This cohort study compares the clinical characteristics, presentation, and outcomes of peripartum cardiomyopathy between African American and non–African American women.
Key Points
Question
What are the differences in presentation and outcomes between African American and non–African American women with peripartum cardiomyopathy?
Findings
In this cohort study of African American women compared with non–African American women with peripartum cardiomyopathy, African American women were diagnosed with peripartum cardiomyopathy later in the postpartum period, presented with more severe disease, recovered less frequently, and took longer to recover compared with their non–African American counterparts.
Meaning
African American women with peripartum cardiomyopathy have a different disease profile and trajectory compared with non–African American women; future studies are needed to explore potential genetic explanations for these differences and to investigate whether personalized therapeutic approaches could benefit African American women with peripartum cardiomyopathy differently.
Abstract
Importance
Peripartum cardiomyopathy (PPCM) disproportionately affects women of African ancestry, but well-powered studies to explore differences in severity of disease and clinical outcomes are lacking.
Objective
To compare the clinical characteristics, presentation, and outcomes of PPCM between African American and non–African American women.
Design, Setting, and Participants
This retrospective cohort study using data from January 1, 1986, through December 31, 2016, performed at the University of Pennsylvania Health System, a tertiary referral center serving a population with a high proportion of African American individuals, included 220 women with PPCM.
Main Outcomes and Measures
Demographic and clinical characteristics and echocardiographic findings at presentation, as well as clinical outcomes including cardiac recovery, time to recovery, cardiac transplant, persistent dysfunction, and death, were compared between African American and non–African American women with PPCM.
Results
A total of 220 women were studied (mean [SD] age at diagnosis, 29.5 [6.6] years). African American women were diagnosed with PPCM at a younger age (27.6 vs 31.7 years, P < .001), were diagnosed with PPCM later in the postpartum period, and were more likely to present with a left ventricular ejection fraction less than 30% compared with non–African American women (48 [56.5%] vs 30 [39.5%], P = .03). African American women were also more likely to worsen after initial diagnosis (30 [35.3%] vs 14 [18.4%], P = .02), were twice as likely to fail to recover (52 [43.0%] vs 24 [24.2%], P = .004), and, when they did recover, recovery took at least twice as long (median, 265 vs 125.5 days; P = .02) despite apparent adequate treatment.
Conclusions and Relevance
In a large cohort of women with well-phenotyped PPCM, this study demonstrates a different profile of disease in African American vs non–African American women. Further work is needed to understand to what extent these differences stem from genetic or socioeconomic differences and how treatment of African American patients might be tailored to improve health outcomes.
Introduction
Peripartum cardiomyopathy (PPCM) is an often devastating form of maternal systolic heart failure that occurs toward the end of pregnancy or in the months after delivery in the absence of a clear cause or preexisting heart disease. Previous research has suggested that PPCM is hormonal and vascular in origin. In addition, recent work has revealed the frequent occurrence in women with PPCM of mutations in the large sarcomeric gene titin (TTN) (OMIM 188840), supporting a strong role for genetics in PPCM. Consistent with a genetic contribution to PPCM, several studies have also suggested that women of African ancestry are disproportionately affected by PPCM and face a worse prognosis. Overall, however, small sample sizes and limited access to medical records have limited the detailed understanding and the generalizability of clinical outcomes between African American and non–African American women. We leveraged the high prevalence of PPCM in the University of Pennsylvania Health System (UPHS), and particularly of women of African ancestry who had PPCM, to explore clinical characteristics at presentation and outcomes in a cohort of 220 women with PPCM, including 121 African American women, who are to our knowledge the largest such cohort to date.
Methods
We retrospectively identified all patients in the UPHS from January 1, 1986, through December 31, 2016, with a potential diagnosis of PPCM based on diagnostic codes (International Classification of Diseases, Ninth Revision codes 674.50-54) or echocardiographic results within 6 months of delivery and manually reviewed electronic medical records. Patients were included if they were diagnosed with PPCM or heart failure toward the end of pregnancy or in the several months after delivery, had echocardiographic documentation of systolic dysfunction (left ventricular ejection fraction [LVEF] <45% or fractional shortening <30%), and had no history of congenital heart disease, valvular disease predating PPCM, radiation or cardiotoxic chemotherapy, or other explanation for their heart failure (eFigure 1 in the Supplement). The University of Pennsylvania Institutional Review Board approved the study and determined that informed consent was not required. Continuous variables were assessed using the 2-tailed t test or Wilcoxon rank sum test. Categorical variables were compared with the χ2 analysis or Fisher exact test. Survival curves used the Kaplan-Meier method. P < .05 (2-tailed) was considered to be statistically significant.
