Significance
Adipose tissue dysfunction such as impaired insulin action in adipocytes is causally implicated in obesity-related metabolic disorders. However, molecular mechanisms underlying obesity-related adipocyte insulin resistance remain unclear. Here we identify a mechanism in the regulation of adipocyte insulin signaling by Fam13a, which is impaired in obesity. We revealed that Fam13a protects IRS1 from proteasomal degradation by recruiting PP2A and consequently accentuates adipocyte insulin signal cascade. We further demonstrated that genetic loss of Fam13a exacerbated obesity-related metabolic disorders, while targeted activation of Fam13a in adipocytes improved it by altering adipose tissue insulin sensitivity. Our data unveiled a previously unknown mechanism underlying obesity-related adipocyte insulin resistance through Fam13a, highlighting Fam13a as a vital player in the pathogenesis of obesity-related metabolic disorders.
Keywords: adipocyte, insulin signaling, IRS1, metabolic disorder, obesity
Abstract
Adipose tissue dysfunction is causally implicated in the impaired metabolic homeostasis associated with obesity; however, detailed mechanisms underlying dysregulated adipocyte functions in obesity remain to be elucidated. Here we searched for genes that provide a previously unknown mechanism in adipocyte metabolic functions and identified family with sequence similarity 13, member A (Fam13a) as a factor that modifies insulin signal cascade in adipocytes. Fam13a was highly expressed in adipose tissue, predominantly in mature adipocytes, and its expression was substantially reduced in adipose tissues of obese compared with lean mice. We revealed that Fam13a accentuated insulin signaling by recruiting protein phosphatase 2A with insulin receptor substrate 1 (IRS1), leading to protection of IRS1 from proteasomal degradation. We further demonstrated that genetic loss of Fam13a exacerbated obesity-related metabolic disorders, while targeted activation of Fam13a in adipocytes ameliorated it in association with altered adipose tissue insulin sensitivity in mice. Our data unveiled a previously unknown mechanism in the regulation of adipocyte insulin signaling by Fam13a and identified its significant role in systemic metabolic homeostasis, shedding light on Fam13a as a pharmacotherapeutic target to treat obesity-related metabolic disorders.
The prevalence of obesity is increasing worldwide (1). Obesity is causally implicated in metabolic disease such as type 2 diabetes and is strongly associated with other pathological conditions including hypertension, hyperlipidemia, and atherosclerosis (2, 3). Therefore, obesity is an established risk of cardiovascular disease, making it a major global health issue. Adipose tissue expands under obese conditions, and pathological adipocyte hypertrophy leads to increased production of fatty acids via lipolysis as well as inflammatory adipokines (4, 5). Fatty acids activate inflammatory signaling in adipocytes and macrophages via Toll-like receptor 4, leading to chronic adipose tissue inflammation in obesity (6). In addition, excessive adipocyte expansion causes imbalanced vascularization and consequent hypoxia, which also triggers inflammation in adipose tissue (7, 8). Chronic inflammation in adipose tissue is closely associated with obesity-related metabolic disorders at least in part through inducing adipocyte insulin resistance (4, 5). Insulin is a major anabolic hormone in the body and tightly regulates energy metabolism through activation of skeletal muscle glucose uptake, inhibition of hepatic gluconeogenesis, and inhibition of adipocyte lipolysis (9). It has been shown that enhancing insulin signaling in adipocytes is sufficient to improve systemic metabolic homeostasis (10); therefore, adipocyte insulin resistance is a promising target in the treatment and prevention of obesity-related metabolic disorders. However, the detailed mechanisms underlying the obesity-mediated defect in insulin action in adipocytes remain unclear.
Here, we identified that family with sequence similarity 13, member A (Fam13a) is a previously unknown player in the insulin signal cascade. Fam13a was highly expressed in healthy adipocytes, and its expression was substantially reduced in the adipose tissue of obese mice, whereas Fam13a preserves insulin signaling in adipocytes by enhancing insulin receptor substrate 1 (IRS1) expression. Our data suggest that down-regulation of Fam13a in obesity plays a key role in a pathological linkage between obesity and adipocyte insulin resistance.
Results
Identification of Fam13a as a Candidate Gene Involved in Obesity-Related Adipocyte Dysfunction.
We performed DNA microarray analysis using RNAs isolated from the white adipose tissue (WAT) of lean and dietary-induced obese mice, aiming to identify genes that could provide previously unknown mechanisms in adipocyte dysfunction associated with obesity. We focused on genes with (i) high expression in the WAT of lean mice and (ii) reduced expression in the WAT of obese mice, because genes that fulfill these conditions are likely involved in adipocyte physiological functions, which collapse under obese conditions. Many genes that satisfy the criteria were implicated in energy metabolism or actin cytoskeleton (SI Appendix, Table S1). Among these genes, we found Fam13a, which has a highly conserved nucleotide sequence among various species but whose biological function remains to be elucidated (SI Appendix, Table S1). A previous study of gene expression profiling in visceral adipose tissue in high-fat diet (HFD)-induced obese mice also reported that Fam13a was one of top 10 down-regulated genes in obesity (11). Of note, the Fam13a gene has been identified as one of the loci associated with waist-to-hip ratio (WHR), which represents body fat distribution, in women (12). Fam13a expression was substantially reduced in the WAT of obese mice fed a HFD for 14 wk compared with that in lean mice, while 4 wk of HFD feeding still did not affect Fam13a expression in WAT (Fig. 1 A and B and SI Appendix, Fig. S1A). Furthermore, we found that Fam13a was highly and preferentially expressed in adipose tissues (Fig. 1C). Fam13a was predominantly expressed in the mature adipocytes of WAT as well as in differentiated 3T3-L1 adipocytes (Fig. 1 D–G). The gene expression patterns of Fam13a were strikingly different from those of its isoforms Fam13b and Fam13c (SI Appendix, Fig. S1 B and C). Treatment with thapsigargin or hydrogen peroxide significantly reduced Fam13a expression in 3T3-L1 adipocytes, suggesting that endoplasmic reticulum and/or oxidative stress are causally involved in the reduction of Fam13a in WAT in obesity (Fig. 1 H and I). Additionally, we found that Fam13a was also highly expressed in human WAT (SI Appendix, Fig. S1D).
