Abstract
Objective
The PROCO RCT is a multicenter, double‐blind, crossover, randomized controlled trial (RCT) that investigated the effects of rate on analgesia in kilohertz frequency (1–10 kHz) spinal cord stimulation (SCS).
Materials and Methods
Patients were implanted with SCS systems and underwent an eight‐week search to identify the best location (“sweet spot”) of stimulation at 10 kHz within the searched region (T8–T11). An electronic diary (e‐diary) prompted patients for pain scores three times per day. Patients who responded to 10 kHz per e‐diary numeric rating scale (ED‐NRS) pain scores proceeded to double‐blind rate randomization. Patients received 1, 4, 7, and 10 kHz SCS at the same sweet spot found for 10 kHz in randomized order (four weeks at each frequency). For each frequency, pulse width and amplitude were titrated to optimize therapy.
Results
All frequencies provided equivalent pain relief as measured by ED‐NRS (p ≤ 0.002). However, mean charge per second differed across frequencies, with 1 kHz SCS requiring 60–70% less charge than higher frequencies (p ≤ 0.0002).
Conclusions
The PROCO RCT provides Level I evidence for equivalent pain relief from 1 to 10 kHz with appropriate titration of pulse width and amplitude. 1 kHz required significantly less charge than higher frequencies.
Keywords: Chronic pain, double‐blind, high frequency, high frequency SCS, kilohertz SCS, PROCO, randomized controlled trial, sub‐perception SCS
Introduction
Based primarily on differences in clinical observations, currently available spinal cord stimulation (SCS) therapies can be broadly categorized into at least two modalities: paresthesia SCS (“classical” SCS) and sub‐perception SCS (e.g., burst, kHz). Paresthesia SCS is generally characterized by programming stimulation parameters (including electric field configuration) such that the patient experiences paresthesia, and the paresthesia topography overlaps the pain topography as much as possible. This modality of stimulation typically results in analgesia in minutes to hours, and in many cases notable decreases in pain are reported during post‐op recovery. Further, the stimulation parameter settings used for paresthesia SCS require relatively less charge per second (as compared to present sub‐perception SCS settings) which can positively affect device longevity (primary cell implantable pulse generators [IPGs]) or patient charging burden (rechargeable IPGs).
In contrast, sub‐perception SCS is characterized by programming parameters that do not cause the patient to feel paresthesia. This modality of stimulation tends to have longer wash‐in and wash‐out times, and typically results in analgesia in several hours to days. The stimulation parameter settings evaluated and published thus far for sub‐perception SCS use relatively more charge per second (as compared to present paresthesia SCS settings) 1, 2, which can negatively affect device longevity (primary cell IPGs) and patient charging burden (rechargeable IPGs). Greater charging burden also increases the risk of patients being less compliant with the therapy due to the additional effort required to maintain therapy through frequent recharging.
These distinct clinical observations of paresthesia SCS and sub‐perception SCS suggest that different mechanisms could underlie these two modalities. Gate control theory 3 was the inspiration for and the putative mechanism underlying paresthesia SCS, though certain inconsistencies indicate that it is incomplete 4. It is not clear whether gate control is engaged directly when using sub‐perception SCS because computational evidence suggests that dorsal columns are apparently not activated 5. Reports that different brain regions may be activated and inhibited with sub‐perception 1 kHz SCS or burst SCS compared to standard rate SCS 6, 7 are consistent with the idea that different mechanisms may be engaged by these two modalities. Preclinical studies also point to the possibility that different mechanisms underlie paresthesia SCS and sub‐perception SCS. γ‐Amino butyric acid appears to play a role in paresthesia SCS 8, 9, but may not be involved in burst SCS 10. The mechanisms put forth thus far for 10 kHz SCS have been deemed unlikely: dorsal column conduction block and dorsal column desynchronization do not occur at clinically relevant amplitudes 5, 11, and the hyperpolarization observed with 10 kHz SCS 12 has been shown to be an artifact of the experimental conditions 13. Thus, a fundamental understanding of the mechanism(s) of action by which sub‐perception SCS acts is still lacking. Further, while the sub‐perception modality has been shown to be effective at very high rates 1, the sensitivity of the modality to the rate parameter is not clear. Understanding the sensitivities of clinical effects to parameters can potentially contribute information that is valuable in the search for mechanistic understanding.
Several clinical studies have shown that 10 kHz SCS can be effective 1, 14, 15. Others have shown that 1 kHz can be effective 16, 17, 18, 19. These results are consistent with preclinical studies showing that 1 and 10 kHz are approximately equally effective in reducing the mechanical hypersensitivity associated with neuropathic pain 20, 21, and that frequencies from 2 to 100 kHz all suppressed wide dynamic range (WDR) neuronal activity 22.
From a physiological perspective, these clinical and preclinical observations are consistent with the notion that effects of stimulation above maximum neuronal firing rates may be similar, because neurons are unable to fire in synchrony with stimuli greater than 800 Hz 23, 24, 25. However, because frequencies from 1 to 10 kHz have not been systematically tested in humans, the question of the sensitivity of pain relief to stimulation frequency remains unanswered. The multicenter, randomized, crossover, double‐blind study described here sought to answer this question by investigating effects of SCS frequency on analgesia in the kilohertz frequency range (1–10 kHz).
Materials and Methods
Details of the PROCO RCT are available on the clinicaltrials.gov registry under identifier NCT02549183. The Multicentre Research Ethics Committee and Research and Development departments at Basildon and Thurrock University Hospitals, Southmead Hospital, and James Cook University Hospital approved this study. This study was sponsored by the Basildon and Thurrock University Hospitals NHS Foundation Trust.
Subject Selection
All subjects provided informed consent and satisfied, among other criteria, key requirements including: 1) persistent or recurrent low back pain, with or without equal or lesser leg pain, for at least 90 days prior to screening; 2) at least 90 days of documented pain management care prior to screening to address the primary pain complaint; 3) mean low back pain intensity ≥ 5; 4) less than 180 mg per day of oral morphine or equivalent; 5) stable opioid medications for 30 days prior to screening; and 6) baseline Oswestry Disability Index score ≥ 20 and ≤ 80.
Key exclusion criteria included 1) back surgery in the previous six months; 2) radiographic evidence of spinal instability requiring fusion; 3) spinal pain secondary to neoplasm, infection, autoimmune disorder with spinal involvement, or a spinal bone metabolic disorder; and 4) any pain‐related diagnosis or medical/psychological condition that a clinician believes might confound reporting of study outcomes. Of note, patients on Employment and Support Allowance or Personal Independence Payment (workers' compensation programs) were not excluded.
