Abstract
Objective
To determine the efficacy, tolerability, and safety of ascending doses of Adhesive Dermally-Applied Microarray (ADAM) zolmitriptan versus placebo for acute migraine treatment.
Background
ADAM is a novel patient-administered system for intracutaneous drug administration. In a phase 1 pharmacokinetic study, zolmitriptan administered using ADAM had much faster absorption than oral administration with higher exposure in the first two hours.
Methods
This was a multicenter, randomized, double-blind, placebo-controlled, parallel-group Phase 2b/3 study evaluating ADAM zolmitriptan 1 mg, 1.9 mg, and 3.8 mg versus placebo. Co-primary endpoints were pain freedom and freedom from most bothersome other migraine-associated symptom 2 hours post-dose.
Results
Of patients treated with ADAM zolmitriptan 3.8 mg or placebo, 41.5% and 14.2%, respectively were pain-free 2 hours post-dose (p = 0.0001) and 68.3% and 42.9% were free from their most bothersome other symptom (p = 0.0009). Due to the fixed sequential testing methodology, formal statistical significance was not established for secondary endpoints. However, the proportion of patients who were photophobia-free, phonophobia-free, and nausea-free at 2 hours post-dose was higher in the ADAM zolmitriptan 3.8 mg group compared with placebo, as were the percentages of patients who were pain-free, and who experienced pain relief up to 48 hours post-dose. Systemic adverse events were consistent with previous triptan trials, and included dizziness, paresthesia, muscle tightness, and nausea, all of which occurred in < 5% of patients in any group. Application site reactions were generally mild and resolved within 48 hours, although erythema and bruising persisted for longer periods in some patients.
Conclusion
ADAM zolmitriptan 3.8 mg provides effective relief of migraine headache and associated most bothersome symptoms compared with placebo, and is well-tolerated.
ClinicalTrials.gov
Keywords: Migraine, headache, triptan, zolmitriptan, drug delivery, intracutaneous, Adhesive Dermally Applied Microarray
Introduction
The triptans, including zolmitriptan, are at the forefront of acute migraine treatment. They have been available in the US market since 1992, and have good evidence in terms of efficacy, tolerability, and safety (1–5). The triptans are available in several formulations: Oral tablet, orally disintegrating tablet, nasal spray, nasal powder, rectal suppository, and subcutaneous delivery with or without needle. The latter three formulations are available for sumatriptan only, and the suppository is only available outside the US.
Migraine attacks are not only associated with gastric symptoms (most notably nausea), but also with gastric dysfunction (gastroparesis), resulting in delayed absorption of enterically-absorbed medications (6). The delayed absorption particularly affects the time to maximum plasma concentration (tmax) and negatively affects efficacy (6,7). Delayed absorption is not only relevant for oral tablets and orally disintegrating tablets, it also pertains to nasal spray formulations as up to 86% of intranasally-administered sumatriptan (8) and 70% of intranasally-administered zolmitriptan (9) is absorbed through the gut.
Adhesive Dermally-Applied Microarray (ADAM) is a new drug delivery system for intracutaneous self-administration. It consists of a 3 cm2 disposable array of drug-coated titanium microprojections on an adhesive backing. It is applied using a low-cost, reusable, handheld applicator. The applicator is designed to ensure that the same energy is applied across multiple uses and is user-independent. The microprojections penetrate the stratum corneum, where the drug coating is rapidly reconstituted by interstitial fluid in the skin, making it available for absorption. The shallow depth of penetration and the minuteness of the microprojections limit the likelihood of stimulating sensory nerve endings. The ADAM adhesive is removed manually after 30 minutes and discarded without the need for “sharps” disposal (10).
Zolmitriptan has been formulated for delivery using ADAM as an investigational product for acute migraine treatment. In a Phase 1 study evaluating the pharmacokinetics of zolmitriptan delivered with ADAM, the median time to maximum serum concentration (tmax) was less than 20 minutes, which was comparable to subcutaneously administered sumatriptan in the same study. Absorption was considerably faster than for oral zolmitriptan, with higher exposure in the first two hours (10). These data suggested that ADAM zolmitriptan may provide a promising new option for providing rapid migraine relief while circumventing some of the limitations of other routes of administration.
Here we describe a randomized, double-blind, placebo-controlled, parallel-group study evaluating ascending doses of ADAM zolmitriptan for the acute treatment of migraine headache and associated symptoms.
Methods
This was a multicenter, randomized, double-blind, placebo-controlled, parallel group Phase 2b/3 study conducted at 36 sites in the US. The protocol was approved by the Quorum Review Institutional Review Board (Seattle, WA). Patients 18–65 years old with a greater than 1-year history of episodic migraine with and/or without aura and with an onset prior to 50 years of age were eligible to participate. All participants gave written informed consent prior to any study procedures being performed. During the 6 months prior to the run-in period, they must have had at least two and no more than eight migraine headaches per month, with no more than 10 headache days per month. At least five attacks must have met the International Classification of Headache Disorders criteria for migraine.