Fig. 1.
Fam13a is highly and preferentially expressed in adipocytes. (A) Quantitative real-time PCR of Fam13a expression in WAT isolated from lean mice fed NC or obese mice fed a HFD for 14 wk (n = 7 for NC; n = 6 for HFD). (B) Immunoblotting for Fam13a and GAPDH in WAT of lean mice fed NC or obese mice fed a HFD for 14 wk. (C) Quantitative real-time PCR of Fam13a expression in various mouse tissues (n = 3 each). (D) Quantitative real-time PCR of Fam13a expression in stromal vascular fraction (SVF) or mature adipocyte isolated from WAT (n = 8 each). (E) Quantitative real-time PCR of Fam13a expression in various types of cells (n = 3 each). 3T3-L1 adipocytes, mature adipocytes differentiated from 3T3-L1 preadipocytes; AEC, mouse aortic endothelial cells; B16, mouse melanoma cell line; C166, mouse endothelial cell line; C2C12, mouse myoblast cell line; EOMA, mouse endothelial cell line; NIH 3T3, mouse fibroblast cell line; RAW, mouse macrophage cell line. (F) Quantitative real-time PCR of Fam13a expression in 3T3-L1 preadipocytes at the indicated time after induction of adipocyte differentiation (n = 3 each). (G) Immunoblotting for Fam13a, FABP4, and GAPDH in 3T3-L1 preadipocytes at the indicated time after induction of adipocyte differentiation. (H) Quantitative real-time PCR of Fam13a expression in 3T3-L1 adipocytes treated with vehicle or thapsigargin (n = 4 each). (I) Immunoblotting for Fam13a in 3T3-L1 adipocytes treated with vehicle or hydrogen peroxide (H2O2) (n = 6 for vehicle; n = 5 for H2O2). Data represent mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001.
Fam13a Accelerates Insulin Signaling.
We then investigated a role of Fam13a in adipocyte functions. Gene silencing of Fam13a significantly reduced insulin-mediated Akt activation in 3T3-L1 adipocytes without affecting their maturity (Fig. 2A and SI Appendix, Fig. S2 A and B), while overexpression of Fam13a accentuated it in HEK293 cells (Fig. 2B). These results strongly suggest that Fam13a directly modulates insulin signaling in a cell-autonomous fashion. Since Fam13a contains two coiled-coil domains (CCDs) that are frequently involved in protein–protein interactions (SI Appendix, Fig. S1B), we investigated a potential association of Fam13a with signaling molecules involved in the insulin signal cascade. Accordingly, we identified that Fam13a was associated with IRS1 in 3T3-L1 adipocytes, while no significant association was detected for phosphatase and tensin homolog deleted on chromosome 10 or Akt (Fig. 2C and SI Appendix, Fig. S2C). To investigate whether CCDs play a role in the association between Fam13a and IRS1, we prepared expression constructs for CCD-deletion mutants of Fam13a. Fam13a associated with IRS1 in HEK293 cells, and deletion of CCD-2 or both CCD-1 and -2 abolished the binding of Fam13a to IRS1, indicating that CCD-2 is essential for the association of Fam13a with IRS1 (Fig. 2D). In contrast to IRS1, Fam13a did not bind to IRS2 (Fig. 2E). We further identified that treatment with NT157, an IRS inhibitor, abolished the enhanced insulin signaling induced by Fam13a, indicating that Fam13a modulates insulin signaling in an IRS-dependent manner (Fig. 2F).
Fig. 2.
Fam13a accelerates insulin signaling in adipocytes. (A) Immunoblotting for insulin signaling in 3T3-L1 adipocytes transfected with either negative control siRNA (Negative siRNA) or siRNA targeting Fam13a. Insulin-mediated Akt activation was quantified (n = 6 each). (B) Immunoblotting for insulin signaling in HEK293 cells transfected with either empty vector (MOCK) or the Fam13a expression construct (Fam13a-OE). Insulin-mediated Akt activation was quantified (n = 3 each). (C) Fam13a in 3T3-L1 adipocytes was immunoprecipitated, and coprecipitation of IRS1 was detected by immunoblotting. (D) FLAG-tagged Fam13a was immunoprecipitated in HEK293 cells transfected with the IRS1 expression construct and either empty vector (MOCK) or plasmid for Fam13a of whole-length or CCD-deletion mutants. Coprecipitation of IRS1 was detected by immunoblotting. (E) FLAG-tagged Fam13a was immunoprecipitated in HEK293 cells transfected with the IRS2 expression construct and empty vector (MOCK) or whole-length Fam13a. Coprecipitation of IRS2 was not detected. (F) HEK293 cells were transfected with either empty vector (MOCK) or the Fam13a expression construct (Fam13a-OE). Fam13a-overexpressing cells were cultured in the presence or absence of NT157. Cells were stimulated with insulin, and insulin signaling was analyzed by immunoblotting. Insulin-mediated Akt activation was quantified (n = 8 each). Data represent mean ± SEM. *P < 0.05, ***P < 0.001, ****P < 0.0001, #not significant.
