Abstract
Background
Colorectal cancers are the third most common cancers in women and men in the US. While dietary and lifestyle factors such as Western diet, physical inactivity, and obesity, have been linked to an increased risk of this malignancy, the mechanisms for these associations are unclear. Gastrointestinal hormones, including ghrelin, are involved in energy balance by mediating appetite and metabolism; however, the association between ghrelin and colorectal cancer has not been studied.
Methods
We conducted a case-control study nested within the all-male Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study of Finnish smokers (aged 50–69 years) to examine serum ghrelin concentration and colorectal cancer risk. Data from 284 colon and 239 rectal cancers and 523 controls (matched on age, date of blood draw and serum availability) were analyzed. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using multivariable (conditional) logistic regression.
Results
Overall, low serum ghrelin was significantly associated with increased risk of colorectal cancer (Q1 vs Q4: OR:1.57, 95% CI: 1.05, 2.34). For individuals developing tumors within 10 years of blood draw, those in the lowest quartile of serum ghrelin concentrations were statistically significantly more likely to develop colorectal cancers than those with higher serum ghrelin concentrations (OR: 10.86, 95% CI: 5.01, 23.55). However, for individuals with tumors developing more than 20 years after blood draw, low serum ghrelin concentrations were associated with a decreased risk of colorectal cancer relative to those with the highest serum ghrelin concentrations (OR: 0.26, 95% CI: 0.11, 0.64).
Conclusion
Low serum ghrelin was associated with an increased colorectal cancer risk within 10 years of blood draw with a decreased risk for developing colorectal cancer more than 20 years after blood draw. These results suggest that ghrelin concentrations may vary across the carcinogenic process.
Keywords: ghrelin, colon cancer, rectal cancer, gastrointestinal hormones
Introduction
Worldwide, colorectal cancers are the third most common incident cancers in men and the second most common in women; 694,000 people died of this cancer in 2012, making it the fourth most common cause of cancer death1. Dietary and lifestyle risk factors, such as Western diet, physical inactivity, and obesity, have been linked to an increased risk of colorectal cancer 2–4; the mechanisms behind these associations are unclear, but metabolic syndrome, insulin resistance, adipoctyokines and other inflammatory agents have been investigated 5.
Less is known about how gastrointestinal hormones may influence, or be influenced by, carcinogenesis in the gastrointestinal tract. Ghrelin is a hormone produced in the stomach. Previous work by our group and others has shown that low serum ghrelin, measured years before diagnosis, is associated with substantially increased risk of oesophageal cancer (both squamous cell carcinoma and adenocarcinoma), oesophagogastric junctional adenocarcinoma, and non-cardia gastric adenocarcinoma 6–8. Ghrelin is known to have a variety of metabolic functions which range from stimulation of gastric acid and regulation of gastrointestinal tract motility to control of appetite 9. Ghrelin may also modulate long-term energy balance, mediating fatty acid metabolism and promoting fat storage 9. Conditions such as chronic inflammation, insulin resistance and diabetes, all associated with an increased risk of colorectal cancer, alter serum ghrelin levels 9,10, which may further promote inflammation and carcinogenesis within the lower gastrointestinal tract.
Though serum ghrelin has been investigated for its role in cachexia among patients with colorectal cancer 11, it is not yet known whether serum ghrelin levels are associated with the development of colorectal cancer. We therefore investigated the association between serum ghrelin concentration and subsequent risk of colorectal cancer in a case-control study nested within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study.
Methods
Subjects were drawn from the ATBC Study, a randomized, double-blind placebo-controlled, primary prevention trial designed to determine whether daily supplementation with alpha-tocopherol (50mg/day), beta-carotene (20mg/day), or both, would reduce the incidence of lung and other cancers in male smokers 12. Between 1985 and 1988, 29,133 eligible Finnish male smokers between 50 and 69 years old were recruited to the ATBC Study. The trial ended in 1993; however, participants continue to be followed as a cohort. This study was approved by the institutional review boards of both the National Institutes of Health in the United States and the National Public Health Institutes in Finland. All participants provided written informed consent.
All case subjects had incident colon or rectal adenocarcinoma (ICD9 codes 153.0–153.4, 153.6–153.9, 154.0–154.3, diagnosed through 12/31/2011) defined according to the International Classification of Diseases, 9th Revision. We selected only first incident primary CRC cases (n=529). For patients diagnosed through April 30th 1999 two study physicians reviewed medical records for diagnostic confirmation and staging, and one pathologist reviewed histopathological or cytological specimens when available. Information on colorectal cancer patients diagnosed since May 1999 was derived solely from the Finnish Cancer Registry, which provides almost 100% tumor ascertainment for the ATBC cohort 13. Controls had to be alive and cancer-free at the time of case diagnosis and had available serum insulin and glucose data. Using incidence density sampling, controls were matched to cases on age at randomization (± 1 year), date of blood draw (± 30 days) and serum sample availability.
