Abstract
Hepatocytes differentiated from human embryonic stem cells (ESCs) could provide a powerful tool for enabling cell-based therapies, studying the mechanisms underlying human liver development and disease, and testing the efficacy and safety of pharmaceuticals. However, currently most in vitro protocols yield hepatocytes with low levels of liver function. In this study, we investigated the potential of Salvianolic acid B (Sal B), an active pharmaceutical compound present in Salvia miltiorrhiza, which has been shown to have an antifibrotic effect in previous studies, to enhance hepatocyte differentiation from human ESCs. After treatment with Sal B, albumin expression and secretion were consistently increased, indicating that Sal B could promote hepatocyte differentiation process. Expression of a large number of important phase 1 and 2 metabolizing enzymes and phase 3 transporters was also increased in treated cells, indicating an enhanced biotransformation function. Our investigations further revealed the activation of Wnt pathway in treated cells, as determined by upregulation of Wnts, which increased amounts of nuclear β-catenin. This increased nuclear β-catenin led in turn to the enhanced expression of T cell factor (TCF) 3 and lymphoid enhancer-binding factor (LEF) 1 which upregulated their downstream targets, cyclin D1 and c-Myc. Notch receptors (Notch1, Notch3), Notch ligand (Jagged2), and Notch receptor targets [hairy and enhancer of split (Hes) 1, 5] were downregulated in treated cells, suggesting that Notch pathway was inhibited. Consistent with the inhibition of Notch pathway, expression of cholangiocyte marker, CK7, was significantly reduced by treatment with Sal B. Numb, a direct transcriptional target of Wnt pathway and a negative regulator of Notch pathway, was upregulated, consistent with activation of Wnt signaling and suppression of Notch signaling. In conclusion, our study demonstrated that Sal B enhanced hepatocyte differentiation from human ESCs through activation of Wnt pathway and inhibition of Notch pathway. Therefore, this study suggests that Sal B can be used as a potential agent to generate more mature hepatocytes for cell-based therapeutics and pharmaceutical studies.
Keywords: : salvianolic acid B, human embryonic stem cells, hepatocyte differentiation, Wnt signaling pathway, Notch signaling pathway
Introduction
Liver disease is a major health problem in the world and it not uncommonly leads to liver failure through progressive cirrhosis of the liver [1]. Liver transplantation is the only effective treatment for end-stage liver disease, but the source of the donor livers for transplantation is limited, and the cost of liver transplantation is very high [2,3]. The use of hepatocytes with extracorporeal bioartificial liver devices and transplantation of human primary hepatocytes may represent an alternative to orthotropic liver transplantation and potentially provide effective treatment for many liver diseases. In addition, effective in vitro models of hepatocyte function are required for drug screening and development. However, human primary hepatocytes are scarce in number; moreover, human primary hepatocytes have limited proliferation in culture, and have rapid functional loss in vitro [4]. As an alternative, human hepatoma cell lines or animal primary hepatocytes are used in drug development; however, these cells have low levels of liver function, especially metabolic function, and usually poorly represent human hepatocytes in vivo [1]. Therefore, developing viable hepatocytes with full metabolic function from other sources is essential not only for use in cell-based therapeutics but also as an accurate test system for pharmacology and toxicology studies.
It has been shown that the use of pluripotent stem cells (PSCs) may be the most effective approach to produce functional hepatocytes for regenerative medicine and for the pharmaceutical industry [5,6]. Thus, hepatocytes differentiated from human PSCs, including human embryonic stem cells (ESCs,) have the potential to overcome the shortage of viable hepatocytes for clinical use and drug development [7]. Many strategies for this purpose have been attempted; however, conditions for generating mature hepatocytes from human PSCs have been not yet fully defined.
Salvianolic acid B (Sal B), a major water soluble component extracted from Chinese herbal, Radix Salviae miltiorrhizae, has been used for treatment of liver diseases [8,9] and to modulate the differentiation of various kinds of stem cells [10–12]. In previous studies, it has been shown that Fuzheng Huayu (FZHY), a Chinese medicine, could significantly reverse hepatic fibrosis and improve the liver function in patients with fibrosis and cirrhosis [13–17]; and our recent study revealed that FZHY could enhance the processes of the differentiation and maturation of human ESC toward hepatocytes [18]. FZHY is a combinatorial compound of different Chinese herbs [18], and 10 single compounds have been identified so far, and Sal B is one of them (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd) [18]. In the present study, we have investigated the effect of this agent on hepatocyte differentiation from human ESCs and found that Sal B could enhance the process of hepatocyte differentiation from human ESCs through activating Wnt signaling pathway and suppressing the Notch pathway.
Materials and Methods
Cell culture
The human ESC line, H9, was purchased from WiCell Research Institute (Madison, WI), maintained and expanded on mouse embryonic fibroblasts (GlobalStem, Rockville, MD) as instructed by the provider.
Hepatocyte differentiation from human ESCs
Hepatocyte differentiation was performed as previously described [5]. Briefly, the induction of definitive endoderm (DE) from human ESCs was initiated by initial induction conditions containing RPMI 1640 medium (Invitrogen, Carlsbad, CA) with 100 ng/mL Activin A (R&D Systems Inc., Minneapolis, MN) without fetal bovine serum (FBS) for 48 h and then the medium was changed to induction conditions, including RPMI medium with 100 ng/mL Activin A, 0.5 mM sodium butyrate, and 1 × B27 supplement (Invitrogen) for up to 6 days. DE cells were then split and reseeded on collagen I-coated six-well plates (BD Biosciences, San Diego, CA) in hepatocyte differentiation medium (HDM) until used. HDM contains Iscove's modified Dulbecco's medium (IMDM; Invitrogen) supplemented with 20% FBS (Invitrogen), 2 mM l-glutamine (Invitrogen), 0.3 mM 1-thioglycerol (Sigma-Aldrich, St. Louis, MO), 0.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich), 0.126 U/mL human insulin (Hospira, Inc.), FGF-4 (20 ng/mL), HGF (20 ng/mL), BMP2 (10 ng/mL), and BMP4 (10 ng/mL; R&D Systems, Inc.).