Use of Electronic Diary
Wrist‐worn electronic diaries (CamNTech LTD, Cambridge, UK) were used to collect pain scores throughout the study. The electronic diary (ED) prompted patients with an alarm for current numerical rating scale (NRS) pain score inputs for each of three pain areas (back, leg, overall), three times per day (morning, afternoon, evening). If patients did not respond within a three min time window, the response was recorded as missed. It was not possible for patients to access or change the response after the window closed. For each stimulation rate, ED‐NRS scores from five consecutive days at the end of each rate randomization period were used for evaluation. The fifteen pain scores (5 days × 3 pain scores per day) per pain area (back, leg, overall) per patient were averaged to yield a mean pain score per pain area for each patient at each rate. A total of 80 data points (4 rates × 20 patients) per pain area were used for statistical analysis. If patients missed an e‐diary entry or if they were aware that they made a mistake in entering the response, they were instructed to note the correct response on paper. Two patients had injuries unrelated to the study during one rate evaluation period, so the five consecutive days prior to injury were used for evaluation. Two other patients forgot to wear the e‐diary during one rate evaluation period, so the five consecutive days prior to neglect of e‐diary were used for evaluation. Missing data were omitted from statistical analyses.
Study Design and Patient Flowchart
The study design is shown in Figure 1 and the patient flowchart in Figure 2. Of the 39 patients consented for the study, 34 patients met inclusion/exclusion criteria and underwent a one week paresthesia trial, after confirming during the implant procedure while the patient was awake that at least 80% paresthesia coverage of pain areas could be achieved. A paresthesia trial was used to ensure that patients could be treated with usual care, which is paresthesia SCS for the participating centers. If a patient did not respond to the paresthesia trial, they underwent a one‐week 10 kHz trial, and responders (per standard of care) continued to the next phase of the study. Per standard of care, patients who verbally reported percent pain relief (PPR) of at least 50% in a trial proceeded to permanent implant (97%, 33 of 34 patients). Nonresponders to a 10 kHz trial did not proceed to permanent implant (3%, 1 of 34 patients).
Figure 1.
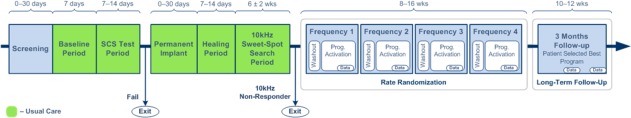
Study design. Patients followed standard of care (green) until rate randomization when they experienced four different frequencies. After rate randomization, the best rate was selected for three month follow‐up.
Figure 2.
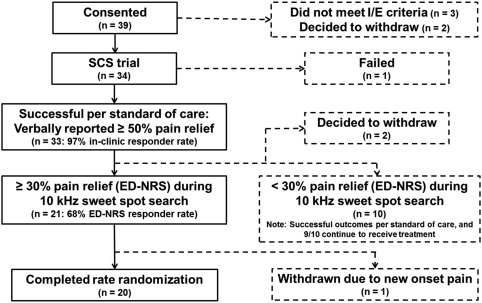
Patient flowchart.
Sweet Spot Search
The 33 patients who were permanently implanted underwent a search for the optimal stimulation location (“sweet spot”). The study provided the opportunity to exhaustively test stimulation bipoles along both leads (up to 14 stimulation locations were tested in eight weeks). The search was done using a frequency of 10 kHz and pulse width of 30 µs, and the amplitude was titrated to optimize therapy. Patients who had at least 30% pain relief per ED‐NRS were considered to be 10 kHz responders (68%, 21 of 31 patients) and proceeded to the rate randomization phase. A criterion of 30% pain relief per ED‐NRS (as opposed to the classical 50% criterion) was used because ED‐NRS is a more rigorous measure of pain relief (see Electronic diary as a rigorous method to assess pain section of Discussion). Two patients chose to withdraw from the study before completing the sweet spot search. Note: The apparent discrepancy between the trial responder rate and the 10 kHz responder rate is due to the difference in the methodology used to assess response (standard of care vs. e‐diary) and is described in detail in the Discussion. The ten patients who did not respond to 10 kHz per ED‐NRS were successful per standard of care, and nine of the ten continue to use SCS (four paresthesia, five multiple waveforms: paresthesia and 1 kHz or burst). 1 was explanted.
A methodological limitation of this study is that the sweet spot search was done only at 10 kHz and only 10 kHz responders proceeded to the rate randomization phase, which may have biased the study in favor of 10 kHz because other frequencies may not have the same optimal stimulation location.
Rate Randomization
The 21 patients who responded to 10 kHz proceeded to the rate randomization phase, where each patient experienced 1, 4, 7, and 10 kHz in randomized order. The same sweet spot identified during the 10 kHz sweet spot search was used for all rates. Before each rate was tested, patients turned off stimulation until their pain returned to 80% of baseline to prevent carryover effects. Washout was typically several hours to a day. Each rate was experienced for four weeks. During the first three weeks of each rate, pulse width and amplitude were adjusted to optimize therapy for the given rate. Pulse widths throughout the available range (different for each frequency) were systematically tested as time permitted. For each pulse width tested, amplitude was adjusted to optimize therapy. Each setting was used for 1–2 days, which allowed 10–20 settings to be evaluated during the three week titration period. The best identified setting was used during the fourth week, the last five days of which were used for the evaluation period. Patients, physicians, and data‐collecting research nurses were blinded to the programmed therapy. By necessity, the programmer was not blinded.
One patient was withdrawn during rate randomization due to new onset pain. The demographics of the 20 patients who completed randomization are shown in Table 1.
Table 1.
Patient Demographics.
| Number of patients who completed randomization | 20 |
|---|---|
| Sex | 9 female, 11 male |
| Age | 32–75 years (range); 53 years (mean) |
| Pain condition | 16 FBSS, 4 chronic radiculopathy |
| Pain duration | 1–27 years (range); 11 years (mean) |
Charge usage at each frequency was assessed by calculating the mean charge delivered per second at the settings used for evaluation at a given rate. Quality of life measurements were assessed at the visit following each rate evaluation period and included Oswestry Disability Index (ODI), EuroQol 5 Dimension 5 Level (EQ‐5D‐5L), Pittsburgh Sleep Quality Index (PSQI), and Patient Global Impression of Change (PGIC).
Three Month Follow‐Up
Patients used the frequency from their preferred period for three months after the end of rate randomization. If patients did not have a preferred period, the frequency that provided the minimum ED‐NRS score was selected. If multiple frequencies provided mean ED‐NRS scores within half a point, the frequency that required the least charge was selected.