Data Collection
Participants completed questionnaires at baseline regarding general risk factors, medical history, and dietary intake. Dietary intake was assessed using a food frequency questionnaire, asking about usual food consumption over the previous year, including 276 common foods and mixed dishes, using a picture booklet to aid estimation of portion size 14,15. The food frequency questionnaire was satisfactorily completed by 27,110 participants (93%) at study entry. The weight and height of all participants was measured by trained study staff. Fasting (overnight) blood samples were collected from participants at baseline (1985–1988) and serum samples were stored at −70°C.
Laboratory Analysis
284 cases of incident colon cancer had adequate serum samples for ghrelin analysis, as did 239 cases of rectal cancer cases and 523 controls. Total ghrelin was measured by radioimmunoassay using reagents obtained from Millipore Linco Research (St. Charles, MO). This assay uses an antibody that is specific for total ghrelin, and 0.1 ml of serum in a 2-day disequilibrium assay. Using 96 blinded quality control samples (6 per batch) from a single serum pool from the ATBC Study, the coefficient of variation across all batches for these assays was calculated as 23%.
Statistical Analysis
Statistical analyses were performed using STATA version 10.1 (Stata Corp., College Station, TX) and all p-values were two-sided. The distributions of baseline characteristics across cancer cases relative to controls were compared using Student’s t tests for continuous variables and Pearson’s χ2 tests for categorical variables. The associations between baseline characteristics and serum ghrelin quartile were determined using a non-parametric Wilcoxon-type test for trend for continuous variables and the Mantel-Haenszel trend test for categorical variables.
Odds ratios (OR) and 95% confidence intervals (95% CI) for associations between serum ghrelin concentration and risk of colorectal cancers, as well as colon and rectum subsites, were calculated using unconditional logistic regression models. Estimates derived from conditional and unconditional logistic regression models were similar, and as such, only the results of the unconditional models, which offered more precise risk estimates, are presented within this manuscript. When we modeled ghrelin as a continuous variable, the estimates were scaled to 176 pg/mL (½ the interquartile range observed in controls: (Q3-Q1)/2). Preliminary analysis included a squared term for ghrelin, however, the fit of this model was equivalent to that of the model with no squared term. Categorical analyses examined the data in quartiles (of serum ghrelin levels in the controls), using the fourth quartile (highest values) as the referent group. Multivariable models of risk were adjusted for: age at randomization, total years of smoking, total cigarettes/day, alcohol (g/day), body mass index (BMI; kg/m2), intake of fruit (g/day) and vegetables (g/day), total dietary fiber (g/day), education (post-primary school), consumption of red meat (g/day) and processed meat (g/day), diabetes (either self-reported history of diabetes or blood glucose ≥ 126 mg/dL), analgesic drug use daily >1 month (yes/no), and ATBC treatment group assignment (alpha-tocopherol, beta-carotene, both or neither). Total caloric intake was included in preliminary models, but did not significantly alter estimates and was therefore omitted in favor of a more parsimonious model.
Ptrend were calculated by performing a nonparametric test for trend (across ghrelin quartiles for each baseline characteristic)16. Stratified analysis examined associations by BMI category (BMI < or ≥ 25 kg/m2) and smoking (</≥ median total cigarettes/day and </≥ median total years of smoking). Lag analysis was also performed: cancer cases were classified according to whether they occurred within 10 years of baseline, between 10 and 20 years of baseline, or more than 20 years after baseline. The food frequency questionnaire was completed satisfactorily by 93% of study participants. Participants who were missing dietary data were included in the analyses with an indicator variable for missing.
Results
Baseline characteristics of the cases and controls are shown in Table 1. Colon and rectal cancer cases were more likely to have a blood glucose level above 126 mg/dL. Colon cancer cases tended to consume more alcohol than controls. Rectal cancer cases were less likely to report a history of diabetes than controls. There were no other differences in baseline characteristics between cases and controls. Baseline characteristics were also examined by serum ghrelin concentration among controls only; this revealed that ghrelin was marginally associated with fruit intake (Table 2) but not with any other characteristics.
Table 1.