Drug preparation
Sal B, an effective water-soluble compound of Radix Salviae miltiorrhizae, was obtained from Shanghai Medical Research Institute, Chinese Academy of Science (Shanghai, China). The purity of Sal B was 99%, molecular weight 718, and molecular formula C36H30O16.
Treatment of differentiated cells with Sal B
To maintain dissolved Sal B in a sterile condition, Sal B was dissolved in DMSO. Sal B was used to treat the differentiating cells at day 8 after the DE cells were reseeded on collagen I-coated plates for differentiation at the final concentration of 1 μM for 6 days. The differentiating control cells were treated with the same concentration of DMSO only. All experiments were repeated at least thrice using independent cell culture.
Enzyme-linked immunosorbent assay analysis
After treatment with Sal B, the medium supernatant was collected every 48 h for determining human albumin (ALB) secretion into the medium using the Human ALB Enzyme-Linked Immunosorbent Assay (ELISA) Quantitation Kit (Bethyl, Inc.). The assay was performed according to the manufacturer's instructions.
Quantitative real-time reverse transcription polymerase chain reaction analysis
Differentiated cells treated with or without Sal B were harvested at day 14, and total RNA isolation, complementary DNA (cDNA) generation, and quantitative real-time polymerase chain reaction (qPCR) were carried out as previously described [5]. Primers or primers/probes used are listed in Supplementary Table S2.
Immunofluorescence staining
To evaluate ALB expression and the proliferation of human ESC-derived hepatocytes, immunofluorescence staining was used to detect ALB or/and Ki67. Briefly, differentiated cells were fixed with 4% paraformaldehyde and incubated at 4°C overnight with primary ALB or/and Ki67 antibodies. Then next day, cells were incubated in Cy3-conjugated donkey anti-goat IgG or Alexa Fluor® 488-conjugated goat anti-mouse IgG at room temperature for 1 h. Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI). Antibodies used are listed in Supplementary Table S3.
Western blot analysis
Differentiated cells were harvested and lysed in RIPA buffer containing 25 mM Tris, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 1% Triton X-100, pH 7–8 plus proteinase inhibitor cocktail, and phosphatase inhibitor cocktail (Millipore, Billerica, MA) at day 14 after differentiation. Thirty to fifty micrograms of total proteins or 5–10 μg of nuclear protein was used for western blot analysis as previously described [19]. Antibodies used are listed in Supplementary Table S3.
PCR array assays
Differentiated cells were harvested at day 14 after treatment with or without Sal B, and total RNA was isolated, and cDNAs were generated using the RT2 First Strand Kit (Qiagen, Valencia, CA). The Signal Transduction PathwayFinder PCR Array (PAHS-014Z; all from SABiosciences, Valencia, CA) was employed using the RT2 SYBR Green qPCR Mastermix (SABiosciences), and data were analyzed as indicated by the manufacturer.
Statistical analysis
All data are summarized as mean plus or minus standard deviation. An Unpaired Student t-test was used to analyze the data. P < 0.05 was considered statistically significant.
Results
Determining the working concentration of Sal B
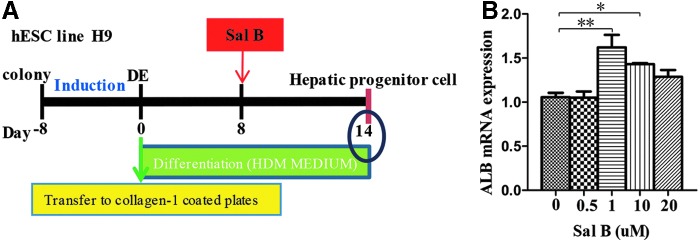
Hepatocyte differentiation of human ESCs was performed as previously described [5]. According to our previous study [18], the differentiating cells after the DE stage were treated at day 8 with Sal B for 6 days at the concentrations of 0.5, 1, 10, and 20 μM, respectively (Fig. 1A). We found that hepatocyte differentiation from human ESCs could be promoted at the concentrations of 1 and 10 μM Sal B, and the best concentration was 1 μM (Fig. 1B), thus, 1 μM Sal B or 1 μM DMSO was used to treat the cells in this study.
FIG. 1.
Treatment of the differentiated cells with different concentrations of Sal B. (A) Illustration of differentiation protocol with the addition of Sal B. (B) qPCR was used to determine the ALB expression of the differentiating cells after DE stage; cells were treated at day 8 after differentiation with Sal B for 6 days at the concentrations of 0.5, 1, 10, and 20 μM. ALB, albumin; DE, definitive endoderm; qPCR, quantitative real-time polymerase chain reaction; Sal B, salvianolic acid B. Data represent mean ± SEM. *P < 0.05, **P < 0.01.
