Abstract
Background
Black patients with lung cancer diagnosed at early stages—for which surgical resection offers a potential cure—experience worse overall survival than do their white counterparts. We undertook a population-based study to estimate the racial and ethnic disparity in death from competing causes and assessed its contribution to the gap in overall survival among patients with early-stage lung cancer.
Methods
We collected survival time data for 105,121 Hispanic, non-Hispanic Asian, non-Hispanic black, and non-Hispanic white patients with early-stage (IA, IB, IIA, and IIB) lung cancer diagnosed between 2004 and 2013 from the Surveillance, Epidemiology, and End-Results registries. We modeled survival time using competing risk regression and included as covariates sex, age at diagnosis, race/ethnicity, stage at diagnosis, histologic type, type of surgical resection, and radiation sequence.
Results
Adjusting for demographic, clinical, and treatment characteristics, non-Hispanic blacks experienced worse overall survival compared with non-Hispanic whites (adjusted hazard ratio [aHR], 1.05; 95% CI, 1.02-1.08), whereas Hispanics and non-Hispanic Asians experienced better overall survival (aHR, 0.93; 95% CI, 0.89-0.98; and aHR, 0.82; 95% CI, 0.79-0.86, respectively). Worse survival from competing causes of death, such as cardiovascular disease and other cancers—rather than from lung cancer itself—led to the disparity in overall survival among non-Hispanic blacks (adjusted relative risk, 1.07; 95% CI, 1.02-1.12).
Conclusions
Narrowing racial and ethnic disparities in survival among patients with early-stage lung cancer will rely on more than just equalizing access to surgical resection and will need to include better management and treatment of smoking-related comorbidities and diseases.
Key words: disparities, lung cancer, surgical oncology
Abbreviations: CVD, cardiovascular disease; NLST, National Lung Screening Trial; SEER, Surveillance, Epidemiology, and End-Results
The disparity in survival among black and white patients with early-stage lung cancer has persisted and widened since at least 1975.1 For example, 5-year relative survival equaled 59.3% for whites diagnosed with localized lung cancer compared with 50.4% for similarly diagnosed blacks between 2005 and 2011.1 Previous research has consistently found that the racial disparity in survival can be explained by lower rates of surgical treatment among blacks compared with whites.2, 3, 4, 5, 6, 7 Surgical treatment considerably improves overall survival by substantially reducing the likelihood of death from lung cancer among patients with early-stage disease.8, 9, 10
We do not know, however, if the disparity in survival among patients with early-stage lung cancer observed between blacks and whites extends to other racial and ethnic groups, such as Hispanics and non-Hispanic Asian and Pacific Islanders. Furthermore, we do not yet know if the rates of surgical treatment in these racial and ethnic groups explain any observed differences in survival. Assessing potential disparities in survival among Hispanics and non-Hispanic Asian patients with lung cancer is important for two reasons. First, lung cancer is the leading and second leading cause of cancer mortality for Hispanic men and women, respectively, and the leading cause of cancer mortality for Asians.1 Second, the size of the Hispanic and Asian populations has grown faster over time than has that of non-Hispanic blacks; Hispanics are now the largest minority population subgroup in the United States.11
In this study, we address this research gap by examining differences in survival among Hispanic, non-Hispanic Asian, non-Hispanic black, and non-Hispanic white patients with early-stage lung cancer. We further assess the source of differences in overall survival: differences in survival from lung cancer and differences in survival from all other causes, including smoking-related diseases such as other cancers, COPD, and cardiovascular disease (CVD).
Methods
We obtained survival time data for patients with lung cancer from the Surveillance, Epidemiology, and End-Results (SEER) 18 registry database from 2004 to 2013. The SEER 18 registries, which cover approximately 25% of the US population, form the largest, most representative, and longest running national cancer incidence database. We analyzed 105,121 Hispanic, non-Hispanic Asian and Pacific Islander (hereafter referred to as Asian), non-Hispanic black (hereafter referred to as black), and non-Hispanic white (hereafter referred to as white) patients diagnosed with stage IA, IB, IIA, and IIB lung cancer and included only the first matching record for each person. We also collected patients’ sex, age at diagnosis (40-44, 45-49, 50-54, 55-59, 60-64, 65-69, 70-74, 75-79, 80-84, and ≥ 85 years), lung cancer histologic type (adenocarcinoma, squamous cell, other non-small cell, and small cell), extent of surgical resection performed (less than one lobe, one lobe or more, no surgery), and radiation sequence with surgery (after surgery, before and after surgery, before surgery, given but sequence unknown, none). We set our study period (2004-2013) based on the first year of available American Joint Committee on Cancer, sixth edition staging.
First, we compared tumor characteristics, treatment, and mortality outcomes by race/ethnicity among patients with early-stage lung cancer using the t test statistic for a difference in proportions. Next, we compared case-fatality rates by race/ethnicity and cause of death standardized to the 2010 US population of adults aged ≥ 40 years. Third, we assessed receipt of surgical treatment by race/ethnicity. We also compared the reasons why no surgery was performed by race/ethnicity. Reasons classified in SEER included surgery not recommended, contraindicated due to other conditions, patient died before recommended surgery, unknown reason, and patient refusal.
Fourth, we fit a Cox proportional hazards model in which survival (from all causes) was a function of sex, age at diagnosis, race/ethnicity, stage at diagnosis, histologic type, type of surgical resection, and radiation sequence. We assessed the proportional hazards assumption by plotting and testing the linearity of the Schoenfeld residuals against survival time for each covariate in the model.
Finally, we modeled survival time using a competing risk regression approach. A patient with lung cancer concurrently faces the risk of death from lung cancer itself and from competing causes of death. Conceptually, the competing risk regression approach models the time from lung cancer diagnosis to death from lung cancer—in the presence of competing causes of death—and accounts for sociodemographic, clinical, and biological factors. Specifically, we fit the semiparametric proportional hazard model for the subdistribution hazard of the cumulative incidence function originally developed by Fine and Gray.12, 13, 14 We fit two such models: (1) death from lung cancer as the outcome of interest with death from all other causes as the competing event and (2) death from all other causes as the outcome of interest and death from lung cancer as the competing event. For each model, we included as covariates sex, age at diagnosis, race/ethnicity, stage at diagnosis, histologic type, type of surgical resection, and radiation sequence. As with the Cox model for all-cause mortality, we assessed the proportional hazards assumption by plotting and testing the linearity of the Schoenfeld residuals against survival time for each covariate in the model. The Dartmouth Committee for the Protection of Human Subjects determined that this study did not involve human subjects research under the purview of the Dartmouth Committee for the Protection of Human Subjects, because any private information in the SEER data is not individually identifiable.