Descriptive characteristics of colorectal cancer cases and controls in a nested case-control study within the ATBC Study.
| Variable | Controls | Colorectal cancer | P value1 | Colon cancer | P value1 | Rectal cancer | P value1 |
|---|---|---|---|---|---|---|---|
| Total N | 523 | 523 | 284 | 239 | |||
| Age at randomization, years, Mean (SD) | 57 (5) | 57 (5) | 0.98 | 57 (5) | 0.73 | 57 (5) | 0.72 |
| Education: post-elementary school, N (%) | 84 (16) | 93 (18) | 0.46 | 50 (18) | 0.57 | 43 (18) | 0.51 |
| Years of smoking, Mean (SD) | 35 (9) | 35 (8) | 0.20 | 36 (8) | 0.09 | 35 (8) | 0.76 |
| Cigarettes/day, Mean (SD) | 19 (9) | 20 (9) | 0.68 | 20 (9) | 0.72 | 20 (9) | 0.75 |
| Physically active, N (%)2 | 113 (22) | 116 (22) | 0.81 | 58 (20) | 0.71 | 58 (24) | 0.41 |
| BMI (Kg/m2), Mean (SD) | 26 (4) | 27 (4) | 0.28 | 27 (4) | 0.08 | 26 (4) | 0.89 |
| Self-reported history of diabetes, N (%) | 17 (3) | 14 (3) | 0.58 | 13 (5) | 0.34 | 1 (0.5) | 0.02 |
| Blood glucose ≥ 126 mg/dL, N (%) | 1 (0.2) | 13 (3) | 0.001 | 6 (2) | 0.005 | 7 (3) | 0.001 |
| Ever daily use of analgesics, N (%)3 | 66 (15) | 51 (13) | 0.33 | 25 (12) | 0.25 | 26 (14) | 0.70 |
| Alcohol, g/day, Mean (SD) | 16 (20) | 19 (21) | 0.03 | 20 (22) | 0.01 | 18 (19) | 0.26 |
| Red meat, g/day, Mean (SD) | 71 (33) | 74 (34) | 0.15 | 74 (35) | 0.28 | 74 (34) | 0.18 |
| Processed meat, g/day, Mean (SD) | 81 (66) | 77 (58) | 0.31 | 74 (57) | 0.14 | 81 (60) | 0.93 |
| Fruit, g/day, Mean (SD) | 228 (218) | 221 (193) | 0.62 | 221 (194) | 0.70 | 221(193) | 0.68 |
| Vegetables, g/day, Mean (SD) | 310 (123) | 303 (112) | 0.39 | 298 (111) | 0.19 | 310 (113) | 0.99 |
| Fiber, g/day, Mean (SD) | 19 (10) | 18 (10) | 0.09 | 18 (10) | 0.07 | 19 (10) | 0.35 |
| Total energy (kcal/day), Mean (SD) | 2729 (746) | 2691 (711) | 0.41 | 2677 (717) | 0.35 | 2708 (706) | 0.71 |
| Serum ghrelin (pg/ml), Mean (SD) | 794 (351) | 761 (385) | 0.15 | 753 (360) | 0.11 | 771 (414) | 0.43 |
P values from Student’s t test and Pearson’s χ2 test, as appropriate, comparing cases to controls.
Physically active: Light or moderate physical activity in leisure time.
Have you ever used any prescribed or over-the-counter analgesic drugs daily for at least a month?
Table 2.
Baseline characteristics (mean or % distribution) by quartiles of serum ghrelin concentration in the 523 controls from the ATBC Study.
| Characteristics | Q1 (< 576 pg/ml) | Q2 (577–713 pg/ml) | Q3 (714–925 pg/ml) | Q4 (> 925 pg/ml) | Ptrend1 |
|---|---|---|---|---|---|
| N | 130 | 131 | 131 | 131 | |
| Age at baseline | 57 | 57 | 57 | 56 | 0.30 |
| Education: post-elementary school, N (%)2 | 18 (21) | 17 (20) | 30 (36) | 19 (23) | 0.41 |
| Years of smoking | 35 | 35 | 34 | 36 | 0.33 |
| Cigarettes/day | 18 | 20 | 18 | 21 | 0.10 |
| Physically active, N (%)2 | 28 (25) | 24 (21) | 38 (34) | 23 (20) | 0.94 |
| BMI, kg/m2 | 26 | 27 | 26 | 26 | 0.31 |
| Self-reported history of diabetes, N (%)2 | 4 (24) | 6 (35) | 5 (29) | 2 (12) | 0.43 |
| Blood glucose ≥ 126 mg/dL, N (%)2 | 0 | 1 | 0 | 0 | 0.65 |
| Ever daily use of analgesics, N (%)2 | 16 (24) | 13 (20) | 18 (27) | 19 (29) | 0.45 |
| Alcohol intake, g/day | 15 | 18 | 14 | 17 | 0.35 |
| Red meat, g/day | 74 | 68 | 71 | 70 | 0.62 |
| Processed meat, g/day | 82 | 83 | 72 | 89 | 0.74 |
| Fruit intake, g/day | 255 | 230 | 219 | 208 | 0.04 |
| Vegetables, g/day | 322 | 307 | 317 | 295 | 0.11 |
| Fiber intake g/day | 20 | 19 | 19 | 20 | 0.65 |
| Total energy intake (kcal) | 2758 | 2718 | 2653 | 2791 | 0.59 |
Mantel-Haenszel trend test for categorical variables and a non-parametric Wilcoxon-type test for trend for continuous variables 16
Percentages are row percents
Lower baseline serum ghrelin (as a continuous variable scaled to 176 pg/mL, ½ the inter-quartile range of serum ghrelin in the controls) was not statistically significantly associated with an increased risk of either colon or rectal cancer (Table 3).
