously described for caries prevention in SD.
Guidelines will be revised as new information becomes available.
KEY POINTS.
Sjögren’s disease (SD) is associated with a high burden of illness, poor quality of life, and increased health care costs.
All SD patients with xerostomia should be given fluoride for caries prophylaxis.
Proper treatment of dry eyes necessitates comprehensive assessment to determine severity level and the relative contributions of aqueous tear deficiency versus meibomian gland dysfunction.
Disease-modifying antirheumatic drugs can be used to treat inflammatory musculoskeletal pain starting with hydroxychloroquine as first-line therapy.
Fatigue is most effectively managed with self-care measures and exercise.
Biological therapy like rituximab is best used in SD patients with serious organ manifestations who fail more conservative treatments.
Footnotes
Conflict of Interest Disclosures: Biogen (F.B. Vivino, S.E. Carson). Coda, Inc; Kala, Inc; Lexitas, Inc; Parion, Inc; Shire Pharmaceuticals, Inc; TearLab, Inc (G. Foulks). None (T.E. Daniels, K.M. Hammitt). UCB; Biogen (A. Parke). Daiichi Sankyo (M.T. Brennan). Alcon; Allergan; Bausch & Lomb; Eleven Biotherapeutics; Nicox; TearScience (S.L. Forstot). UCB (R.H. Scofield).
References
- 1.Valtýsdóttir ST, Gudbjörnsson B, Lindqvist U, et al. Anxiety and depression in patients with primary Sjögren’s syndrome. J Rheumatol. 2000;27(1):165–9. [PubMed] [Google Scholar]
- 2.Vitali C, Tavoni A, Neri R, et al. Fibromyalgia Features in Patients with Primary Sjögren’s Syndrome: Evidence of a Relationship with Psychological Depression. Scand J Rheumatol. 1989;18(1):21–7. doi: 10.3109/03009748909095399. [DOI] [PubMed] [Google Scholar]
- 3.Mishra R, Vivino FB. Diagnosis and management of fatigue. In: Wallace DJ, editor. The Sjögren’s book. New York: The Sjögren’s Syndrome Foundation and Oxford University Press; 2012. pp. 228–34. [Google Scholar]
- 4.Segal B, Bowman SJ, Fox PC, et al. Primary Sjögren’s Syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. 2009;7:46. doi: 10.1186/1477-7525-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strömbeck B, Ekdahl C, Manthorpe R, et al. Health-related quality of life in primary Sjögren’s syndrome, rheumatoid arthritis and fibromyalgia compared to normal population data using SF-36. Scand J Rheumatol. 2000;29(1):20–281. doi: 10.1080/030097400750001761. [DOI] [PubMed] [Google Scholar]
- 6.Sutcliffe N, Stoll T, Pyke S, et al. Functional disability and end organ damage in patients with systemic lupus erythematosus (SLE), SLE and Sjögren’s syndrome (SS) and primary SS. J Rheumatol. 1998;25(1):63–8. [PubMed] [Google Scholar]
- 7.Bowman SJ, St Pierre Y, Sutcliffe N, et al. Estimating indirect costs in primary Sjögren’s syndrome. J Rheumatol. 2010;37(5):1010–5. doi: 10.3899/jrheum.090734. [DOI] [PubMed] [Google Scholar]
- 8.Callaghan R, Prabu A, Allan RB, et al. UK Sjögren’s Interest Group. Direct health-care costs and predictors of costs in patients with primary Sjögren’s syndrome. Rheumatology (Oxford) 2007;46(1):105–11. doi: 10.1093/rheumatology/kel155. [DOI] [PubMed] [Google Scholar]
- 9.Fox PC, Bowman SJ, Segal B, et al. Oral involvement in primary Sjögren’s syndrome. J Am Dent Assoc. 2008;139(12):1592–601. doi: 10.14219/jada.archive.2008.0101. [DOI] [PubMed] [Google Scholar]
- 10.AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AGREE Research Trust. Appraisal of guidelines for research and evaluation II. 2013 Available at: http://www.agreetrust.org/wp-content/uploads/2013/10/AGREE-II-Users-Manual-and-23-item-Instrument_2009_UPDATE_2013.pdf.