Results
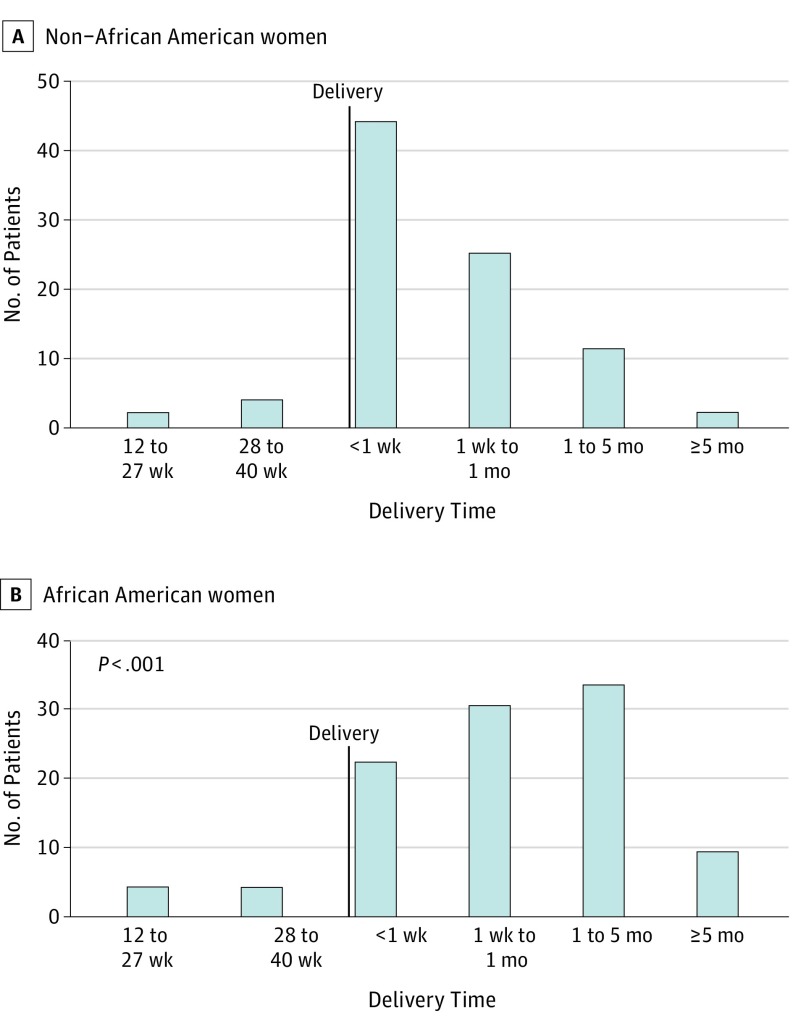
A total of 491 women were identified as having PPCM. After removal of duplicates and those who did not meet the inclusion criteria, 220 women were included (mean [SD] age at diagnosis, 29.5 [6.6] years; 121 African American women and 99 non–African American women [82 white, 6 Asian, 4 other, and 7 unknown]; 8 patients identified as Hispanic/Latina ethnicity, 2 of whom were African American) (eFigure 1 in the Supplement). The Table gives the demographic and clinical characteristics for the overall cohort stratified by race. African American women presented at a younger age than non–African American women, and fewer presented at older than 30 years. Most women were diagnosed post partum, regardless of race. However, although 44 of 88 non–African American women (50.0%) were diagnosed with PPCM within the first week post partum, the time of diagnosis was more spread out for African American women: 22 of 102 (21.6%) in the first week, 30 of 102 (29.4%) in the subsequent 3 weeks, and 33 of 102 (32.4%) in the subsequent 4 months (P < .001 compared with non–African American women) (Figure 1).
