Abstract
Background
Enoxaparin 30 mg twice daily and dalteparin 5,000 units once daily are two common low-molecular-weight heparin (LMWH) thromboprophylaxis regimens used in the trauma population. Pharmacodynamic studies suggest that enoxaparin provides more potent anticoagulation than does dalteparin.
Methods
In 2009, our institution switched its formulary LMWH from enoxaparin to dalteparin followed by a switch back to enoxaparin in 2013. Using a difference in differences design, we contrasted the change in the VTE rate accompanying the LMWH switch with the change in a control group of trauma patients given unfractionated heparin (UFH) during the same period.
Results
The study included 5,880 patients: enoxaparin period (enoxaparin, n = 2,371; UFH, n = 1,539) vs the dalteparin period (dalteparin, n = 1,046; UFH, n = 924). The VTE rate was unchanged in the LMWH group: 3.3/1000 days in the enoxaparin period vs 3.8/1000 days in the dalteparin period: rate ratio (RR), 1.16; 95% CI 0.74-1.81. The rate was also unchanged in the UFH control subjects: 5.7/1,000 days in the enoxaparin period vs 5.2/1,000 days in the dalteparin period: RR, 0.92; 95% CI, 0.61-1.38. After confounding adjustment, the ratio of the change in VTE rate between the LMWH and UFH groups was similar: RR, 1.06; 95% CI 0.71-2.00. A secondary analysis excluding patients with delayed or interrupted prophylaxis (or both) altered this estimate nonsignificantly in favor of enoxaparin: RR, 2.39; 95% CI, 0.80-7.09.
Conclusions
Our results suggest that dalteparin has an effectiveness similar to that of enoxaparin in real-world trauma patients. Future research should investigate how the timing and consistency of prophylaxis affects LMWH effectiveness.
Key Words: comparative effectiveness, dalteparin, enoxaparin, prophylaxis, trauma, VTE
Abbreviations: DID, difference-in-differences; LMWH, low-molecular-weight heparin; PE, pulmonary embolism; PTSF, Pennsylvania Trauma System Foundation; RR, rate ratio; SDF, standardized difference; TFPI, tissue factor pathway inhibitor; UFH, unfractionated heparin
Effective thromboprophylaxis is essential in the trauma population. VTE is the third most common complication in trauma patients,1 and pulmonary embolism (PE) is the third most common cause of death in patients surviving the first 24 hours after an injury.2 Noting its importance, key stakeholders, such as the Centers for Medicare & Medicaid Services, have identified the incidence of VTE as a quality of care metric for all hospitalized patients.3, 4
Enoxaparin and dalteparin are the two most widely used low-molecular-weight heparin (LMWH) agents in North America. Although LMWHs are considered the gold standard for use in trauma patients,5 only enoxaparin has proven efficacy in this population.6, 7, 8 Enoxaparin and dalteparin exhibit important differences in molecular size, clearance, and relative affinity for factor Xa vs factor IIa and are not considered interchangeable by the US Food and Drug administration.9, 10 No randomized trials have tested dalteparin in trauma patients,8 whereas two observational studies comparing the agents provide inconclusive results.11, 12 Pharmacodynamic studies in healthy volunteers suggest that subcutaneous prophylactic doses of enoxaparin provide 30% to 100% greater factor Xa inhibition than does subcutaneous dalteparin on a per-unit basis.13, 14 Importantly, how these data extrapolate to a real-world trauma population is unknown.
Most institutions in the United States carry only one LMWH (either enoxaparin or dalteparin) on formulary at any one time. If these agents have different effectiveness, their interchange in the trauma population could be an important source of excess VTE complications. However, no large-scale study has addressed this important knowledge gap. Formulary changes over time at our institution have resulted in the conversion from enoxaparin to dalteparin and back to enoxaparin for VTE prophylaxis in trauma patients. These changes created a natural experiment that allowed us to compare enoxaparin and dalteparin in a real-world trauma population.
Methods
Study Design
In December 2009, the study institution’s acquisition cost of dalteparin decreased, prompting a formulary switch from enoxaparin to dalteparin. The cost of dalteparin later increased, resulting in a switch back to enoxaparin in February 2013. A study leveraging the resulting natural experiment can provide strong control of confounding by indication,15 because the change in LMWH was due to cost fluctuations and not perceived differences in efficacy. In addition, the removal and reintroduction of enoxaparin reduces bias from temporal variation in risk factors, VTE surveillance,16, 17 and overall quality of care.18 Temporal bias was also reduced by including a concurrent control group of trauma patients who received unfractionated heparin (UFH).18 According to local practice, UFH was prescribed preferentially to trauma patients with traumatic brain injury, spinal cord injury, or reduced renal function, or to those undergoing epidural analgesia. The control patients receiving UFH were cared for by the same providers in the same units and were subject to the same VTE surveillance as the LMWH group. The Institutional Review Board of the University of Pennsylvania approved the study and waived informed consent (IRB No. 7, protocol 818037).
Study Population
Patients were drawn from the Hospital of the University of Pennsylvania, an academic level 1 trauma center in Philadelphia Pennsylvania. Inclusion criteria were admission from January 1, 2004 to March 1, 2014, age ≥ 18 years, receipt of ≥ 24 hours of prophylaxis initiated within 48 hours of admission, and presence of one or more VTE risk factors, including lower-extremity fracture, pelvic fracture, spinal cord injury, traumatic brain injury, transfusion in the ED, age ≥ 40 years, history of prior VTE, malignancy, or ICU admission.5, 19, 20, 21 Patients were excluded for crossover between dalteparin and enoxaparin, receipt of ≥ 24 hours of a second anticoagulant within 48 hours of admission, active VTE on admission, or missing covariate data. This latter criterion was not applied to missing height or weight (or both), given the high prevalence of missingness for these covariates. We addressed missing data through multiple imputation (e-Appendix 1).
