Abstract
Background and purpose
Small fiber neuropathy (SFN) is a common disorder leading to neuropathic pain and autonomic symptoms. The objective of this study was to investigate associated conditions in a large cohort of SFN patients and compare the prevalence to healthy individuals.
Methods
A total of 921 patients with pure SFN were screened according to a standardized comprehensive diagnostic algorithm and compared with literature findings.
Results
No associated condition could be found in 53% of the patients. Autoimmune diseases, sodium channel gene mutations, diabetes mellitus including glucose intolerance, and vitamin B12 deficiencies were more prevalent than reported literature findings, followed by alcohol abuse, chemotherapy, monoclonal gammopathy of undetermined significance, and haemochromatosis. In patients who were already known with a possible underlying condition at screening, additional underlying conditions were still found in another 26.7% of patients.
Conclusions
Based on these results, it is recommended that patients with pure SFN are screened at least for autoimmune diseases, sodium channel gene mutations, diabetes mellitus including glucose intolerance, and vitamin B12 deficiency, even when they already have a potential underlying condition at referral.
Keywords: associated conditions, neuropathic pain, small fiber neuropathy
Introduction
Small fiber neuropathy (SFN) affects the thinly myelinated Aδ‐fibers and the unmyelinated C‐fibers and leads to excruciating neuropathic pain and autonomic symptoms 1 with a negative impact on quality of life expectations 2. The diagnosis of pure SFN is based on typical complaints, combined with abnormal intraepidermal nerve fiber density in skin biopsy and/or abnormal temperature threshold testing levels, without signs of large nerve fiber involvement 1, 3. SFN has been described in several conditions, such as diabetes mellitus and sodium channel gene mutations 1, 4. Management is mostly based on symptomatic treatment. Knowing which conditions are associated with SFN is important, since some conditions are potentially treatable. Most patients diagnosed with SFN undergo many diagnostic tests to find an underlying cause, leading to high burden for patients and high health‐related costs 5. Illustratively, it was shown that Fabry disease was not found in 725 patients with isolated SFN, even though it has been mentioned in the literature as a potential underlying illness 6. This may also apply to other conditions. A better selection of associated conditions may result in a more targeted diagnostic workup with lower costs.
The aims of this study were to investigate associated conditions in a large cohort of patients with pure SFN and to compare the prevalence with literature reports on these conditions in healthy persons. Finally, recommendations are provided for a more targeted diagnostic workup in patients with pure SFN.
Methods
Patients
From January 2010 to December 2015, all consecutive patients fulfilling the diagnosis criteria for SFN at our SFN Center, Maastricht University Medical Center+ (MUMC+), were included in this study. MUMC+ serves as a tertiary referral center for SFN in the Netherlands. Records on complaints and medical history were collected in a standardized fashion as described earlier 7. To confirm the diagnosis of SFN, patients needed to have the typical complaints of SFN combined with a reduced intraepidermal nerve fiber density in skin biopsy 8 and/or abnormal temperature threshold testing 9 without large nerve fiber involvement based on neurological examination (normal muscle strength, vibration sense, and tendon reflexes) and nerve conduction studies.
To find possible underlying conditions, blood and urine analyses, and a chest X‐ray were performed (Table 1). The selection of these additional investigations was based on a literature review 1, 10, 11, 12 and on corresponding diagnostic guidelines (see below).
Table 1.
