Supplemental Digital Content is available in the text
Keywords: glutamate, Glx, Parkinson's disease, proton magnetic resonance spectroscopy (1H-MRS), rehabilitation
Abstract
Rehabilitation interventions represent an alternative strategy to pharmacological treatment in order to slow or reverse some functional aspects of disability in Parkinson's disease (PD). To date, the neurophysiological mechanisms underlying rehabilitation-mediated improvement in PD patients are still poorly understood. Interestingly, growing evidence has highlighted a key role of the glutamate in neurogenesis and brain plasticity. The brain levels of glutamate, and of its precursor glutamine, can be detected in vivo and noninvasively as “Glx” by means of proton magnetic resonance spectroscopy (1H-MRS). In the present pilot study, 7 PD patients with frequent falls and axial dystonia underwent 8-week rehabilitative protocol focused on sensorimotor improvement. Clinical evaluation and Glx quantification were performed before and after rehabilitation. The Glx assessment was focused on the basal ganglia in agreement with their key role in the motor functions. We found that the rehabilitation program improves the static and dynamic balance in PD patients, promoting a better global motor performance. Moreover, we observed that the levels of Glx within the left basal ganglia were higher after rehabilitation as compared with baseline. Thus, we posit that our sensorimotor rehabilitative protocol could stimulate the glutamate metabolism in basal ganglia and, in turn, neuroplasticity processes. We also hypothesize that these mechanisms could prepare the ground to restore the functional interaction among brain areas deputed to motor controls, which are affected in PD.
1. Introduction
Basal ganglia imbalance is associated with a wide range of motor dysfunctions in Parkinson's disease (PD).[1] Among these signs, postural instability, gait abnormalities, and rigidity are accountable for lateral axial dystonia and camptocormia, freezing of gait and a high risk of falls, which worsen the life quality.[2]
Despite a wide range of possible pharmacological interventions in PD, freezing of gait and repeated falls did not respond satisfactorily to dopaminomimetics.[3] Rehabilitation interventions represent an alternative strategy to slow or reverse some functional aspects of disability in PD.[4] In this context, we have recently observed that a rehabilitative program combining a dynamic antigravity postural system (SPAD) and mechanical focal acoustic vibration improves postural instability in patients with progressive supranuclear palsy, an atypical parkinsonism clinically characterized by prominent axial extrapyramidal motor symptoms with frequent falls.[5]
To date, the neurophysiological mechanisms underlying rehabilitation-mediated improvement in PD patients are still poorly understood. However, growing evidence has highlighted a key role of the glutamate in neurogenesis and brain plasticity.[6,7] Interestingly, the brain levels of glutamate could be detected in vivo noninvasively by means of proton magnetic resonance spectroscopy (1H-MRS).[8]
In the present pilot study, 7 PD patients with frequent falls and axial dystonia underwent 1H-MRS before and after treatment with a rehabilitative protocol focused on sensorimotor improvement.
The rehabilitative project is a sensorimotor rehabilitation program combining the motor task with sensory feedback with a multisensory approach in a microgravity environment (SPAD). First, we aimed to test the hypothesis that a specific rehabilitation protocol can improve the static and dynamic balance thus leading to a better global motor performance. Second, by using 1H-MRS, we aimed to investigate whether our rehabilitative program modifies the striatal neurochemistry in PD patients.
2. Methods
2.1. Study sample
Our study was performed in agreement with the Declaration of Helsinki (2013). All subjects involved in the study were informed about the procedure and the aim of the research and signed an informed consent. Seven PD patients were recruited from Neurology Clinics of the University “G. d’Annunzio” of Chieti-Pescara, Italy. The inclusion criteria for the study were as follows: ability to rest in standing position and walk for 20 minutes at least; Hoehn and Yahr stage scores between 1 and 3. Exclusion criteria were as follows: prior history of major medical cardiovascular pathology; head injury or neurological problems which were not correlated to PD; current pregnancy or breastfeeding; history of substance abuse; any contraindication to MRI scanning, including metal implants and claustrophobia; dementia; musculoskeletal impairments or excessive pain in any joint that could limit participation in an exercise program, and insufficient endurance to participate in exercise twice a week for 1 hour per session. For all participating subjects, dosages of dopaminomimetic drugs were kept stable during the course of the study.