Fam13a Recruits PP2A with IRS1 and Protects IRS1 from Proteasomal Degradation.
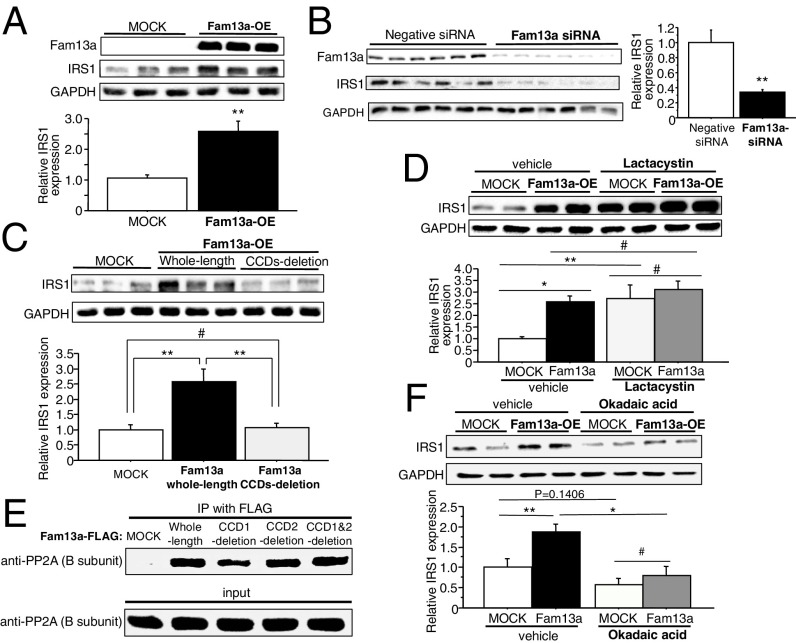
Notably, we found that overexpression of Fam13a increased IRS1 expression in HEK293 cells, whereas gene silencing of Fam13a reduced it in 3T3-L1 adipocytes (Fig. 3 A and B). CCD deletion abolished the Fam13a-mediated increase in IRS1 expression, suggesting that binding to IRS1 is essential for Fam13a to increase its expression (Fig. 3C). Consistent with no binding to IRS2, overexpression of Fam13a did not affect IRS2 expression in HEK293 cells (SI Appendix, Fig. S2D). IRS1 is known to undergo proteasomal degradation, which is triggered by serine/threonine phosphorylation (13, 14). Inhibition of proteasome by lactacystin increased IRS1 expression in cells transfected with empty vector and abrogated the enhanced IRS1 expression induced by Fam13a (Fig. 3D). These results suggest that Fam13a enhances IRS1 expression by inhibiting its proteasomal degradation.
Fig. 3.
Fam13a enhances IRS1 expression by recruiting PP2A and inhibiting the proteasomal degradation. (A) Immunoblotting for IRS1 in HEK293 cells transfected with the IRS1 expression construct and either empty vector (MOCK) or the Fam13a expression construct (Fam13a-OE). IRS1 expression was quantified (n = 6 each). (B) Immunoblotting for IRS1 in 3T3-L1 adipocytes transfected with either negative control siRNA (Negative siRNA) or siRNA targeting Fam13a. IRS1 expression was quantified (n = 6 each). (C) Immunoblotting for IRS1 in HEK293 cells transfected with the IRS1 expression construct and either empty vector (MOCK) or the expression construct for Fam13a of whole-length or CCD1–2-deletion mutant. IRS1 expression was quantified (n = 6 each). (D) Immunoblotting for IRS1 in HEK293 cells transfected with the IRS1 expression construct and either empty vector (MOCK) or the Fam13a expression construct (Fam13a-OE). Cells were treated with vehicle or the proteasome inhibitor lactacystin. IRS1 expression was quantified (n = 4 each). (E) FLAG-tagged Fam13a was immunoprecipitated in HEK293 cells transfected with the IRS1 expression construct and empty vector (MOCK) or plasmid for Fam13a of whole-length or CCD-deletion mutants. Coprecipitation of PP2A B subunit was detected by immunoblotting. The same blot as the one used in Fig. 2D was used. (F) Immunoblotting for IRS1 in HEK293 cells transfected with the IRS1 expression construct and either empty vector (MOCK) or the Fam13a expression construct (Fam13a-OE). Cells were treated with vehicle or the PP2A inhibitor okadaic acid. IRS1 expression was quantified (n = 6 each). Data represent mean ± SEM. *P < 0.05, **P < 0.01, #not significant.
It has been reported that protein phosphatase 2A (PP2A) protects IRS1 against excessive serine/threonine phosphorylation and proteasomal degradation (13, 15). On the other hand, Fam13a has been reported to associate with PP2A and modify Wnt/β-catenin signaling (16, 17); therefore, we explored the Fam13a association with PP2A and found that Fam13a bound to PP2A independently of its CCDs (Fig. 3E). Pharmacological inhibition of PP2A by okadaic acid reduced IRS1 expression, and notably, it abolished the Fam13a-mediated increase in IRS1 expression (Fig. 3F). These data collectively indicate that Fam13a recruits PP2A with IRS1, leading to the protection of IRS1 from proteasomal degradation, which consequently accentuates insulin signal cascade in adipocytes.
Genetic Loss of Fam13a Impairs Systemic Metabolic Health.
