Abstract
This study aimed to examine, at the level of the active muscles, whether the plateau in oxygen (O2) extraction normally observed near the end of a ramp incremental (RI) exercise test to exhaustion is caused by the achievement of an upper limit in O2 extraction. Eleven healthy men (27.3 ± 3.0 yr, 81.6 ± 8.1 kg, 183.9 ± 6.3 cm) performed a RI cycling test to exhaustion. O2 extraction of the vastus lateralis (VL) was measured continuously throughout the test using the near-infrared spectroscopy (NIRS)-derived deoxygenated hemoglobin [HHb] signal. A leg blood flow occlusion was performed at rest (LBFOCC1) and immediately after the RI test (LBFOCC2). The [HHb] values during the resting occlusion (108.1 ± 21.7%; LBFOCC1) and the peak values during exercise (100 ± 0%; [HHb]plateau) were significantly greater than those observed at baseline (0.84 ± 10.6% at baseline 1 and 0 ± 0% at baseline 2) (P < 0.05). No significant difference was found between LBFOCC1 and [HHb]plateau (P > 0.05) or between the baseline measurements (P > 0.05). [HHb] values at LBFOCC2 (130.5 ± 19.7%) were significantly greater than all other time points (P < 0.05). These results support the existence of an O2 extraction reserve in the VL muscle at the end of a RI cycling test and suggest that the observed plateau in the [HHb] signal toward the end of a RI test is not representative of an upper limit in O2 extraction.
Keywords: blood flow, deoxygenated hemoglobin, muscle oxygen extraction, near-infrared spectroscopy
to support the progressive increase in metabolic demand during ramp incremental (RI) exercise, a linear increase in oxygen consumption (V̇o2) is typically observed (5, 33). Based on the Fick equation, increases in muscle V̇o2 rely on the interaction between O2 delivery and O2 extraction. When evaluated from a systemic perspective, the increase in V̇o2 during RI exercise is a result of a linear increase in cardiac output (i.e., systemic blood flow) and a hyperbolic increase in arterial-venous O2 difference (i.e., systemic O2 extraction) until maximal O2 consumption (V̇o2max) is reached (1, 9, 25). However, recent studies have shown that near-infrared spectroscopy (NIRS) measurements of local O2 extraction {inferred from the NIRS-derived deoxygenated hemoglobin ([HHb]) signal} within the active tissues [i.e., vastus lateralis (VL) muscle] do not display the same hyperbolic profile that is associated to systemic measurements, indicating that central and peripheral profiles of O2 extraction and blood flow are different (10, 20). In recent studies, the [HHb] signal during RI exercise has been described to display a linear increase in relation to the increase in metabolic demand (i.e., V̇o2) until a breakpoint leading to a plateau-like response occurs despite the continuous increase in V̇o2 (20, 29). This breakpoint in the [HHb] signal ([HHb]BP), has been indicated as a reliable measure (16) that can be used as the demarcation point for the exercise intensity closely associated with the V̇o2 at the respiratory compensation point (RCP) (11, 18) as well as the electromyography (EMG) threshold (EMGt) (4, 24), and it has been proposed as a tool that can differentiate between heavy and very heavy exercise intensity domains (3, 18).
The mechanisms that control the [HHb]BP are unknown and are a topic of debate. For example, the [HHb]BP has primarily been speculated to 1) be a result of a greater local vasodilatory response within the active tissues, which subsequently leads to an attenuation in the need for O2 extraction to support the increased O2 utilization (20), or 2) represent the point at which O2 extraction in the area of NIRS interrogation has reached its maximal capacity (4). In an attempt to clarify this issue, Spencer et al. (28) examined whether or not an increase in [HHb] beyond that observed subsequent to the [HHb]BP existed in response to a period of blood flow occlusion at rest. Those authors found that the plateau in the [HHb] signal was lower than the maximal [HHb] signal induced during a period of leg blood flow occlusion at rest, which was indicative of a reserve in O2 extraction. However, an adequate evaluation of this response should be made immediately at the end of the RI test and simultaneously with the occurrence of the O2 extraction plateau during the RI test.
Thus, by performing a leg blood flow occlusion (LBFOCC) immediately after the limit of tolerance is reached during a RI test (in addition to that performed at rest), the present study aimed to test whether the plateau in the [HHb] signal ([HHb]plateau; i.e., O2 extraction) that is normally observed near the end of a RI exercise test to exhaustion is caused by the achievement of an upper limit in O2 extraction. If the plateau in the [HHb] signal reflects the upper limit of O2 extraction, then elimination of tissue blood flow should result in no further increase in the [HHb] signal above that achieved after the [HHb]BP. Based on previous suggestions (20), it was hypothesized that an overshoot in the [HHb] signal in relation to the [HHb]plateau will occur after the occlusion of blood flow at the end of the RI test, representing the existence of an O2 extraction reserve.