2.2. Rehabilitation protocol
2.2.1. Data collection
Patients underwent 8-week sensorimotor rehabilitation protocol at the Center of Physical and Rehabilitation Medicine of University of Chieti. Specifically, study participants underwent:
-
-
SPAD (Fig. 1A) last 20 minutes for 3 times a week combined with the use of the metronome that marks rhythmic step of each patient. SPAD is a device for body weight support consisting of a machinery that produces a microgravity environment during gait motion. It acts with a dual action: a mechanical one, which allows a neuromotor retraining with cortical–subcortical learning aimed to the reacquisition of a balanced body schema, which minimizes the energy consumption, needed to maintain the posture, and a proprioceptive one, which acts on the maintenance of automatic and induced over time walking adaptations.[5,9]
-
-
Synergy Viss (Human Tecar Unibell srl, Calco, Italy) (Fig. 1B) last 15 minutes to 3 times a week. It is a device able to produce air waves with a pressure up to 250 mbar, and of a flow modulator which shapes air vibrate with a pressure up to 630 mbar and a frequency up to 980 Hz (a frequency within 300 Hz is recommended) generating mechano-acoustic sinusoidal like a square waves.[10–12]
-
-
The virtual reality system Riablo system (Corehab Trento, Italy) (Fig. 1C–D) last 20 minutes and including 5 training exercises, 2 times a week. It is based on the use of inertial sensors connected to a screen which in real time sent the exact awareness of the carried out motor task to the patient (http://www.corehab.it/).[13]
Figure 1.
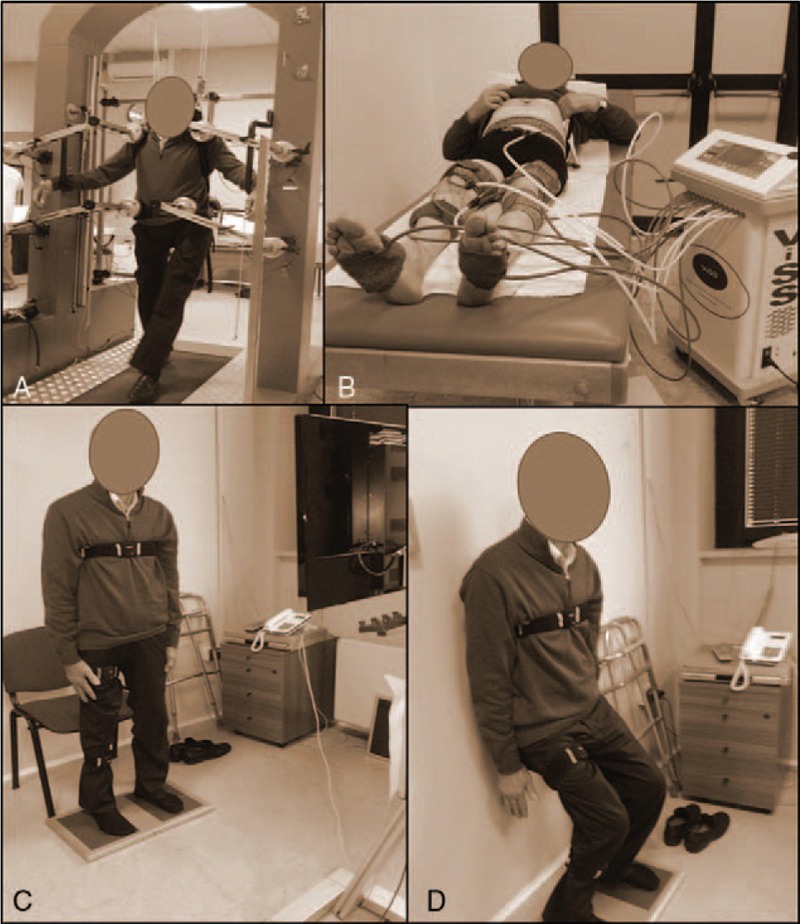
Representative images describing the rehabilitation protocol. In the panel A is shown exercise with SPAD; in the panel B is shown exercise with ViSS; in the panels C–D is shown exercise with Riablo.