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs; conditional logistic regression) for the association between serum ghrelin concentration and colorectal cancer in the ATBC Study.
| Serum ghrelin: continuous1 | Serum ghrelin: quartiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Q1 | Q2 | Q3 | Q4 | |||||||
| OR | 95% CI | Pvalue | OR | 95% CI | OR | 95% CI | OR | 95% CI | Ptrend | ||
| Colorectal cancer (n=523) | |||||||||||
| Case number | n = 185 | n =110 | n =103 | n =125 | |||||||
| Crude | 1.05 | 0.99, 1.12 | 0.11 | 1.65 | 1.15, 2.34 | 0.95 | 0.66, 1.35 | 0.86 | 0.61, 1.21 | REF | 0.01 |
| Multivariable2 | 1.04 | 0.97, 1.11 | 0.29 | 1.57 | 1.05, 2.34 | 0.92 | 0.62, 1.34 | 0.84 | 0.58, 1.20 | REF | 0.03 |
| Colon cancer (n=284) | |||||||||||
| Case number | n = 103 | n = 58 | n =54 | n = 69 | |||||||
| Crude | 1.04 | 0.95, 1.14 | 0.40 | 1.56 | 0.96, 2.54 | 0.80 | 0.50, 1.28 | 0.81 | 0.50, 1.30 | REF | 0.13 |
| Multivariable2 | 1.00 | 0.91, 1.11 | 0.93 | 1.37 | 0.78, 2.40 | 0.66 | 0.39, 1.14 | 0.73 | 0.43, 1.22 | REF | 0.38 |
| Rectal cancer (n=239) | |||||||||||
| Case number | n = 82 | n = 52 | n = 49 | n = 56 | |||||||
| Crude | 1.08 | 0.97, 1.18 | 0.15 | 1.82 | 1.05, 3.15 | 1.18 | 0.68, 2.05 | 0.94 | 0.56, 1.57 | REF | 0.02 |
| Multivariable2 | 1.08 | 0.97, 1.20 | 0.15 | 2.14 | 1.13, 4.03 | 1.27 | 0.70, 2.30 | 0.98 | 0.57, 1.70 | REF | 0.01 |
Continuous ORs are scaled to 176 pg/mL, which is one half of the inter-quartile range in the controls (75th percentile – 25th percentile ÷ 2) and interpreted as the change per unit decrease in serum ghrelin.
Adjusted ORs and 95% CIs were calculated using models which included: education (post-primary school), total years of smoking, total cigarettes/day, BMI (kg/m2), diabetes (either self-reported history of diabetes or blood glucose ≥ 126 mg/dL), daily analgesic use for >1 month (yes/no), alcohol (g/day), red meat consumption (g/day), processed meat consumption (g/day), fruit intake (g/day), vegetable intake (g/day), total dietary fiber (g/day), and ATBC treatment group assignment
However, modeling the data using categorical variables and using the highest quartile as the referent group, the risk of colorectal cancer increased significantly from the highest to the lowest quartile of serum ghrelin. Following multivariable adjustment (which had little effect on our estimates), those in the lowest quartile of serum ghrelin had a statistically significant increase in risk of colorectal cancer relative to those in the highest quartile of serum ghrelin (ORQ1 vs Q4: 1.57; 95% CI: 1.05, 2.34; Ptrend=0.03). This association was significant for rectal cancer (OR Q1 vs Q4: 2.14; 95% CI: 1.13, 4.03, Ptrend=0.01), but not for colon cancer (OR Q1 vs Q4: 1.37; 95% CI: 0.78, 2.40, Ptrend=0.38).
