Abstract
Microparticles are submicron vesicles shed from aging erythrocytes as a characteristic feature of the red blood cell (RBC) storage lesion. Exposure of pulmonary endothelial cells to RBC-derived microparticles promotes an inflammatory response, but the mechanisms underlying microparticle-induced endothelial cell activation are poorly understood. In the present study, cultured murine lung endothelial cells (MLECs) were treated with microparticles isolated from aged murine packed RBCs or vehicle. Microparticle-treated cells demonstrated increased expression of the adhesion molecules ICAM and E-selectin, as well as the cytokine, IL-6. To identify mechanisms that mediate these effects of microparticles on MLECs, cells were treated with microparticles covalently bound to carboxyfluorescein succinimidyl ester (CFSE) and cellular uptake of microparticles was quantified via flow cytometry. Compared with controls, there was a greater proportion of CFSE-positive MLECs from 15 minutes up to 24 hours, suggesting endocytosis of the microparticles by endothelial cells. Co-localization of microparticles with lysosomes was observed via immunofluorescence, indicating endocytosis and endolysosomal trafficking. This process was inhibited by endocytosis inhibitors. SiRNA knockdown of Rab5 signaling protein in endothelial cells resulted in impaired microparticle uptake as compared to nonsense siRNA-treated cells, as well as an attenuation of the inflammatory response to microparticle treatment. Taken together, these data suggest that endocytosis of RBC-derived microparticles by lung endothelial cells results in endothelial cell activation. This response appears to be mediated, in part, by the Rab5 signaling protein.
Keywords: RBC microparticles, Rab5, endocytosis, storage lesion, endotheliopathy
INTRODUCTION
Hemorrhagic shock is a leading cause of death following traumatic injury.1 Severe blood loss is frequently complicated by coagulopathy and organ failure2 and the transfusion of blood and blood products is a life-saving treatment in this patient population. Blood transfusion is not a harmless intervention, however, and exposes its recipients to the risk of transfusion-related complications. Immunologic properties of the donor blood have been implicated in a myriad of adverse reactions, including fever, hemolysis, allergy, anaphylaxis, and lung injury.3, 4 Recent studies have also identified the age of transfused packed red blood cell (pRBC) units as an independent risk factor for mortality among critically ill patients, suggesting that components of the aged erythrocyte may be also be harmful to the recipient.5–7 The mechanisms by which these components interact with the transfusion recipient to cause harm, however, remain poorly understood.
Current US FDA regulations allow pRBC units to be stored up to 42 days following donation.8 During this storage period, aging pRBC units accumulate metabolic, biochemical, and structural changes, collectively termed the erythrocyte storage lesion.9 One characteristic feature of the storage lesion is the shedding of microparticles (MP) from aged RBCs. Previous work from our and other laboratories has demonstrated that RBC-derived MPs induce inflammatory changes during simulated massive transfusion in a small animal model, including pulmonary leukocyte sequestration, neutrophil activation, and acute lung injury.10, 11 While the pathophysiologic effects of RBC-derived MPs on the recipient have been well-described in an animal model, the mechanisms underlying these changes are unknown. An understanding of these mechanisms may provide insight into the harmful effects of aged pRBC units on the transfusion recipient in the clinical setting.
In the current study, we hypothesized that MPs derived from stored pRBC units interact with endothelial cells through endocytosis, leading to a dysregulated activation response. Our data indicate that pulmonary endothelial cells endocytose MPs and traffic these vesicles to endothelial lysosomes. These cells become activated in response to MP exposure, generating a local proinflammatory milieu. Finally, we demonstrate that both MP endocytosis and MP-induced endothelial cell activation are mediated by the Rab5 signaling protein.
MATERIALS AND METHODS
Animal model
Male C57BL/6 mice aged 8–10 weeks were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were fed a standard laboratory diet and water ad libitum. Prior to experimentation, mice were acclimated to a 12-hour light-dark cycle for one week and weighed 21 to 30 g. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati, and performed in accordance with their regulations.
Microparticle isolation protocol
Murine packed RBC units were collected using previously described methods.12 Briefly, male C57BL/6 mice were anesthetized with 0.1 mg/g body weight intraperitoneal pentobarbital. Fresh whole blood was harvested through open cardiac puncture, and anticoagulated using citrate phosphate double dextrose (CP2D) solution in a 1:7 ratio. Anticoagulated whole blood was then centrifuged to separate the RBC pellet from the leukocyte-rich buffy coat and platelet-rich plasma, which were removed. This leukoreduced, platelet-reduced RBC was resuspended in standard erythrocyte storage medium (additive solution-3) in a 2:9 ratio, and stored in light-protected conditions at 4°C. Since current FDA regulations limit human RBC storage at 42 days in humans8, and previous studies have demonstrated that murine blood accumulates metabolic and structural changes at three times the rate of human blood, our murine RBC was stored for a 14-day period.12 After the storage period, MPs were isolated from the stored RBC via serial centrifugation. Specifically, the aged RBCs were centrifuged at 2000 g for 10 minutes to remove the cellular fraction, and the supernatant fluid was collected. This supernatant was centrifuged at 10,000 g for 10 minutes and again, the supernatant fluid was collected. This was centrifuged at 20,000 g for 30 minutes to isolate the MP pellet. The isolated MPs were then resuspended in either cell culture medium for in vitro experiments, or isotonic phosphate-buffered saline solution (PBS) for in vivo experiments. Previous experiments using flow cytometry have confirmed that MPs isolated using this protocol are predominantly RBC-derived.10, 11
Endothelial cell model
Primary murine lung microvascular endothelial cells (MLEC; Cell Biologics, Chicago, IL) isolated from pathogen-free C57BL/6 mouse lung tissue were plated and grown to confluence in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% fetal bovine serum. Cells were cultured in Nunclon™ Delta Surface 24-well plates (Thermo Scientific, Waltham, MA). The culture area was 1.8 cm2 and working volume was one ml. Glass slides were placed in wells and coated with 0.1% Gelatin Solution (ATCC) prior to seeding. MLECs were stored under sterile conditions at 37°C and served as our in vitro model for endothelial cells. All cells were grown to confluent monolayers prior to experimentation.