Diagnostic tests performed in patients referred to the SFN Center
| Kind of test | Disease investigated | Abnormal values | |
|---|---|---|---|
| X‐ray | Chest X‐ray | Sarcoidosis | |
| Blood samples | Glucose | Diabetes mellitus | Two sober plasma levels of ≥ 7.0 mmol/l or the combination of a sober plasma glucose level of ≥ 7.0 mmol/l, a random plasma glucose level of ≥ 11.1 mmol/l with complaints of hyperglycemia, or a level of ≥ 11.1 mmol/l after 120 min |
| Glucose tolerance test | Impaired glucose tolerance | Sober level of < 7.0 mmol/l and a level of ≥ 7.8 and < 11.1 mmol/l after 120 min | |
| Cholesterol | Hypercholesterolemia | Low density lipoprotein value above 3.1 mmol/l, high density lipoprotein value lower than 0.9 mmol/l and triglyceride value above 2.1 mmol/l | |
| Liver function | Hepatic impairment | Increased liver functions | |
| Kidney function | Renal insufficiency | Glomerular filtration rate < 30 | |
| Thyroid function | Hypothyroid or hyperthyroid function | Increased or decreased thyroid stimulating hormone/thyroxin | |
| Vitamin B1 | Vitamin B1 deficiency | <100 nmol/l | |
| Vitamin B6 | Vitamin B6 toxicity | >200 nmol/l | |
| Vitamin B12 | Vitamin B12 deficiency | <148 pmol/l | |
| Anti‐tissue transglutaminase | Coeliac disease | Present | |
| Anti‐extractable nuclear antigen antibodies | Sjogren's disease | Present | |
| Antinuclear antibodies, anti‐neutrophil cytoplasmic antibodies, and soluble Interleukin‐2 receptor | Other autoimmune diseases | Present or soluble IL‐2 receptor above 700 U/l | |
| Monoclonal gammopathy | Monoclonal gammopathy of undetermined significance | Present | |
| Borrelia burgdorferi (immunoglobulin I and M) | Lyme's disease | Present | |
| Anti‐human immunodeficiency virus 1 and 2 | Human immunodeficiency virus | Present | |
| Alpha‐galactosidase A activity and alpha‐galactosidase A gene | Fabry disease | < 30 mmo/l and variants class 3, 4 or 5. | |
| SCN9A, SCN10A and SCN11A gene | Sodium channel gene mutations | Variants with uncertain clinical significance, possibly pathogenic, probably pathogenic or pathogenic variants | |
| Urine sample | Lysosomal globotriaosylceramide | Fabry disease | >0 nmol/mmol creatinine |
SCN, sodium voltage‐gated channels.
Underlying conditions
The following underlying conditions were screened for: alcohol abuse, diabetes mellitus including glucose intolerance, haemochromatosis, autoimmune diseases, monoclonal gammopathy of undetermined significance, sodium channel gene mutations, and vitamin B12 deficiency. The definitions of these underlying conditions are summarized in Appendix S1.
Literature comparison
Literature research on potential conditions related to SFN was performed in the PubMed database using the keywords ‘small fiber neuropathy’, ‘small fibre neuropathy’, ‘neuropathy’, ‘painful neuropathy’, ‘etiology’, in combination with the different known conditions. Also Dutch guidelines were searched for prevalence of the conditions in healthy controls.
Statistics
Patients’ characteristics were expressed as mean with standard deviation when data were normally distributed. When not normally distributed, the median and interquartile range were calculated. Frequencies between two groups were compared by using the chi‐squared test. A stepwise approach was conducted: the prevalence of conditions potentially related to SFN was measured and subsequently compared with reported prevalence in healthy controls. Analyses were performed using SPSS (Version 23.0, SPSS Inc., Chicago, IL, USA).
Standard protocol approvals, registrations and patient consents
The MUMC+'s Medical Ethics Committee and Board of Directors approved this study. According to the Code of Conduct for the Use of Data in Health Research 13, for this type of retrospective study informed consent does not need to be obtained if the data are used anonymously and patients are given the opportunity to object to the use of their medical and personal data for research (which is the case in the MUMC+).
Results
Of 1275 patients screened, the diagnosis of pure SFN could be established in 72% (n = 921; Fig. 1). The characteristics of SFN patients included in the study are shown in Table 2. After the diagnostic workup, no underlying condition was found in 488 patients (53%).
Figure 1.
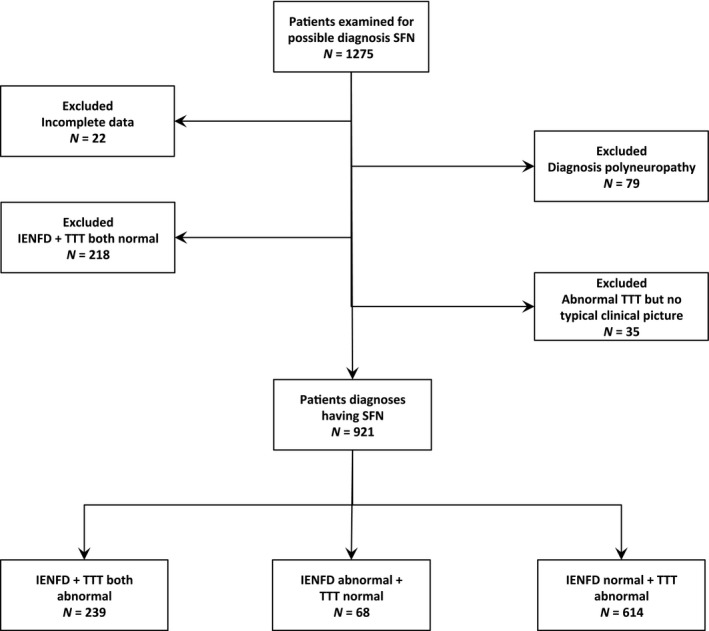
Flowchart of inclusion/exclusion. IENFD, intraepidermal nerve fiber density; SFN, small fiber neuropathy; TTT, temperature threshold testing.