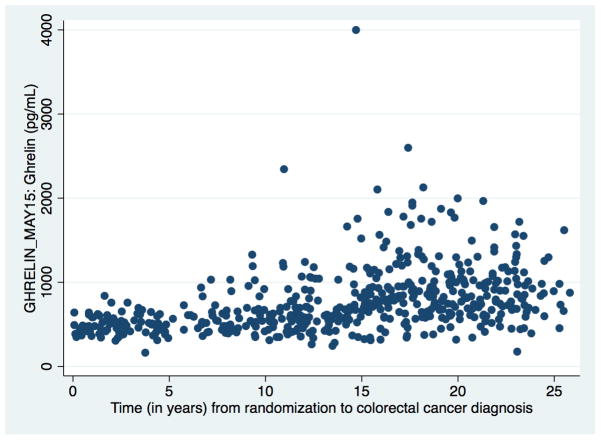
We then undertook lag analyses to assess the association between serum ghrelin levels and colorectal cancer across more than 20 years of follow-up within the ATBC Study (Table 4). Analyses were stratified according to whether tumors developed within 10 years of blood draw, between 10 and 20 years, or more than 20 years after blood draw. Surprisingly we observed substantial differences in the associations between low serum ghrelin and cancers occurring in each time period. Mean serum ghrelin concentrations in colorectal cancer cases varied significantly over time from 541 pg/mL (SD: 172 pg/mL) for tumors developing with in 10 years of blood draw to 824 pg/mL (SD: 439 pg/mL) for tumors developing between 10 and 20 years later and 896 pg/mL (SD: 334 pg/mL) for those developing more than 20 years later (Figure 1: serum ghrelin concentration over time in colorectal cancer cases). Low serum ghrelin (Q1 vs >Q1) was associated with a 21-fold increase in risk of colon cancer (OR: 21.00, 95% CI: 5.08, 86.75) and a 7-fold increase in risk of rectal cancer (OR: 6.80; 95% CI: 2.65, 17.39) occurring within ten years of blood draw (CRC: 10.86; 95% CI: 5.01, 23.55). For cancers occurring between 10 and 20 years of blood draw we found no statistically significant associations between serum ghrelin concentrations and risk of either colon or rectal cancer. However, low serum ghrelin was associated with a statistically significant decrease in risk of both colon (OR: 0.33, 95% CI: 0.11, 1.03) and rectal (OR: 0.18, 95% CI: 0.04, 0.82) cancers occurring more than 20 years after blood draw (CRC:0.26; 95% CI: 0.11, 0.64). We observe consistent associations when examining time between blood draw and diagnosis in two-year intervals; the majority of cases with a shorter length of follow-up were more likely to be in the lowest quartile of serum ghrelin (Table 5). The lag analysis was repeated, this time excluding those cases which developed within 2 years of baseline (n=35) and association remained (CRC: OR: 9.17; 95% CI: 3.96, 21.29). Stratified analyses for BMI (< or ≥ 25 kg/m2) and smoking (< or ≥ median for total cigarettes/day and total years smoking), did not significantly alter risk estimates (data not shown).
Table 4.
Odds ratios (ORs) and 95% confidence intervals (CIs; unadjusted conditional logistic regression) for the association between low (Q1: < 576 pg/ml) or continuous serum ghrelin concentration and colorectal cancer in the ATBC Study by number of years between blood draw and diagnosis.
| Number of years from blood collection to cancer | ||||||
|---|---|---|---|---|---|---|
| < 10 years | 10–20 years | ≥20 years | ||||
| Cases | OR (95% CI)* | Cases | OR (95% CI)* | Cases | OR (95% CI)* | |
| Serum ghrelin < 576 pg/ml (Referent category: ≥ 576 pg/ml) | ||||||
| Colorectal cancer | 97 | 10.86 (5.01, 23.55) | 74 | 1.44 (0.62, 3.38) | 14 | 0.26 (0.11, 0.64) |
| Colon cancer | 57 | 21.00 (5.08, 86.75) | 37 | 1.67 (0.40, 6.97) | 9 | 0.33 (0.11, 1.03) |
| Rectal cancer | 40 | 6.80 (2.65, 17. 39) | 37 | 1.33 (0.46, 3.84) | 5 | 0.18 (0.04, 0.82) |
| Serum ghrelin: continuous1 | ||||||
| Colorectal cancer | 146 | 3.13 (2.17, 4.55) | 262 | 0.94 (0.81, 1.10) | 115 | 0.83 (0.70, 0.98) |
| Colon cancer | 84 | 3.85 (2.13, 7.14) | 143 | 1.00 (0.79, 1.27) | 57 | 0.81 (0.64, 1.02) |
| Rectal cancer | 62 | 2.56 (1.64, 4.17) | 119 | 0.90 (0.73, 1.12) | 58 | 0.85 (0.65, 1.10) |
Continuous ORs are scaled to 176 pg/mL, which is one half of the inter-quartile range in the controls (75th percentile – 25th percentile ÷ 2) and interpreted as the change per unit decrease in serum ghrelin.