Statistical Analyses
Hypothesis Test for Primary Endpoint
The primary statistical objective for this study was to test the hypothesis that mean low back pain relief from baseline for low back pain dominant SCS candidates was not sensitive to stimulation frequencies between 1 and 10 kHz. Let and be the mean low back ED‐NRS pain score at baseline and at a single rate randomization period, respectively. The mean e‐diary percent pain relief (ED‐PPR) from Baseline to the given Rate Randomization period was calculated as
The primary analysis assessed whether the trend line for mean ED‐PPR across the stimulation frequencies 1, 4, 7, and 10 kHz had a slope of 0. Let M be the equivalence margin for the slope. Then the null and alternative hypotheses were
where is the absolute value of the slope of the trend line for mean low back ED‐PPR across stimulation frequencies. The equivalence margin M was 1.67% per kHz, based on an allowed 15% difference in mean low back ED‐PPR across the stimulation frequencies from 1 to 10 kHz. Fifteen percent is well below the minimum clinically important difference estimated to be 33% 26, and is therefore a more conservative definition of equivalence.
A linear mixed model (LMM), a common tool in clinical science research, was used to assess the null hypothesis 27. Specifically, the model included rate as a fixed effect, subject as a random effect, and ED‐PPR as the outcome variable. If the 95% confidence interval for the fixed effect slope fell within (−M, M), then the null hypothesis was rejected and the mean low back ED‐PPR was considered equivalent across the tested frequencies.
Sample Size Calculation and Power Analysis
A sample size calculation and power analysis were performed based on the assumptions in Table 2. Under these assumptions, mean ED‐PPR data were generated for 1000 simulated experiments in which the true slope of mean ED‐PPR across frequencies was 0. A LMM was fitted for each simulated experiment to determine if the confidence interval of the slope fell within the equivalence margin. The Type II error rate (β) was calculated as the percent of simulations in which the interval extended beyond the margin. This process was repeated with different sample sizes until the simulated power (1 − β) reached 80%, yielding a final sample size of 18 subjects. Assuming a 10% attrition rate, this was increased to a final sample size of 20 subjects entering rate randomization.
Table 2.
Population Parameters Assumed to Carry Out Sample Size Calculations and Power Analysis.
| Parameter | Assumed value |
|---|---|
| One‐ or two‐sided test | 2 |
| Significance level (α) | 0.05 |
| Statistical power (1 − β) | 0.8 |
| Mean slope | 0 |
| Slope standard deviation | 1.1 |
| Within‐subject PPR standard deviation | 15 |
| Within‐subject PPR correlation | 0.2 |
| Noninferiority margin (M) | 1.67% per kHz |
| Attrition | 10% |
It was assumed that the true mean slope of mean PPR across stimulation frequencies between 1 and 10 KHz was 0, but that individual subjects' slopes varied around the mean according to the slope standard deviation. Furthermore, the mean PPR values simulated for a specific subject were drawn from a distribution with a mean defined by the subject's trend line slope and a covariance matrix defined by the within‐subject standard deviation and within‐subject correlation across stimulation rates.
Post‐Hoc Analyses
In post‐hoc analyses, the sensitivity of leg and overall pain relief to stimulation rate was assessed using the same method as for low back pain.
Rate randomization sequence (i.e. the order of rates given to each patient), randomization period 1, 2, 3, 4, and the patient's sex, age, pain condition (i.e. diagnosis), and pain duration were explored as possible covariates that helped to further explain the PPR observed during the experiment. These factors were tested for any effect on the ED‐PPR outcome variable by including them one at a time as fixed effects in the LMM described above (in addition to the fixed effect of rate).
Results
Pain Relief
The results of the statistical analysis are presented in Figure 3a which shows mean back pain ED‐NRS scores at baseline, 1, 4, 7, and 10 kHz. The mean back pain ED‐NRS scores decreased from 6.8 ± 0.3 (baseline, mean ± standard error of the mean [SEM]) to 3.2 ± 0.3 (1 kHz), 3.5 ± 0.3 (4 kHz), 3.2 ± 0.3 (7 kHz), and 3.3 ± 0.4 (10 kHz), yielding approximately 50% pain relief across frequencies as measured with ED‐NRS. Importantly, all frequencies provided equivalent back pain relief (p = 0.00002), demonstrating that stimulation rate was not a meaningful determinant of back pain relief. Additionally, mean leg pain ED‐NRS scores (Fig. 3b) decreased from 5.5 ± 0.4 (baseline) to 2.6 ± 0.4 (1 kHz), 2.7 ± 0.4 (4 kHz), 2.7 ± 0.4 (7 kHz), and 2.9 ± 0.4 (10 kHz). Mean overall pain ED‐NRS scores (Fig. 3c) decreased from 6.7 ± 0.3 (baseline) to 3.2 ± 0.3 (1 kHz), 3.5 ± 0.3 (4 kHz), 3.2 ± 0.3 (7 kHz), and 3.3 ± 0.4 (10 kHz). Leg pain relief and overall pain relief were also approximately 50% and equivalent across frequencies (p = 0.003 and p = 0.00002, respectively). Patient compliance with the e‐diary was 91%.
Figure 3.
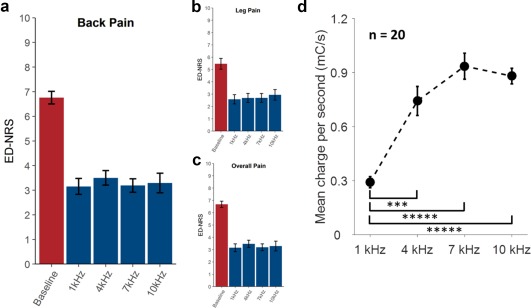
Pain relief and charge required across frequencies. Error bars denote standard error. a. Back pain. b. Leg pain. c. Overall pain. d. Mean charge per second. ***p ≤ 0.001, ***** p ≤ 0.00001.
Charge Usage
Figure 3d shows mean charge per second for each frequency at the amplitude and pulse width settings used by the patient to collect endpoint data. Mean charge per second is calculated as amplitude × pulse width × frequency, and reflects the amount of charge actively delivered to the tissue over time (and not the charge delivered during a single pulse, nor power delivered). One kilohertz required significantly less charge than 4 kHz (p = 0.0002), 7 kHz (p = 0.00001), and 10 kHz (p = 0.00001). Four, seven, and ten kilohertz showed no significant differences in pairwise comparisons (p ≥ 0.1; corrected for multiple comparisons). The shape of the curve indicates that at identified parameters (pulse width and amplitude) for optimal pain relief, the charge per unit time is not constant across frequencies. Further, at optimally identified parameters, the relationship between charge delivered per unit time and frequency appears to be nonlinear, indicating that changes in frequency alone, without titration of pulse width and amplitude, may not yield equivalent pain relief.