Results
Patient Characteristics
Between 2004 and 2013, 4,412 Hispanic, 4,833 Asian, 10,038 black, and 85,838 white patients residing in SEER registry areas were diagnosed with stage IA, IB, IIA, or IIB lung cancer (early stage) (Table 1). The modal age at diagnosis was 70 to 74 years for Hispanics, Asians, and whites and 65 to 69 years for blacks. The proportion of patients with early-stage lung cancer diagnosed as stage IA was highest for whites (46%) and lowest for blacks (41%). Finally, adenocarcinoma was the most commonly diagnosed histologic type across all racial/ethnic groups.
Table 1.
Characteristics of Hispanic, Non-Hispanic Asian, Non-Hispanic Black, and Non-Hispanic White Patients With Lung Cancer Diagnosed With Early-Stage Lung Cancer, 2004-2013
| Characteristic | All | Hispanic | Non-Hispanic Asian |
Non-Hispanic Black |
Non-Hispanic White |
|---|---|---|---|---|---|
| Total, No. | 105,121 | 4,412 | 4,833 | 10,038 | 85,838 |
| Sex, No. (%) | |||||
| Female | 51,410 (49) | 2,227 (50) | 2,164 (45) | 4,665 (46) | 42,354 (49) |
| Male | 53,711 (51) | 2,185 (50) | 2,669 (55) | 5,373 (54) | 43,484 (51) |
| Age at diagnosis, y, No. (%) | |||||
| 40-44 | 669 (1) | 51 (1) | 43 (1) | 104 (1) | 471 (1) |
| 45-49 | 2,066 (2) | 108 (2) | 112 (2) | 319 (3) | 1,527 (2) |
| 50-54 | 4,524 (4) | 200 (5) | 220 (5) | 732 (7) | 3,372 (4) |
| 55-59 | 7,977 (8) | 359 (8) | 355 (7) | 1,159 (12) | 6,104 (7) |
| 60-64 | 12,760 (12) | 477 (11) | 525 (11) | 1,583 (16) | 10,175 (12) |
| 65-69 | 18,257 (17) | 737 (17) | 763 (16) | 1,826 (18) | 14,931 (17) |
| 70-74 | 19,350 (18) | 827 (19) | 911 (19) | 1,632 (16) | 15,980 (19) |
| 75-79 | 18,826 (18) | 787 (18) | 863 (18) | 1,348 (13) | 15,828 (18) |
| 80-84 | 13,316 (13) | 572 (13) | 646 (13) | 866 (9) | 11,232 (13) |
| ≥ 85 | 7,376 (7) | 294 (7) | 395 (8) | 469 (5) | 6,218 (7) |
| Stage at diagnosis, No. (%) | |||||
| IA | 47,055 (45) | 1,883 (43) | 2,017 (42) | 4,080 (41) | 39,075 (46) |
| IB | 38,589 (37) | 1,682 (38) | 1,904 (39) | 3,949 (39) | 31,054 (36) |
| IIA | 4,562 (4) | 183 (4) | 201 (4) | 426 (4) | 3,752 (4) |
| IIB | 14,915 (14) | 664 (15) | 711 (15) | 1,583 (16) | 11,957 (14) |
| Histologic type, No. (%) | |||||
| Adenocarcinoma | 40,053 (38) | 1,841 (42) | 2,490 (52) | 3,851 (38) | 31,871 (37) |
| Other non-small cell | 28,579 (27) | 1,234 (28) | 1,243 (26) | 2,764 (28) | 23,338 (27) |
| Small-cell | 3,977 (4) | 150 (3) | 120 (2) | 361 (4) | 3,346 (4) |
| Squamous cell | 32,512 (31) | 1,187 (27) | 980 (20) | 3,062 (31) | 27,283 (32) |
| Vital status (as of December 31, 2013), No. (%) | |||||
| Alive | 49,739 (47) | 2,298 (52) | 2,751 (57) | 4,550 (45) | 40,140 (47) |
| Dead from lung cancer | 35,610 (34) | 1,393 (32) | 1,378 (29) | 3,582 (36) | 29,257 (34) |
| Dead from other causes | 19,772 (19) | 721 (16) | 704 (15) | 1,906 (19) | 16,441 (19) |
Standardized Mortality Rates by Cause of Death
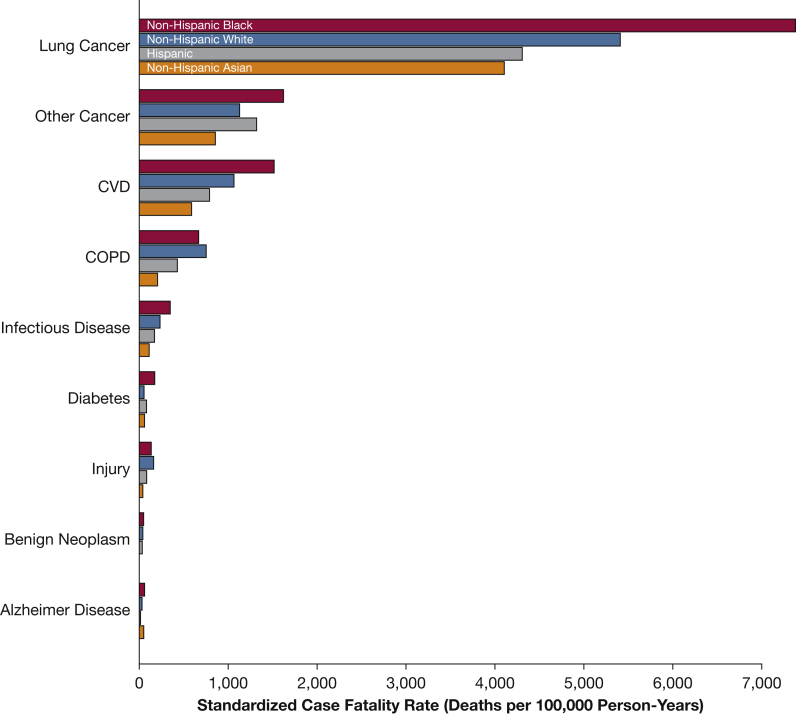
Standardized case-fatality rates for blacks exceeded the corresponding rates for whites for the three leading causes of death among patients with early-stage lung cancer (lung cancer, other cancers, and CVD) (Fig 1). For example, the standardized lung cancer case-fatality rate equaled 7,386 deaths per 100,000 person-years for blacks compared with 5,416 deaths per 100,000 person-years for whites. The standardized case fatality rate for Asians was lowest across racial/ethnic groups for all causes of death.
