This multicenter study examines access to reperfusion and percutaneous coronary intervention during ST-segment elevation myocardial infarction in Indian clinics and hospitals using a hub-and-spoke model.
Key Points
Question
Can access to reperfusion and percutaneous coronary intervention during ST-segment elevation myocardial infarction be achieved in low- to middle-income countries where resources are constrained?
Findings
This multicenter, prospective study of 2420 patients in a quality improvement program in India found that overall reperfusion use and time to reperfusion were similar during the preimplementation and postimplementation phases, but both postfibrinolysis angiography and percutaneous coronary intervention were more commonly performed in the postimplementation phase.
Meaning
A hub-and-spoke model may serve as an example for developing ST-segment elevation myocardial infarction systems of care in low- to middle-income countries.
Abstract
Importance
Challenges to improving ST-segment elevation myocardial infarction (STEMI) care are formidable in low- to middle-income countries because of several system-level factors.
Objective
To examine access to reperfusion and percutaneous coronary intervention (PCI) during STEMI using a hub-and-spoke model.
Design, Setting, and Participants
This multicenter, prospective, observational study of a quality improvement program studied 2420 patients 20 years or older with symptoms or signs consistent with STEMI at primary care clinics, small hospitals, and PCI hospitals in the southern state of Tamil Nadu in India. Data were collected from the 4 clusters before implementation of the program (preimplementation data). We required a minimum of 12 weeks for the preimplementation data with the period extending from August 7, 2012, through January 5, 2013. The program was then implemented in a sequential manner across the 4 clusters, and data were collected in the same manner (postimplementation data) from June 12, 2013, through June 24, 2014, for a mean 32-week period.
Exposures
Creation of an integrated, regional quality improvement program that linked the 35 spoke health care centers to the 4 large PCI hub hospitals and leveraged recent developments in public health insurance schemes, emergency medical services, and health information technology
Main Outcomes and Measures
Primary outcomes focused on the proportion of patients undergoing reperfusion, timely reperfusion, and postfibrinolysis angiography and PCI. Secondary outcomes were in-hospital and 1-year mortality.
Results
A total of 2420 patients with STEMI (2034 men [84.0%] and 386 women [16.0%]; mean [SD] age, 54.7 [12.2] years) (898 in the preimplementation phase and 1522 in the postimplementation phase) were enrolled, with 1053 patients (43.5%) from the spoke health care centers. Missing data were common for systolic blood pressure (213 [8.8%]), heart rate (223 [9.2%]), and anterior MI location (279 [11.5%]). Overall reperfusion use and times to reperfusion were similar (795 [88.5%] vs 1372 [90.1%]; P = .21). Coronary angiography (314 [35.0%] vs 925 [60.8%]; P < .001) and PCI (265 [29.5%] vs 707 [46.5%]; P < .001) were more commonly performed during the postimplementation phase. In-hospital mortality was not different (52 [5.8%] vs 85 [5.6%]; P = .83), but 1-year mortality was lower in the postimplementation phase (134 [17.6%] vs 179 [14.2%]; P = .04), and this difference remained consistent after multivariable adjustment (adjusted odds ratio, 0.76; 95% CI, 0.58-0.98; P = .04).
Conclusions and Relevance
A hub-and-spoke model in South India improved STEMI care through greater use of PCI and may improve 1-year mortality. This model may serve as an example for developing STEMI systems of care in other low- to middle-income countries.
Introduction
ST-segment elevation myocardial infarction (STEMI) is a leading cause of mortality and morbidity worldwide and is associated with a poor prognosis when appropriate treatment is delayed. Numerous reports have documented that patients with STEMI in developing countries more often fail to receive adequate reperfusion or subsequent revascularization compared with those in North America and Europe. In India, for instance, primary percutaneous coronary intervention (PCI) is used for emergency reperfusion in rare situations, and less than 10% of patients with STEMI receive definitive treatment with early postfibrinolysis angiography and PCI (ie, the pharmacoinvasive approach) despite increasing guideline-based support for these strategies. As such, improving access to primary PCI and the pharmacoinvasive approach is an important priority for low- and middle-income countries (LMICs) and offers a key opportunity for their health care systems because cardiovascular diseases assume epidemic proportions, especially in younger, working-age patients.