Quality of Life
There was no evidence that improvements in quality of life measurements from baseline differed across frequencies. Improvements in ODI summary scores (Fig. 4a), EQ‐5D‐5L summary scores (Fig. 4b), and PSQI (Fig. 4c) were similar and not statistically significantly different across frequencies (p ≥ 0.4). Similarly, PGIC (Fig. 4d) measures did not show significant differences across frequencies (p = 0.9). Note that large p values indicate that there was no statistical difference across frequencies in the measured improvements in quality of life.
Figure 4.
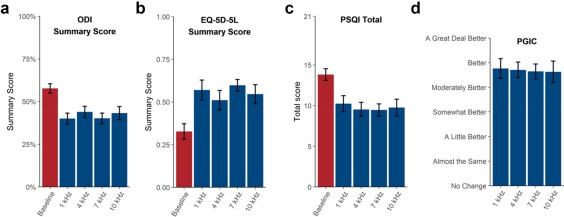
Quality of life metrics across frequencies. Error bars denote SEM. a. ODI summary score. b. EQ‐5D‐5L summary score. c. PSQI total score. d. PGIC.
Three Month Follow‐Up
Figure 5 shows the three month follow‐up mean ED‐NRS scores for back pain (a, 3.1 ± 0.4), leg pain (b, 2.7 ± 0.4), and overall pain (c, 3.1 ± 0.4). The three month follow‐up ED‐NRS scores are approximately the same as the ED‐NRS scores during rate randomization. Figure 5d shows the distribution of rates selected for three month follow‐up: 50% of patients used 1 kHz, 10% used 4 kHz, 25% used 7 kHz, and 15% used 10 kHz during three month follow‐up. Fifteen of 20 patients selected their preferred rate. Five of 20 patients did not have a preference, and the rate was selected as described in the Methods. Of the 15 patients who selected their own rate, 7 patients selected the last rate that they experienced (1 kHz: 2 patients, 4 kHz: 0 patients, 7 kHz: 2 patients, 10 kHz: 3 patients), a larger number than the expected three or four if all periods were equally preferred, but the trend was not statistically significant.
Figure 5.
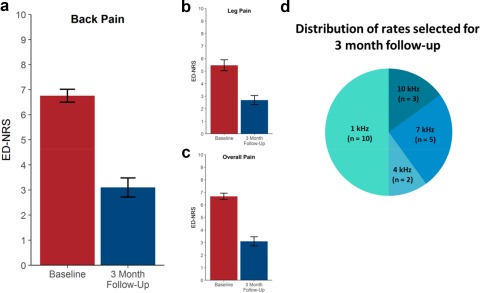
Pain relief three months after completion of rate randomization and rate selected for three month follow‐up. a. Back pain. b. Leg pain. c. Overall pain. d. Distribution of rates selected for three month follow‐up.
Effects of Potential Covariates
No significant effects of rate randomization sequence, randomization period, sex, age, pain condition, and pain duration were detected.
Discussion
This study provides Level 1 evidence 28 that SCS frequencies from 1 to 10 kHz provide equivalent pain relief. That is, no difference in mean pain ED‐NRS scores across the four rates (1, 4, 7, and 10 kHz) for any pain area evaluated (back, leg, overall) was found based on the predefined equivalence bounds. Improvements in quality of life also showed no differences across frequencies. Notably, 1 kHz required significantly less charge to provide the same therapeutic benefits compared to higher frequencies. Providing the same therapy, while using less charge is expected to be beneficial for patients because the charging burden may be reduced. Furthermore, delivering more charge than is required to achieve therapeutic benefit may not be desirable for considerations including device longevity and potential implications on the development of tolerance to stimulation. Drawing an analogy to medication, physicians generally prefer to prescribe the smallest dose of medication that can achieve the desired therapeutic outcomes.
Titration of Pulse Width and Amplitude Is Required to Achieve Pain Relief
A key learning from this study is that SCS at a given frequency requires titration of pulse width and amplitude to achieve maximal pain relief. Figure 6 shows that the variance of ED‐NRS scores during titration (the first three weeks of each rate randomization combined to yield 12 weeks total) was greater than the variance of ED‐NRS during the three month follow‐up in 17 of 20 patients, indicating that pain scores fluctuated more during titration and were more stable on the optimal setting used during three month follow‐up. That is, the larger variance of pain scores during titration is consistent with analgesia being sensitive to changes in parameters, highlighting the importance of titration.
Figure 6.
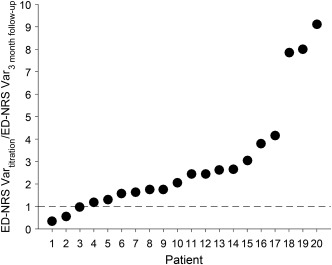
Comparison of variance in ED‐NRS during titration and three month follow‐up. Each symbol represents one patient's variance during titration (ED‐NRS Vartitration) divided by variance during the three month follow‐up (ED‐NRS Var3 month follow‐up). The patients are ordered in increasing ratio of ED‐NRS Vartitration to ED‐NRS Var3 month follow‐up. Seventeen of 20 patients had larger variance in ED‐NRS during titration, indicating that ED‐NRS was more stable on the optimal setting used during three month follow‐up.
As indicated by the apparent nonlinear relationship between charge and stimulation frequency (Fig. 3d), the titration is not as simple as holding charge per time constant. Nor does the data support the idea that decreasing frequency while holding pulse width and amplitude constant would be effective, because the amount of charge required by 10 kHz is three times greater than 1 kHz, not ten times greater as would be expected if changing only frequency were effective. The finding that titration is important is consistent with recent observations that titration of amplitude improves burst SCS outcomes 29, 30 and with findings in preclinical models 31.
Double Blinding Reduces Bias
Double blinding is an important aspect of the study design because expectations of patients and clinicians can have substantial impact on patient outcomes 32, 33, 34. Positive expectations enhance therapeutic effects 35, and negative expectations diminish therapeutic effects 36, 37. Thus, double blinding reduces bias that could confound the results. Double blinding was possible in the PROCO RCT because all parameter sets that were randomized were sub‐perception. The lower mean charge required by 1 kHz could conceivably have differentiated 1 kHz from the other frequencies. However, neither patients nor investigators were aware at study initiation that lower frequency would be associated with lower charge, and therefore it is not expected that patients associated charging with stimulation frequency. Also, during titration, charge usage varied within a single frequency because stimulation pulse width and amplitude were varied. Finally, patients had no expectations of how frequency would affect outcomes, nor did they know which frequency they were using. Rather, they were informed that this study was intended to investigate the effect of frequency on pain relief.