During a 28- to 56-day run-in period to determine eligibility, patients recorded daily headache and migraine symptoms using an e-diary. On the first day of the run-in period, patients declared their most bothersome migraine symptom (MBS) other than pain (nausea [with or without vomiting], photophobia, or phonophobia, which is most bothersome most of the time with their migraine headaches). Eligible patients had experienced two to eight migraine headaches per 28-day period and were randomly assigned in a 2:2:1:2:1 ratio to receive one 1 mg ADAM zolmitriptan, one 1.9 mg ADAM zolmitriptan, one ADAM placebo, two 1.9 mg ADAM zolmitriptan (3.8 mg total), or two ADAM placebo, to treat a single qualifying migraine headache (pain of moderate or severe intensity and presence of MBS). Uncoated titanium microprojections were used in the placebo. A central, permuted block randomization scheme stratified by MBS was generated by an independent statistician.
Patients self-administered ADAM zolmitriptan or placebo to the upper arm, in an outpatient setting. They used an e-diary to record symptom scores (scale: none, mild, moderate, or severe) at 15, 30, 45, and 60 minutes, and 2, 3, 4, 12, 24, and 48 hours post-dosing. The application site was scored for redness, swelling, and bruising at 30 minutes, and 4, 12, 24, and 48 hours post-dose. A follow-up visit occurred 2–7 days after the 48-hour time point.
The co-primary endpoints of the study were the proportion of patients reporting pain freedom at 2 hours post-dose and the proportion reporting freedom from their MBS at 2 hours post-dose. Pain freedom was defined as a score of “none” at 2 hours without the use of rescue medication during the 2-hour post-dose period. Similarly, MBS freedom was defined as the absence of the symptom without the use of rescue medication during the 2-hour post-dose period.
The secondary endpoints were the proportion of patients who achieved pain relief (defined as improvement to mild or none) at 15 and 30 minutes, as well as 3, and 4 hours post-dose; pain freedom at 30 minutes, and 24 and 48 hours post-dose; freedom at 2 hours post-dose from photophobia, phonophobia, or nausea (irrespective of MBS); and the proportion of patients who required rescue medication during the 2-hour post-dose period.
Sample size (n = 360) was determined by estimating that 15% of patients receiving placebo and 35% of those receiving active treatment would achieve freedom from pain or MBS at 2 hours post-dose, and allowing for 15% dropouts. A stratified chi-square test was used to estimate the number used to detect a treatment difference with 80% statistical power and 5% 2-sided significance level.
The primary analysis method was a Cochran-Mantel-Haenszel (CMH) test controlling for the randomization stratification by MBS. Each individual active treatment group was compared to the pooled placebo group in a pairwise manner. Pooling of the placebo groups was planned a priori for statistical simplicity. Post hoc analyses showed no significant differences in outcomes in the 1- or 2-patch placebo groups, supporting the decision to pool these groups. In addition, the Breslow-Day test was performed to assess the homogeneity of the odds ratios across MBS. A fixed sequential testing methodology was applied to control the overall type 1 error. A test was considered statistically significant only if the corresponding CMH test had a p value < 0.05 and all previous tests had a p value < 0.05. Last observation carried forward (LOCF) was used to impute missing data.
All authors had full access to all study data.
Results
Between 16 June 2016 and 10 January 2017, 589 patients were screened, 365 were randomly assigned to receive ADAM zolmitriptan or placebo, and 333 actually applied the study treatment (safety population). Among the 224 who were not randomized, 77 had screening failures, 130 failed to meet the run-in criteria, and data were missing for 17. The most common reasons for screen failures were: 16 (17%) clinically-relevant abnormal findings in the physical exam, vital signs, or laboratory tests; 15 (16%) history or current abuse or dependence on alcohol or drugs; 14 (15%) reason to believe that compliance with study requirements and completion of evaluations would not be possible. The most common reasons for run-in failures were: 64 (50%) had more than 10 headache days in past 28 days; 45 (35%) were not able to use the e-diary; 44 (34%) did not have an average of at least two qualifying migraines per 28-day period, and 17 (18%) did not have confirmation of good general health. Note that a patient could have more than one reason for screening or run-in failures. Of those who applied the study treatment, 321 had at least one post-dose assessment (modified intent to treat [mITT] population). Table 1 presents the disposition of patients in each group and overall.
Table 1.