METHODS
Participants
Eleven healthy men (27.3 ± 3.0 yr, 81.6 ± 8.1 kg, 183.9 ± 6.3 cm) volunteered and gave their written consent to participate in this study after completing the physical activity readiness questionnaire (PARQ+) and being cleared for exercise. Participants ranged from recreationally trained individuals who regularly engage in aerobic training programs to amateur cyclists training 5–6 days/wk. All participants were nonsmokers and nonobese, with no cardiovascular disease, and were not undergoing any medical treatment that could potentially affect their cardiopulmonary and metabolic responses to exercise. The Conjoint Health Research Ethics Board at the University of Calgary approved all procedures included in this study.
Protocol
A single visit to the laboratory was needed for each participant. The visit included two LBFOCC tests and a RI test to exhaustion on an electromagnetically braked cycle ergometer (Velotron Dynafit Pro; Racer Mate, Seattle, WA). The majority (n = 9) of subjects had previously performed maximal testing in our laboratory, and all of them were familiar with performing maximal efforts.
The RI test consisted of a 4-min warmup at 50 W followed by the ramp portion, which was a constant increase in wattage of 30 W/min (1 W every 2 s). Participants were asked to cycle at their preferred cadence between 80 and 90 rpm throughout the test until they reached their limit of exercise tolerance. The RI was stopped when the cadence dropped by 15 rpm or when volitional exhaustion occurred despite strong verbal encouragement.
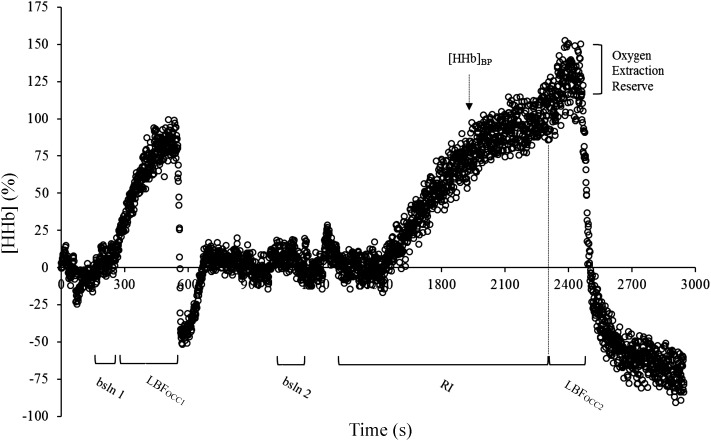
A LBFOCC was performed before and immediately after the cessation of the RI test using an automatic rapid cuff inflation system (Hokanson, Bellevue, WA). A deflated cuff was placed on the highest portion of the thigh. The protocol began with a 4-min resting baseline (bsln) measurement where participants were seated on the bike with their feet secured on the pedals. The pedals were positioned at midrotation (equal height) with the right pedal forward and supported by a block to avoid movement and ensure a relaxed position. The first occlusion (LBFOCC1) consisted of an 8-min period during which the cuff was inflated to a pressure of 300 mmHg; following cuff release an 8-min recovery period was recorded. From the end of LBFOCC1 to the start of the RI test, a minimum of 20 min was always guaranteed to allow for the conditions of the tissues to return to baseline. The second occlusion (LBFOCC2) was performed at the end of the RI exercise. Immediately upon test termination the cuff was rapidly inflated to 300 mmHg, whereas the right pedal was positioned onto the block by the researchers (same position as during LBFOCC1). The occlusion lasted for a minimum of 2 min or until a plateau in O2 extraction was evident. The aim of each occlusion was to achieve the highest level possible of [HHb] for the given metabolic condition. Figure 1 depicts the [HHb] profile during the complete protocol for a representative participant.
Fig. 1.
Deoxygenated hemoglobin ([HHb]) profile from the protocol for a representative participant. bsln, Baseline; LBFOCC, leg blood flow occlusion; RI, ramp incremental test; [HHb]BP, deoxygenated hemoglobin breakpoint.
Measurements
NIRS-derived [HHb] and oxygenated hemoglobin concentration ([HbO2]) were measured in the VL muscle of the right leg by means of NIRS (Oxiplex TS; ISS, Champaign, IL) at a sampling rate of 2 Hz, as previously described (20). Briefly, the system was composed of two channels consisting of eight laser diodes operating at two different wavelengths (λ = 690 and 828 nm), with four at each wavelength that were pulsed in rapid succession, and a photomultiplier tube. The NIRS probe (connected by optical fibers to laser diodes and a photomultiplier tube) consisted of two parallel rows of light-emitting fibers and one detector fiber bundle; the source detector separations for this probe were 2.0, 2.5, 3.0, and 3.5 cm. The [HHb] and total hemoglobin concentration ([HbTOT]), calculated as the sum of [HHb] + [HbO2], were continuously measured throughout the entire experimental procedure. The probe was placed on the belly of the VL muscle midway between the inguinal crease and the proximal border of the patella (the bottom of the probe was ∼17 cm from the proximal border of the patella, with exact distance depending on the length of the upper leg), secured in place by double-sided tape as well as an elastic strap to prevent any movement, and covered by an optically dense, black vinyl sheet and an elastic bandage to minimize both the intrusion of external light and movement. The NIRS system was calibrated on each testing day after a warmup of ≥30 min as per the manufacturer’s recommendations. Skin and adipose tissue thickness (ATT) was measured at the area of the VL interrogated by the NIRS probe using a Harpenden skinfold caliper.