Figure 1.
Standardized case-fatality rates by race/ethnicity and cause of death among patients diagnosed with early-stage lung cancer. Mortality rates standardized to 2010 US population, age ≥ 40 years. CVD = cardiovascular disease.
Receipt of Surgical Treatment
Receipt of surgical treatment among patients with early-stage lung cancer differed by race and ethnicity: 46.8% of blacks did not receive surgical treatment (95% CI, 45.3%-48.2%) compared with 38.1% of Hispanics (95% CI, 35.7%-40.4%), 33.5% of Asians (95% CI, 31.2%-35.8%), and 38.3% of whites (95% CI, 37.8%-38.8%; Table 2). Black patients with early-stage lung cancer were less likely to receive surgical treatment than were other racial and ethnic groups, primarily because surgery was more likely not to be recommended (34.5%; 95% CI, 32.9%-36.0%). In contrast, the proportion of Hispanic, Asian, and white patients with early-stage lung cancer for whom surgery was not recommended was substantially lower: 24.1% for Asians (95% CI, 21.6%-26.5%), 27.7% for whites (95% CI, 27.2%-28.3%), and 29.0% for Hispanics (95% CI, 26.5%-31.5%).
Table 2.
Receipt of Surgical Treatment
| Variable | Hispanic % (95% CI) |
Non-Hispanic Asian % (95% CI) |
Non-Hispanic Black % (95% CI) |
Non-Hispanic White % (95% CI) |
|---|---|---|---|---|
| Type of surgical resection | ||||
| None | 38.1 (35.7-40.4) | 33.5 (31.2-35.8) | 46.8 (45.3-48.2) | 38.3 (37.8-38.8) |
| < 1 lobe | 11.4 (8.6-14.1) | 10.1 (7.4-12.8) | 11.0 (9.2-12.9) | 12.8 (12.2-13.5) |
| ≥ 1 lobe | 50.6 (48.5-52.7) | 56.4 (54.5-58.3) | 42.2 (40.7-43.7) | 48.9 (48.4-49.3) |
| Reason no surgery performed | ||||
| Surgery performed | 62.8 (61.0-64.6) | 67.0 (65.3-68.6) | 53.7 (52.4-55.1) | 62.5 (62.1-62.9) |
| Surgery not recommended | 29.0 (26.5-31.5) | 24.1 (21.6-26.5) | 34.5 (32.9-36.0) | 27.7 (27.2-28.3) |
| Contraindicated due to other conditions | 3.4 (0.5-6.3) | 4.0 (1.2-6.7) | 5.7 (3.8-7.6) | 5.5 (4.9-6.2) |
| Patient died before recommended surgery | 0.1 (0.0-3.0) | 0.1 (0.0-2.9) | 0.1 (0.0-2.1) | 0.1 (0.0-0.8) |
| Unknown reason for no surgery | 2.3 (0.0-5.2) | 1.4 (0.0-4.2) | 2.3 (0.4-4.2) | 1.6 (1.0-2.3) |
| Patient refused | 1.9 (0.0-4.8) | 2.8 (0.1-5.6) | 2.8 (0.8-4.7) | 1.9 (1.3-2.6) |
| Recommended, unknown if performed | 0.5 (0.0-3.4) | 0.6 (0.0-3.4) | 0.6 (0.0-2.5) | 0.3 (0.0-0.9) |
| Unknown if surgery performed | 0.1 (0.0-3.0) | 0.1 (0.0-2.9) | 0.3 (0.0-2.3) | 0.4 (0.0-1.0) |
| Radiation sequence | ||||
| Before surgery | 0.6 (0.0-3.6) | 0.8 (0.0-3.6) | 0.9 (0.0-2.9) | 0.8 (0.1-1.5) |
| Before and after surgery | 0.1 (0.0-3.1) | 0.1 (0.0-2.9) | 0.1 (0.0-2.1) | 0.1 (0.0-0.8) |
| After surgery | 4.4 (1.5-7.3) | 4.3 (1.5-7.1) | 4.9 (3.0-6.8) | 4.8 (4.1-5.4) |
| Given but sequence unknown | 0.0 (0.0-3.0) | 0.0 (0.0-2.9) | 0.1 (0.0-2.0) | 0.1 (0.0-0.7) |
| None | 94.8 (94.1-95.5) | 94.8 (94.1-95.4) | 94.1 (93.6-94.5) | 94.3 (94.1-94.4) |
All-Cause and Competing Risk Model Results
Adjusting for demographic, clinical, and treatment characteristics (including receipt of surgical treatment), the hazard of death (from any cause) was significantly higher for black patients with early-stage lung cancer than for their white counterparts (adjusted hazard ratio [aHR], 1.05; 95% CI, 1.02-1.08) (Table 3). In contrast, the hazard of death (from any cause) was significantly lower for Asian and Hispanic patients with early-stage lung cancer than for their white counterparts (aHR, 0.82; 95% CI, 0.79-0.86; and aHR, 0.93; 95% CI, 0.89-0.98, respectively).
Table 3.