Table. Demographic and Clinical Characteristics at Presentation and Clinical Outcomesa.
| Characteristic or Outcome | Overall (N = 220) | African American Women (n = 121) |
Non–African American Women (n = 99) |
P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 29.5 (6.6) | 27.6 (6.4) | 31.7 (6.2) | <.001 |
| Age >30 y | 115 (52.3) | 45 (37.2) | 70 (70.7) | <.001 |
| Time of diagnosis (117 African American and 96 non–African American women) | ||||
| Ante partum | 17 (8.0) | 10 (8.6) | 7 (7.3) | .74 |
| Post partum | 196 (92.0) | 107 (91.5) | 89 (92.7) | .74 |
| Chronic hypertension (n = 173; 92 African American and 81 non–African American women) | 15 (8.7) | 14 (15.2) | 1 (1.2) | .001 |
| Pregnancy-related hypertension (n = 180; 96 African American and 84 non–African American women) | 80 (44.4) | 46 (47.9) | 34 (40.5) | .32 |
| Parity before PPCM diagnosis | ||||
| Nulliparous | 104 (47.3) | 51 (42.2) | 53 (53.5) | .09 |
| Multiparous | 109 (49.5) | 65 (53.7) | 44 (44.4) | .17 |
| Unknown | 7 (3.2) | 5 (4.1) | 2 (2.0) | .43 |
| Twin pregnancy (n = 215; 118 African American and 97 non–African American women) | 28 (13.0) | 10 (8.5) | 18 (18.6) | .03 |
| Cesarean delivery (n = 209; 114 African American and 95 non–African American women) | 111 (53.1) | 53 (46.5) | 58 (61.1) | .04 |
| Presenting LVEF, mean (SD), % | 29 (13) | 27 (13) | 31 (12) | .06 |
| Presenting LVEF <30% (n = 161; 85 African American and 76 non–African American women) | 78 (35.5) | 48 (56.5) | 30 (39.5) | .03 |
| Follow-up LVEF, mean (SD), % (n = 123; 60 non–African American and 63 African American women) | 39 (14) | 36 (15) | 42 (14) | .02 |
| Worst documented LVEF, mean (SD), % | 27 (13) | 24 (13) | 29 (12) | .02 |
| Worsen after diagnosis (n = 161; 85 African American and 76 non–African American women) | 44 (27.3) | 30 (35.3) | 14 (18.4) | .02 |
| Documentation EF >50% (n = 161; 85 African American and 76 non–African American women) | 144 (89.4) | 69 (57.0) | 75 (75.8) | .004 |
| Time to LVEF >50%, median (interquartile range), d (n = 80; 50 non–African American and 30 African American women) | 167 (67-352) | 265 (89-552) | 125.5 (52-286) | .02 |
Abbreviations: LVEF, left ventricular ejection fraction; PPCM, peripartum cardiomyopathy.
Data are presented as number (percentage) of patients unless otherwise indicated.
Figure 1. Time of Peripartum Cardiomyopathy Diagnosis Stratified by Time of Delivery and Race.
Time of diagnosis of PPCM in relation to time of delivery is indicated for 190 women (88 non–African American and 102 African American).
The overall rate of pregnancy-related hypertension, defined as gestational hypertension or preeclampsia, was higher than the rate in the UPHS obstetric population (44.4% vs 17.5%, P < .001), and no significant difference was noted between African American and non–African American women. A total of 15 women (8.7%) had chronic hypertension, with a significantly higher rate in African American women. Twin gestations occurred in 28 cases (13.0%), which was significantly higher than the number of twins in the UPHS obstetrical population (947 [2.3%] of 40 411 deliveries, P < .001). African American women were less likely to have twin gestations and were less likely to undergo cesarean delivery.
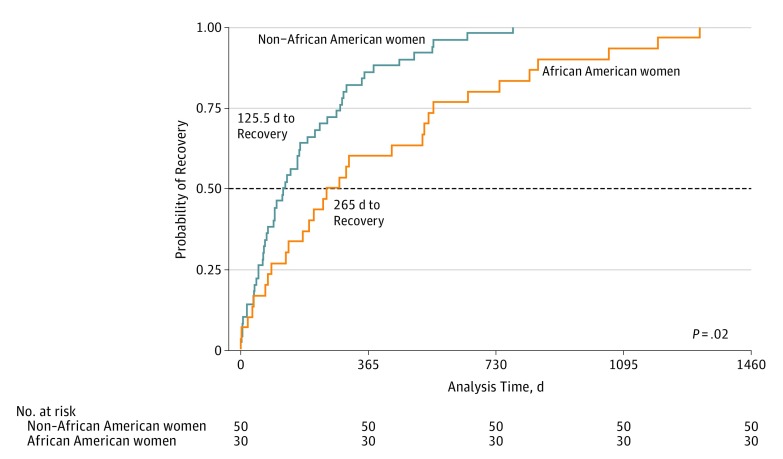
Mean (SD) LVEF at presentation was 29% (13%). African American patients with PPCM tended to present with a lower LVEF and were more likely to present with an LVEF less than 30%. Follow-up LVEF within 6 months was available for 123 patients (63 African American women and 60 non–African American women, P = .02). African American women had a significantly lower LVEF than non–African American women at this follow-up echocardiogram (mean [SD] LVEF, 36% [15%] vs 42% [14%] in African American vs non–African American women). Of the total PPCM population, 144 (65.5%) experienced recovery, defined as an LVEF of 50% on at least 1 occasion. African American women experienced recovery less frequently than their non–African American counterparts (69 [57.0%] vs 75 [75.8%], P = .004). Furthermore, even among those patients who experienced recovery, African American patients recovered more than 2 times more slowly than non–African American patients (median, 265.0 vs 125.5 days to recovery; P = .02) (Figure 2). Some patients worsened after recovery, such that only 112 (54.6%) experienced sustained recovery as their PPCM end point. African American women were less likely to have persistent recovery (52 [47.3%] vs 60 [63.2%], P = .02) and more likely to have continued dysfunction (45 [40.9%] vs 25 [26.3%], P = .03). The rates of PPCM-related mortality and cardiac transplants were similar between groups (eFigure 2 in the Supplement), as were the rates of arrhythmias (eTable 1 in the Supplement). Overall results were similar when limiting to the subset of women for whom all data were available (n = 113) (eTable 2 in the Supplement) or to the more contemporary subset of women diagnosed with PPCM on or after the year 2000 (eTable 3 in the Supplement).