Gas exchange variables and pulmonary ventilation (inspired and expired flow rates) were measured during the RI test using a breath-by-breath metabolic cart (Quark CPET; Cosmed, Rome, Italy). Expired gases were sampled at the mouth and were analyzed for fractional concentrations of O2 and CO2 after calibration with precision-analyzed gas mixtures according to manufacturer specifications. The flowmeter was calibrated using a syringe of known volume (3 liters).
Muscle electrical activity was recorded continuously throughout the RI test at a sampling rate of 1,000 Hz using an electromyography system (Bagnoli; Delsys, Boston, MA) connected to data acquisition hardware (Power Laboratory; ADInstruments, Bella Vista, NSW, Australia) linked to computer software (LabChart 8; ADInstruments). A bipolar surface EMG electrode was placed in close proximity to the NIRS probe. Before placement, the area of skin was prepared by shaving excess hair, gently abrading the epidermal layer with fine sandpaper, and finally, cleaning with an alcohol wipe to minimize impedance.
Data and Statistical Analysis
[HHb] signal.
To determine the [HHb] signal at the time points of interest, bin averaging was used as follows: baseline 1: 45-s average starting at minute 3; baseline 2: 45-s average starting at minute 7 after cuff release post-LBFOCC1; LBFOCC1: highest 10-s average during the last 2 min of the occlusion; [HHb]plateau: average of last 10 s before the RI test termination; LBFOCC2: highest 10-s average during the entire occlusion. Subsequently, the [HHb] values were normalized on an individual basis, with 0% representing baseline 2 and 100% representing the [HHb] value at the [HHb]plateau time point.
As previously described (29), the [HHb]-time relationship related to ramp portion of the RI test was modeled with the following piece-wise “double-linear” model: f = if [x < BP, g(x), h(x)], g(x) = i1 + (s1 × x), i2 = i1 + (s1 × BP), h(x) = i2 + [s2 × (x − BP)], and fit f to y, where f is the double-linear function, x is time, and y is [HHb], BP is the time coordinate corresponding to the interception of the two regression lines (i.e., the [HHb]BP), i1 and i2 are the intercepts of the first and second linear function, respectively, and s1 and s2 are the slopes. Model parameter estimates for each participant were determined by linear least-square regression analysis. The double-linear fit was started at the onset of the systematic increase in the [HHb] signal until the last data point corresponding to the end of the test. Aberrant data that were ±3 SD from the local mean were removed. Specifically, the [HHb]BP was detected as the point at which the increase in the signal would result in an attenuated slope.
Gas exchange parameters.
Breath-by-breath V̇o2 data were individually analyzed: irregular data points that were ± 3 SD from the local mean were removed and linearly interpolated to 1-s intervals. Thereafter, the second-by-second data were time aligned so that the onset of the RI test represented time “zero.” To account for the lag time in the increase in V̇o2 after the onset of the ramp portion, the mean response time (MRT) was calculated (Origin; Origin Laboratory, Northampton, MA) on an individual basis, as previously described (5). Briefly, a double-linear model was fit from baseline to the previously established gas exchange threshold (GET). The MRT corresponded to the time delay between the onset of the RI test (i.e., 240 s) and the intersection of the forward extrapolation of the baseline V̇o2 (slope constrained to “zero”) and backward extrapolation of the linear V̇o2-time relationship from GET.
Two exercise physiologists independently performed a visual inspection of the ventilatory and gas exchange indices to determine the RCP, as previously described (2). Briefly, the RCP corresponded to the second disproportional increase (2nd breakpoint) in the V̇e-V̇o2 relationship, where the end-tidal Pco2 began to fall after a period of isocapnic buffering. The relationship between V̇e and V̇co2 against V̇o2 was also considered for confirmation of the RCP. If the physiologists had a disagreement of >100 ml/min in the result, a second profile evaluation was performed together until a consensus was reached. V̇o2max was defined as the highest V̇o2 computed from a 30-s rolling average. Peak power output (PPO) was the highest power output value obtained at the end of the RI test.
To account for any influence of subcutaneous ATT, a correction factor based on the relationship of ATT and [HbTOT] was utilized as previously described (7, 28).
[HbTOT].
The [HbTOT] signal was selected at time points that corresponded to the time points of interest for the [HHb] signal, as described earlier. The analysis of the signal at baseline 1, baseline 2, and the [HHb]plateau was consistent with that of the [HHb] signal, with the lowest 10-s average selected during LBFOCC1 and LBFOCC2.
EMG signal.
