Abstract
Objective
To study the association between time in therapeutic range (TTR) during warfarin therapy and risk of dementia in a population based cohort of incident atrial fibrillation (AF).
Methods
We conducted an observational population based study of 2800 non-demented patients with incident AF between January 1, 2000 – December 31, 2010. The association of incident dementia with warfarin therapy and TTR was examined using Cox proportional hazards regression model.
Results
Mean age was 71.2 years, 53% were male, and warfarin was prescribed to 50.5% within 90 days of AF diagnosis. Incident dementia diagnosis occurred in 357 (12.8%) patients over a mean±SD follow-up of 5.0±3.7 years. After adjusting for confounders, warfarin therapy was associated with reduced incidence of dementia [HR (95%CI) 0.80(0.64–0.99)]. However, only those in the two highest quartiles of TTR were associated with lower risk of dementia. A 10% increase in TTR with a 10% reduction in time spent in both sub-therapeutic [HR 0.71(0.64–0.79)] and supra-therapeutic [HR 0.67(0.57–0.79)] ranges were associated with decreased risk of dementia.
Conclusions
In the community, warfarin therapy in AF is associated with a 20% reduction in risk of dementia. Increasing TTR on warfarin is associated with reduced risk of dementia. The risk of dementia was reduced with a reduction in time spent in both sub- and supra-therapeutic INR range. Effective anticoagulation may prevent cognitive impairment in AF.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia of clinical significance in adults and is expected to afflict 5.6 million adults in the USA by the year 20501,2. The prevalence of AF increases with age and 45% of affected individuals are over the age of 75 years1. AF is associated with a 40% increased risk of dementia independent of the occurrence of clinical stroke3,4. Dementia in turn is an important cause of morbidity and mortality in the elderly and an enormous public health problem that has major impact both from a personal and socioeconomic standpoint5.
AF is associated with cerebral thromboembolism, a proposed mechanism by which AF may increase the risk for dementia6–8. Oral anticoagulation with warfarin is effective in preventing thromboembolism in AF, but its effect on risk of dementia is unknown. The efficacy of warfarin in preventing thromboembolism is also dependent on intensity of anticoagulation as measured by the international normalized ratio (INR). While sub-therapeutic INR levels can increase the risk of cerebral thromboembolism, supra-therapeutic INR levels can predispose to intracranial hemorrhage, both of which may increase the risk of cognitive decline9–11. The time in therapeutic range (TTR) is a measure of efficacy of warfarin anticoagulation. The aim of our study is to assess the impact of warfarin anticoagulation and the time spent in therapeutic range on the risk of dementia in a community based cohort of incident AF.
Methods
Study population
This study was conducted in Olmsted County, MN, where the majority of health care is provided by Mayo Clinic, Olmsted Medical Center and their affiliated hospitals. All health care related data were retrieved through the records linkage system of the Rochester Epidemiology Project12–14. This study was approved by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center.
The study design and data retrieval are detailed previously15. Briefly, adults (aged 18 years and older) with AF or atrial flutter between January 1, 2000 and December 31, 2010 were identified using International Classification of Diseases, Ninth Revision (ICD-9) codes 427.31 and 427.32 or diagnosis of AF or atrial flutter on an electrocardiogram obtained at Mayo Clinic. Those with AF or atrial flutter diagnosed before 2000 were classified as prevalent AF and excluded. The medical records of the remaining patients were manually reviewed to validate incident AF if electrocardiographic evidence or a physician diagnosis was present. Patients with the only event of AF documented within 30 days of cardiothoracic surgery without any subsequent recurrence were considered to have post-surgical AF and excluded.
Ascertainment of clinical data
Clinical data on comorbid conditions were obtained using ICD-9 codes from inpatient and outpatient encounters. We required at least 2 occurrences of a code (either the same code or different codes within the code set) within the 5 years prior to incident AF to validate the diagnosis. The list of diagnosis codes used to define each comorbidity can be found in Supplemental Table. Stroke risk was assessed by calculating the CHA2DS2-VASc score16. Smoking status, height, and weight were manually abstracted from the medical record at the time of incident AF. Body mass index (BMI) was calculated as weight (in kg) divided by height (in meters) squared.
Diagnosis of dementia
ICD-9 codes 290, 294.1, 294.8, 331.0–331.2, 331.7, and 331.82 were used to identify study subjects diagnosed with dementia, based on previously validated codes.17 If the diagnosis of dementia was first established before or within 6 months of diagnosis of AF, it was considered prevalent dementia and the subject was excluded.