Electronic Diary as a Rigorous Method to Assess Pain
E‐diary is expected to be a more rigorous method to assess pain compared to paper diary and verbal pain assessment in the clinic for several reasons:
When pain is assessed in the clinic, patients are asked to recall their pain intensity over a period of time. Because memory of pain intensity is distorted by many factors, including emotional state, anxiety level, peak pain, and most recent pain 38, 39, 40, recall is an unreliable method of assessing pain. The e‐diary used in the PROCO RCT prompted patients for their current pain, thus obviating the need for recall and enabling a real‐time pain rating.
Compliance with paper diary is only 11% while compliance with e‐diary is 94% 41, which is consistent with the current study (91%). Patients typically fill in paper diary entries based on memory, which is unreliable as discussed in point 1.
Pain fluctuates from day to day and at different times of day 42, 43, 44. Typically, pain is lowest in the morning and increases throughout the day 45. Thus, a single assessment does not sufficiently capture the pain experience. For each frequency, the PROCO RCT averaged five days of pain scores, which were collected three times per day (morning, afternoon, evening), yielding a more accurate evaluation of the overall pain experience.
Finally, when pain is assessed in the clinic, the evaluator could unintentionally influence the pain intensity reported by the patient 46, 47. The use of an e‐diary eliminates the influence of an evaluator during data capture.
One limitation of note is that when pain is assessed in the clinic, PPR is not the only consideration. Functional improvements are also assessed and taken into account when evaluating therapeutic outcomes. ED‐NRS does not capture functional improvements, which makes it a conservative measure of therapeutic efficacy. However, an e‐diary could include questions about functional improvements and satisfaction, which would provide a more complete understanding of patient outcomes.
Direct Comparison of Pain Assessment Using e‐Diary With Pain Assessment in the Clinic
The PROCO RCT trial period provided an opportunity to directly compare pain assessment by e‐diary with pain assessment in the clinic. During the trial, patients used both an e‐diary, and per standard of care, verbally reported their pain relief to the clinical team at the end of the trial. A trial responder rate of 97% was determined when based on in‐clinic verbally reported PPR of at least 50%. However, when ED‐NRS scores were used to calculate PPR in those same patients, the trial responder rate was 62% using a calculated PPR of at least 30%. That is, despite a lower threshold for success (30% vs. 50%), the responder rate was lower (62% vs. 97%) when using ED‐NRS scores compared to in‐clinic evaluation. Figure 7 illustrates the relationship between verbally reported pain relief and ED‐NRS PPR. This result suggests that for the same pain, greater PPR may be reported during in‐clinic verbal assessment than the PPR calculated through an e‐diary capture of current pain.
Figure 7.
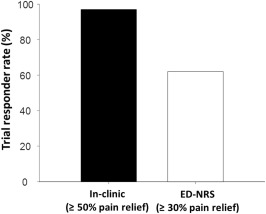
Comparison of responder rate during trial using in‐clinic assessment and ED‐NRS. While the responder rate based on in‐clinic verbal report was 97% (33/34), the responder rate for the same patients based on ED‐NRS was 62% (21/34).
The finding that in‐clinic assessment results in a report of more pain relief compared to e‐diary is consistent with observations in other reports (case series) of comparisons between either 1 or 2 kHz and 10 kHz SCS 48, 49. These reports also suggest equivalent pain relief is achievable with 10 kHz and lower rates (either 1 or 2 kHz), but using in‐clinic pain scores they observed 70–80% improvement from baseline. These findings are also consistent with the SENZA RCT 1 which assessed back pain VAS in the clinic and reported 62.8% mean VAS change from baseline, while also assessing back pain (at rest) via paper diary and reported 56.2% reduction 50. Similarly, the SENZA RCT responder rate based on in‐clinic assessment of pain (78.4%) was greater than the responder rate based on paper diary (65.7%) 50.
Thus, measurements of pain are highly dependent on the method used for assessment, and e‐diary (multiple data points per day over multiple days) appears to be a useful method for rigorous investigation. Attempts to compare results from studies that used different assessment methodologies (e.g., in‐clinic evaluation, paper diary, and e‐diary) must be undertaken with careful consideration of the method used to assess pain among other factors.
Equivalence Margin
The primary endpoint analysis was limited in that it only tested whether the slope of ED‐PPR from 1 to 10 kHz fell within the predefined equivalence bounds (−1.67%, 1.67%). To get a better sense of what the data suggested about possible values for the slope, 99% confidence intervals were calculated for the slope estimated during the primary LMM analysis. Compared to the equivalence bounds of (−1.67%, 1.67%), the 99% confidence interval was notably smaller: (−0.94%, 0.93%). Thus, the data indicated that the slope of ED‐PPR from 1 to 10 kHz was even closer to 0% than the equivalence margin suggested.
Additionally, paired t‐tests comparing ED‐PPR between different pairs of rates provided no evidence of significant differences (t‐test results were Holm‐Bonferroni corrected for multiple comparisons).
Together, these post‐hoc analyses provided evidence that any possible change in PPR per ED‐NRS across rates was much closer to 0% than the equivalence margin could have suggested. In other words, data strongly support the hypothesis that all frequencies provided equivalent pain relief.
Number of Subjects
The PROCO RCT study used a crossover design which greatly enhances the statistical power compared to a parallel design because each subject acts as their own control across the range of stimulation frequencies tested, thereby reducing the variability in the data. Concretely, if a single subject is exposed to each frequency, differences in the subject's outcome data caused by one frequency vs. another will be easier to identify because all other attributes of that subject stay constant. Conversely, in place of this single subject, a parallel design study would require four different subjects experiencing one frequency each; thus, any outcome differences caused by different frequencies must be large enough to stand out from the differences caused by the variability between subjects, which can be sizeable and would reduce the power of the study.
To quantitatively demonstrate the increased power afforded by the crossover design, the sample size calculation described in the methods section was carried out under the assumption that the study was designed with four different groups of subjects in parallel arms. Specifically, the within‐subject outcome correlation across frequencies was removed and no random effect of subject was included in the linear regression models fit to each simulation. This process revealed that to achieve a power of 80%, an overall parallel‐arm sample size of 108 subjects was required (27 at each frequency). This means that had the current study been designed with parallel arms, it would have been necessary to recruit 108 subjects to provide the same statistical power provided here with 20 subjects. Thus, the power gained by crossover, allowing each patient to act as their own control, is a significant strength of the study design. The statistical plan was robust and post‐hoc analysis indicates it was conservative (see Equivalence Margin section of the Discussion).
However, the advantage afforded by the reduction of subjects, adds a significant difficulty in that it requires each subject to provide more data than in a parallel‐arm design, as each subject had to experience each frequency, instead of just one frequency. Therefore, this type of study required collaborative patients to execute, especially because of the large amount of data that each patient was asked to provide.
