Abstract
Scientific reports of sex differences in brain asymmetry – the difference between the two hemispheres – are rather inconsistent. Some studies report no sex differences whatsoever, others reveal striking sex effects, with large discrepancies across studies in the magnitude, direction, and location of the observed effects. One reason for the lack of consistency in findings may be the confounding effects of brain size as male brains are usually larger than female brains. Thus, the goal of this study was to investigate the differential contributions of sex and brain size to asymmetry with a particular focus on gray matter. For this purpose, we applied a well-validated workflow for voxel-wise gray matter asymmetry analyses in a sample of 96 participants (48 males / 48 females), in which a subsample of brains (24 males / 24 females) were matched for size. By comparing outcomes based on three different contrasts – all males vs. all females; all large brains vs. all small brains; matched males vs. matched females – we were able to disentangle the contributing effects of sex and brain size, to reveal true (size-independent) sex differences in gray matter asymmetry: Males show a significantly stronger rightward asymmetry than females within the cerebellum, specifically in lobules VII, VIII, and IX. This finding agrees closely with prior research suggesting sex differences in sensorimotor, cognitive and emotional function, which are all moderated by the respective cerebellar sections. No other significant sex effects were detected across the remainder of the brain.
Keywords: Asymmetry, Gray Matter, MRI, Sex Differences, VBM
1. Introduction
Sex differences in brain anatomy are manifold and have been described in an abundance of studies, in which the most consistent observation is a larger brain size, on average, in males than in females (Giedd, Raznahan, Mills, & Lenroot, 2012; Gong, He, & Evans, 2011; Luders & Toga, 2010; Sacher, Neumann, Okon-Singer, Gotowiec, & Villringer, 2013). Another frequently assessed feature with respect to the sexual dimorphism of the human brain is its asymmetry. Interestingly, while some studies detected no significant differences between male and female brains, others revealed striking sex effects on brain asymmetry, where there are large discrepancies in findings with respect to effect magnitude, direction, and location (Fan et al., 2010; Geschwind & Galaburda, 1985b; Good et al., 2001; Guadalupe et al., 2016; Jancke, Schlaug, Huang, & Steinmetz, 1994; Kovalev, Kruggel, & von Cramon, 2003; Kurth, Jancke, & Luders, 2017; Luders, Gaser, Jancke, & Schlaug, 2004; Luders, Narr, Thompson, et al., 2006; Savic, 2014; Takao et al., 2011; Toga, Narr, Thompson, & Luders, 2009; Toga & Thompson, 2003; Watkins et al., 2001; Wisniewski, 1998; Yucel et al., 2001; Zilles et al., 1997). A few asymmetry studies have specifically focused on mapping gray matter differences between the hemispheres using voxel-based morphometry (VBM). However, outcomes are similarly inconsistent, ranging from no sex differences whatsoever to significant sex differences in various gray matter regions, not necessarily overlapping across studies and with conflicting findings in terms of whether male or female brains are more asymmetric (Fan et al., 2010; Good et al., 2001; Luders et al., 2004; Savic, 2014; Takao et al., 2011; Watkins et al., 2001).
It is not entirely clear if sex-specific gray matter asymmetries reflect sex differences in the performance of tasks that are lateralized (Shaywitz et al., 1995), whether they are a sequel of sex differences in brain connectivity (Ingalhalikar et al., 2014), or both. In addition, there may be yet another reason to expect sex differences in brain asymmetry, namely the sex-specific brain size, which is typically larger in males. According to the Ringo hypothesis (Ringo, 1991; Ringo, Doty, Demeter, & Simard, 1994), larger brains are differently connected than smaller brains, to ensure that computational efforts are distributed most efficiently. In larger brains, for example, this might manifest as more connections within one hemisphere but fewer connections across hemispheres, ultimately resulting in an increased hemispheric specialization and potentially stronger asymmetry (Hanggi, Fovenyi, Liem, Meyer, & Jancke, 2014; Jancke, Staiger, Schlaug, Huang, & Steinmetz, 1997; Jancke & Steinmetz, 2003; Ringo, 1991; Ringo et al., 1994). This hypothesis matches well with some reports that male brains are more asymmetric, in some respects, than female brains (Shaywitz et al., 1995; Toga et al., 2009; Toga & Thompson, 2003). Surprisingly though, while analyses have been conducted to assess sex differences in gray matter asymmetry (Fan et al., 2010; Geschwind & Galaburda, 1985b; Good et al., 2001; Kovalev et al., 2003; Luders et al., 2004; Luders, Narr, Thompson, et al., 2006; Savic, 2014; Takao et al., 2011; Toga et al., 2009; Toga & Thompson, 2003; Watkins et al., 2001; Wisniewski, 1998; Yucel et al., 2001; Zilles et al., 1997), there is a lack of studies systematically investigating how much of this apparent sex difference in gray matter asymmetry is attributable to the typical sex difference in brain size. In other words, it still remains to be addressed if there are any sex differences in gray matter asymmetry after properly accounting for the sex differences in brain size. Similarly, it needs to be resolved if male or female brains show a stronger gray matter asymmetry, and which brain regions are affected in particular.