VTE Prophylaxis
The prophylaxis guideline recommended combined LMWH and mechanical prophylaxis with sequential compression devices. Patients were surveilled for VTE with lower extremity ultrasonography for 3 weeks after injury while in the hospital. Enoxaparin 30 mg twice daily was the standard LMWH during the enoxaparin periods, whereas dalteparin 5,000 units once daily was the standard during the dalteparin period. The standard UFH regimen was 5,000 units three times daily throughout the entire study period. We assumed prophylaxis could not prevent VTE events diagnosed on the day of prophylaxis initiation. Thus, follow-up began 24 hours after the first dose and continued until 48 hours after the last dose, initiation of a different prophylaxis regimen, occurrence of VTE, hospital discharge, death, or until hospital day 30, whichever came first.
Data Sources and Variables
Patients were identified from a local registry that conforms to state reporting guidelines as set forth by the Pennsylvania Trauma System Foundation (PTSF). Specially trained registrars abstract data prospectively according to standardized definitions, and data quality is monitored by PTSF through data audits. The registry is frequently used for research.22, 23, 24 The registry contains demographics, injury characteristics, Injury Severity Score,25 comorbidities, and complications.
Registry data were linked to data from a health system-wide data warehouse26 that contains medications, discharge diagnosis data, orders for sequential compression devices and duplex ultrasonographic examinations, level of care (ICU vs ward), and laboratory results.
Outcomes
The primary outcome was inhospital 30-day VTE, a composite of PE and lower-extremity DVT. VTE was queried from the trauma registry and from hospital discharge diagnosis codes (e-Table 1). All VTEs identified through query were validated with chart review (see e-Appendix 1 for complete definitions). In a prior validation study, we demonstrated complete capture of VTE with this method.27 Secondary outcomes were PE, proximal DVT, all DVT, and a composite of VTE and all-cause hospital mortality (to account for the competing risk of mortality28, 29 and to capture undiagnosed fatal PE).
Statistical Analysis
Baseline covariates were summarized with descriptive statistics. Covariate balance was assessed using standardized differences (SDF),30, 31 with SDF > 0.1 considered an important imbalance.31 VTE occurrence was expressed as the number of incident VTEs/1,000 person-days. A difference-in-differences (DID) analysis18, 32 was used to contrast the change in VTE rate accompanying the LMWH switch with the change in VTE rate in UFH control subjects. Observations from the two enoxaparin periods (January 2004-December 2009 and February 2013-March 2014) were aggregated for comparison with the dalteparin period (December 2009-Febuary 2013). Multivariable Poisson regression with robust variance estimation was used for parameter estimation. The base model was:
where i is the patient, j is the prophylaxis group (LMWH vs UFH), and t is the formulary period (enoxaparin vs dalteparin). Regression coefficients are interpreted as follows: β1 is the difference in VTE between the dalteparin period and the enoxaparin periods, β2 is the difference in VTE between patients receiving LMWH and control subjects receiving UFH, and β3 is the DID term. If the DID term is positive, this implies that the VTE rate increased more among patients receiving LMWH than among the control patients receiving UFH during the dalteparin period. Thus, β3 represents the treatment effect of the formulary switch to dalteparin. Potential confounding was evaluated using a change-in-estimate approach.33, 34 Each covariate (Table 1) was added to the base model individually. Covariates that changed the DID term by ≥ 10% were retained in the final model. Statistical inference was based on the 95% CIs around the DID point estimates.35, 36 All analyses were conducted using Stata/SE, version 14.2 (StataCorp, LLC). Details of model validation procedures can be found in e-Appendix 1.
Table 1.