Time in therapeutic range
International normalized ratio (INR) measurements were obtained to determine patients who were prescribed warfarin and to calculate time in therapeutic range for warfarin. All patients in the county had their INR measured at one of 2 hospitals, providing a complete record of their INRs. The majority of patients in the county are managed at anticoagulation clinics that utilize a standard protocol for warfarin dose adjustment, although the site of anticoagulation management for individual patients in the cohort is not available. Patients with all INRs <1.5 were considered to have never been on warfarin. When a gap of more than 90 days between consecutive INR measurements was observed, we assumed warfarin was discontinued. When warfarin was initiated (or re-initiated after a gap), INRs obtained within the first 7 days were excluded. When multiple INRs were measured on the same day, the mean value was used for that day. An INR was calculated for each day during follow-up using linear interpolation for the days between INR measurements18.
Statistical analysis
Characteristics of the patients at the time of AF diagnosis were described as mean±SD for normally distributed continuous variables, median (25th, 75th percentile) for non-normally distributed continuous variables, and number (%) for categorical variables. Logistic regression was used to test for differences in patient characteristics for those initiated on warfarin within the first 90 days of follow-up and those not on warfarin within the first 90 days. Cox proportional hazard regression models were used to estimate the risk of dementia for those with vs. without warfarin use within the first 90 days after AF diagnosis. Unadjusted, age- and sex-adjusted, and fully-adjusted models adjusting for age, sex, body mass index, history of smoking, hypertension, hyperlipidemia, diabetes, heart failure, ischemic stroke/transient ischemic attack (TIA), hemorrhagic stroke, myocardial infarction, peripheral vascular disease, aortic atherosclerotic disease, chronic pulmonary disease, malignancy, liver disease, and renal disease were modeled (adjusted model 1). Additionally, development of ischemic stroke/TIA or hemorrhagic stroke during follow-up was added as a time-dependent variable to the fully-adjusted model (adjusted model 2). The percent of time in therapeutic range was calculated for each patient as the ratio of the number of days INR was between 2–3 to the total number of days on warfarin. Kaplan-Meier survival curves were plotted by quartiles of percent of TTR. Cox models were run with quartiles of percent of TTR as the exposure (with patients never on warfarin serving as the reference group). Finally, for each day a patient was on warfarin, the INR was categorized as sub-therapeutic (INR<2), therapeutic (INR 2–3) or supra-therapeutic (INR >3). For each period of warfarin use, the percent of time spent in each INR category was calculated. In the Cox models, warfarin use and percent of time spent in sub-therapeutic, therapeutic and supra-therapeutic range were modeled as time-dependent variables with no warfarin as the referent.
Results
Study population and warfarin use
A new diagnosis of AF was determined in 3,344 individuals between January 1, 2000 and December 31, 2010 in Olmsted County, MN. Of these, 544 were excluded due to prevalent dementia (n=319), dementia diagnosis within 6 months of AF (n=86), and incomplete data (n=139). The study cohort consisted of 2800 individuals and the mean±SD age was 71.2±14.6 years, 53.4% were males, and 97.0% were Caucasian. CHA2DS2-VASc score was 0, 1, 2 and >2 in 9.8%, 11.8%, 15.0% and 63.4% of patients, respectively. Overall, 1414 (50.5%) patients were initiated on warfarin within 90 days of the diagnosis of AF and 1772 (63.3%) patients were on warfarin at some point over the course of the study. Only 51 (1.8%) patients were on a novel direct oral anticoagulant (Dabigatran, Apixaban or Rivaroxaban) during follow-up after the year 2011. The use of these novel anticoagulants was not considered in the analysis due to the small proportion of patients on these agents.
The baseline clinical characteristics of the whole cohort and stratified by warfarin therapy within 90 days are presented in Table 1. Patients initiated on warfarin were older and were more likely to have hypertension, diabetes mellitus, hyperlipidemia, myocardial infarction and stroke or TIA at the time of AF diagnosis. Of the patients with CHA2DS2-VASc score ≥2, the level of risk for which anticoagulation is generally recommended, 1178 (53.6%) were initiated on warfarin within 90 days after AF diagnosis; warfarin was initiated in 236 (39.1%) AF patients with CHA2DS2-VASc score <2.
Table 1.
Baseline characteristics of the cohort at the time of atrial fibrillation diagnosis stratified by warfarin therapy.
