Abstract
Health disparities, which can be understood as disadvantages in health associated with one’s social, racial, economic, or physical environment, originate in childhood and persist across an individual’s life course. One’s neighborhood may drive or influence these disparities. Information on neighborhoods that can characterize their risks—what we call place-based risks—is rarely used in patient care. Community-level data, however, could inform and personalize interventions such as arranging for mold removal from the home of a person with asthma from the moment that person’s address is recorded at the site of care. Efficient risk identification could lead to the tailoring of recommendations and targeting of resources, to improve care experiences and clinical outcomes while reducing disparities and costs. In this article we highlight how data on place-based social determinants of health from national and local sources could be incorporated more directly into patient-centered care, adding precision to risk assessment and mitigation. We also discuss how this information could stimulate cross-sector interventions that promote health equity: the attainment of the highest level of health for neighborhoods, patient panels, and individuals. Finally, we draw attention to research questions that focus on the role of geographical place at the bedside.
Imagine that a child is hospitalized for an asthma exacerbation. The child’s care is managed using evidence-based clinical protocols. Soon the child meets discharge criteria, seemingly ready to transition from hospital to home and neighborhood. This transition may prompt an uneventful return to normalcy. Alternatively, it may represent a return to a place with risks that triggered the exacerbation.1 A deeper understanding of this “place” could be a key addition to clinical care, identifying drivers of disparities that the US health care system seeks to eliminate.
Healthy People 2020, which helps guide the federal government’s prevention efforts, defines disparities as health differences “closely linked with social, economic, and/or environmental disadvantage.”2 The concentration of disadvantage greatly affects and drives inequities for health outcomes across the life course. Asthma is but one example; similar patterns have been noted for low birthweight, lead toxicity, and lower respiratory tract infections among children; and hypertension, diabetes, depression, and life expectancy among adults.3–8
These patterns are influenced by social determinants of health. The World Health Organization defines these as “the conditions in which people are born, grow, work, live, and age, and the wider set of forces and systems shaping the conditions of daily life.”9 The American Academy of Pediatrics and Academic Pediatrics Association now both suggest that health care professionals take these determinants into consistent account in their care of patients.10–12
A starting point in turning this suggestion into reality is the recognition that certain people disproportionately experience morbidity. These same people are found in higher concentration in places disproportionately affected by risks related to social determinants of health. Nonetheless, medical care generally focuses more on acute causes of disease and less on factors affecting people’s long-term well-being; patients may be ill repeatedly with the source of morbidity unresolved.13 Data that illuminate the role place plays in unrelenting, inequitable morbidity are available yet rarely woven into clinical care. This is a critical missed opportunity to contextualize and personalize patient care.
In this article we outline ways in which geographic data (contextual/ecological or aggregated individual data, or both) could be brought directly into patient care, using the asthma hospitalization as an illustrative example. We demonstrate how granular data on place-based social determinants of health from national and local sources could facilitate responses to illness that has its origin in one’s geography just as much as one’s biology. We also identify how this information could stimulate cross-sector collaborations to promote health for both populations and patients. Finally, we frame research questions focusing on the role of place at the bedside and in equitable health improvement.