Endothelial cell analysis
Confluent monolayers of MLECs were treated with MPs derived from one mL of murine blood, using cell culture media as a negative control. Our murine blood banking protocol yields approximately 10.7±1.1 × 106 MPs per one mL of murine pRBCs (data under review), whereas human pRBCs have been shown to shed significantly higher concentrations of MPs.11 The concentration of MPs used in the current study, therefore, is relevant to the clinical setting. We simplified this “dose” of MPs to “one mL of murine blood” as this represents the entire circulating volume of one mouse.
After a six-hour interval, cellular supernatant was removed for analysis. These samples were evaluated for interleukin-6 (IL-6) concentration using pre-adsorbed sandwich ELISA kits (Thermo Fisher Scientific, Waltham, MA). Concurrently, treated MLECs were fixed with neutral-buffered formalin, and immunofluorescent staining was performed utilizing antibodies for intercellular adhesion molecule (ICAM) and E-selectin (Abcam, Cambridge, MA). Cells were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). Leukocyte adhesion molecule expression was analyzed under confocal microscopy as previously described.10 Briefly, eight random captures were taken of each slide using software ZEN 2012 version 1.1.2.0 on Axio Imager M2 microscope (Carl Zeiss AG, Jena, Germany). The fluorescent intensity of the adhesion molecule-specific subchannel was quantified using the imaging package ImageJ version 1.49v (Wayne Rasband, National Institutes of Health, USA).
Microparticle endocytosis experiments
Prior to experimentation, erythrocyte-derived MPs were incubated with carboxyfluorescein succinimidyl ester (CFSE) at 37°C. CFSE is a green-fluorescent probe that covalently binds amino acids and allows identification with immunofluorescence and flow cytometry (Sigma-Aldrich, St. Louis, MO).13 CFSE-labelled MPs were washed with 1% bovine serum albumin to remove any unbound CFSE.
MLECs were treated with CFSE-labelled MPs and incubated at 37°C for time intervals ranging from 15 minutes to 24 hours. After the incubation period, MPs were removed from the cell culture supernatant and MLECs were resuspended in PBS. Cellular suspensions were analyzed for CFSE expression using fluorescence-activated cell sorting (FACS) analysis. In separate experiments, MLEC suspensions were treated with 0.4% trypan blue solution prior to FACS analysis, in order to quench non-endocytosed fluorescence.14 The degree of MP endocytosis was quantified through the median fluorescence intensity (MFI) of treated cells.
All FACS experiments were repeated using pharmacological inhibitors of endocytosis. Confluent layers of MLECs were treated with inhibitors as indicated in the results section, incubated for 30 minutes, then treated with CFSE-labelled MPs for 1 hour. After removing the MP-rich supernatant and washing the cells, MLECs were then resuspended in PBS, treated with trypan blue, and analyzed for green fluorescence through flow cytometry. Endocytosis inhibitors utilized include hypertonic sucrose (0.5 M), chloroquine (CQ), methyl-β-cyclodextrin (MBC), phenylarsine oxide (PAO), and Ly294002.
Lysosomal co-localization assay
MLECs were plated and grown to confluent monolayers in endothelial cell medium supplemented with 10% fetal bovine serum. Confluent layers of MLECs were treated with CFSE-labelled MPs derived from 1 mL of murine RBCs. After incubation periods of 2, 4, 8, 24, or 48 hours, the MP-treated MLECs were fixed with neutral-buffered formalin. Lysosomal proteins were stained using CytoPainter Lysosomal Staining Kit (Abcam, Cambridge, MA). Co-localization of lysosomes and CFSE-labelled MPs was determined via confocal microscopy.
Rab5 analysis
Confluent monolayers of MLECs were treated with small interfering ribonucleic acid (siRNA) specific for Rab5 GTPase for 48–72 hours per manufacturer protocol (Santa Cruz Biotechnology, Dallas, TX). Three separate Rab5-specific oligonucleotide sequences (Integrated DNA Technologies, Coralville, IA) were tested: 13.1 (5′-GUAGAAUCAAGUUUCUAAUUCUGAA-3′), 13.2 (5′-UCAAAGGCAAGCAAGUCCUAAUATT-3′), and 13.3 (5′-AAAUUUGGACAUGGCUAAUCGAGGA-3′). The nonsense (NS) siRNA control duplex sequence was used as control. As reported by the manufacturer (Integrated DNA Technologies, Coralville, IA), its genetic sequence is as follows:
5′-CGUUAAUCGCGUAUAAUACGCGUATAUACGCGUAUUAUACGCGAUUAACGAC-3′
Transfection was carried out per manufacturer recommendations. Briefly, cells were grown to 60% confluence at time of transfection. Lipofectamine RNAiMAX Reagent (3 uL) was used as the transfection reagent and diluted in Opti-MEM Medium (50 uL) per manufacturer instructions. A separate vial of Opti-MEM Medium (50 uL) was used to dilute the appropriate siRNA to 10 pmol concentration. Diluted siRNA was then added to diluted Lipofectamine reagent, and incubated for 5 minutes. This siRNA-lipid complex was added to cells and incubated for 2–3 days at 37°C. The transfection efficiency of all sequences was compared with NS siRNA-treated cells using Western blot assay.