Thus, the goal of the current study was to investigate the differential contributions of sex and brain size on gray matter asymmetry. For this purpose, we applied a well-validated workflow for voxel-wise asymmetry analyses (Kurth, Gaser, & Luders, 2015) and compiled a sample of 96 participants (48 males / 48 females), in which a subsample of brains (24 males / 24 females) were matched for size. By contrasting outcomes based on three different contrasts – all males vs. all females; all large brains vs. all small brains; matched males vs. matched females – we were able to disentangle the contributing effects of sex and brain size, revealing true (size-independent) sex differences in gray matter asymmetry.
2. Methods
2.1 Study Sample and Imaging Parameters
High-resolution T1-weighted images (n=153) were obtained from the ICBM database (www.loni.usc.edu/ICBM) of healthy participants rigorously screened and medically evaluated (Mazziotta et al., 2009). To minimize the influence of age-related brain atrophy, participants older than 70 years were excluded for the current study, leaving 145 participants altogether (72 males / 73 females) aged 18-69 years. This sample was then further reduced to 96 participants (48 males / 48 females) as detailed in the next section. All images were acquired on the same Siemens Sonata 1.5 T MRI system at UCLA using an 8-channel head coil and the same T1-weighted MPRAGE sequence with the following parameters: TR = 1900 ms, TE = 4.38 ms, flip angle = 15°, 160 contiguous 1 mm sagittal slices, FOV = 256 × 256 mm2, voxel dimensions = 1.0 × 1.0 × 1.0 mm3. All participants gave informed consent according to UCLA’s Institutional Review Board.
2.2 Brain Sizes and (Sub)Samples
Brain size was estimated using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 Toolbox (http://dbm.neuro.uni-jena.de/vbm.html) as detailed elsewhere (Luders, Gaser, Narr, & Toga, 2009). Briefly, the T1-weighted images were segmented into gray matter, white matter, and cerebrospinal fluid, and the respective tissue volumes were added up to calculate the total intracranial volume (TIV) in milliliters (ml). The resulting TIVs were then used to create the size-matched subsample consisting of 24 males and 24 females; the TIV difference within each matched pair was minute (≤ 5.16 ml). In addition, the resulting TIVs were used to identify 24 extremely small female brains as well as 24 extremely large male brains. Then, by adding the 48 brains of the extreme sample to the 48 brains of the matched sample, we created the whole sample (n=96), which was representative in terms of sex-typical brain sizes (i.e., smaller brains in females, larger brains in males). Table 1 summarizes the TIVs for both matched sample (n=48) and whole sample (n=96).
Table 1.
Sample-specific total intracranial volume (TIV)
| Matched Sample (n=48) | Whole Sample (n=96) | ||
|---|---|---|---|
| 24 Males | 24 Females | 48 Males | 48 Females |
| 1406.57 ± 101.69 | 1406.62 ± 101.41 | 1515.15 ± 139.38 | 1314.10 ± 125.36 |
TIV is shown in ml (mean ± standard deviation).
2.3 Image Preprocessing
Image preprocessing followed an established protocol for voxel-wise asymmetry analyses (Kurth et al., 2015). In short, using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) all images were tissue-segmented and registered to MNI space by applying 12-parameter (affine) transformations. The affine registered gray and white matter segments were flipped at midline, and a DARTEL template was created using the original and flipped affine registered segments (Ashburner, 2007; Kurth et al., 2015). The resulting template represents a symmetric study-specific atlas in MNI space, which was used to perform all subsequent registrations and segmentations (see Section 2.4) as well as to create an explicit brain mask for the final statistical analysis, as detailed elsewhere (Kurth et al., 2015).