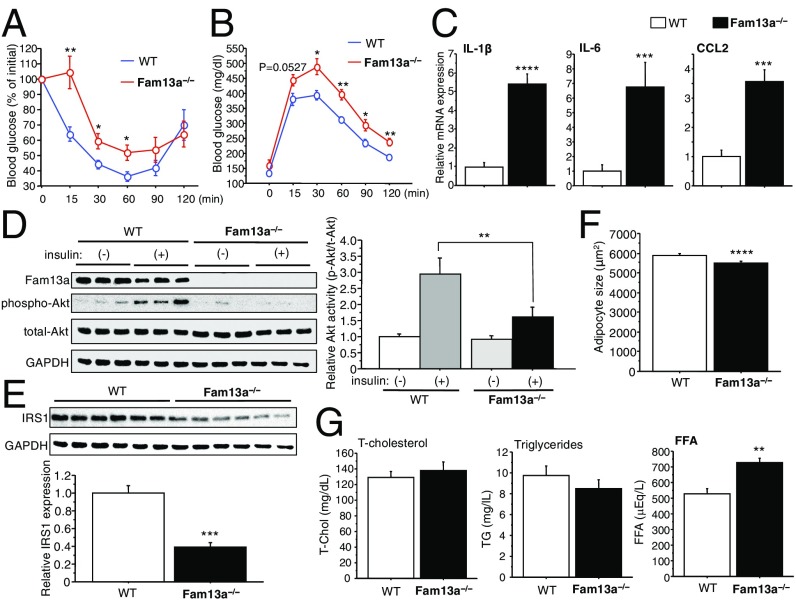
To analyze the role of Fam13a in systemic metabolic homeostasis, we generated mice with a targeted deletion of Fam13a (Fam13a−/−) (SI Appendix, Fig. S3A). Fam13a−/− mice showed body weight and adiposity similar to those in wild-type (WT) mice under normal dietary conditions (Fig. 4A and SI Appendix, Fig. S3B). Body fat distribution in normal chow (NC)-fed Fam13a−/− mice did not differ from that in WT mice in both males and females (Fig. 4 B and C). Nevertheless, modest but significant impairments in insulin sensitivity and glucose tolerance were detected in Fam13a−/− mice (Fig. 4 D and E). Expression of inflammatory cytokines in WAT was similar between WT and Fam13a−/−mice, suggesting that impaired insulin sensitivity in Fam13a−/− mice is not associated with WAT inflammation (Fig. 4F). Consistent with a role of Fam13a in IRS1 expression in vitro, loss of Fam13a caused reduction in IRS1 in WAT but not in skeletal muscle or liver in mice (Fig. 4G and SI Appendix, Fig. S3 C and D). These results suggest that Fam13a is crucially involved in the regulation of IRS1 expression in adipocytes and therefore modifies systemic metabolic health even under normal dietary condition.
Fig. 4.
Targeted deletion of Fam13a impairs metabolic health. (A) Body weight of male WT or Fam13a−/− mice fed NC at the indicated ages in weeks (n = 7 each). (B) Representative pictures for CT analysis were shown. (Scale bars, 10 mm.) (C) Body fat distribution in 17-wk-old male (n = 7 for WT; n = 5 for KO) and 20-wk-old female (n = 8 for WT; n = 9 for KO) mice. (D) ITT in 20-wk-old female WT and Fam13a−/− mice fed NC (n = 7 each). (E) IpGTT in 20-wk-old female WT and Fam13a−/− mice fed NC (n = 8 for WT; n = 9 for KO). (F) Quantitative real-time PCR for inflammatory genes in WAT of WT and Fam13a−/− mice fed NC (n = 8 each). (G) Immunoblotting for IRS1 in WAT of WT and Fam13a−/− mice fed NC (n = 7 for WT; n = 6 for KO). Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
When fed a HFD, body weight, adiposity, and fat distribution were similar between WT and Fam13a−/− mice in both males and females (SI Appendix, Fig. S4 A–C). Nevertheless, HFD-fed Fam13a−/− mice showed exacerbated insulin resistance and glucose intolerance compared with those in WT mice (Fig. 5 A and B and SI Appendix, Fig. S4D). Chronic inflammation in WAT of HFD-fed Fam13a−/− mice appeared to be deteriorated despite similar adiposity (Fig. 5C and SI Appendix, Fig. S4 E and F). Of note, insulin signaling in WAT was significantly impaired in association with reduced IRS1 expression in HFD-fed Fam13a−/− mice (Fig. 5 D and E). IRS1 expression in skeletal muscle and liver in Fam13a−/− mice did not differ from that in WT mice fed a HFD (SI Appendix, Fig. S4 G and H). Insulin potently inhibits lipolysis and promotes fat storage in WAT at least in part by decreasing expression of adipose triglyceride lipase (ATGL), a rate-limiting lipolytic enzyme that catalyzes the first step of fat breakdown (18–20). We found that adipocyte size was modestly reduced, while serum levels of free fatty acid (FFA) were higher in HFD-fed Fam13a−/− mice (Fig. 5 F and G). Serum FFA levels primarily depend on the rate of lipolysis in WAT (18); therefore, increased serum FFA in conjunction with a reduction in adipocyte size suggests enhanced lipolysis in WAT of HFD-fed Fam13a−/− mice. Consistently, HFD-fed Fam13a−/− mice showed higher ATGL expression in WAT than in WT mice fed a HFD (SI Appendix, Fig. S4I). Also, we analyzed the expression of G0/G1 switch gene-2 and alpha-beta hydrase domain 5, which regulates ATGL lipase activity. G0/G1 switch gene-2 expression did not change, while alpha-beta hydrase domain 5 expression was increased in WAT of HFD-fed Fam13a−/− mice compared with WT mice fed a HFD (SI Appendix, Fig. S4J). These results collectively suggest that loss of Fam13a impairs insulin signaling in WAT in association with IRS1 reduction, resulting in enhanced lipolysis, which leads to exacerbated adipose tissue inflammation, higher circulating FFA, and subsequent systemic metabolic disorders under high-fat dietary conditions.
