Abstract
The voiding spot assay (VSA) on filter paper is an increasingly popular method for studying lower urinary tract physiology in mice. However, the ways VSAs are performed differ significantly between laboratories, and many variables are introduced compared with the mouse’s normal housing situation. Rodents are intelligent social animals, and it is increasingly understood that social and environmental stresses have significant effects on their physiology. Surprisingly, little is known about whether change of environment during VSA affects mouse voiding and what the best methodologies are for retaining “natural” micturition patterns. It is well known that stress-related neuropeptide corticotropin-releasing factor is significantly elevated and induces dramatic voiding changes when rodents encounter stresses. Therefore we hypothesized that changes in the environmental situation could potentially alter voiding during VSA. We have examined multiple factors to test whether they affect female mouse voiding patterns during VSA, including cage type, cage floor, water availability, water bottle location, single or group housing, and different handlers. Our results indicate that mice are surprisingly sensitive to changes in cage type and floor surface, water bottle location, and single/group housing, each of which induces significant changes in voiding patterns, indicative of a stress response. In contrast, neither changing handler nor 4 h of water deprivation affected voiding patterns. Our data indicate that VSA should be performed under conditions as close as possible to the mouse’s normal housing. Optimizing VSA methodology will be useful in uncovering voiding alterations in both genetic and disease models of lower urinary dysfunctions.
Keywords: environmental stress, metabolic cage, urinary bladder, voiding pattern quantification
INTRODUCTION
Lower urinary tract symptoms (LUTS) affect ~50% of people aged older than 40, and the underlying mechanisms are poorly understood. This prevents the development of effective therapeutics and causes significant social and economic burdens (5, 9). With many obvious advantages including genetic manipulation, the mouse is the most popular animal model used for LUTS studies. However, because of its size, the evaluation of urinary function in mice tends to be a much more challenging and tedious task. Yet good physiological assays are critical for the evaluation of urinary function and understanding of the underlying pathophysiology. Useful assays have thus been developed in evaluating overall mouse urinary function, including cystometrogram (CMG), metabolic cages, voiding spot assay (VSA), electromyography, and ultrasonography. Each of these assays has its own advantages and disadvantages (13, 24).
Technically, the VSA is the simplest method and one that does not require special equipment. VSA only requires a piece of filter paper to absorb voided mouse urine. The urine stain on the filter paper can then be imaged by the autofluorescence emitted from urine under ultraviolet light, analyzed, and quantified. The urine spots can also be visualized by ninhydrin spray, which is then imaged in bright field and quantified (1). The VSA method has the enormous advantage that it is noninvasive and preserves valuable animals for other purposes. It can be used repeatedly to track changes in voiding function over time. The first VSA application we could find was reported in Science almost half a century ago and convincingly demonstrated that dominant male mice vigorously marked their entire cage floor with numerous small voids, whereas subordinate males typically only have a limited number of large voids at the cage corner, revealing an interesting voiding behavior that is modulated by the social rank of the mouse (6). Since then, the method has been reported in many other studies, including several of our own reports, and has proven to be a simple, reproducible, and useful assay for mouse voiding function evaluation (4, 12, 20, 30).
However, significant questions remain unresolved with regard to VSA, mainly in the following three aspects. First, it lacks a standard protocol, and the way VSA is performed in different laboratories can vary significantly. This inevitably results in inconsistency and incomparability of data generated by different laboratories. Many untested variables have been used in specific VSAs performed in different laboratories including type of cage, housing on solid or metal grid floor, provision or restriction of water, male or female handler, etc. Whether these variables constitute significant stressors and thus affect how the mouse voids is unknown. If they do, then minimizing stressors is important for obtaining the “true” physiological state of mouse voiding function. Second, quantitation methods for VSA differ significantly between laboratories, which will also create difficulties in comparing and interpreting reported data. Third, data generated by VSAs may appear inconsistent with data obtained from other evaluation methods such as CMG or metabolic cages, e.g., voiding frequency or interval and size of voids or bladder capacity. Thus a common question asked is, What is the true physiological voiding or bladder capacity of the mouse, and why do different evaluation methods produce different results even in the same genetic background? Often there is no clear explanation.
Urinary function requires a complex coordination of bladder and nervous system. It not only reflects function of the urinary bladder but also is regulated by social behavior and environmental stresses. Like other mammals and also people, the mouse is a social animal and is very sensitive to different stresses. Recent studies indicated that both social and psychological stresses could result in voiding dysfunction and bladder pathology directly, through increased release of corticotropin-releasing factor (CRF), a stress-related neuropeptide (6, 15, 17, 22, 25, 28). In this study, we have performed VSAs by modifying various experimental factors, including cage types, water, cage floor, housing conditions, etc. We have determined that multiple factors could potentially constitute stressors in mice and thus result in altered voiding patterns.
MATERIALS AND METHODS
Animals.