Table 2.
Characteristics of patients with confirmed diagnosis of SFN
| Total, n = 921 | |
|---|---|
| Female (%) | 532 (57.8) |
| Age at visit, median (IQR) | 53 (44−61.5) |
| Age at onset, median (IQR) | 47 (38−57) |
| Duration of complaints, median (IQR) | 3 (2−7) |
| Diagnosis SFN | |
| Abnormal TTT (%) | 614 (66.7) |
| Abnormal skin biopsy (%) | 68 (7.4) |
| Abnormal TTT and skin biopsy (%) | 239 (26) |
IQR, interquartile range; SFN, small fiber neuropathy; TTT, temperature threshold testing.
Results of the total cohort of patients with pure SFN
In the total cohort, 696 patients (75.6%) did not have known SFN‐related comorbidities before the diagnostic workup.
The diagnostic workup (n = 921) showed immunological conditions in 175 patients (19%), including sarcoidosis (3.0%), Sjogren's disease (1.3%), coeliac disease (0.5%), other autoimmune diseases (8.8%) and non‐specific abnormal immunological laboratory findings (6.1%). Eight patients had two or more of these conditions. The other most frequently found associated conditions with available prevalence numbers in the general population were variants in SCN9A (8.5%), SCN10A (4.8%) and SCN11A (3.4%), diabetes mellitus (7.7%), vitamin B12 deficiency (4.7%), alcohol abuse (3.0%), chemotherapy (2.2%), monoclonal gammopathy of undetermined significance (MGUS) (1.4%) and haemochromatosis (0.3%) (Fig. 2). The MGUS subtypes included IgG‐MGUS (62%), IgM‐MGUS (15%), IgA‐MGUS (15%) and a biclonal‐MGUS (8%, IgG and IgA). The glucose intolerance test was performed in 493 of these patients. In total, 48 patients were found with glucose intolerance (9.7%). The prevalences of the other conditions screened for are shown in Appendix S2. In 488 patients (53%), no underlying conditions were found despite extensive laboratory testing (idiopathic SFN). Of the above‐mentioned conditions, only variants in the SCN9A‐gene were found significantly more often in patients with a non‐length‐dependent pattern of SFN compared to patients with length‐dependent complaints (12.4% vs. 6.9%, P value 0.014), whereas all other conditions showed no differences.
Figure 2.
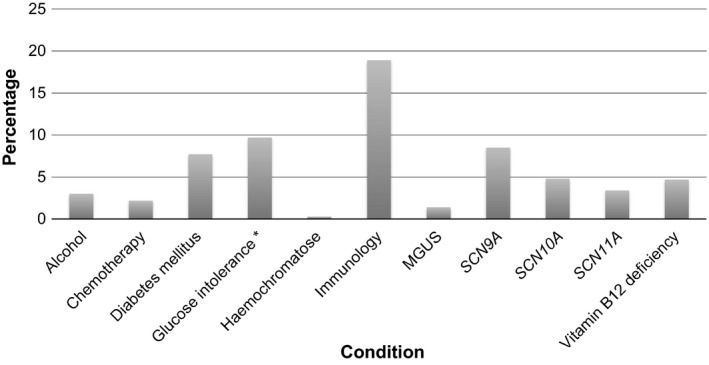
Prevalence of possible underlying causes in patients with SFN (n = 921). *Glucose intolerance was only tested in 493 patients instead of 921. Immunology: sarcoidosis, Sjogren's disease, coeliac disease, other autoimmune diseases and abnormal immunological laboratory findings (antinuclear antibodies, anti‐neutrophil cytoplasmic antibodies, monoclonal gammopathy, soluble interleukin‐2 receptor, anti‐tissue transglutaminase and anti‐extractable nuclear antigen antibodies). MGUS, monoclonal gammopathy of undetermined significance; SCN, sodium voltage‐gated channels.
