Abstract
Peripheral nerve injury (PNI) activates the immune system resulting in increased pro-inflammatory cytokines at the site of injury and in the spinal cord dorsal horn. Exercise modulates the immune system promoting an anti-inflammatory phenotype of macrophages in uninjured muscle, and increases in anti-inflammatory cytokines can promote healing and analgesia. We proposed that PNI will decrease, and treadmill exercise will increase, release of anti-inflammatory cytokines at the site of injury and in the spinal cord. We show two weeks of treadmill exercise improves neuropathic pain behaviors in mice: mechanical hyperalgesia, escape/avoidance behavior, and spontaneous locomotor activity. PNI reduced anti-inflammatory cytokines (IL-4, IL-1ra, IL-5) at the site of nerve injury and in the spinal dorsal horn while exercise restored IL-4, IL-1ra, IL-5 concentrations to pre-injury levels. IL4−/− mice, and mice treated with IL-4 antibody did not develop analgesia to treadmill exercise. Using immunohistochemical staining of the sciatic nerve, treadmill exercise increased the percentage of M2-macrophages (secretes anti-inflammatory cytokines), and decreased M1-macrophages (secretes pro-inflammatory cytokines) when compared to sedentary mice. The increased M2 and decreased M1 macrophages in exercised mice did not occur in IL-4−/− mice. In the spinal cord, PNI increased glial cell activation, BDNF and β-NGF levels, and decreased IL-4 and IL-1ra levels while treadmill exercise suppressed glial cells activation (GFAP and Iba1 immunoreactivity), reduced BDNF and β-NGF, and increased IL-4, IL-1ra, IL-5. Our results suggest IL-4 mediates the analgesia produced by low-intensity exercise by modulating peripheral and central neuroimmune responses in mice with neuropathic pain.
1. Introduction
Neuropathic pain causes a significant loss of function, disability and reduced life quality, and its management is a challenge to clinicians and includes both pharmacological and non-pharmacological strategies [21,30,47,62,66]. Exercise is one strategy, recommended by clinical practice guidelines, that improves function and reduces pain [20,25,41]; however the underlying mechanisms are unclear.
Following peripheral nerve injury (PNI), neuroimmune interactions at both the site of injury and in the spinal cord play a key role in generation of pain [3,29]. Local immune cells, particularly macrophages, play a critical role inflammation and repair [53]. M1 macrophages (classically activated) release pro-inflammatory cytokines (interleukin (IL)-1β, TNF-α and IL-6), are key components of host defense [6], and promote hyperalgesia [24,29,32]. Consistent with this, at the site of injury, there is an infiltration of macrophages and increased release pro-inflammatory cytokines after PNI [3,54,63]. In contrast, M2 macrophages (“alternatively activated”) secrete anti-inflammatory cytokines (IL-10, IL-4 and IL-1ra), promote tissue repair and produce analgesia [29,32,47,49,53,55].
Exercise before or starting after induction of nerve injury reduces hyperalgesia in animal models of neuropathic pain [1,4,5,10,11,13,14,28,37,38,42,48,67]. In parallel, exercise decreases pro-inflammatory cytokines at the site of nerve injury and in the spinal dorsal horn [5,11], suggesting alterations in immune cell phenotype by exercise. A balance between pro-inflammatory and anti-inflammatory cytokines is a key concept to interpretation of immune function. Indeed, we previously show, in uninjured animals, an increase in proportion of muscle macrophages that express an M2 phenotype with wheel running [46]. On the other hand, Grace and colleagues [28] show there are still significant increases in expression of M1 and M2 markers at the nerve injured site. However, it is unclear if the proportion of M1 and M2 phenotypes are altered at the site of nerve injury, and if exercise has the capability of altering phenotype at a site distant from the exercised muscle.
Centrally, PNI activates glial cells in the spinal dorsal horn and increases pro-inflammatory cytokines [3,50,70,73], similar to that described at the site of nerve injury. Upregulated brain-derived neurotrophic factor (BDNF) in the spinal cord and dorsal root ganglia (DRG), and nerve growth factor (NGF) in the DRG after PNI active microglia to enhance inflammatory cytokine release, sensitize dorsal horn neurons, and produce hyperalgesia [12,13,22,29,36,48,56,73]. Exercise decreases expression of markers for microglia (Iba-1, CD11b) and astrocytes (GFAP) in the dorsal horn after PNI [1,14,48], and decreases pro-inflammatory cytokine release [28]. However, it is unclear if anti-inflammatory cytokines are reduced by nerve injury in the spinal cord, and if increased anti-inflammatory cytokines mediate analgesia produced by exercise in animals with PNI.
We hypothesized that PNI reduces, and treadmill exercise restores, anti-inflammatory cytokines at the site of injury and in the spinal cord dorsal horn. We specifically, examined the role of IL-4 in exercise-induced analgesia and induction of macrophage phenotype in animals with PNI since prior studies show IL-4 reduces release of pro-inflammatory cytokines, promotes an anti-inflammatory phenotype, and produced analgesia when injected at the site of injury or in the spinal cord [18,31,40,46,52,69]. To determine this, the present study investigated through pharmacological, behavioral, immunohistochemical and biochemical tools the effect of low-intensity aerobic exercise on the peripheral and central neuroimmune signaling in a mice model of neuropathic pain induced by PNI.
2. Material and methods
2.1 Animals and surgical procedures
Swiss mice (male, 20–30 g) were used for initial experiments. Mice were kept in a room with controlled temperature (22 ± 1° C) and humidity (50% to 80%) with a 12h light-dark cycle (06:00 am–6:00pm lights on). Food and water were provided ad libitum. All procedures were approved by the Institutional Ethics Committee at the Universidade Federal de Santa Catarina (CEUA/UFSC, #PP00681). Balb/cJ (male, 20–30 g, Jackson Laboratories) and congenic Balb/c-IL4tm2Nnt/J (IL-4 knockout, IL-4−/−)(male, 20-30 g, Jackson Laboratories) mice were used for additional experiments at the University of Iowa (Iowa City, IA, USA, #1110229). At the University of Iowa, mice were housed in the animal care facility with a 12h light-dark cycle (07:00 am–7:00 pm, lights on). Mice were acclimatized to the behavior room for 1h prior to testing. Experiments were performed using current guidelines for the care and protection of animals by the European Union and the National Institutes of Health Guidelines.
Mice were anesthetized with deep anesthesia using a premixed solution of ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), followed by a maintenance dose of isoflurane (1%–2%, in 100% O2). An incision was made in the right thigh to expose the sciatic nerve. A crush injury of the sciatic nerve was done by squeezing the nerve for 30s with 2mmwide forceps, 1 cm above the trifurcation of its 3 major branches, as previously described [8]. The skin incision was closed with a 4-O Ethilon suture. Exercise training began after 3 days of recovery from the nerve injury. A control sham group received the same surgical procedures without sciatic nerve crush.
2.2 Treadmill exercise
The animals performed low-intensity aerobic treadmill running (Exer 3/6, Columbus instruments, Columbus, OH, USA or Athletic Advanced 2; Athletic; Joinvile, Brazil) for 30 minutes (10 m/min, no inclination 5 days/week) as described previously [4]. Animals were familiarized with the treadmill for 1 week before induction of nerve injury. Treadmill training began on the 3rd day after nerve injury. The animals in the sham-operated (Sham/Sedentary or WT Sham/Sedentary) and peripheral nerve injury (PNI/Sedentary, WT PNI/Sedentary, IL-4−/− PNI/Sedentary, PNI/IgG1 Control/Sedentary or PNI/Anti-IL-4/Sedentary) groups were handled and placed on the same treadmill but without motion for the same amount of time as the Exercised groups.
2.3 Behavioral tests
2.3.1 Mechanical sensitivity
Mechanical sensitivity of the hindpaw was evaluated as the withdrawal response frequency to 10 applications of a 0.4 g von Frey filament to the ventral surface of the hindpaw (VHF Stoelting, Chicago, OH, USA). Mice were placed in clear plexiglass boxes (9 × 7 × 11 cm) on an elevated wire mesh platform. The number of paw withdrawals was recorded. Data are expressed as a percentage of the number of withdrawals [4].
2.3.2 Escape/Avoidance test
The escape/avoidance test was performed as previously published in mice [58]. This test was used to assess supraspinal processing of nociception. A plexiglas testing box (16 cm × 7 cm × 13 cm) with two chambers placed on top a wire mesh platform. To eliminate preference to one side, one chamber was white with vertical black lines while the other chamber was solid white. Mice were placed in the box for 30 minutes and allowed to move unrestricted between chambers. Mechanical stimulation was applied with a 0.07 g von Frey filament to the plantar surface of the hindpaw. The ipsilateral operated paw (right) was stimulated with an 0.07g von Frey filament (1× per second) when the animal was on one side while the contralateral paw (left) was stimulated when the animal was on the opposite side. The percent time the animal spent in chamber was calculated.
2.3.3 Open field test
In order to examine overall locomotor activity as a possible indication of spontaneous pain we used an open field test. Mice were observed in a circular open field apparatus (62 cm diameter × 29.5 cm height) for 6 minutes. The floor of this apparatus was divided by three concentric circles subdivided by line segments in nineteen similar sized pieces. The number of locomotion units covered by the animal was recorded and expressed as the number of crossings.