Baseline Characteristics
| Variable | LMWH Agents |
SDF | Heparin Control Agents |
SDF | ||
|---|---|---|---|---|---|---|
| Enoxaparin Period, n = 2,371 | Dalteparin Period, n = 1,046 | Enoxaparin Period, n = 1,539 | Dalteparin Period, n = 924 | |||
| Demographics | ||||||
| Age, y | 42 (26-55) | 44 (29-55) | –0.060 | 56 (41-76) | 57 (42-76) | –0.030 |
| Female sex | 702 (29.6) | 313 (29.9) | –0.010 | 546 (35.5) | 316 (34.2) | 0.030 |
| Race | ||||||
| Black | 1,298 (54.7) | 606 (57.9) | –0.060 | 642 (41.7) | 397 (42.9) | –0.030 |
| White | 916 (38.6) | 379 (36.2) | 0.050 | 801 (52.1) | 482 (52.2) | –0.010 |
| Asian | 35 (1.5) | 21 (2.0) | –0.040 | 32 (2.1) | 14 (1.5) | 0.040 |
| Other | 122 (5.2) | 40 (3.8) | 0.060 | 64 (4.2) | 31 (3.4) | 0.040 |
| Weight, kga | 79.4 (68.0-93.4) | 78.5 (68.0-90.7) | 0.070 | 77.1 (65.3-90.7) | 76.0 (65.0-89.8) | 0.060 |
| BMIb | 26.5 (23.2-30.6) | 25.8 (22.9-29.9) | 0.080 | 25.8 (22.7-29.5) | 25.5 (22.3-29.4) | 0.040 |
| Injury characteristics | ||||||
| Injury Severity Score | 10.0 (5-14) | 9.0 (5-12) | 0.180 | 15.5 (5-24) | 13.5 (5-19) | 0.160 |
| Injury type | ||||||
| Blunt | 1,841 (77.7) | 791 (75.6) | 0.050 | 1,379 (89.6) | 834 (90.3) | –0.020 |
| Penetrating | 530 (22.4) | 255 (24.4) | –0.050 | 160 (10.4) | 90 (9.7) | 0.020 |
| Traumatic brain injury | 55 (2.3) | 17 (1.6) | 0.050 | 478 (31.1) | 275 (29.8) | 0.030 |
| Femur fracture | 465 (19.6) | 150 (14.3) | 0.140 | 81 (5.3) | 53 (5.7) | –0.020 |
| Pelvic fracture | 330 (13.9) | 113 (10.8) | 0.090 | 127 (8.3) | 60 (6.5) | 0.070 |
| Spinal cord injury | 34 (1.4) | 8 (0.8) | 0.060 | 107 (6.9) | 66 (7.1) | –0.010 |
| Pulmonary contusion | 187 (7.9) | 73 (6.9) | 0.040 | 165 (10.7) | 85 (9.2) | 0.050 |
| Venous injury | 38 (1.6) | 24 (2.3) | –0.050 | 19 (1.2) | 4 (0.4) | 0.090 |
| Baseline laboratory values | ||||||
| Hemoglobin, g/dL | 12.4 (10.9-13.8) | 12.2 (10.8-13.6) | 0.070 | 11.7 (10.2-13.3) | 11.8 (10.0-13.3) | –0.010 |
| Platelets, ×1011 cells/L | 211 (169-260) | 196 (160-243) | 0.150 | 200 (158-245) | 187 (149-234) | 0.120 |
| Creatinine, mg/dL | 0.9 (0.8-1.1) | 0.9 (0.8-1.1) | 0.070 | 0.9 (0.7-1.2) | 0.9 (0.7-1.2) | –0.050 |
| GFR,c mL/min/1.73 m2 | 89 (73-109) | 91 (74-110) | –0.040 | 81 (58-104) | 81 (55-103) | 0.060 |
| Treatment characteristics | ||||||
| ICU admission | 652 (27.5) | 263 (25.1) | 0.050 | 818 (53.2) | 493 (53.4) | –0.010 |
| Mechanical ventilation | 430 (18.1) | 232 (22.2) | –0.100 | 474 (30.8) | 288 (31.2) | –0.010 |
| Surgery | 664 (28.0) | 234 (22.4) | 0.130 | 270 (17.5) | 86 (9.3) | 0.240 |
| ED transfusion | ||||||
| None | 2,204 (92.9) | 988 (94.5) | –0.060 | 1,454 (94.5) | 884 (95.7) | –0.060 |
| 1 unit | 69 (2.9) | 23 (2.2) | 0.050 | 23 (1.5) | 15 (1.6) | –0.010 |
| ≥ 2 units | 98 (4.1) | 35 (3.4) | 0.040 | 62 (4.0) | 25 (2.7) | 0.070 |
| Mechanical prophylaxis | 1,269 (53.5) | 947 (90.5) | –0.900 | 900 (58.5) | 794 (85.9) | –0.640 |
| Comorbidities | ||||||
| Heart failure | 27 (1.1) | 12 (1.2) | –0.010 | 76 (4.9) | 59 (6.4) | –0.060 |
| Myocardial infarction | 42 (1.8) | 17 (1.6) | 0.010 | 62 (4.0) | 55 (5.9) | –0.090 |
| Atrial fibrillation | 56 (2.4) | 24 (2.3) | 0.010 | 159 (10.3) | 98 (10.6) | –0.010 |
| Hypertension | 596 (25.1) | 275 (26.3) | –0.030 | 649 (42.2) | 445 (48.2) | –0.120 |
| Stroke | 47 (1.9) | 24 (2.3) | –0.020 | 113 (7.3) | 66 (7.1) | 0.010 |
| COPD | 63 (2.7) | 38 (3.6) | –0.060 | 93 (6.0) | 53 (5.7) | 0.010 |
| Liver disease | 16 (0.7) | 7 (0.7) | 0.010 | 20 (1.3) | 13 (1.4) | –0.010 |
| Malignancy | 87 (3.7) | 41 (3.9) | –0.010 | 114 (7.4) | 71 (7.7) | –0.010 |
| Prior thrombosis | 23 (0.9) | 16 (1.5) | –0.050 | 24 (1.6) | 29 (3.1) | –0.100 |
| Thrombophilia | 18 (0.8) | 6 (0.6) | 0.020 | 31 (2.0) | 17 (1.8) | 0.010 |
| ESRD | 7 (0.3) | 1 (0.1) | 0.050 | 49 (3.2) | 30 (3.3) | –0.010 |
| Baseline medications | ||||||
| Antiplatelets | 379 (15.9) | 164 (15.7) | 0.010 | 256 (16.6) | 132 (14.3) | 0.070 |
| RAS inhibitors | 123 (5.2) | 53 (5.07) | 0.010 | 146 (9.5) | 93 (10.1) | 0.020 |
| Vasopressors | 20 (0.8) | 15 (1.4) | –0.060 | 140 (9.1) | 70 (7.6) | 0.060 |
| Statin drugs | 142 (5.9) | 69 (6.6) | –0.030 | 212 (13.8) | 153 (16.6) | –0.080 |
| Epidural analgesia | 11 (0.5) | 5 (0.5) | –0.010 | 110 (7.2) | 54 (5.8) | 0.050 |
Data are presented as median (IQR) or No. (%). ESRD = end-stage renal disease; GFR = glomerular filtration rate; IQR = interquartile range; LMWH = low-molecular-weight heparin; RAS = renin-angiotensin system; SDF = standardized difference; UFH = unfractionated heparin.