The challenges to improving STEMI care in LMICs are formidable because of several nonclinical, system-level factors. The most important limitations arise from poverty, limited health care infrastructure for PCI, and poor accessibility to acute emergency medical services. We previously reported results from the Kovai Erode Pilot STEMI Study, which tested the feasibility of implementing a treatment model for STEMI in a resource-poor setting based on systems of care models currently established in Western countries. This pilot study focused on improving access to PCI through primary PCI or the pharmacoinvasive approach by leveraging recent developments in public health insurance schemes in India, emergency medical services, and health information technology to link several small, peripheral spoke health care centers with a centrally located, PCI-capable hub hospital. On the basis of the promising results from this pilot study, we subsequently expanded this hub-and-spoke model across a broader region of the southern Indian state of Tamil Nadu (TN).
This report describes the primary evaluation and results of the TN-STEMI program, which was a multicenter, prospective study that aimed to improve the delivery of STEMI care in an LMIC by increasing access to reperfusion and revascularization with PCI. The intervention in this study was explicitly designed to be scalable and to address system-level rather than patient-level factors related to quality gaps for advanced STEMI care in India. Thus, the framework of the TN-STEMI program can be extended to other areas of the country and may serve as a practical model for similar resource-poor settings in LMICs.
Methods
Study Design
The TN-STEMI program was developed as a multicenter, prospective study. We used a preimplementation and postimplementation quasi-experimental study design that was developed as part of a community-based treatment program. The design and rationale of the TN-STEMI program have been previously described. In brief, we instituted a hub-and-spoke model that relied on an integrated health care network based on 35 primary care health clinics and small hospitals built around 4 facilities capable of providing advanced cardiovascular services, including PCI. We followed the current Declaration of Helsinki and ethical guidelines for biomedical research on human participants as endorsed by the Indian Council of Medical Research. Ethics committee approval was specifically obtained from the hub hospitals, and written informed consent was obtained from each patient. Identifiers were collected on patients for follow-up; however, the data were analyzed after deidentification, although identifiers remain as part of the study and are protected.
Study Objectives
The primary objectives of the TN-STEMI program were to (1) improve the overall use of reperfusion and reduce the time from first medical contact to treatment in patients with STEMI and (2) increase the rates of postfibrinolysis angiography and PCI in eligible patients presenting to spoke health care centers by means of the pharmacoinvasive approach. Development of the TN-STEMI program involved 3 key partners from the public and private sectors.
TN State Government
The Chief Minister's Comprehensive Health Insurance Scheme in TN was established as government-sponsored social insurance coverage for health care among those below the poverty line (ie, the equivalent of less than Rs72 000 [or US$1100] annual income). However, some patients remained ineligible (eg, out-of-state and migrant workers).
Gunapati Venkata Krishna Emergency Management and Research Institute Ambulance
The Gunapati Venkata Krishna Emergency Management and Research Institute (GVK-EMRI) operates as a public-private partnership and is recognized as a not-for-profit entity. Ambulance services provided by the GVK-EMRI may be activated by a patient with chest pain or a health care facility using the Call 108 system. The GVK-EMRI ambulances are capable of acquiring and transmitting electrocardiograms (ECGs) and transporting patients between hospitals.
STEMI Information Technology Kit
A novel aspect of the TN-STEMI program is the implementation of new hardware and software components to optimize the performance and transmission of real-time clinical data and ECGs across the network of hospitals by paramedics, nurses, and physicians (eFigure 1 in the Supplement).
Study Population, Facilities, and Enrollment Period
Patients 20 years or older with symptoms or signs consistent with an acute coronary syndrome were enrolled after consent was obtained. For entry in the study, an ECG must have evidence of myocardial injury with a 1-mm or greater ST-segment elevation in at least 2 anatomically contiguous limb leads (aVL to III, including aVR), 1-mm or larger ST-segment elevation in precordial lead V4 through V6, 2-mm or larger ST-segment elevation in V1 through V3, or a new left bundle branch block. Cardiac biomarker elevation was not required for diagnosis. Because all patients provided informed consent, we were unable to include those who died before hospital arrival, ECG confirmation, or informed consent was obtained, which may have led to biases in the exclusion of patients who were unstable.