Enhancement of hepatocyte differentiation from human ESCs by Sal B
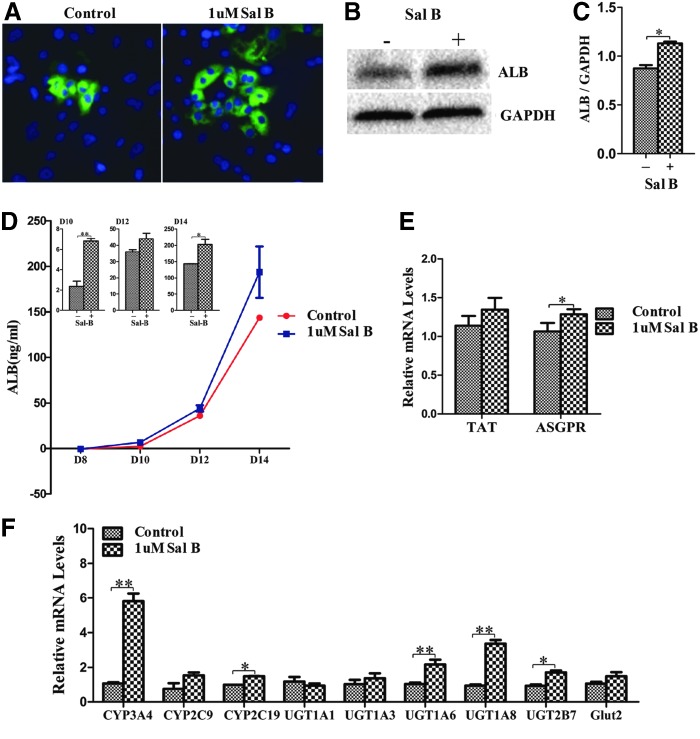
The differentiation process was enhanced with Sal B, as determined by the increase in ALB expression (Fig. 1B). Results of immunofluorescence staining showed that the number of ALB-positive cells was increased in treated cells compared to the untreated cells (Fig. 2A), and the increase of ALB expression was further confirmed by western blot (Fig. 2B, C). The functional assay showed that secreted ALB in the medium was increased during the period of treatment with Sal B as determined by ELISA (Fig. 2D). Expression of asialoglycoprotein receptor (ASGPR), an important marker of mature and functional hepatocytes, and tyrosine aminotransferase (TAT), another important functional enzyme, was also markedly increased in treated cells compared to the cells without treatment (Fig. 2E). Metabolic function is one of the most important actions of hepatocytes, in which metabolizing phase 1 enzyme cytochrome P450 (CYP), phase 2 enzyme UDP-glucuronosyl-S-transferase (UGT), and phase 3 transporters play key roles. We quantitatively evaluated the changes of several important phase 1, phase 2, and phase 3 metabolizing enzymes and proteins by the treatment of Sal B. qPCR results revealed that the expressions of CYP3A4, CYP2C19, UGT1A6, UGT1A8, and UGT2B7 were significantly higher in the treated cells compared to those in cells without treatment (Fig. 2F). Therefore, these results strongly demonstrated that hepatocyte differentiation from human ESCs was markedly enhanced at an early differentiation stage by treatment with Sal B.
FIG. 2.
Enhancement of hepatocyte differentiation from human ESC by Sal B. (A) Immunostaining was performed to detect the expression of ALB at day 14 after differentiation in the untreated (control) and the treated cells. (B) The expression of ALB protein was determined by western blot in the untreated and treated cells at day 14 after differentiation. (C) The expression of ALB protein was measured using histogram normalized to GAPDH based on the results of western blot. (D) The amount of secreted ALB in the medium was measured by ELISA analysis at days 8, 10, 12, and 14 after differentiation, and the secretion of ALB in treated cells exhibited significantly higher than those in untreated cells, which were shown by the panels of column graphs. (E) Relative expression of TAT and ASGPR was determined by qPCR in treated cells compared to untreated cells. (F) Relative expression levels of phase 1, 2, and 3 enzymes and proteins were determined by qPCR in the treated cells and compared to untreated cells. Data represent mean ± SEM. *P < 0.05, **P < 0.01. ASGPR, asialoglycoprotein receptor; ELISA, enzyme-linked immunosorbent assay; ESC, embryonic stem cell; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SEM, standard error of the mean; TAT, tyrosine aminotransferase.
Sal B enhanced the canonical Wnt signaling pathway
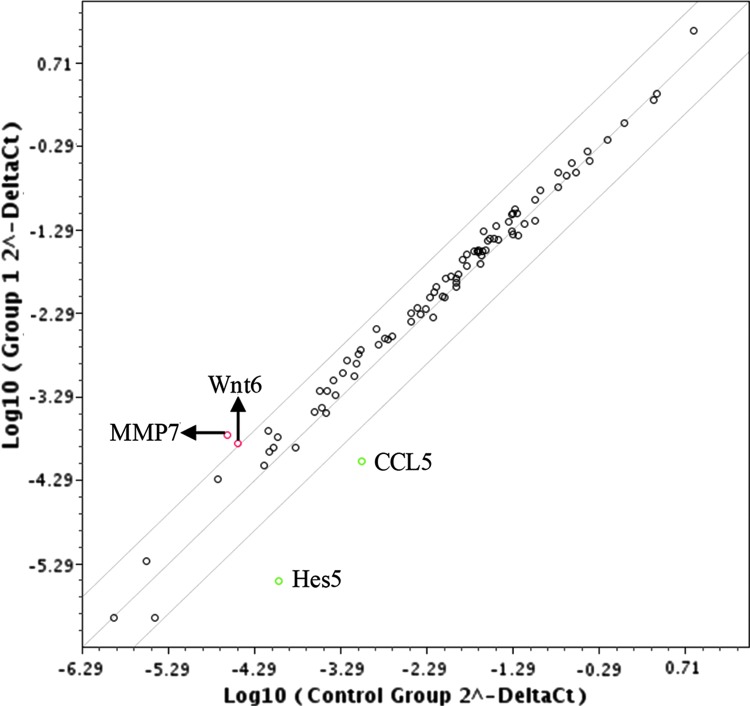
To investigate whether specific signaling pathways are involved in hepatocyte differentiation from human ESCs in the presence of Sal B, the Signal Transduction PathwayFinder PCR Array containing 84 genes representing 10 pathways was used to assess the effects of Sal B treatment. We found that the Wnt signaling- related proteins, Wnt6 and MMP7, were upregulated in Sal B treated cells (Fig. 3 and Supplementary Fig. S1), while Hes5 and CCL5, transcription factors of the Notch signaling pathway, were downregulated by Sal B. Normally Wnt and Notch signaling pathways are involved in proliferation and differentiation of stem cells; thus we sought to further characterize how these pathways were affected by Sal B. qPCR results showed that Wnt1, Wnt2, Wnt3, and Wnt7a were more highly expressed in Sal B-treated cells (Fig. 4A), and western blot results showed that Wnt1 was highly expressed in Sal B-treated cells (Fig. 4B, C), suggesting that Sal B treatment enhanced Wnt signaling pathways during the process of hepatocyte differentiation.