Rab5-knockdown experiments
After incubating confluent monolayers of MLECs with Rab5 or NS siRNA, cells were treated with CFSE-labelled MPs and flow cytometry experiments were performed as previously described. The degree of MP endocytosis was again quantified through MFI. In separate experiments, Rab5-knockdown (KD) cells were treated with RBC-derived MPs and incubated for six hours. Cellular ICAM and E-selectin expression was determined via immunofluorescent assay, and IL-6 secretion was measured using ELISA kits as described above.
Statistical analysis
Results are reported as the mean and standard deviation where applicable. All experiments were performed in quadruplicate to ensure veracity of findings. Student t-test was used to compare groups. Statistical analysis was performed using SigmaPlot 13.0 (Systat Software Inc., Chicago, IL). Probability value of less than 0.05 was used to determine statistical significance.
RESULTS
Microparticles activate endothelial cells
After an incubation period of six hours, endothelial cells treated with RBC-derived MPs demonstrated increased expression of the cell adhesion molecules ICAM (26.0±12.0% increase) and E-selectin (20.3±3.2% increase) as compared with vehicle (Figure 1a and b). IL-6 levels in the media were also higher in cells treated with MPs as compared with vehicle (171.0±59.0% increase, Figure 1c). Taken together, these data confirm our recent work indicating that treatment of endothelial cells with MPs from stored RBC units leads to endothelial cell activation.10
Figure 1.
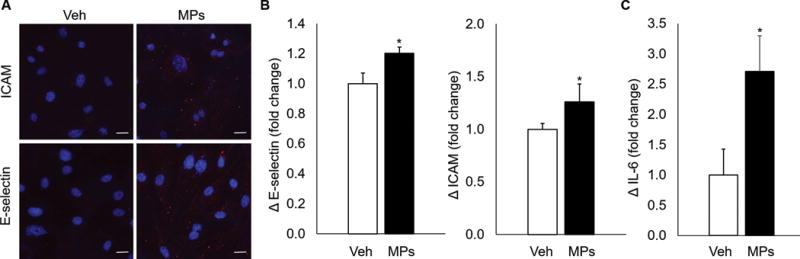
Endothelial cells are activated after treatment with RBC-derived MPs. (A) MLECs treated with MPs demonstrate increased expression of ICAM and E-selectin as determined by immunofluorescence. (B) Quantification of adhesion molecule upregulation. (C) IL-6 expression, as determined by ELISA, is increased after MP treatment as compared with vehicle. *p<0.05 vs control.
Microparticles are endocytosed by endothelial cells
The mechanism by which erythrocyte-derived MPs activate endothelial cells is unknown. Previous studies have suggested that MPs derived from other cell types, such as platelets and endothelial cells, may activate endothelial cells via endocytosis.15, 16 In order to determine if RBC-derived MPs act on lung endothelial cells through endocytosis, flow cytometry was used to determine possible endocytosis of CFSE-labelled MPs from stored pRBC units. Endothelial cells treated with vehicle only served as negative control and exhibited minimal fluorescence (16.8±0.3k MFI; Figure 2a and c). After treating cells with MPs, MLECs demonstrated a temporal increase in fluorescence after 15 min (88.9±9.1k MFI), 30 min (194.3±12.8k MFI), 1 hour (324.6±36.0k MFI), 2 hours (420.0±44.2k MFI), 4 hours (464.9±49.2k MFI), 8 hours (442.7±45.4k MFI), and 24 hours (295.2±24.7k MFI) of incubation (Figure 2a and 2c; each p<0.05 vs control).
Figure 2.
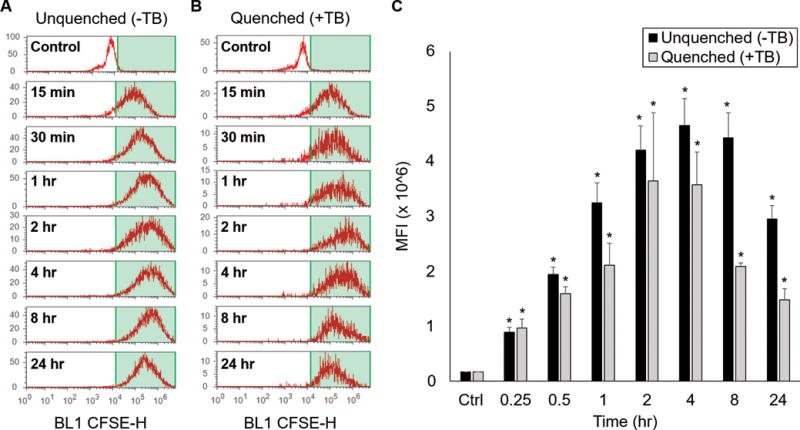
RBC-derived MPs undergo endocytosis by endothelial cells in a time-dependent fashion. (A) Flow cytometry was used to quantify CFSE fluorescence after treatment with CFSE-labelled pRBC MPs. (B) Experiments were repeated using 0.4% trypan blue (TB) to quench surface fluorescence. (C) Quantification of endocytosis from median fluorescent intensity (MFI) of treated cells. *p<0.05 vs control.