The hub hospitals were 4 large tertiary care hospitals: 3 with the capability to perform emergency coronary angiography and PCI around the clock and 1 with the capability to perform PCI only between 8 am and 4 pm. All participating units committed to complying with the study protocol and were within the catchment area for the GVK-EMRI ambulances. Each hub hospital was linked to between 3 and 15 referring spoke health care centers for a total of 35, with most spoke health care centers located in small towns and rural areas and staffed by generalist physicians. (A total of 40 spoke health care centers were initially recruited, but 5 failed to enroll a single patient.) A full list of the participating spoke health care centers and PCI hub hospitals organized by their classes is presented in eTable 1, eTable 2, eFigure 2, and eFigure 3 in the Supplement.
Data Collection, Study Timeline, and Outcomes
Study personnel captured data prospectively using an electronic data capture application that collected information on demographic characteristics, personal history, and medical history. Data relating to processes of care were also prospectively obtained and included the mode of transportation and intervals of onset of chest pain, time of arrival at the hospital, time taken to perform ECG, and time when coronary angiography and/or PCI (if performed) were started. Clinical examination findings, medications, cardiac catheterization details, and in-hospital outcomes were abstracted from hospital records.
Data were collected from the 4 clusters before implementation of the program (preimplementation data). We required a minimum of 12 weeks for the preimplementation data, with the period extending from August 7, 2012, through January 5, 2013, for a mean period of 15 weeks per cluster. The program was then implemented in a sequential manner across the 4 clusters, and data were collected in the same manner (postimplementation data) from June 12, 2013, through June 24, 2014, for a mean 32-week period.
Our primary outcomes were based on key process measures. They included (1) proportion of patients with STEMI receiving reperfusion, with fibrinolysis or primary PCI; (2) timely reperfusion defined as door-to-balloon time of 90 minutes or less in patients with STEMI treated with primary PCI or door-to-needle times of 30 minutes or less in patients with STEMI treated with fibrinolytic therapy; and (3) proportion of patients undergoing primary PCI or postfibrinolysis angiography with potential PCI as part of the pharmacoinvasive approach. As a secondary outcome, we evaluated in-hospital and 1-year all-cause mortality. Follow-up data on 1-year mortality after discharge were obtained through scheduled outpatient clinic follow-up appointments and telephone surveys and were available for 2020 patients (83.5%). An additional secondary outcome was use of aspirin, P2Y12 inhibitors, and statins at discharge.
Statistical Analysis
Data from all 4 clusters were combined for analysis. Continuous variables were summarized using descriptive statistics and categorical data as the percentage of participants in each category. Comparison between patients treated during preimplementation and postimplementation phases were tested using 2-tailed, unpaired t tests; nonparametric Mann-Whitney tests; and χ2 tests based on the outcome. To account for potential changes over time in the assessment of the secondary outcome of mortality, multivariable logistic regression models were constructed. We compared in-hospital and 1-year mortality between preimplementation and postimplementation phases after adjusting for age, sex, diabetes, hypertension, smoking, prior PCI, initial systolic blood pressure, heart rate, anterior MI location, and the site of entry in the STEMI system. For these models, we used multiple imputation analyses to account for missing data for systolic blood pressure, heart rate, and anterior MI location. This approach assumed missing data at random; relied on age, sex, diabetes, hypertension, smoking, prior PCI, and the outcome variable for prediction of imputed values; and generated 10 imputed data sets for analyses. Standard statistical models were fit to the 10 imputed data sets, and overall estimates of association were averaged using STATA’s mi command. Our results were qualitatively similar when we used complete cases only. All statistical analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc) and STATA software, version 14.1 (StataCorp). Statistical significance was set at a 2-tailed P < .05.
Results
A total of 2420 consecutive patients presenting with STEMI (898 in the preimplementation phase and 1522 in the postimplementation phase) were enrolled from August 7, 2012, through June 24, 2014. Of these, 1367 (56.5%) presented directly to the 4 hub hospitals and 1053 (43.5%) to the 35 spoke health care centers. Baseline characteristics of the study population are listed in Table 1. The mean (SD) age was 53.9 (12.3) years in the preimplementation phase and 55.2 (12.1) years in the postimplementation phase. Additional baseline differences in risk factors were observed. Patients in the preimplementation phase were more likely to be current smokers but less likely to have diabetes, hypertension, prior PCI, and anterior wall infarctions. There was a significant difference in the proportion of patients transferred from spoke health care centers to the hub hospitals between the preimplementation and postimplementation phases (18 of 484 [3.7%] vs 190 of 564 [33.5%]; P < .001). A total of 737 (48.4%) of the 1522 patients in the postimplementation phase were living below the poverty line.