Framework Linking Public Health And Clinical Medicine
The current health care climate increasingly emphasizes and incentivizes equity and prevention, “value over volume.”14–18 The National Academy of Medicine recently articulated a vision for “healthy communities,” using fifteen core social determinants of health (such as education, housing, and air and water quality).19 This vision aligns with the Accountable Health Communities program under way through the Centers for Medicare and Medicaid Services, which seeks sustainable funding models that promote “data systems that bridge health and community services.”20
We see data systems that use place-based measures as key to population health management (online Appendix Exhibit 1).21 We categorize measures into relevant health services, physical, economic, and psychosocial environments. The health services environment includes, but is not limited to, accessibility to primary care, emergency departments or hospitals, and pharmacies. The physical environment involves quality of housing, access to green space, and exposure to pollutants. The economic environment relates to measures of socioeconomic status, including income, education, and employment. Finally, the psychosocial environment highlights community supports, access to mental health resources, and exposure to crime.22–25
To date, such data have been used primarily in research and public health. The Public Health Disparities Geocoding Project has shown clear associations between place-based measures and multiple health outcomes.26 Marie Lynn Miranda and colleagues linked geographic data with health system data to gain a “multidimensional understanding of individual and community health status and vulnerabilities,” guiding public health programs and community partnerships.27
Translating data into action at the bedside remains a critical challenge and opportunity. If used effectively, these data could simulate a visit to a patient’s home community, informing care in real time.28–30 They could “take the pulse” of communities of interest, identifying the acuity (and distribution) of risk to warn providers of need for action. Just as abnormal laboratory tests or vital signs warn of a patient’s potential for clinical deterioration, so too may place-based “community vital signs” warn of social determinants of health–related concerns.31,32 Place-based insights could add nuance to how risk is assessed and treatments are pursued. It also could support the identification of community partners, and development of partnerships, for populations and individual patients.28,33–36
Bringing Geomarkers And Community Vital Signs Into Clinical Care
Biomedical research is increasingly creating opportunities for personalized medical care.37 Biomarkers, a key area of focus, “can be measured in the body or its products and influence or predict the incidence of outcome or disease.”38 We define geomarkers similarly, as “any objective, contextual, or geographic measure” that influences or predicts the incidence of outcome or disease.39 By complementing biology with geography, we are able to tap into health-relevant data that generally exist in isolation from clinical care. Exhibit 1 provides examples of readily available, potentially useful geomarkers.
EXHIBIT 1.
Community-level geomarkers specific to the health service, physical, economic, and psychosocial environments and potential interventions that could be relevant at the population or patient level
| Health service environment | Physical environment | Economic environment | Psychosocial environment |
|---|---|---|---|
|
GEOMARKERS
| |||
| Distance to pharmacy Live within “pharmacy desert” Pharmacy quality metric Distance to primary care Live within undeserved area Vehicle availability Public transport availability |
Housing code violations Vacancy rate Renter rate Home value Crowding/population density Exposure to pollution |
Poverty rate Household income Home ownership Car ownership Educational attainment |
Crime rate Mental health access |
|
| |||
| INTERVENTIONS | |||
|
| |||
| Medication delivery Care coordination Community health worker Home nurse visitation Medicaid rides Telemedicine |
Housing inspection Legal advocacy Air conditioning or filtration Development of affordable housing |
Financial services Medicaid rides Legal advocacy Public benefit procurement Community health worker Community agency referrals |
Community health worker Community agency referrals Resilience training Community partnerships |
SOURCE Authors’ contributions.
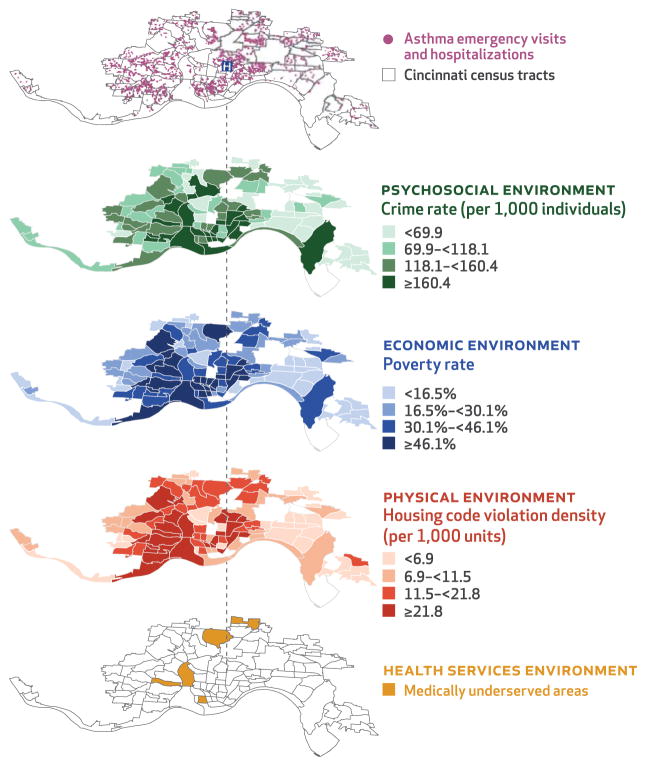
Exhibit 2 displays how place-based, social determinants of health–relevant geomarkers could be put into practice. The first of the five map layers depicts all asthma-related emergency visits and hospitalizations in Cincinnati, Ohio, over a three-year period, superimposed on the city’s census tracts or neighborhoods. The remaining four layers illustrate specific aspects of the psychosocial, economic, physical, and health service environments that affect the health of neighborhood residents. Each of these layers represents a geomarker specific to these environments. These geomarkers, including measures of crime, poverty, suboptimal housing, and limited health care access, could inform additional assessments and interventions for populations or patients.