Female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) aged 12–16 wk old were used in this study with the approval of the Beth Israel Deaconess Medical Center (BIDMC) Institutional Animal Care and Use Committee. Male mice were not used since the goal of the study is to evaluate the effect of different VSA conditions on voiding patterns and the dominant and subordinate social behavior in male mice complicates interpretation of the data. Mice were housed in standard polycarbonate cages and maintained on a 12:12-h light-dark cycle at 25°C with free access to food and water.
Cage types.
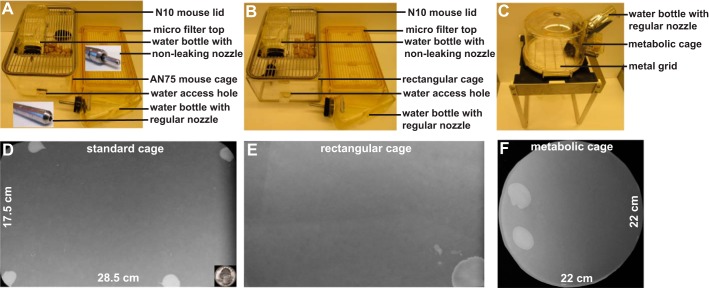
Three types of cages were used. The first type was the polycarbonate AN75 mouse cage with N10 mouse lid and microfilter top (Ancare, Bellmore, NY; Fig. 1A). It is transparent with dimensions ~28.5 cm (length) × 17.5 cm (width) × 12 cm (height). This type of cage is used in the BIDMC animal facility for daily mouse housing. Thus mice are familiar with this type of cage as it represents their usual home. We call this the “standard cage.” The second type was a custom-built cage using clear 3-mm-thick transparent Makrolon polycarbonate sheets, and we call this the “new rectangular cage.” This cage has the same dimensions as the standard cage, but all sides and the floor meet at sharp right angles, unlike the standard cage with rounded corners and edges (Fig. 1B). Similar types of custom-made cages with vertically sharp corners and edges have also been used or reported by other laboratories or are commercially available. Third, we used Techniplast metabolic cages (Braintree Scientific, Braintree, MA) for VSA (Fig. 1C). This is a cylindrical cage with dimensions ~11 cm (radius) × 12 cm (height), and VSA has often been performed by other investigators using this type of cage.
Fig. 1.
Different types of cages used for voiding spot assay and representative images of voided filter papers. A: standard cage used for daily mouse housing in animal facility. B: custom-built new rectangular cage with sharp cage corners and edges, which differs from the standard cage. C: traditional Techniplast metabolic cage with metal grid floor; filter paper can be placed either above the grid or below the grid, in which case mice will walk on the grid. D: representative image of voided filter paper from a standard cage. E: representative image of voided filter paper from a new rectangular cage. F: representative image of voided filter paper from a metabolic cage. The quarter dollar coin at bottom right of D (surface area of 462 mm2) serves as the size standard.
Experimental design.
In general, VSA was performed during the day with free access to food in a quiet room for 4 h. In this study, we varied the following factors. 1) We used three types of cages as described, to test whether the new rectangular cage and the metabolic cage, with which mice are not familiar, constitute an environmental stressor and thereby cause different voiding patterns. 2) Since mice in metabolic cages stand on metal grids in many reported VSA studies, the grid itself, independent of the cage, constitutes an additional potential stress factor. We therefore performed VSA with mice both on the grid and directly on filter paper placed on top of the grid (solid floor). 3) Water is an important factor that could impact urine production and therefore voiding patterns. In our previous reports, VSA was performed in standard cages with water deprivation (for 4 h) because of the problems associated with dripping water, which dilutes and spreads any urine it contacts rendering quantitation impossible. To understand whether the deprivation of water for 4 h could impact voiding patterns, we provided water to the mouse by replacing the regular water bottle nozzle with a new nonleaking push-type nozzle. In this method, the water bottle sits on the N10 mouse lid where it is normally located. Alternatively, we put the water bottle with the regular nozzle on the outside of cage (like the water bottle location in metabolic cages). In this way, the mouse could access water through an 8-mm-radius hole on the cage wall with no chance of water damage. 4) Mice are social animals and normally live in groups. Isolation is a potential stressor for mice that could impact their voiding patterns. Normally, mice are tested singly. In this study, we performed VSA by putting two or three mice in the same cage to test whether this changes their voiding pattern. 5) We tested the influence of different handlers, and VSA was performed by both a man and a woman. Neither handler wore perfume or cologne during the VSA experiment. This allowed us to test the repeatability of our VSAs.
VSA.