FIG. 3.
The determination of affected signaling pathways by the treatment with Sal B. PCR Array revealed upregulation of the Wnt signaling pathway and downregulation of the Notch signaling pathway (the details of expression of 84 genes representing 10 signaling pathways are listed in Supplementary Fig. S1).
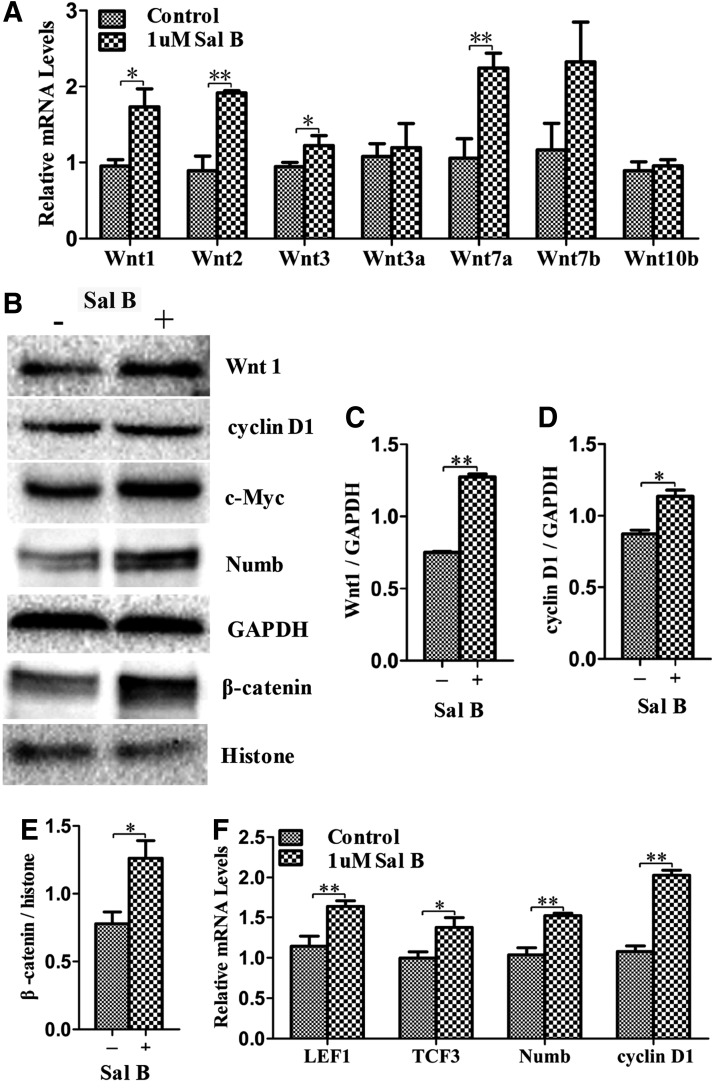
FIG. 4.
Wnt/β-catenin pathway was activated by Sal B during differentiation. (A) Relative expression of genes from Wnt ligands was determined by qPCR at day 14 of differentiation in the treated and untreated cells. (B) Expression of Wnt1, cyclin D1, c-Myc, Numb, and β-catenin was detected by western blot in the treated and untreated cells. (C, D) Wnt1 and cyclin D1 protein expression was measured using histogram normalized to GAPDH after calculation based on the results of western blot. (E) The amount of nuclear beta-catenin protein was measured using histogram normalized to histone protein after calculation based on the results of western blot. (F) Relative expressions of LEF1, TCF3, Numb, and cyclin D1 were determined by qPCR in the treated cells and compared to the untreated cells at day 14 of differentiation. Data represent mean ± SEM. *P < 0.05, **P < 0.01. LEF, lymphoid enhancer-binding factor; TCF, T cell factor.
β-catenin nuclear translocation and TCF and LEF upregulation have been shown to be involved in canonical Wnt pathways [20], and in our studies, western blot results showed that the amount of β-catenin protein in nuclei was increased in treated cells (Fig. 4B, E) and that the expression of LEF1 and TCF3, β-catenin target genes, was also increased (Fig. 4F). We further evaluated TCF3 downstream targets such as cyclin D1 and c-Myc, which facilitate cell differentiation. Both qPCR results and western blots revealed that both cyclin D1 and c-Myc were more highly expressed in treated cells compared to untreated cells (Fig. 4B, D, F). Numb mediates the interaction between Wnt and Notch as an interruption point of the Wnt-Notch signaling cycle [21,22]. Western blot and qPCR analysis showed that the expression of Numb was upregulated (Fig. 4B, F), and this upregulation is consistent with the activation of canonical Wnt signaling and suppression of Notch signaling.
The Notch signaling pathway was suppressed by Sal B
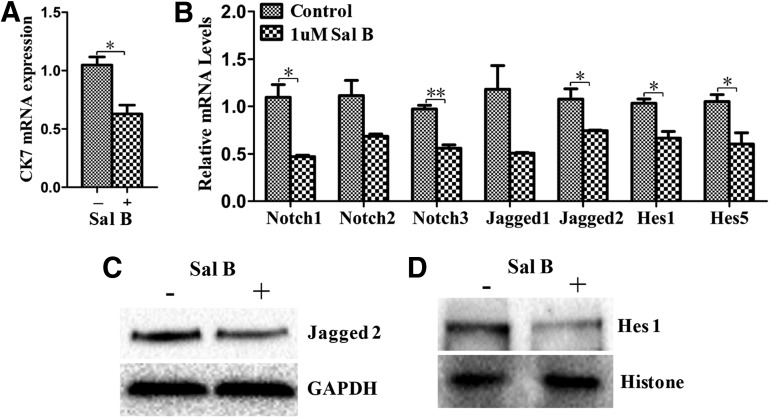
Consistent with the result of the Signal Transduction PathwayFinder PCR Array, the expression of Hes5 was decreased in treated cells as determined by qPCR (Fig. 5B). We further determined the proteins associated with the Notch signaling pathway. Notch receptor, Notch1 and Notch3, Notch ligand, Jagged2, and the Notch target gene, Hes1, were downregulated in treated cells compared to those in the untreated cells (Fig. 5B). Western blot results further confirmed that Hes1 and Jagged2 were downregulated in treated cells (Fig. 5C, D); these results suggest that the Notch signaling was suppressed in the Sal B-treated cells. The Notch signaling pathway is necessary for specification of the biliary tree, and Notch pathway inhibition results in failure of hepatoblast specification into cholangiocytes [23,24]. Thus, we investigated the expression of cholangiocyte marker, CK7, by qPCR, which was significantly decreased in the treated cells compared to the untreated cells (Fig. 5A). These results suggest that one of the mechanisms of promoting hepatocyte differentiation by Sal B may be the inhibition of the cholangiocyte fate through suppression of the Notch pathway.