EXHIBIT 2. Examples of how census tract–level information can be linked to health outcomes in a staged manner by displaying geomarkers.
SOURCE Authors’ analysis of data from the Cincinnati Children’s Hospital Medical Center (asthma emergency visits and hospitalizations); Health Resources and Services Administration, US Department of Health and Human Services (medically underserved areas); Cincinnati Area Geographic Information System (housing code violation density); US Census Bureau (census tracts, poverty rate); and Cincinnati Police Department (crime rate). NOTES This exhibit shows census tract–level information specific to the health service, physical, economic, and psychosocial environments in Cincinnati, Ohio. Geomarkers such as those shown could inform community health assessments and be amenable to population- or patient-level interventions.
Complementary “bottom up” (public health) and “top down” (clinical) strategies could support the tailoring of services to communities and patients. A “bottom up,” community-focused approach is key to understanding inequities across the region. Public health interventions could then be targeted community by community, perhaps starting with those that have the highest utilization rates. After identifying target communities, one may move upward through the layers, gaining a deeper appreciation of that community’s health service, physical, economic, and psychosocial environments.
A “top down,” patient-focused approach starts with an individual. Recall the patient hospitalized for asthma. Drawing a line from this patient to his or her home neighborhood helps identify place-based risks that may have contributed to their exacerbation (and their risk for future exacerbations). Perhaps knowledge of place-based risks would have prompted more in-depth risk assessment at the time of treatment. For example, knowing that a child lives in a neighborhood characterized by high rates of crime, poverty, suboptimal housing, and limited health care access may prompt directed screening focusing on competing needs, stressors, and exposures. The clinical team could then deploy a community health worker to support parental mental health and a legal aid advocate to assist with public benefit enrollment. They could refer to a home inspector and connect to pharmacies providing medication delivery programs.
Geographic data elements could improve patient history taking by prompting deeper, more targeted assessments and by directly facilitating implementation of care plans. This clinical use of geomarkers is especially relevant because clinicians often report a lack of time to adequately screen for social and environmental risks.40 Having the right information conveyed by geo-markers could help overcome these barriers, giving clinicians a critical head start.
Evidence Supporting Place-Based Assessments
Medication adherence is related to asthma hospitalization but can be very difficult to assess. We developed a geomarker of asthma-related medication adherence based on data from a pharmacy chain and tested it against asthma utilization. Specifically, we calculated a Pharmacy-level Asthma Medication Ratio (Ph-AMR), which examined the balance of rescue versus preventive medication use. Our ratio runs parallel to the patient-level Asthma Medication Ratio, a nationally recognized quality metric,41 but is based on the census tracts in which pharmacies were located. For each of twenty-seven Greater Cincinnati pharmacies, all preventive medication fills (for example, inhaled corticosteroid) were divided by all preventive plus rescue medication fills (for example, inhaled beta agonist) to yield a ratio ranging from 0 to 1. A higher Ph-AMR reflected more prevention and less rescue. Adjusted analyses illustrated that census-tract Ph-AMR was inversely related to population-level asthma utilization rates (emergency visits plus hospitalizations). For every 0.1 increase in Ph-AMR, the census-tract asthma utilization rate decreased by approximately 10 events per 1,000 children.