The EMG signal was amplified, band-pass filtered, and rectified (5–500 Hz), and the root mean square (RMS) was calculated on a 1-s average. Two exercise physiologists then independently analyzed the EMG profiles to detect the EMGt (14, 15). Finally, the RMS recorded during the last 2 min of the baseline (50 W) was used to normalize the entire EMG profiles. This is a more specific and reproducible approach than the normalization performed against the “classical” maximal voluntary contraction (30).
The averaged and time-aligned [HHb] and EMG data were then plotted against the absolute V̇o2 data. After being left-shifted according to the previously determined MRT, the V̇o2 associated with the [HHb]BP and the EMGt was calculated (11).
All statistics were performed using SPSS version 23 (SPSS, Chicago, IL). Descriptive data are presented as means ± SD. A repeated-measure ANOVA was used to detect differences in the absolute and normalized [HHb] as well as in the [HbTOT] signal at baseline 1, baseline 2, LBFOCC1, [HHb]plateau, and LBFOCC2. A paired t-test was also used to compare the V̇o2 associated with the RCP and [HHb]BP. The statistical significance was set at P < 0.05.
RESULTS
PPO and V̇o2max at the end of the RI test were 431 ± 41 W and 4.60 ± 0.50 l/min (57.0 ± 5.2 ml·kg−1·min−1), respectively. No difference (P > 0.05) was found between the V̇o2 associated with the RCP (3.85 ± 0.40 l/min) and the [HHb]BP (3.90 ± 0.60 l/min). The RCP and the [HHb]BP were found to occur at 82.1 ± 6.7 and 83.2 ± 8.1% of V̇o2max and 73.1 ± 4.0 and 73.3 ± 9.5% of PPO, respectively.
Table 1 shows the normalized [HHb] values at each time point of interest. Findings of statistical significance were the same for both the absolute and normalized [HHb] data. No difference was found between baseline 1 and baseline 2 (P > 0.05) in the [HHb] signal; the [HHb] values at LBFOCC1 and [HHb]plateau were significantly greater than those observed at baseline 1 and baseline 2 (P < 0.05) but not different between each other (P > 0.05). However, the [HHb] value at LBFOCC2 was significantly greater than all other points of interest (P < 0.05).
Table 1.
Percent [HHb] values during baseline, LBFOCC, and at [HHb]plateau
| %[HHb] | bsln 1 | LBFOCC1 | bsln 2 | [HHb]plateau | LBFOCC2 |
|---|---|---|---|---|---|
| Mean | 0.8 | 108.1# | 0.0 | 100.0# | 130.5* |
| SD | 10.6 | 21.7 | 0.0 | 0.0 | 19.7 |
%[HHb], %deoxygenated hemoglobin; bsln, baseline; LBFOCC, leg blood flow occlusion; [HHb]PLATEAU, deoxygenated hemoglobin plateau. [HHb] values are expressed as a percentage relative to the [HHb]plateau; statistics were run on absolute values.
Significantly different from bsln 1 and bsln 2;
significantly different from all other time points.
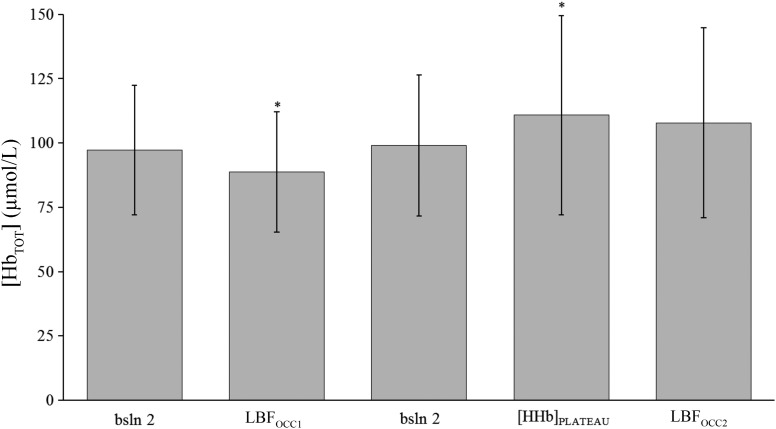
Figure 2 depicts the [HbTOT] signal corresponding to each time point as selected for analysis of the [HHb] signal. The [HbTOT] at LBFOCC1 and at [HHb]plateau were different from all other time points (P < 0.05). No difference was found between [HbTOT] at LBFOCC2 compared with baseline measurements (bsln 1 and 2; P > 0.05).
Fig. 2.
Mean total hemoglobin concentration ([HbTOT]) at the selected time points during the protocol. bsln, Baseline; LBFOCC, leg blood flow occlusion; [HHb]plateau, deoxygenated hemoglobin plateau. *Significant difference from all time points.