2.2.2. Data assessment
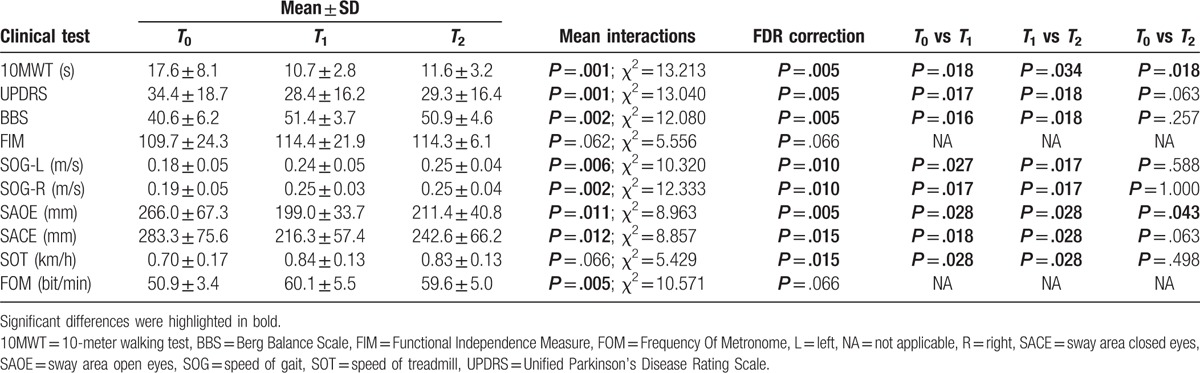
The effects of the rehabilitative protocols on each patient performance were clinically evaluated before (T0), after the 8 weeks of rehabilitative protocols (T1) and after 3 months (T2). The Berg Balance Scale (BBS) was administered to measure balance and risk of falls.[14] The Unified Parkinson's Disease Rating Scale (UPDRS) was used for monitor global disability and impairment.[15] The 10-m walking test (10 MWT), measuring the time required to walk 10 m, was utilized to evaluate walking speed and gait function. [16] Functional Independence Scale (FIM) was used to assess motor and cognitive progress during rehabilitation protocol.[17] Gait analysis (speed of gait for left and right food) was applied as predictor of improvement of the gait performance and dynamic balance.[18] Stabilometry was used for an objective balance assessment with open and closed eyes.[19] Treadmill speed and metronome frequency were collected by SPAD and they were used as indexes of the improved motor performance, gait cycle, and postural stability.
2.3. MR protocol
2.3.1. MR data acquisition
Each participant underwent 2 separate MR sessions, which, in turn, was performed at T0 and T1. MR data were acquired with a Philips Achieva 3 tesla scanner (Philips Medical System, Best, The Netherlands) equipped with 8-channel receiver coil. For each patient, 3-dimensional T1-weighted images were acquired by using Turbo Field-Echo sequence (TFE; TR/TE = 11/5 ms, slice thickness of 0.8 mm). Two 1H-MRS voxels of 2.0 × 1.5 × 1.5 cm3 were accurately placed on right and left striatum, respectively (Fig. 2). Point-resolved spectroscopy (PRESS) sequences (TR/TE = 2000/39 ms 16-step phase-cycle and an averages for 128 scan) with and without water suppression were performed by using chemically shift selective (CHESS) pulses. A total of 1024 points were acquired with a spectral width of 2000 Hz.
Figure 2.
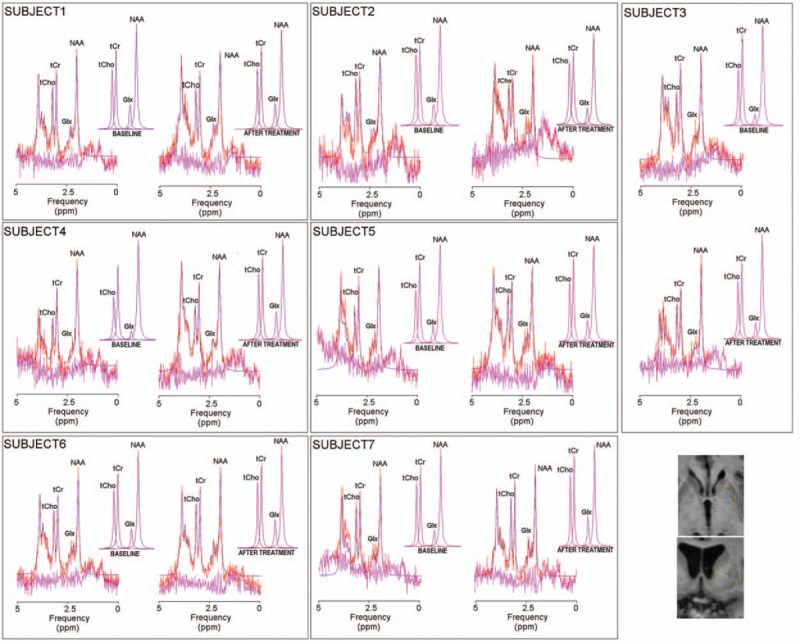
Proton Magnetic Resonance Spectroscopy (1H-MRS). Spectra in the left striatum, at baseline (T0) and after rehabilitation (T1) are shown for each one of the 7 subjects. JMRUI estimated signals (red), were reported on original signals (violet). Residue was shown in fuchsia. At the bottom, right corner, a representative image reports the positioning of a voxel of 2.0 × 1.5 × 1.5 mm3 on left striatum using T1-weighted image as anatomical reference. Abbreviations: Glx = glutamate + glutamine (2.35 ppm), tCho = total choline (3.22 ppm), tCr = total creatine (3.03 ppm).