Despite these findings, patients sent home to census tracts that have half the preventive medication adherence are discharged in the same manner as those discharged to tracts with much higher average adherence. While additional analyses are needed to determine the relationship between the Ph-AMR and medication adherence and morbidity among individual patients, we expect that targeted medication adherence interventions (for example, home delivery or self-management programs) could be developed for those in high-risk areas. Hospital-pharmacy partnerships could also be expanded to direct attention toward both high-risk populations and individual patients.42
Dima Qato and colleagues described an analogous example of “pharmacy deserts.” They found that racially and socioeconomically segregated neighborhoods in Chicago, Illinois, had more limited pharmacy access, promulgating lower rates of medication adherence in these same neighborhoods. They recommended policy changes to provide incentives for a more equitable distribution of pharmacies.43 We suggest that the pharmacy desert classification they presented could itself be a geomarker. If a patient lives in such a desert, changes in the way non-adherence risk is assessed and medications are dispensed may be prudent.
Another example draws on established associations between housing and asthma. To approximate exposure to asthma-related housing risks, we calculated a housing code violation density metric from Greater Cincinnati Building, Health, and Property Maintenance data. This metric was significantly associated with population-level asthma utilization rates. After adjustment for patients’ age, sex, race, insurance, and census-tract poverty, hospitalized children from tracts with a high density of housing code violations were found to have more than 80 percent increased odds of reutilization within twelve months of an index hospitalization compared to those living in census tracts with a low density of violations.39 Knowing a child is from a high-density area could prompt referrals for home inspections or legal advocacy.44,45 Boston’s Breathe Easy at Home program does just this, providing a web-based linkage between pediatric care providers and housing inspectors. Not surprisingly, two Boston neighborhoods have both the highest asthma hospitalization rates and referral rates for housing inspection.46 A clinician armed with the knowledge that a child lives in one of these neighborhoods (or a similar neighborhood) may more reliably discuss these types of programs in the exam room.
Like measures of medication adherence and housing quality, similar associations have been noted between asthma morbidity and measures of crime, socioeconomic status, and traffic.23,25,47 Such geomarkers have value individually; they may also be informative when packaged into summary scores or indices. For example, we previously linked children hospitalized for asthma exacerbations to a “geo-risk” index constructed using census-tract measures of home values, poverty, and adult educational attainment (that is, geomarkers of the physical and economic environments). Children at highest geo-risk were 80 percent more likely to have a reutilization event than those at lowest risk. They were also more likely to live in households reporting potentially modifiable financial hardships and psychological distress.23 Other indices, such as the Child Opportunity Index, hold similar promise.48
Implications Of A Place-Based Approach
Just as clinicians change treatment plans based on abnormal laboratory tests or vital signs, so too might they change practice based on abnormal geomarkers. With advances in electronic health records,31,49 place-based, contextual information could be inserted into a patient’s chart in real time, providing an efficient means by which providers assess or stratify that patient’s risk level. Immediate linkages to evidence-based, upstream assessments and interventions could follow (for example, linkages to community health navigators, transportation supports, or telemedicine).34,50
Health care reform, and the move toward payment for value, makes this focus timely and important. The Department of Health and Human Services recently outlined goals to expand the link between reimbursements and outcomes.15 The accountable care organization and patient-centered medical home models represent two potential approaches. Both incentivize improvements in how patients experience care, the health of the covered population, and the cost of care provided.51 Incentives promote a focus on the distribution of medical and social risks within the covered population. This then guides the delivery of scarce or costly resources in ways that maximize benefit, or value.14,31 There exists a key opportunity to evaluate whether geomarkers aid in such a process.17
Place-based data also have utility in the development and expansion of cross-sector collaborations. The Health Policy Institute of Ohio recently introduced a framework connecting public health and clinical activities (from the statehouse to the community to the bedside). Data are at the core, informing assessments of need, development and deployment of interventions, and tracking of outcomes.52
Research Gaps And Future Directions
Many industries and community social service agencies use place-based data to connect the right person to the right resource at the right time. They do this by knowing as much as they can about a person simply by knowing where that person lives. Although geography is clearly linked to health, less is known about how this linkage can be leveraged to provide better, less costly patient care. Studies that address this would be of great benefit and relevance as care is reoriented toward payment for value. Of course, the distribution and types of risks in certain populations may vary. As a result, different settings may need to define risk thresholds (and determine which variables to include) in ways that are relevant to their patient panels and the resources available. Some may also need to rely more on individual-level social histories.12
Given the ubiquity of “big data,” efforts that support linking isolated information streams should be pursued and evaluated. Subsequent studies could assess associations among a range of health outcomes. Assessments with patient- or family-reported risks may be just as if not more important,53 highlighting factors that may be more proximal, and potentially more actionable.