Unless otherwise specified, mice were transported in their home cage to a quiet nearby testing room in the same corridor of the BIDMC Research North Animal Research Facility. In this study, each condition (experiment) was repeated on different groups of mice (at least 2) to get consistent results. Mice might be used for multiple experiments, but each mouse was evaluated by VSA in a standard cage before a new experiment (such as the new rectangular cage or metabolic cage) to ensure the mouse was in its “normal” condition. Mice were allowed to rest for up to a week between two experiments. For a particular experiment, each mouse was tested twice for the VSA on 2 consecutive days. Individual mice were gently placed in a mouse cage (standard cage, new rectangular cage, or metabolic cage) with Blick Cosmos Blotting Paper (cat. no. 10422-1005) cut to size and placed on the floor, for 4 h. Mice were given standard dry mouse chow for the duration of the assay. Water was withheld or provided as described. After 4 h, mice were returned to their home cages, the filter paper was allowed to dry and was recovered, and the cage was cleaned by paper towel for VSA repeat.
Image acquisition and analysis.
Filters were imaged under ultraviolet light at 365 nm in a UVP Chromato-Vue C-75 system (UVP, Upland, CA) that incorporates an onboard Canon digital single-lens reflex camera (EOS Rebel T3, 12 megapixels). Overlapping voiding spots were visually examined and manually separated by outlining, copying, and then pasting to a nearby empty space using ImageJ software (http://fiji.sc/Fiji; v. 2.0.0-rc-41/1.50d; build 266f61cf6d). In some cases, voiding spots were chewed away because of urine soaking, which makes the filter paper soft. Using Fiji software, parts chewed away were manually filled by use of the drawing tool and then filled with pixel intensity the same as the edge of the torn part that was still fluorescent, thereby allowing us to quantify all spots. Images were analyzed by UrineQuant software developed by us in collaboration with the Harvard Imaging and Data Core. The results table, which contains the area of each individual voiding spot and total number of spots, was imported into Excel software for further statistical processing. A volume-area standard curve on this paper determined that 1 mm2 is equal to 0.283 µl of urine. Voiding spots with an area ≥80 mm2 are considered as primary voiding spots (PVS), and this cutoff was arrived at on the basis of urine distribution patterns analyzed from hundreds of mice (20, 30).
Statistical analyses.
Data were analyzed using Student's t-test between two groups or one-way analysis of variance for comparison among groups. Bonferroni’s multiple-comparison post hoc tests were used where necessary, and P < 0.05 was considered to be significant.
RESULTS
Mice void differently in new types of cages.
Mice were tested in different types of cages as shown in Fig. 1, for 4 h with water deprivation, and representative filter papers are shown. In the standard cage, mice usually have ~3.5 PVS on average with the average size of PVS ~376 mm2. To our surprise, mice had only ~2 PVS on average in both the new rectangular cage and the metabolic cage, and the average sizes of PVS in these cages were increased significantly to 500–700 mm2 (Table 1). In the new rectangular cage, the total urine area is significantly less than that of the standard cage. In the metabolic cage, the total urine area is also significantly less than that of the standard cage when the filter paper was suspended below the metal grid (5 mm) and the mice stood on the metal grid during VSA. These data indicate a significant alteration to voiding patterns when mice were housed in unfamiliar cages (Table 1).
Table 1.
Effect of cage types on voiding patterns
| Metabolic Cage |
||||
|---|---|---|---|---|
| Standard Cage (n = 20) | New Rectangular Cage (n = 32) | Filter Top (n = 20) | Filter Below (n = 20) | |
| PVS per filter, n | 3.55 ± 0.92 | 1.69 ± 0.79* | 2.05 ± 1.03* | 1.9 ± 0.7* |
| Size of PVS, mm2 | 376.74 ± 169.53 (n = 71) | 588.75 ± 256.38* (n = 54) | 690.13 ± 422.92* (n = 41) | 523.81 ± 210.18* (n = 38) |
| Total area of PVS per filter, mm2 | 1,337.43 ± 342.68 | 993.52 ± 335.14* | 1,420.85 ± 638.72 | 995.24 ± 447.94* |
| Total area of all spots per filter, mm2 | 1,380.18 ± 372.65 | 1,025.68 ± 349.20* | 1,449.76 ± 646.97 | 1,040.99 ± 465.99* |
| PVS per total area, % | 97.27 ± 3.48 | 97.15 ± 4.21 | 98.30 ± 2.98 | 95.85 ± 5.53 |
Values are means ± SD. PVS, primary voiding spots.
P < 0.05.
Effect of water deprivation for 4 h and water bottle location on mouse voiding pattern.
For obvious reasons, water deprivation might affect a mouse’s voiding pattern. To investigate, we performed the following experiments.
1) In standard cages, water was provided at the regular site with a nonleaking nozzle. In this case, voiding patterns showed no difference from those of water-deprived mice. Surprisingly, when the water bottle was placed at a new location (outside the cage but accessible) with the regular nozzle, the average number of PVS decreased, and the average area of PVS increased significantly, which is similar to the changes observed with mice in new cages, suggesting that the change of water bottle location and access might be a stressor for the mice (Table 2).