Results for the group of SFN patients without known associated conditions at presentation
Of the patients without comorbidity at presentation (n = 696), abnormal immunological laboratory findings were present in 5.9%, variants in SCN9A in 9.6%, SCN10A in 4.5%, SCN11A in 3.4%, diabetes mellitus in 3%, vitamin B12 deficiency in 0.75% and MGUS in 0.6%. In 379 of these patients, glucose intolerance was tested and this was diagnosed in 35 patients (9.2%). In total, associated conditions were found in 208 of these patients (29.9%) with additional diagnostic tests.
Results for the group with known comorbidities at presentation
In the group of patients who were already known to have one or more comorbidities (n = 225) at presentation, new abnormalities were found: abnormal immunological laboratory findings (5.8%), variants in SCN9A (4.9%), SCN10A (5.8%) and SCN11A (3.1%), and diabetes mellitus (2.7%). In 114 of these patients, glucose intolerance was tested and was diagnosed in 13 patients (11.4%). In total, additional associated conditions were found in 60 patients (26.7%) with our diagnostic panel.
Discussion
In our cohort of 921 patients with pure SFN, an underlying condition was found in 433 patients (47%). The most prevalent conditions were immunological conditions, sodium channel gene mutations, diabetes mellitus including glucose intolerance, and vitamin B12 deficiency, and at least these entities are suggested to be tested in the diagnostic workup of potential SFN. Even if comorbidity was present at presentation, still other associated conditions were found in 26.7% after diagnostic testing. After thorough workup, 53% of patients had no underlying cause (idiopathic pure SFN), which is in conformity with the literature 11, 14. A recent study also described a high prevalence of immunological abnormalities, but no association with diabetes mellitus or vitamin B12 deficiency, possibly due to a smaller sample size and different criteria for the diagnosis of SFN 15.
Immunological abnormalities
Immunological conditions may affect nerve fibers 16 and were found in 12.9% (n = 119) of patients, whereas another 6.1% of patients had one or more abnormal immunological laboratory findings. The overall prevalence of autoimmune diseases in the Netherlands is around 3%−6% 17, which is much lower than in our cohort.
Also the prevalence of sarcoidosis is higher in our cohort than in the general European population (3.0% vs. 0.005%−0.03%) 18. However, the prevalence of sarcoidosis in our cohort might be an overestimation, because until 5 years ago MUMC+ was a tertiary referral center for patients with sarcoidosis.
Sjogren's disease was present in 1.3% of our SFN patients compared to a prevalence in the general population of 0.1%−4.8%, and thus within the reported limits in the general population 19. Of our patients, 0.5% had coeliac disease, with a prevalence of recognized coeliac disease of 0.016% and non‐recognized coeliac disease around 0.35% in the Netherlands 20.
Sodium channel gene mutations
In 16.7% of SFN patients a sodium channel gene variant was found. The sodium channels Nav1.7, Nav1.8 and Nav1.9, coded by SCN9A, SCN10A and SCN11A respectively, are all preferentially expressed in peripheral nerves 21. Mutations in the SCN9A‐, SCN10A‐, and SCN11A‐gene, showing electrophysiological changes in the corresponding channel, have been described by others and us in patients with SFN 7, 22, 23. Although the exact mechanism for axonal degeneration is not completely clear, it is plausible that DRG neuron hyperexcitability results in neuropathic pain 24. The results in this cohort are comparable with the results that were published earlier in a smaller cohort (n = 393) 4, although prevalence is lower than the results for SCN9A‐variants in a small cohort of patients with biopsy‐confirmed idiopathic SFN 7. SCN9A‐variants were more frequently found in the patients with a non‐length‐dependent pattern, which is in line with the description of different clinical patterns in patients with SCN9A‐mutations and SFN 25.
Diabetes mellitus
Diabetes mellitus was found in 7.7%, of which most (90.1%) were type 2 diabetes. Peripheral neuropathy is the most common complication of diabetes mellitus with lifetime prevalence up to 50% 26. The prevalence of diabetes is around 6% in the Netherlands 27. Our proportion found in SFN (7.7%) is higher than the prevalence of patients in the Netherlands. The prevalence in our cohort probably is an underestimation, as most patients with painful diabetic neuropathy will not be referred to our center because painful neuropathy is a well‐known complication of diabetes mellitus.