Fig. 5.
Loss of Fam13a exacerbates obesity-related metabolic disorder. (A) ITT in male WT and Fam13a−/− mice fed a HFD for 12 wk (n = 7 each). (B) IpGTT in male WT or Fam13a−/− mice fed a HFD for 12 wk (n = 6 for WT; n = 5 for KO). (C) Quantitative real-time PCR for inflammatory genes in WAT of WT and Fam13a−/− mice fed a HFD for 14 wk (n = 5 each). (D) Immunoblotting for insulin signaling in WAT of WT and Fam13a−/− mice fed a HFD for 12 wk. Insulin was s.c. administered, and WAT was isolated 20 min after insulin injection. Insulin-mediated Akt activation was quantified [n = 8 for WT-insulin (−); n = 11 for WT-insulin (+); n = 7 for KO-insulin (−); n = 12 for KO-insulin (+)]. (E) Immunoblotting for IRS1 in WAT of WT and Fam13a−/− mice fed a HFD for 12 wk. IRS1 expression was quantified (n = 6 each). (F) Adipocyte size in WAT of WT and Fam13a−/− mice fed a HFD for 14 wk (n = 4,500 adipocytes each). (G) Serum lipid profiles in WT and Fam13a−/− mice fed a HFD for 14 wk (n = 6 each). Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Targeted Activation of Fam13a in Adipocytes Improves Systemic Metabolic Health.
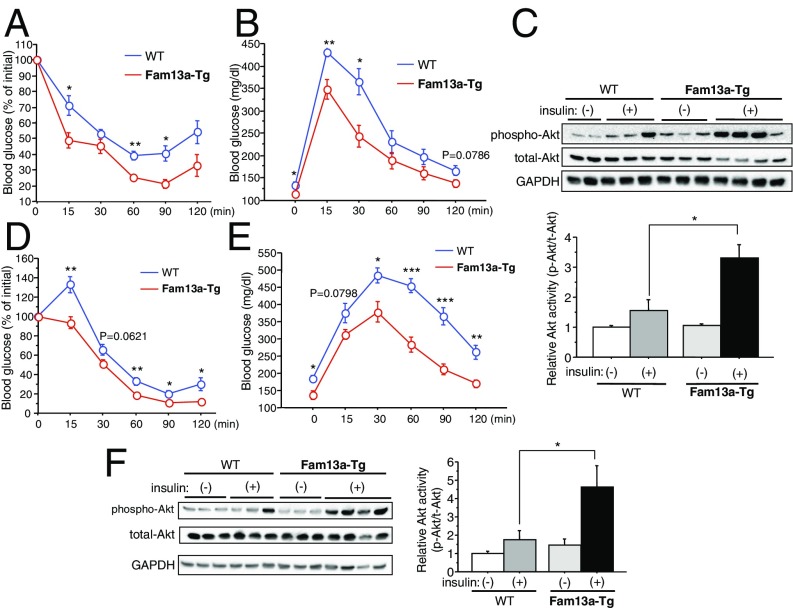
We then generated mice with a targeted activation of Fam13a in adipocytes (aP2-Fam13a-Tg) to further analyze the Fam13a function in adipocytes (SI Appendix, Fig. S5A). aP2-Fam13a-Tg mice showed higher insulin sensitivity and better glucose tolerance than WT mice despite similar body weight and body fat distribution even while on an NC diet (Fig. 6 A and B and SI Appendix, Fig. S5 B and C). Insulin signaling was accentuated in the WAT of aP2-Fam13a-Tg mice relative to WT mice fed NC (Fig. 6C). Expression of inflammatory cytokines, adiponectin, and leptin in WAT were not different between these mice (SI Appendix, Fig. S5 D and E). When fed a HFD, male aP2-Fam13a-Tg mice exhibited a slight increase in body weight and body fat ratio compared with WT mice, though it was not statistically significant (SI Appendix, Fig. S6 A and B). Body fat distribution was similar among HFD-fed WT and aP2-Fam13a-Tg mice in both males and females (SI Appendix, Fig. S6C). Expression of genes involved in thermogenesis and fatty acid oxidation in brown adipose tissue was not different between the mice (SI Appendix, Fig. S7), and there were no changes in food intake, body temperature, activity, respiratory exchange ratio, and energy expenditure between male WT and aP2-Fam13a-Tg mice fed a HFD (SI Appendix, Fig. S8). Obesity-related insulin resistance and glucose intolerance were ameliorated in aP2-Fam13a-Tg mice fed a HFD in association with preserved insulin signaling in WAT (Fig. 6 D–F and SI Appendix, Fig. S9 A and B). In contrast to Fam13a−/− mice, adipocyte size was modestly but significantly increased in conjunction with lower serum FFA levels in HFD-fed aP2-Fam13a-Tg mice relative to WT mice, suggesting reduced lipolysis in the WAT of aP2-Fam13a-Tg mice (SI Appendix, Fig. S9 C and D). Consistently, expression of ATGL was significantly reduced in the WAT of HFD-fed aP2-Fam13a-Tg mice (SI Appendix, Fig. S9E). Expression of inflammatory genes was modestly reduced in the WAT of HFD-fed aP2-Fam13a-Tg mice compared with WT mice fed a HFD (SI Appendix, Fig. S10). These data demonstrate that preserved expression of Fam13a in adipocytes sufficiently ameliorates adipose tissue insulin resistance and systemic metabolic disorders associated with obesity.
Fig. 6.