2.4 Enzyme Linked Immunosorbent Assay
Twenty-four hours after the last exercise session, mice were anesthetized with isoflurane (2-3%, with 100% O2) and euthanized by decapitation. Both the injured sciatic nerve and the lumbar spinal cord (L1-L6) were removed. Tissue was homogenized in a Ultra-Turrax Homogenizer (T-18, IKA Works, Wilmington, NC, USA) with a PBS (phosphate-buffered saline) solution containing: Tween 20 (0.05 %), phenylmethylsulphonyl fluoride (PMSF, 0.1 mM), ethylenediaminetetraacetic acid (EDTA, 10 mM), Aprotinin (2 ng/ml) and benzamethonium chloride (0.1 mM). After transferring homogenates to Eppendorfs tubes they were centrifuged at 3000 × g (10 min, 4 °C) and supernatants collected and stored at −80°C until further analyses. The total protein content of the supernatant was measured using the Bradford method. Levels of IL-4, IL-1ra, IL-5 and IL-6 cytokines and BDNF and β-NGF neurotrophins were measured using 100 μL sample with commercially available mouse ELISA kits (R&D Systems, Minneapolis, MN, USA). The level of cytokines and neurotrophic factors were estimated by interpolation from a standard curve. Measurements (pg/mg of protein) were done on an ELISA using ELISA plate reader (Berthold Technologies – Apollo 8 – LB 912, Pforzheim, Germany) at 450 nm with a correction wavelength of 540 nm.
The most common ELISA kits available have been validated for use with serum, plasma or culture supernatants. To assess the reliability of the IL-4 kit for proteins in tissue extracts we performed a validation test. We show linear quantification to IL-4 cytokine (see Figure 1a–d, Supplementary Digital Content 1). Thus, data obtained with the IL-4 ELISA Kit strongly indicates that commercially available ELISA kits can be used to quantify proteins in tissue extracts.
Figure 1.
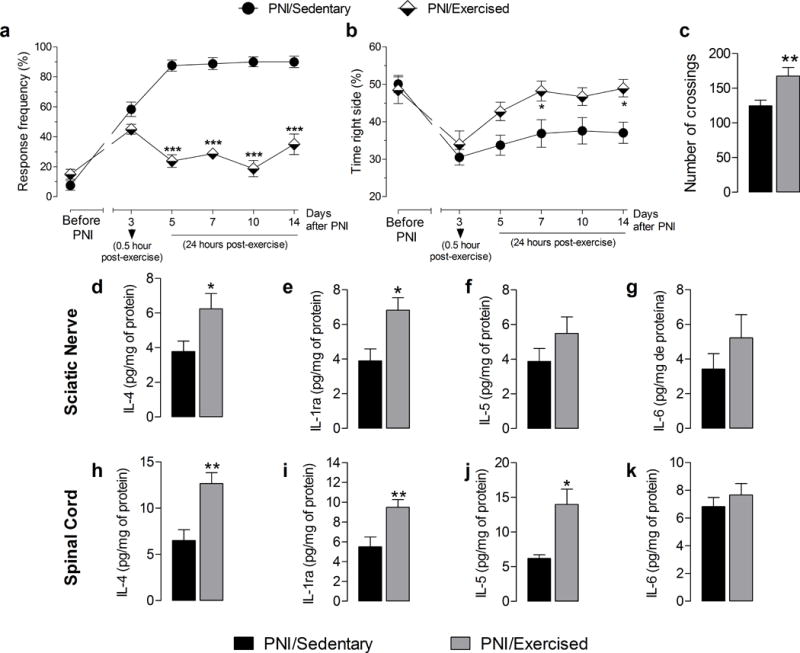
Effect of exercise on mechanical hyperalgesia, escape/avoidance test, spontaneous locomotor activity, and peripheral and central neuroimmunomodulation in Swiss mice sedentary or exercised after peripheral nerve-injury (PNI). (a) Withdrawal response frequency of mouse hind paw to application of a 0.4 g von Frey filament before and after PNI for up to 14 days post-injury. In the PNI/Exercised group evaluations were performed 0.5 h after exercise on 3rd after-PNI day or 24h after exercise on days 5 to 14 after-PNI. (b) Graph shows results of the escape/avoidance test and represents the time spent on the right side of the chamber when the right paw was stimulated, before and after PNI or exercise for up to 14 days post-injury. In the PNI/Exercised group evaluations were performed 0.5 h after exercise on 3rd after-PNI day or 24h after exercise on days 5 to 14 after-PNI. (c) Graph shows results of the open field test and represents the number of locomotion units covered by the animal in six minutes, expressed as the number of crossings on day 14 post-PNI. (d, e, f and g) Graphs show IL-4, IL-1ra, IL-5 and IL-6 cytokine concentrations in the sciatic nerve tissue, respectively. (h, i, j and k) Graphs show IL-4, IL-1ra, IL-5 and IL-6 cytokine concentrations in lumbar (L1 to L6) spinal cord, respectively. Each point represents the mean of five to eight animals and error bars indicate ± SEM. Asterisks denote significance levels of the PNI/Sedentary group when compared with PNI/Exercised group (*P<.05, **P<.01 and ***P<.001).
2.5 Neutralization of IL-4 cytokine-receptor interactions
To determine the effects of IL-4 on analgesia produced by exercise, we injected mice with an IL-4 antibody (anti-IL-4). Mice that had PNI were treated with IL-4 antibody (50 μg/mice in 100 μL intraperitoneal injection; In Vivo Ready™ Anti-Mouse IL-4, Tonbo Biosciences, San Diego, CA, USA) 30 minutes before beginning the exercise program on day 3 and again on days 6, 11 and 14 after PNI. A nerve injured sedentary control group also received IL-4 antibody on the same days: 3, 6, 11 and 14 days after PNI. Control animals received injection of isotype control antibody (50 μg/mice in 100 μL intraperitoneal injection, In Vivo Ready™ Rat IgG1 Isotype Control, clone HRPN, Tonbo Biosciences, San Diego, CA, USA). Behavioral tests examined mechanical sensitivity before PNI and 30 minutes after exercise on day 3 after PNI, and 24h after exercise from the 4th to 14th day after PNI. The experimenter was blinded to group.
2.6 Immunohistochemistry
Immunohistochemistry of macrophage phenotype was examined in the injured sciatic nerve according to procedures previously published by us [46]. In another set of experiments glial cell activity in the spinal cord dorsal horn was examined by immunohistochemistry. All immunohistochemistry evaluations were performed 15 days after PNI. Mice were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with heparinized saline followed by 4% paraformaldehyde (PFA). Both the injured sciatic nerve and lumbar spinal cord (L1-L6) were removed and post-fixed in 4% PFA overnight (4°C), followed by 30% sucrose (4°C) for 24h. Samples were frozen in optimal cutting temperature compound (OCT) in cryomolds and stored at −20°C until processed (Day 3). Frozen tissue were sectioned on a cryostat and placed on slides in serial order. The sciatic nerve was cut at 10-12 μm thickness and the lumbar spinal cord (L1 to L6) was cut at 20 μm. To reduce variability in staining, sections from all mice were stained simultaneously for each marker.
For sections from sciatic nerves, macrophages in the sciatic nerve were co-stained using an M1 marker (hamster anti-mouse CD11c, Abdserotec, 1:100) or an M2 marker (biotinylated rat anti-mouse αCD206, Abdserotec, 1:200) and a general macrophage marker (rat anti-mouse F4/80, Abdserotec, 1:250, 3h). Antibodies were diluted in 1% NGS with 0.1% Triton X-100. Sections were initially blocked with Fc receptor block and 10% normal goat serum (NGS) to reduce non-specific staining. Primary antibodies to M1 or M2 markers were applied overnight at room temperature. The next day, sections were incubated in the primary antibody to F4/80 followed by incubation in secondary antibodies (M1: goat anti-hamster IgG-Rhod-Red-X, 1:500, Jackson Immuno Research; M2, Streptavidin-Alexafluor-568, 1:5000, Vector Labs; F4/80: Fab fragment goat anti-rat Dylight488, 1:500, Jackson Immuno Research).
For immunohistochemistry in the lumbar spinal cord, sections were blocked with 3% NGS and Avidin/Biotin blocking kit. Sections were incubated overnight with primary antibodies overnight at room temperature. For astrocyte staining sections were incubated in the monoclonal antibody mouse anti-Glial Fibrillary Acidic Protein (GFAP) (Millipore, 1:1000). For microglial staining sections were incubated in the primary polyclonal rabbit anti-Iba-1 (Wako, 1:250). On the second day, sections were incubated with Biotinylated Goat anti-Mouse or Goat anti-Rabbit IgG (Invitrogen, 1:1000, 1 hour) according to host primary antibody, followed by Strepavidin-568 (1:1000, 1h). Antibodies were diluted in 1% NGS with 0.1% Triton-X 100 and 1:100 sodium azide.
Slides were cover slipped with Vectashield and imaged using a confocal laser scanning microscope (MRC1024, BioRad, Hercules, CA, USA). Sections were chosen visually using a 20× objective and then captured using both 20× and 60× objective lens. All images for each stain and amplification were taken using the same exposure and settings (iris, gain and offset). Images were stored for off-line analysis and were not manipulated for publication. Twelve images per slide from each animal were obtained from three entire nerve sections and analyzed for macrophage content. For lumbar spinal cord, five images on the ipsilateral and contralateral sides of the dorsal horn were obtained from each animal and analyzed.