LMWH, n = 3,162; UFH, n = 2,113.
LMWH, n = 2,697; UFH, n = 1,957.
LMWH, n = 2,697; UFH, n = 1,957.
Sensitivity Analyses
We examined the robustness of our findings in several sensitivity analyses: (1) repeating analysis using negative binomial regression, (2) restricting analysis to patients who underwent one or more surveillance ultrasonographic examinations, (3) censoring follow-up at the time of the last dose, (4) excluding patients admitted before January 2007, (5) including patients with missing data using multiple imputation (e-Appendix 1), (6) restricting analysis to patients who received prophylaxis within 24 hours of admission, (7) restricting analysis to patients who missed < 20% of scheduled doses (post hoc analysis), and (8) restricting analysis to patients who missed < 20% of scheduled doses who also received prophylaxis within 24 hours of admission (post hoc analysis). Missed-dose tabulation methods and results are detailed in e-Appendix 1, e-Table 2.
Results
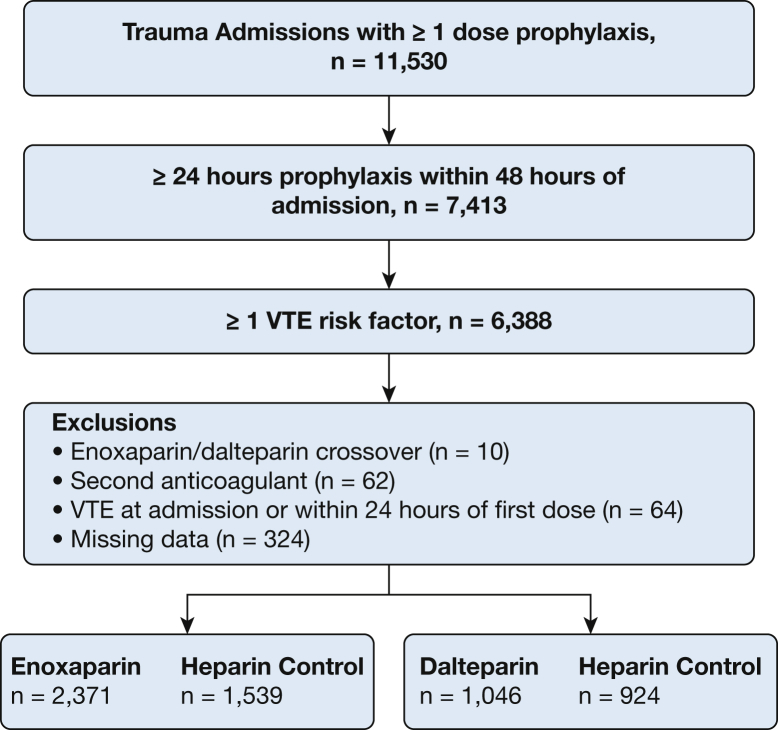
We included 5,880 patients (Fig 1): 3,910 patients during the enoxaparin period (enoxaparin, n = 2,371; UFH control subjects, n = 1,539) and 1,970 patients during the dalteparin period (dalteparin, n = 1,046; UFH control subjects, n = 924). Patients receiving enoxaparin and those receiving dalteparin had similar age, sex, body size, injury mechanism, baseline renal function, and comorbid illnesses (Table 1). As expected, the control patients receiving UFH differed from the patients receiving LMWH, reflecting the different indications for UFH vs LMWH as dictated by the prophylaxis guideline. UFH control subjects had a higher prevalence of traumatic brain injury, spinal cord injury, epidural analgesia, and lower baseline renal function. However, the characteristics of the UFH control subjects generally changed little over time. The control patients receiving UFH in the enoxaparin vs dalteparin periods showed similar age, sex, body size, injury mechanism, baseline renal function, and comorbid illnesses. Some characteristics changed over time in both the LMWH and UFH control groups: Injury Severity Score, baseline platelet count, use of mechanical prophylaxis, and incidence of surgical procedures. However, the magnitude and direction of the temporal changes were similar between the LMWH and UFH control groups. Finally, a change in VTE surveillance over time was similar in the LMWH and UFH control groups (e-Table 3).
Figure 1.
Patient flowchart.