Table 1. Baseline Characteristics of the 2420 Patients in the Studya .
| Characteristic | Preimplementation Phase (n = 898) |
Postimplementation Phase (n = 1522) |
P Value | Missing, No. (%) |
|---|---|---|---|---|
| Age, mean (SD), y | 53.9 (12.3) | 55.2 (12.1) | .01 | NA |
| Females | 152 (16.9) | 234 (15.4) | .31 | NA |
| Risk factors | ||||
| Diabetes | 191 (21.3) | 430 (28.3) | <.001 | NA |
| Hypertension | 206 (22.9) | 395 (26.0) | .10 | NA |
| Current smoker | 419 (46.7) | 553 (36.3) | <.001 | NA |
| Prior PCI | 4 (0.5) | 19 (1.3) | .049 | NA |
| Features at presentation | ||||
| Blood pressure, mean (SD), mm Hg | ||||
| Systolic | 131.9 (28.9) | 129.0 (26.4) | .02 | 213 (8.8) |
| Diastolic | 84.8 (17.8) | 82.7 (16.2) | .003 | 224 (9.3) |
| Heart rate, mean (SD), /min | 84.6 (35.7) | 82.5 (17.8) | .10 | 223 (9.2) |
| Location of infarct | ||||
| Anterior | 466 (53.0) | 733 (58.1) | .009 | 279 (11.5) |
| Inferior | 298 (33.9) | 415 (32.9) | ||
| Posterolateral | 30 (3.4) | 26 (2.1) | ||
| Other | 86 (9.8) | 87 (6.9) | ||
| Ambulance use | ||||
| Private | 111 (12.4) | 99 (6.5) | <.001 | NA |
| EMRI | 0 | 6 (0.4) | ||
| Transfer to hub hospital, No. (%)b | 18 (3.7) | 190 (33.5) | <.001 | NA |
Abbreviations: EMRI, Emergency Management and Research Institute; NA, not applicable; PCI, percutaneous coronary intervention.
Data are presented as number (percentage) of study participants unless otherwise indicated.
There were 485 patients in the preimplementation phase and 568 patients in the postimplementation phase.
Primary Outcomes: Process Measures
Table 2 lists the key process measures during the preimplementation and postimplementation phases. The proportion of patients receiving reperfusion was high and similar between the preimplementation and postimplementation phases (795 [88.5%] vs 1372 [90.1%]; P = .21). When referred for early invasive evaluation with coronary angiography and PCI as part of a pharmacoinvasive approach, patients had shorter times to treatment during the postimplementation phase (median, 39.2 [interquartile range, 7.1-109.2] vs 17.3 [interquartile range, 9.0-23.0] hours; P = .003).
Table 2. Process Measure Differences Between the Preimplementation and Postimplementation Phasesa.
| Metric | Preimplementation Phase (n = 898) |
Postimplementation Phase (n = 1522) |
P Value |
|---|---|---|---|
| Time from symptom onset to first medical contact, median (IQR), min | 170 (90-300) | 174.5 (90-310) | .91 |
| Time from first medical contact to ECG, median (IQR), min | 7 (5-13) | 5 (5-10) | .02 |
| Time from ECG to fibrinolysis, median (IQR), min | 30 (10-80) | 25 (15-45) | .19 |
| Time from fibrinolysis to PCI, median (IQR), hb | 39.2 (7.1-109.2) | 17.3 (9.0-23.0) | .003 |
| Door-to-balloon time for primary PCI, median (IQR), minc | 100 (84-143) | 105 (80-145) | .56 |
| Any reperfusion | 795 (88.5) | 1372 (90.1) | .21 |
| Fibrinolytic use | 599 (66.7) | 753 (49.5) | <.001 |
| Fibrinolytic use in spoke health center patientsd | 386 (79.6) | 423 (74.5) | .05 |
| Primary PCI | 196 (21.8) | 619 (40.7) | <.001 |
| Coronary angiography | 314 (35.0) | 925 (60.8) | <.001 |
| Coronary angiography in spoke health center patientsd | 17 (3.5) | 178 (31.3) | <.001 |
| All PCI | 265 (29.5) | 707 (46.5) | <.001 |
| All PCI in spoke health center patientsd | 15 (3.1) | 117 (20.6) | <.001 |
Abbreviations: ECG, electrocardiography; IQR, interquartile range; PCI, percutaneous coronary intervention.