Patient disposition.
| Treatment group |
|||||
|---|---|---|---|---|---|
| ADAM zolmitriptan |
|||||
| Placeboa | 1 mg | 1.9 mg | 3.8 mg | Total | |
| Randomized, n | 91 | 90 | 92 | 92 | 365 |
| Treated, n (%) | 83 (91.2) | 80 (88.9) | 87 (94.6) | 83 (90.2) | 333 (91.2) |
| Not treated, n (%) | 8 (8.8) | 10 (11.1) | 5 (5.4) | 9 (9.8) | 32 (8.8) |
| Reason: | |||||
| No qualifying migraine, n (%) | 7 (7.7) | 7 (7.8) | 5 (5.4) | 6 (6.5) | 25 (6.8) |
| Other, n (%) | – | 2 (2.2) | – | – | 2 (0.5) |
| Lost-to-follow-up, n (%) | – | 1 (1.1) | – | 2 (2.2) | 3 (0.8) |
| Patient request, n (%) | 1 (1.1) | – | – | 1 (1.1) | 2 (0.5) |
| Randomized and completed, n (%) | 83 (91.2) | 79 (87.8) | 87 (94.6) | 83 (90.2) | 332 (91.0) |
| Randomized and withdrawn early, n (%) | – | 1 (1.1) | – | – | 1 (0.3) |
| Lost to follow-up, n (%) | – | 1 (1.1) | – | – | 1 (0.3) |
Consisted of pooled 1-patch and 2-patch placebo groups.
The majority of patients were female (87%), and the mean age was 41 ± 11.3 years. Demographic characteristics were generally similar among groups, except for the ADAM zolmitriptan 1.9 mg group, in which a smaller percentage were Caucasian (Table 2). The characteristics of the qualifying migraine headaches pre-treatment are shown in Table 3. The proportion of patients with severe migraine pain was higher in the ADAM zolmitriptan 1.9 mg group than in the other treatment groups.
Table 2.
Demographics and baseline characteristics.
| n (%) | Treatment group (mITT population) |
||||
|---|---|---|---|---|---|
| ADAM zolmitriptan |
|||||
| Placeboa n = 77 | 1 mg n = 79 | 1.9 mg n = 83 | 3.8 mg n = 82 | Total n = 321 | |
| Female | 69 (89.6) | 70 (88.6) | 73 (88.0) | 68 (82.9) | 280 (87.2) |
| Age, mean (SD) years | 42.7 (11.5) | 41.7 (11.6) | 40.1 (10.9) | 41.0 (11.4) | 41.3 (11.3) |
| Race | |||||
| White | 59 (76.6) | 58 (73.4) | 54 (65.1) | 67 (81.7) | 238 (74.1) |
| MBS | |||||
| Nausea | 20 (26.0) | 17 (21.5) | 19 (22.9) | 17 (20.7) | 73 (22.7) |
| Phonophobia | 21 (27.3) | 21 (26.6) | 22 (26.5) | 22 (26.8) | 86 (26.8) |
| Photophobia | 36 (46.8) | 41 (51.9) | 42 (50.6) | 43 (52.4) | 162 (50.5) |
Consisted of pooled 1-patch and 2-patch placebo groups.
Table 3.
Characteristics of qualifying migraine.
| Treatment group |
|||||
|---|---|---|---|---|---|
| ADAM zolmitriptan |
|||||
| n (%) | Placeboa n = 77 | 1 mg n = 79 | 1.9 mg n = 83 | 3.8 mg n = 82 | Total n = 321 |
| Severity of pain | |||||
| Moderate | 44 (57.1) | 37 (46.8) | 33 (39.8) | 43 (52.4) | 157 (48.9) |
| Severe | 33 (42.9) | 42 (53.2) | 50 (60.2) | 39 (47.6) | 164 (51.1) |
| Nausea present | 51 (66.2) | 56 (70.9) | 60 (72.3) | 59 (72.0) | 226 (70.4) |
| Vomiting present | 6 (7.8) | 3 (3.8) | 7 (8.4) | 5 (6.1) | 21 (6.5) |
| Photophobia present | 75 (97.4) | 73 (92.4) | 77 (92.8) | 78 (95.1) | 303 (94.4) |
| Phonophobia present | 72 (93.5) | 70 (88.6) | 75 (90.4) | 71 (86.6) | 288 (89.7) |
| Aura present | 23 (29.9) | 26 (32.9) | 35 (42.2) | 35 (42.7) | 119 (37.1) |
| Woke up with headache | 44 (57.1) | 41 (51.9) | 43 (51.8) | 36 (43.9) | 164 (51.1) |
Consisted of pooled 1-patch and 2-patch placebo groups.