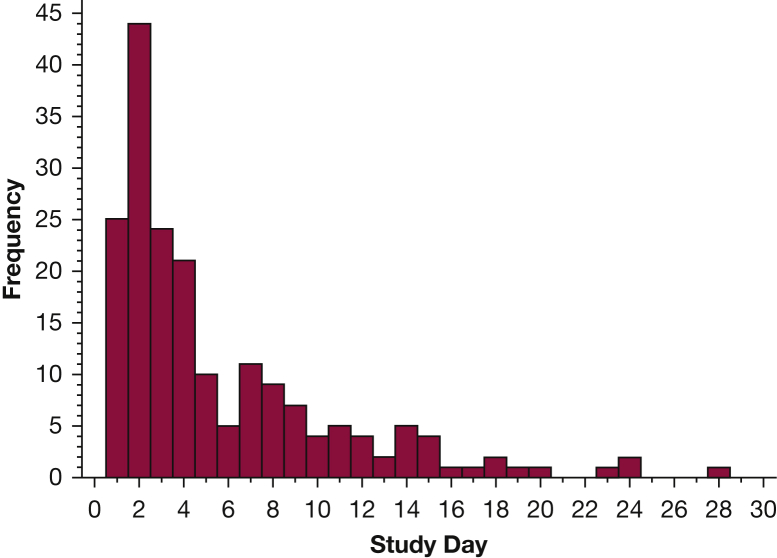
There were 190 VTE events, with most (65.3%) occurring during the first 5 days (Fig 2). PE occurred in 46 patients, whereas isolated DVT occurred in 144 patients, with 73 of these being proximal events. Seven patients with PE had concomitant DVT. Thirty-day inpatient mortality was higher in patients with VTE than in those without: 11 of 190 patients (5.8%) vs 105 of 5,690 patients (1.9%); relative risk, 3.3; 95% CI, 1.7-5.7. The primary analysis is shown in Table 3. The unadjusted DID estimate was 1.25 (95% CI, 0.69-2.29). Adjustment for confounding attenuated the estimate to 1.06 (95% CI, 0.71-2.00), consistent with a negligible difference in effectiveness between enoxaparin and dalteparin, albeit with a fairly wide CI. Similar patterns were observed for the secondary outcomes (Table 3).
Table 2.
Primary Outcome Difference-in-Differences Analysis
| Group | Enoxaparin Period, Rate/1,000 d | Dalteparin Period, Rate/1,000 d | RR (95% CI) |
|---|---|---|---|
| LMWH group | |||
| Unadjusted | 3.31 | 3.84 | 1.16 (0.74-1.81) |
| Adjusteda | … | … | 1.03 (0.63-1.69) |
| UFH control group | |||
| Unadjusted | 5.72 | 5.28 | 0.92 (0.62-1.38) |
| Adjusteda | … | … | 0.97 (0.64-1.48) |
| Difference-in-differencesb | |||
| Unadjusted | … | … | 1.25 (0.69-2.29) |
| Adjusteda | … | … | 1.06 (0.71-2.00) |
RR = rate ratio. See Table 1 legend for expansion of other abbreviations.
Adjusted for Injury Severity Score, mechanical ventilation, surgery, spinal cord injury, femur facture, mechanical prophylaxis, ICU admission, and venous injury.
The difference-in-differences parameter represents the relative difference in the RR between treatment groups. It is interpreted as follows: the relative increase of VTE rate in LMWH-exposed patients after the switch was 6% larger than the relative increase in UFH control subjects.
Figure 2.
Distribution of time to VTE.
Table 3.
Secondary Outcomes and Sensitivity Analyses
| Analysis | Difference-in-Differences (95% CI)a |
|---|---|
| Secondary end points | |
| VTE + mortality | 0.98 (0.57-1.72) |
| Pulmonary embolism | 1.21 (0.33-4.45) |
| All DVT | 0.97 (0.46-2.03) |
| Proximal DVT | 0.98 (0.31-3.08) |
| Sensitivity analyses | |
| Negative binomial regression model | 1.09 (0.57-2.08) |
| At least 1 ultrasonographic scanb | 0.99 (0.53-1.85) |
| Censor follow-up at last dose | 1.00 (0.52-1.91) |
| Admission after 2007c | 1.08 (0.56-2.11) |
| Initiation within 24 h of admissiond | 1.57 (0.61-4.09) |
| Missed < 20% of scheduled dosese | 1.39 (0.68-2.82) |
| Initiation within 24 h and missed < 20% of scheduled dosesf | 2.39 (0.80-7.09) |
| Multiple imputation modelg | 0.99 (0.53-1.86) |
See e-Appendix 1 for details of multivariable model specifications.
The difference-in-differences parameter represents the relative difference in the rate ratio between treatment groups.
n = 184 VTEs in 3,184 patients.
n = 148 VTEs in 4,265 patients.
n = 73 VTEs in 3,203 patients.
n = 150 VTEs in 4,625 patients.
n = 55 VTEs in 2,457 patients.
n = 191 VTEs in 6,204 patients.
Results were robust regarding the choice of statistical model, length of follow-up after the last dose, changes in period, and changes in ultrasonographic surveillance (Table 3). Our results were also similar in the multiple imputation analysis (e-Appendix 1, e-Tables 4-7). The DID estimate increased when we restricted analysis to patients who had prophylaxis initiated within 24 hours of admission (n = 3,203) or to patients who missed < 20% of scheduled doses (n = 4,625). Further restriction to patients who received prophylaxis within 24 hours of admission while also missing < 20% of scheduled doses (n = 2,457) resulted in a DID of 2.39 (95% CI, 0.80-7.09).
Discussion
The goal of comparative effectiveness research is to examine the benefits and harms of treatments in real-world settings.37 Implicit in this aim is a recognition that findings from highly controlled trials or preclinical settings, or both, may not translate to everyday practice.37 This idea is particularly relevant to thromboprophylaxis in trauma, in which patients with a high risk for thrombosis may also experience delayed or interrupted prophylaxis because of surgery or bleeding. Against this backdrop, we compared the two most widely used LMWH regimens in the trauma population. Despite pharmacodynamic data suggesting substantial differences in anticoagulant potency, we observed similar rates of VTE with enoxaparin and dalteparin. Exclusion of patients with prophylaxis delays or interruptions (or both) produced estimates in favor of enoxaparin. Although these secondary findings should be viewed as hypothesis generating only, they suggest that differences in potency may be relevant only in patients who receive early and consistent prophylaxis.