FIG. 5.
Notch signaling pathway was suppressed by Sal B to inhibit the formation of cholangiocytes during differentiation. (A) The relative expression of cholangiocyte marker CK7 was determined by qPCR in the treated cells and compared to the untreated cells at day 14 of differentiation. (B) The relative expression of Notch receptor, Notch1, Notch 2, and Notch3, Notch ligand, Jagged1 and Jagged2, and Notch target genes, Hes1 and Hes5, was detected by qPCR in the treated cells and compared to the untreated cells at day 14 of differentiation. (C, D) The protein expressions of Jagged2 (C) and Hes1 (D) were detected by western blot in the treated cells and the untreated cells at day 14 of differentiation. Data represent mean ± SEM. *P < 0.05, **P < 0.01. Hes, hairy and enhancer of split.
Promotion of hepatocyte proliferation by Sal B
Human primary hepatocytes lose the proliferative capacity rapidly in in vitro culture; this phenomenon hinders the use of hepatocytes. In the aforementioned analysis of the activation of Wnt signaling, TCF1 upregulated its downstream target genes, cyclin D1 and c-myc; both genes facilitate cell proliferation, suggesting that Sal B potentially promoted human ESC-derived hepatocytes. After treatment with Sal B, the proliferative capacity of human ESC-derived hepatocytes was enhanced in the treated cells as indicated by increasing numbers of cells that were double positive for ALB and Ki67 at day 14 after the differentiation, as determined by immunofluorescence staining (Fig. 6); thus, the result of immunostaining assay was consistent with those of the analysis for the activation of Wnt pathway.
FIG. 6.
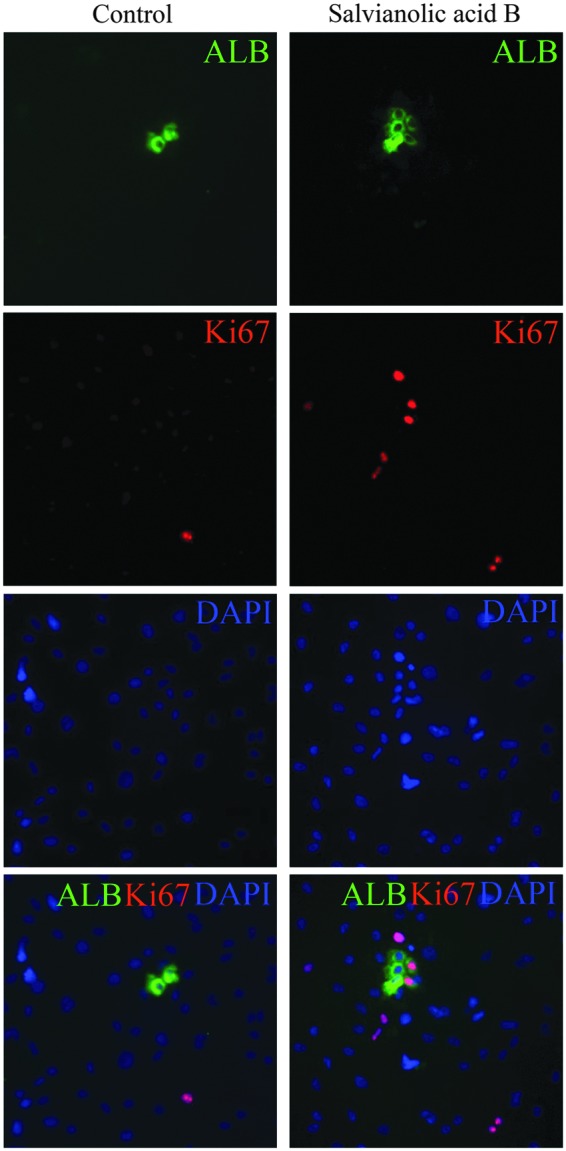
Enhancement of hepatocyte proliferation by Sal B. Double-immunostaining was performed to detect coexpression of ALB and Ki67 at days 14 after the differentiation in the untreated cells and the treated cells. Left panel: control—the untreated cells; right panel: salvianolic acid B—the treated cells with Sal B.
Discussion
Mimicking liver development, we have developed an efficient protocol to generate metabolically functioning hepatocytes from human ESCs [5], and these hepatocytes exhibit in vivo function shown by engrafting and proliferating in mouse livers [25]. Our results are encouraging; however, the differentiated cells are not completely mature hepatocytes. Therefore, we and others turned to the study of optimizing the protocol of hepatocyte differentiation from human ESCs to obtain more mature hepatocytes by small molecules [26], Chinese medicine [18], coculture with other liver cells [27], three-dimensional differentiation configurations [28,29], etc. Recently, we found that the traditional Chinese Medicine FZHY not only promoted hepatocyte differentiation and maturation from human ESCs but also enhanced hepatocyte proliferation [18], suggesting that some traditional Chinese medicines may contribute to enhanced hepatocyte differentiation and maturation from human ESCs.
