Abstract
OBJECTIVES
This study sought to investigate whether patients with mental stress–induced myocardial ischemia will have high resting and post–mental stress high-sensitivity cardiac troponin I (hs-cTnI).
BACKGROUND
Hs-cTnI is a marker of myocardial necrosis, and its elevated levels are associated with adverse outcomes. Hs-cTnI levels may increase with exercise in patients with coronary artery disease. Mental stress–induced myocardial ischemia is also linked to adverse outcomes.
METHODS
In this study, 587 patients with stable coronary artery disease underwent technetium Tc 99m sestamibi–single-photon emission tomography myocardial perfusion imaging during mental stress testing using a public speaking task and during conventional (pharmacologic/exercise) stress testing as a control condition. Ischemia was defined as new/worsening impairment in myocardial perfusion using a 17-segment model.
RESULTS
The median hs-cTnI resting level was 4.3 (interquartile range [IQR]: 2.9 to 7.3) pg/ml. Overall, 16% and 34.8% of patients developed myocardial ischemia during mental and conventional stress, respectively. Compared with those without ischemia, median resting hs-cTnI levels were higher in patients who developed ischemia either during mental stress (5.9 [IQR: 3.9 to 8.3] vs. 4.1 [IQR: 2.7 to 7.0] pg/ml; p < 0.001) or during conventional stress (5.4 [IQR: 3.9 to 9.3] vs. 3.9 [IQR: 2.5 to 6.5] pg/ml; p < 0.001). Patients with high hs-cTnI (cutoff of 4.6 pg/ml for men and 3.9 pg/ml for women) had greater odds of developing mental (odds ratio [OR]: 2.4; 95% confidence interval [CI]: 1.5 to 3.9; p < 0.001) and conventional (OR: 2.4; 95% CI: 1.7 to 3.4; p < 0.001) stress-induced ischemia. Although there was a significant increase in 45-min post–treadmill exercise hs-cTnI levels in those who developed ischemia, there was no significant increase after mental or pharmacological stress test.
CONCLUSIONS
In patients with coronary artery disease, myocardial ischemia during either mental stress or conventional stress is associated with higher resting levels of hs-cTnI. This suggests that hs-cTnI elevation is an indicator of chronic ischemic burden experienced during everyday life. Whether elevated hs-cTnI levels are an indicator of adverse prognosis beyond inducible ischemia or whether it is amenable to intervention requires further investigation.
Keywords: high-sensitivity troponin I, mental stress, myocardial ischemia, physical stress
Cardiac troponin is a recognized marker of myocardial injury and it is elevated in several clinical conditions besides myocardial infarction, including myocarditis, severe heart failure, hypertensive crises, and pulmonary embolism (1,2). Development of sensitive assays has enabled determination of hitherto undetectable levels of troponin. Recent reports using high-sensitivity assays indicate that troponin can be released in the setting of myocardial ischemia induced by rapid atrial pacing and exercise, even in the absence of necrosis (2–4). Elevated levels of high-sensitivity cardiac troponin (hs-cTn) in subjects without acute coronary syndromes are also associated with adverse long-term outcomes, and thus it is important to determine the mechanisms underlying these changes (1,5–7).
Psychological stress may precipitate myocardial ischemia. In a laboratory setting, mental stress-induced myocardial ischemia (MSIMI) is associated with adverse cardiovascular outcomes in patients with coronary artery disease (CAD), although the mechanisms of this increased risk remain unknown (8). MSIMI may occur even in the absence of ischemia during physical stress (9). Abnormal vasomotion, depression, platelet reactivity, vitamin D deficiency, inflammation, and metabolic risk factors have been associated with MSIMI, but the exact physiological mechanisms are still unclear (9). Although physical stress has been associated with release of troponin, whether MSIMI will also lead to a similar change remains unknown (3,10). In a large sample of patients with chronic stable CAD, we measured high-sensitivity cardiac troponin I (hs-cTnI) levels at rest and after both conventional and mental stress testing to investigate the effects of ischemia on hs-cTnI levels. We hypothesized that subjects with both forms of ischemia, with mental stress and with conventional stress, will have higher resting and/or post-stress hs-cTnI levels. This may help us understand whether recurrent episodes of myocardial ischemia result in hs-cTn elevation.
METHODS
STUDY SAMPLE
Patients were enrolled into the MIPS (Mental Stress Ischemia Prognosis Study), a prospective study that recruited patients with stable CAD between June 23, 2011 and August 5, 2014 at Emory University-affiliated hospitals. Presence of CAD was defined by an abnormal coronary angiogram demonstrating evidence of atherosclerosis with at least luminal irregularities, documented previous percutaneous or surgical coronary revascularization, documented myocardial infarction, or a positive nuclear stress test. Patients with acute coronary syndrome or decompensated heart failure during the previous 2 months, end-stage renal disease, or unstable psychiatric conditions were excluded. Clinical information including previous CAD events, CAD risk factors, coronary angiography results, and current medications were documented using standardized questionnaires and chart reviews. The research protocol was approved by the institutional review board of our institution and all participants provided informed consent. As described previously (11), patients were tested in the morning after a 12-h fast. Antianginal medications (beta-blockers, calcium-channel blockers, and long-acting nitrates), xanthine derivatives, and caffeine-containing products were withheld for 24 h prior to stress testing (conventional and mental). Current and lifetime diagnosis of major depression and other psychiatric diagnoses were assessed with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (12). Estimated creatinine clearance was calculated by means of the Chronic Kidney Disease Epidemiology Collaboration equation. Angiographic CAD severity was calculated using the Gensini score for 511 patients with a median time between the angiogram and enrolment of 2.1 years (interquartile range [IQR]: 1.0 to 4.7 years) (11).