Although no randomized trials have addressed this question, two retrospective studies are relevant. Slavik et al11 studied 135 trauma patients and found a numeric imbalance suggesting a higher rate of VTE with dalteparin than with enoxaparin: 9.7% vs 1.6%, respectively (difference, 8.1% [95% CI, –0.6% to 15.6%]; P = .1), but the small sample size precluded definitive conclusions. A second study of 610 trauma patients showed similar effectiveness (enoxaparin, 5.1% vs dalteparin, 4.5%; difference, 0.5% [95% CI, –2.9% to 4.0%]; P = .85), but the study included patients with delayed prophylaxis, potentially blunting differences in effectiveness.12 Our results extend these findings, suggesting that the enoxaparin and dalteparin regimens provide similar protection against VTE in a real-world trauma setting.
LMWH is presumed to inhibit coagulation primarily through inhibition of factor Xa.10 Our primary results thus seem inconsistent with the apparent differences in anti-FXa activity produced by the two LMWH regimens.9, 10, 13, 14, 38 Collignon et al14 showed that prophylactic doses of enoxaparin provided 2.4-fold higher anti-FXa activity per 1,000 international units vs dalteparin.14 Similar results were observed in a study by Bendz et al.38 The higher anti-FXa activity of enoxaparin would be expected to reduce VTE rates. However, the antithrombotic effect of LMWHs may be mediated by other mechanisms.9, 10 LMWHs cause release of tissue factor pathway inhibitor (TFPI),39 and the degree of TFPI release does not mirror anti-FXa activity.10, 38 LMWHs also have variable effects on factor IIa, von Willebrand factor, and thrombin-activatable fibrinolysis inhibitor.10, 40, 41 Consequently, the global antithrombotic effects of the two LMWHs may be similar despite differences in measured anti-FXa activity.
Our primary analysis was designed to measure effectiveness, which focuses on real-world effects as opposed to effects under ideal conditions (ie, efficacy).37 Thus, patients were included regardless of missed or delayed prophylaxis initiation (or both), factors that may substantially reduce the benefit of LMWH prophylaxis.42, 43, 44 Restriction of our analysis to patients who received early (within 24 hours) and consistent (> 80% of scheduled doses) treatment with LMWH produced an estimate that favored enoxaparin. This result could be viewed as a measure of the comparative efficacy of the two regimens, rather than effectiveness, as it derives from a population that more closely represents “ideal conditions.” It should be noted, however, that the CIs around these post hoc sensitivity estimates are considerably wider, overlapping with the results of the primary analysis. These caveats notwithstanding, the findings generate plausible hypotheses that may help to explain discrepancies between efficacy and effectiveness in studies of VTE thromboprophylaxis in trauma patients.
VTE can occur very early after injury,45 with most cases occurring by day 5 in our study. Consequently, thrombosis may have already been developing at the time of prophylaxis initiation in many patients, blunting differences in anticoagulation efficacy provided by the two LMWH regimens. Missed doses may similarly blunt differences in efficacy by allowing clot formation during periods of low exposure after a missed dose. The cumulative effect of such gaps in prophylaxis could potentially mask differences in anticoagulation potency between the enoxaparin and dalteparin regimens. Although these factors represent targets for intervention, the extent to which they are modifiable is unclear. Our results suggest that additional research is needed to understand whether and how the timing and consistency of prophylaxis alters LMWH comparative effectiveness.
Our study has limitations. Although our primary results are most consistent with similar effectiveness, the CIs do not rule out potentially important differences. Additional studies in larger populations are needed to increase precision. It is possible that our results are biased by unmeasured factors that changed differentially over time in the LMWH group vs the UFH control group. We minimized such bias by virtue of our study design and analysis approach. In addition, we adjusted for important VTE risk factors. However, as with any nonrandomized design, the possibility of residual bias cannot be eliminated. Our results stem from a single center, potentially limiting generalizability. In addition, we could not distinguish symptomatic vs asymptomatic VTE. Ideally, the analysis would focus on symptomatic events, but such an approach was not feasible for multiple reasons. First, adequate documentation of the requisite information on symptoms is largely absent in the electronic medical record. Second, the signs and symptoms of VTE are very nonspecific in trauma and other critically ill populations. Additional research in this area is needed. Finally, we could not measure bleeding because of a lack of validated algorithms to identify bleeding retrospectively. In addition, data on transfusions were only available after the year 2010 in our database, precluding the use of this variable as an outcome in the analysis. Alternatively, we could have derived a variable related to drops in hemoglobin values; however, we judged this approach to lack sufficient specificity in the trauma population, which experiences drops in hemoglobin values for reasons other than bleeding (eg, anemia of critical illness, dilutional effects of fluid resuscitation). Prior evidence suggests that bleeding rates are low with both regimens.8
Conclusions
We observed similar effectiveness for dalteparin vs enoxaparin in a real-world trauma population. Future research should investigate how the timing and consistency of prophylaxis delivery affects LMWH effectiveness.