Primary outcome measures
ADAM zolmitriptan 3.8 mg was superior to the pooled placebo group for the co-primary endpoints of proportion of patients who were pain-free at 2 hours (41.5% vs. 14.3%; p = 0.0001) and the proportion who were MBS-free at 2 hours (68.3% vs. 42.9%; p = 0.0009) (Figure 1). ADAM zolmitriptan 1.9 mg was also superior to placebo for pain freedom (27.7% vs. 14.3%; p = 0.0351), but the comparison for MBS at 2 hours was not significant. Because both co-primary endpoints are required to be significant at the 2-sided alpha level of 0.05, the 1.9 mg group was not considered to be statistically significantly different from placebo. In addition, all subsequent significance tests (including secondary endpoints) were not considered to be statistically significant, and any p values provided are for descriptive purposes only (nominal p values are shown).
Figure 1.
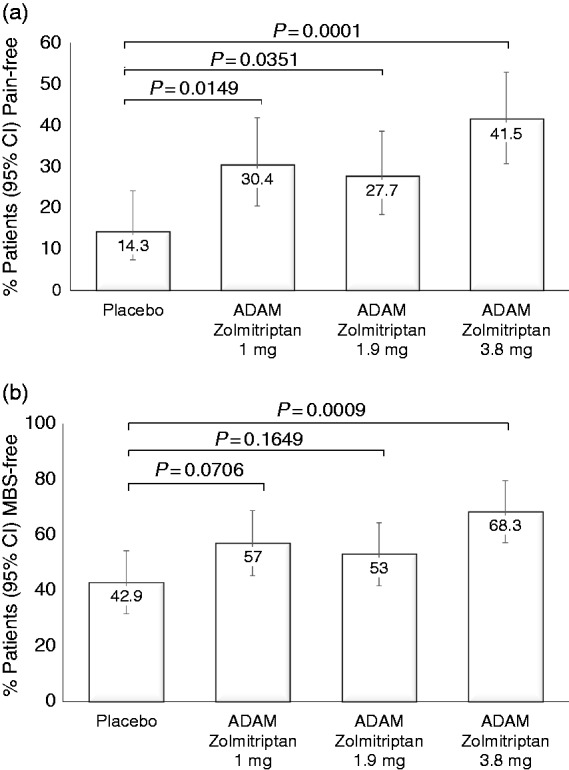
Primary endpoints. Proportion of patients who were (a) pain-free or (b) free of their most bothersome other symptom at 2 hours post-dose. Error bars: 95% confidence intervals.
Secondary outcome measures
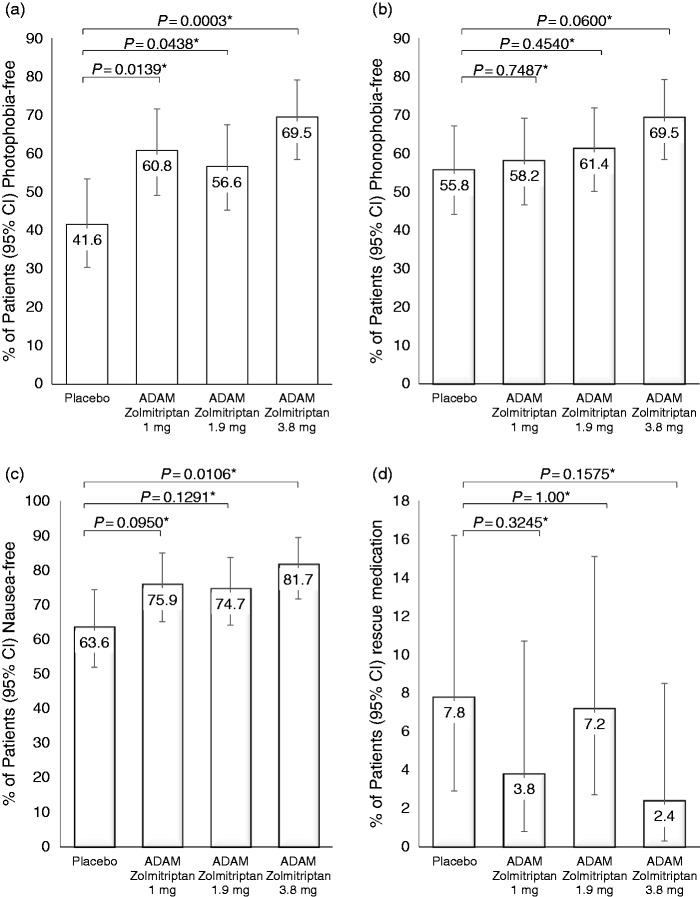
The proportion of patients who were photophobia-free, phonophobia-free, and nausea-free at 2 hours post-dose was higher in all ADAM zolmitriptan groups compared with placebo (Figure 3(a), (b), (c)). In the ADAM zolmitriptan 3.8 mg group, 69.5% were photophobia-free versus 41.6% in the placebo group (nominal p = 0.0003). Corresponding values for phonophobia-free were 69.5% and 55.8% (nominal p = 0.0060). For nausea-free, the corresponding values were 81.7% versus 63.6% (nominal p = 0.0106). The percentage of patients who took rescue medication within 2 hours post-dose was small in all groups (2.4–7.8%) with no discernable differences among them (Figure 3(d)).