Table 2.
Effect of water and water bottle location on voiding patterns in standard cages
| Standard Cage |
|||
|---|---|---|---|
| Water Restricted (n = 20) | Water With Regular Nozzle (n = 22) | Water With Nonleaking Nozzle (n = 23) | |
| PVS per filter, n | 3.55 ± 0.92 | 2.24 ± 0.97* | 3.47 ± 1.06 |
| Size of PVS, mm2 | 376.74 ± 169.53 (n = 71) | 567.31 ± 295.07* (n = 47) | 396.67 ± 163.81 (n = 80) |
| Total area of PVS per filter, mm2 | 1,337.43 ± 342.68 | 1,287.58 ± 547.66 | 1,379.69 ± 442.84 |
| Total area of all spots per filter, mm2 | 1,380.18 ± 372.65 | 1,309.13 ± 517.61 | 1,430.34 ± 463.98 |
| PVS per total area, % | 97.27 ± 3.48 | 97.35 ± 7.45 | 96.52 ± 2.43 |
Values are means ± SD. PVS, primary voiding spots.
P < 0.05.
2) In the new rectangular cage, the voiding pattern does not show a difference between water deprived and water provided with the regular nozzle at the new position. However, when water was provided at the regular site with a nonleaking nozzle, the average number of PVS increased, and the average size of PVS significantly decreased compared with those of water deprived, consistent with the hypothesis that changing water bottle location is a stress factor (Table 3).
Table 3.
Effect of water and water bottle location on voiding pattern in new rectangular cages
| New Rectangular Cage |
|||
|---|---|---|---|
| Water Restricted (n = 51) | Water With Regular Nozzle (n = 50) | Water With Nonleaking Nozzle (n = 51) | |
| PVS per filter, n | 1.73 ± 0.79 | 2.14 ± 1.13 | 2.76 ± 1.23* |
| Size of PVS, mm2 | 555.36 ± 246.11 (n = 88) | 474.85 ± 261.80 (n = 107) | 377.01 ± 179.69* (n = 141) |
| Total area of PVS per filter, mm2 | 947.39 ± 309.31 | 1,011.38 ± 377.43 | 1,042.33 ± 360.24 |
| Total area of all spots per filter, mm2 | 991.90 ± 316.84 | 1,063.88 ± 406.05 | 1,094.52 ± 360.31 |
| PVS per total area, % | 95.58 ± 5.78 | 95.68 ± 5.87 | 94.81 ± 4.91 |
Values are means ± SD. PVS, primary voiding spots.
P < 0.05.
3) In metabolic cages, water was only provided at the side of the cage with the regular nozzle. Availability of water made no difference for mouse voiding pattern when filter paper was placed on the metal grid. When the filter paper was placed below the metal grid, the availability of water increased the average number of PVS, and other parameters of voiding patterns were unchanged (Tables 4 and 5).
Table 4.
Effect of water and water bottle location on voiding pattern in metabolic cage with filter paper above the metal grid
| Metabolic Cage Filter Paper on Top |
||
|---|---|---|
| Water Restricted (n = 20) | Water Provided (n = 23) | |
| PVS per filter, n | 2.05 ± 1.03 | 2.26 ± 1.33 |
| Size of PVS, mm2 | 690.13 ± 422.92 (n = 41) | 594.92 ± 287.61 (n = 52) |
| Total area of PVS per filter, mm2 | 1,420.85 ± 638.72 | 1,345.04 ± 662.94 |
| Total area of all spots per filter, mm2 | 1,449.76 ± 646.97 | 1,381.99 ± 666.97 |
| PVS per total area, % | 98.30 ± 2.98 | 95.63 ± 9.96 |
Values are means ± SD. PVS, primary voiding spots.
Table 5.
Effect of water and water bottle location on voiding pattern in metabolic cage with filter paper below the metal grid
| Metabolic Cage Filter Paper Below |
||
|---|---|---|
| Water Restricted (n = 20) | Water Provided (n = 20) | |
| PVS per filter, n | 1.9 ± 0.7 | 2.50 ± 1.20* |
| Size of PVS, mm2 | 523.81 ± 210.18 (n = 38) | 523.09 ± 226.17 (n = 50) |
| Total area of PVS per filter, mm2 | 995.24 ± 447.94 | 1,307.73 ± 577.70 |
| Total area of all spots per filter, mm2 | 1,040.99 ± 465.99 | 1,308.407 ± 578.56 |
| PVS per total area, % | 95.85 ± 5.53 | 99.97 ± 0.12* |
Values are means ± SD. PVS, primary voiding spots.
P < 0.05.
Overall, there was not a significant change in total urine area, indicating that water deprivation for 4 h during VSA does not decrease the total urine output or voided volume. These data also indicate that water deprivation for 4 h does not affect the voiding pattern dramatically but more likely the location of the water bottle might be a stress factor and does affect the voiding pattern significantly.