In addition, as has been suggested in the literature, the glucose tolerance test was also abnormal in 9.7% of the patients, adding to the underlying conditions of SFN 28, 29.
Vitamin B12 deficiency
Vitamin B12 deficiency was present in 4.7% of SFN patients. The prevalence of vitamin B12 deficiency is less than 3% in the general population aged between 20 and 39 years, and increases gradually up to 10% or higher in people of 70 years or older. The prevalence of vitamin B12 deficiency in our SFN population was higher than in the general population. According to general guidelines, vitamin B12 deficiency was diagnosed when serum vitamin B12 was below 148 pmol/l or when there was a history of vitamin B12 deficiency for which patients were treated. Homocysteine or methylmalonic acid was not assessed, which may have led to underestimation of functional vitamin B12 in patients with serum vitamin B12 of 148−258 pmol/l.
Alcohol abuse
In our cohort 3.0% of the patients (n = 28) reported an alcohol consumption of >5 IU/day. As people tend to underreport their alcohol intake, this may be an underestimation as well 30. The typical presentation of alcohol‐related peripheral neuropathy is a painful, burning neuropathy and autonomic instability 31. In the Netherlands, the estimated prevalence of alcohol abuse is 0.75% of adults between 18 and 65 years old 30. The prevalence of alcohol abuse is higher in our cohort of patients with pure SFN suggesting an association.
Chemotherapy
Chemotherapy‐induced peripheral neuropathy is a well‐known adverse event of several chemotherapeutic agents, and was present in 2.2% (n = 20) of our patients with pure SFN. Different prevalences are mentioned, between 17% and 88%, for different ages, different grades of neuropathies and different agents 32. The prevalence of chemotherapy found in our cohort is probably underestimated, as these patients are usually not referred because the neuropathy is considered an expected adverse event of the treatment.
Monoclonal gammopathy of undetermined significance
In our cohort, 1.4% of patients were known with MGUS. The etiology of MGUS in peripheral neuropathy is not very well understood 33. In healthy subjects (above 45−50 years old) MGUS is found in 3.2%−3.5% 34, 35. The prevalence of monoclonal gammopathy increases with age 36. The prevalence of MGUS in our population is lower than the prevalence found in the general population. However, the overall prevalence is based on subjects between 45 and 50 years old, and in our population there were patients ranging from 11 to 85 years.
Haemochromatosis
Three patients (0.3%) had haemochromatosis. In northern European countries, 0.4% of the people are homozygotes for the C282Y allele 37. This means that the prevalence in the whole population is equal to the prevalence in patients with SFN, which makes the association between SFN and haemochromatosis less likely.
Patients with associated conditions at presentation
Despite having an associated condition at presentation, additional associated conditions were found in 26.7% of our SFN cohort. Finding other diseases might lead to new treatment possibilities for these patients, and possibly relieve complaints or prevent disease progression.
Pathophysiology
This study shows that some of the associated conditions are more prevalent in patients with pure SFN compared to healthy persons. However, the underlying pathophysiology is still unclear in most of these conditions. It would be of interest to investigate these specific conditions in detail in animal models, to search for underlying mechanisms. This knowledge would also stimulate the development of targeted therapy. Better treatments would lead to reduction of pain, and therefore to lower health costs 5.
Conclusion
Autoimmune diseases, sodium channel gene mutations, diabetes mellitus including glucose intolerance, and vitamin B12 deficiency are the most common underlying conditions in patients with pure SFN; despite a thorough workup no underlying condition could be found in 53% of the SFN patients. The prevalence of alcohol abuse, autoimmune diseases, diabetes mellitus including glucose intolerance, and vitamin B12 deficiencies seems higher in our population of patients with pure SFN than found in the general population. Moreover, the prevalence of SFN is much higher in patients who received chemotherapy compared to the prevalence of SFN in the Netherlands. For these conditions a causal relationship with small nerve fiber damage is suspected. Further research is needed to explore the exact pathophysiological mechanisms. Although some patients are already known with an underlying condition at presentation, it is still recommended that all patients with pure SFN are tested for diabetes mellitus including glucose intolerance, autoimmune diseases, sodium channel gene mutations, and vitamin B12 deficiency. Testing for rarer underlying conditions can be considered in SFN, based on specific signs or symptoms.