- 12.Richardson WS, Wilson MC, Nishikawa J, et al. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12–3. [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist G, et al. for the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–66. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preferred practice guidelines: dry eye. American Academy of Ophthalmology; 2012. [Accessed August 19, 2014]. Available at: http://one.aao.org/summary-benchmark-detail/summary-benchmarks-complete-set-october-2012. [Google Scholar]
- 15.Christensen LB, Petersen PE, Thorn JJ, et al. Dental caries and dental health behavior of patients with primary Sjögren syndrome. Acta Odontol Scand. 2001;59(3):116–20. doi: 10.1080/000163501750266684. [DOI] [PubMed] [Google Scholar]
- 16.Zero DT, Brennan MT, Daniels TE, et al. Clinical practice guidelines for oral management of Sjögren disease: Dental caries prevention. J Am Dent Assoc. 2016;147(4):295–305. doi: 10.1016/j.adaj.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Quality of life impact of Sjögren’s syndrome. The Moisture Seekers. 2006;24:1–3. [Google Scholar]
- 18.Sjögren’s syndrome foundation breakthrough goal survey. Polaris Marketing Research, Inc; 2012. [Google Scholar]
- 19.Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients’ lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005;46:46–50. doi: 10.1167/iovs.03-0915. [DOI] [PubMed] [Google Scholar]
- 20.Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–9. doi: 10.1016/S0161-6420(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 21.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan BD, Crews LA, Messmer EM, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92:161–6. doi: 10.1111/aos.12012. [DOI] [PubMed] [Google Scholar]
- 23.Foulks GN, Forstot SL, Donshik PC, et al. Clinical Guidelines for Management of Dry Eye Associated with Sjögren Disease. Ocul Surf. 2015;13(2):118–32. doi: 10.1016/j.jtos.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Smedby KE, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029–38. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carsons SE, Parke A, Vivino FB, et al. Treatment guidelines for rheumatologic and systemic manifestations of Sjögren’s: Use of biologics, management of fatigue and inflammatory musculoskeletal pain. Arthritis Care Res. doi: 10.1002/acr.22968. in press. [DOI] [PubMed] [Google Scholar]
- 26.Gottenberg JE, Ravaud P, Puéchal X, et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA. 2014;312(3):249–58. doi: 10.1001/jama.2014.7682. [DOI] [PubMed] [Google Scholar]
- 27.Fox RI, Chan E, Benton L, et al. Treatment of primary Sjögren’s syndrome with hydroxychloroquine. Am J Med. 1988;85(4A):62–7. doi: 10.1016/0002-9343(88)90365-8. [DOI] [PubMed] [Google Scholar]
- 28.Fox RI, Dixon R, Guarrassi V, et al. Treatment of primary Sjögren’s syndrome with hydroxychloroquine: a retrospective, open label study. Lupus. 1996;5(Suppl 1):S31–6. [PubMed] [Google Scholar]
- 29.Tishler M, Yaron I, Shirazi I, et al. Hydroxychloroquine treatment for primary Sjögren’s syndrome: its effect on salivary and serum inflammatory markers. Ann Rheum Dis. 1999;58(4):253–6. doi: 10.1136/ard.58.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fauchais AL, Ouattara B, Gondran G, et al. Articular manifestations in primary Sjögren’s syndrome: clinical significance and prognosis of 188 patients. Rheumatology (Oxford) 2010;49(6):1164–72. doi: 10.1093/rheumatology/keq047. [DOI] [PubMed] [Google Scholar]
- 31.Skopouli FN, Jagiello P, Tsifetaki N, et al. Methotrexate in primary Sjögren’s syndrome. Clin Exp Rheumatol. 1996;14(5):555. [PubMed] [Google Scholar]