MENTAL STRESS PROCEDURE
In a quiet dimly lit, temperature-controlled (21 °C to 23 °C) room, after a 30-min rest period, vital signs were measured and mental stress was induced by a standardized public speaking task. Briefly, patients were asked to imagine a situation in which a close relative had been mistreated in a nursing home. Patients were given 2 min to prepare their speech and 3 min to deliver their speech in front of an evaluative audience. Blood pressure and heart rate were recorded. Mental stress testing was performed by trained and experienced staff to standardize the psychophysiological stress-provoking elements of the test. During mental stress testing, 20 to 30 mCi of technetium Tc 99m radioisotope was given 1 min into the speech.
CONVENTIONAL STRESS TESTING
On a separate day, and within 1 week of the mental stress, conventional stress testing was performed using the Bruce protocol, and when contraindicated, pharmacologic testing with regadenoson. The radioisotope injection was given at peak exertion during the exercise test or immediately after the regadenoson injection.
MYOCARDIAL PERFUSION IMAGING AND SPECT IMAGES INTERPRETATION
Myocardial perfusion imaging with technetium Tc 99m sestamibi-single-photon emission computed tomography (SPECT) was performed at rest and 30 to 60 min after mental and conventional (exercise/pharmacologic) stress according to standard protocols (11). Studies were interpreted by 2 experienced readers blinded to the stressor (mental or exercise/pharmacological) and without prior knowledge of severity of CAD or other patient medical history. Discrepancies in interpretation of SPECT images were resolved by consensus. Rest and stress images were visually compared for number and severity of perfusion defects using a 17-segment model. Each segment was scored from 0 to 4, with 0 being normal uptake and 4 no uptake. Ischemia was defined as a new impairment with a score ≥2 in any segment, or as worsening of a pre-existing impairment by at least 2 points if in a single segment, or by at least 1 point if in 2 or more contiguous segments (13). In addition to individual segment scores, we calculated summed scores in a conventional fashion, including a summed stress score, a summed rest score (SRS), and a summed difference score (SDS), the latter representing a semiquantitative measure of inducible ischemia (13). We calculated the percentage of myocardium with resting perfusion defects as (SRS ÷ 68) × 100 and percent of ischemic myocardium as (SDS ÷ 68) × 100, as previously described (12). Only a limited number of patients had echocardiogram performed within 1 year of enrollment, thus ejection fraction was evaluated using SPECT imaging from the resting scan.
HS-cTn ASSAY
Patients had fasting venous blood drawn at rest, 45 min, and 90 min after mental stress test (n = 587, 542, and 533, respectively), and 45 min after conventional stress testing (n = 461). Samples were processed and stored at −80°C. Plasma hs-cTnI was measured using the ARCHITECT STAT Hs-cTnI assay (Abbott Laboratories, Abbott Park, Illinois), which has a limit of detection of 1.2 pg/ml and an interassay coefficient of variation of <10% at 4.7 pg/ml. The upper reference limit (99th centile) ranges between 24 pg/ml and 30 pg/ml in healthy populations (14–16), with a sex-specific upper reference range of 36 pg/ml for men and 15 pg/ml for women (17).
STATISTICAL ANALYSES
To examine the differences between assigned groups, 2-sample Student t tests and Kruskal-Wallis tests were used for continuous variables and chi-square tests were used for categorical variables. Hs-cTnI levels are reported as median (IQR). The natural logarithmic transformation was used for non-normally distributed variables (troponin and Gensini score). Pearson and Spearman rank correlations and linear regression analysis were used to study factors associated with resting hs-cTnI. Linear mixed models of the natural logarithm of hs-cTnI were used to investigate change during mental or conventional stress. Logistic regression analysis was used to derive odds ratios (OR) for the association between resting hs-cTnI levels and presence of MSIMI or conventional stress-induced myocardial ischemia (CSIMI) after adjusting for factors that affect resting hs-cTnI. Hs-cTnI was studied as continuous and binary variables. Because there is no clinically established cutoff for the association between hs-cTnI and ischemia, we used high hs-cTnI using cutoffs that have been linked to adverse cardiovascular outcomes in the JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial: 4.6 pg/ml for men; 3.9 pg/ml for women (7).
RESULTS
A total of 587 patients with stable CAD were included in this study. Mean age was 63 ± 9 years, 76% were male, 32% had diabetes mellitus, and 31% had a history of previous myocardial infarction (Table 1). Conventional stress testing was performed with a treadmill test in 407 patients and the remaining had a pharmacological stress test (Online Table 1 compares patients who had exercise vs. pharmacological stress tests). Incidence of MSIMI and CSIMI was 16% (n = 94) and 34.8% (n = 204), respectively. No significant differences in demographic features or risk factor profiles were observed between patients with ischemia (mental or conventional) compared with those without ischemia. Most patients who developed MSIMI also developed CSIMI (74.5%). In those with ischemia during both stress tests, the severity of ischemia was lower during mental stress than during the conventional stress (median perfusion defect of 4% [IQR: 3% to 7%] vs. 9% [IQR: 5% to 13%] of left ventricle; p < 0.001).