Data are presented as number (percentage) of participants unless otherwise indicated.
There were 52 patients in the preimplementation phase and 162 in the postimplementation phase.
There were 182 patients in the preimplementation phase and 536 in the postimplementation phase.
There were 485 patients in the preimplementation phase and 568 patients in the postimplementation phase.
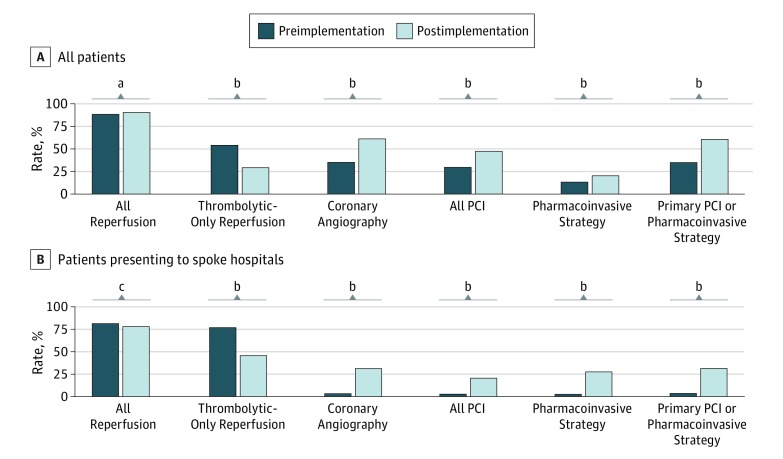
Coronary angiography (314 [35.0%] vs 925 [60.8%]; P < .001) and PCI (265 [29.5%] vs 707 [46.5%]; P < .001) were more commonly performed during the postimplementation phase. For spoke health care center patients, these differences were further magnified for coronary angiography (17 of 485 [3.5%] vs 178 of 568 [31.3%]; P < .001) and PCI (15 [3.1%] vs 117 [20.6%]; P < .001). Primary PCI was pursued at higher rates during the postimplementation phase in all patients overall (196 [21.8%] vs 619 [40.7%]; P < .001), largely driven by a greater increase in the use of primary PCI in hub hospitals during the postimplementation phase (191 of 413 [46.3%] vs 601 of 954 [63.0%]; P < .001). The use of primary PCI or the pharmacoinvasive approach increased during the postimplementation phase (314 [35.0%] vs 925 [60.8%]; P < .001), which was especially prominent for patients presenting to spoke hospitals first (17 of 485 [3.5%] vs 178 of 568 [31.3%]; P < .001) (Figure).
Figure. Rates of Reperfusion Therapy and Percutaneous Coronary Intervention (PCI) Among Patients With ST-Segment Elevation Myocardial Infarction (STEMI) During the Preimplementation and Postimplementation Phases.
Rates of reperfusion and PCI in all patients and those presenting to spoke hospitals in the preimplementation and postimplementation phases.
aP = .21.
bP < .001.
cP = .23.
Secondary Outcomes: Mortality and Medication Prescription
No differences were observed between the preimplementation and postimplementation phases for cardiogenic shock (35 [3.9%] vs 49 [3.2%]; P = .38), stroke (4 [0.5%] vs 3 [0.2%]; P = .27), or in-hospital mortality (52 [5.8%] vs 85 [5.6%]; P = .83). In contrast, 1-year mortality was lower in the postimplementation phase (134 [17.6%] vs 179 [14.2%]; P = .04) (Table 3). In-hospital mortality was significantly lower in the hub hospital patients compared with patients initially evaluated at the spoke health care centers during the preimplementation phase (15 [3.6%] vs 37 [7.6%]; P = .01), but this difference was not present during the postimplementation phase (49 [5.1%] vs 36 [6.3%]; P = .42). The unadjusted odds ratios for the postimplementation vs preimplementation phase were 0.96 (95% CI, 0.67-1.37; P = .83) for in-hospital mortality and 0.78 (95% CI, 0.61-0.99; P = .04) for 1-year mortality. The adjusted odds ratios for the postimplementation vs preimplementation phase, with multiple imputation of missing values, were 0.89 (95% CI, 0.61-1.29; P = .54) for in-hospital mortality and 0.76 (95% CI, 0.58-0.98; P = .04) for 1-year mortality. In the postimplementation phase, we also saw an increase in prescriptions for aspirin, statins, and dual antiplatelet therapy (eTable 3 in the Supplement).