| Clinical characteristic | All AF patients (N=2800) | AF patients on warfarin within 90 days (N=1414) | AF patients not on warfarin within 90 days (N=1386) | P |
|---|---|---|---|---|
| Age, mean±SD | 71.2±14.6 | 71.9±12.0 | 70.4±16.8 | .005 |
| Male gender, n (%) | 1495 (53.4) | 777 (55.0) | 718 (51.8) | .10 |
| BMI, mean±SD | 29.6±7.3 | 30.3±7.3 | 28.8±7.2 | <.001 |
| History of smoking, n (%) | 1498 (53.9) | 781 (55.7) | 717 (52.0) | .06 |
| Comorbidities prior to AF diagnosis | ||||
| Hypertension, n (%) | 1889 (67.5) | 1011 (71.5) | 878 (63.4) | <.001 |
| Diabetes mellitus, n (%) | 616 (22.0) | 354 (25.0) | 262 (18.9) | <.001 |
| Hyperlipidemia, n (%) | 1487 (53.1) | 807 (57.1) | 680 (49.1) | <.001 |
| Myocardial infarction, n (%) | 358 (12.8) | 200 (14.1) | 158 (11.4) | .03 |
| Peripheral vascular disease, n (%) | 212 (7.6) | 120 (8.5) | 92 (6.6) | .07 |
| Aortic atherosclerotic disease, n (%) | 155 (5.5) | 79 (5.6) | 76 (5.5) | .91 |
| Heart failure, n (%) | 553 (19.8) | 312 (22.1) | 241 (17.4) | .002 |
| Ischemic stroke/TIA, n (%) | 343 (12.3) | 214 (15.1) | 129 (9.3) | <.001 |
| Hemorrhagic stroke, n (%) | 25 (0.9) | 11 (0.8) | 14 (1.0) | .52 |
| Chronic pulmonary disease, n (%) | 432 (15.4) | 229 (16.2) | 203 (14.7) | .26 |
| Malignancy, n (%) | 548 (19.6) | 277 (19.6) | 271 (19.6) | .98 |
| Liver disease, n (%) | 37 (1.3) | 23 (1.6) | 14 (1.0) | .16 |
| Renal disease, n (%) | 172 (6.1) | 90 (6.4) | 82 (5.9) | .62 |
| Assessment of stroke risk | ||||
| CHA2DS2-VASc score, median (25th–75th centile) | 3 (2–4) | 3 (2–5) | 3 (1–4) | <.001 |
| CHA2DS2-VASc score ≥2, n (%) | 2196 (78.4) | 1178 (83.3) | 1018 (73.5) | <.001 |
AF = atrial fibrillation; TIA = transient ischemic attack; BMI = body mass index.
Warfarin therapy and dementia in AF
During a mean±SD follow-up of 5.0±3.7 years, dementia was diagnosed in 357 (12.8%) patients 6 months or more after the diagnosis of AF. The cumulative incidence (%, 95% CI) of dementia at 2 and 5 years was 4.4 (3.5–5.2)% and 11.6 (10.2–13.0)% respectively. The incidence of dementia in the group with warfarin therapy within 90 days after AF diagnosis was 4.1 (3.0 – 5.3)% and 11.2 (9.2 – 13.1)% at 2 and 5 years respectively. Dementia was diagnosed in 4.6 (3.4 – 5.9)% and 12.1 (9.9 – 14.1)% of patients without warfarin within 90 days at 2 and 5 years (P=.58 comparing incidence of dementia for those with and without warfarin therapy within 90 days). The associations between warfarin therapy within 90 days post AF and dementia in unadjusted and adjusted models are presented in Table 2. In the unadjusted model, warfarin therapy was not associated with risk of dementia. However, after adjusting for potential confounders in model 1, warfarin use was associated with a 22% reduced risk of dementia [hazard ratio (HR) 0.78 (95% CI 0.64–0.97)]. It is notable that this association persisted after adjusting for incident clinical stroke/TIA and hemorrhagic stroke during follow-up (model 2).
Table 2.
Associations between warfarin therapy within 90 days after atrial fibrillation diagnosis and incident dementia
| Model | HR (95% CI) | P |
|---|---|---|
| Unadjusted | 0.94 (0.77–1.16) | .58 |
| Age- and sex-adjusted | 0.83 (0.68–1.10) | .08 |
| Adjusted model 1a | 0.78 (0.64–0.97) | .03 |
| Adjusted model 2b | 0.80 (0.64–0.99) | .04 |
Adjusted for age, sex, body mass index, history of smoking, hypertension, hyperlipidemia, diabetes, heart failure, ischemic stroke/transient ischemic attack, hemorrhagic stroke, myocardial infarction, peripheral vascular disease, aortic atherosclerotic disease, chronic pulmonary disease, malignancy, liver disease, and renal disease.
Adjusted for variables in model 1 plus ischemic stroke/transient ischemic attack and hemorrhagic stroke modeled as time-dependent variables.
CI = confidence interval; HR = hazard ratio
The association between warfarin therapy within 90 days and dementia was then examined in the cohort stratified by CHA2DS2-VASc score. Amongst patients with CHA2DS2-VASc score ≥2, warfarin was associated with a lower risk of dementia in the unadjusted model [HR 0.77 (95% CI 0.62–0.95), P=.02] with no significant association in the adjusted models [model 1 HR 0.82 (95% CI 0.66–1.02), P=.08; model 2 HR 0.85 (95% CI 0.68–1.06), P=.16]. There was no significant association between warfarin use and dementia in patients with CHA2DS2-VASc score <2 in the unadjusted [HR 1.24 (95% CI 0.51–2.99), P=.63] and adjusted models [model 1 HR 0.84 (95% CI 0.33–2.12), P=.71; model 2 HR 0.82 (95% CI 0.30–2.23), P=.69].