More quantitative work is needed to determine which geomarkers have the most meaning and how they can best be packaged. Level of geography (for example, ZIP code, census tract, or census block group) also merits consideration, as areas of different sizes or definitions may characterize risk quite differently. Also, not all patients who live in at-risk areas will be at risk themselves (nor will all in low-risk areas be free of risk); perhaps smaller geographic levels are more homogenous, thereby limiting the degree to which area-based measures misclassify those who live within an area. Qualitative analyses may also be prudent to determine how key stakeholders, including providers and patients (alongside community partners and policy makers), view the use of place-based data within clinical settings. Additionally, an adaptable information technology backbone will be critical, ideally one that can facilitate real-time, user-friendly, and reliable geocoding and data linkages while also protecting privacy.54 Finally, training providers and support staff to respond appropriately to identified risks will be essential to translating knowledge into action.31,32,55
Conclusion
Imagine, once again, the child hospitalized for asthma exacerbation. Ready for discharge, the child goes home with interventions informed by a deeper understanding of his neighborhood. The pharmacy delivers the child’s medications that evening, a community health worker visits in the morning, and a home inspector comes within days to assess for mold in the rental apartment. In parallel, policies are put into place that enhance data collection and sharing to drive community-minded care delivery. With the right programs and policies in place, person-centered care begins the moment patients provide their address, promoting improved, equitable health outcomes.
Acknowledgments
Support was provided, in part, through the National Institutes of Health (Grant No. 1K23AI112916) and the AcademyHealth Community Health Peer Learning Program (Office of the National Coordinator for Health Information Technology, Department of Health and Human Services, Grant No. 90CL0001.01-00, sub-award 375.90CL.006).
Footnotes
Concepts from this work were presented during a webinar, “Partnering with Lawyers to Address the Social Determinants of Health,” as part of the Spotlight on Health series (sponsored by Healthy People 2020, Office of Disease Prevention and Health Promotion, Department of Health and Human Services, March 10, 2016).
The funders played no role in the preparation, review, or approval of the manuscript.
Contributor Information
Andrew F. Beck, Assistant professor of pediatrics at the Cincinnati Children’s Hospital Medical Center, in Ohio
Megan T. Sandel, Associate professor of pediatrics at the Boston University School of Medicine, in Massachusetts
Patrick H. Ryan, Associate professor of pediatrics at the Cincinnati Children’s Hospital Medical Center
Robert S. Kahn, Professor of pediatrics at the Cincinnati Children’s Hospital Medical Center
NOTES
- 1.Beck AF, Solan LG, Brunswick SA, Sauers-Ford H, Simmons JM, Shah S, et al. Socioeconomic status influences the toll paediatric hospitalisations take on families: a qualitative study. BMJ Qual Saf. 2017;26(4):304–11. doi: 10.1136/bmjqs-2016-005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Disparities [Internet] Washington (DC): HHS; 2015. [cited 2017 May 2]. Available from: http://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities. [Google Scholar]