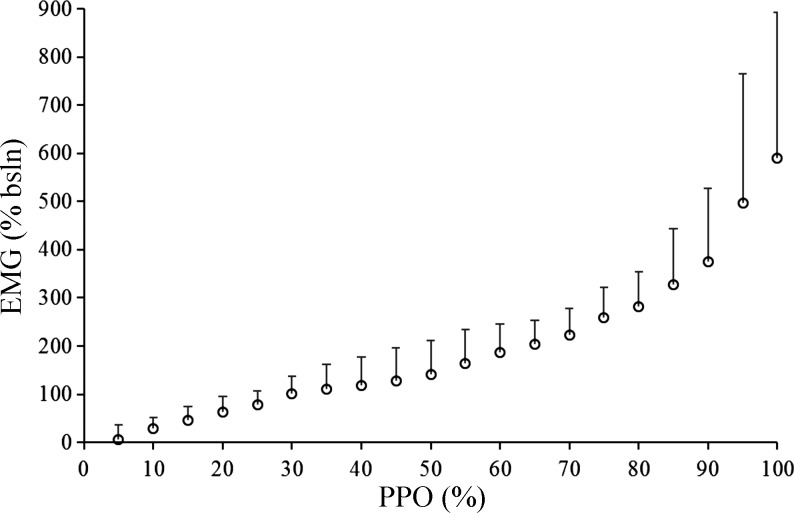
The overall group mean EMG profile is displayed in Fig. 3. Average muscle activation at maximal exercise normalized against baseline cycling was 613 ± 295%. V̇o2 at the EMGt was 4.0 ± 0.7 l/min (86.3 ± 9.5% V̇o2max, 78.3 ± 7.9% PPO). In four participants, the EMGt was not detectable, as their profiles did not display any clear breakpoint, although a continued increase in muscle activity was still observed.
Fig. 3.
Changes in electromyography (EMG) signal for the vastus lateralis throughout the ramp incremental test. The signal was normalized to the baseline cycling intensity of 50 W. bsln, Baseline; PPO, peak power output.
DISCUSSION
The primary aim of this study was to investigate whether the plateau in O2 extraction ([HHb]) normally observed near the end of a RI exercise test to exhaustion reflects the upper limit of O2 extraction or whether further extraction is possible beyond the plateau. The results of this study demonstrated that it is possible to achieve a further increase in O2 extraction as [HHb] increased (∼30%) above its plateau value during a period of leg blood flow occlusion commencing immediately after the limit of tolerance was reached during a RI protocol. As hypothesized, this supports the existence of an O2 extraction reserve and suggests that the observed plateau in the [HHb] signal test is not associated with an upper limit in O2 extraction.
A novel and important aspect of this study is that it was the first to evaluate an O2 extraction reserve immediately after the limit of tolerance was reached at the end of a RI test and to show that a potential for an increase in O2 extraction exists above that achieved during a resting occlusion despite the plateau that is typically observed toward the end of the RI test in this signal. Spencer et al. (28) compared peak levels of the [HHb] signal subsequent to a blood flow occlusion during rest to the [HHb] response observed toward the end of a RI exercise in the proximal and distal regions of the VL muscle. Using this model, these authors found that the [HHb] response was significantly higher during the blood flow occlusion in the distal but not in the proximal regions of the muscle and concluded that the observed reserve might reflect abundant microvascular blood flow or insufficient time to allow for equilibration between capillary and muscle fibers (28). Thus, these results are contrary to the findings of the current study, in which no significant difference was found between the [HHb] signal at LBFOCC1 and the signal at [HHb]plateau (see Fig. 1 and Table 1). However, it should be noted that the probe placement in the current study fell in between the locations described by Spencer et al. (28), albeit slightly closer to that of the distal probe. Thus, this interpretation should be made with caution.
Importantly, the blood flow occlusion in the study of Spencer et al. (28) was performed only at rest, and thus it was likely insufficient to fully account for the influence of the metabolic state of the muscle on total amplitude of the [HHb] response. For example, when the oxidative metabolic demand is at its peak, the intracellular oxidative “machinery” is fully activated and the mitochondria are operating closer to their critical partial pressure of O2, then the potential for O2 extraction will be at its highest level. In this scenario, the amount of increase in the [HHb] signal that is needed to support a given resting V̇o2 in the presence of a blood flow occlusion does not necessarily need to be greater than that needed to support the metabolic demands of maximal exercise. Therefore, the level of the [HHb] during LBFOCC1 (at rest) may not necessarily need to reach the level achieved during LBFOCC2 (at the end of the RI test), as the potential for O2 extraction might be lower at rest compared with peak exercise. Thus, the presence or absence of an O2 extraction reserve during blood flow occlusions performed at rest might be arbitrary in relation to the [HHb] response observed near exhaustion. For that reason, the present study was designed to evaluate the maximal capacity for [HHb], as LBFOCC2 was performed at the highest metabolic rate (and at the highest potential for O2 extraction) that each individual could sustain. Under these conditions, a clear O2 extraction reserve was observed in all participants. It should be noted that some papers have discussed the use of a blood flow occlusion period before (28) or subsequent to (but not immediately after) exercise (23, 27). In those studies, the blood flow occlusion was used as a physiological calibration for the [HHb] signal. The present results indicate that if the goal is to detect a true maximal [HHb], this type of calibration should be performed based on the metabolic demand being examined or simply immediately after the maximal metabolic demand has been reached.