Time in therapeutic range on warfarin and incident dementia
Amongst patients on warfarin, the median (25th, 75th percentile) number of INR measurements per person during follow-up was 52 (15, 121); the mean±SD interval between INR measurements was 14.3±12.0 days. The mean±SD percent of time patients were on warfarin during follow-up was 55.4%±39.0%. During follow-up, 762 (43.0%) remained on warfarin throughout follow-up, 436 (24.6%) discontinued warfarin use and never resumed, 397 (22.4%) discontinued warfarin and resumed use at least once and were on warfarin at the time of dementia/last follow-up, and 177 (10.0%) discontinued warfarin and resumed use at least once and were not on warfarin at the time of dementia/last follow-up. The mean±SD percent of time patients spent in therapeutic range was 52.2%±26.4%. To assess the possible effect of preexisting cognitive decline on percent of TTR, we also examined the percent of TTR within 1 year prior to the diagnosis of dementia. In patients who developed dementia, the percent of TTR within 1 year prior to dementia diagnosis [mean±SD 51.6%±26.5%] was lower than the percent of TTR >1 year prior to dementia [52.2% ±24.8%] and was also lower than the percent of TTR in patients without dementia [52.5%±26.7%]. However, the absolute differences in the percent of TTR noted above may not be clinically meaningful.
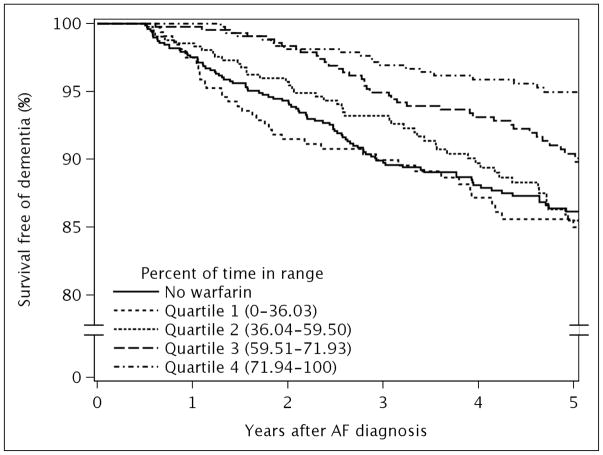
Patients were divided into quartiles of percent of TTR (quartile 1: 0–36.03%; quartile 2: 36.04–59.50%; quartile 3: 59.51–71.93%; quartile 4: 71.94–100%) and the incidence of dementia was examined in each quartile. Mean±SD percent of TTR in each quartile was 12.8%±12.7%, 49.6% ± 6.9%, 66.2% ±3.7%, and 80.0% ± 6.5%. Figure 1 presents survival free of dementia in each quartile of percent of TTR. When compared to the group without warfarin therapy, quartiles 3 and 4 for percent of TTR were associated with a 46% and 72% reduced risk of dementia, respectively, in the fully adjusted model 2 (Table 3). In contrast, quartile 1 and 2 of percent of TTR had similar risk of dementia compared to those not treated with warfarin.
Figure 1.
Survival free of dementia stratified by quartiles of percent of time spent in therapeutic range.
Table 3.
Associations between quartiles of percent of time in therapeutic range on warfarin and incident dementia among atrial fibrillation patients
| Model | No warfarin HR (95% CI) | Quartile 1(0–36.03%) HR (95% CI) | Quartile 2(36.04%–59.50%) HR (95% CI) | Quartile 3(59.51%–71.93%) HR (95% CI) | Quartile 4(71.94%–100%) HR (95% CI) | P for trend |
|---|---|---|---|---|---|---|
| Unadjusted | 1.00 (ref) | 1.12 (0.82–1.53) | 1.07 (0.80–1.42) | 0.74 (0.55–1.00) | 0.34 (0.23–0.51) | <.001 |
| Age- and sex-adjusted | 1.00 (ref) | 1.00 (0.73–1.36) | 0.99 (0.75–1.32) | 0.60 (0.44–0.81) | 0.30 (0.20–0.44) | <.001 |
| Adjusted model 1a | 1.00 (ref) | 0.95 (0.69–1.31) | 0.95 (0.71–1.27) | 0.54 (0.40–0.73) | 0.29 (0.20–0.42) | <.001 |
| Adjusted model 2b | 1.00 (ref) | 0.94 (0.67–1.31) | 0.93 (0.69–1.25) | 0.54 (0.39–0.73) | 0.28 (0.19–0.42) | <.001 |
Adjusted for age, sex, body mass index, history of smoking, hypertension, hyperlipidemia, diabetes, heart failure, ischemic stroke/transient ischemic attack, hemorrhagic stroke, myocardial infarction, peripheral vascular disease, aortic atherosclerotic disease, chronic pulmonary disease, malignancy, liver disease, and renal disease.