We used ImageJ software (NIH) for quantification and the experimenter was blinded to treatment conditions. For sections from sciatic nerve, we manually counted total number of macrophages (F4/80) and the number of F4/80-positive macrophages that were also labeled for either the M1 (CD11c) or the M2 (αCD206) marker as previously published by us [46]. Data are presented as the total number of macrophages, and the percentage of M1-positive and M2-positive macrophages out of the total number of macrophages for each animal.
For sections from lumbar spinal cord, we measured the optical density generated by the labeling with Iba-1 and GFAP antibodies (fluorescence intensity in arbitrary units) separately in laminae I and II, or deep laminae (III-VI) from the ipsilateral (right) and contralateral (left) dorsal horn of the spinal cord. ImageJ software (National Institutes of Health, Bethesda, MD) was used to quantify density of immunoreactivity as previously described by us [33]. Initially each picture was converted to 8-bit gray scale. We then calibrated each section suing the “uncalibrated OD” function. Pixels ranged from 0 to 255. Density values in each area, lamina I/II and deep lamina (III-VI) were calculated as pixels per area. Values are expressed as arbitrary units. The average of density values from 5 images was averaged for each site and animal.
2.7 Experimental protocol
2.7.1 Experiment 1
The first experiment examined the anti-hyperalgesic effects of physical exercise in male Swiss mice subjected to PNI to confirm our previous data [4] and extend our results to additional behavioral tests. The following groups were compared: i) Sham/Sedentary (sham-operated sedentary, n= 8), ii) PNI/Sedentary (injured sedentary mice, n= 8), iii) PNI/Exercised (injured exercised mice, n= 8). Behavioral tests examined response frequency to mechanical stimuli, escape/avoidance behavior, and spontaneous locomotor activity. Response frequency and escape/avoidance tests were performed before PNI, and on days 3, 5, 7, and 14 after PNI, 24 h after the exercise session. Spontaneous locomotor activity using an open field test was performed once 24h after the last exercise session on the 15th day after PNI. Behavioral tests were performed with the experimenter blinded to group.
2.7.2 Experiment 2
Previously, we showed that low-intensity exercise after PNI reduced proinflammatory cytokines, TNF-α and IL-1β, at the nerve injury site and in the spinal cord [4,5]. To examine the effects of PNI and exercise on anti-inflammatory cytokines, we quantified the levels of IL-4, IL-1ra, IL-5, and IL-6 by ELISA in the sciatic nerve and lumbar spinal cord on the 15th day after PNI. Additional analysis examined if there were alterations in the glial cell activators, BDNF and β-NGF, in the lumbar spinal cord using ELISA on the 15th day after PNI. It is important to note, as a methodological limitation of the study, that the dorsal and ventral spinal cord were not dissected and that ELISA analyzes were performed using the whole lumbar portion of the spinal cord (L1-L6). We used the same animals from Experiment 1: i) Sham/Sedentary (n= 8), ii) PNI/Sedentary (n= 8), iii) PNI/Exercised (n= 8).
2.7.3 Experiment 3
Since IL-4 levels in the sciatic nerve and spinal cord were increased by exercise, we examined if genetic deletion of IL-4 would prevent the anti-hyperalgesia produced by physical exercise. Commercially available IL-4−/− mice are congenic on a Balb/cJ strain. Therefore, preliminary experiments confirmed that treadmill exercise produced anti-hyperalgesia in Balb/cJ mice (Wild-type, WT). We then compared the following groups: i) WT Sham/Sedentary (Balb/cJ sham-operated sedentary mice, n= 5); ii) WT PNI/Sedentary (Balb/cJ injured sedentary mice, n= 5); iii) WT PNI/Exercised (Balb/cJ injured exercised mice, n= 5); iv) IL-4−/− PNI/Exercised (IL-4−/− injured exercised mice, n= 6) and v) IL-4−/− PNI/Sedentary (IL-4−/− injured sedentary mice, n= 6). Behavioral tests examining mechanical hyperalgesia were performed before PNI and 24h after physical exercise from 3rd to 14th post-PNI day with the experimenter was blinded to group.
2.7.4 Experiment 4
To confirm the results found in IL-4−/− mice, we used a functional assay by administering an IL-4 antibody in Swiss mice prior to exercise. The anti-hyperalgesic effect of physical exercise was measured in the following groups: i) PNI/IgG1 Control/Sedentary (injured sedentary mice, pretreated with IgG1 control antibody, n= 5); ii) PNI/IgG1 Control/Exercised (injured exercised mice, pretreated with IgG1 control antibody, n= 5); iii) PNI/Anti-IL-4/Exercised (injured exercised mice, pretreated with IL-4 antibody, n= 6) and iv) PNI/Anti-IL-4/Sedentary (injured sedentary mice pretreated with IL-4 antibody, n= 6). Behavioral tests examining response frequency to mechanical stimulation were performed before PNI and 24h after physical exercise from 3rd to 14th post-PNI day with the experimenter blinded to group.
2.7.5 Experiment 5
We previously showed that physical exercise can modulate macrophage phenotype in exercising muscle increasing M2 macrophages [46], which can produce anti-inflammatory cytokines like IL-4 [26,49,72]. We therefore examined the total number and phenotype of macrophages in the sciatic nerve after PNI and two-weeks of exercise in WT and IL-4−/− mice. The sciatic nerve was removed and immunohistochemically stained for macrophages with F4/80, and double labeled these with the M1 marker CD11c, or the M2 marker αCD206 in animals at the end of Experiment 3, in the same groups: i) WT Sham/Sedentary (n= 5); ii) WT PNI/Sedentary (n= 5); iii) WT PNI/Exercised (n= 5); iv) IL-4−/− PNI/Exercised (n= 6) and v) IL-4−/− PNI/Sedentary (n= 6). The experimenter blinded to group.
2.7.6 Experiment 6
Since anti-inflammatory cytokines such as IL-4, IL-1ra and IL-5 were increased by physical exercise in the spinal cord after PNI, and glial cells modulate release of cytokines [29,73], we examined glial activation in the dorsal horn of the spinal cord 15 days after PNI. We immunohistochemically stained the spinal cord for GFAP (astrocyte) and Iba-1 (microglia), and quantified the density of staining. Animals (Swiss mice) from the following groups were compared: i) Sham/Sedentary, ii) PNI/Sedentary, iii) PNI/Exercised, (n= 4–6). The experimenter blinded to group.
2.8 Statistical analyses
Statistical analysis was performed using the GraphPad Prism Software (GraphPad Software, La Jolla, CA, USA). Data are presented as the mean ± SEM for each group. Behavioral tests were analyzed using repeated-measures analysis of variance (ANOVA) (Experiment 1) or two-way repeated-measures ANOVA (Experiments 3 and 4) for difference across time and between groups. A post hoc Bonferroni test examined for differences between groups. Biochemical data was analyzed using independent Student t test (Experiment 2). Assessment of immunohistochemistry results were performed using a two-way ANOVA followed by Bonferroni test (Experiment 5) or independent Student t test (Experiment 6). Normality was confirmed using the Kolmogorov-Smirnov analysis. P < 0.05 were considered statistically significant.
3 Results
3.1 Treadmill exercise improves neuropathic pain behaviors
There were no differences between animals assigned to the PNI/Sedentary and Sham/Sedentary in their baseline (preoperative) responsiveness to the 0.4 g force applied to their paw (see Figure 2a and b, Supplemental Digital Content 1). PNI/sedentary group significantly (P < 0.001) increased withdrawal frequency to mechanical stimulation applied to the hind paw from the 3rd to 14th day after nerve injury compared to the Sham/sedentary group (see Figure 2a, Supplemental Digital Content 1). Besides, PNI/sedentary group also significantly decreased the time spent on the side of the box where the injured paw was stimulated 3 (P < 0.001), 5 (P < 0.001), 7 (P < 0.01), 10 (P < 0.01), and 14 (P < 0.001) days after nerve injury in the escape/avoidance test compared to the Sham/sedentary group (see Figure 2b, Supplemental Digital Content 1). In the open field test, there was a significant reduction (P < 0.01) in locomotor activity 15 days after injury in PNI/sedentary group compared to the Sham/sedentary group (see Figure 2c, Supplemental Digital Content 1).
Figure 2.