Table 3. Clinical Outcomes Between the Preimplementation and Postimplementation Phases Stratified by Hub Hospitals and Spoke Health Centers.
| Outcome | Hub Hospitals, No. (%) (n = 1367) |
Spoke Health Centers, No. (%) (n = 1053) |
Overall, No. (%) | P Value | |||
|---|---|---|---|---|---|---|---|
| Preimplementation Phase (n = 413) |
Postimplementation Phase (n = 954) |
Preimplementation Phase (n = 485) |
Postimplementation Phase (n = 568) |
Preimplementation Phase (n = 898) |
Postimplementation Phase (n = 1522) |
||
| In-hospital mortality (n = 2420) | 15 (3.6) | 49 (5.1) | 37 (7.6) | 36 (6.3) | 52 (5.8) | 85 (5.6) | .83 |
| Stroke (n = 2420) | 1 (0.2) | 1 (0.1) | 3 (0.6) | 2 (0.4) | 4 (0.5) | 3 (0.2) | .27 |
| Cardiogenic shock (n = 2420) | 8 (1.9) | 23 (2.4) | 27 (5.6) | 26 (4.6) | 35 (3.9) | 49 (3.2) | .38 |
| Symptomatic ischemia (n = 2420) | 1 (0.2) | 6 (0.6) | 15 (3.1) | 10 (1.8) | 16 (1.8) | 16 (1.1) | .13 |
| 1-Year mortality (n = 2020) | 48 (13.3) | 100 (12.1) | 86 (21.5) | 79 (18.2) | 134 (17.6) | 179 (14.2) | .04 |
Discussion
India is a large country with a high burden of noncommunicable disease, particularly cardiovascular diseases. The low rates of health care spending by the state and national governments, uneven distribution of basic health care facilities, and inability of large populations of rural and urban poor to access high-quality care make the challenge of developing a viable STEMI system of care in India a daunting task. Added to this situation is the disproportionately high prevalence of coronary artery disease in lower socioeconomic classes and consequent higher mortality attributable to poor access to affordable, quality health care. All of these challenges focused toward acute care need to be balanced against additional health priorities focused toward prevention, and this scenario in India represents a prototypical case for many LMICs.
To develop a system of care in LMICs, it is important to understand that focusing on primary PCI as the exclusive mode of reperfusion is not feasible despite its dominant treatment role in many Western countries. The Treatment and Outcomes of Acute Coronary Syndromes in India (CREATE) registry found that only 8% of patients with STEMI underwent PCI at any point during hospitalization. The China Patient-Centered Evaluative Assessment of Cardiac Events (PEACE)–Retrospective Acute Myocardial Infarction Study analyzed data from 13 815 patients treated for STEMI at 162 hospitals and found limited use of PCI despite an increase from 10.2% in 2001 to 27.6% in 2011. The situations in other LMICs outside India and China are less certain (because data are sparse) but suggest similar practical challenges.
In this context, our findings of stable reperfusion rates and times to treatment combined with increases in primary PCI and the pharmacoinvasive approach after the implementation of the TN-STEMI program are noteworthy because of several of its key features. First, our high rates of reperfusion at baseline (approximately 90%) may reflect the institution of care processes around measurement in the preimplementation phase. Although we did not see an improvement, this outcome may have occurred because of the ceiling effect that reflected increased attention on this important process-of-care measure. Second, we found that establishing transfer of patients with STEMI using ambulance services with paramedics was important for their safe transportation. Although taken for granted in developed countries, LMICs do not always have similar prehospital emergency medical services. Third, out-of-pocket payment for medical care is the most common payment method for medical services in India, where insurance coverage is low. Because medical care is one of the most common causes of debt for the rural poor, a key strategy was to make this program inclusive by linking it to government-sponsored social insurance. Indeed, we found that most spoke health care centers were not accredited for insurance reimbursement in the preimplementation period, and hence patients paid out of pocket for treatment. In the postimplementation phase, 35% of the patients in the PCI hubs and 59% in the spoke health care centers received coverage through the Chief Minister’s Comprehensive Health Insurance Scheme. Fourth, use of the STEMI Information Technology Kit for diagnosis and monitoring was a unique feature that allowed for communication across the network.