Multivariable Regression Results: Cox Proportional Hazard (All-Cause) and Competing Risk (Death From Lung Cancer and Death From Competing Causes)
| Covariate | Death from All Causesa |
Death from Lung Cancerb |
Death from Competing Causesb |
|||
|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | Adjusted RR | 95% CI | Adjusted RR | 95% CI | |
| Race/ethnicity (reference: Non-Hispanic white) | ||||||
| Hispanic | 0.93 | 0.89-0.98 | 0.95 | 0.90-1.01 | 0.87 | 0.80-0.94 |
| Non-Hispanic Asian | 0.82 | 0.79-0.86 | 0.87 | 0.83-0.92 | 0.77 | 0.71-0.83 |
| Non-Hispanic black | 1.05 | 1.02-1.08 | 1.00 | 0.96-1.04 | 1.07 | 1.02-1.12 |
| Female sex (reference: Male sex) | 0.79 | 0.77-0.80 | 0.89 | 0.87-0.91 | 0.77 | 0.75-0.8 |
| Age at diagnosis, y (reference: 40-44 y) | ||||||
| 45-49 | 1.22 | 1.05-1.42 | 1.14 | 0.96-1.35 | 1.17 | 0.88-1.57 |
| 50-54 | 1.36 | 1.18-1.56 | 1.22 | 1.04-1.43 | 1.23 | 0.94-1.61 |
| 55-59 | 1.51 | 1.32-1.74 | 1.33 | 1.14-1.55 | 1.40 | 1.08-1.82 |
| 60-64 | 1.70 | 1.48-1.95 | 1.44 | 1.23-1.67 | 1.55 | 1.19-2.01 |
| 65-69 | 1.87 | 1.64-2.14 | 1.49 | 1.28-1.73 | 1.76 | 1.36-2.28 |
| 70-74 | 2.17 | 1.89-2.48 | 1.57 | 1.35-1.83 | 2.11 | 1.63-2.73 |
| 75-79 | 2.56 | 2.24-2.93 | 1.73 | 1.48-2.01 | 2.49 | 1.93-3.22 |
| 80-84 | 2.80 | 2.44-3.20 | 1.83 | 1.58-2.14 | 2.60 | 2.01-3.37 |
| ≥ 85 | 3.26 | 2.85-3.74 | 1.85 | 1.58-2.16 | 2.90 | 2.24-3.76 |
| Histologic type (reference: adenocarcinoma) | ||||||
| Other non-small cell | 1.25 | 1.22-1.28 | 1.10 | 1.07-1.13 | 1.33 | 1.29-1.38 |
| Small cell | 1.30 | 1.24-1.35 | 1.39 | 1.32-1.46 | 0.96 | 0.88-1.04 |
| Squamous cell | 1.22 | 1.20-1.25 | 1.12 | 1.09-1.15 | 1.27 | 1.22-1.31 |
| Stage at diagnosis (reference: IA) | ||||||
| IB | 1.47 | 1.44-1.50 | 1.72 | 1.68-1.77 | 0.88 | 0.85-0.91 |
| IIA | 1.59 | 1.53-1.66 | 1.93 | 1.83-2.02 | 0.91 | 0.84-0.97 |
| IIB | 2.00 | 1.95-2.05 | 2.59 | 2.51-2.67 | 0.74 | 0.7-0.77 |
| Type of surgical resection (reference: none) | ||||||
| < 1 lobe | 0.46 | 0.45-0.48 | 0.50 | 0.48-0.52 | 0.92 | 0.88-0.96 |
| ≥ 1 lobe | 0.33 | 0.32-0.34 | 0.38 | 0.37-0.39 | 0.78 | 0.76-0.81 |
| Radiation sequence (reference: none) | ||||||
| Before surgery | 1.07 | 1.03-1.11 | 1.22 | 1.17-1.28 | 0.88 | 0.81-0.94 |
| Before and after surgery | 1.24 | 0.97-1.58 | 1.40 | 1.07-1.83 | 0.92 | 0.55-1.54 |
| After surgery | 1.15 | 1.05-1.26 | 1.20 | 1.08-1.34 | 1.12 | 0.94-1.33 |
| Given but sequence unknown | 1.35 | 0.98-1.84 | 1.66 | 1.17-2.36 | 0.79 | 0.4-1.54 |
RR = relative risk.
Cox proportional hazard (all-cause) model.
Competing risk (death from lung cancer and death from competing causes) model.
Next, we determined whether higher all-cause mortality for blacks—accounting for receipt of surgical treatment—was the result of higher mortality from lung cancer or higher mortality from all other causes. Accounting for surgical treatment, the relative risk of death from lung cancer (conditional on no death from other causes) was not significantly higher for black patients with early-stage lung cancer than for their white counterparts (adjusted relative risk [aRR] 1.00; 95% CI, 0.96-1.04). In contrast, the relative risk of death from other causes (conditional on no death from lung cancer) was significantly higher for blacks than for whites (aRR, 1.07; 95% CI, 1.02-1.12). Thus black patients with early-stage lung cancer who received surgical treatment experienced higher all-cause mortality because of higher mortality from all other causes, rather than higher mortality from lung cancer.
In contrast to blacks, Hispanic and Asian patients with early-stage lung cancer experienced lower all-cause mortality compared with their white counterparts because of both lower mortality from lung cancer and lower mortality from all other causes. Accounting for surgical treatment, the relative risk of death from lung cancer (conditional on no death from other causes) was lower for Asians (aRR, 0.87; 95% CI, 0.83-0.92) than for whites, although not significantly lower for Hispanics (aRR, 0.95; 95% CI, 0.90-1.01) than for whites. The relative risk of death from other causes (conditional on no death from lung cancer) was also lower for both Asians (aRR, 0.77; 95% CI, 0.71-0.83) and Hispanics (aRR, 0.87; 95% CI, 0.80-0.94) than for whites.
Discussion
Our study assessed racial and ethnic disparities in survival, both overall and by cause (lung cancer and all other diseases) among patients with early-stage lung cancer. First, surgery was recommended less often for black patients with early-stage lung cancer than for other racial/ethnic groups. Second, black patients with early-stage lung cancer who received lung cancer surgery still experienced worse overall survival than did their whites counterparts. Third, the source of this disparity in overall survival—accounting for receipt of surgical treatment—was worse survival from other causes rather than from lung cancer itself. Fourth, the racial disparity in survival between blacks and whites did not extend to Hispanics and Asians. Both Hispanic and Asian patients with early-stage lung cancer experienced higher overall survival than did whites because of higher survival from all other causes death.