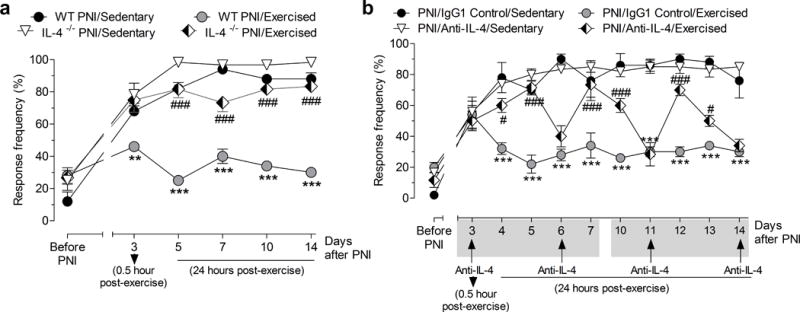
Effect of exercise on mechanical hyperalgesia after peripheral nerve-injury (PNI) is dependent on IL-4. (a) Withdrawal response frequency of the paw in Balb/cJ (Wild-type, WT) and IL-4−/− mice before and after PNI in sedentary or exercised mice. Evaluations were performed 0.5 h after exercise on 3rd after-PNI day or 24h after exercise from days 5 to 14 after-PNI. (b) Withdrawal response frequency of the paw in Swiss mice pretreated with IgG1 control antibody or IL-4 antibody (Anti-IL-4), before and after PNI in sedentary or exercised mice. Evaluations were performed 0.5 h after exercise on 3rd after-PNI day or 24h after exercise from days 5 to 14 after-PNI. Anti-IL-4 or control IgG1 antibodies were given systemically (i.p.) on days 3, 6, 11 and 14 after PNI. Each point represents the mean of five to six animals and error bars indicate ± SEM. Hash marks denote significance levels, when compared with the WT PNI/Exercised or PNI/IgG1 Control/Exercised group (#P<.005 and ###P<.001). Asterisks denote significance levels when compared with WT PNI/Sedentary or PNI/IgG1 Control/Sedentary group (**P<.01 and ***P<.001).
Figure 1a and b show that there were no differences in baseline (preoperative) responsiveness to the force of 0.4 g applied to the paw of the animals assigned to the PNI/sedentary and PNI/exercised group. In addition, treadmill running for 2 weeks after PNI significantly (P < 0.001) reduced the mechanical withdrawal frequency of the paw on days 5 to 14 after PNI of the PNI/exercised group compared to the PNI/sedentary group (Fig. 1a). For the escape/avoidance test, PNI/exercised group significantly (P < 0.05) increased their time spent on the side of the box ipsilateral to the injury on day 7 and 14 after PNI when compared to PNI/sedentary group (Fig. 1b). In addition, PNI/exercised group also showed a significant (P < 0.01) increase in spontaneous locomotor activity in the open field test when compared to the PNI/sedentary group (Fig. 1c).
3.2 Anti-inflammatory cytokines levels decrease after PNI, and increase with exercise
Previously, we demonstrated that sciatic nerve injury increases proinflammatory cytokine concentration (TNF-α and IL-1β) in the injured nerve and spinal cord [5], and anti-inflammatory cytokines negatively modulates the release of pro-inflammatory cytokines, we tested if PNI reduced anti-inflammatory cytokines in the sciatic nerve and spinal cord. Initially, we showed that fifteen days after nerve injury the concentration of anti-inflammatory cytokines IL-4 (P <0.05) and IL-1ra (P <0.001) and IL-5 (P <0.05) were significantly lower in the sciatic nerve and in the spinal cord of the PNI/sedentary group compared to the Sham/sedentary group (see Figure 2d–f and h–j, Supplemental Digital Content 1). However, there were no changes in IL-6 concentration in the sciatic nerve in both PNI/sedentary and Sham/sedentary groups (see Figure 2g and k, Supplemental Digital Content 1).
Figure 1d and e show that treadmill running for 2 weeks after PNI significantly increased IL-4 (P < 0.05) and IL-1ra (P < 0.05) concentrations in the sciatic nerve of the PNI/exercised group compared to the PNI/sedentary group. No changes were observed in IL-5 or IL-6 concentrations in the sciatic nerve between PNI/Sedentary or PNI/Exercised groups (Fig. 1f and g). Furthermore, treadmill exercise increased the anti-inflammatory cytokines IL-4 (P < 0.01), IL-1ra (P <0.01) and IL-5 (P < 0.5) concentrations in the spinal cord of the PNI/exercised group compared to the PNI/sedentary group (Fig. 1h–j). Similar to the sciatic nerve, there were no changes in IL-6 concentration in the spinal cord in both PNI/sedentary and Sham/sedentary groups (Fig. 1k).
3.3 IL-4−/− mice do not show analgesia to treadmill exercise
Because we found that treadmill exercise increased IL-4 concentration in the sciatic nerve and spinal cord, we tested if IL-4 plays a role in the analgesia produced by treadmill exercise using IL-4−/− mice (Fig. 2a and b). Since IL-4−/− were congenic on Balb/cJ strain (Wild-type, WT), we initially confirmed the effects of exercise on frequency of paw withdrawals to von Frey filament. First, we showed that Balb/cJ PNI (WT PNI) mice developed a similar and significant (P<0.001) increase in withdrawal frequency of the paw when compared to Swiss PNI mice (Fig. 1a and Fig. 2a). Secondly, we also show there was no difference in baseline (preoperative) responsiveness to the 0.4 g force applied to their paw between Balb/cJ mice assigned to the WT PNI/Sedentary group and WT Sham/Sedentary group (Figure 3, Supplemental Digital Content 1). However, WT PNI/sedentary group significantly (P <0.001) increased the mechanical withdrawal frequency of the paw on days on days 5 to 14 after PNI compared to the WT Sham/sedentary group (see Figure 3, Supplemental Digital Content 1). Similar to Swiss mice, two weeks of treadmill exercise using the Balb/cJ strain significantly (P < 0.001) reduced the mechanical withdrawal frequency of the paw on days 3 to 14 after PNI of the WT PNI/Exercised group compared to the WT PNI/sedentary group (Fig. 2a).
Figure 3.
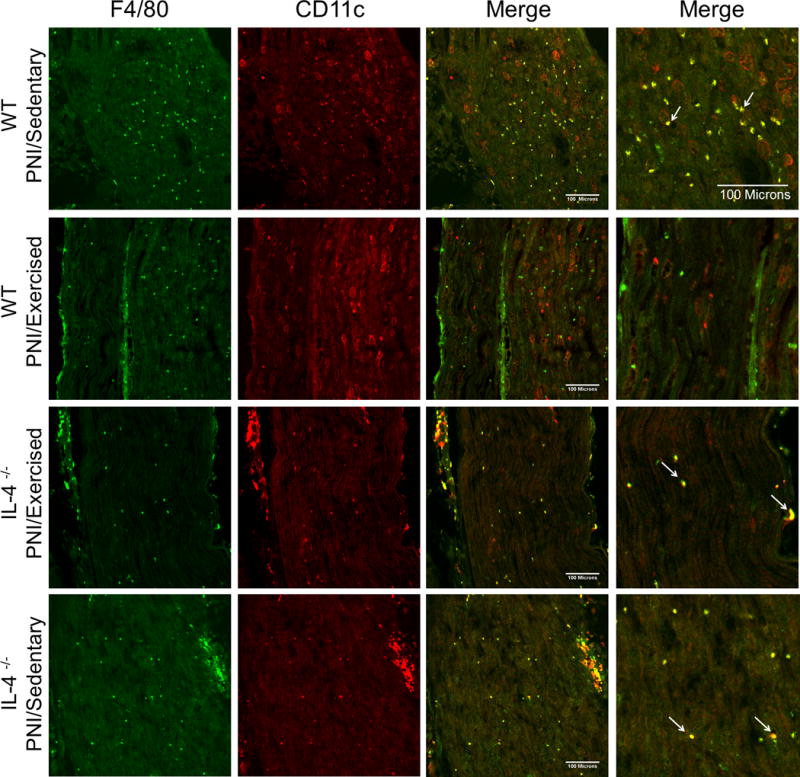
Confocal micrographs of the sciatic nerve in Balb/cJ wild-type mice (WT) (groups: PNI/Sedentary and PNI/Exercised) and IL-4−/− mice (groups: IL-4−/− PNI/Exercised and IL-4−/− PNI/Sedentary), two weeks after peripheral nerve-injury (PNI) and exercise. Sections show immunohistochemical labeling for macrophages with F4/80 (green) and labeling for M1 macrophages with CD11c (red), merged pictures in columns 3 and 4 (higher magnification) show the co-localization of F4/80 and CD11c as yellow (arrows). Scale bar: 100 μm.
IL-4−/− PNI/Exercised mice that performed treadmill exercise showed a significantly (P <0.001) greater withdrawal frequency of the paw when compared to WT PNI/Exercised mice after PNI (Fig. 2a). This increase in response frequency after PNI in IL-4−/− PNI/Exercised mice was similar to that observed in WT sedentary mice (Fig. 2a).
3.4 IL-4 antibody prevents analgesia produced by treadmill exercise
Because IL-4 concentration increased in sciatic nerve and spinal cord by treadmill exercise and treadmill exercise did not produce analgesia in IL-4−/− mice, we assessed if IL-4 antibody treatment could prevent or reverse the analgesia produced by treadmill exercise in Swiss mice (Fig. 2b). First, IL-4 antibody treatment on day 3 after PNI, before starting treadmill exercise in the PNI/Anti-IL-4/Exercised group, significantly (P < 0.05 and P < 0.001, days 4 and 5, respectively) prevented the analgesic effect of treadmill exercise for two consecutive days when compared to PNI/IgG1 Control/Exercised group treated with IgG1 control antibody (Fig. 2b). Similarly, on day 6 and 11, IL-4 antibody, but not IgG1 control antibody, temporarily reversed the analgesia produced by treadmill exercise (P < 0.001, days 7, 10 and 12; P < 0.05, day 13; Fig. 2b).