2.3.2. 1H-MRS analysis
1H-MRS data were processed by using JMRUI version 4.0.[20] In spectra with water suppression, residual water was removed using the Hankel Lanczos Singular Values Decomposition (HLSVD) algorithm. 1H-MRS data were preprocessed by applying autophasing, baseline, and frequency shifts correction. A priori knowledge database (N-acetil-aspartate = NAA, 2.02 ppm; glutamate + glutamine, Glx = 2.35 ppm; total creatine = tCr, 3.03 ppm; total choline = tCho, 3.22 ppm) was generated to set constraints on the Advanced Magnetic Resonance (AMARES) fitting algorithm within jMRUI package. Peak shifts were confined to ±5 ppm of the theoretical location. For applicability to the clinical practice and because several 1H-MRS studies demonstrated stable tCr levels in neurodegenerative disorders,[21,22] we normalized the metabolites of interest relatively to tCr. However, we performed absolute quantification of tCr to corroborate that it was effectively unchanged among groups. In depth, using spectra without water suppression, we determined the area of the water peak and utilized it as an internal standard reference for tCr quantification.[22–27]
2.4. Statistics
The demographic, clinical, and imaging outcomes are reported as mean ± SD.
For clinical outcomes, nonparametric Friedman test was carried out to assess statistical differences among T0, T1, and T2. In case of significant mean interactions among groups, Wilcoxon post hoc tests were applied to investigated the difference between T0 and T1, between T0 and T2, between T1 and T2. For metabolites/tCr, nonparametric Wilcoxon test was used to investigate the significant differences between T0 and T1.
Spearman's correlations were used to assess the relationship between age, deltaT0–T1 for Glx/tCr and demographic/clinical outcomes. Specifically, the deltaT0–T1 expresses the variation of imaging and clinical values between T0 and T1.
3. Results
3.1. Rehabilitation protocol
Table 1 shows demographic and clinical data. Table 2 shows statistics on clinical outcomes. As compared with baseline, the UPDRS scores and the surface of the sway area with closed eyes were significantly reduced at T1. The BBS scores, the speed of gait (left and right), and the frequency of metronome were significantly increased at T1. Of note, no significant changes were observed between T1 and T2 for the UPDRS and BBS scores, speed of gait (left and right), frequency of metronome, and surface of the sway area with closed eyes. Otherwise, the 10-MWT (the time required to walk 10 m) and the surface of the sway area with open eyes were significantly increased between T1 and T2. No significant difference was found for FIM scores and SPAD treadmill speed among T0, T1, and T2.
Table 1.
Demographic and clinical characteristics.
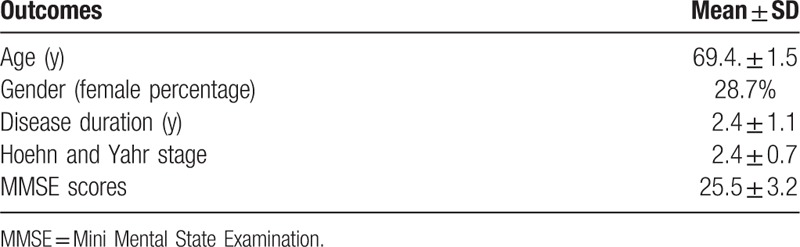
Table 2.
Rehabilitation protocol results.
3.2. 1H-MRS
Table 3 summarizes 1H-MRS results. The spectra of each subject at T0 and T1 were reported in Figure 2. At T1, greater Glx/tCr was observed in the left striatum of PD patients as compared with T0 (Table 3). Conversely, the NAA/tCr, tCho/tCr, and tCr/water in the left and right striata were unchanged between T0 and T1 (Suppl. Table 1).
Table 3.
1H-MRS results.

No significant correlation was found between age, the deltaT0–T1 relative to Glx/tCr, NAA/tCr, tCho/tCr, and clinical outcomes (Suppl. Fig. 1).