Voiding patterns differ in singly tested and group-tested mice.
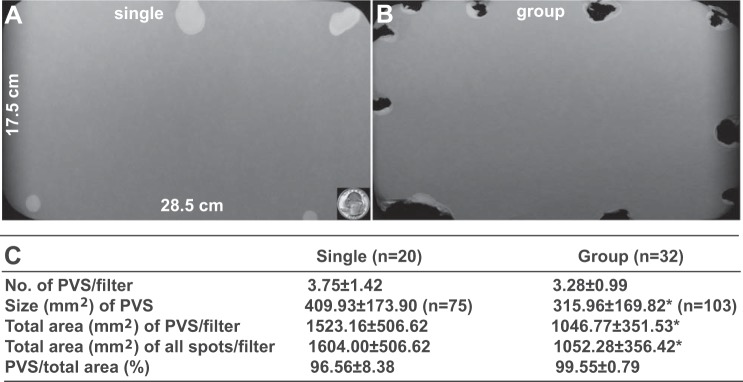
Mice are social animals, and in their daily activities they urinate in the presence of companions in their cage. Usually, VSA is performed with isolated animals. We were therefore curious as to whether voiding patterns and volumes would be different if the VSA was performed with two or three mice housed together in a standard cage. Interestingly, our data indicate that mouse voiding patterns were significantly different when they were tested in groups (Fig. 2). Their average PVS size was significantly smaller than that of singly tested mice. More interestingly, their average urine area voided in 4 h was also significantly reduced. A remarkable phenomenon in group-tested mice is that they were more active and thus the voided urine spots were almost totally chewed away, while in singly housed mice (same batch of mice), the voided urine spots were mostly intact, indicating significant behavioral differences, which might play an important role in regulating their different voiding patterns.
Fig. 2.
Representative images of voided filter papers for voiding spot assay (VSA) of single- and group-housed mice with water deprivation. A: representative image of voided filter paper from a standard cage with a single mouse. The quarter dollar coin at bottom right (surface area of 462 mm2) serves as the size standard. B: representative image of voided filter paper from a standard cage with three group-housed mice. Voided spots on this filter paper were mostly chewed away during VSA. C: quantitated data from multiple trials. Values are means ± SD. PVS, primary voiding spots. *P < 0.05.
Change of handlers does not change VSA pattern.
To test the repeatability of VSAs and also understand whether different handlers would have different impacts on mouse voiding patterns, such as through interomone (pheromone affecting other species) signaling mechanisms (18, 19), a male (~30 yr old) and a female handler (~30 yr old) performed VSA in standard and new rectangular cages. Our data indicated that in both types of cages the handler had no effect on voiding patterns (Tables 6 and 7).
Table 6.
Effect of handler on voiding pattern in standard cages
| Standard Cage |
||
|---|---|---|
| Handler 1 (n = 20) | Handler 2 (n = 20) | |
| PVS per filter, n | 3.55 ± 0.92 | 3.20 ± 1.33 |
| Size of PVS, mm2 | 376.74 ± 169.53 (n = 71) | 378.38 ± 179.90 (n = 64) |
| Total area of PVS per filter, mm2 | 1,337.43 ± 342.68 | 1,135.13 ± 601.90 |
| Total area of all spots per filter, mm2 | 1,380.18 ± 372.65 | 1,236.08 ± 530.77 |
| PVS per total area, % | 97.27 ± 3.48 | 97.14 ± 6.15 |
Values are means ± SD. PVS, primary voiding spots.
Table 7.
Effect of handler on voiding pattern in new rectangular cages
| New Rectangular Cage |
||
|---|---|---|
| Handler 1 (n = 32) | Handler 2 (n = 19) | |
| PVS per filter, n | 1.69 ± 0.79 | 1.74 ± 0.78 |
| Size of PVS, mm2 | 588.75 ± 256.38 (n = 54) | 500.73 ± 217.53 (n = 33) |
| Total area of PVS per filter, mm2 | 993.52 ± 335.14 | 869.68 ± 240.89 |
| Total area of all spots per filter, mm2 | 1,025.68 ± 349.20 | 935.00 ± 242.76 |
| PVS per total area, % | 97.15 ± 4.21 | 92.94 ± 6.97* |
Values are means ± SD. PVS, primary voiding spots.
P < 0.05.
DISCUSSION
Because of its simplicity and proven utility the VSA has been growing in popularity. This is especially true since mouse genetics has allowed highly targeted approaches to the study of bladder and LUTS physiology. However, laboratories use many different approaches in performing the assay, and whether any of these variables change how and when mice urinate has not been investigated. Since mice are social animals with acute sensitivity to environmental stresses and also have significant intelligence and curiosity, we hypothesized that unfamiliar environmental factors during VSA could be potential stressors and thus impact voiding behavior.