3.5 Treadmill exercise alters macrophage phenotype in sciatic nerve
Since we show that IL-4 increases at the site of sciatic nerve injury during treadmill exercise, genetic deletion or blockade of IL-4 prevents treadmill exercise-induced analgesia, and prior studies show that IL-4 polarizes macrophages toward an M2 phenotype to promote healing [40], we tested if treadmill exercise changed macrophages phenotype in sciatic nerve after injury using immunohistochemistry (Fig. 3 and 4). Macrophages expressing the M1 and M2 markers, CD11c and αCD206, respectively, were co-labeled with the macrophage marker F4/80 in the sciatic nerve of mice (WT and IL-4−/−) that had undergone 2 weeks of treadmill exercise and compared to sedentary mice after PNI.
Figure 4.
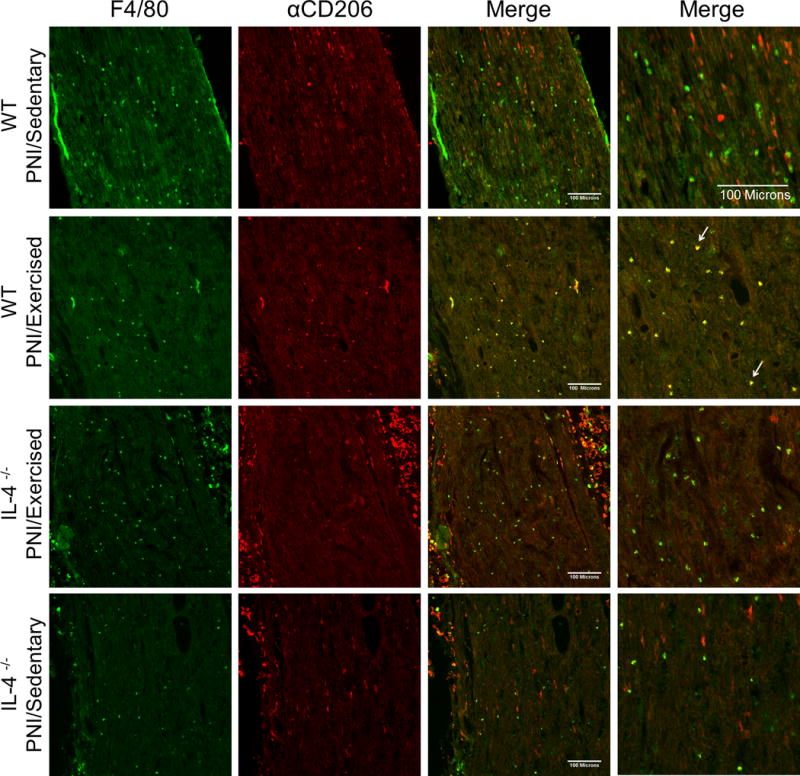
Confocal micrographs of the sciatic nerve in Balb/cJ wild-type mice (WT) (groups: PNI/Sedentary and PNI/Exercised) and IL-4−/− mice (groups: IL-4−/− PNI/Exercised and IL-4−/− PNI/Sedentary), two weeks after peripheral nerve-injury (PNI) and exercise. Sections show immunohistochemical labeling for macrophages with F4/80 (green) and labeling for M2 macrophages with αCD206 (red), merged pictures in columns 3 and 4 (higher magnification) show the co-localization of F4/80 and αCD206 as yellow (arrows). Scale bar: 100 μm.
Figure 3 shows sciatic nerve tissue stained for F4/80 and CD11c, and Figure 4 shows sciatic nerve tissue stained for F4/80 and αCD206. First, we showed that there was a significant (P < 0.001) increase in the number of F4/80+ macrophages in sciatic nerve after PNI of the WT PNI/Sedentary group (62.9 ± 13.1) compared with non-injured WT Sham/sedentary group (11.0 ± 2.8) (see Figure 4a–c, Supplemental Digital Content 1). Secondly, we also showed that 2 weeks after injury there was a significantly greater percentage of M1 (P < 0.001) and lower percentage of M2 (P < 0.001) macrophages in WT PNI/Sedentary group compared with non-injured WT Sham/sedentary group (see Figure 4a, b and d, Supplemental Digital Content 1). Furthermore, the percentage of M1 macrophages in injured nerve from WT PNI/Exercised group was markedly (P < 0.001) reduced by two weeks of treadmill exercise compared with WT PNI/Sedentary group (Fig. 3 and 5b). On the other hand, the percentage of M2 macrophages in injured nerve from WT PNI/Exercised group was significantly (P <0.001) increased after two weeks of treadmill exercise when compared to WT PNI/Sedentary group (Fig. 3 and 5b).
Figure 5.
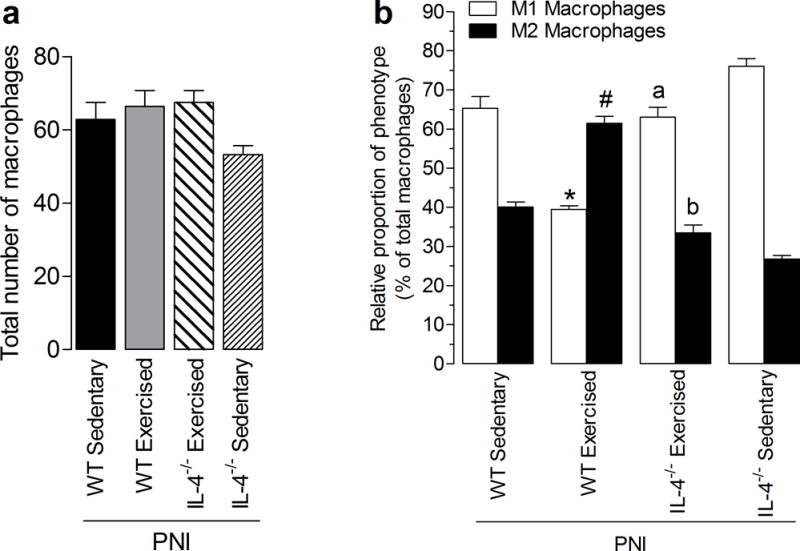
Effect of treadmill exercise on total and differential macrophages on the sciatic nerve in Balb/cJ wild-type mice (WT) (groups: PNI/Sedentary and PNI/Exercised) and IL-4−/− mice (groups: IL-4−/− PNI/Exercised and IL-4−/− PNI/Sedentary), two weeks after peripheral nerve-injury (PNI). (a) Graphs show the quantification of the total number of macrophages per group and (b) percentage of macrophage types, respectively that are labeled as M1 or M2. Each column represents the mean of six mice ± SEM. Asterisk denotes the level of significance compared with WT PNI/Sedentary M1 macrophages group (*P <.001). Hash mark denotes the level of significance compared with WT PNI/Sedentary M2 macrophages group (#P <.001). Letter a denotes the level of significance compared with WT PNI/Exercised M1 macrophages group (aP <.001). Letter b denotes the level of significance compared with WT PNI/Exercised M2 macrophages group (bP <.001).
In contrast, this change in proportion of M1 and M2 phenotypes of the WT PNI/Exercised group did not occur in the IL-4−/− PNI/Exercised group that performed treadmill exercise. There was a similar profile between IL-4−/− PNI/Sedentary group with WT PNI/Sedentary group (Fig. 3, 4 and 5b). Specifically, for M1 macrophages, there was a significant (P < 0.001) increase in the proportion of CD11c-positive cells in IL-4−/− PNI/Exercised group when compared with WT PNI/Exercised group and for M2 macrophages, there was a significant (P < 0.001) decreases in the proportion of αCD206-positive cells in IL-4−/− PNI/Exercised group compared to WT PNI/Sedentary group (Fig. 3, 4 and 5b). Further, there was a significant increase in the number of F4/80+ macrophages in the sciatic nerve after the nerve injury in sedentary animals, regardless of genotype when compared with WT Sham/Sedentary group (Fig. 4c, Supplemental Digital Content 1).
3.6 Treadmill exercise reduces glial activation in the spinal cord after PNI
There is substantial evidence that activation of glial cells in the spinal cord plays a significant role in the transmission of nociceptive information after nerve injury [3,29,50,51], and recent studies show alterations in spinal glia after exercise [1,14,38,48]. We therefore examined effects of treadmill running on glial cell activation in the spinal cord.
We initially confirmed by immunofluorescence that sciatic nerve injury promotes increased immunostaining for GFAP (astrocytes marker) or Iba-1 (microglia marker) in the dorsal horn of the spinal cord on the ipsilateral side to injury in the PNI/Sedentary group compared to the Sham/Sedentary group (See Fig. 5a–d, Supplemental Digital Content 1). Further, the quantification showed a significant increased (P <0.05) in GFAP immunostaining in the superficial (I-II) and deep (III-VI) laminae of the dorsal horn in the PNI/Sedentary group when compared to the Sham/Sedentary group (See Fig. 5c, Supplemental Digital Content 1). Similarly, in PNI/Sedentary group there is a significant increased (P <0.01) in Iba-1 immunostaining bilaterally in the superficial dorsal horn (I-II) and ipsilaterally in the deep dorsal horn (III-VI) when compared to Sham/Sedentary group (see Figure 5d, Supplemental Digital Content 1).