Figure 2. Time to Recovery Stratified by Race.
Kaplan-Meier estimates of median time to recovery for 80 women (50 non–African American and 30 African American).
Finally, there were no significant differences in prescription rates of angiotensin-converting enzyme inhibitors (African American vs non–African American women: 99 [84.6%] vs 74 [77.9%], P = .20) or β-blockers (African American vs non–African American women: 92 [78.6%] vs 58 [61.1%], P = .50) by race. However, African American patients were more likely than non–African American patients to be prescribed some other antihypertensive (92 [78.6%] vs 58 [61.1%], P = .005), consistent with the higher prevalence of chronic hypertension in the African American patients.
Discussion
To our knowledge, this retrospective review of 220 women represents the largest study of PPCM cases to date and in particular of African American women with PPCM. Previously well-established epidemiologic observations were recapitulated, including the relative advanced age of women, presentation largely in the first few weeks after delivery, and high prevalence of twin pregnancies and gestational hypertension.
Striking observations were made in racial differences. African American patients were diagnosed younger, consistent with prior reports, and likely reflected a younger African American obstetric population at the UPHS (mean age for African American women vs non–African American women, 23.2 vs 30.5 years). African American patients were diagnosed later post partum than non–African American patients (Figure 1), a finding not previously reported likely because of insufficient cohort sizes. African American patients were also more likely to present with an LVEF less than 30%. Late diagnosis and low LVEF at presentation may reflect a delay in presentation, allowing disease to progress before receiving appropriate treatment, or may instead reflect a different pathophysiologic phenotype with later development of clinical heart failure in African American patients.
African American patients had higher rates of chronic hypertension, a difference not seen in the general UPHS obstetrical population. However, rates of gestational hypertension were remarkably high in both groups, in keeping with the well-established strong association between pregnancy-related hypertension and PPCM. This observation suggests that pathophysiologic differences between PPCM in African American and non–African American patients are unlikely to be caused by a different burden of gestational hypertension.
Despite their younger age at diagnosis, African American women with PPCM had worse outcomes than non–African American women. African American women were twice as likely to experience worsening of their LVEF after diagnosis, had significantly lower worst documented LVEF, and were twice as likely to fail to recover to an LVEF of 50% or higher. Furthermore, even among those who ultimately recovered, the time to recovery for African American patients was more than twice as long as that for non–African American patients (Figure 2) despite a similar rate of treatment with β-blockers and angiotensin-converting enzyme inhibitors.
Together, these findings indicate that African American patients are affected with a pathophysiologically more severe form of PPCM or there are environmental factors that lead to more severe presentation and disease progression. Racial disparities in health care, socioeconomics, and prevalence of classic cardiovascular disease risk factors, such as obesity, have been widely studied and well established. Although our study was unable to explore many of these socioeconomic variables, we assessed at least 1 surrogate of access to care, medication use, and did not note any different use of angiotensin-converting enzyme inhibitors and β-blockers, suggesting similar access to health care at least to this extent.