Increased fluorescence under these conditions may indicate MP endocytosis by endothelial cells, MP adherence to endothelial cells, or a combination of these events. To differentiate between these events, these experiments were repeated with trypan blue treatment. Trypan blue has previously been shown to effectively quench surface fluorescence with minimal side-effects to the treated cells, indicating that that any residual fluorescence is intracellular in location.17 In these experiments, cells were treated with trypan blue after exposure to MPs. Cells treated with vehicle served as negative control, and exhibited minimal fluorescence (17.0±0.3k MFI). After MP and trypan blue treatment, a temporal increase in endothelial cell fluorescence was again observed after 15 min (96.7±16.3k MFI), 30 min (158.9±13.1k MFI), 1 hour (210.9±40.3k MFI), 2 hours (364.1±124.1k MFI), 4 hours (357.3±59.7k MFI), 8 hours (208.9±6.3k MFI) and 24 hours (147.8±20.6k MFI) of incubation (Figure 2b and 2c; each p<0.05 vs control). These data demonstrate that the increased endothelial cell fluorescence is predominantly intracellular in location, indicating that MPs undergo endocytosis by endothelial cells.
To determine the intracellular destination for MPs following endocytosis, MLECs treated with CFSE-labelled MPs were analyzed utilizing confocal microscopy. We found that there was an increase in intracellular CFSE within endothelial cells in a time-dependent manner. After staining for lysosomal proteins, CFSE-labelled MPs co-localized with lysosomes within the murine lung endothelial cells, indicating endolysosomal trafficking of endocytosed MPs (Figure 3). Taken together, these data indicate that MPs from stored pRBC units undergo endocytosis by endothelial cells and are subsequently trafficked to lysosomal vesicles.
Figure 3.
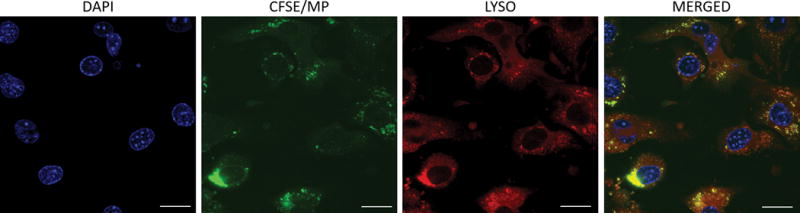
MPs undergo endocytosis and co-localize with lysosomes. Cultured endothelial cells were treated with CFSE-labelled MPs (green) and incubated for 24 hours. Cells were then fixed and stained with lysosomal stain (red) and DAPI (nuclei, blue). Images were captured with Axio Imager M2 microscope with a 40× objective. Yellow fluorescence on merged confocal images reflects co-localization of MPs and lysosomes.
In additional experiments, we utilized endocytosis inhibitors to attempt to inhibit uptake of RBC-derived MPs by endothelial cells. Five inhibitors were investigated. Hypertonic sucrose (0.5 M) targets clathrin-mediated endocytosis (CME) by trapping clathrin mobility.18 CQ affects CME by altering the function of clathrin-coated vehicles.19 The mechanism of PAO is less clear, but has also been shown to block CME.20 MBC sequesters cholesterol from the cellular membrane, affecting lipid raft mobility.21 Finally, Ly294002 is a specific inhibitor of phosphatidylinositol-3-kinase, and blocks both endocytosis and intracellular vesicle transport.22
Endothelial cells exposed to CFSE-labelled MPs without any inhibitor served as positive controls. Cells were treated with MPs for one hour prior to analysis. Among the positive controls, 55.7±2.1% of treated cells exhibited intracellular fluorescence. Each inhibitor impaired MP endocytosis to varying degrees (Figure 4), including hypertonic sucrose (16.0±3.2% of total cells), CQ (22.2±4.0%), PAO (22.4±1.3%), MBC (39.3±3.0%), and Ly294002 (22.1±5.6%; each p<0.05).
Figure 4.
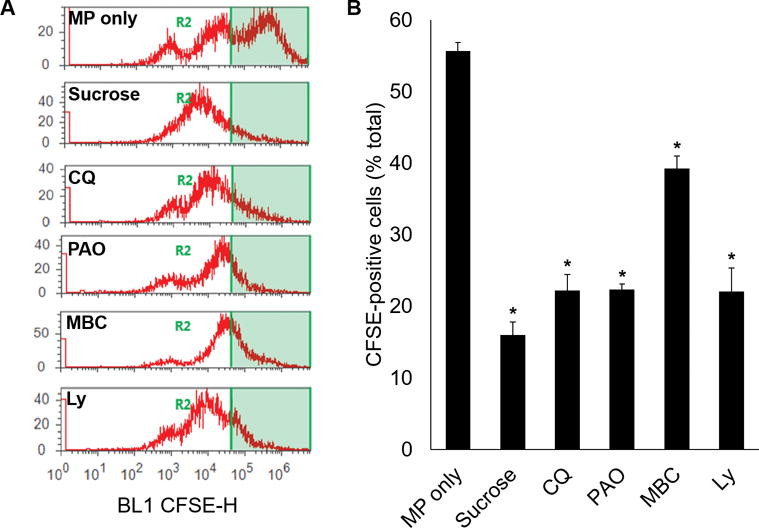
Pharmacological inhibitors of endocytosis inhibit uptake of MPs by endothelial cells. Cultured endothelial cells were treated with endocytosis inhibitors prior to incubation with CFSE-labelled MPs. All samples were treated with 0.4% trypan blue to remove surface fluorescence. Flow cytometry was utilized to quantify the percentage of treated cells containing CFSE. Endothelial cells exposed to MPs without inhibitors served as positive control. *p<0.05 vs control.