TABLE 1.
Patient Characteristics
| MSIMI Negative (n = 493) |
MSIMI Positive (n = 94) |
p Value* | CSIMI Negative (n = 383) |
CSIMI Positive (n = 204) |
p Value* | |
|---|---|---|---|---|---|---|
| Age, yrs | 63 ± 9 | 62 ± 9 | 0.54 | 63 ± 9 | 63 ± 9 | 0.58 |
| Male | 371 (75) | 76 (81) | 0.24 | 282 (74) | 165 (81) | 0.05 |
| African American | 131 (27) | 30 (32) | 0.29 | 103 (27) | 58 (28) | 0.69 |
| Body mass index, kg/m2 | 30 ± 5 | 30 ± 5 | 0.42 | 30 ± 5 | 30 ± 5 | 0.65 |
| Hypertension | 335 (68) | 63 (67) | 0.86 | 259 (68) | 139 (68) | 0.9 |
| Hyperlipidemia | 394 (80) | 77 (82) | 0.66 | 304 (79) | 167 (82) | 0.47 |
| Diabetes | 150 (30) | 36 (38) | 0.13 | 112 (29) | 74 (36) | 0.08 |
| Current/former smoking | 288 (59) | 62 (66) | 0.19 | 221 (58) | 129 (63) | 0.23 |
| Myocardial infarction | 176 (36) | 38 (40) | 0.38 | 142 (37) | 72 (35) | 0.67 |
| Congestive heart failure | 63 (14) | 13 (15) | 0.69 | 46 (13) | 30 (16) | 0.30 |
| Depression | 136 (28) | 19 (20) | 0.13 | 106 (28) | 49 (25) | 0.37 |
| Creatinine clearance, ml/min per 1.73 m2 | 78 (67–91) | 81 (69–92) | 0.43 | 78 (67–91) | 81 (69–91) | 0.18 |
| Ejection fraction | 69 ± 13 | 65 ± 14 | 0.01 | 70 ± 13 | 66 ± 14 | <0.001 |
| Resting perfusion defects, % of LV | 0 (0–6) | 9 (2–19) | <0.001 | 0 (0–4) | 6 (0–16) | <0.001 |
| Aspirin | 422 (86) | 82 (87) | 0.71 | 328 (86) | 176 (87) | 0.73 |
| ACE inhibitor | 218 (44) | 51 (54) | 0.08 | 169 (44) | 100 (49) | 0.24 |
| Beta-blockers | 364 (74) | 71 (76) | 0.75 | 281 (73) | 154 (76) | 0.51 |
| Statins | 419 (85) | 80 (85) | 0.99 | 325 (85) | 174 (86) | 0.78 |
| Antidepressant | 121 (25) | 21 (22) | 0.64 | 97 (25) | 45 (22) | 0.4 |
Values are mean ± SD, n (%), or median (IQR).
The p values compare positive vs. negative ischemia.
ACE = angiotensin-converting enzyme; CSIMI = conventional stress–induced myocardial ischemia; IQR = interquartile range; LV = left ventricle; MSIMI = mental stress–induced myocardial ischemia.
The median resting hs-cTnI level was 4.3 pg/ml (IQR: 2.9 to 7.3 pg/ml). Only 24 patients (4.3%) had levels above 24 pg/ml (99th percentile). Men had higher median hs-cTnI levels in comparison to women (5.4 pg/ml [IQR: 3.9 to 9.2 pg/ml] vs. 3.9 pg/ml [IQR: 2.5 to 6.5 pg/ml]; p < 0.001). Using a multivariate linear regression model that includes CAD risk factors, we found age, African American race, diabetes mellitus, lower creatinine clearance, heart failure, lower ejection fraction, and CAD severity to be all independently associated with higher resting hs-cTnI levels (Online Table 2).
RESTING hs-cTnI LEVELS IN PATIENTS WITH ISCHEMIA DURING STRESS TESTING
Patients with MSIMI had significantly higher median resting hs-cTnI levels compared with those without MSIMI (5.9 pg/ml [IQR: 3.8 to 8.1 pg/ml] vs. 4.1 pg/ml [IQR: 2.8 to 7.1 pg/ml]; p < 0.0001). Similarly, patients with CSIMI during either physical or pharmacological stress testing had significantly higher median resting hs-cTnI levels compared with those without CSIMI (5.4 pg/ml [IQR: 3.8 to 9.4 pg/ml] vs. 3.9 pg/ml [IQR: 2.6 to 6.6 pg/ml]; p < 0.0001). Subgroup analysis showed that both men and women with ischemia had higher resting hs-cTnI levels in comparison to those who were nonischemic (Online Figure 1).