Figure 3.
Secondary endpoints. Proportion of patients who, at 2 hours post-dose, were (a) photophobia-free, (b) phonophobia-free, (c) nausea-free, or (d) took rescue medications within 2 hours post-dose. Error bars: 95% confidence intervals. *p values are nominal.
The percentages of patients who were pain-free at the pre-specified time points of 30 minutes, 24 hours and 48 hours post-dose were 7.3%, 69.5% and 64.6%, respectively, for the ADAM zolmitriptan 3.8 mg group, and 2.6%, 39.0%, and 39.0% for the placebo group (nominal p = 0.1768 at 30 minutes and p < 0.01 for both 24 and 48 hours). The time course of pain freedom from 30 minutes to 24 hours is shown in Figure 2. The percentages of patients who experienced pain relief (improvement to mild or none), was also higher for the zolmitriptan 3.8 mg group than the placebo group at all time points (Figure 2). However, among the pre-specified time points of 15 minutes, 30 minutes, and 4 hours, nominal significance was only achieved at 4 hours (nominal p < 0.0001).
Figure 2.
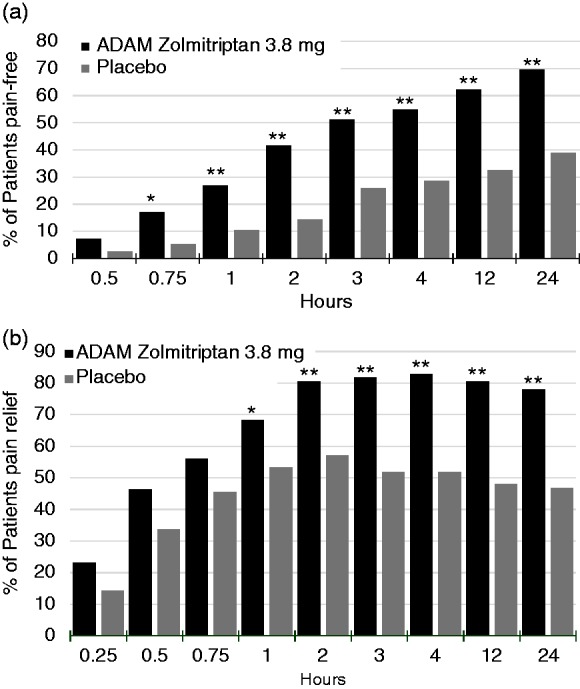
Time course of pain freedom and pain relief. Proportion of patients who were (a) pain-free or (b) had pain relief, in the first 24 hours following dosing. Nominal p values: *p < 0.05, **p < 0.01.
Post-hoc analysis
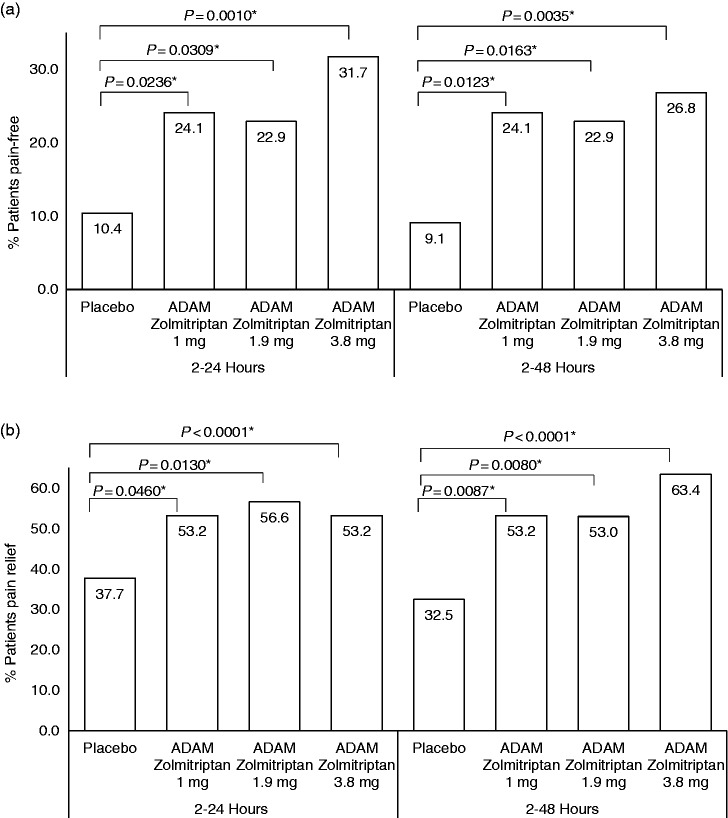
A post-hoc analysis showed that pain freedom was sustained, as 31.7% of patients in the ADAM zolmitriptan 3.8 mg group were free from pain (without the use of rescue medications) at all time points between 2 and 24 hours versus 10.4% in the placebo group (nominal p = 0.001). Sustained pain freedom between 2 and 48 hours was observed in 26.8% in the ADAM zolmitriptan 3.8 mg group and 9.1% in the placebo group (nominal p = 0.0035). Pain relief was sustained 2–24 hours for 68.3% in the ADAM zolmitriptan 3.8 mg group and 37.7% in the placebo group (nominal p = < 0.0001). Corresponding values for sustained pain relief 2–48 hours were 63.4% and 32.5% (nominal p < 0.0001) (Figure 4).
