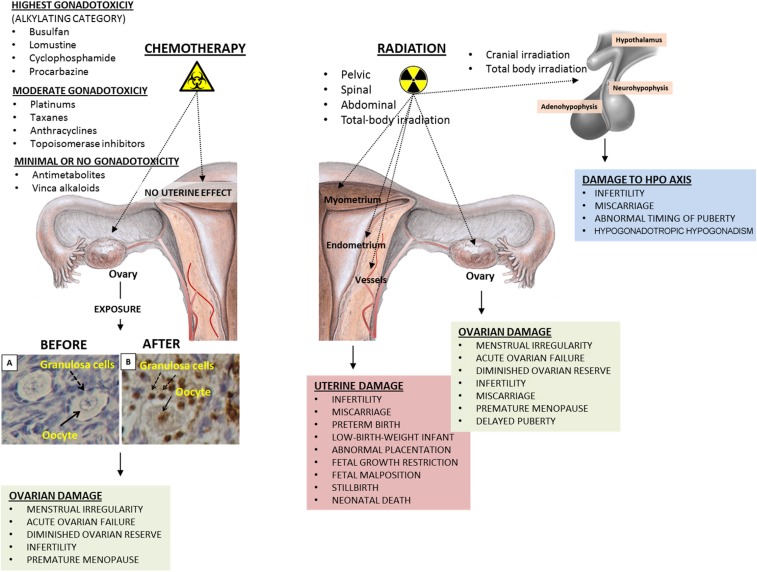
This article provides a narrative update on the impact of chemotherapy and radiation on the ovarian and uterine function in female survivors exposed to chemotherapy and radiation during childhood.
Keywords: Childhood cancer survivors, Ovarian function, Uterine function, Fertility preservation, Pediatric cancers, Reproductive outcome, Obstetrical outcome, Chemotherapy, Radiation
Abstract
Adult survivors of childhood cancers are more prone to developing poor reproductive and obstetrical outcomes than their siblings and the general population as a result of previous exposure to chemotherapy and radiation during childhood. Chemotherapy drugs exert cytotoxic effects systemically and therefore can damage the ovaries, leading to infertility, premature ovarian failure, and, to a lesser extent, spontaneous abortions. They have very limited or no deleterious effects on the uterus that can be recognized clinically. By contrast, radiation is detrimental to both the ovaries and the uterus, thereby causing a greater magnitude of adverse effects on the female reproductive function. These include infertility, premature ovarian failure, miscarriage, fetal growth restrictions, perinatal deaths, preterm births, delivery of small‐for‐gestational‐age infants, preeclampsia, and abnormal placentation. Regrettably, the majority of these adverse outcomes arise from radiation‐induced uterine injury and are reported at higher incidence in the adult survivors of childhood cancers who were exposed to uterine radiation during childhood in the form of pelvic, spinal, or total‐body irradiation. Recent findings of long‐term follow‐up studies evaluating reproductive performance of female survivors provided some reassurance to female cancer survivors by documenting that pregnancy and live birth rates were not significantly compromised in survivors, including those who had been treated with alkylating agents and had not received pelvic, cranial, and total‐body irradiation. We aimed in this narrative review article to provide an update on the impact of chemotherapy and radiation on the ovarian and uterine function in female survivors of childhood cancer.
Implications for Practice.
Adult survivors of childhood cancers are more prone to developing a number of poor reproductive and obstetrical outcomes than their siblings and the general population as a result of previous exposure to chemotherapy and radiation during childhood. The impact of radiation therapy on the female genital system is greater than chemotherapy regimens because radiation is detrimental to both the uterus and the ovaries, whereas toxic effects of chemotherapy drugs are confined to the ovaries. Therefore, radiation‐induced uterine damage accounts for most poor obstetrical outcomes in the survivors. These include infertility, miscarriages, stillbirths, fetal growth restrictions, preeclampsia, and preterm deliveries.
Introduction
The most common cancer types in children up to 14 years of age are leukemias (acute lymphocytic leukemia), tumors of the nervous system (21%), soft tissue sarcomas (neuroblastoma and rhabdomyosarcoma), renal (Wilms) tumors (5%), and non‐Hodgkin lymphoma (4%) [1]. The overall 5‐year relative survival rate for all childhood cancers (in children aged birth to 14 years) has significantly improved over the last 30 years, from 58% to 83%, because of new and improved treatments [2]. The most significant increase in the survival rate was observed in non‐Hodgkin lymphoma (increased from 43% to 88%, a difference of 45%), followed by acute myeloid leukemia (41%) and acute lymphocytic leukemia (31%). Overall, Hodgkin lymphoma has the highest 5‐year survival rate among all pediatric cancers (98%) [3]. Improved survival has given rise to a special population: adult survivors of childhood cancer. Regrettably, a wide range of adverse health conditions emerge in these survivors, varying from metabolic and endocrine problems to cognitive defects, as a result of previous exposure to cytotoxic chemotherapy regimens and radiation [4], [5]. Ovarian insufficiency and other poor reproductive and obstetrical outcomes are other long‐term sequelae of cancer treatment in survivors. Cytotoxic chemotherapy regimens and radiotherapy induce genomic damage and apoptosis in the oocytes and somatic cells in the ovary, leading to early exhaustion of the follicle stockpile and premature ovarian failure [6]. Unfortunately, end organ damage after cancer treatment is not limited to the ovary in the female reproductive system. The uterus and pelvic structures are also impacted during pelvic, spinal, and total‐body irradiation (TBI). As a result, there is an increased risk of pregnancy complications, including early pregnancy loss, preterm birth, fetal growth restrictions, and delivery of low‐ or very low‐birth‐weight infants in fertile survivors of childhood cancers who were exposed to uterine radiation [7]. Besides cancers, some pediatric patients with certain precancerous and nonmalignant illnesses, such as myelodysplasia, aplastic anemia, thalassemia, and systemic lupus erythematosus, need to undergo high‐dose gonadotoxic chemotherapy with or without TBI for the treatment of their primary diseases [4]. Therefore, preservation of gonadal function and fertility has become one of the major quality‐of‐life issues for pediatric and adult patients with cancer. Accordingly, clinical guidelines on fertility preservation strategies in young cancer survivors have been issued by the different societies of oncology and reproductive medicine [8], [9], [10], [11]. We aimed to provide in this article a narrative update on the impact of chemotherapy and radiation on the ovarian and uterine function in female survivors exposed to chemotherapy and radiation during childhood.
Materials and Methods
We searched published studies in the PubMed database using the relevant keywords (adult female survivors, pediatric cancers, chemotherapy, radiation, ovarian function, uterine function, fertility preservation, fertility). Full‐text articles of case control or cohort studies and their follow‐up interim reports were included. Furthermore, reference lists of main reports and systematic and narrative review articles were also reviewed to identify additional relevant publications.