Our results differ from those of Bach et al,3 who found that black patients with early-stage lung cancer who received lung cancer surgery experienced nearly identical survival as their white counterparts. In contrast, we found that black patients with early-stage lung cancer who received lung cancer surgery still experienced worse overall survival because of lower survival from other causes. Our results may differ from those of Bach et al3 because we considered a broader age range of patients (≥ 40 years for our study vs ≥ 65 years and older for the study of Bach et al3) and the time period considered (2004-2013 for our study vs 1985-1993 for Bach et al3). Although lobectomy through a thoracotomy was the most common lung cancer surgery in the earlier period, contemporary procedures include sublobar resection and lobectomy through video-assisted thoracoscopic surgery (VATS). These advancements in the treatment of early-stage lung cancer yield survival outcomes equivalent to that of lobectomy through thoracotomy, with fewer complications, faster recovery time, and higher postsurgical quality of life.15, 16, 17, 18 Harrison et al19 found that black patients with lung cancer who received VATS experienced more than twice the in-hospital mortality as nonwhite patients with lung cancer who also received VATS: 3.0% vs 1.4%.19 Moreover, white patients with lung cancer who received lung cancer surgery, regardless of technique, were less likely to experience postsurgical complications and postsurgical mortality than were blacks.3, 5, 7, 19, 20, 21, 22, 23
Our results also differ from those of Wisnivesky et al,4 who found that Hispanic patients with early-stage lung cancer who received lung cancer surgery experienced equivalent survival as their white counterparts. In contrast, we observed that Hispanic patients with early-stage lung cancer who received lung cancer surgery experienced better overall survival because of higher survival from other causes. Nativity may partly explain the survival advantage for Hispanic patients. A previous population-based study using the California Cancer Registry concluded that foreign-born Hispanic patients with lung cancer experienced better survival than non-Hispanic whites, whereas US-born Hispanics experienced survival equivalent to that of non-Hispanic whites.24 Additionally, lower rates of cigarette smoking among Hispanics, compared with non-Hispanic whites, may partially explain the survival advantage from other causes. In 2014, for example, 11.2% of Hispanic adults were current cigarette smokers (14.8% of men and 7.6% of women) compared with 18.3% of non-Hispanic adults (19.4% of men and 17.3% of women).25 Thus, Hispanic patients with lung cancer, especially women, may experience less smoking-related comorbidity than non-Hispanic whites. Finally, Hispanic patients may experience higher survival from other causes, compared with non-Hispanic patients, because of lower prevalence of several chronic conditions, including coronary heart disease, hypertension, and COPD.26
Our results support those of Ou et al,27 who found that Asian patients with non-small cell lung cancer experienced better survival than did non-Asians. This survival advantage may result from a comparatively high percentage of never cigarette smokers among Asian patients with lung cancer who, therefore, do not experience smoking-related comorbidity. The molecular profile of lung cancer tumors differs between never cigarette smokers and cigarette smokers.28, 29 For example, mutations in the EGFR gene are more common among never cigarette smokers than among cigarette smokers, whereas the opposite pattern occurs for mutations in the KRAS gene.30 EGFR-positive and KRAS-negative patients with lung cancer experience lower rates of lung cancer recurrence.31, 32
Many of the same factors that create racial and ethnic disparities in health for the general population may also contribute to lower survival from competing causes of death among black patients with early-stage lung cancer. These factors include socioeconomic status, discrimination, racial bias and racism, access to primary care, and quality of health care. Socioeconomic status affects health through multiple and well-documented pathways.33, 34, 35, 36, 37 For example, the chronic and everyday discrimination that is faced more often by blacks than by whites is associated with coronary artery calcification, higher nocturnal blood pressure, and activation of the sympathetic nervous system.38, 39, 40 Additionally, access to primary care is also lower among both blacks and Hispanics than among whites.41 In 2012, for example, 84% of non-Hispanic blacks and 73% of Hispanics reported a usual source of health care compared with 86% of non-Hispanic whites.26 Notably, the rate of health insurance coverage increased more among Hispanics than among non-Hispanic blacks and whites between 2013 and 2014 (from 76% in 2013 to 80% in 2014); this increase in coverage was likely due to continued expansion of health insurance coverage enabled by the Patient Protection and Affordable Care Act.42 Even among adults with access to primary care, this care for black patients is largely concentrated among a subset of US health care providers who are, on average, less well trained.43 Hospital-based care for black patients is similarly concentrated.44 Providers serving black patients also tend to be fragmented from the rest of the health care system,33 and black patients experience more difficulty obtaining specialty and subspecialty care, diagnostic imaging, and nonemergency hospital admission.43, 45 Black patients may also receive suboptimal lung cancer care.46 Epstein et al47 found that black patients with lung cancer were 16 percentage points less likely to receive lung cancer surgery performed by surgeons with high surgical volume at hospitals with high surgical volume. Dimick et al22 also found that black patients were more likely to receive lung cancer surgery at low-quality hospitals (as measured by procedure-specific mortality) than were white patients, even in areas of low racial segregation. Ultimately, lesser access to primary care and lower quality of care may interact to produce higher perioperative mortality and worse postoperative surveillance for recurrence of lung cancer, detection of other cancers, and treatment of comorbid conditions.
The lack of racial disparities in overall and cause-specific survival among patients with early-stage lung cancer who receive care in the Veterans Affairs healthcare system further suggests that unequal access to health care may contribute to racial disparities in survival.48 The lack of racial disparities in overall survival was also observed among participants randomized to the treatment arm (low-dose CT) in the multicenter randomized National Lung Screening Trial (NLST).49 This observation in the NLST suggests that the increased access to health care that resulted from screening improved the management and treatment of chronic diseases for black trial participants.49
In addition to the aforementioned health-care system level factors, differences in comorbidity level may partly explain racial disparities in the receipt of lung cancer surgery. In a study of > 1,300 patients with early-stage non-small cell lung cancer, the prevalence of several chronic conditions (eg, hypertension, liver disease, poor performance status) was higher for blacks than for whites.50 A medical record review of nearly 600 patients with cancer concluded that greater comorbidity among black patients with early-stage lung cancer, compared with white patients, explained some of the difference in receipt of surgery.51 These higher levels of comorbidity may explain, in part, why surgery was recommended less often for black patients compared with other racial/ethnic groups. Moreover, physicians may not detect higher levels of comorbidity if patients do not receive comprehensive preoperative physiological evaluations of cardiovascular risk and respiratory function. If the racial disparity identified in receipt of diagnostic services52 extends to preoperative evaluation (eg, cardiopulmonary exercise tests), a higher proportion of underlying comorbidity may remain undetected among black patients with lung cancer. A second factor for the difference in receipt of surgery may be higher rates of refusal for lung cancer surgery among black patients compared with white patients.51 This refusal may have cultural origins; for example, black patients were more likely to believe that tumors spread when exposed to air than were white patients surveyed in pulmonary and lung cancer clinics in three large medical centers.53 Based on SEER data in our analysis, a higher proportion of black patients refused lung cancer surgery than did white patients (2.8% for blacks vs 1.9% for whites).