Adjusted for variables in model 1 plus ischemic stroke/transient ischemic attack and hemorrhagic stroke modeled as time-dependent variables.
CI = confidence interval; HR = hazard ratio
We performed additional modeling to determine the impact of percent of time spent in therapeutic, sub- and supra-therapeutic ranges on dementia risk. Combining the daily INR results over follow-up for all patients, the cohort on warfarin spent a total of 19%, 67% and 14% of their time on warfarin in sub-therapeutic, therapeutic and supra-therapeutic ranges, respectively. These proportions were considered the referent. Modeling warfarin use and percent of time in sub-therapeutic, therapeutic and supra-therapeutic ranges as time-dependent variables, the unadjusted and adjusted models are presented in Table 4. A 10% absolute increase in the percent of TTR was associated with a significant decrease in the risk of dementia. The risk reduction was comparable if the increase in percent of TTR occurred in conjunction with a reduction in percent of time spent in the supra-therapeutic range [model 2 HR 0.67 (95% CI 0.57–0.79)] or sub-therapeutic range [HR 0.71 (95% CI 0.64–0.79)].
Table 4.
Associations between percent of time in sub-therapeutic, therapeutic and supra-therapeutic INR range and risk of dementia among atrial fibrillation patients.
| Proportion of time in each INR category | ||||
|---|---|---|---|---|
|
| ||||
| INR categories | Reference category | Scenario 1a | Scenario 2b | Scenario 3c |
|
| ||||
| Sub-therapeutic INR (<2) | 19% | 9% | 14% | 19% |
| Therapeutic INR (2 – 3) | 67% | 77% | 77% | 77% |
| Supra-therapeutic INR (>3) | 14% | 14% | 9% | 4% |
|
| ||||
| Models | Referent | HR (95% CI) | HR (95% CI) | HR (95% CI) |
|
| ||||
| Unadjusted model | 1.00 (ref) | 0.71 (0.65–0.78) | 0.74 (0.66–0.82) | 0.76 (0.65–0.89) |
| Age- and sex-adjusted | 1.00 (ref) | 0.70 (0.64–0.77) | 0.70 (0.62–0.78) | 0.69 (0.59–0.81) |
| Adjusted model 1d | 1.00 (ref) | 0.70 (0.63–0.77) | 0.69 (0.61–0.77) | 0.68 (0.58–0.80) |
| Adjusted model 2e | 1.00 (ref) | 0.71 (0.64–0.79) | 0.69 (0.62–0.77) | 0.67 (0.57–0.79) |
Scenario 1 represents a 10% absolute increase in time in therapeutic range with a corresponding 10% absolute decrease in the proportion of time spent in sub-therapeutic range of INR.
Scenario 2 represents a 10% absolute increase in time in therapeutic range with a corresponding 5% absolute decrease in the proportion of time spent in sub-therapeutic range and a 5% absolute decrease in the proportion of time spent in supra-therapeutic range of INR.
Scenario 3 represents a 10% absolute increase in time in therapeutic range with a corresponding 10% absolute decrease in the proportion of time spent in supra-therapeutic range of INR.
Adjusted for age, sex, body mass index, history of smoking, hypertension, hyperlipidemia, diabetes, heart failure, ischemic stroke/transient ischemic attack, hemorrhagic stroke, myocardial infarction, peripheral vascular disease, aortic atherosclerotic disease, chronic pulmonary disease, malignancy, liver disease, and renal disease.
Adjusted for variables in model 1 plus ischemic stroke/transient ischemic attack and hemorrhagic stroke modeled as time-dependent variables.
CI = confidence interval; HR = hazard ratio
Discussion
In a population based cohort of patients with incident AF, anticoagulation with warfarin was associated with an approximately 20% lower risk of dementia. Amongst patients initiated on warfarin, greater efficacy of warfarin anticoagulation, measured by the time in therapeutic range, was significantly associated with lower risk of dementia. Only those in the two highest quartiles of percent of TTR had associated decline in risk of dementia. The incidence of dementia in patients with TTR below the median was similar to those not prescribed warfarin. Increase in TTR as a result of decrease in time spent in both sub-therapeutic and supra-therapeutic INR was associated with a reduction in incidence of dementia.