- 3.Kressin NR, Wang F, Long J, Bokhour BG, Orner MB, Rothendler J, et al. Hypertensive patients’ race, health beliefs, process of care, and medication adherence. J Gen Intern Med. 2007;22(6):768–74. doi: 10.1007/s11606-007-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson DL, Puterman E, Bibbins-Domingo K, Matthews KA, Adler NE. Race, life course socioeconomic position, racial discrimination, depressive symptoms, and self-rated health. Soc Sci Med. 2013;97:7–14. doi: 10.1016/j.socscimed.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, et al. The association between income and life expectancy in the United States, 2001–2014. JAMA. 2016;315(16):1750–66. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: the Public Health Disparities Geocoding Project (US) J Epidemiol Community Health. 2003;57(3):186–99. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck AF, Florin TA, Campanella S, Shah SS. Geographic variation in hospitalization for lower respiratory tract infections across one county. JAMA Pediatr. 2015;169(9):846–54. doi: 10.1001/jamapediatrics.2015.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck AF, Moncrief T, Huang B, Simmons JM, Sauers H, Chen C, et al. Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. J Pediatr. 2013;163(2):574–80. doi: 10.1016/j.jpeds.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Social determinants of health [Internet] Geneva: WHO; 2015. [cited 2017 May 2]. Available from: http://www.who.int/social_determinants/en/ [Google Scholar]
- 10.Council on Community Pediatrics. Poverty and child health in the United States. Pediatrics. 2016;137(4):e20160339. doi: 10.1542/peds.2016-0339. [DOI] [PubMed] [Google Scholar]
- 11.Dreyer B, Chung PJ, Szilagyi P, Wong S. Child poverty in the United States today: introduction and executive summary. Acad Pediatr. 2016;16(3, Suppl):S1–5. doi: 10.1016/j.acap.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Chung EK, Siegel BS, Garg A, Conroy K, Gross RS, Long DA, et al. Screening for social determinants of health among children and families living in poverty: a guide for clinicians. Curr Probl Pediatr Adolesc Health Care. 2016;46(5):135–53. doi: 10.1016/j.cppeds.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DR, Costa MV, Odunlami AO, Mohammed SA. Moving upstream: how interventions that address the social determinants of health can improve health and reduce disparities. J Public Health Manag Pract. 2008;14(Suppl):S8–17. doi: 10.1097/01.PHH.0000338382.36695.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacker K, Walker DK. Achieving population health in accountable care organizations. Am J Public Health. 2013;103(7):1163–7. doi: 10.2105/AJPH.2013.301254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burwell SM. Setting value-based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–9. doi: 10.1056/NEJMp1500445. [DOI] [PubMed] [Google Scholar]
- 16.Galloway I. Using pay-for-success to increase investment in the non-medical determinants of health. Health Aff (Millwood) 2014;33(11):1897–904. doi: 10.1377/hlthaff.2014.0741. [DOI] [PubMed] [Google Scholar]
- 17.Stine NW, Chokshi DA, Gourevitch MN. Improving population health in US cities. JAMA. 2013;309(5):449–50. doi: 10.1001/jama.2012.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong WF, LaVeist TA, Sharfstein JM. Achieving health equity by design. JAMA. 2015;313(14):1417–8. doi: 10.1001/jama.2015.2434. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal D, McGinnis JM. Measuring vital signs: an IOM report on core metrics for health and health care progress. JAMA. 2015;313(19):1901–2. doi: 10.1001/jama.2015.4862. [DOI] [PubMed] [Google Scholar]
- 20.Alley DE, Asomugha CN, Conway PH, Sanghavi DM. Accountable Health Communities—addressing social needs through Medicare and Medicaid. N Engl J Med. 2016;374(1):8. doi: 10.1056/NEJMp1512532. [DOI] [PubMed] [Google Scholar]