When interaction with type of ischemia was studied, patients who had isolated CSIMI (n = 134), isolated MSIMI (n = 24), or both types of ischemia (n = 70) had similar elevations in median hs-cTnI levels (5.2 pg/ml [IQR: 3.6 to 9.2 pg/ml], 5.2 pg/ml [IQR: 3.7 to 6.9 pg/ml], and 6.2 pg/ml [IQR: 4.1 to 9.6 pg/ml]; p = NS) compared with those without ischemia (3.8 [IQR: 2.5 to 6.5] pg/ml).
RESTING hs-cTnI LEVELS AND THE MAGNITUDE OF RESTING PERFUSION DEFECTS
There was a significant correlation between the resting perfusion defects (myocardial scar) and ejection fraction (Spearman rank correlation of −0.56; p < 0.001), as well as CAD severity (Spearman rank correlation of 0.28; p < 0.001). There was a moderate correlation between the resting and stress-induced perfusion defects (Spearman rank correlation of 0.3 for both mental and conventional stress; p < 0.001).
Increasing magnitude of myocardial scar and stress-induced perfusion defects were both associated with higher resting levels of hs-cTnI, an association that remained significant even after adjustment for age, sex, African American race, diabetes, heart failure, and creatinine clearance (Figure 1). The estimated hs-cTnI level based on linear regression models that include both resting and stress-induced perfusion defects are shown in Figure 2. Combined information on resting and stress-induced perfusion defects explained nearly 13% to 18% of the variability in resting hs-cTnI (R2 = 0.13 and 0.18 for mental and conventional stress, respectively). Thus, patients with a larger myocardial scar who had inducible ischemia, also had higher estimated resting hs-cTnI.
FIGURE 1. Association Between Resting hs-cTnI and Perfusion Defects.
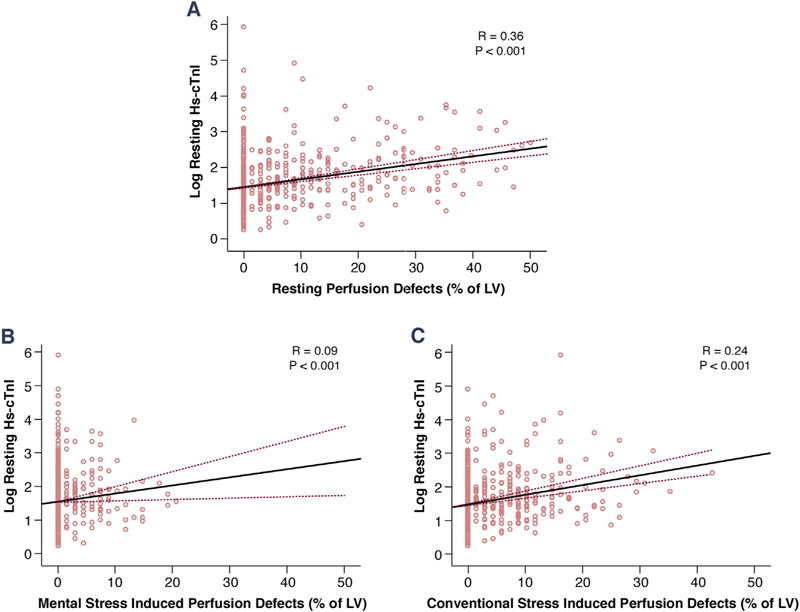
Scatter plots showing the association of log-transformed resting high-sensitivity cardiac troponin I (hs-cTnI) levels and (A) resting perfusion defects, (B) mental stress–induced perfusion defects, and (C) conventional stress–induced perfusion defects. The graphs show linear regression correlation coefficients (R), and p values. LV = left ventricle.
FIGURE 2. Estimated Resting hs-cTnI Levels by Resting and Stress-Induced Perfusion Defects.
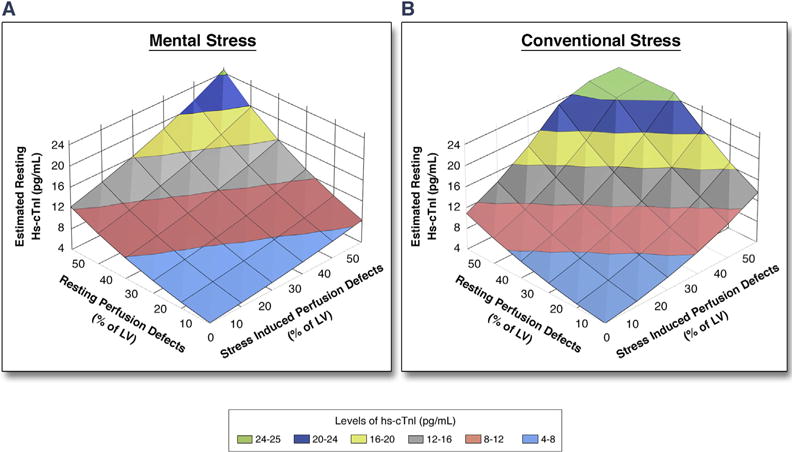
The levels of log-transformed hs-cTnI were estimated with a linear regression model using the extent of resting and stress-induced perfusion defects. The y-axis was back-transformed into regular units of hs-cTnI levels (pg/ml). The estimated levels of hs-cTnI are illustrated by different colors as shown in the color key. For example, with 10% resting perfusion defect, the estimated resting hs-cTnI is 4.7 pg/ml, which increases to 6.1 pg/ml in the presence of concomitant 10% ischemia during conventional stress. Abbreviations as in Figure 1.