Evidence of just how sensitive the mice were was provided by the observation that voiding patterns changed significantly when VSA was performed in the new rectangular cage as well as in metabolic cages. These two types of cages are often used in rodent studies but it has not been reported that they affect mouse micturition patterns, and in general, we believe that the field is unaware of this important effect (7, 8, 27, 29). In particular, metabolic cages with computerized systems are popular for measuring rodent voiding patterns. In these systems, mice or rats dwell on metal grids or mesh whereas their daily housing has a solid floor with bedding. Previous studies showed that laboratory rats preferred to dwell on a solid floor rather than on grids, particularly when resting (16, 26). Moreover, metabolic cage confinement results in 5–10% fall in body weight. It also leads to significant changes in mouse behavior and physiology that are consistent with a stress response with significant plasma and urinary biochemical changes (23). A recent study further indicated that mice housed in metabolic cages for 3 wk were still not acclimated to it. These mice excreted high amounts of corticosterone metabolites in feces and urine throughout the study, indicating oxidative stress, increased muscle catabolism, and stress-induced hyperthermia (11). Consistent with these observations, our data also indicate that new cages might induce significant stress responses in mice, resulting in reduced voiding frequency, increased volume per void, and reduced overall urine output. We have noticed that void spot sizes reported for control mice measured by metabolic cages are often significantly larger (~2–4-fold) than when measured by VSA or CMG (27, 29). Our results shed light on this inconsistency and indicate that it is likely stress related. Therefore data obtained from metabolic cages cannot be considered representative of normal physiological voiding.
It has been reported that water deprivation for 24–48 h in mice and rats induced significant weight loss with increased plasma osmolality and plasma corticosterone concentration (3, 14, 21). In the simplest form of VSA using regular mouse cages, it is necessary to restrict water for 4 h to avoid water damage to the urine and filter paper. We were interested to note that this had no effect on voiding patterns or volumes, which therefore suggests that 4 h of water deprivation does not cause significant dehydration of the mice and reassures investigators that their data are not impacted if they restrict water for this duration. In contrast, however, simply changing the location of the water bottle impacted voiding patterns significantly. It is intriguing that such a subtle alteration could cause significant changes, and we speculate that this represents another stress factor for mice, since regular food and water access is extremely important for survival.
Mouse housing density has been known to play an important role in regulating organ physiology, and both overcrowding and isolation are significant stress factors (2, 10). Social isolation has been reported to have deleterious effects on health. In rat and mouse models, singly housed mice or rats, especially in the presence of other environmental stress factors, exhibited altered immune-endocrine and cardiovascular function with increased levels of norepinephrine and corticosteroids (2). To date, the effect of single or group testing on mouse voiding behavior has not been investigated, and we are the first to report data on this issue. The significant differences between single and group housing suggest that single housing represents another stress factor for voiding outputs.
We quantitated the chewing behavior in our study: 1) standard cage with a single mouse, chewed spots were seen in 22% of filters (n = 20); total chewed PVS in all filter papers, 36% (n = 75); 2) standard cage with two to three mice, filter paper with chewed spot, 75% (n = 32); total chewed PVS in all filter papers, 81% (n = 105); 3) new rectangular cage with single mouse, filter paper with chewed spot, 0% (n = 32); total chewed PVS in all filter papers, 0% (n = 54); and 4) metabolic cage with single mouse, filter paper with chewed spot, 0% (n = 20); total chewed PVS in all filter papers, 0% (n = 41). This striking chewing behavior in group-housed mice suggests that they are more active and less stressed than when alone or in unfamiliar cages. This parameter is very consistent with our observed urine quantitation data and indicates that chewing might be a sensitive index to reflect the degree of stress that mice are experiencing. Of course, mice are normally housed on absorbent bedding chips, so the move to a cage with an unfamiliar surface is likely to be a stressor in itself. Unfortunately, this cannot be avoided for the assay to be performed.
An interesting and consistent phenomenon was that when stressed, mice exhibited reduced PVS number and increased PVS area, often with reduced total urine volume. One exception was the group-housed mice, which exhibited reduced total voided urine, suggesting that because of greater activity they might have elevated metabolism and water loss through skin and respiration (Fig. 2). Stress has been known to induce voiding dysfunction for decades. Social defeat stress causes marked changes in bladder structure and function through the stress-related neuropeptide CRF in Barrington’s nucleus, which regulates the micturition reflex and has an inhibitory influence in this pathway (15, 17, 28). In our hands, modifying VSA variables causes voiding changes similar to those seen with social defeat stress and potentially may also induce significant CRF expression in Barrington’s nucleus.