Our most prominent finding was greater use of primary PCI and the pharmacoinvasive approach. This greater use was particularly striking for those admitted to spoke health care centers and, importantly, did not lead to diminished rates of reperfusion or delays in times to treatment. In our study, use of primary PCI and the pharmacoinvasive approach increased most notably in the spoke health care centers by almost 10-fold from 3.5% to 31.3%. Recent data from the Strategic Reperfusion Early After Myocardial Infarction (STREAM) trial suggest that the pharmacoinvasive approach may be comparable to primary PCI, whereas a meta-analysis suggests that postfibrinolysis angiography and PCI lead to significant clinical benefits when compared with stand-alone fibrinolysis. Application of the pharmacoinvasive approach has also been studied in the Indian context. In the present study, 53.6% of patients in the preimplementation phase had stand-alone fibrinolysis, which was reduced to 29.4% in the postimplementation phase. The spoke health care centers had an even greater reduction in stand-alone fibrinolysis from 77.1% to 46.3%.
At 1 year, we found all-cause, risk-adjusted mortality to be lower in the postimplementation group compared with the preimplementation group, but this finding was not statistically significant and was a secondary outcome. We urge caution when interpreting this result. However, this finding is consistent with the potential benefits of reperfusion and revascularization after STEMI seen in Western countries. It also may be related to improvements in prescriptions for key medications, such as statins and dual antiplatelet therapy.
Limitations
Our study also has important limitations. First, this was an observational study that examined key processes and outcomes before and after implementation of a number of interventions. Our ability to causally link any specific part of our program to the benefits we observed is limited and subject to temporal trends; the strategy we incorporated to increase primary PCI and the pharmacoinvasive approach has been evaluated in randomized clinical trials in other populations, suggesting that our results have face validity. Second, we required informed consent for enrollment in the intervention to collect data. Therefore, although we tried to capture consecutive patients, it is likely that we missed many who died before arriving at the hospital, confirming ECGs, or obtaining informed consent. This requirement for infomred consent also may have led to our observation of a less sick cohort than would be expected by an all-comers study design as demonstrated by lower-than-expected rates of shock and mortality. Third, we implemented a STEMI system of care, leveraging several ongoing efforts that may not be universally available. For example, we were able to use a popular health insurance scheme for patients living below the poverty line to fund many of the services provided in the TN-STEMI program, and we benefited from the concomitant increase in GVK-EMRI ambulance services. These types of investments by public organizations and the government are critical aspects for creating sustainable quality improvement efforts in LMICs. In addition, such programs remain imperfect (eg, partial insurance coverage and ongoing costs may not be fully covered for all patients). Regardless, based on the TN-STEMI program, the adjacent state of Telangana is providing government support to develop a similar hub-and-spoke model for its population. Finally, the high overall rates of reperfusion seen in our registry are not typical for those observed in India and other LMICs but highlight the potential of a program such as ours in streamlining STEMI care even in resource-poor settings.
Conclusions
The hub-and-spoke model of a STEMI system of care that connects peripherally located spoke health care centers with large PCI hub hospitals is a feasible and effective model for STEMI reperfusion in LMICs, such as India. This model improves access to primary PCI and the pharmacoinvasive approach without reducing or delaying reperfusion and may improve 1-year mortality.