Mechanism of Detrimental Effects of Chemotherapy and Radiation on the Female Reproductive System
Chemotherapy
The ovary is the most sensitive organ to chemotherapy drugs in the female reproductive system (Fig. 1). By contrast, uterine function does not appear to be compromised by chemotherapy agents. Among chemotherapy agents, those in the alkylating category have the most detrimental effects on the ovary. They cause massive follicle loss by inducing genomic damage in the oocyte and somatic cells of dormant primordial follicles and growing follicles [12], [13]. Emerging data suggest that there are some other mechanisms that contribute to the gonadotoxic effects of chemotherapy drugs on the ovary, in addition to their direct toxic effects on the oocyte. One of these proposed mechanisms involves vascular damage. It was shown by different groups that chemotherapy drugs injure blood vessels in the cortical and medullary portions of the human ovary, which results in obliteration, fibrosis, and decreases in the expression of vascular endothelial growth factor and microvascular intensity [14], [15]. These pathological alterations may further aggravate follicle loss and accelerate aging of the ovary. The second mechanism is the so‐called burn‐out phenomenon. It was hypothesized that cyclophosphamide exposure activates the phosphatidylinositol 3‐kinase signaling pathway, which in turn causes premature activation of primordial follicles and hence “burns out” or causes early depletion of the follicle pool [16]. It was also suggested as a third possible mechanism, at least in the mouse ovary, that chemotherapy drugs may induce different mechanisms of follicle loss. Exposure to cisplatin caused oocyte‐specific damage, whereas doxorubicin preferentially induced damage to the mitotic granulosa cells of secondary follicles. In support of the burn‐out theory, the investigators also observed that primordial follicles were lost after exposure to both drugs, and only a few of them were positive after staining with apoptosis marker terminal deoxynucleotidyl transferase (TdT) dUTP nick‐end labeling (TUNEL), suggesting that the reduction in primordial follicle numbers after chemotherapy exposure is due to accelerated growth initiation rather than a direct toxic effect of the drugs [17]. Last, an autopsy series of young girls who had been treated with cytotoxic drugs for leukemia and other childhood cancers demonstrated no development or arrested follicle growth, atresia of small‐sized follicles, and a marked decrease in the size and number of antral follicles [18]. Taken together, these findings suggest that there could be multiple distinct pathogenetic mechanisms underlying chemotherapy‐induced gonadotoxicity and ovarian failure.
Figure 1.
The impact of chemotherapy drugs and radiation on the female reproductive system. Chemotherapy drugs, particularly those in the alkylating category, are detrimental to the ovaries. Genomic damage in the oocyte and surrounding granulosa cells results in apoptotic death of the follicle, leading to a decrease or total exhaustion of the follicle pool, depending upon the magnitude of ovarian damage. Uterine function does not appear to be affected by chemotherapy regimens, regardless of their cytotoxic potential. By contrast, radiation impacts not only the ovary but also the uterus and the HPO axis. Uterine exposure to radiation during pelvic, spinal, abdominal, or total‐body irradiation causes irreversible damage to the endometrium, myometrial layer, and vascular structures, causing a wide range of adverse reproductive and obstetrical outcomes. The functions of the HPO axis are perturbed after total‐body and cranial irradiation, which may result in abnormal timing of puberty, infertility, and hypogonadotropic hypogonadism.
Abbreviation: HPO, hypothalamic‐pituitary ovarian.
Alkylating agents, such as busulfan, cyclophosphamide, lomustine, and procarbazine, have the highest gonadotoxic potential. Cytotoxic actions of drugs in this category are not cell‐cycle specific. Therefore, they are able to produce a greater magnitude of damage by affecting cells at every stage of the cell cycle. They alter base pairs, produce DNA cross‐links (guanosine adducts), and cause single‐ and double‐strand breaks in DNA [19]. Unfortunately, many of these alkylating drugs are included in the first‐line therapy of many solid and hematological malignancies affecting children, such as Hodgkin lymphoma [20]. High‐dose cyclophosphamide (120–200 mg/kg) with and without busulfan (8–16 mg/kg) is commonly used as conditioning therapy before bone marrow transplantation [21]. DNA interstrand cross‐linking drugs (cisplatin and carboplatin) closely follow the alkylating agents with regard to gonadotoxicity and are called “alkylating‐like agents.” Drugs in these categories destroy both the primordial follicle pool and the growing follicle fraction in the human ovary, thereby creating more extensive gonadal damage [15]. By contrast, microtubule depolymerization inhibitors (taxanes) and DNA intercalating agents (anthracyclines) are less toxic than alkylating agents and platinum compounds and are therefore considered to have moderate gonadotoxicity. The members of the antimetabolite group, such as methotrexate and 5‐fluorouracil, and vinca alkaloids (vincristine and vinblastine) have minimal or no gonadotoxicity. The growing follicle fraction, preantral and antral follicles with their mitotic granulosa cell layer, is more sensitive to the cytotoxic actions of mitosis‐specific drugs, possibly due to their higher mitotic rate and increased metabolic demand. Chemotherapy drugs are generally administered in combinations, precluding the estimation of individual effects of each drug in a combination regimen. Total dose, dose density, and ovarian reserve of the patient prior to initiating chemotherapy are other confounders that modify the gonadotoxic effect of a given drug. In other words, drugs that are thought to have minimal or moderate gonadal toxicity can induce a significant degree of ovarian damage and jeopardize future fertility if they are used for longer period of time and/or at higher doses, especially in patients with poor ovarian reserve prior to initiating chemotherapy.
Radiation
Direct cytotoxic action of radiation on the genome of the cell is the predominant mechanism of damage for particle radiation. Genomic damage mainly occurs as double‐strand breaks in DNA and leads to irreversible loss of vital homeostatic functions and eventual death of the cell. There are also indirect cytotoxic actions of radiation that come from its interaction with other substances in the cell, such as water. Radiolysis of cellular water leads to formation of free radicals, which further amplifies the damage in the DNA and organelles of the cell. This mechanism is particularly true for ionizing radiation, such as x‐rays.
Mitotically active cells are generally more vulnerable to cytotoxic actions of radiation because of active DNA replication, whereas those with low metabolic and mitotic division rates and hypoxic cells are more resistant to it. The oocyte is one notable exception to this principle. Even though the cell cycle is arrested at the diplotene stage of prophase I of the first meiotic division in the human oocyte, it is somehow exquisitely sensitive to radiation. Lethal dose (LD50), the radiation dose required to destroy 50% of the human oocytes, is ∼2 Gy [22]. It is currently unclear why such an interphase nucleus is so sensitive to radiation. It was believed in the past that the oocyte does not have the enzymatic repair capacity to counteract the modifications caused to its DNA by ionizing radiation and/or chemicals. But emerging evidence from animal studies in mouse and guinea pig ovaries showed that the mammalian oocyte is indeed able to repair DNA damage induced by radiation and that radiosensitivity of ovarian follicles differs depending upon their developmental stages [23], [24]. There is also evidence that, like other mammalian species, the human oocyte expresses several DNA repair genes [25], but their role in repair of radiation‐induced genomic damage in the human oocyte is still unclear. Radiation induces genomic damage in the oocyte and the surrounding granulosa cells, leading to atresia of the follicle. Depending upon the extent of damage, the ovarian follicle pool is either decreased or totally exhausted. Autopsy series of young girls who had been treated with radiation for abdominal tumors showed that their ovaries were severely damaged, the proportion of atretic small follicles was increased, and follicle growth was arrested in comparison with healthy girls who died of accidents [26]. Wallace et al. utilized the Faddy‐Gosden model in order to predict the age at which ovarian failure is likely to occur after exposure to radiation by considering age and radiation dose. The model incorporates decay as an instantaneous rate of temporal change based on the remaining population pool. They showed that the effective sterilizing dose (ESD), or dose of fractionated radiotherapy at which ovarian failure occurs immediately after treatment in 97.5% of patients, decreases with increasing age at treatment. The estimated ESD at birth was calculated to be 20.3 Gy; at 10 years, 18.4 Gy; at 20 years, 16.5 Gy; and at 30 years, 14.3 Gy. This model can be a helpful guide to estimate the likelihood of ovarian failure from birth to 50 years after exposure to radiation at any given dose. It should be noted that there is considerable individual variability in ovarian reserve among patients of the same age. In other words, chronological age of a given patient may not necessarily match her ovarian age. This fact is likely to account for the differences in the onset of premature ovarian failure among patients treated at the same age. Ovarian damage occurs by the ovary's direct exposure to radiation during pelvic or low abdominal, lumbo‐sacral, spinal, and total‐body irradiation [4]. Furthermore, scatter radiation may induce significant ovarian damage even if the ovaries are not within the radiation field. The risk of ovarian damage and premature ovarian failure increases with incremental doses of radiation. Single dose appears to be more toxic than fractionated doses [22]. The probability of pregnancy in female survivors is 0.56 if the ovaries are exposed to 5–10 Gy radiation. It further decreases to 0.18 if the ovarian radiation dose exceeds 10 Gy [27]. Unfortunately, the detrimental effect of radiation in the female reproductive system is not limited to the ovary.