We believe that an important contribution from this study relates to the analysis of data. Researchers have generally adopted two different approaches for data interpretation: 1) arbitrarily group different sizes of voiding spots with certain intervals and then compare differences between control and intervention groups (12, 30) or 2) visually grade void spot patterns by scoring from 1 to 5, with 1 indicating a few large voiding spots and 5 indicating many scattered small spots (7, 8). By plotting large numbers of voiding spots from many mice as a frequency distribution chart, we have recently determined that the vast majority of voided volume is contained in spots that are larger than 80 mm2 in our system (~23 µl), and 80 mm2 seems to form a natural volume separation point. Therefore we have defined all spots larger than this number as primary voiding spots [PVS; further details may be found in our recent publication (20)]. On the basis of this well-supported cutoff, we have quantified the following parameters in studying VSA, which we believe are sufficient to capture the key elements of voiding in most cases: 1) PVS number, which directly indicates bladder activity; 2) mean PVS size, indicating the volume of each void, which directly relates to bladder capacity and activity; 3) total area of all spots, indicating the total volume voided, which at least partially reflects kidney function; 4) ratio of PVS area to total urine area, which indicates urinary continence. We have used these parameters in analyzing many genetically modified or bladder disease models (unpublished data), and it has proven to be a simple and useful approach for categorizing bladder phenotypes. Depending on the model being studied, in some cases a detailed spot area-frequency distribution chart might be helpful (20).
In the last several years we have spent significant effort in characterizing and optimizing VSA (4, 30). We have also developed new analytic methods for data interpretation (20). Here, we have further shown that voiding behavior is very sensitive to environment and have identified multiple factors, including cage types, water bottle locations, and housing conditions, that affect voiding patterns significantly. On the basis of these observations, we suggest performing the VSA in a situation as close to the mouse’s normal housing as possible. For example, we recommend using standard cages with water deprivation for 4 h as an effective protocol, which has great reproducibility and minimizes stress. Finally, we want to point out that this study was performed on female mice and that voiding behavior in male mice might be complicated by dominant and subordinate social behavior; thus VSA data interpretation in male mice may require additional caution.
GRANTS
We acknowledge funding received from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant 95922 (to W. Yu). This study also continues initiatives begun under NIDDK Grant 97818-02S3.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.C. and W.Y. conceived and designed research; H.C., L.Z., and W.Y. performed experiments; H.C., L.Z., and W.Y. analyzed data; H.C., L.Z., W.G.H., and W.Y. interpreted results of experiments; H.C. and W.Y. prepared figures; H.C. and W.Y. drafted manuscript; H.C., L.Z., W.G.H., and W.Y. edited and revised manuscript; H.C., L.Z., W.G.H., and W.Y. approved final version of manuscript.
REFERENCES
- 1.Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev 32: 1236–1248, 2008. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartolomucci A, Palanza P, Sacerdote P, Ceresini G, Chirieleison A, Panerai AE, Parmigiani S. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology 28: 540–558, 2003. doi: 10.1016/S0306-4530(02)00039-2. [DOI] [PubMed] [Google Scholar]
- 3.Bekkevold CM, Robertson KL, Reinhard MK, Battles AH, Rowland NE. Dehydration parameters and standards for laboratory mice. J Am Assoc Lab Anim Sci 52: 233–239, 2013. [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorling DE, Wang Z, Vezina CM, Ricke WA, Keil KP, Yu W, Guo L, Zeidel ML, Hill WG. Evaluation of voiding assays in mice: impact of genetic strains and sex. Am J Physiol Renal Physiol 308: F1369–F1378, 2015. doi: 10.1152/ajprenal.00072.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, Kopp ZS, Chapple CR, Kaplan S, Tubaro A, Aiyer LP, Wein AJ. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int 104: 352–360, 2009. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- 6.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939–941, 1973. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 7.Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R, Vezina CA, Sarma AV, Macoska JA. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate 73: 1123–1133, 2013. doi: 10.1002/pros.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodges SJ, Zhou G, Deng FM, Aboushwareb T, Turner C, Andersson KE, Santago P, Case D, Sun TT, Christ GJ. Voiding pattern analysis as a surrogate for cystometric evaluation in uroplakin II knockout mice. J Urol 179: 2046–2051, 2008. doi: 10.1016/j.juro.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 108: 1132–1138, 2011. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishida H, Mitsui K, Nukaya H, Matsumoto K, Tsuji K. Study of active substances involved in skin dysfunction induced by crowding stress. I. Effect of crowding and isolation on some physiological variables, skin function and skin blood perfusion in hairless mice. Biol Pharm Bull 26: 170–181, 2003. doi: 10.1248/bpb.26.170. [DOI] [PubMed] [Google Scholar]