Comparison With Previous Clinical Studies
In two previous RCTs investigating kilohertz frequencies, Perruchoud et al. 51 found that 5 kHz was no more effective than sham, and Al‐Kaisy et al. 52 found that 5.882 kHz was more effective than 1.2 and 3.030 kHz, which were no different from sham. However, methodological differences in programming may account for these apparent inconsistencies with PROCO RCT results. First, in the Perruchoud et al. RCT, pulse width at 5 kHz was fixed at 60 µs and amplitude set just below perception threshold, while the PROCO RCT found that titrating pulse width and amplitude on an individual patient basis was required for achieving maximal pain relief (see Titration of pulse width and amplitude is required to achieve pain relief section of the Discussion). Second, the active electrodes in the Perruchoud et al. RCT were selected based on paresthesia coverage rather than on an anatomical search, as was done in the PROCO RCT. Similarly, the Al‐Kaisy et al. RCT used a fixed pulse width at each frequency, and it is unclear whether amplitude was titrated. However, at 1.2 kHz Al‐Kaisy et al. reported a mean charge per second more than double than that associated with optimal pain relief at 1 kHz in this study, so different outcomes may have been a result of different program settings.
Consistent with the results of the PROCO RCT, other RCTs have indicated that 1 kHz stimulation is effective 16, 17. Kriek et al. compared 40, 500, 1200 Hz, burst, and placebo SCS, finding that the four stimulation programs were equally effective and they were all significantly more effective than placebo. In the Kriek et al. RCT, the electrode configuration used for 500, 1200 Hz, and burst was identical to the electrode configuration providing paresthesia coverage. North et al. showed that 1 kHz SCS was effective in SCS patients with limited response to paresthesia SCS. Like the Kriek et al. RCT, electrode configuration in the North et al. RCT was based on paresthesia coverage.
Consistent with the PROCO RCT, the SENZA RCT showed that sub‐perception SCS at 10 kHz can be effective. SENZA did report a larger NRS decrease (∼70%) 1 than the ED‐NRS decrease (∼50%) reported by PROCO, but this difference is likely explained by the different methods used in each study to measure pain (see Discussion section Direct comparison of pain assessment using e‐diary with pain assessment in the clinic). Also, patients and clinicians in the SENZA RCT knew which treatment was being applied, and multiple studies have shown that positive and negative patient expectations can significantly impact pain, sleep, depression, and physical function 53, 54, 55, 56. Because all stimulation comparisons in the PROCO RCT were sub‐perception, double blinding was able to be used to avoid this effect.
Comparison With Preclinical Studies
The PROCO RCT results are also consistent with preclinical studies. Schechter et al. 20 used a spinal nerve ligation model of pain and tested amplitudes of 20, 40, and 80% motor threshold (MT), showing that 1 and 10 kHz were equally effective in reducing mechanical hypersensitivity, and efficacy increased with amplitude. Song et al. 21 used a spared nerve injury model of pain and tested amplitudes of 40–50% MT, also showing that 1 and 10 kHz yielded equivalent improvement in paw withdrawal threshold. Another preclinical study 22 found that frequencies from 2 to 100 kHz suppressed WDR neuronal activity. Though these preclinical studies have begun investigating mechanisms of high frequency SCS, the mechanisms remain unclear.
Study Limitations
A limitation of this study is that the sweet spot search was done only at 10 kHz and the optimal topographical target found from the sweet spot search at 10 kHz was always used for all frequencies. It is possible that different frequencies have different sweet spots. Also, only 10 kHz responders proceeded to rate randomization to experience all rates, guaranteeing that 10 kHz, but not other frequencies, would provide pain relief. These two aspects of the study design could have biased the results in favor of 10 kHz.
A potential criticism of this study is that it was not sham‐controlled. The PROCO RCT focused on the rate comparison rather than evaluating therapy efficacy because 10 kHz had already been shown to be effective 1, 14. From an ethical standpoint, we could not be sure that patients would respond to frequencies below 10 kHz, so including a sham phase could have prolonged the time patients did not experience pain relief. Although the methods used in PROCO could be used to better understand placebo effects in SCS, the question that the PROCO RCT aimed to answer was “What is the effect of rate on analgesia?”
Another potential criticism of this study is that patients experienced paresthesia first during the trial, which could have biased the patients to prefer paresthesia. However, the ED‐NRS responder rate for the paresthesia‐based trial (62%) and the 10 kHz sweet spot search (68%) were similar, suggesting that such a bias did not likely exist. In addition, to maintain clinical equipoise among treatments, patients were informed that paresthesia and sub‐perception SCS were two options and that both could be effective. Further, any bias would have affected all frequencies equally because the comparison was between kilohertz frequencies, not paresthesia.
Another limitation of this study is that patients experienced each rate for only four weeks. However, over the course of the study, patients experienced kilohertz frequency SCS for eight to nine months with consistent pain relief, as shown by the results of the three month follow‐up at the selected frequency after rate randomization.
Future Directions
A natural question arising from this work is “What is the sensitivity of analgesia to frequency outside the range of 1–10 kHz?” In particular, the possibility that frequencies below 1 kHz may require even less charge while providing equivalent pain relief without paresthesia is intriguing. Long pulse width burst SCS at 500 Hz has been shown to have an effect 2, 7, further suggesting that lower frequencies of SCS may be effective in the sub‐perception modality (although, whether or not the mechanisms of action underlying burst SCS and kilohertz frequency SCS are the same remains an open question). Other questions include whether the mechanisms of action for these two broad modalities of SCS (paresthesia and sub‐perception) overlap in frequency range, and whether they can be engaged simultaneously to potentially yield an additive effect that further improves therapy. Finally, simple bipoles were used in this study, but optimizing field shape may further improve therapy. Just as in paresthesia therapies, more advanced and flexible electrode combinations can significantly improve outcomes 57, so it can be speculated that the availability of such flexibility of electrode combinations may also further improve kilohertz frequency SCS outcomes.
As fundamental understanding of mechanisms of action increases, continued optimization is likely. In the meantime, empirical optimization is expected to support improved therapy, and increased empirical understanding of “what works” may inform mechanistic understanding.
Conclusions
The PROCO RCT is the first study to provide Level I evidence on the effect of frequencies from 1 to 10 kHz on analgesia. The study showed equivalent pain relief and improvement in quality of life across all evaluated frequencies. However, 1 kHz required significantly less charge than higher frequencies, which is beneficial to patients because it reduces charging burden and exposure to stimulation.