The presence of a reserve in O2 extraction above the values observed during the [HHb]plateau may provide indirect support for the previously speculated idea that a large redistribution in blood flow to the active tissues is the mechanism responsible for the mitigation of a further rise in O2 extraction despite a further increase in V̇o2 (20). As shown recently, the V̇o2 corresponding to the [HHb]BP is closely associated with the V̇o2 at the RCP, and at the maximal lactate steady state (MLSS) (11, 18), which denote the upper boundaries of sustainable exercise intensity. Beyond this metabolic boundary, changes in the environment of the muscle (i.e., decrease in , increase in ) (32), as well as accumulation of metabolites and the subsequent release of vasodilatory compounds (i.e., ATP, ADP, [K+], [La], [H+], nitric oxide, etc.) (6, 19, 34), are likely to contribute to local vasodilation. In support of this idea of increased local blood flow at higher intensities of exercise, Calbet et al. (9) have shown that leg blood flow continues to increase in parallel with cardiac output as exercise intensity increases up to the end of an incremental test to exhaustion.
The [HbTOT] results from the present study also indirectly support the notion of a redistribution of blood to the active tissues, as the level of [HbTOT] corresponding to the [HHb]plateau was significantly greater than at all other time points (Fig. 2). Thus, it is likely that the local vasodilatory signals associated with this upper metabolic boundary results in increased local blood flow. It may be debated that the significant increase in [HHb] observed during the second occlusion period is somewhat related to the decrease in [HbTOT] after cuff inflation. However, if a greater increase in [HHb] observed during LBFOCC2 was associated to a lower [HbTOT], then it would be expected that during LBFOCC1 (where [HbTOT] was the lowest likely due to the fourfold longer occlusion time compared with LBFOCC2) the highest [HHb] values would have been observed. However, that was not the case.
Contrary to the idea that increased O2 availability in the active tissues explains the [HHb]plateau, other concepts can also be considered. It has been described that to maintain systemic blood pressure and perfusion, there is sympathetically mediated vasoconstriction (26) that might reduce blood flow to the active tissues. However, it has been observed that this sympathetic vasoconstriction is progressively blunted with increases in exercise intensity, which suggests that there is an exercise intensity dependence of functional sympatholysis within the exercising muscles (31). Moreover, it has been demonstrated that vasodilation triggered by the infusion of metabolites can override sympathetic vasoconstriction in the exercising limbs, resulting in further increases in blood flow during maximal exercise (8). Furthermore, some studies (4, 21) have proposed, among other hypotheses, that the lack of further increase in the [HHb] signal near the end of an incremental test to exhaustion indicates that O2 extraction has reached a ceiling. Data from the present study contradict this view, as all participants showed an O2 extraction “reserve,” which demonstrates that the upper limit for O2 extraction was not achieved.
Another factor to consider, as Spencer et al. (28) mentioned, is that the results of this study cannot rule out the possibility that capillary transit time, at near-maximal exercise intensities, becomes too short for the superficial muscles to sustain a high rate of O2 extraction (28). However, a study by Richardson et al. (25) found that, despite a shortened capillary transit time due to high red blood cell velocity, O2 extraction continued to rise hyperbolically with increased work rate. This finding is supported by other studies in which, through the use of catheters, O2 extraction does not plateau with increases in exercise intensity but rather continues to demonstrate small increases (9, 12). Although these results seem to be in stark contrast with the [HHb]BP discussed in the current study, the difference in methodology of O2 extraction measurement must be considered. In these aforementioned studies, O2 extraction was measured via catheterization during which there is a mixing of blood from other nonactive tissues, which leads to the inability to decisively determine O2 extraction solely from active muscles, as acknowledged by González-Alonso and Calbet (12). Furthermore, because the NIRS deoxyhemoglobin signal is limited to the area of interrogation of the probe, it is thought to be more reflective of O2 extraction within the capillaries of the muscle being examined.
Finally, a potential mechanism supporting a plateau in O2 extraction toward the end of a RI test revolves around the notion that muscle V̇o2 might not demonstrate a further increase; rather, the continued rise in V̇o2 measured at the level of the mouth is due to increased O2 utilization of the respiratory muscles. For example, by increasing the amount of work of the respiratory muscles, it has been shown that the rise in leg blood flow and O2 transport is attenuated (13). However, the stress induced in the respiratory muscles in this type of study is likely much greater than what is typically observed during a RI test. Additionally, Calbet et al. (9) demonstrated that as cardiac output increases there is a parallel increase in V̇o2 and blood flow to the legs, which receive a greater percentage of cardiac output. Moreover, data from the present study provide support to the idea of a continued rise in metabolic demand, and thus O2 consumption, toward the end of exercise through the EMG data, which indicates steeper increases in the activation of the VL beyond the [HHb]plateau (Fig. 3).
Technical Considerations
Spatial heterogeneities have been proposed, and type I fibers have been shown to be more predominant in deeper regions of the human quadriceps (17), with the implication that, at higher exercise intensities (i.e., above MLSS or RCP), more superficial muscle fibers are recruited (22). Given that type II fibers are characterized by poorer capillarization and O2 provision, the finding that O2 extraction plateaus toward the end of the exercise and that an O2 extraction reserve exists (implying increase local blood flow), even if more type II fibers were measured, is remarkable.