Microparticle endocytosis is mediated by Rab5
Rab5 is a small GTPase that regulates endosomal biogenesis and fusion of endocytic vesicles to endosomes.23–26 Given the role of Rab5 in mediating endocytosis, we hypothesized that MP endocytosis is mediated by Rab5. MLECs were transfected with nonsense siRNA as a negative control or three different nucleic acid sequences of Rab5 siRNA (13.1, 13.2, 13.3). Western blot was then used to quantify Rab5 transfection efficiency (Figure 5a and 5b). MLECs transfected with Rab5 siRNA demonstrated decreased protein expression with all three sequences. The greatest decrease was observed with the 13.1 sequence. The 13.1 sequence was therefore used in all subsequent experiments.
Figure 5.
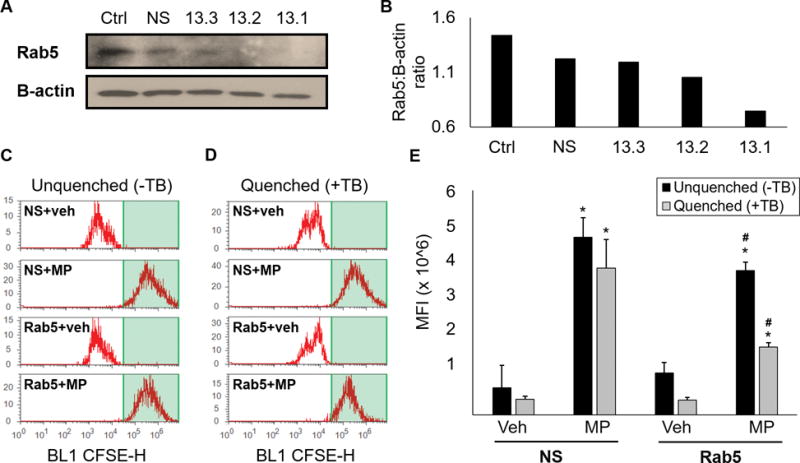
Knockdown of Rab5 inhibits endocytosis of RBC-derived MPs. (A) Cultured endothelial cells were treated with nonsense siRNA (NS) as a negative control, or three separate Rab5 siRNA oligonucleotide sequences (13.3, 13.2, 13.1). After incubation, Rab5 protein was determined by Western blot analysis. Note: both lanes were different exposures of the same gel, as β-actin expression required less exposure as compared with Rab5. (B) Quantitation of Rab5 to B-actin protein expression. (C) Cultured endothelial cells were transfected with nonsense (NS) or Rab5 siRNA 13.1, then treated with vehicle or CFSE-labelled MPs and analyzed via flow cytometry for the presence of CFSE. (D) Experiments were repeated with trypan blue. (E) Quantification of MFI from data presented in C and D. *p<0.05, vehicle vs MP; #p<0.05, NS+MP vs Rab5+MP.
To determine the role of Rab5 in MP endocytosis by endothelial cells, confluent monolayers of MLECs were transfected with Rab5 or nonsense siRNA per manufacturer protocol. Cell populations were then treated with either vehicle or CFSE-labelled MPs and analyzed via flow cytometry (Figure 5c and 5e). Cells treated with nonsense siRNA then treated with MPs demonstrated increased fluorescence intensity as compared with vehicle (73.0±59.8k vs 474.3±52.5k MFI, p<0.05). This increase in fluorescence persisted after treatment with 0.4% trypan blue, indicating that treatment with nonsense siRNA did not inhibit MP endocytosis (42.0±8.7k vs 391.8±77.2k MFI, p<0.05; Figure 5d and 5e). Cells treated with Rab5 siRNA then MPs also demonstrated increased fluorescence intensity after MP exposure (112.0±28.6k vs 385.1±22.6k MFI, p<0.05). Trypan blue treatment led to significantly decreased fluorescence intensity in cells treated with Rab5 siRNA (39.5±7.2k vs 181.5±11.1k MFI, p<0.05) indicating that MPs were adherent to endothelial cells under these conditions but did not undergo endocytosis. Taken together, these findings demonstrate that Rab5 siRNA treated cells have an attenuated capacity for MP endocytosis as compared with nonsense treated cells, suggesting that endocytosis of MPs is regulated by the Rab5 GTPase.
Rab5 mediates microparticle-associated endothelial activation
In a final series of experiments, we investigated the role of Rab5 on endothelial cell activation after MP treatment. Nonsense siRNA treated cells served as a negative control. Cells pretreated with Rab5 siRNA, then exposed to MPs, demonstrated decreased expression of ICAM, E-selectin, and IL-6 as compared to cells treated with nonsense siRNA, prior to MP exposure (Figure 6). Taken together, these results demonstrate that Rab5 plays a key role in endothelial activation by RBC-derived MPs.