Figure 6a and c show that treadmill running for 2 weeks after PNI significantly (P < 0.05) reduced GFAP immunostaining bilaterally in the superficial (I-II) and deep (III-VI) dorsal horn in PNI/Exercised group compared to PNI/Sedentary animals. In addition, PNI/Exercised group showed lower Iba-1 immunostaining bilaterally in the superficial (I-II) dorsal horn (P < 0.05), and ipsilaterally in the deep (III-VI) (P < 0.01) dorsal horn, compared to PNI/Sedentary group (Fig. 6b and d).
Figure 6.
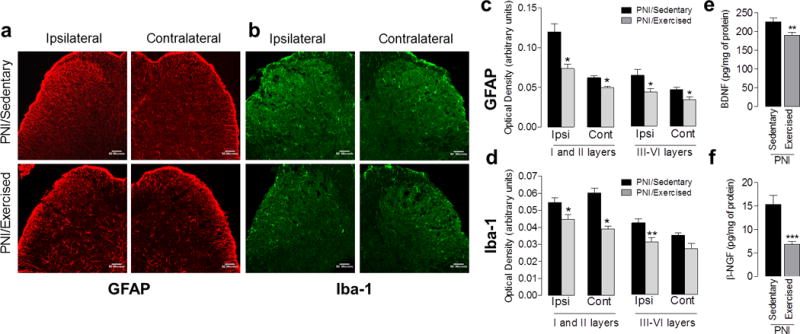
Effects of treadmill exercise on the glial cells activation and modulation of neurotrophins in the spinal cord of Swiss mice, two weeks after peripheral nerve-injury (PNI) in sedentary and exercised animals. (a and b) Confocal micrographs of the spinal cord dorsal horn show immunohistochemical labeling for GFAP (red) and Iba-1 (green), respectively, for the ipsilateral and contralateral sides of the spinal cord dorsal horn. (c and d) Graphs show the quantification of the GFAP and Iba-1 optical density (arbitrary units), respectively, for the ipsilateral and contralateral sides of the spinal cord dorsal horn. Quantification using optical density readings was done for the superficial laminae (I and II) and in the deep laminae (III - VI). (e and f) Graphs show BDNF and β-NGF levels in lumbar spinal cord, respectively. Each column represents the mean of six to eight mice ± SEM. Asterisks denote significance levels of the PNI/Sedentary group when compared with PNI/Exercised group (*P<.05, **P<.01 and ***P <.001). Scale bar: 50 μm.
3.7 PNI increases, and treadmill exercise reduces, BDNF and β-NGF in the spinal cord after PNI
Since neurotrophins are released centrally after nerve injury, activate glial cells in the spinal cord dorsal horn [65], and exercise negatively modulates BDNF [1,28,48], we examined if treadmill running altered neurotrophins concentrations in the spinal cord after sciatic nerve injury. First, we confirmed by the ELISA assay that the sciatic nerve injury promotes a significant increase in the concentration of BDNF (P < 0.001) and β-NGF (P < 0.05) in the spinal cord in PNI/Sedentary group compared to Sham/Sedentary group (See Fig. 5e and f, Supplemental Digital Content 1). Furthermore, treadmill exercise significantly reduced both BDNF (P < 0.01) and β (P < 0.001) concentrations in the spinal cord of the PNI/exercised group compared to the PNI/sedentary group (Fig. 6e and f).
4 Discussion
The current study shows 2 weeks of exercise after PNI produces analgesia through negative modulation of the neuroimmune system, peripherally and centrally, in favor of anti-inflammatory cytokines, particularly IL-4. Specifically, exercise reduced nociceptive behaviors after nerve injury, consistent with prior studies from us and others for effects on mechanical hyperalgesia [1,4,5,11,13,14,28,37,38,42,48,67]. We extended these studies and also show normalization of the injury-induced pain behaviors using escape/avoidance and open field tests, suggesting exercise reduces the affective dimension pain, in addition to the sensory component. Mechanistically, our data show, for the first time that exercise increased concentrations of IL-4, IL-1ra, and IL-5 in the sciatic nerve, and induced a phenotypic switch in macrophages at the site of injury with a greater proportion of M2s and a lower proportion of M1s. Further, exercised IL-4−/− and anti-IL-4 antibody treated mice showed reduced analgesic effects, and IL-4−/− mice do not produce the phenotypic switch in sciatic nerve macrophages. Finally, exercise reduced glial cell activation (astrocyte and microglia) and neurotrophin release, and increased IL-4, IL-1ra, and IL-5 in the spinal cord. Together these data suggest that exercise produces analgesia by increasing anti-inflammatory cytokines at the site of injury and in the spinal cord by altering macrophage phenotype and glial function (See Fig. 7).
Figure 7.
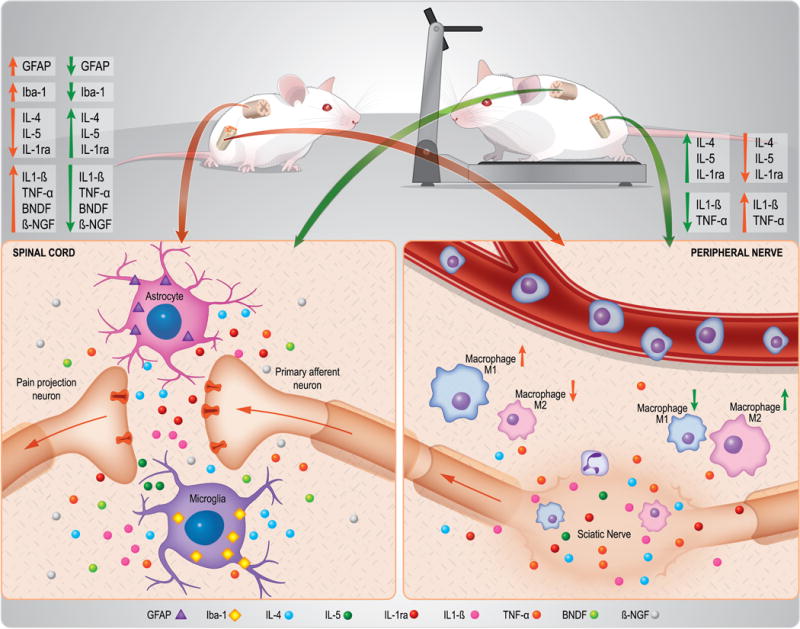
Schematic representation showing that 2 weeks of the low-intensity exercise produces analgesia through modulation of the neuroimmune system, peripherally and centrally, in favor of anti-inflammatory cytokines, particularly IL-4. Mechanistically, our data show, for the first time that treadmill exercise increased concentrations of IL-4, IL-1ra, and IL-5 in the sciatic nerve, and induced a phenotypic switch in macrophages at the site of injury with a greater proportion of M2 and a lower proportion of M1 after peripheral nerve-injury. Further, exercise reduced glial cell activation (astrocyte and microglia) and neurotrophin (BDNF and NGF) release, while increasing IL-4, IL-1ra, and IL-5 in the spinal cord after peripheral nerve-injury. Orange and green lines represent the changes observed in sedentary and exercised mice after sciatic nerve injury, respectively. The up arrows represent increase and down decrease.
4.1 Neuroimmune modulation at the site of nerve injury
Macrophages produce predominately a pro-inflammatory or anti-inflammatory state depending on the balance of M1 and M2 phenotypes [53]. The current study shows that nerve injury results in increased infiltration of macrophages to the site of injury with a greater proportion of these being an M1s phenotype and a lower proportion being M2s. This phenotype distribution (M1>M2) is consistent with increased pro-inflammatory cytokines we previously published (TNF, IL-1β) [5]. Additionally, the current study showed decreased anti-inflammatory cytokines (IL-4, IL-5, IL-1ra) in nerve injured mice (See Fig. 7). Similar to our results, Grace and colleagues [28] show increases in macrophage infiltration to the site of nerve injury; however they show increases in expression of both M1 phenotype (iNOS) and M2 markers (Arginine-1) at the nerve injury site. The differences could be related to methodology, as we counted the percentage of the total macrophage population that expressed either an M1 or M2 marker, and Grace and colleagues [28] examined density of immunoreactivity for each marker.
The current study shows that 2 weeks of treadmill exercise, beginning after PNI, increased the proportion of M2 macrophages and decreased the proportion of M1 macrophages at the site of nerve injury. These results are consistent with previous studies demonstrating that exercise promotes an M2 phenotype in adipose tissue of obese mice, muscle tissue of uninjured mice, and in the dorsal root ganglia of rats with chronic constriction injury [28,35,39,46]. As above, these data are again in contrast to the prior study by Grace et al. [28] who showed no change at the site of nerve injury in M1 or M2 markers with exercise using density readings of immunohistochemistry data. Prior studies show that the phenotypic switch in macrophages occurs with both 1) a single-bout of treadmill running (20 m/min, 90 min) in normal mice as well as with longer duration tasks including wheel running (6-8 weeks), and 2) within the exercising muscle as well as at sites distant to the exercising muscle [35,39,46].