Differences in the rate of tobacco cessation after diagnosis may explain, in part, the racial and ethnic disparities in survival from competing causes of death. Although black participants in the NLST were more likely than white participants to report a 24-h or 7-day attempt to quit, 6 months of continuous abstinence was equally likely across racial groups in NLST.54 In community-based studies, however, black smokers were less likely to attempt to quit smoking than were their white counterparts.55, 56 Black smokers may be less likely to attempt to quit smoking and successfully quit smoking than white smokers because of greater use of mentholated cigarettes, which may contribute to higher levels of nicotine addiction.57 Use of mentholated cigarettes may also attenuate the effectiveness of sustained-release bupropion.58 Hispanic smokers are less likely to use nicotine replacement therapy or bupropion when attempting to quit smoking compared with non-Hispanic white smokers.59, 60
Our study has some potential limitations. First, our results may be subject to misclassification bias of the underlying cause of death on death certificates. Earlier studies conclude that the agreement in the cause of death between medical records and death certificates for lung cancer exceeded 93% and was among the highest across all cancers types.61 Second, the SEER 18 registries do not capture the entire US population. Our results may not be generalizable to the national population of patients with lung cancer to the extent that the SEER 18 registries fail to represent more general lung cancer mortality outcomes. Our results may also not be generalizable because the racial and ethnic distribution and geographic distribution of patients in the SEER 18 registries differs from that of the entire US population. For example, blacks represent 11.6% of SEER patients compared with 12.6% of the national population,62, 63 and 21.6% of SEER patients reside in the South compared with 37.1% of the US population.62, 64 The racial disparity in competing causes of death may be greater among patients residing in the South, where rates of diabetes, obesity, and stroke are higher than in the rest of the United States,65, 66 than in patients residing in the West. Thus the skewed geographic distribution of the SEER data may yield a conservative estimate of the US-wide disparity in competing causes of death among black and white patients with lung cancer.
Our study identified an important—and potentially modifiable—source of racial and ethnic disparities in overall survival among patients with lung cancer, namely, survival from competing causes of death. We posit several patient-, provider-, and health-care system level reasons why survival from competing causes of death may differ across racial and ethnic groups. Greater biological susceptibility to lung cancer and other tobacco-associated diseases may also contribute to worse survival for specific populations. Genetic studies yield inconsistent conclusions about whether mutational frequencies in lung cancer tumors differ between black and white patients.67, 68, 69, 70 Future genetic studies will need to include more racial and ethnic minorities to definitively ascertain differences in mutational frequencies across populations.71 Genotyping and genomic profiling may enable patients—regardless of their race or ethnicity—to receive personalized lung cancer treatments that extend survival.72
Conclusions
We found that worse survival from competing causes of death, rather than from lung cancer itself, led to disparities in overall survival among black and white patients with early-stage lung cancer. In contrast, better survival from competing causes of death led to the overall survival advantage of Hispanic and Asian patients with early-stage lung cancer compared with their white counterparts. Narrowing racial disparities in survival among patients with early-stage lung cancer will rely on more than just equalizing access to surgical resection. Rather, narrowing these racial disparities will require equal access—across race—to high-quality medical care that appropriately manages and treats smoking-related chronic conditions and diseases and other determinants of health.
Acknowledgments
Author contributions: S. S., G. A. S., N. T. T., C. S. L., and W. B. were involved in the study design and preparation of the article. S. S. was involved in the data collection and statistical analysis.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health (R21-CA197912 to S. S.) and the American Lung Association (to S. S.).
References
- 1.Howlader N., Noone A., Krapcho M. National Cancer Institute; Bethesda, MD: 2015. SEER cancer statistics review, 1975-2012. [Google Scholar]
- 2.Greenwald H.P., Polissar N.L., Borgatta E.F., McCorkle R., Goodman G. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health. 1998;88(11):1681–1684. doi: 10.2105/ajph.88.11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach P.B., Cramer L.D., Warren J.L., Begg C.B. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 4.Wisnivesky J.P., McGinn T., Henschke C., Hebert P., Iannuzzi M.C., Halm E.A. Ethnic disparities in the treatment of stage I non-small cell lung cancer. Am J Respir Crit Care Med. 2005;171(10):1158–1163. doi: 10.1164/rccm.200411-1475OC. [DOI] [PubMed] [Google Scholar]
- 5.Lathan C.S., Neville B.A., Earle C.C. Racial composition of hospitals: effects on surgery for early-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(26):4347–4352. doi: 10.1200/JCO.2007.15.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy D., Liu C.-C., Xia R. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115(10):2199–2211. doi: 10.1002/cncr.24248. [DOI] [PubMed] [Google Scholar]
- 7.Cykert S., Dilworth-Anderson P., Monroe M.H. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303(23):2368–2376. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields T.W. Surgical therapy for carcinoma of the lung. Clin Chest Med. 1993;14(1):121–147. [PubMed] [Google Scholar]
- 9.Ettinger D.S., Cox J.D., Ginsberg R.J. NCCN non-small-cell lung cancer practice guidelines. J Natl Compr Canc Netw. 1996;10(11 suppl):81–111. [Google Scholar]
- 10.Lam W.K. Management of non-small cell lung cancer according to staging—an update. Respirology. 1998;3(1):51–54. doi: 10.1046/j.1440-1843.1998.d01-8.x. [DOI] [PubMed] [Google Scholar]