RESTING hs-cTnI IN PREDICTING MSIMI AND CSIMI
Increasing levels of resting hs-cTnI were also associated with higher risk of MSIMI and CSIMI (Table 2). For easier clinical application, we dichotomized hs-cTnI using previously published cutoff from the JUPITER trial (cutoff of 4.6 pg/ml for men and 3.9 pg/ml for women) (7). Patients with levels above the cutoff were more likely to develop both MSIMI (OR: 2.4; 95% confidence interval [CI]: 1.5 to 3.9; p < 0.001) and CSIMI (OR: 2.4; 95% CI: 1.7 to 3.4; p < 0.001) compared with those below the cutoff (Table 2). These associations were unchanged after adjustment for factors associated with higher resting hs-cTnI, including age, race, diabetes, creatinine clearance, heart failure, and ejection fraction. Adjusting for severity of underlying CAD did not have an impact on the results. Even after adjustment for resting perfusion defects and conventional stress-induced perfusion defects, the association between MSIMI and hs-cTnI level remained statistically significant (Table 2).
TABLE 2.
Associations Between Resting hs-cTnI Levels (≥ and < Cutoff) and MSIMI and CSIMI Using Logistic Regression Analyses
| MSIMI | CSIMI | |||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| Unadjusted, hs-cTnI, (ln) as continuous variable (per SD increment) | 1.4 (1.1–1.7) | 0.002 | 1.7 (1.5–2.1) | <0.001 |
| Unadjusted, above vs. below hs-cTnI cutoff | 2.4 (1.5–3.9) | <0.001 | 2.4 (1.7–3.4) | <0.001 |
| Adjusted, model 1 | 2.3 (1.4–3.8) | 0.001 | 2.2 (1.5–3.2) | <0.001 |
| Adjusted, model 2 | 2.2 (1.3–3.7) | 0.002 | 2.2 (1.5–3.2) | <0.001 |
| Adjusted, model 3 | 1.8 (1.03–3.0) | 0.040 | 2.0 (1.3–3.1) | 0.001 |
| Adjusted, model 4 | 1.9 (1.1–3.2) | 0.029 | 1.8 (1.2–2.8) | 0.006 |
Cutoffs were 3.9 pg/ml and 4.6 pg/ml for women and men, respectively. Adjusted model 1 was adjusted for age, sex, race, diabetes, heart failure, creatinine clearance, and ejection fraction. Adjusted model 2 was adjusted for model 1 + resting perfusion defects. Adjusted model 3 was adjusted model 2 + percentage of ischemic myocardium during conventional stress for MSIMI model and during mental stress for CSIMI model, respectively. Adjusted model 4 was adjusted model 3 + CAD severity (available only for 511 patients).
CAD = coronary artery disease; CI = confidence interval; hs-cTnI = high-sensitivity cardiac troponin I; OR = odds ratio; other abbreviations as in Table 1.
CHANGE IN hs-cTnI LEVELS WITH CONVENTIONAL AND MENTAL STRESS TESTING
In the entire group, there was no significant change in the median hs-cTnI levels 45 and 90 min after the mental stress (4.2 [IQR: 2.8, 6.9] pg/ml and 4.3 [IQR: 2.9, 7.1] pg/ml, respectively; p = 0.14 compared with at rest). Patients who had exercise stress test had lower resting median hs-cTnI compared with those who underwent pharmacological stress test (4.0 pg/ml [IQR: 2.6 to 6.4 pg/ml] vs. 5.6 pg/ml [IQR: 3.5 to 9.9 pg/ml]; p < 0.001). Forty-five minutes after either stress test, there was no significant change in hs-cTnI levels (median post-stress hs-cTnI levels of 3.8 pg/ml [IQR: 2.5 to 6.3 pg/ml] and 6.0 pg/ml [IQR: 3.2 to 9.2 pg/ml]; p = 0.91 and 0.15, for exercise and pharmacological stress test, respectively).
When hs-cTnI change after stress testing was stratified by ischemia status, no significant difference was observed between the change in hs-cTnI level in those with MSIMI compared with those without (p = 0.6 for the interaction between ischemia status and time) (Figure 3A). However, patients who developed ischemia during treadmill stress had a significant rise in hs-cTnI levels (p = 0.012 for the interaction with time) (Figure 3B). In patients who had pharmacological stress test, no significant difference was observed between the change in hs-cTnI level in those with ischemia compared with those without (p = 0.79 for the interaction with time) (Figure 3C).
FIGURE 3. hs-cTnI Response to Different Types of Stress Test.