Apart from the ovaries, the uterus is also targeted by radiation. Radiation‐induced damage in the endometrium, myometrium, and vascular structures in the uterus can significantly compromise its functions and potentially lead to infertility and adverse pregnancy outcomes such as miscarriages, still births, fetal growth restrictions, preeclampsia, and preterm deliveries in survivors exposed to uterine radiation during childhood (Fig. 1) [4], [7], [28], [29]. A multitude of different pathophysiological mechanisms play a role in the development of uterine dysfunction after exposure to radiation. First, radiation causes damage, sclerosis, and obliterations in the vascular structures of the myometrial and endometrial layers of the uterus, leading to decreased uterine blood flow. If pregnancy occurs, this will potentially impair the invasion of the cytotrophoblast in the placental bed and result in defective placentation, decreased fetal‐placental blood flow, and fetal growth restriction [4], [30]. Radiation‐induced damage in the endometrium may also prevent normal decidualization and lead to disorders of placental attachment, such as placenta accreta, if pregnancy can be achieved [30], [31], [32], [33], [34]. Second, uterine elasticity and volume can be decreased by radiation‐induced myometrial damage, which can lead to preterm labor and delivery [31]. Third, radiation‐induced damage in the glandular and stromal components of the endometrium with particular destruction of progenitor basal cell layer may cause infertility by inducing endometrial atrophy and preventing implantation [34]. Survivors of childhood cancers are more likely to suffer from clinical infertility and have prolonged time to achieve pregnancy (relative risk [RR] 1.48, 95% confidence interval [CI] 1.23–1.78; p < .0001) than their siblings even when they retain ovarian function after exposure to radiation [35]. Pathological changes in the uterus induced by radiation may, at least in part, account for subfertility observed in these survivors.
Ovarian Function in Survivors of Childhood Cancers
There are different clinical manifestations of ovarian damage induced by chemotherapy drugs and radiation (Fig. 1). These may vary from temporary menstrual irregularity to permanent ovarian failure, depending upon the magnitude of ovarian damage and reduction in the follicle stockpile. Acute damage to growing follicles, particularly at the preantral and antral stages of development, may cause temporary menstrual irregularity by interfering with recruitment of antral follicles for further growth, dominant follicle selection, and ovulation processes. This damage is usually short term and reverts back to normal with replenishment of the antral cohort from the dormant follicle pool in the following months. If ovarian damage is massive and results in total depletion of the follicle reserve, acute ovarian failure (AOF) develops, defined as the loss of ovarian function that develops during or shortly after the completion of cancer therapy. The other form is premature menopause, which is described as the development of premature ovarian failure (POF) before age 40. Typically, a subset of survivors who retain ovarian function after the completion of gonadotoxic chemotherapy or radiation develop POF after a window of normal ovarian functioning. In these patients, diminished ovarian reserve cannot sustain a normal reproductive life span [36].
Acute Ovarian Failure
According to the reports of the Childhood Cancer Survivor Study, 6% of the female participants who were older than 18 years of age developed AOF [36], [37]. It is also worthwhile to note that these survivors with AOF were more frequently diagnosed with Hodgkin lymphoma, older at the time of diagnosis, and more likely to have been treated with abdominal or pelvic radiotherapy than the survivors without AOF. Half of these survivors were exposed to at least 10 Gy of ovarian irradiation. Increasing ovarian irradiation dose and exposure to procarbazine and cyclophosphamide at ages 13–20 years were independent risk factors for the development of AOF. The ovaries of younger individuals are more resistant to damage from irradiation than the ovaries of older individuals because younger patients harbor higher number of primordial follicles in their ovaries; therefore, they are more likely to retain some residual ovarian function after radiotherapy than older patients [12], [20], [38], [39]. As an example, 6 Gy radiation might be sufficient to cause permanent irreversible ovarian failure in women older than 40 years of age, in contrast to 10–20 Gy doses needed to produce the same effect in the majority of females treated during childhood [40], [41]. The most devastating effect of radiation on the ovary is observed in patients who receive high‐dose TBI, such that all patients older than 10 years and half of girls before age 10 developed acute ovarian failure following TBI for bone marrow transplantation, as one study showed [42]. TBI given as a single dose or fractionated (10–15 Gy) is often used in combination with gonadotoxic cyclophosphamide or melphalan. Unfortunately, such combinations further increase the risk of permanent ovarian failure. As a striking example, in a study of 144 patients <25 years old who were treated with TBI with cyclophosphamide for bone marrow transplantation, all of the patients (100%) developed amenorrhea in the first 3 years of treatment, and menses returned in only 9 patients 3–7 years after transplant [43]. Similarly, five of ten patients with Fanconi anemia (median age at transplantation was 12 years [range 5–17 years]) undergoing hematopoietic stem cell transplantation developed signs of ovarian failure during follow‐up [44]. Unfortunately, compromised ovarian function or failure occurring after exposure of prepubertal ovaries to high‐dose radiation (>20 Gy) in the form of TBI or whole abdominal radiotherapy may interfere with pubertal development. As a striking example, a case series study reported that 27 out of 38 patients treated with whole abdominal radiation (20–30 Gy) for intra‐abdominal tumors failed to undergo or complete pubertal development [45]. In contrast to this effect of radiation, pubertal development in male and female patients is not affected by chemotherapy administration [46].
The most devastating effect of radiation on the ovary is observed in patients who receive high‐dose total body irradiation (TBI), such that all patients older than age 10 years and half of the girls before age 10 developed acute ovarian failure following TBI for bone marrow transplantation as one study showed.
Premature Ovarian Failure
Premature ovarian failure is another manifestation of previous ovarian exposure to gonadotoxic chemotherapy regimens and radiation. It may develop in childhood cancer survivors who did not develop AOF and retained some ovarian function after cancer treatment was completed. In this group of patients, permanent loss of ovarian function develops gradually in the years after completion of cancer therapy, following a period of normal functioning. The Childhood Cancer Survivor Study in 2006 evaluated long‐term ovarian function in 2,819 survivors of childhood cancer who were diagnosed at a median age of 7 and followed up for up to 40 years of age, in comparison with 1,065 sibling controls [47]. The cumulative incidence of premature menopause was found to be significantly higher for survivors than for siblings (8% vs. 0.8%, p < .001). The study identified age, exposure to increasing doses of radiation to the ovaries, increasing alkylating agent score (calculated based on the number of alkylating agents received and their cumulative doses), and a diagnosis of Hodgkin lymphoma as the risk factors for premature menopause. The RRs for the development of POF were 2.3 (95% CI 1.08–4.9) and 5.78 (95% CI 2.9–11.55) in the survivors, whose alkylating agent scores were 1–2 and 3, respectively, compared with nonexposed individuals. Similarly, the RRs were 4.3 (95% CI 1.2–15.4), 5.7 (95% CI 1.12–28.9), and 105.6 (95% CI 28.15–426.7) for the survivors exposed to ovarian radiation at 1–99, 100–999 and >1,000 cGY doses, respectively. The long‐term follow‐up of the same study in 2009 reported the same POF incidence in the survivors (8% of participants vs. 0.8% of siblings [RR 13.21, 95% CI 3.26–53.51; p < .001) [37]. Whole abdominal radiation is associated with a higher risk of ovarian failure in comparison with flank radiation. For instance, 10 out of 38 patients who received total abdominal radiotherapy (20–30 Gy) developed POF (median age 23.5 years), whereas only 1 of 15 patients treated with flank radiation (20–30 Gy) developed POF [45].