M2 macrophages secrete anti-inflammatory cytokines (IL-10, IL-4) [26,49,72], which is consistent with the increases in IL-4 and IL-1ra observed at the site of nerve injury in the current study. Since anti-inflammatory cytokines inhibit production of pro-inflammatory cytokines (e.g. IL-1β, TNF-α) [9,51,60,70], the decreases in pro-inflammatory cytokines by exercise could be a direct result of the increases in anti-inflammatory cytokines. The current study further showed that genetic deletion or blockade of IL-4 prevents analgesia to treadmill running, which is consistent with prior studies showing that local application of IL-4 dose-dependently inhibits hyperalgesia to intraplantar injections of carrageenan, TNF, IL-1β, IL-8 and PGE2 [15], intraperitoneal injection of acetic acid [71], and intra-articular injection of zymosan [71]. Further, overexpression of IL-4 in primary afferent fibers reversed hyperalgesia in animals with nerve injury [31]. Similar to the current study, we previously show an increase in M2 macrophages in muscle with wheel running, and the anti-inflammatory cytokine IL-10 mediated the analgesia in a model of chronic muscle pain [46]. It is likely that exercise produces its effects by modulating and increasing release of multiple cytokines including IL-4 and IL-10, and thus could be more effective than a therapeutic agent against a single target. In support, a recent report shows a fusion protein with IL-4 and IL-10 had a greater effect on hyperalgesia than either protein alone in animals with inflammation or nerve injury [18]. Our data further show that the switch in phenotype to a greater proportion of M2s does not occur in IL-4−/− mice, suggesting that IL-4 mediates the phenotypic switch. Consistent with our data, prior studies show treatment of macrophages with IL-4 downregulates expression of M1-specific molecules while upregulating expression of M2-specific molecules [39]. The analgesic properties of IL-4 could result from decreasing inflammatory mediators at the site of injury. Alternatively, since IL-4 is involved in healing and repair [52], exercise could reduce damage to the nerve and promote repair. Indeed, our prior study shows less nerve damage in the exercise groups with the same two-week treadmill training [5]. While studies are consistent with macrophages producing IL-4 and providing the analgesic effect, it does not rule out the involvement of other immune cells in exercise-induced analgesia. Thus, we suggest that treadmill exercise modulates hyperalgesia by promoting an M2 macrophage phenotype which increases anti-inflammatory cytokines that reduces pro-inflammatory cytokines promote healing; however, future studies are needed to clarify this hypothesis.
4.2 Neuroimmune modulation in the spinal cord
The current study shows a decrease in the spinal cord anti-inflammatory cytokines concentrations after nerve injury, which together with earlier work showing an increase in pro-inflammatory cytokines [5,28], suggests an altered balance in pro-inflammatory and anti-inflammatory cytokines in the spinal cord after nerve injury. It is well-established that astrocytes and microglia in the dorsal horn are activated by PNI and contribute to increased excitability of dorsal horn neurons through release of pro-inflammatory molecules, such as TNF-α, IL-1β, and BDNF [3,12,17,29,50]. In this context, we demonstrated that treadmill exercise prevented the PNI-induced activation of microglia and astrocytes in the dorsal horn of the spinal cord and is consistent with prior studies [1,14,48]. In parallel to the immune changes at the site of nerve injury, the current study showed treadmill running normalized of the PNI-reduction in anti-inflammatory cytokines and the PNI-increase in neurotrophins in the spinal cord. BDNF can directly activate central glial cells, and promote increases in inflammatory cytokines in the spinal cord [65]. Our result for BDNF is consistent with prior studies on exercise-induced analgesia [1,13,28,48]. Prior studies have shown alterations in NGF with PNI and exercise in the DRG [1,13,48]; however, we show these changes in NGF extend to the spinal cord. Interestingly, mRNA and IL-4 protein have been found in astrocytes and microglia [34,57] and, although little is known about the role of IL-5 in dorsal horn, it has been identified as a cytokine secreted in vitro by astrocytes and microglia to result in a mitogenic effect on microglia [59,61].
Anti-inflammatory cytokines, such as IL-4 and IL-10, activate glial cells to promote neuronal survival and reverse hyperalgesia in animals with nerve injury [27,50]. Intrathecal injection of the IL-4 at the time of surgery prevented nerve injury-induced inflammation and hyperalgesia [69], and intrathecal injection of an IL-4-IL-10 fusion proteins reversed nerve-injury induced hyperalgesia and glial cell activation [18]. IL-4 downregulates and reduces release of inflammatory mediators in the spinal cord including IL-1β, TNF-α and PGE2, MCP-1, and iNOS [23,31,45,69]. Interestingly, mRNA and IL-4 protein have been found in astrocytes and microglia [34,57] and, although little is known about the role of IL-5 in dorsal horn, it has been identified as a cytokine secreted in vitro by astrocytes and microglia to result in a mitogenic effect on microglia [59,61]. Thus, we propose that exercise promotes a decrease in glial activation and inflammatory cytokines by decreasing release of neurotrophins spinally and simultaneously increasing anti-inflammatory cytokines, creating an endogenous anti-inflammatory and neuroprotective environment in the central nervous system to reduce nociceptive behaviors (See Fig. 7).
4.3 Conclusions
Greater levels of physical activity and exercise are associated with a lower risk of developing chronic pain [16,43,44,64]. On the other hand, people with chronic pain have reduced levels of physical activity [7,19]. While exercise is part of the guidelines for treatment of individuals with neuropathic pain, there is little clinical evidence to support its effectiveness [2,68]. Understanding the mechanisms of exercise could lead to new therapeutic targets aimed not only at improving pain, but also improving healing and modifying the underlying pathology. Further, these studies will provide a rationale for both clinicians and patients to prescribe and adhere to an exercise program. The current study, in combination with prior studies suggests that low-intensity exercise positively modulates the state of the immune system at multiple sites to favor anti-inflammatory cytokines over pro-inflammatory cytokines increases to prevent development of neuropathic pain.
Supplementary Material
Supplemental Digital Content 1. Figures demonstrate behavioral, biochemical and immunohistochemistry changes after peripheral nerve injury (PNI) in sedentary mice when compared to sham-injured sedentary mice.
Acknowledgments
The authors acknowledge the following sources of funding: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Programa Ciência sem Fronteiras, Programa INCT-INOVAMED (465430/2014-7), and National Institutes of Health (NIH) RO1 AR061371 (KAS). The authors thank Jing Danielson, Sandra J. Kolker and Lynn Rasmussen for excellent technical assistance, mainly with immunohistochemistry. Also, the authors would like to thank Juliete Palandi, Jessica Probst and Róli Simões for their assistance in behavioral experiments.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare. Authors (K.A.S. and A.R.S.S.) contributed equally to this work.
References
- 1.Almeida C, DeMaman A, Kusuda R, Cadetti F, Ravanelli MI, Queiroz AL, Sousa TA, Zanon S, Silveira LR, Lucas G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain. 2015;156(3):504–513. doi: 10.1097/01.j.pain.0000460339.23976.12. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose KR, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best Pract Res Clin Rheumatol. 2015;29(1):120–130. doi: 10.1016/j.berh.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229(1–2):26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Bobinski F, Ferreira TA, Cordova MM, Dombrowski PA, da Cunha C, Santo CC, Poli A, Pires RG, Martins-Silva C, Sluka KA, Santos AR. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain. 2015;156(12):2595–2606. doi: 10.1097/j.pain.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobinski F, Martins DF, Bratti T, Mazzardo-Martins L, Winkelmann-Duarte EC, Guglielmo LG, Santos AR. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neuroscience. 2011;194:337–348. doi: 10.1016/j.neuroscience.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12(1):64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 7.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA, Hertl C. Nerve crush injuries–a model for axonotmesis. Exp Neurol. 1994;127(2):284–290. doi: 10.1006/exnr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain CS, Leiferman EM, Frisch KE, Brickson SL, Murphy WL, Baer GS, Vanderby R. Interleukin expression after injury and the effects of interleukin-1 receptor antagonist. PloS one. 2013;8(8):e71631. doi: 10.1371/journal.pone.0071631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YW, Chiu CC, Hsieh PL, Hung CH, Wang JJ. Treadmill training combined with insulin suppresses diabetic nerve pain and cytokines in rat sciatic nerve. Anesth Analg. 2015;121(1):239–246. doi: 10.1213/ANE.0000000000000799. [DOI] [PubMed] [Google Scholar]
- 11.Chen YW, Li YT, Chen YC, Li ZY, Hung CH. Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve. Anesth Analg. 2012;114(6):1330–1337. doi: 10.1213/ANE.0b013e31824c4ed4. [DOI] [PubMed] [Google Scholar]