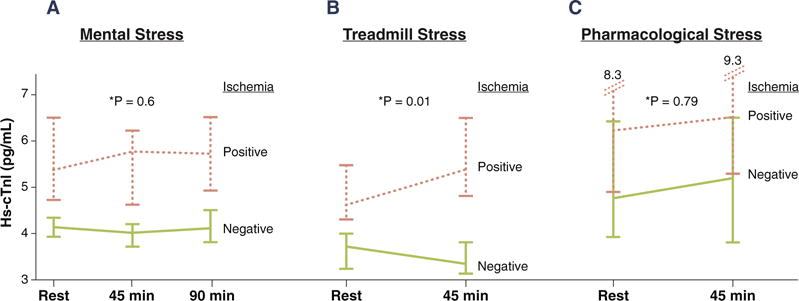
Change in hs-cTnI level in patients with and without ischemia during: (A) mental stress; (B) treadmill stress; and (C) pharmacological stress test. Error bars represent 95% confidence interval of the median. *The p value represents the interaction between ischemia status and time. hs-cTnI = high-sensitivity cardiac troponin I.
The change in hs-cTnI level during different stress tests was not related to the change in rate-pressure product (Pearson r = 0.07, p = 0.12; r = −0.001, p = 0.99; and r = 0.10, p = 0.24 for mental, exercise, and pharmacological stresses, respectively) nor to resting perfusion defects (Spearman r = 0.04, p = 0.35; r = 0.01, p = 0.83; and r = 0.03, p = 0.71 for mental, exercise, and pharmacological stress tests, respectively).
DISCUSSION
In the largest mental stress study to date performed in patients with stable CAD, we found that those developing myocardial ischemia during either mental and/or conventional stress testing to have elevated resting levels of hs-cTnI. There was a graded increase in resting hs-cTnI levels with increasing magnitude of ischemia. Importantly, these increases were independent of demographic variables, renal function, heart failure, ejection fraction, underlying severity of CAD, or the type of ischemia (mental or conventional). These findings are important because elevated circulating hs-cTnI levels are associated with increased risk of adverse cardiovascular events (1,5–7). Whether elevated hs-cTnI levels are a prognostic indicator above and beyond inducible ischemia or whether inducible ischemia is amenable to intervention requires further investigation.
A novel finding of our study is the elevation of resting levels of hs-cTnI in patients with MSIMI. MSIMI is usually silent, occurs at a lower workload than exercise-induced ischemia does, is associated with transient left ventricular dysfunction, is independent of the underlying burden of CAD, and is known to be associated with adverse cardiovascular outcomes (8,9,18,19). Postulated mechanisms for MISMI include coronary microcirculatory dysfunction (limited dilation and/or constriction) in the setting of vascular endothelial and/or smooth muscle dysfunction, increased oxidant stress and inflammation, dysregulation of the hypothalamic-pituitary-adrenal axis, and platelet hyper-reactivity (11,18,19). Although troponin increase after exposing atherosclerotic mice to mental stress has been observed, our study and a previous investigation in a small sample of heart failure patients found no change in hs-cTnI levels after mental stress (20,21). Thus, although we found higher resting hs-cTnI levels in those with MSIMI, there was no demonstrable increase in hs-cTnI levels 45 and 90 min after mental stress. One reason for not observing an increase in hs-cTnI with MSIMI may be because in comparison to CSIMI, MSIMI is generally less intense and shorter in duration (2,11).
Conditions known to be associated with hs-cTnI elevation include myocarditis, severe heart failure, coronary calcification, hypertensive crises and pulmonary embolism (1,2). We demonstrated that persistent elevations in hs-cTnI levels are also present in CAD patients with inducible myocardial ischemia during both mental and exercise stress. It is likely that repeated episodes of myocardial ischemia during daily life, that are often silent, contribute to the observed sustained increase in resting hs-cTnI levels (22). Similar findings were observed before in patients with exercise stress ischemia (23). Although cardiac troponins are largely structural (bound) proteins, about 6% to 8% of troponin T and 3.5% of troponin I are freely available in the cytoplasm (24). It is likely that repeated episodes of myocardial ischemia during daily life, that are often silent, increase cell wall permeability due to recurrent wall injury/stress leading to release of cytosolic troponin (2). It is also possible that ischemia, which is usually due to obstructive plaque, results in microinfarcts (25) that may cause sustained structural troponin degradation (26,27). Although the half-life of troponin I in blood is approximately 6 h, it can be detected in the circulation for up to 2 weeks after myocardial infarction, possibly due to gradual degradation of myofibrils and release of troponin complex (2,26,27).
Our observations of chronically elevated resting hs-cTnI levels in those with inducible myocardial ischemia is particularly notable in view of the fact that non-hs-cTnI measurements are conventionally used to distinguish myocardial infarction from myocardial ischemia in patients presenting with chest pain. It now appears that there is a continuum of troponin elevation from reversible ischemia to infarction, and changes in patients with ischemia may be only detectable using the high-sensitivity assays. Despite that the elevated levels of hs-cTnI in those with ischemia are far below the 99th percentile cutoff normally used to define myocardial infarction (7,15,28), such small levels have been linked previously to adverse cardiovascular outcomes (1,5–7,28). When we applied a previously published cutoff to our population (7), we found that patients with levels above the cutoff had higher incidence of both MSIMI and CSIMI. Whether the previously described association between hs-cTnI and adverse cardiovascular outcomes (7) is due to the presence of myocardial ischemia needs to be further investigated.