Other studies documented the importance of Hodgkin lymphoma and exposure to alkylating agents as treatment‐related risk factors for premature menopause as well. For instance, using the National Cancer Registry of The Netherlands, a cohort study conducted among 518 female 5‐year survivors of Hodgkin lymphoma survivors (median age 25 years) showed that 97 women (19%) had entered menopause before age 40 after a median follow‐up of 9.4 years. Exposure to procarbazine (hazard ratio [HR] 8.1) and cyclophosphamide (HR 3.5) were identified as the variables that showed the strongest associations with the occurrence of premature menopause. Doubling the cumulative dose of procarbazine (from ≤4.2 g/m2 to >8.4 g/m2) increased the risk of premature menopause from 15% to 65% 10 years after treatment [48]. Notably, the cumulative risk of menopause at age 40 did not differ much according to age in this study, but time to premature menopause was much longer in women treated at early ages. The investigators in that study did not observe any significant effects of treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and epirubicin, bleomycin, vinblastine, and prednisone regimens on premature menopause [48]. The ABVD regimen remains a common and successful first‐line treatment protocol for young people with Hodgkin lymphoma. This combination is far less toxic than alkylating regimens. In support of this notion, previous studies had already shown comparable follicle densities in the ovaries of the patients undergoing ovarian tissue freezing before and after receiving the ABVD regimen [12], [49].
Chemotherapy Drugs in the Alkylating Category Do Not Exert the Same Level of Gonadotoxicity on the Ovary
Alkylating chemotherapy drugs are more toxic to the gonads than the drugs of all other types. But do all alkylating drugs exert the same level of gonadotoxicity? Recent evidence suggests that they may not be equally toxic to the ovaries and that there might be a difference between XX and XY gonads in terms of their sensitivity to cytotoxic chemotherapy regimens. The Childhood Cancer Survival Study analyzed live birth and pregnancy rates in 5,298 female survivors and 5,640 male survivors who were treated with chemotherapy drugs but did not receive pelvic and cranial radiation. In male survivors, upper tertile doses of cyclophosphamide (>7,412 mg/m2, HR 0.60, 95% CI 0.51–0.71; p < .0001), ifosfamide (>53,000 mg/m2, HR 0.42, 95% CI 0.23–0.79; p = .0069), procarbazine (>5,060 mg/m2, HR 0.30, 95% CI 0.20–0.46; p < .0001), and cisplatin (>488 mg/m2, HR 0.56, 95% CI 0.39–0.82; p = .0023) were associated with reduced likelihood of siring a pregnancy. By contrast, only busulfan (<450 mg/m2, HR 0.22, 95% CI 0.06–0.79; p = .020; ≥450 mg/m2, HR 0.14, 95% CI 0.03–0.55; p = .0051) and doses of lomustine equal to or greater than 411 mg/m2 (HR 0.41, 95% CI 0.17–0.98; p = .046) were significantly associated with reduced probability of pregnancy in female survivors. These findings suggest that except for busulfan and lomustine, other alkylating agents and DNA interstrand cross‐linking drugs might not be associated with reduced pregnancy or live birth rates in female survivors unless they are administered at very high cumulative doses. Nevertheless, female survivors still had a decreased likelihood of having a pregnancy (HR 0.87, 95% CI 0.81–0.94; p < .0001) and live birth (HR 0.82, 95% CI 0.76–0.89; p < .0001) versus siblings. Furthermore, if pregnancy did not occur by age 30 in the survivors, the likelihood of ever becoming pregnant by age 45 years was further reduced in them versus siblings [50]. These recent findings may provide some reassurance by demonstrating that the impact of chemotherapy on the occurrence of pregnancy might be lower than expected in female survivors who were treated with alkylating chemotherapy drugs at conventional doses and were not exposed to pelvic radiation. But it should not obviate the need for consideration of fertility preservation in pediatric and adolescent patients with newly diagnosed cancer in order to maximize and maintain their reproductive potential, because chemotherapy‐induced ovarian damage and the resultant decrease in follicle pool will eventually shorten the reproductive life span of many of these survivors. The different odds ratios of the pregnancy and live birth rates of the female survivors exposed to different alkylating regimens probably come from the differences in the residual ovarian reserve retained after exposure to chemotherapy. Some drugs, such as busulfan and lomustine, are now pronounced as more gonadotoxic because the remaining follicle pool is lower, and therefore pregnancy and live birth rates are more severely reduced in the survivors exposed to these drugs during childhood. In support of this notion, another study evaluated 105 survivors who were treated for Hodgkin lymphoma during childhood and found that exposure to procarbazine but not cyclophosphamide or ifosfamide dose was associated with reduced ovarian reserve, antral follicle counts, and anti‐Müllerian hormone (AMH) and higher follicle‐stimulating hormone (FSH) levels [51].
Ovarian Reserve Tests
Assessment of ovarian reserve with AMH measurements, as well as antral follicle counts, can give a better estimate of ovarian reserve before and after exposure to chemotherapy and/or radiation than FSH. AMH is produced by the granulosa cells of growing preantral and small antral follicles as a dimeric glycoprotein [52]. In fetal ovaries, AMH is first detected at 36 weeks of gestation in granulosa cells of developing preantral follicles [53], reaches its highest levels in puberty, and becomes undetectable after menopause [54], [55]. Serum AMH levels are significantly lower in female survivors who were treated with alkylating regimens or with abdominal or total‐body irradiation during childhood [56], [57], [58], [59], [60], [61].Therefore, AMH can be used to screen adult and prepubertal female survivors for a quantitative estimation of residual ovarian reserve after exposure to chemotherapy and radiation but also may help identify those at risk for delayed puberty. On the other hand, the prognostic value of AMH in estimating the probability of live birth in female survivors is unknown.
Uterine Function and Pregnancy Outcomes in Survivors of Childhood Cancers
Chemotherapy drugs have minimal or no detrimental effect on the uterus that can be recognized clinically. By contrast, uterine exposure to pelvic, spinal, abdominal, and total‐body irradiation during childhood may significantly compromise uterine function and cause a number of early‐ and late‐onset adverse obstetrical outcomes in survivors (Fig. 1). These include infertility, miscarriage, fetal growth restrictions, fetal malposition, preterm births, perinatal deaths, delivery of small‐for‐gestational‐age infants, preeclampsia, and abnormal placentation [7], [10], [29], [30], [31], [34], [37], [43], [62], [63], [64].
The most devastating clinical consequences are observed in survivors who were treated with TBI, exposing the uterus to radiation of >20 Gy during childhood. A significant reduction in uterine size is observed in the survivors with ovarian failure secondary to TBI (20–30 Gy). But uterine atrophy is not related to hypoestrogenic state induced by ovarian failure, because hormone replacement therapy does not increase uterine size, blood flow, or endometrial thickness in these survivors [32], [64], [65]. First‐ and mid‐trimester pregnancy losses and preterm births are drastically increased in survivors treated with TBI. Sanders et al. reported that if conditioning prior to hematopoietic stem cell transplantation was made with TBI, the risk of first‐trimester loss significantly increased to 37% compared with survivors conditioned with cyclophosphamide (7%, p = .02). Preterm delivery rate was significantly higher in female patients conditioned with TBI versus cyclophosphamide (63% vs. 18%, p = .01). The overall incidence of preterm birth was markedly higher than the expected incidence in the general population (25% vs. 6.5%, p < .001) [43]. In addition to first‐trimester abortions, mid‐trimester pregnancy losses are also more frequently observed in female survivors exposed to uterine radiation than in the general population [45], [64]. Wallace et al. reported that pregnancy was achieved in only 4 of 38 patients who had received whole‐body irradiation (20–30 Gy) during childhood and that all resulted in mid‐trimester miscarriage [45]. Larsen et al. evaluated the impact of radiation on uterine volume in 100 childhood survivors using transvaginal sonography. While the median uterine volumes were 47 mL for control patients and 40 mL for the patients exposed to radiation above the diaphragm, volumes were 34 mL and 19 mL, respectively, in those treated with radiation below the diaphragm and with uterine radiation. They found that mid‐trimester pregnancy loss was substantially increased in the survivors with reduced uterine size [64]. The chance of full‐term births decreases with increasing doses of radiation to the uterus. For instance, while 82.7% of the survivors who had been exposed to uterine radiation at 0–10 cGy had full‐term births, only half of those treated with >500 cGy had full‐term births [37], [62]. In a similar manner, the risk of preterm birth increases in a dose‐dependent manner after uterine irradiation (odds ratio [OR] 1.8 for 0.05–2.50 Gy, 2.3 for 2.5–5.0 Gy, and 3.5 for 5 Gy). The increase in the dose of uterine radiation >5 Gy was associated with a further increase in the risk of birth of small‐for‐gestational‐age babies (birth weight ≤10 percentile for gestational age; OR 4.0, 95% CI 1.6–9.8; p = .003) [37], [62].