Disclosure of conflicts of interest
Bianca T.A. de Greef reports a grant from the Prinses Beatrix Spierfonds (W.OR12‐01). Janneke G.J. Hoeijmakers reports no disclosure. Carla M.L. Gorissen‐Brouwers reports no disclosure. Margot Geerts reports no disclosure. Catharina G. Faber reports grants from the European Union 7th Framework Programme (grant agreement no. 602273, 2013), the European Union's Horizon 2020 research and innovation programme Marie Sklodowska‐Curie (grant no. 721841), the Prinses Beatrix Spierfonds (W.OR12‐01), and from Grifols and Lamepro for a trial on IVIg in small fibre neuropathy. Participation in advisory boards for Biogen and Vertex (C.G. Faber). Ingemar S.J. Merkies reports grants from GBS/CIDP Foundation International and Talents Program for PeriNomS study and from the European Union 7th Framework Programme (grant agreement no. 602273, 2013); participation in steering committees of the Talecris ICE Study, CSL Behring, LFB, Novartis, Biotest and Octapharma (a research foundation at the University of Maastricht received the honoraria on behalf of Dr Merkies), outside the submitted work. He received a grant from the Grifols Investigator‐Sponsored Research (ISR) Program.
Supporting information
Appendix S1. Definitions of underlying conditions.
Appendix S2. Prevalence of other conditions.
Acknowledgements
Aline Kosten and Ilse Driesmans are thanked for their dedicated assistance with the skin biopsies. The work was supported by a grant of the Prinses Beatrix Spierfonds (W.OR12‐01).
References
- 1. Hoeijmakers JG, Faber CG, Lauria G, et al Small‐fibre neuropathies − advances in diagnosis, pathophysiology and management. Nat Rev Neurol 2012; 8: 369–379. [DOI] [PubMed] [Google Scholar]
- 2. Bakkers M, Faber CG, Hoeijmakers JG, et al Small fibers, large impact: quality of life in small‐fiber neuropathy. Muscle Nerve 2014; 49: 329–336. [DOI] [PubMed] [Google Scholar]
- 3. Tesfaye S, Boulton AJ, Dyck PJ, et al Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brouwer BA, Merkies IS, Gerrits MM, et al Painful neuropathies: the emerging role of sodium channelopathies. J Peripher Nerv Syst 2014; 19: 53–65. [DOI] [PubMed] [Google Scholar]
- 5. Schaefer C, Mann R, Sadosky A, et al Health status, function, productivity, and costs among individuals with idiopathic painful peripheral neuropathy with small fiber involvement in the United States: results from a retrospective chart review and cross‐sectional survey. J Med Econ 2014; 17: 394–407. [DOI] [PubMed] [Google Scholar]
- 6. de Greef BT, Hoeijmakers JG, Wolters EE, et al No Fabry disease in patients presenting with isolated small fiber neuropathy. PLoS One 2016; 11: e0148316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faber CG, Hoeijmakers JG, Ahn HS, et al Gain of function NaV1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012; 71: 26–39.21698661 [Google Scholar]
- 8. Lauria G, Bakkers M, Schmitz C, et al Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010; 15: 202–207. [DOI] [PubMed] [Google Scholar]
- 9. Bakkers M, Faber CG, Reulen JP, et al Optimizing temperature threshold testing in small‐fiber neuropathy. Muscle Nerve 2015; 51: 870–876. [DOI] [PubMed] [Google Scholar]
- 10. Hovaguimian A, Gibbons CH. Diagnosis and treatment of pain in small‐fiber neuropathy. Curr Pain Headache Rep 2011; 15: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devigili G, Tugnoli V, Penza P, et al The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131: 1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lauria G. Small fibre neuropathies. Curr Opin Neurol 2005; 18: 591–597. [DOI] [PubMed] [Google Scholar]
- 13. Federa . http://www.federa.org/codes-conduct (accessed 18/05/2017).