The differences in disease severity and prognosis are so profound that they suggest a potential difference based on genetics. Approximately 10% of patients with PPCM have truncating genetic variants in the gene TTN, encoding for the critical sarcomeric protein titin. These variants were found in both African American and non–African American women, indicating that the observed clinical differences are unlikely to be explained by different burdens of mutations in TTN. The findings suggest, instead, that other propensity genetic or epigenetic modifiers exist in the African American population, such as common polymorphisms that associate with severity of outcome and are more prevalent in the African American population.
Limitations
This study is not without limitations. As a retrospective review of medical records, not all demographic and clinical variables could be captured. Furthermore, some of the patients with PPCM were referred from outside hospitals, possibly introducing some bias. However, because the referral population contains relatively few African American patients and because referrals likely represent patients with more severe disease, we suspect that removal of this bias would, if anything, strengthen our findings. We were also unable to capture details of symptom severity. Finally, 7 women whose race was unknown were analyzed as non–African American, which could represent misclassification; however, if some of these women were African American, this misclassification would have biased us away from detecting the differences observed.
Conclusions
This retrospective review of 220 patients demonstrates that African American women with PPCM fare markedly worse than non–African American patients. These women are diagnosed later post partum, they present with more severe systolic dysfunction, their cardiac function worsens after diagnosis twice as often, their cardiac function ultimately recovers half as often, and, when cardiac function recovers, it does so in at least twice as much time as in non–African American patients with PPCM. Therefore, African American patients with PPCM, at the very least, need to be counseled differently than non–African American patients. Future studies will be needed to determine whether these differences are primarily genetic or socioeconomic in origin and whether personalized therapeutic approaches could benefit these patients.
eFigure 1. Inclusion Flowchart
eFigure 2. PPCM End Points
eTable 1. Incidence of Arrhythmias
eTable 2. Presenting Demographic and Clinical Characteristics and Clinical Outcomes in Subset of Women for Whom All Data Are Available (n = 113)
eTable 3. Presenting Demographic and Clinical Characteristics and Clinical Outcomes in the Contemporary Subset of Women Presenting on or After the Year 2000 (n = 190)
References
- 1.Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. ; Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy . Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767-778. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133(14):1397-1409. [DOI] [PubMed] [Google Scholar]
- 3.Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283(9):1183-1188. [DOI] [PubMed] [Google Scholar]
- 4.Bello NA, Arany Z. Molecular mechanisms of peripartum cardiomyopathy: a vascular/hormonal hypothesis. Trends Cardiovasc Med. 2015;25(6):499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485(7398):333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilfiker-Kleiner D, Sliwa K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol. 2014;11(6):364-370. [DOI] [PubMed] [Google Scholar]
- 7.Ware JS, Li J, Mazaika E, et al. ; IMAC-2 and IPAC Investigators . Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374(3):233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: population-based birth prevalence and 7-year mortality. Obstet Gynecol. 2012;120(5):1013-1019. [DOI] [PubMed] [Google Scholar]
- 9.McNamara DM, Elkayam U, Alharethi R, et al. ; IPAC Investigators . Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66(8):905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goland S, Modi K, Hatamizadeh P, Elkayam U. Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. J Card Fail. 2013;19(4):214-218. [DOI] [PubMed] [Google Scholar]
- 11.Levine LD, Bogner HR, Hirshberg A, Elovitz MA, Sammel MD, Srinivas SK. Term induction of labor and subsequent preterm birth. Am J Obstet Gynecol. 2014;210(4):354.e1-354.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bello N, Rendon IS, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62(18):1715-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Multi-Ethnic Study of Atherosclerosis. Circulation. 2012;126(1):50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Ezzati M, Rimm EB, Hajifathalian K, Ueda P, Danaei G. Sick populations and sick subpopulations: reducing disparities in cardiovascular disease between blacks and whites in the United States. Circulation. 2016;134(6):472-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheppard R, Hsich E, Damp J, et al. ; IPAC Investigators . GNB3 C825T polymorphism and myocardial recovery in peripartum cardiomyopathy: results of the Multicenter Investigations of Pregnancy-Associated Cardiomyopathy Study. Circ Heart Fail. 2016;9(3):e002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Inclusion Flowchart
eFigure 2. PPCM End Points
eTable 1. Incidence of Arrhythmias
eTable 2. Presenting Demographic and Clinical Characteristics and Clinical Outcomes in Subset of Women for Whom All Data Are Available (n = 113)
eTable 3. Presenting Demographic and Clinical Characteristics and Clinical Outcomes in the Contemporary Subset of Women Presenting on or After the Year 2000 (n = 190)