Targeted activation of Fam13a in adipocytes improves systemic metabolic health. (A) ITT in female WT and aP2-Fam13a-Tg mice fed NC at the age of 18 wk old (n = 6 each). (B) IpGTT in female WT and aP2-Fam13a-Tg mice fed NC at the age of 18 wk old (n = 5 each). (C) Immunoblotting for insulin signaling in WAT of WT and aP2-Fam13a-Tg mice fed NC at the age of 20 wk old. Insulin-mediated Akt activation was quantified [n = 5 for insulin (−) group; n = 6 for insulin (+) group]. (D) ITT in female WT and aP2-Fam13a-Tg mice fed a HFD for 12 wk (n = 5 each). (E) IpGTT in female WT and aP2-Fam13a-Tg mice fed a HFD for 12 wk (n = 5 each). (F) Immunoblotting for insulin signaling in WAT of WT and aP2-Fam13a-Tg mice fed a HFD for 14 wk. Insulin-mediated Akt activation was quantified (n = 5 each). Data represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study, we show that Fam13a plays an important role in the pathogenesis of obesity-related metabolic disorders by modulating adipocyte insulin signaling. The nucleotide sequence of the Fam gene is well preserved among various species, but its biological functions remain largely unknown. To our knowledge, there are no previous reports on the role of Fam13a in the regulation of adipocyte function and metabolic homeostasis. Genome-wide association studies have revealed that the Fam13a locus is associated with WHR in humans, especially women. WHR is an easily accessible measure of body fat distribution, and a larger WHR indicates more visceral fat deposition, which is associated with higher risk for type 2 diabetes and cardiovascular disease. Our data demonstrated that gain- or loss-of-function of Fam13a did not affect fat distribution, while we revealed that Fam13a regulates adipocyte insulin signaling and systemic metabolic homeostasis in both male and female mice fed either NC or a HFD. Therefore, Fam13a modifies systemic metabolic health independently of fat distribution in mice, and further investigation is needed to elucidate a potential role of Fam13a in body fat distribution in humans. In addition, genome-wide association studies also showed that a variant of the Fam13a gene is associated with increased risk of chronic obstructive pulmonary disease (17). Very recently, it was reported that Fam13a might be involved in the pathogenesis of cigarette smoke-induced emphysema in mice by promoting β-catenin degradation (21). On the other hand, Fam13a has also been reported to stabilize β-catenin and activate Wnt signaling (16). We therefore examined expression of total and active (nonphosphorylation at Ser33/Ser37/Thr41) β-catenin in WAT and found no difference between WT and Fam13a−/− mice, suggesting that β-catenin is unlikely to be involved in the Fam13a-mediated regulation of adipocyte functions (SI Appendix, Fig. S11). Nevertheless, direct analysis for β-catenin transcriptional activity using TOPflash assay is preferred to clarify a possible role of Fam13a in Wnt/β-catenin signaling in adipocytes.
We used HEK293 cells to analyze insulin signaling in vitro. Although HEK293 cells have been used to investigate the biological effects of insulin, they also express insulin-like growth factor (IGF) receptor that binds to insulin with lower affinity (22, 23). Insulin and IGF receptor display extensive structural homologies, and both receptors utilize IRS-1/2 to activate Akt. Because HEK293 cells showed higher sensitivity to IGF than to insulin (23), insulin-mediated Akt activation might largely be elicited through an IGF receptor in HEK293 cells.
Insulin resistance in WAT is closely associated with obesity-related metabolic disorders (4, 24). Notably, enhancing insulin signaling in WAT was sufficient for systemic metabolic improvements, although the definitive underlying mechanism remains unclear (10). WAT is a major source of serum FFA through lipolysis, which is potently inhibited by insulin. Therefore, adipocyte insulin resistance probably increases the serum FFA levels due to enhanced lipolysis and consequently impairs systemic metabolic homeostasis. However, mechanisms underlying adipocyte insulin resistance in obesity remain largely unknown. Our in vitro and in vivo data sufficiently indicate that Fam13a accelerates insulin signaling in adipocytes and potentially inhibits lipolysis under a high-fat dietary condition. Insulin-mediated activation of the insulin receptor triggers recruitment and phosphorylation of IRS1 at multiple tyrosine residues that form binding sites for the following signaling molecules containing Src-homology 2, represented by the p85 subunit of phosphoinositide 3-kinase that further activates Akt (25). Therefore, IRS1 is a key molecule in the insulin signal cascade. In this study, we demonstrated that Fam13a preserves IRS1 expression levels in adipocytes by promoting PP2A-mediated protection from proteasomal degradation. Furthermore, we revealed that gain- or loss-of-function for Fam13a modified systemic metabolic health in mice. Additionally, we found that IRS1 expression in WAT was modestly reduced in diet-induced obese mice (SI Appendix, Fig. S12). Nevertheless, our data do not indicate a causal relevance between IRS1 expression levels in WAT and systemic metabolic homeostasis in general. We found that HFD-fed Fam13a−/− mice showed increased ATGL expression in WAT and higher circulating FFA levels compared with those in WT mice. However, it remains unclear whether ATGL enzymatic activity is enhanced in WAT of Fam13a−/− mice and if increased ATGL expression in WAT is causally implicated in the higher serum FFA levels in Fam13a−/− mice under high-fat dietary condition. Further investigations are required to elucidate molecular mechanisms underlying the Fam13a-mediated metabolic changes in mice. It has been reported that Fam13a showed nuclear-cytoplasmic shuttling depending on Ser-322 phosphorylation in HEK293 cells (16). Although further analysis is required to validate Fam13a nuclear-cytoplasmic shuttling in adipocytes, this shuttling may regulate Fam13a function in insulin signaling because Fam13a needs to localize in cytoplasm to interact with IRS1. Given that Fam13a expression is substantially down-regulated in WAT under obese condition, Fam13a may causally be implicated in obesity-related adipocyte insulin resistance. Because insulin resistance in WAT is primarily involved in obesity-related metabolic disorders, Fam13a might be an attractive pharmacotherapeutic target in the treatment and/or prevention of metabolic disease associated with obesity.