Timing of uterine exposure to radiation appears to be a critical determinant of the magnitude of damage and poor obstetric outcomes. Women exposed to radiation after puberty have a larger uterus and greater likelihood of live birth than those exposed prepubertally. Uterine irradiation at doses greater than 10 Gy significantly increased risk of stillbirth and neonatal death after puberty (OR 9.1, 95% CI 3.4–24.6). But, for girls treated before menarche, radiation doses as low as 1–2.5 Gy were associated with a significant increase in the risk of stillbirth and neonatal deaths (OR 4.7, 95% CI 1.2–19) [66], [67]. These findings suggest that prepubertal exposure to radiation has more detrimental effects on the uterus than postpubertal exposure.
Certain pregnancy complications might be more closely related to the type of radiation used in specific treatment groups [63]. A striking example of this is Wilms tumor. After initial reports brought this issue to attention by showing an abnormally high incidence of perinatal deaths and low‐birth‐weight infants born to mothers who had received radiation during childhood for Wilms’ tumor [68], [69], the National Wilms Tumor Long‐Term Follow‐Up Study evaluated 1,021 pregnancies that resulted in 955 live‐born singletons. The study showed that pregnancies complicated by hypertension, preterm labor and delivery, low birth weight, and fetal malposition were observed at increased rates with increasing flank radiation dose in the female survivors of Wilms’ tumor [70].
Aside from the adverse effects of uterine exposure to radiation, cranial irradiation (RR 1.4, 95% CI 1.02–1.94) also appears to increase the relative risk of miscarriage—although the risk is lower than in those treated with craniospinal irradiation (RR 2.22, 95% CI 1.36–3.64) compared with those who received no radiation therapy [37]. Cranial irradiation may also cause abnormal pubertal timing, alterations of the hypothalamic‐pituitary‐ovarian interaction, and other endocrinopathies [71].
In contrast to the well‐documented detrimental effect of radiation on uterine function and pregnancy outcomes, most chemotherapy drugs do not appear to have deleterious effects on the uterus that can be recognized clinically [72]. Prior treatment with doxorubicin and daunorubicin was linked to low birth weight (RR 2.14, 95% CI 1.43–3.21; p = .0002) independently of pelvic radiation [37]. On the other hand, live birth and stillbirth rates of the survivors who had been treated with any specific chemotherapy drug were found to be comparable to those who had not received that chemotherapy agent. Furthermore, when the cumulative doses of chemotherapeutic agents were divided into tertiles, no significant differences were found in the rate of live birth, miscarriage, or medical abortion by tertiles [37]. Despite increased risks of adverse pregnancy outcomes after exposure to cytotoxic chemotherapy and radiation regimens, there is no evidence for an increase in the incidence of congenital malformations, cytogenetic syndromes, or single‐gene defects in the offspring of the female survivors compared with their siblings, according to the results of the Childhood Cancer Survival Study, linked cancer‐birth registry analysis, British Childhood Cancer Survivor Study, Danish nationwide cohort study, and Norway Cancer and Birth Registry Data [37], [73], [74], [75], [76], [77], [78]
In summary, radiation has a greater negative impact on reproductive functions in female survivors than chemotherapy agents. Female survivors who were exposed to uterine radiation during childhood are more prone to developing a number of adverse obstetrical outcomes than their siblings and general population. Their offspring are more likely to be premature, have a low birth weight, and be small for gestational age. The risk of miscarriage was increased among women who were exposed to uterine, high‐dose cranial, and craniospinal irradiation. The relative risks for these adverse obstetrical outcomes increase with incremental doses of uterine radiation. Prior treatment with doxorubicin or daunorubicin may increase the risk of low birth weight independent of pelvic irradiation. But the rates of live birth and stillbirth for the patients treated with any particular chemotherapeutic agent are not different from those who have not been treated with that agent. Relative risks of malformations and congenital anomalies among the children of cancer survivors are not significantly different from those of their siblings.
How to Preserve the Fertility of Pediatric Cancer Patients
Large‐scale studies assessing the reproductive performance of female survivors documented that many of them eventually achieve pregnancy and live births unless they had been exposed to high‐dose pelvic radiation and alkylating agents during childhood [27], [50], [79]. Even though these data provide reassurance to female survivors, fertility preservation before cancer treatment still remains an important issue in order to protect and maximize the reproductive potential of the survivors. Therefore, young female patients diagnosed with cancer or other diseases requiring the use of cytotoxic chemotherapy regimens and radiation should be informed at the time of diagnosis about their fertility prognosis and the options to preserve it [9], [80], [81], [82]. Strategies to preserve the fertility of pediatric patients are limited, mainly due to sexual immaturity. In fact, the young age of females diagnosed with cancer appears to be one of the main reasons for not discussing fertility preservation options, as shown by a study assessing the attitude and clinical practice of physicians on this issue in the U.K. [82]. There are currently three evidence‐based recommendations for fertility preservation in young female patients with cancer, two produced by the American Society of Clinical Oncology [8], [9] and one by the International Society of Fertility Preservation [83]. Unfortunately, a considerable percentage of pediatric patients with cancer and their parents are not counseled about the adverse effects of cancer therapies on reproductive functions, future childbearing potential, and current fertility preservation strategies [84]. One study showed that fertility preservation options were offered to only 38 out of 463 girls (8.2%) diagnosed with cancer. The most common reasons given for not discussing fertility preservation options included “not at significant risk” in 29% of cases, “too young” in 27%, “techniques unproven” in 22%, “no facilities” in 10%, and “no funding” in 8% [82]. A recent survey demonstrated that none of the 136 young female patients diagnosed with childhood cancers older than age 13 were counseled about fertility preservation options [85]. Currently, ovarian tissue cryopreservation and transposition (oophoropexy) are the only methods to preserve the fertility of sexually immature children. Both procedures are invasive, requiring laparoscopic surgery.
The most common reasons given for not discussing fertility preservation options included ‘not at significant risk’ in 29% of cases, ‘too young’ in 27%, ‘techniques unproven’ in 22%, ‘no facilities’ in 10% and ‘no funding’ in 8%. A recent survey demonstrated that none of the 136 young females diagnosed with childhood cancers older than age 13 were counseled about fertility preservation options.
Ovarian Tissue Cryopreservation
There are 60 live births reported to date after transplantation of frozen‐thawed ovarian cortical pieces [86]. However, ovarian tissue banking is considered still experimental and therefore should be performed under institutional review board approval and guidance [87]. Common indications for ovarian tissue freezing in pediatric patients are summarized in Table 1. As can be seen, the indications extend beyond cancer because some other benign illnesses, such as systemic lupus erythematosus and Wegener vasculitis, may require the administration of cytotoxic chemotherapy regimens and/or radiation for immunosuppression or stem cell transplantation. One ovary is generally removed laparoscopically for freezing. Bilateral gonadectomy is indicated in the case of Y‐chromosome mosaicism in the ovary due to the increased risk of malignant germ‐cell tumor development. If the indication for ovarian tissue freezing is an intrinsic oocyte defect such as Turner syndrome or galactosemia, which is characterized by accelerated follicle atresia and early depletion of the follicle stockpile in the ovary, cryopreservation of both ovaries can also be considered. If pubertal transition is completed, oocyte freezing should be considered instead of ovarian tissue cryopreservation. Pathological examination of removed ovaries is a prerequisite to rule out any microscopic tumor invasion in the ovaries, especially in cancers with a high risk of ovarian metastasis, such as leukemia, neuroblastoma, and genital rhabdomyosarcoma [88]. Slow freezing is the conventional method for cryopreservation of the ovarian tissue [89]. Almost all of the ongoing pregnancies and live births reported to date were achieved from transplantation of slow‐frozen ovaries. There is also a report of two pregnancies after engraftment of vitrified and warmed ovaries [86].