Figure 4.
Proportions of patients who had sustained pain freedom and relief. (a) percentage of patients who were free from headache pain for the entire period of 2–24 hours (left panel) or 2–48 hours (right panel). (b) Percentage of patients who had pain relief for the entire period of 2–24 hours (left panel) or 2–48 hours (right panel). *p values are nominal.
Safety
A high proportion of patients applied the treatment for the full prescribed 30 minutes: 98.8% in the placebo group, 98.8% in the ADAM zolmitriptan 1 mg group, 97.7% in the ADAM zolmitriptan 1.9 mg group, and 97.6% in the ADAM zolmitriptan 3.8 mg group. Table 4 provides a summary of treatment-emergent adverse events (TEAEs). Most TEAEs were mild or moderate in severity. The exceptions were three patients in the ADAM 3.8 mg group who reported a severe TEAE, one with application site bruising, one with application site pain, and one with muscle tightness. Dosing was discontinued in two patients in the ADAM zolmitriptan 3.8 mg group due to TEAEs. There were no serious TEAEs and no TEAEs of special interest (defined as any application site reactions requiring further evaluation or care or resulting in burns or scars as determined by clinical review). The most commonly occurring adverse event was application site erythema, followed by application site bruising. There were no pigmentation reactions. Table 5 presents TEAEs that occurred in ≥ 2% of patients in any ADAM zolmitriptan treatment group.
Table 4.
Summary of treatment-emergent adverse events (safety population).
| Treatment group |
|||||
|---|---|---|---|---|---|
| Placeboa n = 83 | ADAM zolmitriptan |
||||
| n (%) | 1 mg n = 80 | 1.9 mg n = 87 | 3.8 mg n = 83 | Total n = 333 | |
| Patients with at least one TEAE | 15 (18.1) | 26 (32.5) | 37 (42.5) | 43 (51.8) | 121 (36.3) |
| Treatment-relatedb | 14 (16.9) | 24 (30.0) | 33 (37.9) | 43 (50.6) | 113 (33.9) |
| Patients with at least one TEAE within 24 hours of ADAM application | 12 (14.5) | 17 (21.3) | 32 (36.8) | 38 (45.8) | 99 (29.7) |
| Patients with at least one TEAE by severityc | |||||
| Mild | 11 (13.3) | 24 (30.0) | 33 (37.9) | 33 (39.8) | 101 (30.3) |
| Moderate | 4 (4.8) | 2 (2.5) | 4 (4.6) | 7 (8.4) | 17 (5.1) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (3.6) | 3 (0.9) |
TEAE: treatment-emergent adverse event.
Consisted of pooled 1-patch and 2-patch placebo groups.
Possibly or probably treatment-related. If a patient reported an event multiple times with differing relationships to study medication, only the one most related to study medication was counted.
If a patient reported an event multiple times with differing severities, only the most severe was counted.
Table 5.
Treatment-emergent adverse events occurring in ≥ 2% of patients in any active treatment group (safety -population).
| Treatment group |
|||||
|---|---|---|---|---|---|
| Placeboa n = 83 | ADAM zolmitriptan |
||||
| n (%) | 1 mg n = 80 | 1.9 mg n = 87 | 3.8 mg n = 83 | Total n = 333 | |
| Application site erythema | 9 (10.8) | 13 (16.3) | 17 (19.5) | 22 (26.5) | 61 (18.3) |
| Application site bruise | 3 (3.6) | 5 (6.3) | 12 (13.8) | 12 (14.5) | 32 (9.6) |
| Application site pain | 1 (1.2) | 2 (2.5) | 2 (2.3) | 8 (9.6) | 13 (3.9) |
| Application site hemorrhage | 0 (0.0) | 3 (3.8) | 5 (5.7) | 4 (4.8) | 12 (3.6) |
| Application site swelling/edema | 3 (3.6) | 2 (2.6) | 6 (6.8) | 4 (4.8) | 15 (4.5) |
| Dizziness | 0 (0.0) | 1 (1.3) | 0 (0.0) | 4 (4.8) | 5 (1.5) |
| Paresthesia | 1 (1.2) | 0 (0.0) | 0 (0.0) | 2 (2.4) | 3 (0.9) |
| Muscle tightness | 0 (0.0) | 0 (0.0) | 1 (1.1) | 2 (2.4) | 3 (0.9) |
| Nausea | 0 (0.0) | 2 (2.5) | 1 (1.1) | 1 (1.2) | 4 (1.2) |
Consisted of pooled 1-patch and 2-patch placebo groups.