Since the NIRS signal is limited to the area of interrogation of the probe, there may be redistribution of blood to other tissues during the occlusion, resulting in a decrease in blood volume that would be reflected in the [HbTOT] signal. However, as explained here in the discussion, if the overshoot in the [HHb] signal was attributed to blood flow redistribution, then the [HHb] signal should have reached the maximum level during the resting occlusion, where a greater decrease and the lowest [HbTOT] signal was observed.
Conclusions
This study is the first to demonstrate the existence of an O2 extraction reserve in the VL muscle at the end of a RI cycling test. This indicates that the plateau observed in the NIRS-derived [HHb] signal (i.e., O2 extraction) does not represent the achievement of an upper limit in O2 extraction and that further extraction beyond this plateau is possible.
Perspectives and Significance
The results of this study contribute to the understanding of the mechanisms responsible for the observed plateau in the near-infrared spectroscopy-derived deoxyhemoglobin signal during a ramp incremental exercise test and provides insight into the cardiovascular adjustments to exercise at the peripheral level. Future directions should include the evaluation of the oxygen extraction reserve in other muscles as well as the assessment of potential differences due to sex and training status.
GRANTS
This study was supported by Natural Sciences and Engineering Research Council of Canada. E. Calaine Inglis was supported by the Natural Sciences and Engineering Research Council Master’s Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.C.I., D.I., and J.M.M. conceived and designed research; E.C.I. and D.I. performed experiments; E.C.I. analyzed data; E.C.I., D.I., and J.M.M. interpreted results of experiments; E.C.I. and D.I. prepared figures; E.C.I. drafted manuscript; E.C.I., D.I., and J.M.M. edited and revised manuscript; E.C.I., D.I., and J.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We express gratitude to the participants in this study and acknowledge Dr. Daniel Keir for valuable feedback.
REFERENCES
- 1.Astrand PO, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaximal and maximal work. J Appl Physiol 19: 268–274, 1964. [DOI] [PubMed] [Google Scholar]
- 2.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 60: 2020–2027, 1986. [DOI] [PubMed] [Google Scholar]
- 3.Bellotti C, Calabria E, Capelli C, Pogliaghi S. Determination of maximal lactate steady state in healthy adults: can NIRS help? Med Sci Sports Exerc 45: 1208–1216, 2013. doi: 10.1249/MSS.0b013e3182828ab2. [DOI] [PubMed] [Google Scholar]
- 4.Boone J, Barstow TJ, Celie B, Prieur F, Bourgois J. The interrelationship between muscle oxygenation, muscle activation, and pulmonary oxygen uptake to incremental ramp exercise: influence of aerobic fitness. Appl Physiol Nutr Metab 41: 55–62, 2016. doi: 10.1139/apnm-2015-0261. [DOI] [PubMed] [Google Scholar]
- 5.Boone J, Bourgois J. The oxygen uptake response to incremental ramp exercise: methodogical and physiological issues. Sports Med 42: 511–526, 2012. doi: 10.2165/11599690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Boone J, Vandekerckhove K, Coomans I, Prieur F, Bourgois JG. An integrated view on the oxygenation responses to incremental exercise at the brain, the locomotor and respiratory muscles. Eur J Appl Physiol 116: 2085–2102, 2016. doi: 10.1007/s00421-016-3468-x. [DOI] [PubMed] [Google Scholar]
- 7.Bowen TS, Rossiter HB, Benson AP, Amano T, Kondo N, Kowalchuk JM, Koga S. Slowed oxygen uptake kinetics in hypoxia correlate with the transient peak and reduced spatial distribution of absolute skeletal muscle deoxygenation. Exp Physiol 98: 1585–1596, 2013. doi: 10.1113/expphysiol.2013.073270. [DOI] [PubMed] [Google Scholar]
- 8.Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on VO2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol 291: R447–R453, 2006. doi: 10.1152/ajpregu.00746.2005. [DOI] [PubMed] [Google Scholar]
- 9.Calbet JA, Gonzalez-Alonso J, Helge JW, Søndergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol (1985) 103: 969–978, 2007. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira LF, Koga S, Barstow TJ. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J Appl Physiol (1985) 103: 1999–2004, 2007. doi: 10.1152/japplphysiol.01414.2006. [DOI] [PubMed] [Google Scholar]
- 11.Fontana FY, Keir DA, Bellotti C, De Roia GF, Murias JM, Pogliaghi S. Determination of respiratory point compensation in healthy adults: Can non-invasive near-infrared spectroscopy help? J Sci Med Sport 18: 590–595, 2015. doi: 10.1016/j.jsams.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 12.González-Alonso J, Calbet JA. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation 107: 824–830, 2003. doi: 10.1161/01.CIR.0000049746.29175.3F. [DOI] [PubMed] [Google Scholar]