- 14. Peters MJ, Bakkers M, Merkies IS, et al Incidence and prevalence of small‐fiber neuropathy: a survey in the Netherlands. Neurology 2013; 81: 1356–1360. [DOI] [PubMed] [Google Scholar]
- 15. Lang M, Treister R, Oaklander AL. Diagnostic value of blood tests for occult causes of initially idiopathic small‐fiber polyneuropathy. J Neurol 2016; 263: 2515–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lehmann HC, Meyer Zu Horste G, Kieseier BC, et al Pathogenesis and treatment of immune‐mediated neuropathies. Ther Adv Neurol Disord 2009; 2: 261–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rijkers GT, Batstra MR, Allebes W, et al Auto‐immuunziekten. Ned Tijdschr Klin Chem Labgeneesk 2006; 31: 246–248. [Google Scholar]
- 18. Mekkes JR. Sarcoidosis. 2014. http://www.huidziekten.nl/zakboek/dermatosen/stxt/Sarcoidose.htm (accessed 18/05/2017).
- 19. Tincani A, Andreoli L, Cavazzana I, et al Novel aspects of Sjogren's syndrome in 2012. BMC Med 2013; 11: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maag‐Darm‐Leverartsen NVv . Guideline coeliac disease and dermatitis herpetiformis. 2008.
- 21. Dib‐Hajj SD, Tyrrell L, Black JA, et al NaN, a novel voltage‐gated Na channel, is expressed preferentially in peripheral sensory neurons and down‐regulated after axotomy. Proc Natl Acad Sci U S A 1998; 95: 8963–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faber CG, Lauria G, Merkies IS, et al Gain‐of‐function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci U S A 2012; 109: 19444–19449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang J, Han C, Estacion M, et al Gain‐of‐function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain 2014; 137: 1627–1642. [DOI] [PubMed] [Google Scholar]
- 24. Hoeijmakers JG, Faber CG, Merkies IS, et al Painful peripheral neuropathy and sodium channel mutations. Neurosci Lett 2015; 596: 51–59. [DOI] [PubMed] [Google Scholar]
- 25. Estacion M, Han C, Choi JS, et al Intra‐ and interfamily phenotypic diversity in pain syndromes associated with a gain‐of‐function variant of NaV1.7. Mol Pain 2011; 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boulton AJ, Vinik AI, Arezzo JC, et al Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962. [DOI] [PubMed] [Google Scholar]
- 27. National Public Health Compass − Diabetes Mellitus. 2014. http://www.nationaalkompas.nl/gezondheid-en-ziekte/ziekten-en-aandoeningen/endocriene-voedings-en-stofwisselingsziekten-en-immuniteitsstoornissen/diabetes-mellitus (accessed 21/09/2017).
- 28. Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve 2001; 24: 1225–1228. [DOI] [PubMed] [Google Scholar]
- 29. Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001; 24: 1448‐1453. [DOI] [PubMed] [Google Scholar]
- 30. National Public Health Compass − Alcohol dependence. 2010. http://www.nationaalkompas.nl/gezondheid-en-ziekte/ziekten-en-aandoeningen/psychische-stoornissen/afhankelijkheid-van-alcohol-drugs-of-andere-middelen/afhankelijkheid-van-alcohol (accessed 18/05/2017).
- 31. Mellion ML, Silbermann E, Gilchrist JM, et al Small‐fiber degeneration in alcohol‐related peripheral neuropathy. Alcohol Clin Exp Res 2014; 38: 1965–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brewer JR, Morrison G, Dolan ME, et al Chemotherapy‐induced peripheral neuropathy: current status and progress. Gynecol Oncol 2016; 140: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramchandren S, Lewis RA. An update on monoclonal gammopathy and neuropathy. Curr Neurol Neurosci Rep 2012; 12: 102–110. [DOI] [PubMed] [Google Scholar]
- 34. Eisele L, Durig J, Huttmann A, et al Prevalence and progression of monoclonal gammopathy of undetermined significance and light‐chain MGUS in Germany. Ann Hematol 2012; 91: 243–248. [DOI] [PubMed] [Google Scholar]
- 35. Kyle RA, Therneau TM, Rajkumar SV, et al Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006; 354: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 36. Crawford J, Eye MK, Cohen HJ. Evaluation of monoclonal gammopathies in the ‘well’ elderly. Am J Med 1987; 82: 39–45. [DOI] [PubMed] [Google Scholar]
- 37. European Association for the Study of the Liver . EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol 2010; 53: 3–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Definitions of underlying conditions.
Appendix S2. Prevalence of other conditions.