The mechanisms of cognitive impairment in AF are likely multifactorial. A role for cerebral infarcts due to thromboembolism and cerebral hypoperfusion due to reduced cardiac output has been proposed6,7,19. The majority of cerebral infarcts detected by magnetic resonance imaging (MRI) do not manifest as clinical stroke. These silent cerebral infarcts have been associated with higher risk of cognitive impairment and dementia both in the general population and in patients with AF6,7,20. Graff-Radford et al reported a 30% prevalence of MRI detected cerebral infarct in community dwelling individuals with AF, a significantly higher prevalence compared to those without AF [odds ratio 1.87 (95% CI 1.25–2.81)]7. The risk of cognitive impairment in AF patients with MRI evidence of infarction was three times higher than AF patients without cerebral infarct and non-AF patients7. Barber et al reported serum markers of increased thrombin generation and fibrin turnover in subjects with AF who developed dementia, suggestive of a predisposition for thrombosis21. Oral anticoagulation with warfarin has been shown to reduce the incidence of thromboembolism and stroke in AF,22 and is a potential mechanism for the reduction in risk of dementia associated with anticoagulation that we observed in this study. We also observed that the effect of warfarin therapy on dementia was more pronounced in those with CHA2DS2-VASc score ≥2, a subset with higher risk of thromboembolism for whom anticoagulation is recommended by guideline16.
Warfarin has a narrow therapeutic window for the prevention of stroke with the greatest efficacy and safety when INR is maintained between 2 and 3. There is a 5-fold increased risk of ischemic stroke with INR<2 and a 3 fold increased risk of major bleeding with INR>3.9 The risk of stroke, myocardial infarction, major bleeding and death increases on a continuum with the decline in TTR9,23,24. Our study suggests that the benefits of maintaining INR in the therapeutic range between 2 and 3 may extend beyond stroke prevention to better neurocognitive outcomes. However, due to the observational design of the study, conclusions regarding cause and effect cannot be drawn and these findings are hypothesis generating only. The novel direct oral anticoagulants may provide a more steady state of anticoagulation, thus theoretically avoiding the fluctuations in anticoagulation intensity associated with warfarin. Whether this translates to reduced risk of cognitive impairment in patients treated with a novel anticoagulant requires future prospective study. Due to the time frame of the current study, the use of these novel anticoagulants was not prevalent.
Prevention of thromboembolism can potentially explain the reduction in incident dementia in association with a reduction in sub-therapeutic INR. A striking finding however, is the reduction in dementia risk noted with decline in time spent in supra-therapeutic INR. Warfarin anticoagulation is associated with increased risk of intracerebral hemorrhage, with a progressive increase with every 0.5 elevation in INR25. Clinically manifest intracerebral hemorrhage is associated with an increased risk for cognitive decline, but the incidence is low even in anticoagulated patients10,11. Cerebral microbleeds, which are associated with amyloid angiopathy and arteriolosclerosis, have emerged as a predictor of future intracerebral hemorrhage and cognitive decline26–28. Both AF and oral anticoagulation have been associated with increased prevalence of cerebral microbleeds29,30. While cerebral microbleeds are more likely to occur in those treated with an anticoagulant, microbleeds in turn increase the risk of warfarin-associated cerebral hemorrhage26,28,31,32. The presence of cerebral microbleeds and hemorrhage may be a potential explanation for the elevated risk of dementia with supra-therapeutic anticoagulation. It is possible that at a threshold INR of 3, the risk of dementia in association with cerebral bleed may outweigh any benefit from prevention of thromboembolism. Neuroimaging was however not performed as part of this study and this hypothesis should be verified in future studies.
This study presents the first comprehensive longitudinal study on risk of dementia in AF patients with and without oral anticoagulation in a community setting and adds to previous reports. Barber et al reported a trend towards reduced incidence of dementia in AF treated with warfarin21. In contrast, the Birmingham Atrial Fibrillation Treatment of the Aged Study, a randomized controlled trial of warfarin vs. aspirin in AF did not show a significant difference in Mini-Mental State Examination scores between the groups, although a trend in favor of warfarin was noted at 33 months33. Both studies were limited by smaller sample size and shorter follow-up. Jacobs et al reported increasing incidence of dementia with lower TTR in a cohort of warfarin treated AF patients34. In contrast to our study, however, there was no differential effect of sub- and supra-therapeutic INR on incident dementia. An increased incidence of dementia in AF patients with supra-therapeutic INR and combination therapy with an anti-platelet agent has been reported35.
Only 64% of patients in the cohort were initiated on warfarin, which is comparable to data from national registries that show less than two thirds of eligible patients with AF are on anticoagulation36. TTR in our study is lower than that reported in randomized controlled trials of anticoagulation in AF, but comparable to that in multiple other observational studies and represents real world experience and also illustrates the potential for improvement24,37,38. Challenges to maintaining therapeutic INR are frequently multifactorial and the impact of novel oral anticoagulants on cognitive outcomes warrant investigation.