- 21.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 22.Auger KA, Kahn RS, Davis MM, Beck AF, Simmons JM. Medical home quality and readmission risk for children hospitalized with asthma exacerbations. Pediatrics. 2013;131(1):64–70. doi: 10.1542/peds.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck AF, Simmons JM, Huang B, Kahn RS. Geomedicine: area-based socioeconomic measures for assessing risk of hospital reutilization among children admitted for asthma. Am J Public Health. 2012;102(12):2308–14. doi: 10.2105/AJPH.2012.300806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenfeld L, Rudd R, Chew GL, Emmons K, Acevedo-García D. Are neighborhood-level characteristics associated with indoor allergens in the household? J Asthma. 2010;47(1):66–75. doi: 10.3109/02770900903362676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck AF, Huang B, Ryan PH, Sandel MT, Chen C, Kahn RS. Areas with high rates of police-reported violent crime have higher rates of childhood asthma morbidity. J Pediatr. 2016;173:175–182e1. doi: 10.1016/j.jpeds.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–23. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda ML, Ferranti J, Strauss B, Neelon B, Califf RM. Geographic health information systems: a platform to support the “Triple Aim”. Health Aff (Millwood) 2013;32(9):1608. doi: 10.1377/hlthaff.2012.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolf SH, Purnell JQ. The good life: working together to promote opportunity and improve population health and well-being. JAMA. 2016;315(16):1706–8. doi: 10.1001/jama.2016.4263. [DOI] [PubMed] [Google Scholar]
- 29.DeVoe JE, Bazemore AW, Cottrell EK, Likumahuwa-Ackman S, Grandmont J, Spach N, et al. Perspectives in primary care: a conceptual framework and path for integrating social determinants of health into primary care practice. Ann Fam Med. 2016;14(2):104–8. doi: 10.1370/afm.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sessums LL, McHugh SJ, Rajkumar R. Medicare’s vision for advanced primary care: new directions for care delivery and payment. JAMA. 2016;315(24):2665–6. doi: 10.1001/jama.2016.4472. [DOI] [PubMed] [Google Scholar]
- 31.Bazemore AW, Cottrell EK, Gold R, Hughes LS, Phillips RL, Angier H, et al. “Community Vital Signs”: incorporating geocoded social determinants into electronic records to promote patient and population health. J Am Med Inform Assoc. 2016;23(2):407–12. doi: 10.1093/jamia/ocv088. [DOI] [PubMed] [Google Scholar]
- 32.Hughes LS, Phillips RL, Jr, DeVoe JE, Bazemore AW. Community Vital Signs: taking the pulse of the community while caring for patients. J Am Board Fam Med. 2016;29(3):419–22. doi: 10.3122/jabfm.2016.03.150172. [DOI] [PubMed] [Google Scholar]
- 33.Henize AW, Beck AF, Klein MD, Adams M, Kahn RS. A road map to address the social determinants of health through community collaboration. Pediatrics. 2015;136(4):e993–1001. doi: 10.1542/peds.2015-0549. [DOI] [PubMed] [Google Scholar]
- 34.Sandel M, Faugno E, Mingo A, Cannon J, Byrd K, Garcia DA, et al. Neighborhood-level interventions to improve childhood opportunity and lift children out of poverty. Acad Pediatr. 2016;16(3, Suppl):S128–35. doi: 10.1016/j.acap.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Keddem S, Barg FK, Glanz K, Jackson T, Green S, George M. Mapping the urban asthma experience: using qualitative GIS to understand contextual factors affecting asthma control. Soc Sci Med. 2015;140:9–17. doi: 10.1016/j.socscimed.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 36.Lindau ST, Makelarski J, Abramsohn E, Beiser DG, Escamilla V, Jerome J, et al. Community Rx: a population health improvement innovation that connects clinics to communities. Health Aff (Millwood) 2016;35(11):2020–9. doi: 10.1377/hlthaff.2016.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363(4):301–4. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 38.International Programme on Chemical Safety (INCHEM) Biomarkers in risk assessment: validity and validation. Geneva: World Health Organization; 2001. [cited 2017 May 2]. [Environmental Health Criteria 222]. Available from: http://www.inchem.org/documents/ehc/ehc/ehc222.htm. [Google Scholar]