Table 1. Diseases requiring counseling for fertility preservation in children.

The most common indications for fertility preservation in pediatric patients are listed. In addition to malignancies, some precancerous, autoimmune, and benign diseases are also treated with cytotoxic chemotherapy drugs and/or radiation, either to induce disease remission or to suppress immune system as myeloablative preconditioning treatment before hematopoietic stem cell transplantation.
Abbreviation: HSCT, hematopoietic stem cell transplantation.
More than half of the dormant primordial follicles are lost during the ischemic period until revascularization is established in the grafted cortical pieces. Given that prepubertal ovaries harbor more follicles, they are more likely to retain more follicles after ischemic insult than are adult ovaries. There is one live birth reported from an ovarian graft taken from a patient at age 14, proving that cryopreservation of pre‐ or peripubertal ovaries may protect the fertility of pediatric patients [90]. Transplantation of frozen‐thawed ovarian tissue samples has also been successfully utilized to induce puberty in two girls in whom pubertal development was achieved after transplantation of a couple of pieces of frozen ovarian cortical strips [91], [92]. Some argued against the use of ovarian tissue samples for this purpose based on the grounds that pubertal development can be easily achieved by the administration of exogenous sex hormones, and the main purpose of ovarian tissue transplantation is to restore fertility, not to induce puberty [93]. To date, seven case series of ovarian tissue freezing were reported in a total of 266 pediatric and adolescent patients (age range 8 months to 21 years) [94], [95], [96], [97], [98], [99], [100].
Ovarian Transposition
Transposing the ovaries out of the radiation field is an option to preserve gonadal function, but it does not deter the deleterious effects of radiation on the uterus, which may have a substantial negative impact on the future reproductive success and obstetrical outcome of the survivors. If no chemotherapy is planned, the ovaries can be surgically moved with their vascular pedicle outside the pelvis (transposed) to shield them from radiation therapy. Ovarian function can be retained in the majority of young girls and adolescent females if the ovaries are transposed before radiation [40], [101]. Spontaneous pregnancies and live births have been reported after transposition of the ovaries [102]. The success rate of retaining ovarian function with ovarian transposition prior to radiotherapy varies between 16% and 90% and depends upon the magnitude of scatter radiation, the presence of vascular compromise in the pedicle, the age of the patient, dose of radiation, whether the ovaries were shielded, and whether concomitant chemotherapy was used [99]. Infarction, chronic ovarian pain, ovarian cyst formation, and migration of ovaries back to their original position before radiotherapy is completed are reported complications [103]. Patients undergoing pelvic radiation must be informed about the fact that ovarian transposition will not circumvent the harmful effects of radiation on the uterus and other pelvic structures that might prevent them from carrying a pregnancy.
Conclusion
The options to preserve potential fertility in pediatric patients with cancer who are at risk of gonadal failure are more limited than in adults and currently include ovarian tissue cryopreservation and ovarian transposition. Ovarian failure may not develop immediately after chemotherapy and/or radiotherapy, but these patients are at much higher risk of diminished primordial follicle reserve, infertility, premature ovarian failure, poor reproductive outcomes, and reproductive end organ damage later in life. Ovarian transposition will not prevent the adverse effects of radiation on the uterus. Overall, the risk of premature ovarian failure is several times higher in the survivors of childhood cancers compared with their siblings. In addition, uterine exposure to radiation during pelvic, spinal, or total‐body irradiation increases the risk of adverse pregnancy outcomes in female survivors of childhood cancers, such as fetal growth restriction, low birth weight, preterm labor, spontaneous abortions, and perinatal death. There are no methods currently available that have been demonstrated to protect the reproductive organs or the primordial follicle pool from the cytotoxic effects of chemotherapy or radiation. Therefore, appropriate counselling on the options for fertility preservation should be offered to all patients in order to maximize their reproductive potential.
Author Contributions
Conception/design: Ozgur Oktem
Collection and/or assembly of data: Ozgur Oktem, Samuel S. Kim, Ugur Selek, Glenn Schatmann, Bulent Urman
Manuscript writing: Ozgur Oktem, Samuel S. Kim, Ugur Selek, Glenn Schatmann, Bulent Urman
Final approval of manuscript: Ozgur Oktem
Disclosures
The authors indicated no financial relationships.
References
- 1. Jemal A, Siegel R, Xu J et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- 2. Miller KD, Siegel RL, Lin CC et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 4. Oktem O, Oktay K. Preservation of menstrual function in adolescent and young females. Ann NY Acad Sci 2008;1135:237–243. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong GT, Sklar CA, Hudson MM et al. Long‐term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol 2007;25:4477–4489. [DOI] [PubMed] [Google Scholar]
- 6. Oktem O, Oktay K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res 2007;67:10159–10162. [DOI] [PubMed] [Google Scholar]
- 7. Critchley HO, Wallace WH. Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr 2005:64–68. [DOI] [PubMed] [Google Scholar]
- 8. Lee SJ, Schover LR, Partridge AH et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917–2931. [DOI] [PubMed] [Google Scholar]
- 9. Loren AW, Mangu PB, Beck LN et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee opinion no. 607: Gynecologic concerns in children and adolescents with cancer. Obstet Gynecol 2014;124:403–408. [DOI] [PubMed] [Google Scholar]
- 11.Practice Committee of American Society for Reproductive Medicine . Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil Steril 2013;100:1214–1223. [DOI] [PubMed] [Google Scholar]
- 12. Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007;110:2222–2229. [DOI] [PubMed] [Google Scholar]
- 13. Plowchalk DR, Mattison DR. Phosphoramide mustard is responsible for the ovarian toxicity of cyclophosphamide. Toxicol Appl Pharmacol 1991;107:472–481. [DOI] [PubMed] [Google Scholar]
- 14. Meirow D, Dor J, Kaufman B et al. Cortical fibrosis and blood‐vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod 2007;22:1626–1633. [DOI] [PubMed] [Google Scholar]
- 15. Bildik G, Akin N, Senbabaoglu F et al. GnRH agonist leuprolide acetate does not confer any protection against ovarian damage induced by chemotherapy and radiation in vitro. Hum Reprod 2015;30:2912–2925. [DOI] [PubMed] [Google Scholar]
- 16. Kalich‐Philosoph L, Roness H, Carmely A et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med 2013;5:185ra162. [DOI] [PubMed] [Google Scholar]
- 17. Morgan S, Lopes F, Gourley C et al. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS One 2013;8:e70117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Himelstein‐Braw R, Peters H, Faber M. Morphological study of the ovaries of leukaemic children. Br J Cancer 1978;38:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Epstein RJ. Drug‐induced DNA damage and tumor chemosensitivity. J Clin Oncol 1990;8:2062–2084. [DOI] [PubMed] [Google Scholar]
- 20. Oktem O, Urman B. Options of fertility preservation in female cancer patients. Obstet Gynecol Surv 2010;65:531–542. [DOI] [PubMed] [Google Scholar]