- 12.Cheng CF, Cheng JK, Chen CY, Lien CC, Chu D, Wang SY, Tsaur ML. Mirror-image pain is mediated by nerve growth factor produced from tumor necrosis factor alpha-activated satellite glia after peripheral nerve injury. Pain. 2014;155(5):906–920. doi: 10.1016/j.pain.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Cobianchi S, Casals-Diaz L, Jaramillo J, Navarro X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol. 2013;240:157–167. doi: 10.1016/j.expneurol.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Cobianchi S, Marinelli S, Florenzano F, Pavone F, Luvisetto S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience. 2010;168(1):273–287. doi: 10.1016/j.neuroscience.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Cunha FQ, Poole S, Lorenzetti BB, Veiga FH, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-4. Br J Pharmacol. 1999;126(1):45–50. doi: 10.1038/sj.bjp.0702266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Front Cell Neurosci. 2014;8:102. doi: 10.3389/fncel.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008;135(1–2):37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Eijkelkamp N, Steen-Louws C, Hartgring SA, Willemen HL, Prado J, Lafeber FP, Heijnen CJ, Hack CE, van Roon JA, Kavelaars A. IL4-10 Fusion Protein Is a Novel Drug to Treat Persistent Inflammatory Pain. J neurosci. 2016;36(28):7353–7363. doi: 10.1523/JNEUROSCI.0092-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellingson LD, Shields MR, Stegner AJ, Cook DB. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain. 2012;13(2):195–206. doi: 10.1016/j.jpain.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellingson LD, Stegner AJ, Schwabacher IJ, Koltyn KF, Cook DB. Exercise Strengthens Central Nervous System Modulation of Pain in Fibromyalgia. Brain Sci. 2016;6(1) doi: 10.3390/brainsci6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J neurosci. 2001;21(13):4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furlan R, Bergami A, Lang R, Brambilla E, Franciotta D, Martinelli V, Comi G, Panina P, Martino G. Interferon-beta treatment in multiple sclerosis patients decreases the number of circulating T cells producing interferon-gamma and interleukin-4. J Neuroimmunol. 2000;111(1–2):86–92. doi: 10.1016/s0165-5728(00)00377-5. [DOI] [PubMed] [Google Scholar]
- 24.Gong WY, Abdelhamid RE, Carvalho CS, Sluka KA. Resident Macrophages in Muscle Contribute to Development of Hyperalgesia in a Mouse Model of Noninflammatory Muscle Pain. J Pain. 2016;17(10):1081–1094. doi: 10.1016/j.jpain.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon R, Bloxham S. A Systematic Review of the Effects of Exercise and Physical Activity on Non-Specific Chronic Low Back Pain. Healthcare (Basel) 2016;4(2) doi: 10.3390/healthcare4020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Goss JR, Goins WF, Glorioso JC. Gene therapy applications for the treatment of neuropathic pain. Expert Rev Neurother. 2007;7(5):487–506. doi: 10.1586/14737175.7.5.487. [DOI] [PubMed] [Google Scholar]
- 28.Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, Galer EL, Walker FR, Greenwood BN, Maier SF, Fleshner M, Watkins LR. Prior voluntary wheel running attenuates neuropathic pain. Pain. 2016;157(9):2012–2023. doi: 10.1097/j.pain.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haanpaa M. The assessment of neuropathic pain patients. Pain Manag. 2013;3(1):59–65. doi: 10.2217/pmt.12.71. [DOI] [PubMed] [Google Scholar]
- 31.Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa-Moriyama M, Kurimoto T, Nakama M, Godai K, Kojima M, Kuwaki T, Kanmura Y. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuates inflammatory pain through the induction of heme oxygenase-1 in macrophages. Pain. 2013;154(8):1402–1412. doi: 10.1016/j.pain.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 33.Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci. 2003;23(13):5437–5445. doi: 10.1523/JNEUROSCI.23-13-05437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulshof S, Montagne L, De Groot CJ, Van Der Valk P. Cellular localization and expression patterns of interleukin-10, interleukin-4, and their receptors in multiple sclerosis lesions. Glia. 2002;38(1):24–35. doi: 10.1002/glia.10050. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda S, Tamura Y, Kakehi S, Takeno K, Kawaguchi M, Watanabe T, Sato F, Ogihara T, Kanazawa A, Fujitani Y, Kawamori R, Watada H. Exercise-induced enhancement of insulin sensitivity is associated with accumulation of M2-polarized macrophages in mouse skeletal muscle. Biochem Biophys Res Commun. 2013;441(1):36–41. doi: 10.1016/j.bbrc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kami K, Taguchi Ms S, Tajima F, Senba E. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol Pain. 2016;12 doi: 10.1177/1744806916629059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kami K, Taguchi S, Tajima F, Senba E. Histone Acetylation in Microglia Contributes to Exercise-Induced Hypoalgesia in Neuropathic Pain Model Mice. J Pain. 2016;17(5):588–599. doi: 10.1016/j.jpain.2016.01.471. [DOI] [PubMed] [Google Scholar]
- 39.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 40.Kiguchi N, Kobayashi Y, Saika F, Sakaguchi H, Maeda T, Kishioka S. Peripheral interleukin-4 ameliorates inflammatory macrophage-dependent neuropathic pain. Pain. 2015;156(4):684–693. doi: 10.1097/j.pain.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 41.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, Sharma NK, Wright DE. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26(5):424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8(12):989–997. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152(10):2241–2247. doi: 10.1016/j.pain.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 44.Landmark T, Romundstad P, Dale O, Borchgrevink PC, Kaasa S. Estimating the prevalence of chronic pain: validation of recall against longitudinal reporting (the HUNT pain study) Pain. 2012;153(7):1368–1373. doi: 10.1016/j.pain.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Ledeboer A, Breve JJ, Poole S, Tilders FJ, Van Dam AM. Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. 2000;30(2):134–142. doi: 10.1002/(sici)1098-1136(200004)30:2<134::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Leung A, Gregory NS, Allen LA, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain. 2016;157(1):70–79. doi: 10.1097/j.pain.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137(3):473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 48.López-Álvarez VM, Modol L, Navarro X, Cobianchi S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain. 2015;156(9):1812–1825. doi: 10.1097/j.pain.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51(2):240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33(34):8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, Kiefer R. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest. 2003;83(2):175–185. doi: 10.1097/01.lab.0000056993.28149.bf. [DOI] [PubMed] [Google Scholar]
- 55.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguchi K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101(1–2):65–77. doi: 10.1016/s0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- 57.Park KW, Lee DY, Joe EH, Kim SU, Jin BK. Neuroprotective role of microglia expressing interleukin-4. J Neurosci Res. 2005;81(3):397–402. doi: 10.1002/jnr.20483. [DOI] [PubMed] [Google Scholar]
- 58.Pratt D, Fuchs PN, Sluka KA. Assessment of avoidance behaviors in mouse models of muscle pain. Neurosci. 2013;248:54–60. doi: 10.1016/j.neuroscience.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ringheim GE. Mitogenic effects of interleukin-5 on microglia. Neurosci Lett. 1995;201(2):131–134. doi: 10.1016/0304-3940(95)12153-6. [DOI] [PubMed] [Google Scholar]
- 60.Rocken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17(5):225–231. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- 61.Sawada M, Suzumura A, Itoh Y, Marunouchi T. Production of interleukin-5 by mouse astrocytes and microglia in culture. Neurosci Lett. 1993;155(2):175–178. doi: 10.1016/0304-3940(93)90701-l. [DOI] [PubMed] [Google Scholar]
- 62.Schaefer C, Sadosky A, Mann R, Daniel S, Parsons B, Tuchman M, Anschel A, Stacey BR, Nalamachu S, Nieshoff E. Pain severity and the economic burden of neuropathic pain in the United States: BEAT Neuropathic Pain Observational Study. Clinicoecon Outcomes Res. 2014;6:483–496. doi: 10.2147/CEOR.S63323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen ZL, Lassner F, Bader A, Becker M, Walter GF, Berger A. Cellular activity of resident macrophages during Wallerian degeneration. Microsurgery. 2000;20(5):255–261. doi: 10.1002/1098-2752(2000)20:5<255::aid-micr6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 64.Simons LE, Kaczynski KJ, Conroy C, Logan DE. Fear of pain in the context of intensive pain rehabilitation among children and adolescents with neuropathic pain: associations with treatment response. J Pain. 2012;13(12):1151–1161. doi: 10.1016/j.jpain.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siniscalco D, Giordano C, Rossi F, Maione S, de Novellis V. Role of neurotrophins in neuropathic pain. Curr Neuropharmacol. 2011;9(4):523–529. doi: 10.2174/157015911798376208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siniscalco D, Rossi F, Maione S. Molecular approaches for neuropathic pain treatment. Curr Med Chem. 2007;14(16):1783–1787. doi: 10.2174/092986707781058913. [DOI] [PubMed] [Google Scholar]
- 67.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T., Jr Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114(4):940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sullivan AB, Scheman J, Venesy D, Davin S. The role of exercise and types of exercise in the rehabilitation of chronic pain: specific or nonspecific benefits. Curr Pain Headache Rep. 2012;16(2):153–161. doi: 10.1007/s11916-012-0245-3. [DOI] [PubMed] [Google Scholar]
- 69.Sun S, Chen D, Lin F, Chen M, Yu H, Hou L, Li C. Role of interleukin-4, the chemokine CCL3 and its receptor CCR5 in neuropathic pain. Mol Immunol. 2016;77:184–192. doi: 10.1016/j.molimm.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105(3):838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- 71.Vale ML, Marques JB, Moreira CA, Rocha FA, Ferreira SH, Poole S, Cunha FQ, Ribeiro RA. Antinociceptive effects of interleukin-4, -10, and -13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther. 2003;304(1):102–108. doi: 10.1124/jpet.102.038703. [DOI] [PubMed] [Google Scholar]
- 72.Vogel DY, Heijnen PD, Breur M, de Vries HE, Tool AT, Amor S, Dijkstra CD. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J Neuroinflamm. 2014;11:23. doi: 10.1186/1742-2094-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82(4):981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Figures demonstrate behavioral, biochemical and immunohistochemistry changes after peripheral nerve injury (PNI) in sedentary mice when compared to sham-injured sedentary mice.