- 32.van Woerkom JM, Kruize AA, Geenen R, et al. Safety and efficacy of leflunomide in primary Sjögren’s syndrome: a phase II pilot study. Ann Rheum Dis. 2007;66(8):1026–32. doi: 10.1136/ard.2006.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan O, Carsons S. Occurrence of rheumatoid arthritis requiring oral and/or biological disease-modifying antirheumatic drug therapy following a diagnosis of primary Sjögren syndrome. J Clin Rheumatol. 2012;18(7):356–8. doi: 10.1097/RHU.0b013e31826d2abb. [DOI] [PubMed] [Google Scholar]
- 34.Segal B. Fatigue in primary Sjögren’s syndrome. In: Ramos-Casals M, editor. Sjögren’s syndrome: diagnosis and therapeutics. London: Springer Verlag; 2012. pp. 129–43. [Google Scholar]
- 35.Strombeck BE, Theander E, Jacobsson LT. Effectiveness of exercise on aerobic capacity and fatigue in women with Primary Sjögren’s syndrome. Rheumatology (Oxford) 2007;46(5):868–71. doi: 10.1093/rheumatology/kem004. [DOI] [PubMed] [Google Scholar]
- 36.Virkki LM, Porola P, Forsbladd’Elia H, et al. Dehydroepiandrosterone (DHEA) substitution treatment for severe fatigue in DHEA-deficient patients with primary Sjögren’s syndrome. Arthritis Care Res (Hoboken) 2010;62(1):118–24. doi: 10.1002/acr.20022. [DOI] [PubMed] [Google Scholar]
- 37.Hartkamp A, Geenen R, Godaert GL, et al. Effect of dehydroepiandrosterone administration on fatigue, well-being, and functioning in women with primary Sjögren syndrome: a randomised controlled trial. Ann Rheum Dis. 2008;67(1):91–7. doi: 10.1136/ard.2007.071563. [DOI] [PubMed] [Google Scholar]
- 38.Sankar V, Brennan MT, Kok MR, et al. Etanercept in Sjögren’s syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum. 2004;50(7):2240–538. doi: 10.1002/art.20299. [DOI] [PubMed] [Google Scholar]
- 39.Mariette X, Ravaud P, Steinfeld S, et al. Inefficacy of infliximab in primary Sjögren’s syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjögren’s Syndrome (TRIPSS) Arthritis Rheum. 2004;50(4):1270–6. [Google Scholar]
- 40.Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, et al. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med. 2014;160(4):233–42. doi: 10.7326/M13-1085. [DOI] [PubMed] [Google Scholar]
- 41.Faustman DL, Vivino FB, Carsons SE. Treatment of primary Sjögren’s syndrome with rituximab: Comment on Devauchelle et al. 2014. Ann Intern Med. 2014;161(5):376–7. doi: 10.7326/L14-5017-3. [DOI] [PubMed] [Google Scholar]
- 42.Meijer JM, Meiners PM, Vissink A, et al. Effectiveness of rituximab treatment in primary Sjögren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(4):960–8. doi: 10.1002/art.27314. [DOI] [PubMed] [Google Scholar]
- 43.Carubbi F, Cipriani P, Marrelli A, et al. Efficacy and safety of rituximab treatment in early primary Sjögren’s syndrome: a prospective, multi-center, follow-up study. Arthritis Res Ther. 2013;15(5):R172. doi: 10.1186/ar4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pijpe J, van Imhoff GW, Vissink A, et al. Rituximab treatment in patients with primary Sjögren’s syndrome: an open-label phase II study. Arthritis Rheum. 2005;52(9):2740–50. doi: 10.1002/art.21260. [DOI] [PubMed] [Google Scholar]
- 45.Seror R, Sordet C, Guillevin L, et al. Tolerance and efficacy of rituximab and changes in serum B cell biomarkers in patients with systemic complications of primary Sjögren’s syndrome. Ann Rheum Dis. 2007;66(3):351–7. doi: 10.1136/ard.2006.057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorson KC, Natarajan N, Ropper AH, et al. Rituximab treatment in patients with IVIg-dependent immune polyneuropathy: a prospective pilot trial. Muscle Nerve. 2007;35(1):66–9. doi: 10.1002/mus.20664. [DOI] [PubMed] [Google Scholar]
- 47.Gottenberg JE, Guillevin L, Lambotte O, et al. Club Rheumatismes et Inflammation (CRI). Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis. 2005;64(6):913–20. doi: 10.1136/ard.2004.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]