- 21. Antal Z, Sklar CA. Gonadal function and fertility among survivors of childhood cancer. Endocrinol Metab Clin North Am 2015;44:739–749. [DOI] [PubMed] [Google Scholar]
- 22. Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod 2003;18:117–121. [DOI] [PubMed] [Google Scholar]
- 23. Ashwood‐Smith MJ, Edwards RG. DNA repair by oocytes. Mol Hum Reprod 1996;2:46–51. [DOI] [PubMed] [Google Scholar]
- 24. Adriaens I, Smitz J, Jacquet P. The current knowledge on radiosensitivity of ovarian follicle development stages. Hum Reprod Update 2009;15:359–377. [DOI] [PubMed] [Google Scholar]
- 25. Jaroudi S, Kakourou G, Cawood S et al. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum Reprod 2009;24:2649–2655. [DOI] [PubMed] [Google Scholar]
- 26. Himelstein‐Braw R, Peters H, Faber M. Influence of irradiation and chemotherapy on the ovaries of children with abdominal tumours. Br J Cancer 1977;36:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Green DM, Kawashima T, Stovall M et al. Fertility of female survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol 2009;27:2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sudour H, Chastagner P, Claude L et al. Fertility and pregnancy outcome after abdominal irradiation that included or excluded the pelvis in childhood tumor survivors. Int J Radiat Oncol Biol Phys 2010;76:867–873. [DOI] [PubMed] [Google Scholar]
- 29. Beneventi F, Locatelli E, Giorgiani G et al. Gonadal and uterine function in female survivors treated by chemotherapy, radiotherapy, and/or bone marrow transplantation for childhood malignant and non‐malignant diseases. BJOG 2014;121:856–865; discussion 865. [DOI] [PubMed] [Google Scholar]
- 30. Hawkins MM, Smith RA. Pregnancy outcomes in childhood cancer survivors: Probable effects of abdominal irradiation. Int J Cancer 1989;43:399–402. [DOI] [PubMed] [Google Scholar]
- 31. Knopman JM, Papadopoulos EB, Grifo JA et al. Surviving childhood and reproductive‐age malignancy: Effects on fertility and future parenthood. Lancet Oncol 2010;11:490–498. [DOI] [PubMed] [Google Scholar]
- 32. Critchley HO, Bath LE, Wallace WH. Radiation damage to the uterus – review of the effects of treatment of childhood cancer. Hum Fertil (Camb) 2002;5:61–66. [DOI] [PubMed] [Google Scholar]
- 33. Norwitz ER, Stern HM, Grier H et al. Placenta percreta and uterine rupture associated with prior whole body radiation therapy. Obstet Gynecol 2001;98:929–931. [DOI] [PubMed] [Google Scholar]
- 34. Teh WT, Stern C, Chander S et al. The impact of uterine radiation on subsequent fertility and pregnancy outcomes. Biomed Res Int 2014;2014:482968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barton SE, Najita JS, Ginsburg ES et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2013;14:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chemaitilly W, Mertens AC, Mitby P et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 2006;91:1723–1728. [DOI] [PubMed] [Google Scholar]
- 37. Green DM, Sklar CA, Boice JD Jr et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: Results from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horning SJ, Hoppe RT, Kaplan HS et al. Female reproductive potential after treatment for Hodgkin's disease. N Engl J Med 1981;304:1377–1382. [DOI] [PubMed] [Google Scholar]
- 39. Lushbaugh CC, Casarett GW. The effects of gonadal irradiation in clinical radiation therapy: A review. Cancer 1976;37:1111–1125. [DOI] [PubMed] [Google Scholar]
- 40. Thibaud E, Ramirez M, Brauner R et al. Preservation of ovarian function by ovarian transposition performed before pelvic irradiation during childhood. J Pediatr 1992;121:880–884. [DOI] [PubMed] [Google Scholar]
- 41. Sarafoglou K, Boulad F, Gillio A et al. Gonadal function after bone marrow transplantation for acute leukemia during childhood. J Pediatr 1997;130:210–216. [DOI] [PubMed] [Google Scholar]
- 42. Sklar C. Growth and endocrine disturbances after bone marrow transplantation in childhood. Acta Paediatr Suppl 1995;411:57–61; discussion 62. [DOI] [PubMed] [Google Scholar]
- 43. Sanders JE, Hawley J, Levy W et al. Pregnancies following high‐dose cyclophosphamide with or without high‐dose busulfan or total‐body irradiation and bone marrow transplantation. Blood 1996;87:3045–3052. [PubMed] [Google Scholar]
- 44. Nabhan SK, Bitencourt MA, Duval M et al. Fertility recovery and pregnancy after allogeneic hematopoietic stem cell transplantation in Fanconi anemia patients. Haematologica 2010;95:1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wallace WH, Shalet SM, Crowne EC et al. Ovarian failure following abdominal irradiation in childhood: Natural history and prognosis. Clin Oncol (R Coll Radiol) 1989;1:75–79. [DOI] [PubMed] [Google Scholar]
- 46. Sklar CA. Growth and pubertal development in survivors of childhood cancer. Pediatrician 1991;18:53–60. [PubMed] [Google Scholar]
- 47. Sklar CA, Mertens AC, Mitby P et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J Natl Cancer Inst 2006;98:890–896. [DOI] [PubMed] [Google Scholar]
- 48. De Bruin ML, Huisbrink J, Hauptmann M et al. Treatment‐related risk factors for premature menopause following Hodgkin lymphoma. Blood 2008;111:101–108. [DOI] [PubMed] [Google Scholar]
- 49. Seshadri T, Gook D, Lade S et al. Lack of evidence of disease contamination in ovarian tissue harvested for cryopreservation from patients with Hodgkin lymphoma and analysis of factors predictive of oocyte yield. Br J Cancer 2006;94:1007–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chow EJ, Stratton KL, Leisenring WM et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2016;17:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomas‐Teinturier C, Allodji RS, Svetlova E et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod 2015;30:1437–1446. [DOI] [PubMed] [Google Scholar]
- 52. Visser JA, Themmen AP. Anti‐Mullerian hormone and folliculogenesis. Mol Cell Endocrinol 2005;234:81–86. [DOI] [PubMed] [Google Scholar]
- 53. Rajpert‐De Meyts E, Jørgensen N, Graem N et al. Expression of anti‐Müllerian hormone during normal and pathological gonadal development: Association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 1999;84:3836–3844. [DOI] [PubMed] [Google Scholar]
- 54. Hudson PL, Dougas I, Donahoe PK et al. An immunoassay to detect human müllerian inhibiting substance in males and females during normal development. J Clin Endocrinol Metab 1990;70:16–22. [DOI] [PubMed] [Google Scholar]
- 55. de Vet A, Laven JS, de Jong FH et al. Antimüllerian hormone serum levels: A putative marker for ovarian aging. Fertil Steril 2002;77:357–362. [DOI] [PubMed] [Google Scholar]
- 56. Lie Fong S, Laven JS, Hakvoort‐Cammel FG et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti‐Müllerian hormone. Hum Reprod 2009;24:982–990. [DOI] [PubMed] [Google Scholar]
- 57.J BH, M S, T D et al. Retroperitoneal sarcomas: A single center experience [in French]. Cancer Radiother 2008;12:331–335. [DOI] [PubMed] [Google Scholar]
- 58. Dillon KE, Sammel MD, Ginsberg JP et al. Pregnancy after cancer: Results from a prospective cohort study of cancer survivors. Pediatr Blood Cancer 2013;60:2001–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Charpentier AM, Chong AL, Gingras‐Hill G et al. Anti‐Müllerian hormone screening to assess ovarian reserve among female survivors of childhood cancer. J Cancer Surviv 2014;8:548–554. [DOI] [PubMed] [Google Scholar]
- 60. Brougham MF, Crofton PM, Johnson EJ et al. Anti‐Müllerian hormone is a marker of gonadotoxicity in pre‐ and postpubertal girls treated for cancer: A prospective study. J Clin Endocrinol Metab 2012;97:2059–2067. [DOI] [PubMed] [Google Scholar]
- 61. Lunsford AJ, Whelan K, McCormick K et al. Antimüllerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertil Steril 2014;101:227–231. [DOI] [PubMed] [Google Scholar]