- 11.Kalliokoski O, Jacobsen KR, Darusman HS, Henriksen T, Weimann A, Poulsen HE, Hau J, Abelson KS. Mice do not habituate to metabolism cage housing: a three week study of male BALB/c mice. PLoS One 8: e58460, 2013. doi: 10.1371/journal.pone.0058460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanasaki K, Yu W, von Bodungen M, Larigakis JD, Kanasaki M, Ayala de la Pena F, Kalluri R, Hill WG. Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. FASEB J 27: 1950–1961, 2013. doi: 10.1096/fj.12-223404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keil KP, Abler LL, Altmann HM, Bushman W, Marker PC, Li L, Ricke WA, Bjorling DE, Vezina CM. Influence of animal husbandry practices on void spot assay outcomes in C57BL/6J male mice. Neurourol Urodyn 35: 192–198, 2016. doi: 10.1002/nau.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleiner SM. Water: an essential but overlooked nutrient. J Am Diet Assoc 99: 200–206, 1999. doi: 10.1016/S0002-8223(99)00048-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee UJ, Ackerman AL, Wu A, Zhang R, Leung J, Bradesi S, Mayer EA, Rodríguez LV. Chronic psychological stress in high-anxiety rats induces sustained bladder hyperalgesia. Physiol Behav 139: 541–548, 2015. doi: 10.1016/j.physbeh.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 16.Manser CEE, Elliott H, Morris TH, Broom DM. The use of a novel operant test to determine the strength of preference for flooring in laboratory rats. Lab Anim 30: 1–6, 1996. doi: 10.1258/002367796780744974. [DOI] [PubMed] [Google Scholar]
- 17.McFadden K, Griffin TA, Levy V, Wolfe JH, Valentino RJ. Overexpression of corticotropin-releasing factor in Barrington’s nucleus neurons by adeno-associated viral transduction: effects on bladder function and behavior. Eur J Neurosci 36: 3356–3364, 2012. doi: 10.1111/j.1460-9568.2012.08250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGlone JJ, Thompson G, Devaraj S. A natural interomone 2-methyl-2-butenal stimulates feed intake and weight gain in weaned pigs. Animal 11: 306–308, 2017. doi: 10.1017/S1751731116001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGlone JJ, Thompson WG, Guay KA. Case study: the pig pheromone androstenone, acting as an interomone, stops dogs from barking. Prof Anim Sci 30: 105–108, 2014. doi: 10.15232/S1080-7446(15)30091-7. [DOI] [Google Scholar]
- 20.Rajandram R, Ong TA, Razack AH, MacIver B, Zeidel M, Yu W. Intact urothelial barrier function in a mouse model of ketamine-induced voiding dysfunction. Am J Physiol Renal Physiol 310: F885–F894, 2016. doi: 10.1152/ajprenal.00483.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowland NE. Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comp Med 57: 149–160, 2007. [PubMed] [Google Scholar]
- 22.Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S, Lee U, Golovatscka V, Pothoulakis C, Bradesi S, Mayer EA, Rodríguez LV. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology 78: 967.e1–967.e7, 2011. doi: 10.1016/j.urology.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stechman MJ, Ahmad BN, Loh NY, Reed AA, Stewart M, Wells S, Hough T, Bentley L, Cox RD, Brown SD, Thakker RV. Establishing normal plasma and 24-hour urinary biochemictry ranges in C3H, BALB/c and C57BL/6J mice following acclimatization in metabolic cages. Lab Anim 44: 218–225, 2010. doi: 10.1258/la.2010.009128. [DOI] [PubMed] [Google Scholar]
- 24.Sugino Y, Kanematsu A, Hayashi Y, Haga H, Yoshimura N, Yoshimura K, Ogawa O. Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn 27: 548–552, 2008. doi: 10.1002/nau.20552. [DOI] [PubMed] [Google Scholar]
- 25.Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder-brain connection: putative role of corticotropin-releasing factor. Nat Rev Urol 8: 19–28, 2011. doi: 10.1038/nrurol.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whittaker AL, Lymn KA, Howarth GS. Effects of metabolic cage housing on rat behavior and performance in the social interaction test. J Appl Anim Welf Sci 19: 363–374, 2016. doi: 10.1080/10888705.2016.1164048. [DOI] [PubMed] [Google Scholar]
- 27.Wood R, Eichel L, Messing EM, Schwarz E. Automated noninvasive measurement of cyclophosphamide-induced changes in murine voiding frequency and volume. J Urol 165: 653–659, 2001. doi: 10.1097/00005392-200102000-00089. [DOI] [PubMed] [Google Scholar]
- 28.Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol 296: R1671–R1678, 2009. doi: 10.1152/ajpregu.91013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshiyama M, Mochizuki T, Nakagomi H, Miyamoto T, Kira S, Mizumachi R, Sokabe T, Takayama Y, Tominaga M, Takeda M. Functional roles of TRPV1 and TRPV4 in control of lower urinary tract activity: dual analysis of behavior and reflex during the micturition cycle. Am J Physiol Renal Physiol 308: F1128–F1134, 2015. doi: 10.1152/ajprenal.00016.2015. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG, Zeidel ML. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol 306: F1296–F1307, 2014. doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]