4. Discussion
In this pilot study we found that a rehabilitation program based on sensorimotor recovery improves the static and dynamic balance in PD patients, promoting a better global motor performance. Moreover, the 1H-MRS findings revealed the occurrence of neurochemical changes within basal ganglia after treatment, suggesting that the processes of motor recovery could be mediated by glutamate and glutamine.
The use of this specific protocol is based on previous evidence showing that training treadmill with SPAD improves gait, leading to a reduction of the variability of kinetic and cinematic characteristics of the step[5]. Furthermore, it reduces freezing and risk of falls, at least partly ascribed to alterations of sensory perception and proprioceptive integration and the consequent abnormality of internal body representation.[28–33]
SPAD allows to correct the gait and to correct the asymmetry of the 2 hemisomata, leading to a more physiological gait, which is critical in PD patients. In the present study, the advantages shown by SPAD were amplified by the use of the metronome during the training session, which combines the effects of proprioceptive blocks and the visual cue given by the mirror to improve the executive, visuospatial, and attention functions.
Our rehabilitation protocol allows improving automatic and cognitive process and enhances attentional strategies facilitating integration of proprioceptive, visual, and vestibular information more efficiently through the cerebellum, which then would influence the brain stem and spinal cord to improve motor control processes.[34] Furthermore, the oscillations produced by Synergy Viss are able to activate proprioceptors (mainly Pacinian corpuscles and muscle spindles) and to activate afferent nerve fibers, inducing a long-term potentiation not only on the segmental levels, but also on a central circuits.[35,36] Steyvers et al[37] have demonstrated that muscle vibration leads to an increased neuronal cortical excitability supported by the study of motor evoked potentials.
The results suggest that multisensory training determines an improvement of the kinetic and cinematic characteristics of gait. Furthermore, data relating to the velocity of treadmill and frequency of metronome reflect the improvement of sensorimotor integration. The balance improvement is evident at scores of BBS and to stabilometry at T1. Probably the significant reduction in sway area with open eyes (T1 and T2) is expression of improvement in the overall balance related to improvement of attentional and integration of visual input. The relationship of BWST and muscle tone modulation with Synergy Viss had a positive impact on general motor performance of patients detectable to significant improvement of data collected with 10 MWT both at T1 and T2. The improvements obtained are persistent in the immediate short term (weeks) after the end of the rehabilitation protocol probably in relation to motor re-learning processes. Therefore, it can be assumed that the rehabilitation process is to be repeated over time to consolidate the improvement obtained.
By using 1H-MRS, we found that the Glx/tCr content within the striatum in PD patients was significantly increased after treatment as compared with baseline. Supplementary analyses demonstrated that NAA/tCr, tCho/tCr, and tCr/water remained stable. In addition, no correlation was found between the levels of Glx/tCr and NAA/Cr and between the levels of Glx/tCr and NAA/Cr. The NAA and Cho express neuronal/axonal integrity and cellular membrane breakdown, respectively.[21] Therefore, the Glx change is independent of tCr concentration and it is not significantly associated to neurodegeneration processes or excitotoxicity.
The Glx complex expresses the content of glutamate and glutamine. The glutamate is the main excitatory neurotransmitter in the brain and it is largely involved in neuroplasticity and synaptic strengthening.[38] Interestingly, it was shown that exercise protects from toxin-induced striatal dopamine depletion and substantia nigra (pars compacta) degeneration in animal models.[39,40] The striatal dopamine depletion induces hyperexcitability of the indirect pathway that, in turn, is triggered by alterations of the glutamatergic receptor expression and by the neurotransmitter release.[41] On animal PD models, it was shown that an intensive exercise restores the glutamate receptor expression[42] and increases the glutamate storage and release in presynaptic terminals, improving the circuit function and diminishing the increased inhibitory drive of the dopamine-depleted striatum (which are cause of motor impairment).[43,44] Recently, it was also suggested that physical exercise improves the brain functions by increasing the neuron activity and the expression of neurotrophic factors including brain-derived neurotrophic factor (BDNF) and glial cell-line-derived neurotrophic factor (GDNF).[45] The rise of this neurotrophic factors seem to play a key role on neuron survival and on plasticity in the striatum.[46] In this context, recent evidences hypothesized that the BDNF could increase the glutamate pre-synaptic release and the glutamate receptors expression in the striatum.[46–48] Furthermore, it was observed that the increase of the glutamate release after exercise induces a long-term potentiation (LTP), enhancing the motor skill learning.[48,49] Thus, through this mechanism, the exercise could restore the synapse balance within the injured striatum improving the motor learning and behavior in PD patients. Furthermore, recent evidences suggested that the exercise might improve the homeostasis within motor circuitry by improving the synaptic strength which results from increased dopamine and glutamate content within the basal ganglia and from augmented dendritic spine formation.[39] Therefore, we suggest that the higher glutamate content in the striatum after rehabilitation could also contribute to LTP generation, improving the motor performance through learning.