eFigure 1. STEMI Information Technology Kit
eFigure 2. Four Hub Hospitals and Their Primary Spoke Centers (Including Dropouts With No Enrollment)
eFigure 3. Map of Tamil Nadu Showing the 4 PCI Hub Hospitals
eTable 1. PCI Hub Hospitals in the TN-STEMI Program
eTable 2. List of Spoke Centers That Contributed Patients to the Study and Their PIs in the TN-STEMI Program
eTable 3. Medication Prescription at Discharge
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. N Engl J Med. 2007;357(16):1631-1638. [DOI] [PubMed] [Google Scholar]
- 3.Kaifoszova Z, Kala P, Alexander T, et al. Stent for Life Initiative: leading example in building STEMI systems of care in emerging countries. EuroIntervention. 2014;10(suppl T):T87-T95. [DOI] [PubMed] [Google Scholar]
- 4.Xavier D, Pais P, Devereaux PJ, et al. ; CREATE Registry Investigators . Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371(9622):1435-1442. [DOI] [PubMed] [Google Scholar]
- 5.O’Gara PT, Kushner FG, Ascheim DD, et al. ; ACCF/AHA Task Force . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):529-555. [DOI] [PubMed] [Google Scholar]
- 6.Steg PG, James SK, Atar D, et al. ; Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology (ESC) . ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619. [DOI] [PubMed] [Google Scholar]
- 7.Alexander T, Mullasari AS, Narula J. Developing a STEMI system of care for low- and middle-income countries: the STEMI-India model. Glob Heart. 2014;9(4):419-423. [DOI] [PubMed] [Google Scholar]
- 8.Vedanthan R, Seligman B, Fuster V. Global perspective on acute coronary syndrome: a burden on the young and poor. Circ Res. 2014;114(12):1959-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nallamothu BK. A race for the base: ST-segment-elevation myocardial infarction systems of care in low- and middle-income countries. Circ Cardiovasc Qual Outcomes. 2013;6(1):5-6. [DOI] [PubMed] [Google Scholar]
- 10.Baliga RR, Bahl VK, Alexander T, et al. Management of STEMI in low- and middle-income countries. Glob Heart. 2014;9(4):469-510. [DOI] [PubMed] [Google Scholar]
- 11.Alexander T, Mehta S, Mullasari A, Nallamothu BK. Systems of care for ST-elevation myocardial infarction in India. Heart. 2012;98(1):15-17. [DOI] [PubMed] [Google Scholar]
- 12.Alexander T, Victor SM, Mullasari AS, Veerasekar G, Subramaniam K, Nallamothu BK; TN-STEMI Programme Investigators . Protocol for a prospective, controlled study of assertive and timely reperfusion for patients with ST-segment elevation myocardial infarction in Tamil Nadu: the TN-STEMI programme. BMJ Open. 2013;3(12):e003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinath Reddy K, Shah B, Varghese C, Ramadoss A. Responding to the threat of chronic diseases in India. Lancet. 2005;366(9498):1744-1749. [DOI] [PubMed] [Google Scholar]
- 16.Reddy KS. India wakes up to the threat of cardiovascular diseases. J Am Coll Cardiol. 2007;50(14):1370-1372. [DOI] [PubMed] [Google Scholar]
- 17.Jeemon P, Reddy KS. Social determinants of cardiovascular disease outcomes in Indians. Indian J Med Res. 2010;132:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy KS, Patel V, Jha P, Paul VK, Kumar AK, Dandona L; Lancet India Group for Universal Healthcare . Towards achievement of universal health care in India by 2020: a call to action. Lancet. 2011;377(9767):760-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Li X, Wang Q, et al. ; China PEACE Collaborative Group . ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet. 2015;385(9966):441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razzak JA, Kellermann AL. Emergency medical care in developing countries: is it worthwhile? Bull World Health Organ. 2002;80(11):900-905. [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom DE, Cafiero-Fonseca ET, Candeias V, et al. Economics of Non-Communicable Diseases in India: The Costs and Returns on Investment of Interventions to Promote Healthy Living and Prevent, Treat, and Manage NCDs. Boston, MA: World Economic Forum, Harvard School of Public Health; 2014. [Google Scholar]
- 22.Mahal A, Karan A, Engalgua M. The Economic Implications of Non-Communicable Disease for India Study. Health Nutrition and Population Discussion Paper. Washington, DC: The World Bank; 2010.
- 23.Armstrong PW, Gershlick AH, Goldstein P, et al. ; STREAM Investigative Team . Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368(15):1379-1387. [DOI] [PubMed] [Google Scholar]
- 24.D’Souza SP, Mamas MA, Fraser DG, Fath-Ordoubadi F. Routine early coronary angioplasty versus ischaemia-guided angioplasty after thrombolysis in acute ST-elevation myocardial infarction: a meta-analysis. Eur Heart J. 2011;32(8):972-982. [DOI] [PubMed] [Google Scholar]
- 25.Victor SM, Subban V, Alexander T, et al. A prospective, observational, multicentre study comparing tenecteplase facilitated PCI versus primary PCI in Indian patients with STEMI (STEPP-AMI). Open Heart. 2014;1(1):e000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. STEMI Information Technology Kit
eFigure 2. Four Hub Hospitals and Their Primary Spoke Centers (Including Dropouts With No Enrollment)
eFigure 3. Map of Tamil Nadu Showing the 4 PCI Hub Hospitals
eTable 1. PCI Hub Hospitals in the TN-STEMI Program
eTable 2. List of Spoke Centers That Contributed Patients to the Study and Their PIs in the TN-STEMI Program
eTable 3. Medication Prescription at Discharge