Figure 6.
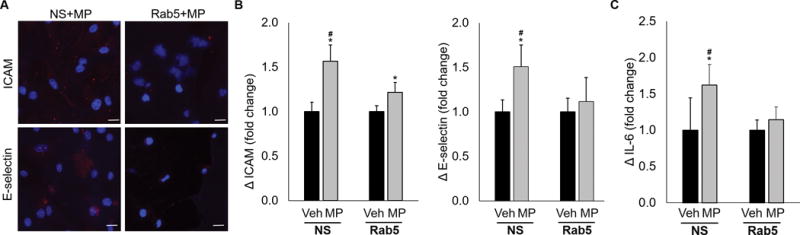
Knockdown of Rab5 in endothelial cells attenuates the endothelial activation response after treatment with RBC-derived MPs. Nonsense (NS) siRNA was used as a control for all experiments. (A) Cells treated with NS siRNA, then MPs demonstrate increased ICAM and E-selectin expression. This effect was attenuated in Rab5-knockdown cells. (B) Quantification of adhesion molecule upregulation data. (C) NS cells treated with MPs also demonstrated increased concentrations of IL-6 as compared to Rab5-knockdown cells. This effect was inhibited in cells treated with Rab5 siRNA, then MPs. *p<0.05, vehicle vs MP; #p<0.05, NS+MP vs Rab5+MP.
DISCUSSION
In the present study, we investigated the mechanism by which RBC-derived MPs activate pulmonary endothelial cells. Our results demonstrate that MPs from aged pRBC units undergo endocytosis by lung endothelial cells and are trafficked to lysosomal vesicles. Both endocytosis and endothelial cell activation appear to be regulated by the Rab5 signaling protein, suggesting that these processes may be linked to a common pathway. Together, these data describe one mechanism by which components of aged blood interact with the transfusion recipient, and provide novel insight into the potential harms of stored blood.
Traumatic injury is a leading cause of death and disability worldwide, with uncontrolled hemorrhage accounting for over 30% of trauma-associated mortality.1, 27 While resuscitation strategies are an area of ongoing research, strategic transfusion of blood products remains the standard of care in this critically ill patient population.28, 29 Blood transfusion is not without risk, however, and several studies have suggested that resuscitation with older blood products may harm the transfusion recipient. According to recent literature, critically ill and complex surgical patients appear to be most vulnerable to harm related to old blood.5, 30, 31 Although the reasons for this are poorly understood, previous studies suggest that elements of the erythrocyte storage lesion may be accountable.32 MPs are 0.1–1.0 μm vesicles shed from the aging RBCs as a key element of this storage lesion, and have been shown to inflict harm in animal models of transfusion.10, 11 Even though over 20 million blood products are transfused each year in the United States33, surprisingly little is known about how MPs from aged pRBCs affect the transfusion recipient.
Previous research from our and other laboratories have discovered several key characteristics regarding RBC-derived MPs. First, MPs accumulate within the pRBC unit as a function of storage duration. Aged murine and human pRBC units contain a substantially higher number of MPs as compared to freshly isolated pRBC units.11 Second, the physiologic function of MPs is incompletely understood, but data suggests that these vesicles play a role in RBC self-preservation by selectively sequestering damaged proteins and lipids prior to shedding.34 Third, removal of MPs prior to transfusion, either through cell washing or bioactive lipid modulation, attenuates the proinflammatory response in the transfusion recipient.35 Fourth, as part of this proinflammatory response, RBC-derived MPs promote endothelial cell activation and transfusion-related lung injury in a murine model of transfusion.10, 11
Our findings elucidate one mechanism by which RBC-derived MPs promote the dysregulated activation of endothelial cells. Endothelial activation is a well-defined process characterized by upregulation of leukocyte adhesion molecules (e.g., ICAM, E-selectin) and a proinflammatory response (e.g., IL-6).36, 37 Under normal physiologic conditions, these changes serve to promote leukocyte diapedesis and transmigration to the site of injury. Dysregulated endothelial activation, however, can lead to an exaggerated immunologic response and contribute to the pathogenesis of acute lung injury.10 The current data confirm our previous findings of MP-induced endothelial activation, and provide strong evidence that endothelial cells endocytose RBC-derived MPs. These MP-laden endosomes appear to be trafficked to lysosomal vesicles after endocytosis. We also demonstrate that the Rab5 signaling protein mediates both processes, in part, suggesting that MP endocytosis and endothelial activation may share a common pathway.
Previous investigations from our laboratory have elucidated different cellular interactions by which pRBC-derived MPs may harm the transfusion recipient. As mentioned, MPs have been shown to activate lung endothelial cells following transfusion.38 These activated endothelial cells upregulate their expression of adhesion molecules, which leads to the pulmonary sequestration of leukocytes.38 MPs from pRBC units have also been shown to prime neutrophils, as evidenced by superoxide generation and increased surface expression of CD11b.11 Together, these interactions contribute the development of transfusion-associated lung inflammation and injury.11, 35 While previous efforts have focused on the removal of MPs in order to reduce this inflammatory response35, 39, in the current study, we provide further insight into the mechanisms by which pRBC-derived MPs harm the transfusion recipient. Future studies may consider the endocytic pathways of the host endothelium as potential targets for intervention.