All dermal adverse events were followed to resolution and most resolved within 7 days. However, some took 8–14 days to resolve (in the placebo, 1.0 mg, 1.9 mg and 3.9 mg groups, redness was seen in zero, two, five, and six patients, respectively, and bruising was seen in zero, two, five, and three patients, respectively), and three took more than 14 days to fully resolve (one patient in the placebo group with redness and two patients in the 1 mg group with bruising). Most patients with prolonged time to resolution of redness or bruising were taking medications that inhibit platelet function (NSAIDs, including aspirin, or fish oil).
Conclusions and discussion
In this randomized, double-blind, placebo-controlled study, ADAM zolmitriptan 3.8 mg was effective and well-tolerated for the acute treatment of migraine. Nearly 42% of patients treated with ADAM zolmitriptan 3.8 mg were pain-free 2 hours after treatment and nearly 70% were free from their most bothersome headache-associated migraine symptom. Efficacy was dose dependent, with the 3.8 mg dose providing a better response than the 1.9 mg and 1 mg doses.
Due to the statistical approach in this study (fixed sequential testing), no formal statistical significance could be assigned for the secondary endpoints. However, the proportion of patients treated with ADAM zolmitriptan 3.8 mg who were free from photophobia, phonophobia, and nausea was higher than for placebo, with nominal significance achieved for photophobia and nausea.
Sustained pain freedom is considered the ideal migraine treatment response (11). It is notable that in a post-hoc analysis efficacy was sustained, with many patients remaining pain-free out to 48 hours. The percentage of patients who had sustained pain freedom in the first 24 hours was 31.7% for ADAM zolmitriptan 3.8 mg, which compares favorably with the 15–25% historically seen in controlled trials of triptans in migraine treatment (12). Onset of pain freedom and pain relief was apparent much earlier than 2 hours, with nominally-significant differences between ADAM zolmitriptan 3.8 mg and placebo beginning at 45 minutes and 1 hour, respectively.
For some parameters, such as pain relief, a higher than expected placebo response was observed. The fact that the drug-device combination is new and innovative, and that there is some sensation when the device is applied, may have led some participants to believe they were receiving the active agent. Additionally, because three different doses of zolmitriptan were investigated, patients had a 75% probability of receiving active therapy, a fact of which they were aware. The odds in favor of receiving a drug that is known to be effective may have also elevated the placebo response (13). Nonetheless, a clear separation was observed between active treatment and placebo for pain relief.
As noted for the efficacy endpoints, a dose-response effect was also observed for the occurrence of TEAEs, which were mild to moderate in intensity. The most common adverse events reported were those related to the application site, and they generally resolved within 48 hours. None occurred in more than 10% of patients. Additional adverse events were consistent with those commonly seen in previous trials with triptans (12).
The design of this study was in accordance with the 2014 Guidance for Industry in the design of trials for the acute treatment of migraine (14). Importantly, the four co-primary endpoints of pain, nausea, photophobia, and phonophobia have been replaced with the two co-primary endpoints of freedom from pain and freedom from MBS, both at 2 hours. At the time of writing, we believe this may be the first completed pivotal trial reported using MBS as a co-primary efficacy endpoint. It is important to recognize these key differences in interpreting the current data in the context of prior clinical studies in migraine.
Key findings
Intracutaneous zolmitriptan delivery using an Adhesive Dermally-Applied Microarray (ADAM) system was effective and well-tolerated for the acute treatment of migraine with or without aura.
ADAM zolmitriptan was significantly more efficacious than placebo for achieving freedom from pain and from most bothersome other migraine-associated symptoms at 2 hours post-dose.
Most adverse events were mild to moderate in intensity and most were related to the application site and resolved within 48 hours.