Acknowledgments
Author contributions: T. A. M. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and assumes full responsibility for the integrity of the submission as a whole from inception to published article. T. A. M. contributed substantially to conception and study design, acquisition of data, analysis and interpretation of data, drafting of the manuscript and all subsequent revisions, and has approved the final version. A. D. and J. D. C. contributed substantially to study design, analysis, and interpretation of data, as well as critical revision of the manuscript for important intellectual content, and approved the final version. N. M. contributed substantially to the acquisition of data, analysis and interpretation of the data, and critical revision of the manuscript for important intellectual content and approved the final version. B. S. contributed substantially to study design and critical revision of the manuscript for important intellectual content and approved the final version. A. T. contributed substantially to study design and critical revision of the manuscript for important intellectual content and approved the final version. W. G. contributed substantially to study design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content and approved the final version. S. H. has contributed substantially to conception and study design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and approved the final version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. C. has served as a consultant for Biogen-Idec, Diagnostica Stago, and Genzyme and has received research support from Bayer, Biogen-Idec, Spark Therapeutics, Shire, and T2 Biosystems. S. H. has served as a consultant for Sanofi and also directs a center that receives educational support from Pfizer. None declared (T. A. M., J. D. C., N. M., B. S., A. T. M., W. G.) .
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by grants from the National Institutes of Health [grants F32HL124914 to T. A. M., T32GM075766 to T. A. M. and S. H., HL115354 to J. D. C., and HL087115 to J. D. C.].
Supplementary Data
References
- 1.National Trauma Databank. Annual reports. https://www.facs.org/quality-programs/trauma/ntdb/docpub. Accessed June 12, 2017.
- 2.Acosta J.A., Yang J.C., Winchell R.J. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186(5):528–533. doi: 10.1016/s1072-7515(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 3.Agency for Healthcare Research and Quality. Preventing hospital-associated venous thromboembolism. http://www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/index.html. Accessed March 13, 2016.
- 4.Centers for Medicaid & Medicaid Services. Hospital-acquired conditions (present of admissions indicator). http://cms.gov/HospitalAcqCond/06_Hospital-Acquired_Conditions.asp. Accessed March 13, 2016.
- 5.Gould M.K., Garcia D.A., Wren S.M. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl):e227S–e277S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geerts W.H., Jay R.M., Code K.I. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335(10):701–707. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- 7.Spinal Cord Injury Thromboprophylaxis Investigators Prevention of venous thromboembolism in the acute treatment phase after spinal cord injury: a randomized, multicenter trial comparing low-dose heparin plus intermittent pneumatic compression with enoxaparin. J Trauma. 2003;54(6):1116–1126. doi: 10.1097/01.TA.0000066385.10596.71. [DOI] [PubMed] [Google Scholar]
- 8.Barrera L.M., Perel P., Ker K., Cirocchi R., Farinella E. Morales Uribe CH. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013;3(3):CD008303. doi: 10.1002/14651858.CD008303.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Frydman A. Low molecular weight heparins: an overview of pharmacokinetics, pharmacodynamics and metabolism in humans. Haemostasis. 1996;26(suppl 2):24–38. doi: 10.1159/000217270. [DOI] [PubMed] [Google Scholar]
- 10.Fareed J., Jeske W., Hoppensteadt D., Clarizio R., Walenga J.M. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L–10L. doi: 10.1016/s0002-9149(98)00105-2. [DOI] [PubMed] [Google Scholar]
- 11.Slavik R.S., Chan E., Gorman S.K. Dalteparin versus enoxaparin for venous thromboembolism prophylaxis in acute spinal cord injury and major orthopedic trauma patients: ‘DETECT’ trial. Trauma. 2007;62(5):1075–1081. doi: 10.1097/TA.0b013e31804fa177. [DOI] [PubMed] [Google Scholar]
- 12.Okoye O.T., Gelbard R., Inaba K. Dalteparin versus enoxaparin for the prevention of venous thromboembolic events in trauma patients. Eur J Trauma Emerg Surg. 2014;40(2):183–189. doi: 10.1007/s00068-013-0333-z. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson B.I., Soderberg K., Wedlund L., Vandeli B., Tengborn L., Risberg B. A comparative study of three low molecular weight heparins (LMWH) and unfractionated heparin (UFH) in healthy volunteers. Thromb Haemost. 1995;73(3):398–401. [PubMed] [Google Scholar]
- 14.Collignon F., Frydman A., Caplain H. Comparison of the pharmacokinetic profiles of three low molecular mass heparins—dalteparin, enoxaparin and nadroparin—administered subcutaneously in healthy volunteers (doses for prevention of thromboembolism) Thromb Haemost. 1995;73(4):630–640. [PubMed] [Google Scholar]
- 15.Strom B.L., Schinner R., Hennessy S. Comparative effectiveness research. In: Strom B.L., Kimmel S.E., Hennessy S., editors. Pharmacoepidemiology. 5th ed. Wiley-Blackwell; Hoboken, NJ: 2012. pp. 561–581. [Google Scholar]
- 16.Haut E.R., Noll K., Efron D.T. Can increased incidence of deep vein thrombosis (DVT) be used as a marker of quality of care in the absence of standardized screening? The potential effect of surveillance bias on reported DVT rates after trauma. J Trauma. 2007;63(5):1132–1137. doi: 10.1097/TA.0b013e31814856ad. [DOI] [PubMed] [Google Scholar]