Materials and Methods
Cell Culture.
3T3-L1 preadipocytes were obtained from Health Science Research Resources Bank. Adipogenesis was induced as described previously (26). Briefly, confluent 3T3-L1 preadipocytes were treated with insulin, dexamethasone, and isobutylmethylxanthine at day 0 for 48 h, followed by treatment with insulin for another 48 h. Afterward, cells were cultured in DMEM supplemented with 10% FBS. In some experiments, 3T3-L1 adipocytes at day 10–12 postdifferentiation were treated with thapsigargin (0.5 μM) for 24 h or hydrogen peroxide (0.5 mM) for 48 h.
HEK293 cells were cultured in DMEM supplemented with 10% FBS. Fam13a expression constructs were transfected into HEK293 cells using lipofectamine 3000 (Thermo) as the manufacturer recommended. The medium was changed with fresh growth medium 18 h after transfection, and cells were incubated for another 24 h before use in the experiments. For insulin signal analysis, cells were incubated in serum-free medium for 3 h and then stimulated with 100 nM insulin, followed by protein extraction 20 min after insulin treatment. For proteasome inhibition experiments, cells were treated with 5 μM lactacystin 18 h after transfection and further incubated for 24 h, followed by protein extraction. For IRS inhibition experiments, cells were treated with 5 μM NT157 at 18 h posttransfection and incubated for 24 h, followed by insulin signal analysis. For PP2A inhibition experiments, cells were treated with 10 nM okadaic acid 4 h after transfection and thereafter cultured in the presence of 10 nM okadaic acid for another 24 h, followed by protein extraction.
Short interfering (si)RNAs for Fam13a were purchased from Dharmacon (SMARTpool siGENOME, M-041073-01) and transfected into mature 3T3-L1 adipocytes in suspension as previously reported (27). Briefly, 3T3-L1 adipocytes at day 10 postdifferentiation were detached from culture plates using trypsin EDTA. Transfection mixture was prepared using 100 nM siRNA and 5.6 μL DharmaFECT Duo (Dharmacon) in 160 μL Opti-MEM (Gibco). Subsequently, 2 × 105 3T3-L1 adipocytes in 800 μL DMEM/10% FBS were combined with the transfection mixture and replated into 12-well plates. The medium was changed with fresh growth medium 24 h after transfection, and cells were incubated for another 48 h before use in the experiments.
Animal Study.
All experimental protocols were approved by the Ethics Review Committee for Animal Experimentation of Kobe Pharmaceutical University. Fam13a−/− mice [Fam13atm1e(KOMP)Wtsi; C57BL6N background] were obtained from the Knockout Mouse Project (KOMP) at UC Davis. Transgenic mice overexpressing Fam13a in adipocytes (aP2-Fam13a-Tg) were generated (C57BL6J background). The pBS aP2 promoter (5.4 kb) polyA was a gift from Ronald Kahn, Joslin Diabetes Center, Boston, (Addgene plasmid 11424). The aP2-Fam13a-Tg mice were propagated as heterozygous Tg animals by breeding with WT C57BL6J mice.
Mice were fed either NC (containing 23.1% protein and 5.1% fat) or a HFD (Oriental Bio HFD-60) containing 35% fat, 25.3% carbohydrates, and 23% protein with ad libitum access to water and food. For the HFD feeding, 6-wk-old mice were maintained on a HFD for up to 14 wk. The insulin and glucose tolerance tests (ITT and ipGTT) were performed as previously described (28, 29). For the ipGTT, mice fasted for 6 h, and 1.5 g/kg d-glucose was intraperitoneally administered. For the ITT, mice were given 1 IU/kg human insulin by s.c. injection without fasting. Blood glucose was measured by the glucose oxidase method (Johnson & Johnson K.K.). Visceral perigonadal WAT and interscapular BAT were used for all analyses. Before blood sampling, mice fasted for 6 h.
To analyze insulin signaling in WAT, mice were given 1 IU/kg human insulin by s.c. injection without fasting, and visceral perigonadal WAT was extracted 20 min after insulin injection, followed by protein extraction and SDS/PAGE.
Immunoprecipitation.
Immunoprecipitation was performed as previously described with a minor modification (30). 3T3-L1 adipocytes were lysed with CelLytic M lysis buffer (Sigma), followed by precleaning with IgG-agarose (Sigma). Subsequently, cell lysates were incubated with anti-Fam13a antibody (Sigma HPA038108) or normal rabbit IgG (Santa Cruz) at 4 °C overnight, followed by incubation with protein G-agarose (Thermo) at 4 °C for 2 h. After three washes with lysis buffer, immunoprecipitated proteins were released by boiling in the protein sample buffer, followed by SDS/PAGE. Coprecipitation of IRS1 was detected by immunoblotting using anti-IRS1 antibody (Santa Cruz sc-559).