Limitations
This is an observational study and the decision to treat with warfarin was not randomized. While known confounders were adjusted for, residual confounding due to unmeasured risk factors such as alcohol consumption cannot be excluded. The duration, pattern (paroxysmal vs persistent) and treatment (rate vs rhythm control) of AF could not be reliably determined and may impact the risk of dementia and the decision to anticoagulate. Factors beyond the risk of stroke can influence the prescription of anticoagulants including the diagnosis of dementia, risk for falls and nursing home residence39,40. Cognitive impairment represents a continuous spectrum of disorders and patients with milder forms of the disease may remain undiagnosed. Patients with cognitive impairment may have a lower rate of initiation and higher rate of discontinuation of oral anticoagulant. Discontinuation of warfarin could not be definitely determined in this cohort. Anticoagulated patients with mild cognitive impairment may also have lower TTR due to medication non-compliance and inability to follow dietary restrictions, which may influence the observations of this study. The exclusion of patients with prevalent dementia and incident dementia within 6 months of AF diagnosis reduces this bias but does not eliminate the possibility of undiagnosed milder forms of cognitive dysfunction impacting prescription patterns or the TTR. TTR in the year prior to dementia diagnosis was noted to be slightly lower than that in the preceding years, but the absolute difference may not be clinically significant. However, the TTR greater than 1 year prior to the diagnosis of dementia was comparable to those who did not develop dementia suggesting that undiagnosed dementia likely did not have a significant effect on the majority of INR measurements obtained in the study.
Patients prescribed warfarin had more co-morbidities than those not prescribed warfarin, many of which have been known to affect the risk of dementia. While statistical methods to adjust for these confounders were undertaken, we cannot rule out the possibility of residual confounding resulting in a more conservative estimate of the association between warfarin use and dementia.
The reason for the prescription of oral anticoagulation was not available and indications other than AF cannot be excluded, especially in lower risk patients with CHA2DS2-VASc score <2. Standardized neurocognitive testing to establish the diagnosis of dementia was not performed and dementia may be underdiagnosed. The use of over-the-counter drugs such as aspirin and NSAIDs could not be ascertained and may affect the outcome. Finally, INR values vary from day to day and the use of linear interpolation between two measurements may not accurately reflect these day to day variations in the intensity of anticoagulation.
Conclusions
Oral anticoagulation with warfarin was associated with reduced incidence of dementia in a community based cohort of incident AF, with a more prominent effect in those with CHA2DS2-VASc score ≥2. These data support and extend the potential benefits of current recommendations to anticoagulate high risk AF patients. Furthermore, among patients on warfarin more time in the narrow therapeutic window of warfarin was associated with a reduced risk of dementia compared to patients with less time in therapeutic range. Since novel direct oral anticoagulants are associated with less variations in the intensity of anticoagulation, prospective studies on the role of both warfarin and the novel oral anticoagulants in preventing cognitive decline in AF patients are needed.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the American Heart Association (11SDG7260039) and the National Institute on Aging (R01 AG034676). The funding sources played no role in the design, conduct, or reporting of this study.
Abbreviations
- AF
Atrial fibrillation
- INR
International normalized ratio
- TIA
Transient ischemic attack
- TTR
Time in therapeutic range
Footnotes
Relationship with industry: none for all authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Jama. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158(5 Pt 1):338–346. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyasaka Y, Barnes ME, Petersen RC, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur Heart J. 2007;28(16):1962–1967. doi: 10.1093/eurheartj/ehm012. [DOI] [PubMed] [Google Scholar]
- 5.Plassman BL, Langa KM, McCammon RJ, et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011;70(3):418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaita F, Corsinovi L, Anselmino M, et al. Prevalence of Silent Cerebral Ischemia in Paroxysmal and Persistent Atrial Fibrillation and correlation with cognitive function. J Am Coll Cardiol. 2013;62(21):1990–1997. doi: 10.1016/j.jacc.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 7.Graff-Radford J, Madhavan M, Vemuri P, et al. Atrial fibrillation, cognitive impairment, and neuroimaging. Alzheimers Dement. 2016;12(4):391–398. doi: 10.1016/j.jalz.2015.08.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34(5):1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds MW, Fahrbach K, Hauch O, et al. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis. Chest. 2004;126(6):1938–1945. doi: 10.1378/chest.126.6.1938. [DOI] [PubMed] [Google Scholar]