- 11.Colby S., Ortman J. US Census Bureau; Washington, DC: 2014. Projections of the size and composition of the U.S. population: 2014 to 2060. [Google Scholar]
- 12.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 13.Scrucca L., Santucci A., Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transpl. 2007;40(4):381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 14.Scrucca L., Santucci A., Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transpl. 2010;45(9):1388–1395. doi: 10.1038/bmt.2009.359. [DOI] [PubMed] [Google Scholar]
- 15.Balduyck B., Hendriks J., Lauwers P., Van Schil P. Quality of life evolution after lung cancer surgery: a prospective study in 100 patients. Lung Cancer. 2007;56(3):423–431. doi: 10.1016/j.lungcan.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Yan T.D., Black D., Bannon P.G., McCaughan B.C. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non–small-cell lung cancer. J Clin Oncol. 2009;27(15):2553–2562. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 17.Kates M., Swanson S., Wisnivesky J.P. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer <=1 cm in size: a review of SEER data. Chest. 2011;139(3):491–496. doi: 10.1378/chest.09-2547. [DOI] [PubMed] [Google Scholar]
- 18.Paoletti L., Pastis N.J., Denlinger C.E., Silvestri G.A. A decade of advances in treatment of early-stage lung cancer. Clin Chest Med. 2011;32(4):827–838. doi: 10.1016/j.ccm.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison M.A., Hegarty S.E., Keith S.W., Cowan S.W., Evans N.R., III Racial disparity in in-hospital mortality after lobectomy for lung cancer. Am J Surg. 2015;209(4):652–658. doi: 10.1016/j.amjsurg.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Bach P.B., Cramer L.D., Schrag D., Downey R.J., Gelfand S.E., Begg C.B. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345(3):181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 21.Lucas F.L., Stukel T.A., Morris A.M., Siewers A.E., Birkmeyer J.D. Race and surgical mortality in the United States. Ann Surg. 2006;243(2):281–286. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimick J., Ruhter J., Sarrazin M.V., Birkmeyer J.D. Black patients more likely than whites to undergo surgery at low-quality hospitals in segregated regions. Health Aff (Millwood) 2013;32(6):1046–1053. doi: 10.1377/hlthaff.2011.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris A.M., Rhoads K.F., Stain S.C., Birkmeyer J.D. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211(1):105–113. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Patel M.I., Wang A., Kapphahn K. Racial and ethnic variations in lung cancer incidence and mortality: results from the Women’s Health Initiative. J Clin Oncol. 2016 Feb 1;34(4):360–368. doi: 10.1200/JCO.2015.63.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel R.L., Fedewa S.A., Miller K.D. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2015;65(6):457–480. doi: 10.3322/caac.21314. [DOI] [PubMed] [Google Scholar]
- 26.Blackwell D., Lucas J., Clarke T. Centers for Disease Control and Prevention, National Center for Health Statistics; Atlanta, GA: 2014. Summary health statistics for U.S. adults: national health interview survey, 2012. [PubMed] [Google Scholar]
- 27.Ou S.-H.I., Ziogas A., Zell J.A. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol. 2009;4(9):1083–1093. doi: 10.1097/JTO.0b013e3181b27b15. [DOI] [PubMed] [Google Scholar]
- 28.Thun M.J., Hannan L.M., Adams-Campbell L.L. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5(9):e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudin C.M., Avila-Tang E., Harris C.C. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin Cancer Res. 2009;15(18):5646–5661. doi: 10.1158/1078-0432.CCR-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun S., Schiller J.H., Gazdar A.F. Lung cancer in never smokers—a different disease. Nat Rev Cancer. 2007;7(10):778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 31.Helland Å., Skaug H.M., Kleinberg L. EGFR gene alterations in a Norwegian cohort of patients with lung cancer selected for surgery. J Thorac Oncol. 2011;6(5):947–950. doi: 10.1097/JTO.0b013e31820db209. [DOI] [PubMed] [Google Scholar]
- 32.Kadota K., Sima C.S., Arcila M.E. KRAS mutation is a significant prognostic factor in early-stage lung adenocarcinoma. Am J Surg Pathol. 2016;40(12):1579–1590. doi: 10.1097/PAS.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smedley B.D., Stith A.Y., Nelson A.R., editors. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 34.Williams D.R., Sternthal M. Understanding Racial-ethnic disparities in health sociological contributions. J Health Soc Behav. 2010;51(1 suppl):S15–S27. doi: 10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams D.R., Mohammed S.A., Leavell J., Collins C. Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186(1):69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams D., Mohammed S.A. Racism and health I: pathways and scientific evidence. Am Behav Sci. 2013;57(8):1152–1173. doi: 10.1177/0002764213487340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams D.R., Wyatt R. Racial bias in health care and health: challenges and opportunities. JAMA. 2015;314(6):555–556. doi: 10.1001/jama.2015.9260. [DOI] [PubMed] [Google Scholar]
- 38.Lewis T.T., Everson-Rose S.A., Powell L.H. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: the SWAN Heart Study. Psychosom Med. 2006;68(3):362–368. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- 39.Brondolo E., Brady N., Thompson S. Perceived racism and negative affect: analyses of trait and state measures of affect in a community sample. J Soc Clin Psychol. 2008;27(2):150–173. doi: 10.1521/jscp.2008.27.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawyer P.J., Major B., Casad B.J., Townsend S.S.M., Mendes W.B. Discrimination and the stress response: psychological and physiological consequences of anticipating prejudice in interethnic interactions. Am J Public Health. 2012;102(5):1020–1026. doi: 10.2105/AJPH.2011.300620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi L., Chen C.-C., Nie X., Zhu J., Hu R. Racial and socioeconomic disparities in access to primary care among people with chronic conditions. J Am Board Fam Med. 2014;27(2):189–198. doi: 10.3122/jabfm.2014.02.130246. [DOI] [PubMed] [Google Scholar]
- 42.Smith J, Medalia C. Health insurance coverage in the United States: 2014. Washington DC: US Census Bureau; 2015.