Our findings must be considered in light of three limitations. The first limitation to relates to the clinical relevance of RBC-derived MPs. While transfusion of MPs themselves have no clinical indication, these vesicles likely become relevant with larger volumes of blood transfusion. Previous studies have shown that the biological effects of MPs on the transfusion recipient are more pronounced with higher concentrations11, and the overall concentration of MPs present in human blood units far exceeds those isolated from our murine model.35 Thus, patients receiving multiple units of aged packed RBCs are frequently exposed to large concentration of potentially harmful MPs. The second limitation is our exclusive use of a murine model. We used a small animal model due the variability of storage lesion development in humans. A recent publication describes aspects of the storage lesion, including MP formation, as being significantly affected by poorly understood characteristics of the pRBC unit donor.40 Separate studies have demonstrated that different patients have different “microvesicle phenotypes”, which further introduces additional variables with the use of a human model.41 In the current study, the use of genetically similar small animals maximizes our ability to investigate endocytosis without introducing additional variables into our experimental design. Our third limitation is the exclusion of female mice from our experiments. Previous studies have documented the impact of mouse gender on erythrocyte characteristics, including membrane integrity42, ability to resist mechanical stress43, and storage-related hemolysis.44 Therefore, we focused on male mice intentionally, to decrease gender-related variability in the development of the storage lesion.
In conclusion, MPs are submicron vesicles shed from aging erythrocytes during the storage period. These MPs are endocytosed by lung endothelial cells in a Rab5-mediated mechanism and result in activation of endothelial cells. Blood transfusion is a common practice in modern medicine, and yet the manifold relationships between the transfused RBCs and the transfusion recipient are incompletely understood. Through our experiments on the impact of aged blood, future investigations may elucidate mechanisms and potential strategies for minimizing adverse effects in the transfusion-dependent patient.
Acknowledgments
Grants and financial support: This work was supported by National Institutes of Health (NIH) grants R01 GM107625 (TAP) and T32 GM008478-24 (YK).
ABBREVIATIONS
- CFSE
carboxyfluorescein succinimidyl ester
- CME
clathrin-mediated endocytosis
- CP2D
citrate phosphate double dextrose
- CQ
chloroquine
- DAPI
4′,6-diamidino-2-phenylindole dihydrochloride
- DMEM
Dulbecco’s Modified Eagle Medium
- FACS
fluorescent-activated cell sorting
- FDA
Food and Drug Administration
- GTPase
guanine triphosphatase
- ICAM
intercellular adhesion molecule
- IL-6
interleukin 6
- KD
knockdown
- MBC
methyl-β-cyclodextrin
- MFI
median fluorescence intensity
- MLEC
mouse lung endothelial cell
- MP
microparticle
- NS
nonsignificant
- PAO
phenylarsine oxide
- PBS
phosphate-buffered saline
- pRBC
packed red blood cell
- siRNA
small interfering ribonucleic acid
Footnotes
Conflicts of interest: All authors have no conflicts of interest to disclose.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Ledgerwood AM, Lucas CE. A review of studies on the effects of hemorrhagic shock and resuscitation on the coagulation profile. J Trauma. 2003;54:S68–74. doi: 10.1097/01.TA.0000064513.59253.70. [DOI] [PubMed] [Google Scholar]
- 3.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 4.Osterman JL, Arora S. Blood product transfusions and reactions. Emerg Med Clin North Am. 2014;32:727–38. doi: 10.1016/j.emc.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Goel R, Johnson DJ, Scott AV, et al. Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion. 2016 doi: 10.1111/trf.13559. [DOI] [PubMed] [Google Scholar]
- 6.Rawn J. The silent risks of blood transfusion. Curr Opin Anaesthesiol. 2008;21:664–8. doi: 10.1097/ACO.0b013e32830f1fd1. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Amini N, Gani F, et al. Age of Transfused Blood Impacts Perioperative Outcomes Among Patients Who Undergo Major Gastrointestinal Surgery. Ann Surg. 2017;265:103–10. doi: 10.1097/SLA.0000000000001647. [DOI] [PubMed] [Google Scholar]
- 8.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–61. [PubMed] [Google Scholar]
- 9.Hoehn RS, Jernigan PL, Chang AL, et al. Molecular mechanisms of erythrocyte aging. Biol Chem. 2015;396:621–31. doi: 10.1515/hsz-2014-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang AL, Kim Y, Seitz AP, et al. Erythrocyte Derived Microparticles Activate Pulmonary Endothelial Cells in a Murine Model of Transfusion. Shock. 2016 doi: 10.1097/SHK.0000000000000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belizaire RM, Prakash PS, Richter JR, et al. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J Am Coll Surg. 2012;214:648–55. doi: 10.1016/j.jamcollsurg.2011.12.032. discussion 56–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makley AT, Goodman MD, Friend LA, et al. Murine blood banking: characterization and comparisons to human blood. Shock. 2010;34:40–5. doi: 10.1097/SHK.0b013e3181d494fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grisendi G, Finetti E, Manganaro D, et al. Detection of microparticles from human red blood cells by multiparametric flow cytometry. Blood Transfus. 2015;13:274–80. doi: 10.2450/2014.0136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava GK, Reinoso R, Singh AK, et al. Trypan Blue staining method for quenching the autofluorescence of RPE cells for improving protein expression analysis. Exp Eye Res. 2011;93:956–62. doi: 10.1016/j.exer.