- 10.Benedictus MR, Hochart A, Rossi C, et al. Prognostic Factors for Cognitive Decline After Intracerebral Hemorrhage. Stroke. 2015;46(10):2773–2778. doi: 10.1161/STROKEAHA.115.010200. [DOI] [PubMed] [Google Scholar]
- 11.Garcia PY, Roussel M, Bugnicourt JM, et al. Cognitive impairment and dementia after intracerebral hemorrhage: a cross-sectional study of a hospital-based series. J Stroke Cerebrovasc Dis. 2013;22(1):80–86. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamberlain AM, Gersh BJ, Alonso A, et al. Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med. 2015;128(3):260–267. e261. doi: 10.1016/j.amjmed.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St Germaine-Smith C, Metcalfe A, Pringsheim T, et al. Recommendations for optimal ICD codes to study neurologic conditions: a systematic review. Neurology. 2012;79(10):1049–1055. doi: 10.1212/WNL.0b013e3182684707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. [PubMed] [Google Scholar]
- 19.Lavy S, Stern S, Melamed E, Cooper G, Keren A, Levy P. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke; a journal of cerebral circulation. 1980;11(1):35–38. doi: 10.1161/01.str.11.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 21.Barber M, Tait RC, Scott J, Rumley A, Lowe GD, Stott DJ. Dementia in subjects with atrial fibrillation: hemostatic function and the role of anticoagulation. Journal of thrombosis and haemostasis: JTH. 2004;2(11):1873–1878. doi: 10.1111/j.1538-7836.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- 22.Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet. 1994;343(8899):687–691. [PubMed] [Google Scholar]
- 23.Jones M, McEwan P, Morgan CL, Peters JR, Goodfellow J, Currie CJ. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91(4):472–477. doi: 10.1136/hrt.2004.042465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007;167(3):239–245. doi: 10.1001/archinte.167.3.239. [DOI] [PubMed] [Google Scholar]
- 25.Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999;53(6):1319–1327. doi: 10.1212/wnl.53.6.1319. [DOI] [PubMed] [Google Scholar]
- 26.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010;41(6):1222–1228. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 27.Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78(5):326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 28.van Etten ES, Auriel E, Haley KE, et al. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke. 2014;45(8):2280–2285. doi: 10.1161/STROKEAHA.114.005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horstmann S, Mohlenbruch M, Wegele C, et al. Prevalence of atrial fibrillation and association of previous antithrombotic treatment in patients with cerebral microbleeds. Eur J Neurol. 2015;22(10):1355–1362. doi: 10.1111/ene.12608. [DOI] [PubMed] [Google Scholar]
- 30.Song TJ, Kim J, Song D, et al. Association of cerebral microbleeds with mortality in stroke patients having atrial fibrillation. Neurology. 2014;83(15):1308–1315. doi: 10.1212/WNL.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 31.Lee SH, Ryu WS, Roh JK. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology. 2009;72(2):171–176. doi: 10.1212/01.wnl.0000339060.11702.dd. [DOI] [PubMed] [Google Scholar]
- 32.Orken DN, Uysal E, Timer E, Kuloglu-Pazarci N, Mumcu S, Forta H. New cerebral microbleeds in ischemic stroke patients on warfarin treatment: two-year follow-up. Clin Neurol Neurosurg. 2013;115(9):1682–1685. doi: 10.1016/j.clineuro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Mavaddat N, Roalfe A, Fletcher K, et al. Warfarin versus aspirin for prevention of cognitive decline in atrial fibrillation: randomized controlled trial (Birmingham Atrial Fibrillation Treatment of the Aged Study) Stroke; a journal of cerebral circulation. 2014;45(5):1381–1386. doi: 10.1161/STROKEAHA.113.004009. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs V, Woller SC, Stevens S, et al. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart rhythm: the official journal of the Heart Rhythm Society. 2014;11(12):2206–2213. doi: 10.1016/j.hrthm.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs V, Woller SC, Stevens SM, et al. Percent Time With a Supratherapeutic INR in Atrial Fibrillation Patients Also Using an Antiplatelet Agent Is Associated With Long-Term Risk of Dementia [published online ahead of print August 13, 2015] J Cardiovasc Electrophysiol. 2015 doi: 10.1111/jce.12776. [DOI] [PubMed] [Google Scholar]
- 36.Hsu JC, Maddox TM, Kennedy K, et al. Aspirin Instead of Oral Anticoagulant Prescription in Atrial Fibrillation Patients at Risk for Stroke. J Am Coll Cardiol. 2016;67(25):2913–2923. doi: 10.1016/j.jacc.2016.03.581. [DOI] [PubMed] [Google Scholar]
- 37.Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118(20):2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 38.Dlott JS, George RA, Huang X, et al. National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation. 2014;129(13):1407–1414. doi: 10.1161/CIRCULATIONAHA.113.002601. [DOI] [PubMed] [Google Scholar]
- 39.Holt TA, Hunter TD, Gunnarsson C, Khan N, Cload P, Lip GY. Risk of stroke and oral anticoagulant use in atrial fibrillation: a cross-sectional survey. Br J Gen Pract. 2012;62(603):e710–717. doi: 10.3399/bjgp12X656856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tjia J, Field TS, Mazor KM, et al. Dementia and risk of adverse warfarin-related events in the nursing home setting. The American journal of geriatric pharmacotherapy. 2012;10(5):323–330. doi: 10.1016/j.amjopharm.2012.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.