- 17.Haut E.R., Chang D.C., Pierce C.A. Predictors of posttraumatic deep vein thrombosis (DVT): hospital practice versus patient factors—an analysis of the National Trauma Data Bank (NTDB) J Trauma. 2009;66(4):994–1001. doi: 10.1097/TA.0b013e3181991adc. [DOI] [PubMed] [Google Scholar]
- 18.Shadish W.R., Cook T.D., Campbell D.T. Houghton Mifflin; Boston: 2002. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. [Google Scholar]
- 19.Geerts W., Code K., Jay R. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601–1606. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 20.Knudson M., Ikossi D., Khaw L. Thromboembolism after trauma. An analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240(3):490–498. doi: 10.1097/01.sla.0000137138.40116.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortel T.L. Acquired thrombotic risk factors in the critical care setting. Crit Care Med. 2010;38(suppl):S43–S50. doi: 10.1097/CCM.0b013e3181c9ccc8. [DOI] [PubMed] [Google Scholar]
- 22.Earl-Royal E., Kaufman E.J., Hsu J.Y., Wiebe D.J., Reilly P.M., Holena D.N. Age and preexisting conditions as risk factors for severe adverse events and failure to rescue after injury. J Surg Res. 2016;205(2):368–377. doi: 10.1016/j.jss.2016.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew S., Smith B.P., Cannon J.W., Reilly P.M., Schwab C.W., Seamon M.J. Temporary arterial shunts in damage control: experience and outcomes. J Trauma Acute Care Surg. 2017;82(3):512–517. doi: 10.1097/TA.0000000000001334. [DOI] [PubMed] [Google Scholar]
- 24.Morris D.S., Reilly P., Rohrbach J., Telford G., Kim P., Sims C.A. The influence of unit-based nurse practitioners on hospital outcomes and readmission rates for patients with trauma. J Trauma Acute Care Surg. 2012;73(2):474–478. doi: 10.1097/TA.0b013e31825882bb. [DOI] [PubMed] [Google Scholar]
- 25.Baker S.P., O'Neill B., Haddon W., Long W.B. The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care.”. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 26.Penn Medicine. Data Analytics Center. Penn data store. http://www.med.upenn.edu/dac/penn-data-store-warehouse.html. Accessed November 8, 2015.
- 27.Miano T.A., Abelian G., Smith B. American College of Surgeons Clinical Congress; Washington DC: October 18, 2016. Whose benchmark is right? Validating venous thromboembolism events between trauma registries and hospital administrative databases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry S.D., Ngo L., Samelson E.J., Kiel D.P. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783–787. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Cook D., Meade M., Guyatt G. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364(14):1305–1314. doi: 10.1056/NEJMoa1014475. [DOI] [PubMed] [Google Scholar]
- 30.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. [Google Scholar]
- 32.Dimick J.B., Ryan A.M. The differences-in-differences approach. JAMA. 2014;312(22):2401–2402. doi: 10.1001/jama.2014.16153. [DOI] [PubMed] [Google Scholar]
- 33.Mickey R.M., Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 34.Maldonado G., Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 35.Wasserstein R.L., Lazar N.A. The ASA's statement on p-values: context, process, and purpose. Am Stat. 2016;70(2):129–133. [Google Scholar]
- 36.Greenland S., Senn S.J., Rothman K.J. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31(4):337–350. doi: 10.1007/s10654-016-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conway P.H., Clancy C. Comparative-Effectiveness research—implications of the Federal Coordinating Council’s report. N Engl J Med. 2009;361(4):328–330. doi: 10.1056/NEJMp0905631. [DOI] [PubMed] [Google Scholar]
- 38.Bendz B., Andersen T.O., Sandset P.M. Dose-dependent release of endogenous tissue factor pathway inhibitor by different low molecular weight heparins. Blood Coagul Fibrinolysis. 2000;11(4):343–348. doi: 10.1097/00001721-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Wood J.P., Ellery P.E.R., Maroney S.A., Mast A.E. Biology of tissue factor pathway inhibitor. Blood. 2014;23(19):2934–2943. doi: 10.1182/blood-2013-11-512764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montalescot G., Collet J.P., Lison L. Effects of various anticoagulant treatments on von Willebrand factor release in unstable angina. J Am Coll Cardiol. 2000;36(1):110–114. doi: 10.1016/s0735-1097(00)00695-1. [DOI] [PubMed] [Google Scholar]
- 41.Florian-Kjawski M., Hoppensteadt D., Maddineni J., Ziegler H., Fareed J. Differential regulation of thrombin activatable fibrinolytic inhibitor by low molecular weight heparins. Pharmacologic implications. Int Angiol. 2004;23(4):346–354. [PubMed] [Google Scholar]
- 42.Nathens A.B., McMurray M.K., Cuschieri J. The practice of venous thromboembolism prophylaxis in the major trauma patient. J Trauma. 2007;62(3):557–563. doi: 10.1097/TA.0b013e318031b5f5. [DOI] [PubMed] [Google Scholar]
- 43.Louis S.G., Sato M., Geraci T. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149(4):365–370. doi: 10.1001/jamasurg.2013.3963. [DOI] [PubMed] [Google Scholar]
- 44.Salottolo K., Offner P., Levy A.S., Mains C.W., Slone D.S., Bar-Or D. Interrupted pharmacologic thromboprophylaxis increases venous thromboembolism in traumatic brain injury. J Trauma. 2011;70(1):19–26. doi: 10.1097/TA.0b013e318207c54d. [DOI] [PubMed] [Google Scholar]
- 45.Owings J.T., Kraut E., Battistella F., Cornelius J.T., O’Malley R. Timing of the occurrence of pulmonary embolism in trauma patients. Arch Surg. 1997;132(8):862–867. doi: 10.1001/archsurg.1997.01430320064010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.