Considering also the glutamine contribute in the Glx signals, we highlight that glutamine is involved in energy production throughout the body[50] and in the neurotransmission by modulating the recycling of extracellular glutamate.[51] Furthermore, previous animal studies showed that task-specific rehabilitative exercises induce neuroplasticity of cortico-striatal circuits with an increase of amount of dopamine D2 receptors, number of synaptic connections, diminished in PD, and increase of glutamine.[52]
Therefore, we suppose that motor performance improvement in PD patients after this rehabilitation protocols could be explained by cortical motor relearning phenomena that result in an increased glutamatergic stimulation on striatal with an improvement of cortico-striatal circuits essential for motor control.
This study has some limitations: the sample size is small and the control data to check the effect of drug alone are missing. Particularly, even though every patient underwent MRI protocol before the assumption of dopaminomimetic drugs to avoid the contamination of MRS spectra by acute drug assumption and the dosages of dopaminomimetics were kept stable during the course of the study, based on our data, we cannot exclude that the chronic pharmacological treatment could be a confounding factor when interpreting the MRS results. Anyway, our findings are supported by compelling evidence from literature reporting no changes of Glx within the putamen during drug-off or drug-on condition in patients with PD.[53]
In conclusion, we posit that our sensorimotor rehabilitation protocol could stimulate the glutamate metabolism in basal ganglia and, in turn, neuroplasticity processes. We also hypothesized that these mechanisms could prepare the ground to restore the functional interaction among brain areas deputed to motor controls, which are affected in PD. In this context, functional magnetic resonance imaging (fMRI) studies reported reduced functional connectivity between putamen and cortical areas including supplementary and motor areas which were linked to motor deficits in PD.[54] However, interesting evidences on idiopathic PD patients reported a significant increase of striatum resting-state functional connectivity after automatic mechanical peripheral stimulation.[55] Further investigations combining 1H-MRS and fMRI will be crucial to better clarify whether/how the glutamate striatal changes after rehabilitation influences the restoring of motor circuit in PD.
Supplementary Material
Footnotes
Abbreviations: 1H-MRS = proton magnetic resonance spectroscopy, AMARES = advanced magnetic resonance, BBS = berg balance scale, CHESS = chemically shift selective, FIM = Functional Independence Scale, fMRI = functional magnetic resonance imaging, Glx = glutamate+glutamine, HLSVD = Hankel Lanczos Singular Values Decomposition, MWT = 10-m walking test, NAA = N-Acetylaspartic acid, PD = Parkinson's disease, SPAD = dynamic antigravity postural system, tCho = total choline, tCr = total creatine, TE = echo time, TFE = Turbo Field-Echo, TR = repetition time, UPDRS = unified Parkinson's disease rating scale.
LB and RS equally contributed to this work.