- 43.Bach P.B., Pham H.H., Schrag D., Tate R.C., Hargraves J.L. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351(6):575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 44.Jha A.K., Orav E.J., Li Z., Epstein A.M. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med. 2007;167(11):1177–1182. doi: 10.1001/archinte.167.11.1177. [DOI] [PubMed] [Google Scholar]
- 45.Cook N.L., Hicks L.S., O’Malley A.J., Keegan T., Guadagnoli E., Landon B.E. Access to specialty care and medical services in community health centers. Health Aff (Milwood) 2007;26(5):1459–1468. doi: 10.1377/hlthaff.26.5.1459. [DOI] [PubMed] [Google Scholar]
- 46.Esnaola N.F., Ford M.E. Racial differences and disparities in cancer care and outcomes: where’s the rub? Surg Oncol Clin N Am. 2012;21(3):417–1437. doi: 10.1016/j.soc.2012.03.012. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epstein A.J., Gray B.H., Schlesinger M. Racial and ethnic differences in the use of high-volume hospitals and surgeons. Arch Surg. 2010;145(2):179–186. doi: 10.1001/archsurg.2009.268. [DOI] [PubMed] [Google Scholar]
- 48.Williams C.D., Salama J.K., Moghanaki D., Karas T.Z., Kelley M.J. Impact of race on treatment and survival among U.S. veterans with early-stage lung cancer. J Thorac Oncol. 2016;11(10):1672–1681. doi: 10.1016/j.jtho.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 49.Tanner N.T., Gebregziabher M., Hughes Halbert C., Payne E., Egede L.E., Silvestri G.A. Racial differences in outcomes within the National Lung Screening Trial. Implications for widespread implementation. Am J Respir Crit Care Med. 2015;192(2):200–208. doi: 10.1164/rccm.201502-0259OC. [DOI] [PubMed] [Google Scholar]
- 50.Williams C.D., Stechuchak K.M., Zullig L.L., Provenzale D., Kelley M.J. Influence of comorbidity on racial differences in receipt of surgery among US Veterans with early-stage non–small-cell lung cancer. J Clin Oncol. 2013;31(4):475–481. doi: 10.1200/JCO.2012.44.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landrum M.B., Keating N.L., Lamont E.B., Bozeman S.R., McNeil B.J. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer. 2012;118(13):3345–3355. doi: 10.1002/cncr.26628. [DOI] [PubMed] [Google Scholar]
- 52.Colla C.H., Morden N.E., Sequist T.D., Schpero W.L., Rosenthal M.B. Choosing wisely: prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2015;30(2):221–228. doi: 10.1007/s11606-014-3070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margolis M.L., Christie J.D., Silvestri G.A., Kaiser L., Santiago S., Hansen-Flaschen J. Racial differences pertaining to a belief about lung cancer surgery: results of a multicenter survey. Ann Intern Med. 2003;139(7):558–563. doi: 10.7326/0003-4819-139-7-200310070-00007. [DOI] [PubMed] [Google Scholar]
- 54.Kumar P., Gareen I.F., Lathan C. Racial differences in tobacco cessation and treatment usage after lung screening: an examination of the National Lung Screening Trial. The Oncologist. 2016;21(1):40–49. doi: 10.1634/theoncologist.2015-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bacio G.A., Guzman I.Y., Shapiro J.R., Ray L.A. Differences in quit attempts between non-hispanic black and white daily smokers: the role of smoking motives. Addict Behav. 2014;39(12):1769–1772. doi: 10.1016/j.addbeh.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulak J.A., Cornelius M.E., Fong G.T., Giovino G.A. Differences in quit attempts and cigarette smoking abstinence between whites and African Americans in the United States: literature review and results from the International Tobacco Control US Survey. Nicotine Tob Res. 2016;18(suppl 1):S79–S87. doi: 10.1093/ntr/ntv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gandhi K.K., Foulds J., Steinberg M.B., Lu S.-E., Williams J.M. Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract. 2009;63(3):360–367. doi: 10.1111/j.1742-1241.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- 58.Okuyemi K.S., Ahluwalia J.S., Ebersole-Robinson M., Catley D., Mayo M.S., Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction. 2003;98(10):1387–1393. doi: 10.1046/j.1360-0443.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- 59.Levinson A.H., Pérez-Stable E.J., Espinoza P., Flores E.T., Byers T.E. Latinos report less use of pharmaceutical aids when trying to quit smoking. Am J Prev Med. 2004;26(2):105–111. doi: 10.1016/j.amepre.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Levinson A.H., Borrayo E.A., Espinoza P., Flores E.T., Pérez-Stable E.J. An exploration of Latino smokers and the use of pharmaceutical aids. Am J Prev Med. 2006;31(2):167–171. doi: 10.1016/j.amepre.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 61.German R.R., Fink A.K., Heron M. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;35(2):126–131. doi: 10.1016/j.canep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 62.National Cancer Institute. Surveillance, Epidemiology and End Results Program. Number of persons by race and hispanic ethnicity (2010 census data). http://seer.cancer.gov/registries/data.html. Accessed January 22, 2016.
- 63.Humes K., Jones N., Ramirez R. US Census Bureau; Suitland, MD: 2011. Overview of race and Hispanic origin: 2010. [Google Scholar]
- 64.Mackun P., Wilson S. U.S. Census Bureau; Suitland, MD: 2011. Population distribution and change: 2000 to 2010. [Google Scholar]
- 65.Liao Y., Greenlund K.J., Croft J.B., Keenan N.L., Giles W.H. Factors explaining excess stroke prevalence in the US stroke belt. Stroke. 2009;40(10):3336–3341. doi: 10.1161/STROKEAHA.109.561688. [DOI] [PubMed] [Google Scholar]
- 66.Barker L.E., Kirtland K.A., Gregg E.W., Geiss L.S., Thompson T.J. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40(4):434–439. doi: 10.1016/j.amepre.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 67.Leidner R.S., Fu P., Clifford B. Genetic abnormalities of the EGFR pathway in African American patients with non–small-cell lung cancer. J Clin Oncol. 2009;27(33):5620–5626. doi: 10.1200/JCO.2009.23.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bollig-Fischer A., Chen W., Gadgeel S.M. Racial diversity of actionable mutations in non-small cell lung cancer. J Thorac Oncol. 2015;10(2):250–255. doi: 10.1097/JTO.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Araujo L.H., Timmers C., Bell E.H. Genomic characterization of non-small-cell lung cancer in African Americans by targeted massively parallel sequencing. J Clin Oncol. 2015;33(17):1966–1973. doi: 10.1200/JCO.2014.59.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell JD, Lathan C, Sholl L, et al. Comparison of prevalence and types of mutations in lung cancers among black and white populations [published online ahead of print January 19, 2017]. JAMA Oncol. http://dx.doi.org/10.1001/jamaoncol.2016.6108. [DOI] [PMC free article] [PubMed]
- 71.Spratt D.E., Chan T., Waldron L. Racial/ethnic disparities in genomic sequencing. JAMA Oncol. 2016;2(8):1070–1074. doi: 10.1001/jamaoncol.2016.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li T., Kung H.-J., Mack P.C., Gandara D.R. Genotyping and genomic profiling of non–small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31(8):1039–1049. doi: 10.1200/JCO.2012.45.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]