Sal B is the ingredient of greatest concentration and with the most activity of the phenolic acids of Radix Salviae miltiorrhizae [30]. Moreover, it has been reported to regulate the proliferation and differentiation of many kinds of stem cells. Gao et al. found that when Sal B was combined with 5-azacytidine and cardiomyocyte lysis medium, it improved cardiomyocyte differentiation from mesenchymal stem cells [31]. Xu et al. revealed that Sal B promoted osteogenesis of human mesenchymal stem cells through activating the ERK signaling pathway [32]. Zhang et al. reported that Sal B facilitated the survival of bone marrow derived-neural stem cells (BM-NSCs), reduced lactate dehydrogenase leakage, inhibited apoptosis, and induced brain-derived neurotrophic factor production by BM-NSCs. And it was thought to be beneficial for the cells' survival and differentiation in an unfavorable environment [12]. Zhuang et al. demonstrated that Sal B helped maintain the self-renewal of neural stem/progenitor cells, and it also promoted proliferation [10].
In this study, we determined that Sal B promoted the process of hepatocyte differentiation from human ESCs under our differentiation conditions, including an increase of ALB expression as determined by qPCR, western blot, and immunofluorescence staining. These results were further confirmed by an increase of ALB secretion into the media. After treatment with Sal B, the ASGPR expression was also markedly increased. Our results also showed that the expression of the metabolizing enzymes of differentiated cells was significantly increased in the Sal B-treated cells, indicating that they were more mature.
The Wnt/β-catenin signaling pathway appears to play an important role in regulating the stemness, proliferation, and differentiation of stem cells [33,34], especially in liver development. However, the effect of the Wnt signaling pathway on liver development is very complex [35]. For example, the activation or inhibition of the Wnt pathway has been used to direct differentiation of human PSCs into intestinal cells [36] and foregut derivatives [37], respectively. And investigations in rodents elucidated the role of Wnt1 in directing stem cells to differentiate to hepatocytes during liver regeneration after liver injury in rats [38]. Our latest study revealed that traditional Chinese medicine Fuzheng Huayu can enhance hepatocyte differentiation and maturation from human ESCs by the activation of the canonical Wnt signaling pathway [18].
In this study, it was found that the expressions of Wnt1, Wnt2, Wnt3, and Wnt7a were markedly increased after treatment with Sal B and that the level of β-catenin in nuclei was increased in treated cells. Upon Wnt activation, cytoplasmic β-catenin is stabilized and enters the nucleus, where it associates with transcription factors, notably TCF and LEF, to regulate the transcription of target genes, which in turn are prominently involved in regulating cell differentiation [39,40]. As expected, the expression of LEF1 and TCF3, and the target genes, cyclin D1 and c-Myc, which facilitate cell proliferation [38], as well as Numb, were significantly increased in Sal B-treated cells. These results suggest that Sal B enhances both the hepatocyte differentiation from human ESCs and proliferation through activation of the Wnt/β-catenin signaling pathway (Figs. 6 and 7). The hepatocytes lack the proliferative capacity in in vitro culture, and this hinders its use in in vitro analysis; thus, Sal B would potentially help produce proliferating hepatocytes for in vitro analysis.
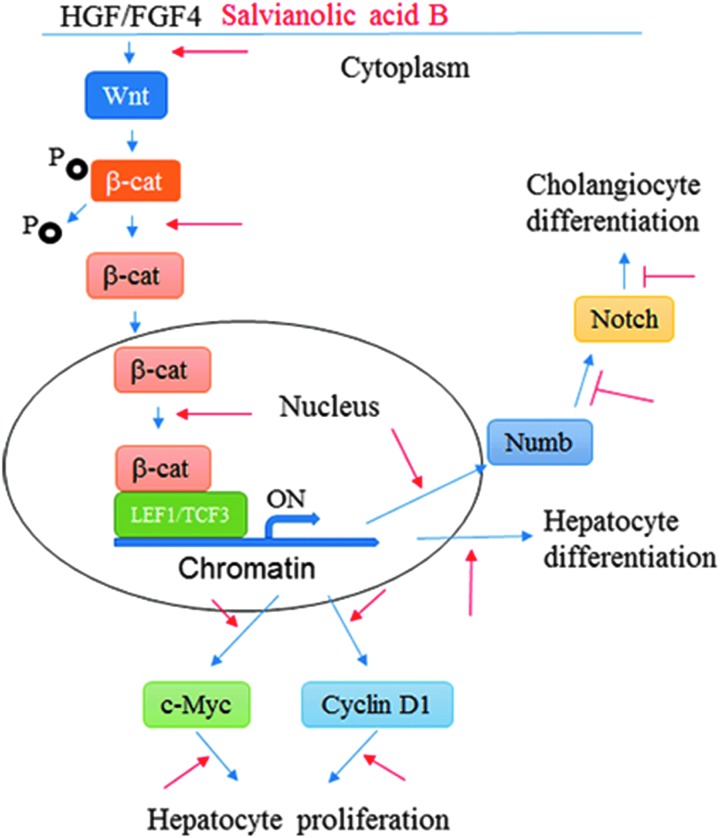
FIG. 7.
Illustration of the effects of Sal B on hepatocyte differentiation from human ESC. Under the culture conditions with HGF and FGF4, the Wnt signaling pathway was activated to regulate hepatocyte differentiation from human ESC. Free β-catenin after dephosphorylation was translocated into the nucleus where β-catenin binds to LEF1/TCF3 to trigger the expressions of the downstream genes, including those that promote hepatocyte differentiation, c-Myc and cyclin D1, which facilitate cell proliferation, as well as Numb, which acts as a negative regulator of the Notch signaling pathway to inhibit cholangiocyte differentiation, is also upregulated. Thus, Sal B treatment upregulates the Wnt signaling pathway (red arrow) to enhance hepatocyte differentiation and downregulates the Notch signaling pathway to inhibit cholangiocyte differentiation (red T).