Although previous studies have shown a significant increase in hs-cTn levels after endurance and intense physical exercise (marathon runners), examination of troponin levels change during treadmill exercise stress testing has yielded inconsistent results (3,10,23,29–31). Specifically, a few studies have reported increased cardiac troponin levels 2 to 3 h after exercise, particularly in those who developed ischemia and without evidence of necrosis, findings consistent with our data acquired during physical stress (3,10,30–32). However, other studies have found the opposite (23,29). Part of the inconsistency may be due to differences in the studied population and/or exercise intensity.
We also found a robust association between resting hs-cTnI and resting perfusion defects or low ejection fraction that may represent/reflect infarction, hibernating myocardium, and structural abnormality (1,33). Here, high levels of hs-cTnI could be related to CAD burden, systolic dysfunction, and ongoing myocardial remodeling (2,34). Thus, both a previous myocardial infarction and intermittent inducible ischemia contribute to hs-cTnI elevation.
STUDY LIMITATIONS
This is the largest study to date to investigate the effects of inducible ischemia during mental and conventional stress testing on a marker of myocardial stress/necrosis. The diversity of the population studied and use of state-of-the-art myocardial perfusion imaging are important strengths. In light of the delayed release and clearance of troponin, a limitation may be that the timing of blood sample collection post-mental stress may have been too early to observe stress-induced changes in hs-cTnI levels. However, this is unlikely as previous studies have reported elevation in hs-cTn as early as 1 h after myocardial ischemia (4,35). Finally, at lower levels of hs-cTnI, the imprecision of the assay increases, thus a smaller release of troponin after mental stress might be missed.
CONCLUSIONS
Patients with CAD and inducible ischemia during mental or conventional stress testing have elevated resting hs-cTnI levels. This likely reflects the burden of myocardial ischemia, often silent, with both psychological and physical triggers during everyday life. Further studies are needed to assess whether interventions such as revascularization, pharmacological, or cognitive behavioral therapies will modify hs-cTnI levels and ultimately influence cardiovascular outcomes in those with MSIMI.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Mental stress–induced myocardial ischemia is associated with elevated resting levels of hs-cTnI, probably due to repeated ischemic episodes during daily life. This elevation was similar to that observed in patients who have ischemia with conventional stress testing.
TRANSLATIONAL OUTLOOK
Stable CAD patients with high levels of hs-cTnI have increased risk of adverse cardiovascular outcomes. Whether elevated hs-cTnI levels have a prognostic indicator above and beyond inducible ischemia or whether inducible ischemia is amenable to intervention requires further investigation.
Acknowledgments
This work was supported by the National Institutes of Health (grants P01 HL101398, P20HL113451-01, P01HL086773-06A1, R56HL126558-01, R01 HL109413, R01HL109413-02S1, UL1TR000454, KL2TR000455, K24HL077506, and K24 MH076955). The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Garcia has received consulting honoraria from Syntermed; and has received royalties from the sale of Emory Cardiac Toolbox software, which was used for some analyses in this study. Drs. Vaccarino and Quyyumi have received research support from the National Institutes of Health.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CI
confidence interval
- CSIMI
conventional stress–induced myocardial ischemia
- hs-cTn
high-sensitivity cardiac troponin
- hs-cTnI
high-sensitivity cardiac troponin I
- IQR
interquartile range
- MSIMI
mental stress–induced myocardial ischemia
- OR
odds ratio
- SPECT
single-photon emission computed tomography
APPENDIX
For supplemental material, please see the online version of this paper.
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Beatty AL, Ku IA, Christenson RH, DeFilippi CR, Schiller NB, Whooley MA. High-sensitivity cardiac troponin T levels and secondary events in outpatients with coronary heart disease from the Heart and Soul Study. JAMA Intern Med. 2013;173:763–9. doi: 10.1001/jamainternmed.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57:2406–8. doi: 10.1016/j.jacc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J. 2009;30:162–9. doi: 10.1093/eurheartj/ehn504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol. 2011;57:2398–405. doi: 10.1016/j.jacc.2010.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everett BM, Brooks MM, Vlachos HE, et al. Troponin and cardiac events in stable ischemic heart disease and diabetes. N Engl J Med. 2015;373:610–20. doi: 10.1056/NEJMoa1415921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett BM, Zeller T, Glynn RJ, Ridker PM, Blankenberg S. High-sensitivity cardiac troponin I and B-type natriuretic peptide as predictors of vascular events in primary prevention: impact of statin therapy. Circulation. 2015;131:1851–60. doi: 10.1161/CIRCULATIONAHA.114.014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei J, Rooks C, Ramadan R, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol. 2014;114:187–92. doi: 10.1016/j.amjcard.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaccarino V. Mental stress-induced myocardial ischemia. In: Baune TB, Tully JP, editors. Cardiovascular Diseases and Depression: Treatment and Prevention in Psychocardiology. Cham, Switzerland: Springer International Publishing; 2016. pp. 105–21. [Google Scholar]