Authorship Statements
Dr. Thomson, Dr. Tavakkolizadeh, Mr. Doan, and Dr. Moffitt conceived and designed the study. Dr. Thomson, Dr. Tavakkolizadeh, Dr. Love‐Jones, and Mr. Patel contributed to data acquisition. Dr. Gu programmed the patients. Drs. Gu and Bains analyzed the data and prepared the manuscript draft with important intellectual input from Drs. Thomson, Love‐Jones, and Moffitt. All authors approved the final manuscript.
COMMENT
Use of high frequency stimulation is not new but this study demonstrates that with 1kHz frequency a patient can produce similar analgesia as 10kHz while using 60–70% less energy. This is a substantial saving of energy and increases the duration between charges of an IPG.
Vikram Patel, MD
Algonquin, IL, USA
Comments not included in the Early View version of this paper.
Acknowledgements
The authors would like to acknowledge Sam Eldabe of South Tees Hospitals and Ashish Gulve of South Tees Hospitals for enrolling patients in the study; Carol Alves of Basidon and Thurrock University Hospitals for coordinating the study; Anne Nicholson of Basidon and Thurrock University Hospitals, Kellie Allen of Basidon and Thurrock University Hospitals, and Ann Rich of Southmead Hospital for assistance with collecting data; and Ramsey Samara of Boston Scientific for assistance with writing and editing the manuscript. Boston Scientific provided funding for this study.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: Research grant to BTUH R&D from Boston Scientific.
Conflict of Interest: Dr. Thomson and Dr. Love‐Jones are consultants for Boston Scientific. Mr. Patel has received an honorarium from Boston Scientific. The remaining authors have no conflicts of interest to disclose.
References
- 1. Kapural L, Yu C, Doust MW et al. Novel 10‐kHz high‐frequency therapy (HF10 Therapy) is superior to traditional low‐frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA‐RCT randomized controlled trial. Anesthesiology 2015;123:851–860. [DOI] [PubMed] [Google Scholar]
- 2. Deer T, Slavin KV, Amirdelfan K et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation 2018;21:56–66. [DOI] [PubMed] [Google Scholar]
- 3. Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:970–979. [DOI] [PubMed] [Google Scholar]
- 4. Zhang TC, Janik JJ, Peters RV et al. Spinal sensory projection neuron responses to spinal cord stimulation are mediated by circuits beyond gate control. J Neurophysiol 2015;114:284–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lempka SF, McIntyre CC, Kilgore KL et al. Computational analysis of kilohertz frequency spinal cord stimulation for chronic pain management. Anesthesiology 2015;122:1362–1376. [DOI] [PubMed] [Google Scholar]
- 6. Rauck R. Differential mechanisms of action between paresthesia and paresthesia‐free SCS: a PET study. NANS 19th Annual Meeting, December 10–13, 2015, Las Vegas, NV.
- 7. De Ridder D, Plazier M, Kamerling N et al. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013;80:642–649.e1. [DOI] [PubMed] [Google Scholar]
- 8. Stiller CO, Cui JG, O'Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of [gamma]‐aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery 1996;39:367–375. [DOI] [PubMed] [Google Scholar]
- 9. Cui JG, Linderoth B, Meyerson BA. Effects of spinal cord stimulation on touch‐evoked allodynia involve GABAergic mechanisms. An experimental study in the mononeuropathic rat. Pain 1996;66:287–295. [DOI] [PubMed] [Google Scholar]
- 10. Crosby ND, Weisshaar CL, Smith JR, Zeeman ME, Goodman‐Keiser MD, Winkelstein BA. Burst and tonic spinal cord stimulation differentially activate GABAergic mechanisms to attenuate pain in a rat model of cervical radiculopathy. IEEE Trans Biomed Eng 2015;62:1604–1613. [DOI] [PubMed] [Google Scholar]
- 11. Crosby ND, Janik JJ, Grill WM. Modulation of activity and conduction in single dorsal column axons by kilohertz‐frequency spinal cord stimulation. J Neurophysiol 2017;117:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: hypothetical mechanisms of action and comments on outcomes. Neuromodulation 2017;20:525–533. [DOI] [PubMed] [Google Scholar]
- 13. Prescott SA. Electrophysiological artifacts during high‐frequency SCS and optical methods to circumvent them. INS 13th Annual Meeting, 2017, Edinburgh, Scotland.
- 14. Al‐Kaisy A, Van Buyten J‐P, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high‐frequency spinal cord stimulation for patients with chronic, low back pain: 24‐month results of a prospective multicenter study. Pain Med 2014;15:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russo M, Verrills P, Mitchell B, Salmon J, Barnard A, Santarelli D. High frequency spinal cord stimulation at 10 kHz for the treatment of chronic pain: 6‐month Australian clinical experience. Pain Phys 2016;19:267–280. [PubMed] [Google Scholar]
- 16. North JM, Hong KSJ, Cho PY. Clinical outcomes of 1 kHz subperception spinal cord stimulation in implanted patients with failed paresthesia‐based stimulation: results of a prospective randomized controlled trial. Neuromodulation 2016;19:731–737. [DOI] [PubMed] [Google Scholar]
- 17. Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen FJPM. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double‐blind, randomized and placebo‐controlled crossover trial. Eur J Pain 2017;21:507–519. [DOI] [PubMed] [Google Scholar]
- 18. Haider N, Miller N, Gilmore C. Spinal cord stimulation (SCS) trial outcomes after conversion to a multiple waveform SCS System. NANS 20th Annual Meeting, 2017, Las Vegas, NV. [DOI] [PubMed]
- 19. Pyles S, Lechleiter K, Huynh D et al. Improved spinal cord stimulation outcomes associated with percutaneous lead placement and multiple waveform programming technique. NANS 20th Annual Meeting, 2017, Las Vegas, NV.
- 20. Shechter R, Yang F, Xu Q et al. Conventional and kilohertz‐frequency spinal cord stimulation produces intensity‐ and frequency‐dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology 2013;119:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song Z, Viisanen H, Meyerson BA et al. Efficacy of kilohertz‐frequency and conventional spinal cord stimulation in rat models of different pain conditions. Neuromodulation 2014;17:226–235. [DOI] [PubMed] [Google Scholar]
- 22. Cuellar JM, Alataris K, Walker A et al. Effect of high‐frequency alternating current on spinal afferent nociceptive transmission. Neuromodulation 2013;16:318–327. [DOI] [PubMed] [Google Scholar]
- 23. Blight AR, Someya S. Depolarizing afterpotentials in myelinated axons of mammalian spinal cord. Neuroscience 1985;15:1–12. [DOI] [PubMed] [Google Scholar]
- 24. Kiernan MC, Bostock H. Depolarizing afterpotentials in myelinated axons of mammalian spinal cord. Brain 2000;123:2542–2551. 11099455 [Google Scholar]
- 25. Woolf CJ, Salter M. Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765–1768. [DOI] [PubMed] [Google Scholar]
- 26. Farrar JT, Portenoy RK, Berlin JA et al. Defining the clinically important difference in pain outcome measures. Pain 2000;88:287–294. [DOI] [PubMed] [Google Scholar]
- 27. West B, Welch K, Galecki A. Linear mixed models: a practical guide using statistical software. Boca Raton: Chapman & Hall/CRC, 2007. [Google Scholar]
- 28. Canadian Task Force on the Periodic Health Examination . The periodic health examination. CMA J 1979;121:1193–1254. [PMC free article] [PubMed] [Google Scholar]
- 29. Tavel E, Amirdelfan K, Phillips G et al. Programming optimization strategies for burst may improve outcomes. NANS 20th Annual Meeting, 2017, Las Vegas, NV.