The Notch signaling pathway is necessary for specification of the biliary tree, and Notch pathway ablation results in the failure of hepatoblast specification to cholangiocytes [24,41]. Touboul et al. found that inhibition of the Notch signaling pathway in the process of differentiation of human ESCs into hepatocytes was associated with rapid upregulation of ALB expression and maintenance of high levels of CEBPA and HNF1a expression, as well as the downregulation of the cholangiocyte-associated transcription factor SOX9 [7]. Notch receptors (Notch1–4) and Notch ligands (DDLs, Jagged1 and 2) are highly expressed during biliary regeneration of mouse hepatic progenitor cells, indicating that the Notch pathway was activated [42], and this activation was confirmed by greater expression of the Notch receptor targets Hes1 and Hes5 during biliary regeneration in comparison to hepatocyte regeneration [41].
In our study, the Notch signaling pathway was suppressed by Sal B during the hepatocyte differentiation from human ESCs based on the finding that the expression of Notch1, Notch3, Jagged2, Hes1, and Hes5 was markedly downregulated in the Sal B-treated cells. Furthermore, we found that Sal B inhibited the differentiation of cholangiocyte as demonstrated by a decrease of CK7 expression. Numb is a direct transcriptional target of the Wnt signaling pathway and is a negative regulator of the Notch pathway [42,43]; thus, Numb is a key mediator of the coordinated interaction of the Wnt and Notch pathways [21,22]. In our study, upon Wnt/β-catenin signaling pathway activation after treatment with Sal B, the Numb expression was upregulated, further negatively regulating the Notch pathway, which in turn resulted in inhibition of the biliary differentiation of human ESCs (Fig. 7).
In conclusion, the results of this study demonstrated that Sal B treatment can enhance hepatocyte differentiation and proliferation from human ESCs through the activation of the Wnt/β-catenin signaling pathway and the inhibition of the Notch pathway to suppress the formation of cholangiocytes. In addition, Numb, a key mediator of the coordinated interaction of the Wnt and Notch pathways, was upregulated by Sal B. Therefore, the present study suggests that Sal B may be used as a potential agent to generate more mature hepatocytes for cell-based therapeutics for liver diseases and for pharmaceutical study.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant DK075415 (to M.A.Z.), by Research Agreement among Shanghai Sundise Traditional Chinese Medicine Co., Ltd., Shanghai University of Traditional Chinese Medicine, and the Regents of the University of California (agreement no. 201302245; to M.A.Z.), by National Natural Science Foundation of China (grant no. 81530101; to P.L.), and by National Natural Science Foundation of China (grant no. 81603465; to J.C.). All work in this study was performed at the University of California, Davis.
Author Disclosure Statement
Competing financial interests exist: M.A.Z. had a sponsored research agreement among Shanghai Sundise Traditional Chinese Medicine Co., Ltd. (a commercial source), Shanghai University of Traditional Chinese Medicine, and the Regents of the University of California, in which Shanghai Sundise Traditional Chinese Medicine Co., Ltd. provided some funding for this research. There has been no perceived conflict of interest in the work yielding this article.
References
- 1.Bataller R. and Brenner DA. (2005). Liver fibrosis. J Clin Invest 115:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jadlowiec CC. and Taner T. (2016). Liver transplantation: current status and challenges. World J Gastroenterol 22:4438–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coilly A, Roche B, Duclos-Valléé JC. and Samuel D. (2016). News and challenges in the treatment of hepatitis C in liver transplantation. Liver Int 36 (Suppl. 1):34–42 [DOI] [PubMed] [Google Scholar]
- 4.Cho CH, Berthiaume F, Tilles AW. and Yarmush ML. (2008). A new technique for primary hepatocyte expansion in vitro. Biotechnol Bioeng 101:345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan Y, Ma X, Wang C, Bahbahan IS, Ahuja TP, Tolstikov V. and Zern MA. (2010). Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells 28:674–686 [DOI] [PubMed] [Google Scholar]
- 6.Ma X, Duan Y, Tschudy-Seney B, Garrett R, Behbahan IS, Ahujia TP, Tolstikov V, Wang C, McGee J, et al. (2013). Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem Cells Transl Med 2:409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touboul T, Chen S, To CC, Mora-Castilla S, Sabatini K, Tukey RH. and Laurent LC. (2016). Stage-specific regulation of the WNT/beta-catenin pathway enhances differentiation of hESCs into hepatocytes. J Hepatol 64:1315–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Wang L, Yan X, Wang Q, Tao Y, Li J, Peng Y, Liu P. and Liu C. (2012). Salvianolic acid B attenuates rat hepatic fibrosis via downregulating angiotensin II signaling. Evid Based Complement Alternat Med 2012:160726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P, Hu YY, Liu C, Zhu DY, Xue HM, Xu ZQ, Liu CH, Gu HT. and Zhang ZQ. (2002). Clinical observation of salvianolic acid B in treatment of liver fibrosis in chronic hepatitis B. World J Gastroenterol 8:679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang P, Zhang Y, Cui G, Bian Y, Zhang M, Zhang J, Liu Y, Yang X, Isaiah AQ, Lin Y. and Jian Y. (2012). Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS One 7:e35636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang CY, Chen SY, Fu RH, Huang YC, Chen SY, Shyu WC, Lin SZ. and Li SP. (2015). Differentiation of embryonic stem cells into cardiomyocytes used to investigate the cardioprotective effect of salvianolic acid B through BNIP3 involved pathway. Cell Transplant 24:61–71 [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Kang T, Xia Y, Wen Q, Zhang X, Liu H, Hu Y, Hao H, Zhao D, et al. (2012). Effects of salvianolic acid B on survival, self-renewal and neuronal differentiation of bone marrow derived neural stem cells. Eur J Pharmacol 697:32–39 [DOI] [PubMed] [Google Scholar]