Figure 1.
Baseline serum ghrelin concentration in colorectal cancer cases by years of ATBC follow-up accrued before diagnosis.
Table 5.
Time between blood draw and tumor diagnosis among colorectal cancer by quartile of serum ghrelin concentration at blood draw.
| Number of years from blood draw to diagnosis: case numbers (%) | Serum Ghrelin Quartile | Total cases | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Controls | 130 | 131 | 131 | 131 | |
| Cases | |||||
| <2 | 25 (71) | 9 (26) | 1 (3) | 0 | 35 |
| 2–4 | 24 (77) | 5 (16) | 2 (6) | 0 | 31 |
| 4–6 | 18 (82) | 3 (14) | 1 (5) | 0 | 22 |
| 6–8 | 14 (52) | 9 (33) | 2 (7) | 2 (7) | 27 |
| 8–10 | 16 (52) | 7 (23) | 3 (10) | 5 (16) | 31 |
| 10–12 | 27 (57) | 12 (26) | 4 (9) | 4 (9) | 47 |
| 12–14 | 19 (43) | 14 (32) | 4 (9) | 7 (16) | 44 |
| 14–16 | 10 (18) | 11 (20) | 19 (34) | 16 (29) | 56 |
| 16–18 | 10 (18) | 4 (7) | 20 (35) | 23 (40) | 57 |
| 18–20 | 8 (14) | 13 (22) | 15 (26) | 22 (38) | 58 |
| 20–22 | 6 (11) | 12 (21) | 15(27) | 23 (41) | 56 |
| 22–24 | 7 (16) | 8 (18) | 12 (27) | 18 (40) | 45 |
| 24–26 | 1 (7) | 3 (21) | 5 (36) | 5 (36) | 14 |
| 26–28 | 0 | 0 | 0 | 0 | 0 |
| 523 | |||||
Discussion
In this nested case-control study within the ATBC Study cohort of male smokers aged between 50 and 69 years old, we report a statistically significant increase in risk of developing colorectal cancer for individuals with lower levels of serum ghrelin at baseline. This statistically significant association was confined to cancers which occurred earlier (within 10 years of blood draw) in the study, conversely there was a statistically significant positive association for colorectal cancers occurring later in the study (>20 years from blood draw). These results suggest that the association between ghrelin concentrations and colorectal cancer risk may vary across the carcinogenic process.
We can find no prospective studies of ghrelin and colorectal cancer in the literature; in fact we have identified just 4 previous studies of ghrelin and any gastrointestinal cancers. A study nested within a multiphasic health check-up cohort in the Kaiser Permanente Medical Care Program (California) analyzed serum ghrelin concentrations among oesophageal adenocarcinoma cases and matched controls and found a five-fold increase in risk of oesophageal adenocarcinoma for those in the lowest quartile of serum ghrelin 8. In a previous prospective analysis from the ATBC Study cohort, we reported a five-fold increase in risk of gastric non-cardia adenocarcinoma and oesophagogastric junction adenocarcinoma among individuals in the lowest quartile of serum ghrelin, compared to those in the highest 7. Further, we identified close to a seven-fold increase in risk of developing oesophageal squamous cell carcinoma, again in the ATBC Study, for those individuals in the lowest relative to the highest quartile of serum ghrelin 6. In this previous study, the baseline serum ghrelin concentrations in controls were almost identical in range to those seen here. Lastly, a cross-sectional case-control study of gastric cancer (non-cardia and cardia), oesophageal adenocarcinoma and oesophageal squamous cell carcinoma within the Aras Gastrointestinal Health Survey (Iran)17, also reported a statistically significant inverse relationship between serum ghrelin and gastric cancer (both non-cardia and cardia) and oesophageal squamous cell carcinoma, although no significant association was found with oesophageal adenocarcinoma. Together these reports indicate that low serum ghrelin levels are significantly associated with risk of all four major upper gastrointestinal cancers: non-cardia gastric adenocarcinoma, gastric cardia/oesophagogastric junctional adenocarcinoma, oesophageal adenocarcinoma and oesophageal squamous cell carcinoma, despite differing etiologic and risk factor profiles across these four sites. Our current analysis suggests that ghrelin may have a more nuanced role in the etiology of lower gastrointestinal cancers relative to upper gastrointestinal cancers.