- 13.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 82: 1573–1583, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Hug F, Faucher M, Kipson N, Jammes Y. EMG signs of neuromuscular fatigue related to the ventilatory threshold during cycling exercise. Clin Physiol Funct Imaging 23: 208–214, 2003. doi: 10.1046/j.1475-097X.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- 15.Hug F, Laplaud D, Savin B, Grélot L. Occurrence of electromyographic and ventilatory thresholds in professional road cyclists. Eur J Appl Physiol 90: 643–646, 2003. doi: 10.1007/s00421-003-0949-5. [DOI] [PubMed] [Google Scholar]
- 16.Iannetta D, Qahtani A, Mattioni Maturana F, Murias JM. The near-infrared spectroscopy-derived deoxygenated haemoglobin breaking-point is a repeatable measure that demarcates exercise intensity domains. J Sci Med Sport 20: 873–877, 2017. doi: 10.1016/j.jsams.2017.01.237. [DOI] [PubMed] [Google Scholar]
- 17.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18: 111–129, 1973. doi: 10.1016/0022-510X(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 18.Keir DA, Fontana FY, Robertson TC, Murias JM, Paterson DH, Kowalchuk JM, Pogliaghi S. Exercise intensity thresholds: Identifying the boundaries of sustainable performance. Med Sci Sports Exerc 47: 1932–1940, 2015. doi: 10.1249/MSS.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 19.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 20.Murias JM, Spencer MD, Keir DA, Paterson DH. Systemic and vastus lateralis muscle blood flow and O2 extraction during ramp incremental cycle exercise. Am J Physiol Regul Integr Comp Physiol 304: R720–R725, 2013. doi: 10.1152/ajpregu.00016.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okushima D, Poole DC, Rossiter HB, Barstow TJ, Kondo N, Ohmae E, Koga S. Muscle deoxygenation in the quadriceps during ramp incremental cycling: Deep vs. superficial heterogeneity. J Appl Physiol (1985) 119: 1313–1319, 2015. doi: 10.1152/japplphysiol.00574.2015. [DOI] [PubMed] [Google Scholar]
- 22.Poole DC, Burnley M, Vanhatalo A, Rossiter HB, Jones AM. Critical power: an important fatigue threshold in exercise physiology. Med Sci Sports Exerc 48: 2320–2334, 2016. doi: 10.1249/MSS.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porcelli S, Marzorati M, Lanfranconi F, Vago P, Pisot R, Grassi B. Role of skeletal muscles impairment and brain oxygenation in limiting oxidative metabolism during exercise after bed rest. J Appl Physiol (1985) 109: 101–111, 2010. doi: 10.1152/japplphysiol.00782.2009. [DOI] [PubMed] [Google Scholar]
- 24.Racinais S, Buchheit M, Girard O. Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Front Physiol 5: 142, 2014. doi: 10.3389/fphys.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol (1985) 75: 1911–1916, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol 24: 117–125, 1997. doi: 10.1111/j.1440-1681.1997.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 27.Salvadego D, Domenis R, Lazzer S, Porcelli S, Rittweger J, Rizzo G, Mavelli I, Simunic B, Pisot R, Grassi B. Skeletal muscle oxidative function in vivo and ex vivo in athletes with marked hypertrophy from resistance training. J Appl Physiol (1985) 114: 1527–1535, 2013. doi: 10.1152/japplphysiol.00883.2012. [DOI] [PubMed] [Google Scholar]
- 28.Spencer MD, Amano T, Kondo N, Kowalchuk JM, Koga S. Muscle O2 extraction reserve during intense cycling is site-specific. J Appl Physiol (1985) 117: 1199–1206, 2014. doi: 10.1152/japplphysiol.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer MD, Murias JM, Paterson DH. Characterizing the profile of muscle deoxygenation during ramp incremental exercise in young men. Eur J Appl Physiol 112: 3349–3360, 2012. doi: 10.1007/s00421-012-2323-y. [DOI] [PubMed] [Google Scholar]
- 30.Torres-Peralta R, Losa-Reyna J, González-Izal M, Perez-Suarez I, Calle-Herrero J, Izquierdo M, Calbet JA. Muscle activation during exercise in severe acute hypoxia: role of absolute and relative intensity. High Alt Med Biol 15: 472–482, 2014. doi: 10.1089/ham.2014.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasserman K, Whipp BJ, Koyl SN, Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol 35: 236–243, 1973. [DOI] [PubMed] [Google Scholar]
- 33.Whipp BJ, Davis JA, Torres F, Wasserman K. A test to determine parameters of aerobic function during exercise. J Appl Physiol Respir Environ Exerc Physiol 50: 217–221, 1981. [DOI] [PubMed] [Google Scholar]
- 34.Whipp BJ, Davis JA, Wasserman K. Ventilatory control of the ‘isocapnic buffering’ region in rapidly-incremental exercise. Respir Physiol 76: 357–367, 1989. doi: 10.1016/0034-5687(89)90076-5. [DOI] [PubMed] [Google Scholar]