Acknowledgements
Medical writing support provided by Pamela Foreman and funded by Zosano Pharma. Statistical analysis provided by Clinical Trial Data Services (CTDS) and funded by Zosano Pharma. The authors wish to thank all investigators and the patients who participated in this trial.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ELHS received research grants from Alder Biopharmaceuticals, Allergan, Amgen, Breaburn, Daiichi Sankyo, Dr. Reddy’s Laboratories, Eli Lilly, Pfizer, Teva Pharmaceutical Industries, and Zosano Pharma. ELHS has received consulting honoraria from Alder Biopharmaceuticals, Arteus, Eli Lilly, and Teva Pharmaceutical Industries. JLB serves on the advisory boards of Amgen, Supernus, and Lilly. She is a speaker for Depomed, Supernus, Pernix and Avanir; has received research support from Amgen, Teva, Arteus, Alder, Zosano, Allergan, Dr. Reddy, and Clinvest. DBK serves on the advisory boards of Amgen, Alder and Eli Lilly. DBK is a principal investigator for Amgen, Alder, Eli Lilly, Teva, Zosano, CoLucid, Eisai, Roche-Genentech, VM Biopharma, and Allergan. JRW is a speaker for Avanir Pharmaceuticals and Teva Pharmaceutical Industries. JRW performs research (without personal compensation) for Alder Biopharmaceuticals, Allergen, Amgen, Avanir Pharmaceuticals, Eli Lilly, Teva Pharmaceutical Industries, and Zosana Pharma. PCS and DJK are employees of Zosano Pharma. SJT serves as a consultant for Acorda Therapeutics, Alder Biopharmaceuticals, Allergan, Amgen, Autonomic Technologies, Inc., Avanir Pharmaceuticals, Biovision, electroCore, Eli Lilly, eNeura, Kimberly-Clark, Pernix Therapeutics, Pfizer, Teva Pharmaceutical Industries, and Zosano Pharma. SJT serves on the advisor’s board for Alder Biopharmaceuticals, Allergan, Amgen, Autonomic Technologies, Inc, Avanir Pharmaceuticals, Charleston Laboratories, Dr. Reddy’s, Kimberly-Clark, Pfizer, Scion Neurostim, Teva Pharmaceutical Industries, and Zosano Pharma. SJT performs research (without personal compensation) for Alder Biopharmaceuticals, Allergan, Amgen, Autonomic Technologies, Inc., Avanir Pharmaceuticals, electroCore, eNeura, Scion Neurostim, Teva Pharmaceutical Industries, and Zosano Pharma. SJT has received stock options from Autonomic Technologies, Inc. SJT receives royalties from University of Mississippi Press and Springer. SJT receives salary compensation from Dartmouth-Hitchcock Medical Center and the American Headache Society.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Zosano Pharma, Inc.
References
- 1.Gilmore B, Michael M. Treatment of acute migraine headache. Am Fam Phys 2011; 83: 271–280. [PubMed] [Google Scholar]
- 2.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55: 3–20. [DOI] [PubMed] [Google Scholar]
- 3.Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine – revised report of an EFNS task force. Eur J Neurol 2009; 16: 968–981. [DOI] [PubMed] [Google Scholar]
- 4.Lipton RB, Stewart WF, Stone AM, et al. Stratified care vs step care strategies for migraine: The Disability in Strategies of Care (DISC) Study: A randomized trial. Jama 2000; 284: 2599–2605. [DOI] [PubMed] [Google Scholar]
- 5.Tepper SJ, Dahlof CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: Data from the Landmark Study. Headache 2004; 44: 856–864. [DOI] [PubMed] [Google Scholar]
- 6.Volans GN. Migraine and drug absorption. Clin Pharm 1978; 3: 313–318. [DOI] [PubMed] [Google Scholar]
- 7.Dahlof CG. Non-oral formulations of triptans and their use in acute migraine. Curr Pain Head Rep 2005; 9: 206–212. [DOI] [PubMed] [Google Scholar]
- 8.Fuseau E, Petricoul O, Moore KH, et al. Clinical pharmacokinetics of intranasal sumatriptan. Clin Pharm 2002; 41: 801–811. [DOI] [PubMed] [Google Scholar]
- 9.Syrett N, Abu-Shakra S, Yates R. Zolmitriptan nasal spray: Advances in migraine treatment. Neurology 2003; 61: S27–S30. [DOI] [PubMed] [Google Scholar]
- 10.Kellerman DJ, Ameri M and Tepper SJ. Rapid systemic delivery of zolmitriptan using an adhesive dermally applied microarray. Pain Manage. Epub ahead of print 26 July 2017. DOI: 10.2217/pmt-2017-0036. [DOI] [PubMed]
- 11.Tfelt-Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: Third edition. A guide for investigators. Cephalalgia 2012; 32: 6–38. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: Detailed results and methods of a meta-analysis of 53 trials. Cephalalgia 2002; 22: 633–658. [DOI] [PubMed] [Google Scholar]
- 13.Geraud G, Olesen J, Pfaffenrath V, et al. Comparison of the efficacy of zolmitriptan and sumatriptan: Issues in migraine trial design. Cephalalgia 2000; 20: 30–38. [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration (FDA) Guidance for Industry. Migraine: Developing drugs for acute treatment. https://www.fda.gov/downloads/drugs/guidances/ucm419465.pdf (accessed 14 June 2017).