Surprisingly, however, we observed a complicated association between ghrelin and colorectal cancer. Whereas low ghrelin was associated with an increased risk among cancers occurring within 10 years of blood draw, it was associated with a decreased risk in cancers occurring more than 20 years after blood draw. Although such associations are intriguing, they should be interpreted cautiously: not only are they a surprise, but ours is the first study to investigate the association and it could be a chance finding. Each of our previous investigations (oesophageal squamous cell carcinoma, gastric cardia- and non-cardia adenocarcinoma) had outlined a very consistent inverse association for each time period. However, we do note that there is prior evidence of ghrelin having both a pro- and anti- proliferative effect in tissue culture studies based on varied cell lines and varying conditions 18. It seems possible that ghrelin might act differently, in terms of inflammation and proliferation, depending on the stage of tissue carcinogenesis: normal tissue, adenoma, or tumor. It is also possible that the metabolic repercussions of low serum ghrelin may contribute to a pro-carcinogenic environment during cancer initiation while the pro-inflammatory effect of low serum ghrelin may enhance subsequent tumor development. Low ghrelin may also be the result of reverse causation, if the tumor is somehow inhibiting the secretion of ghrelin as a result of altered hormonal signals as a consequence of changing histology.
The prospective design of our study is one of its major strengths because it not only allowed the use of baseline prediagnostic serum, obtained following an overnight fast, for analysis of ghrelin, but it also provides more than two decades of follow-up to assess cancer development. The ATBC Study also includes substantial covariate information that allowed for adjustment for potential confounders. Additionally, Finland has an excellent population-wide cancer registry so that loss-to-follow-up is minimal; similarly, migration within this cohort is also minimal. Our study also had limitations. The ATBC Study only recruited male smokers, and as such, the generalizability of our findings may be limited, but in this study, we found that smoking (either intensity or duration) did not alter the observed associations (data not shown). Clinical characteristics such as tumor stage and grade were unavailable for the majority of colorectal cancer cases in our study. Also, we measured total ghrelin, but there are two isoforms of ghrelin: acylated ghrelin, which binds to the growth hormone secretagogue receptor 1a, and des-acylated ghrelin. In the stomach, des-acylated ghrelin is present in levels that are three times higher than acylated ghrelin. Des-acyl ghrelin was initially thought to be biologically inactive, but recent evidence suggests that this is not the case 19. Further studies are needed to determine the significance of acylated and des-acylated ghrelin in the context of gastrointestinal cancer disease. The sub-group analyses of colon and rectal tumors by lag-time were underpowered and should be interpreted with caution, although results were similar for both sites, future studies are needed to replicate these findings. Lastly, we measured ghrelin at one time-point only, and our results emphasize the need for measurement at multiple time-points over very long periods of time, which we hope may be addressed in future studies. We note that a previous study in (obese) children found that fasting serum ghrelin concentrations were remarkably consistent when measured one year apart20.
In this prospective investigation, we found that low serum ghrelin concentrations were associated with a statistically significant increase in risk of both colon and rectal cancer for tumors developing within 10 years of blood draw, and a statistically significant decrease in risk of cancer for those developing more than 20 years after blood draw. These intriguing results suggest that ghrelin concentrations may vary across the carcinogenic process and that in addition to an etiologic role, ghrelin may serve as a marker of altered gastrointestinal physiology.
Summary Box.
The gastrointestinal tract is the largest endocrine organ in the body, though studies exploring the association between gastrointestinal hormones and gastrointestinal carcinogenesis are limited
Ghrelin is a hormone produced in the stomach which is known to have a variety of metabolic functions which range from stimulation of gastric acid and regulation of motility, to control of appetite. Ghrelin concentrations might be altered in conditions of chronic inflammation
Low serum ghrelin concentrations have been associated with an increased risk of development of esophageal and gastric cancers in prospective studies
Individuals with low serum ghrelin concentrations had a 10-fold increase in risk of colorectal cancers for tumors developing within 10 years of blood draw
Conversely, for individuals with tumors developing more than 20 years later, low serum ghrelin concentration was associated with a significantly decreased risk of colorectal cancer
Ghrelin concentrations may vary across the carcinogenic process, and this variation may have clinical utility. Further studies are required to replicate these findings and explore the association between ghrelin across the natural history of colorectal cancer.
Abbreviations
- ATBC Study
Alpha-Tocopherol, Beta-Carotene Cancer Prevention
- BMI
body mass index
- CI
confidence intervals
- OR
odds ratio
Footnotes
Disclosure of potential conflicts of interest: None to declare.