Expression constructs for FLAG-tagged Fam13a in whole-length or with CCD deletion(s) and IRS1/2 were cotransfected into HEK293 cells using lipofectamine 3000 (Thermo). Amino acids at positions 343−396 and 617−693 were deleted to generate CCD1 and CCD2 deletion mutants for Fam13a, respectively. The pBS human IRS1 (Addgene plasmid 11359) and pBABE puro mouse IRS2 myc (Addgene plasmid 11373) were gifts from Ronald Kahn, Joslin Diabetes Center, Boston. cDNAs for IRS1 and IRS2 were subcloned into pcDNA3 to prepare expression constructs. FLAG-tagged Fam13a was immunoprecipitated using anti-FLAG M2 affinity gel (Sigma), followed by SDS/PAGE. Coprecipitation of IRS1, IRS2, and PP2A was detected by immunoblotting using anti-IRS1 antibody, anti-IRS2 antibody (Santa Cruz sc-1555), and anti-PP2A B subunit antibody (Cell Signaling Technology 2290), respectively.
Statistical Analysis.
All data are presented as mean ± SE. Differences between groups were analyzed using two-tailed Student’s t test or Mann–Whitney U test. Comparisons among more than three groups were assessed for significance by nonrepeated ANOVA with post hoc analysis of Fisher’s protected least significant difference. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by grant-in-aid for scientific research KAKENHI 16K09524, Novartis Foundation for the Promotion of Science, Hyogo Science and Technology Association, and Kobayashi International Scholarship Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720475115/-/DCSupplemental.
References
- 1.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberger J, Daniels SR. American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) American Heart Association Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Obesity, insulin resistance, diabetes, and cardiovascular risk in children: An American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- 3.Zanella MT, Kohlmann O, Jr, Ribeiro AB. Treatment of obesity hypertension and diabetes syndrome. Hypertension. 2001;38:705–708. doi: 10.1161/01.hyp.38.3.705. [DOI] [PubMed] [Google Scholar]
- 4.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun K, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akakabe Y, et al. Ecscr regulates insulin sensitivity and predisposition to obesity by modulating endothelial cell functions. Nat Commun. 2013;4:2389. doi: 10.1038/ncomms3389. [DOI] [PubMed] [Google Scholar]
- 9.Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93:S52–S59. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]
- 10.Morley TS, Xia JY, Scherer PE. Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements. Nat Commun. 2015;6:7906. doi: 10.1038/ncomms8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi MS, et al. High-fat diet decreases energy expenditure and expression of genes controlling lipid metabolism, mitochondrial function and skeletal system development in the adipose tissue, along with increased expression of extracellular matrix remodelling- and inflammation-related genes. Br J Nutr. 2015;113:867–877. doi: 10.1017/S0007114515000100. [DOI] [PubMed] [Google Scholar]
- 12.Shungin D, et al. ADIPOGen Consortium CARDIOGRAMplusC4D Consortium CKDGen Consortium GEFOS Consortium GENIE Consortium GLGC ICBP International Endogene Consortium LifeLines Cohort Study MAGIC Investigators MuTHER Consortium PAGE Consortium ReproGen Consortium New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: Possible regulation by tyrosine phosphorylation. Diabetes. 2001;50:24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- 14.Zhande R, Mitchell JJ, Wu J, Sun XJ. Molecular mechanism of insulin-induced degradation of insulin receptor substrate 1. Mol Cell Biol. 2002;22:1016–1026. doi: 10.1128/MCB.22.4.1016-1026.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandavia C, Sowers JR. Phosphoprotein phosphatase PP2A regulation of insulin receptor substrate 1 and insulin metabolic signaling. Cardiorenal Med. 2012;2:308–313. doi: 10.1159/000343889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin Z, et al. Regulation of nuclear-cytoplasmic shuttling and function of family with sequence similarity 13, member A (Fam13a), by B56-containing PP2As and Akt. Mol Biol Cell. 2015;26:1160–1173. doi: 10.1091/mbc.E14-08-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho MH, et al. Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat Genet. 2010;42:200–202. doi: 10.1038/ng.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakrabarti P, et al. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway. Mol Cell Biol. 2013;33:3659–3666. doi: 10.1128/MCB.01584-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haemmerle G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Z, et al. A chronic obstructive pulmonary disease susceptibility gene, FAM13A, regulates protein stability of β-catenin. Am J Respir Crit Care Med. 2016;194:185–197. doi: 10.1164/rccm.201505-0999OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schumacher R, Mosthaf L, Schlessinger J, Brandenburg D, Ullrich A. Insulin and insulin-like growth factor-1 binding specificity is determined by distinct regions of their cognate receptors. J Biol Chem. 1991;266:19288–19295. [PubMed] [Google Scholar]
- 23.El-Shewy HM, Lee MH, Obeid LM, Jaffa AA, Luttrell LM. The insulin-like growth factor type 1 and insulin-like growth factor type 2/mannose-6-phosphate receptors independently regulate ERK1/2 activity in HEK293 cells. J Biol Chem. 2007;282:26150–26157. doi: 10.1074/jbc.M703276200. [DOI] [PubMed] [Google Scholar]
- 24.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. doi: 10.1016/j.biochi.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6:a009191. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamori Y, et al. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes. J Biol Chem. 1998;273:19740–19746. doi: 10.1074/jbc.273.31.19740. [DOI] [PubMed] [Google Scholar]
- 27.Kilroy G, Burk DH, Floyd ZE. High efficiency lipid-based siRNA transfection of adipocytes in suspension. PLoS One. 2009;4:e6940. doi: 10.1371/journal.pone.0006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oike Y, et al. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005;11:400–408. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- 29.Tabata M, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178–188. doi: 10.1016/j.cmet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Koide M, et al. Apoptosis regulator through modulating IAP expression (ARIA) controls the PI3K/Akt pathway in endothelial and endothelial progenitor cells. Proc Natl Acad Sci USA. 2011;108:9472–9477. doi: 10.1073/pnas.1101296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.