- 39.Beck AF, Huang B, Chundur R, Kahn RS. Housing code violation density associated with emergency department and hospital use by children with asthma. Health Aff (Millwood) 2014;33(11):1993–2002. doi: 10.1377/hlthaff.2014.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123(Suppl 3):S174–84. doi: 10.1542/peds.2008-2233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yong PL, Werner RM. Process quality measures and asthma exacerbations in the Medicaid population. J Allergy Clin Immunol. 2009;124(5):961–6. doi: 10.1016/j.jaci.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Beck AF, Bradley CL, Huang B, Simmons JM, Heaton PC, Kahn RS. The Pharmacy-level Asthma Medication Ratio and population health. Pediatrics. 2015;135(6):1009–17. doi: 10.1542/peds.2014-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qato DM, Daviglus ML, Wilder J, Lee T, Qato D, Lambert B. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood) 2014;33(11):1958–65. doi: 10.1377/hlthaff.2013.1397. [DOI] [PubMed] [Google Scholar]
- 44.Beck AF, Simmons JM, Sauers HS, Sharkey K, Alam M, Jones C, et al. Connecting at-risk inpatient asthmatics to a community-based program to reduce home environmental risks: care system redesign using quality improvement methods. Hosp Pediatr. 2013;3(4):326–34. doi: 10.1542/hpeds.2013-0047. [DOI] [PubMed] [Google Scholar]
- 45.Beck AF, Klein MD, Schaffzin JK, Tallent V, Gillam M, Kahn RS. Identifying and treating a substandard housing cluster using a medical-legal partnership. Pediatrics. 2012;130(5):831–8. doi: 10.1542/peds.2012-0769. [DOI] [PubMed] [Google Scholar]
- 46.Reid M, Fiffer M, Gunturi N, Ali A, Irish D, Sandel M. Breathe Easy at Home: a web-based referral system linking clinical sites with housing code enforcement for patients with asthma. J Environ Health. 2014;76(7):36–9. [PubMed] [Google Scholar]
- 47.Newman NC, Ryan PH, Huang B, Beck AF, Sauers HS, Kahn RS. Traffic-related air pollution and asthma hospital readmission in children: a longitudinal cohort study. J Pediatr. 2014;164(6):1396–1402e1. doi: 10.1016/j.jpeds.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acevedo-Garcia D, McArdle N, Hardy EF, Crisan UI, Romano B, Norris D, et al. The Child Opportunity Index: improving collaboration between community development and public health. Health Aff (Millwood) 2014;33(11):1948–57. doi: 10.1377/hlthaff.2014.0679. [DOI] [PubMed] [Google Scholar]
- 49.Comer KF, Grannis S, Dixon BE, Bodenhamer DJ, Wiehe SE. Incorporating geospatial capacity within clinical data systems to address social determinants of health. Public Health Rep. 2011;126(Suppl 3):54–61. doi: 10.1177/00333549111260S310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raphael JL, Rueda A, Lion KC, Giordano TP. The role of lay health workers in pediatric chronic disease: a systematic review. Acad Pediatr. 2013;13(5):408–20. doi: 10.1016/j.acap.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berwick DM, Nolan TW, Whittington J. The Triple Aim: care, health, and cost. Health Aff (Millwood) 2008;27(3):759–69. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 52.Aly R, Rohling Mcgee A, Bush Stevens A. Improving population health planning in Ohio [Internet] Columbus (OH): Health Policy Institute of Ohio; 2016. [cited 2017 May 2]. [Prepared for the Ohio Governor’s Office of Health Transformation, Ohio Department of Health, and Ohio Department of Medicaid]. Available from: http://www.healthpolicyohio.org/wp-content/uploads/2016/01/SIMreport_Final_01112016.pdf. [Google Scholar]
- 53.Auger KA, Kahn RS, Simmons JM, Huang B, Shah AN, Timmons K, et al. Using address information to identify hardships reported by families of children hospitalized with asthma. Acad Pediatr. 2017;17(1):79–87. doi: 10.1016/j.acap.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes AE, Pruitt SL. The utility of EMR address histories for assessing neighborhood exposures. Ann Epidemiol. 2017;27(1):20–6. doi: 10.1016/j.annepidem.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck AF, Klein MD. Moving from social risk assessment and identification to intervention and treatment. Acad Pediatr. 2016;16(2):97–8. doi: 10.1016/j.acap.2016.01.001. [DOI] [PubMed] [Google Scholar]