- 30. Jennings J, Bourke C, Baranidharan G. Burst spinal column stimulation, Leeds experience in optimisation of programming to enhance patient outcome and satisfaction. NANS 20th Annual Meeting, 2017, Las Vegas, NV.
- 31. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Conventional versus burst spinal cord stimulation on mechanical hypersensitivity in a rat model of chronic neuropathic pain: effect of amplitude. Neuromodulation 2018;21:19–30. [DOI] [PubMed] [Google Scholar]
- 32. Colagiuri B. Participant expectancies in double‐blind randomized placebo‐controlled trials: potential limitations to trial validity. Clin Trials 2010;7:246–255. [DOI] [PubMed] [Google Scholar]
- 33. Enck P, Klosterhalfen S, Weimer K et al. The placebo response in clinical trials: more questions than answers. Phil Trans R Soc B 2011;366:1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med 2010;16:1277–1283. [DOI] [PubMed] [Google Scholar]
- 35. Quessy SN, Rowbotham MC. Placebo response in neuropathic pain trials. Pain 2008;138:479–483. [DOI] [PubMed] [Google Scholar]
- 36. Rief W, Bingel U, Schedlowski M et al. Mechanisms involved in placebo and nocebo responses and implications for drug trials. Clin Pharmacol Ther 2011;90:722–726. [DOI] [PubMed] [Google Scholar]
- 37. Vase L, Vollert J, Finnerup NB et al. Predictors of the placebo analgesia response in randomized controlled trials of chronic pain: a meta‐analysis of the individual data from nine industrially sponsored trials. Pain 2015;156:1795–1802. [DOI] [PubMed] [Google Scholar]
- 38. Broderick JE, Schwartz JE, Vikingstad G et al. The accuracy of pain and fatigue items across different reporting periods. Pain 2008;139:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Redelmeier DA, Kahneman D. Patients' memories of painful medical treatments: real‐time and retrospective evaluations of two minimally invasive procedures. Pain 1996;66:3–8. [DOI] [PubMed] [Google Scholar]
- 40. Eich E, Reeves J, Jaeger B et al. Memory for pain: relation between past and present pain intensity. Pain 1985;23:375–379. [DOI] [PubMed] [Google Scholar]
- 41. Stone AA, Broderick JE, Schwartz JE et al. Intensive momentary reporting of pain with an electronic diary: reactivity, compliance, and patient satisfaction. Pain 2003;104:343–351. [DOI] [PubMed] [Google Scholar]
- 42. Affleck G, Tennen H, Urrows S et al. Person and contextual features of daily stress reactivity: individual differences in relations of undesirable daily events with mood disturbance and chronic pain intensity. J Pers Soc Psychol 1994;66:329–340. [DOI] [PubMed] [Google Scholar]
- 43. Stone A, Broderick J, Porter L et al. The experience of rheumatoid arthritis pain and fatigue: examining momentary reports and correlates over one week. Arthritis Care Res 1997;10:185–193. [DOI] [PubMed] [Google Scholar]
- 44. Affleck G, Tennen H, Keefe F et al. Everyday life with osteoarthritis or rheumatoid arthritis: independent effects of disease and gender on daily pain, mood, and coping. Pain 1999;83:601–609. [DOI] [PubMed] [Google Scholar]
- 45. Folkard S, Glynn CJ, Lloyd JW. Diurnal variation and individual differences in the perception of intractable pain. J Psychosom Res 1976;20:289–301. [DOI] [PubMed] [Google Scholar]
- 46. Kállai I, Barke A, Voss U. The effects of experimenter characteristics on pain reports in women and men. Pain 2004;112:142–147. [DOI] [PubMed] [Google Scholar]
- 47. Levine FM, De Simone LL. The effects of experimenter gender on pain report in male and female subjects. Pain 1991;44:69–72. [DOI] [PubMed] [Google Scholar]
- 48. Koulousakis A, Matis G, Bara G et al. 10 kHz and 1.2 kHz Comparison concerning clinical outcomes and charge burden. NANS 20th Annual Meeting, 2017, Las Vegas, NV.
- 49. Ibrahim R, Sackerer D. Comparative analysis of patient preference for 10 kHz and 2 kHz SCS in patients with chronic neuropathic back and leg pain. INS 13th Annual Meeting, 2017, Edinburgh, Scotland.
- 50. FDA Summary of Safety and Effectiveness Data, Senza Spinal Cord Stimulation System, PMA P130022, page 44.
- 51. Perruchoud C, Eldabe S, Batterham AM et al. Analgesic efficacy of high‐frequency spinal cord stimulation: a randomized double‐blind placebo‐controlled study. Neuromodulation 2013;16:363–369. [DOI] [PubMed] [Google Scholar]
- 52. Al‐Kaisy A, Palmisani S, Sanderson K et al. SCS frequency study: subject therapy preference post randomized phase in a spinal cord stimulation study using higher frequencies. NANS 20th Annual Meeting, 2017, Las Vegas, NV.
- 53. Kam‐Hansen S, Jakubowski M, Kelley JM et al. Labeling of medication and placebo alters the outcome of episodic migraine attacks. Sci Transl Med 2014;6:218ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. Am J Psychiatry 2013;170:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schedlowski M, Enck P, Rief W et al. Neuro‐bio‐behavioral mechanisms of placebo and nocebo responses: implications for clinical trials and clinical practice. Pharmacol Rev 2015;67:697–730. [DOI] [PubMed] [Google Scholar]
- 56. Weimer K, Colloca L, Enck P. Placebo effects in psychiatry: mediators and moderators. Lancet Psychiatry 2015;2:246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Veizi E, Hayek SM, North J et al. Spinal cord stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS‐LUMINA study. Pain Med 2017;18:1534–1548. [DOI] [PubMed] [Google Scholar]