- 62. Signorello LB, Cohen SS, Bosetti C et al. Female survivors of childhood cancer: Preterm birth and low birth weight among their children. J Natl Cancer Inst 2006;98:1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol 2010;116:1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Larsen EC, Schmiegelow K, Rechnitzer C et al. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand 2004;83:96–102. [DOI] [PubMed] [Google Scholar]
- 65. Holm K, Nysom K, Brocks V et al. Ultrasound B‐mode changes in the uterus and ovaries and Doppler changes in the uterus after total body irradiation and allogeneic bone marrow transplantation in childhood. Bone Marrow Transplant 1999;23:259–263. [DOI] [PubMed] [Google Scholar]
- 66. Bath LE, Wallace WH, Critchley HO. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG 2002;109:107–114. [DOI] [PubMed] [Google Scholar]
- 67. Signorello LB, Mulvihill JJ, Green DM et al. Stillbirth and neonatal death in relation to radiation exposure before conception: A retrospective cohort study. Lancet 2010;376:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li FP, Gimbrere K, Gelber RD et al. Outcome of pregnancy in survivors of Wilms’ tumor. JAMA 1987;257:216–219. [PubMed] [Google Scholar]
- 69. Green DM, Fine WE, Li FP. Offspring of patients treated for unilateral Wilms’ tumor in childhood. Cancer 1982;49:2285–2288. [DOI] [PubMed] [Google Scholar]
- 70. Green DM, Lange JM, Peabody EM et al. Pregnancy outcome after treatment for Wilms tumor: A report from the national Wilms tumor long‐term follow‐up study. J Clin Oncol 2010;28:2824–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chemaitilly W, Sklar CA. Endocrine complications in long‐term survivors of childhood cancers. Endocr Relat Cancer 2010;17:R141–R159. [DOI] [PubMed] [Google Scholar]
- 72. Critchley HO. Factors of importance for implantation and problems after treatment for childhood cancer. Med Pediatr Oncol 1999;33:9–14. [DOI] [PubMed] [Google Scholar]
- 73. Boice JD Jr., Tawn EJ, Winther JF et al. Genetic effects of radiotherapy for childhood cancer. Health Phys 2003;85:65–80. [DOI] [PubMed] [Google Scholar]
- 74. Chow EJ, Friedman DL, Yasui Y et al. Timing of menarche among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2008;50:854–858. [DOI] [PubMed] [Google Scholar]
- 75. Mueller BA, Chow EJ, Kamineni A et al. Pregnancy outcomes in female childhood and adolescent cancer survivors: A linked cancer‐birth registry analysis. Arch Pediatr Adolesc Med 2009;163:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Reulen RC, Zeegers MP, Wallace WH et al. Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev 2009;18:2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Langagergaard V, Horvath‐Puho E, Nørgaard M et al. Hodgkin's disease and birth outcome: A Danish nationwide cohort study. Br J Cancer 2008;98:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stensheim H, Klungsøyr K, Skjaerven R et al. Birth outcomes among offspring of adult cancer survivors: A population‐based study. Int J Cancer 2013;133:2696–2705. [DOI] [PubMed] [Google Scholar]
- 79. Brämswig JH, Riepenhausen M, Schellong G. Parenthood in adult female survivors treated for Hodgkin's lymphoma during childhood and adolescence: A prospective, longitudinal study. Lancet Oncol 2015;16:667–675. [DOI] [PubMed] [Google Scholar]
- 80. Wallace WH, Kelsey TW, Anderson RA. Fertility preservation in pre‐pubertal girls with cancer: The role of ovarian tissue cryopreservation. Fertil Steril 2016;105:6–12. [DOI] [PubMed] [Google Scholar]
- 81. Wallace WH, Critchley HO, Anderson RA. Optimizing reproductive outcome in children and young people with cancer. J Clin Oncol 2012;30:3–5. [DOI] [PubMed] [Google Scholar]
- 82. Anderson RA, Weddell A, Spoudeas HA et al. Do doctors discuss fertility issues before they treat young patients with cancer? Hum Reprod 2008;23:2246–2251. [DOI] [PubMed] [Google Scholar]
- 83. Jadoul P, Kim SS, ISFP Practice Committee . Fertility considerations in young women with hematological malignancies. J Assist Reprod Genet 2012;29:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schover LR, Rybicki LA, Martin BA et al. Having children after cancer. A pilot survey of survivors' attitudes and experiences. Cancer 1999;86:697–709. [DOI] [PubMed] [Google Scholar]
- 85. Salih SM, Elsarrag SZ, Prange E et al. Evidence to incorporate inclusive reproductive health measures in guidelines for childhood and adolescent cancer survivors. J Pediatr Adolesc Gynecol 2015;28:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet 2015;32:1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Practice Committee of American Society for Reproductive Medicine . Ovarian tissue cryopreservation: A committee opinion. Fertil Steril 2014;101:1237–1243. [DOI] [PubMed] [Google Scholar]
- 88. Oktem O, Sonmezer M, Oktay K. Ovarian tissue cryopreservation and other fertility preservation strategies In: Gardner DK, Weissman A, Howles CM. et al., eds. Textbook of Assisted Reproductive Techniques: Laboratory and Clinical Perspectives. 2nd ed. Boca Raton, FL: CRC Press, 2004:315–328. [Google Scholar]
- 89. Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: Report of an ongoing experience. Fertil Steril 2010;93:762–768. [DOI] [PubMed] [Google Scholar]
- 90. Demeestere I, Simon P, Dedeken L et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod 2015;30:2107–2109. [DOI] [PubMed] [Google Scholar]
- 91. Ernst E, Kjærsgaard M, Birkebæk NH et al. Case report: Stimulation of puberty in a girl with chemo‐ and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer 2013;49:911–914. [DOI] [PubMed] [Google Scholar]
- 92. Poirot C, Abirached F, Prades M et al. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet 2012;379:588. [DOI] [PubMed] [Google Scholar]
- 93. Anderson RA, Hindmarsh PC, Wallace WH. Induction of puberty by autograft of cryopreserved ovarian tissue in a patient previously treated for Ewing sarcoma. Eur J Cancer 2013;49:2960–2961. [DOI] [PubMed] [Google Scholar]
- 94. Feigin E, Abir R, Fisch B et al. Laparoscopic ovarian tissue preservation in young patients at risk for ovarian failure as a result of chemotherapy/irradiation for primary malignancy. J Pediatr Surg 2007;42:862–864. [DOI] [PubMed] [Google Scholar]
- 95. Anderson RA, Wallace WH, Baird DT. Ovarian cryopreservation for fertility preservation: Indications and outcomes. Reproduction 2008;136:681–689. [DOI] [PubMed] [Google Scholar]
- 96. Revel A, Revel‐Vilk S, Aizenman E et al. At what age can human oocytes be obtained? Fertil Steril 2009;92:458–463. [DOI] [PubMed] [Google Scholar]
- 97. Borgström B, Hreinsson J, Rasmussen C et al. Fertility preservation in girls with turner syndrome: Prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab 2009;94:74–80. [DOI] [PubMed] [Google Scholar]
- 98. Jadoul P, Dolmans MM, Donnez J. Fertility preservation in girls during childhood: Is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update 2010;16:617–630. [DOI] [PubMed] [Google Scholar]
- 99. Oktay K, Oktem O. Fertility preservation medicine: A new field in the care of young cancer survivors. Pediatr Blood Cancer 2009;53:267–273. [DOI] [PubMed] [Google Scholar]
- 100. Poirot CJ, Martelli H, Genestie C et al. Feasibility of ovarian tissue cryopreservation for prepubertal females with cancer. Pediatr Blood Cancer 2007;49:74–78. [DOI] [PubMed] [Google Scholar]
- 101. Cowles RA, Gewanter RM, Kandel JJ. Ovarian repositioning in pediatric cancer patients: Flexible techniques accommodate pelvic radiation fields. Pediatr Blood Cancer 2007;49:339–341. [DOI] [PubMed] [Google Scholar]
- 102. Terenziani M, Piva L, Meazza C et al. Oophoropexy: A relevant role in preservation of ovarian function after pelvic irradiation. Fertil Steril 2009;91:935.e915–935.e16. [DOI] [PubMed] [Google Scholar]
- 103. Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update 2001;7:535–543. [DOI] [PubMed] [Google Scholar]