Author Contribution
Gwen Murphy: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis; study supervision
Amanda J. Cross, Neal D. Freedman: analysis and interpretation of data; critical revision of the manuscript for important intellectual content; study supervision
Sanford M. Dawsey, Farin Kamangar, Christian C. Abnet: analysis and interpretation of data; critical revision of the manuscript for important intellectual content;
Stephanie J. Weinstein, Philip R. Taylor, Satu Männistö, Demetrius Albanes: acquisition of data; critical revision of the manuscript for important intellectual content; material support;
Frank Z. Stanczyk: critical revision of the manuscript for important intellectual content; technical support;
References
- 1.Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. Journal of the National Cancer Institute. 2005;97(22):1679–87. doi: 10.1093/jnci/dji375. 97/22/1679 [pii] [published Online First: 2005/11/17] [DOI] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 4.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–60. e16. doi: 10.1053/j.gastro.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62(6):933–47. doi: 10.1136/gutjnl-2013-304701. [published Online First: 2013/03/14] [DOI] [PubMed] [Google Scholar]
- 6.Murphy G, Kamangar F, Albanes D, et al. Serum ghrelin is inversely associated with risk of subsequent oesophageal squamous cell carcinoma. Gut. 2012;61(11):1533–7. doi: 10.1136/gutjnl-2011-300653. [published Online First: 2011/12/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy G, Kamangar F, Dawsey SM, et al. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. Journal of the National Cancer Institute. 2011;103(14):1123–9. doi: 10.1093/jnci/djr194. [published Online First: 2011/06/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Martel C, Haggerty TD, Corley DA, et al. Serum ghrelin levels and risk of subsequent adenocarcinoma of the esophagus. Am J Gastroenterol. 2007;102(6):1166–72. doi: 10.1111/j.1572-0241.2007.01116.x. AJG1116 [pii] [published Online First: 2007/03/24] [DOI] [PubMed] [Google Scholar]
- 9.Korbonits M, Goldstone AP, Gueorguiev M, et al. Ghrelin--a hormone with multiple functions. Front Neuroendocrinol. 2004;25(1):27–68. doi: 10.1016/j.yfrne.2004.03.002. S0091302204000032 [pii] [published Online First: 2004/06/09] [DOI] [PubMed] [Google Scholar]
- 10.Higgins SC, Gueorguiev M, Korbonits M. Ghrelin, the peripheral hunger hormone. Ann Med. 2007;39(2):116–36. doi: 10.1080/07853890601149179. 777131636 [pii] [published Online First: 2007/04/25] [DOI] [PubMed] [Google Scholar]
- 11.Huang Q, Fan YZ, Ge BJ, et al. Circulating ghrelin in patients with gastric or colorectal cancer. Dig Dis Sci. 2007;52(3):803–9. doi: 10.1007/s10620-006-9508-3. [published Online First: 2007/01/25] [DOI] [PubMed] [Google Scholar]
- 12.The ATBC cancer prevention study group. The alpha-tocopherol, beta-carotene lung cancer prevention study: Design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 13.Korhonen P, Malila N, Pukkala E, et al. The Finnish Cancer Registry as follow-up source of a large trial cohort--accuracy and delay. Acta Oncol. 2002;41(4):381–8. doi: 10.1080/028418602760169442. [DOI] [PubMed] [Google Scholar]
- 14.Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments. II. A qualitative food frequency questionnaire. Am J Epidemiol. 1988;128(3):667–76. doi: 10.1093/oxfordjournals.aje.a115014. [published Online First: 1988/09/01] [DOI] [PubMed] [Google Scholar]
- 15.Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128(3):655–66. doi: 10.1093/oxfordjournals.aje.a115013. [published Online First: 1988/09/01] [DOI] [PubMed] [Google Scholar]
- 16.Cuzick J. A Wilcoxon-type test for trend. Statistics in medicine. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [published Online First: 1985/01/01] [DOI] [PubMed] [Google Scholar]
- 17.Sadjadi A, Yazdanbod A, Lee YY, et al. Serum ghrelin; a new surrogate marker of gastric mucosal alterations in upper gastrointestinal carcinogenesis. PLoS One. 2013;8(9):e74440. doi: 10.1371/journal.pone.0074440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu JT, Kral JG. Ghrelin: integrative neuroendocrine peptide in health and disease. Annals of surgery. 2004;239(4):464–74. doi: 10.1097/01.sla.0000118561.54919.61. [published Online First: 2004/03/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baatar D, Patel K, Taub DD. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol. 2011;340(1):44–58. doi: 10.1016/j.mce.2011.04.019. S0303-7207(11)00227-9 [pii] [published Online First: 2011/05/14] [DOI] [PubMed] [Google Scholar]
- 20.Reinehr T, Roth CL, Alexy U, et al. Ghrelin levels before and after reduction of overweight due to a low-fat high-carbohydrate diet in obese children and adolescents. Int J Obes (Lond) 2005;29(4):362–8. doi: 10.1038/sj.ijo.0802913. [DOI] [PubMed] [Google Scholar]