- 10.Axelsson A, Ruwald MH, Dalsgaard M, Rossing K, Steffensen R, Iversen K. Serial measurements of high-sensitivity cardiac troponin T after exercise stress test in stable coronary artery disease. Biomarkers. 2013;18:304–9. doi: 10.3109/1354750X.2013.776635. [DOI] [PubMed] [Google Scholar]
- 11.Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, Quyyumi AA. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2013;2:e000321. doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaccarino V, Wilmot K, Al Mheid I, et al. Sex differences in mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003630. pii:e003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17:941–73. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 14.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–81. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 15.Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–93. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 16.Zeller T, Tunstall-Pedoe H, Saarela O, et al. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. Eur Heart J. 2014;35:271–81. doi: 10.1093/eurheartj/eht406. [DOI] [PubMed] [Google Scholar]
- 17.Aw TC, Phua SK, Tan SP. Measurement of cardiac troponin I in serum with a new high-sensitivity assay in a large multi-ethnic Asian cohort and the impact of gender. Clin Chim Acta. 2013;422:26–8. doi: 10.1016/j.cca.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Ersbøll M, Al Enezi F, Samad Z, et al. Impaired resting myocardial annular velocities are independently associated with mental stress–induced ischemia in coronary heart disease. J Am Coll Cardiol Img. 2014;7:351–61. doi: 10.1016/j.jcmg.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepine CJ, Petersen JW, Bairey Merz CN. A microvascular-myocardial diastolic dysfunctional state and risk for mental stress ischemia: a revised concept of ischemia during daily life. J Am Coll Cardiol Img. 2014;7:362–5. doi: 10.1016/j.jcmg.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Caligiuri G, Levy B, Pernow J, Thorén P, Hansson GK. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 1999;96:6920–4. doi: 10.1073/pnas.96.12.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wawrzyniak AJ, Dilsizian V, Krantz DS, et al. High concordance between mental stress-induced and adenosine-induced myocardial ischemia assessed using SPECT in heart failure patients: hemodynamic and biomarker correlates. J Nucl Med. 2015;56:1527–33. doi: 10.2967/jnumed.115.157990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quyyumi AA, Panza JA, Diodati JG, Callahan TS, Bonow RO, Epstein SE. Prognostic implications of myocardial ischemia during daily life in low risk patients with coronary artery disease. J Am Coll Cardiol. 1993;21:700–8. doi: 10.1016/0735-1097(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress: experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94:2402–9. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 24.Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart. 2006;92:987–93. doi: 10.1136/hrt.2005.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oemrawsingh RM, Cheng JM, Garcia-Garcia HM, et al. High-sensitivity troponin T in relation to coronary plaque characteristics in patients with stable coronary artery disease; results of the ATHEROREMO-IVUS study. Atherosclerosis. 2016;247:135–41. doi: 10.1016/j.atherosclerosis.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Katus HA, Remppis A, Scheffold T, Diederich KW, Kuebler W. Intracellular compart-mentation of cardiac troponin T and its release kinetics in patients with reperfused and non-reperfused myocardial infarction. Am J Cardiol. 1991;67:1360–7. doi: 10.1016/0002-9149(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien PJ, Smith DE, Knechtel TJ, et al. Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab Anim. 2006;40:153–71. doi: 10.1258/002367706776319042. [DOI] [PubMed] [Google Scholar]
- 28.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–77. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 29.Kurz K, Giannitsis E, Zehelein J, Katus HA. Highly sensitive cardiac troponin T values remain constant after brief exercise- or pharmacologic-induced reversible myocardial ischemia. Clin Chem. 2008;54:1234–8. doi: 10.1373/clinchem.2007.097865. [DOI] [PubMed] [Google Scholar]
- 30.Mouridsen MR, Nielsen OW, Pedersen OD, et al. Diagnostic value of exercise-induced changes in circulating high sensitive troponin T in stable chest pain patients. Biomarkers. 2013;18:726–33. doi: 10.3109/1354750X.2013.854835. [DOI] [PubMed] [Google Scholar]
- 31.Røsjø H, Kravdal G, Høiseth AD, et al. Troponin I measured by a high-sensitivity assay in patients with suspected reversible myocardial ischemia: data from the Akershus Cardiac Examination (ACE) 1 study. Clin Chem. 2012;58:1565–73. doi: 10.1373/clinchem.2012.190868. [DOI] [PubMed] [Google Scholar]
- 32.Mousavi N, Czarnecki A, Kumar K, et al. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol. 2009;103:1467–72. doi: 10.1016/j.amjcard.2009.01.294. [DOI] [PubMed] [Google Scholar]
- 33.Shaw LJ, Hendel RC, Heller GV, Borges-Neto S, Cerqueira M, Berman DS. Prognostic estimation of coronary artery disease risk with resting perfusion abnormalities and stress ischemia on myocardial perfusion SPECT. J Nucl Cardiol. 2008;15:762–73. doi: 10.1007/BF03007357. [DOI] [PubMed] [Google Scholar]
- 34.Moreno V, Hernandez-Romero D, Vilchez JA, et al. Serum levels of high-sensitivity troponin T: a novel marker for cardiac remodeling in hypertrophic cardiomyopathy. J Card Fail. 2010;16:950–6. doi: 10.1016/j.cardfail.2010.07.245. [DOI] [PubMed] [Google Scholar]
- 35.Reichlin T, Twerenbold R, Wildi K, et al. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ. 2015;187:E243–52. doi: 10.1503/cmaj.141349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