The work has been supported by the Italian Ministry of Health (grant no. GR-2010-2313418).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Magrinelli F, Picelli A, Tocco P, et al. Pathophysiology of motor dysfunction in Parkinson's disease as the rationale for drug treatment and rehabilitation. Parkinsons Dis 2016;2016: 9832839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mak MK, Pang MY, Mok V. Gait difficulty, postural instability, and muscle weakness are associated with fear of falling in people with Parkinson's disease. Parkinsons Dis 2012;2012: 901721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Varanese S, Birnbaum Z, Rossi R, et al. Treatment of advanced Parkinson's disease. Parkinsons Dis 2011;2010: 480260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seppi K, Weintraub D, Coelho M, et al. The movement disorder society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson's disease. Mov Disord 2011;26(Suppl. 3):S42–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Di Pancrazio L, Bellomo RG, Franciotti R, et al. Combined rehabilitation program for postural instability in progressive supranuclear palsy. NeuroRehabilitation 2013;32:855–60. [DOI] [PubMed] [Google Scholar]
- [6].Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philos Trans R Soc Lond B Biol Sci 2003;358:829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Suzuki M, Nelson AD, Eickstaedt JB, et al. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci 2006;24:645–53. [DOI] [PubMed] [Google Scholar]
- [8].Modrego PJ, Fayed N, Artal J, et al. Correlation of findings in advanced MRI techniques with global severity scales in patients with Parkinson disease. Acad Radiol 2011;18:235–41. [DOI] [PubMed] [Google Scholar]
- [9].Saggini R, Cancelli F, Di Bonaventura V, et al. Efficacy of two micro-gravitational protocols to treat chronic low back pain associated with discal lesions: a randomized controlled trial. Eura Medicophys 2004;40:311–6. [PubMed] [Google Scholar]
- [10].Pietrangelo T, Mancinelli R, Toniolo L, et al. Effects of local vibrations on skeletal muscle trophism in elderly people: mechanical, cellular, and molecular events. Int J Mol Med 2009;24:503–12. [DOI] [PubMed] [Google Scholar]
- [11].Iodice P, Bellomo RG, Gialluca G, et al. Acute and cumulative effects of focused high-frequency vibrations on the endocrine system and muscle strength. Eur J Appl Physiol 2011;111:897–904. [DOI] [PubMed] [Google Scholar]
- [12].Iodice P, Bellomo RG, Migliorini M, et al. Flexible flatfoot treatment in children with mechanical sound vibration therapy. Int J Immunopathol Pharmacol 2012;25:9S–15S. [DOI] [PubMed] [Google Scholar]
- [13].Giggins OM, Persson UM, Caulfield B. Biofeedback in rehabilitation. J Neuroeng Rehabil 2013;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Qutubuddin AA, Pegg PO, Cifu DX, et al. Validating the Berg Balance Scale for patients with Parkinson's disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil 2005;86:789–92. [DOI] [PubMed] [Google Scholar]
- [15].Fahn SER. Unified Parkinson's Disease Rating Scale. Florham Park, NJ: Macmillan Healthcare; 1987. [Google Scholar]
- [16].Combs SA, Diehl MD, Filip J, et al. Short-distance walking speed tests in people with Parkinson disease: reliability, responsiveness, and validity. Gait Posture 2014;39:784–8. [DOI] [PubMed] [Google Scholar]
- [17].Dickson HG, Kohler F. Interrater reliability of the 7-level functional independence measure (FIM). Scand J Rehabil Med 1995;27:253–6. [PubMed] [Google Scholar]
- [18].Farley BG, Koshland GF. Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson's disease. Exp Brain Res 2005;167:462–7. [DOI] [PubMed] [Google Scholar]
- [19].Nardone A, Schieppati M. Balance in Parkinson's disease under static and dynamic conditions. Mov Disord 2006;21:1515–20. [DOI] [PubMed] [Google Scholar]
- [20].Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. Magma 2001;12:141–52. [DOI] [PubMed] [Google Scholar]
- [21].Kantarci K. Proton MRS in mild cognitive impairment. J Magn Reson Imaging 2013;37:770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Delli Pizzi S, Franciotti R, Taylor JP, et al. Thalamic involvement in fluctuating cognition in dementia with lewy bodies: magnetic resonance evidences. Cereb Cortex 2015;25:3682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Christiansen P, Henriksen O, Stubgaard M, et al. In vivo quantification of brain metabolites by 1H-MRS using water as an internal standard. Magn Reson Imaging 1993;11:107–18. [DOI] [PubMed] [Google Scholar]
- [24].Delli Pizzi S, Madonna R, Caulo M, et al. MR angiography, MR imaging and proton MR spectroscopy in-vivo assessment of skeletal muscle ischemia in diabetic rats. PLoS One 2012;7:e44752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Delli Pizzi S, Rossi C, Di Matteo V, et al. Morphological and metabolic changes in the nigro-striatal pathway of synthetic proteasome inhibitor (PSI)-treated rats: a MRI and MRS study. PLoS One 2013;8:e56501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Delli Pizzi S, Padulo C, Brancucci A, et al. GABA content within the ventromedial prefrontal cortex is related to trait anxiety. Soc Cogn Affect Neurosci 2016;11:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Padulo C, Delli Pizzi S, Bonanni L, et al. GABA levels in the ventromedial prefrontal cortex during the viewing of appetitive and disgusting food images. Neuroscience 2016;333:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord 2003;18:231–40. [DOI] [PubMed] [Google Scholar]