- 13.Jia Y, Wang RQ, Mi HM, Kong LB, Ren WG, Li WC, Zhao SX, Zhang YG, Wu WJ, Nan YM. and Yu J. (2012). Fuzheng Huayu recipe prevents nutritional brosing steatohepatitis in mice. Lipids Health Dis 11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang QL, Yuan JL, Tao YY, Zhang Y, Liu P. and Liu CH. (2010). Fuzheng Huayu recipe and vitamin E reverse renal interstitial brosis through counteracting TGF-beta1-induced epithelial-to-mesenchymal transition. J Ethnopharmacol 127:631–640 [DOI] [PubMed] [Google Scholar]
- 15.Yang T, Shen DP, Wang LQ, Tao YY. and Liu CH. (2013). Investigation of the absorbed and metabolized components of Danshen from Fuzheng Huayu recipe and study on the anti-hepatic fibrosis effects of these components. J Ethnopharmacol 148:691–700 [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Hu Y, Xu L, Liu C. and Liu. P. Effect of Fuzheng Huayu formula and its actions against liver brosis. Chin Med 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L. and Schuppan D. (2014). Traditional Chinese medicine (TCM) for brotic liver disease: hope and hype. J Hepatol 61:166–168 [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Gao W, Zhou P, Ma X, Tschudy-Seney B, Liu C, Zern MA, Liu P. and Duan Y. (2016). Enhancement of hepatocyte differentiation from human embryonic stem cells by Chinese medicine Fuzhenghuayu. Sci Rep 6:18841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaret KS. and Grompe M. (2008). Generation and regeneration of cells of the liver and pancreas. Science 322:1490–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clevers H. and Nusse R. (2012). Wnt/beta-catenin signaling and disease. Cell 149:1192–1205 [DOI] [PubMed] [Google Scholar]
- 21.Cheng X, Huber TL, Chen VC, Gadue P P. and Keller GM. (2008). Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development 135:3447–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh M. and Katoh M. (2006). NUMB is a break of WNT-Notch signaling cycle. Int J Mol Med 18:517–521 [PubMed] [Google Scholar]
- 23.Tanimizu N. and Miyajima A. (2004). Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci 117 (Pt. 15):3165–3174 [DOI] [PubMed] [Google Scholar]
- 24.McCright B, Lozier J. and Gridley T. (2002). A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development 129:1075–1082 [DOI] [PubMed] [Google Scholar]
- 25.Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gambhir SS. and Zern MA. (2007). Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells 25:3058–3068 [DOI] [PubMed] [Google Scholar]
- 26.Magner NL, Jung Y, Wu J, Nolta JA. Zern MA. and Zhou P. (2013). Insulin and IGFs enhance hepatocyte differentiation from human embryonic stem cells via the PI3K/AKT pathway. Stem Cells 31:2095–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soto-Gutierrez A, Navarro-Alvarez N, Zhao D, Rivas-Carrillo JD, Lebkowski J, Tanaka N, Fox IJ. and Kobayashi N. (2007). Differentiation of mouse embryonic stem cells to hepatocyte-like cells by co-culture with human liver nonparenchymal cell lines. Nat Protoc 2:347–356 [DOI] [PubMed] [Google Scholar]
- 28.Yao R, Wang J, Li X, Jung Jung D, Qi H, Kee KK. and Du Y. (2014). Hepatic differentiation of human embryonic stem cells as microscaled multilayered colonies leading to enhanced homogeneity and maturation. Small 10:4311–4323 [DOI] [PubMed] [Google Scholar]
- 29.Takayama K, Kawabata K, Nagamoto Y, Kishimoto K, Tashiro K, Sakurai F, Tachibana M, Kanda K, Hayakawa T, Furue MK. and Mizuguchi H. (2013). 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 34:1781–1789 [DOI] [PubMed] [Google Scholar]
- 30.Cao W, Guo XW, Zheng HZ, Li DP, Jia GB. and Wang J. (2012). Current progress of research on pharmacologic actions of salvianolic acid B. Chin J Integr Med 18:316–320 [DOI] [PubMed] [Google Scholar]
- 31.Gao Q, Guo M, Jiang X, Hu X, Wang Y. and Fan Y. (2014). A cocktail method for promoting cardiomyocyte differentiation from bone marrow-derived mesenchymal stem cells. Stem Cells Int 2014:162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu D, Xu L, Zhou C, Lee WY, Wu T, Cui L. and Li G. (2014). Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int J Biochem Cell Biol 51:1–9 [DOI] [PubMed] [Google Scholar]
- 33.Niehrs C. (2012). The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 13:767–779 [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Zhao L, Fish M, Logan CY. and Nusse R. (2014). Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 524:180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Si-Tayeb K, Lemaigre FP. and Duncan SA. (2010). Organogenesis and development of the liver. Dev Cell 18:175–189 [DOI] [PubMed] [Google Scholar]
- 36.Fordham RP, Yui S, Hannan NR, Soendergaard C, Madgwick A, Schweiger PJ, Nielsen OH, Vallier L, Pedersen RA, et al. (2013). Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13:734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loh KM, Ang LT, Zhang J, Kumar V, Ang J, Auyeong JQ, Lee KL, Choo SH, Lim CY, et al. (2014). Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell 14:237–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams JM, Oh SH, Jorgensen M, Steiger N, Darwicher H, Shupe T. and Petersen BE. (2010). The role of the Wnt family of secreted proteins in rat oval “stem” cell-based liver regeneration: Wnt1 drives differentiation. Am J Pathol 176:2732–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray J. and Hartmann C. (2012). WNTing embryonic stem cells. Trends Cell Biol 22:159–168 [DOI] [PubMed] [Google Scholar]
- 40.Reya T. and Clevers H. (2005). Wnt signalling in stem cells and cancer. Nature 434:843–850 [DOI] [PubMed] [Google Scholar]
- 41.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, et al. (2012). Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGill MA, Dho SE, Weinmaster G. and McGlade CJ. (2009). Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem 284:26427–26438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spana EP. and Doe CQ. (1996). Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17:21–26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.