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Andrews AM, Rizzo V. Microparticle-Induced Activation of the Vascular Endothelium Requires Caveolin-1/Caveolae. PLoS One. 2016;11:e0149272. doi: 10.1371/journal.pone.0149272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faille D, El-Assaad F, Mitchell AJ, et al. Endocytosis and intracellular processing of platelet microparticles by brain endothelial cells. J Cell Mol Med. 2012;16:1731–8. doi: 10.1111/j.1582-4934.2011.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Amersfoort ES, Van Strijp JA. Evaluation of a flow cytometric fluorescence quenching assay of phagocytosis of sensitized sheep erythrocytes by polymorphonuclear leukocytes. Cytometry. 1994;17:294–301. doi: 10.1002/cyto.990170404. [DOI] [PubMed] [Google Scholar]
- 18.Hansen SH, Sandvig K, van Deurs B. Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J Cell Biol. 1993;123:89–97. doi: 10.1083/jcb.123.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–17. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson AE, Noel RJ, Herlihy JT, Ward WF. Phenylarsine oxide inhibition of endocytosis: effects on asialofetuin internalization. Am J Physiol. 1989;257:C182–4. doi: 10.1152/ajpcell.1989.257.2.C182. [DOI] [PubMed] [Google Scholar]
- 21.Kilsdonk EP, Yancey PG, Stoudt GW, et al. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–6. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 22.Takac T, Pechan T, Samajova O, Samaj J. Vesicular trafficking and stress response coupled to PI3K inhibition by LY294002 as revealed by proteomic and cell biological analysis. J Proteome Res. 2013;12:4435–48. doi: 10.1021/pr400466x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbieri MA, Roberts RL, Mukhopadhyay A, Stahl PD. Rab5 regulates the dynamics of early endosome fusion. Biocell. 1996;20:331–8. [PubMed] [Google Scholar]
- 24.de Hoop MJ, Huber LA, Stenmark H, et al. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 25.Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113(Pt 2):183–92. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- 26.Zeigerer A, Gilleron J, Bogorad RL, et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature. 2012;485:465–70. doi: 10.1038/nature11133. [DOI] [PubMed] [Google Scholar]
- 27.Gruen RL, Jurkovich GJ, McIntyre LK, et al. Patterns of errors contributing to trauma mortality: lessons learned from 2,594 deaths. Ann Surg. 2006;244:371–80. doi: 10.1097/01.sla.0000234655.83517.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvath KA, Acker MA, Chang H, et al. Blood transfusion and infection after cardiac surgery. Ann Thorac Surg. 2013;95:2194–201. doi: 10.1016/j.athoracsur.2012.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spadaro S, Reverberi R, Fogagnolo A, et al. Transfusion of stored red blood cells in critically ill trauma patients: a retrospective study. Eur Rev Med Pharmacol Sci. 2015;19:2689–96. [PubMed] [Google Scholar]
- 32.Koch CG, Figueroa PI, Li L, et al. Red blood cell storage: how long is too long? Ann Thorac Surg. 2013;96:1894–9. doi: 10.1016/j.athoracsur.2013.05.116. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y, Chang AL, Wima K, et al. The impact of morbid obesity on resource utilization after renal transplantation. Surgery. 2016 doi: 10.1016/j.surg.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosman GJ, Lasonder E, Groenen-Dopp YA, et al. The proteome of erythrocyte-derived microparticles from plasma: new clues for erythrocyte aging and vesiculation. J Proteomics. 2012;76 doi: 10.1016/j.jprot.2012.05.031. Spec No.:203–10. [DOI] [PubMed] [Google Scholar]
- 35.Hoehn RS, Jernigan PL, Japtok L, et al. Acid Sphingomyelinase Inhibition in Stored Erythrocytes Reduces Transfusion-Associated Lung Inflammation. Ann Surg. 2017;265:218–26. doi: 10.1097/SLA.0000000000001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma-induced coagulopathy. Blood. 2016;128:1043–9. doi: 10.1182/blood-2016-01-636423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunt BJ, Jurd KM. Endothelial cell activation. A central pathophysiological process. BMJ. 1998;316:1328–9. doi: 10.1136/bmj.316.7141.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web–based Injury Statistics Query and Reporting System (WISQARS) [online] Accessed October 25, 2016. http://http://www.cdc.gov/injury/wisqars/fatal.html.
- 39.Belizaire RM, Makley AT, Campion EM, et al. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg. 2012;73:S128–33. doi: 10.1097/TA.0b013e3182606301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion. 2016;56:1274–86. doi: 10.1111/trf.13582. [DOI] [PubMed] [Google Scholar]
- 41.Matijevic N, Wang YW, Holcomb JB, et al. Microvesicle phenotypes are associated with transfusion requirements and mortality in subjects with severe injuries. J Extracell Vesicles. 2015;4:29338. doi: 10.3402/jev.v4.29338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeVenuto F, Wilson SM. Distribution of progesterone and its effect on human blood during storage. Transfusion. 1976;16:107–12. doi: 10.1046/j.1537-2995.1976.16276155103.x. [DOI] [PubMed] [Google Scholar]
- 43.Raval JS, Waters JH, Seltsam A, et al. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011;100:418–21. doi: 10.1111/j.1423-0410.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- 44.Kanias T, Gladwin MT. Nitric oxide, hemolysis, and the red blood cell storage lesion: interactions between transfusion, donor, and recipient. Transfusion. 2012;52:1388–92. doi: 10.1111/j.1537-2995.2012.03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