- [29].Pohl M, Rockstroh G, Ruckriem S, et al. Immediate effects of speed-dependent treadmill training on gait parameters in early Parkinson's disease. Arch Phys Med Rehabil 2003;84:1760–6. [DOI] [PubMed] [Google Scholar]
- [30].Frenkel-Toledo S, Giladi N, Peretz C, et al. Treadmill walking as an external pacemaker to improve gait rhythm and stability in Parkinson's disease. Mov Disord 2005;20:1109–14. [DOI] [PubMed] [Google Scholar]
- [31].Herman T, Giladi N, Gruendlinger L, et al. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson's disease: a pilot study. Arch Phys Med Rehabil 2007;88:1154–8. [DOI] [PubMed] [Google Scholar]
- [32].Miyai I, Fujimoto Y, Ueda Y, et al. Treadmill training with body weight support: its effect on Parkinson's disease. Arch Phys Med Rehabil 2000;81:849–52. [DOI] [PubMed] [Google Scholar]
- [33].Protas EJ, Mitchell K, Williams A, et al. Gait and step training to reduce falls in Parkinson's disease. NeuroRehabilitation 2005;20:183–90. [PubMed] [Google Scholar]
- [34].Yen CY, Lin KH, Hu MH, et al. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther 2011;91:862–74. [DOI] [PubMed] [Google Scholar]
- [35].Murillo N, Valls-Sole J, Vidal J, et al. Focal vibration in neurorehabilitation. Eur J Phys Rehabil Med 2014;50:231–42. [PubMed] [Google Scholar]
- [36].Saggini R, Bellomo RG. Integration to focal vibration in neurorehabilitation. Eur J Phys Rehabil Med 2015;51:508. [PubMed] [Google Scholar]
- [37].Steyvers M, Levin O, Van Baelen M, et al. Corticospinal excitability changes following prolonged muscle tendon vibration. Neuroreport 2003;14:1901–5. [DOI] [PubMed] [Google Scholar]
- [38].McEntee WJ, Crook TH. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology 1993;111:391–401. [DOI] [PubMed] [Google Scholar]
- [39].Petzinger GM, Fisher BE, McEwen S, et al. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol 2013;12:716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Petzinger GM, Holschneider DP, Fisher BE, et al. The effects of exercise on dopamine neurotransmission in Parkinson's disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast 2015;1:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Calabresi P, Picconi B, Tozzi A, et al. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 2007;30:211–9. [DOI] [PubMed] [Google Scholar]
- [42].VanLeeuwen JE, Petzinger GM, Walsh JP, et al. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci Res 2010;88:650–68. [DOI] [PubMed] [Google Scholar]
- [43].Calabresi P, Pisani A, Centonze D, et al. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev 1997;21:519–23. [DOI] [PubMed] [Google Scholar]
- [44].Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron 2008;60:543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 2009;5:311–22. [DOI] [PubMed] [Google Scholar]
- [46].Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002;25:295–301. [DOI] [PubMed] [Google Scholar]
- [47].Carvalho AL, Caldeira MV, Santos SD, et al. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol 2008;153(Suppl. 1):S310–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chang HC, Yang YR, Wang SG, et al. Effects of treadmill training on motor performance and extracellular glutamate level in striatum in rats with or without transient middle cerebral artery occlusion. Behav Brain Res 2009;205:450–5. [DOI] [PubMed] [Google Scholar]
- [49].Richter-Levin G, Canevari L, Bliss TV. Long-term potentiation and glutamate release in the dentate gyrus: links to spatial learning. Behav Brain Res 1995;66:37–40. [DOI] [PubMed] [Google Scholar]
- [50].Aledo JC. Glutamine breakdown in rapidly dividing cells: waste or investment? Bioessays 2004;26:778–85. [DOI] [PubMed] [Google Scholar]
- [51].Shen J. Modeling the glutamate-glutamine neurotransmitter cycle. Front Neuroenergetics 2013;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Petzinger GM, Fisher BE, Van Leeuwen JE, et al. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Mov Disord 2010;25(Suppl. 1):S141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mazuel L, Chassain C, Jean B, et al. Proton MR spectroscopy for diagnosis and evaluation of treatment efficacy in Parkinson disease. Radiology 2016;278:505–13. [DOI] [PubMed] [Google Scholar]
- [54].Barbic F, Galli M, Dalla Vecchia L, et al. Effects of mechanical stimulation of the feet on gait and cardiovascular autonomic control in Parkinson's disease. J Appl Physiol 2014;116:495–503. [DOI] [PubMed] [Google Scholar]
- [55].Quattrocchi CC, de Pandis MF, Piervincenzi C, et al. Acute modulation of brain connectivity in Parkinson disease after automatic mechanical peripheral stimulation: a pilot study. PLoS One 2015;10:e0137977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