2.4 Local Gray Matter Asymmetry
While a methodically stringent analysis stream is essential for assessing the influence of sex and brain size on local gray matter asymmetry, different asymmetry measures may impact and bias results substantially. Two different asymmetry measures have been mainly used in existing asymmetry VBM studies: the simple right-left difference1 and the more complex asymmetry index: The simple right-left difference still seems to be the preferred asymmetry measure, perhaps due to its straightforward calculation (i.e., right minus left), especially on a voxel-level. Unfortunately, as illustrated in Figure 1, the simple right-left difference might bias study outcomes as a mere change in overall brain size can result in artificial changes in asymmetry (i.e., the simple right-left difference scales with overall brain size). Importantly, such false effects do not only occur if brains vary in size globally but also locally (e.g., when the size difference manifests only within the temporal lobes). In addition to producing false sex differences, simple right-left difference measures may lead to the concealment of true (size-independent) sex differences, as also recently demonstrated in a PET study on amyloid-β burden asymmetry in Alzheimer’s disease (Frings et al., 2015). The asymmetry index, on the other hand, is impervious to scaling on a global as well as local level, and as such seems to be more suitable for asymmetry analyses than the simple right-left difference. Thus, the asymmetry index was the measure of choice in the current study.
Figure 1.
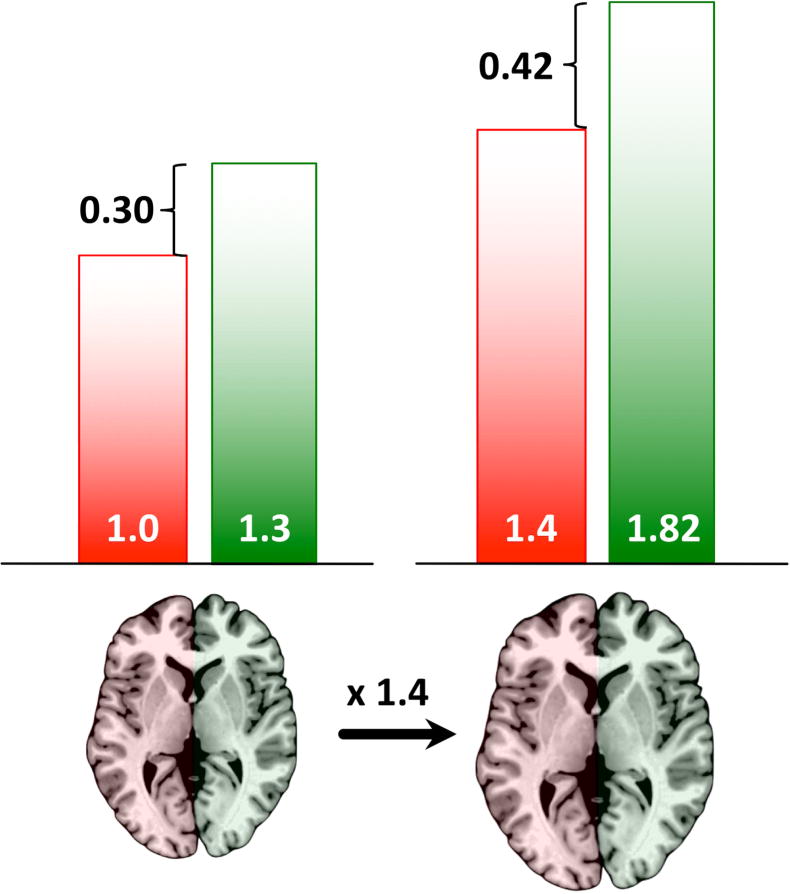
The effect of brain size on hemispheric right-left differences (the difference is exaggerated for illustration purposes). Left Panel: Subtracting the left from the right hemisphere reveals a hemispheric difference of 0.3. This indicates a rightward lateralization (i.e., larger on the right). Right Panel: If the same brain was 1.4 times bigger (but otherwise identical), subtracting the left from the right hemisphere would reveal a hemispheric difference of 0.42. This still indicates a rightward asymmetry but one that seems to be 1.4 times bigger than in the original (smaller) brain. In other words, even though both hemispheres were enlarged by exactly the same factor (1.4), the calculated hemispheric difference has changed, indicating a seemingly 1.4 times larger rightward asymmetry in the bigger brain.
To calculate the voxel-wise asymmetry index, the 12-parameter (affine) registered flipped and original gray matter segments (created as described in Section 2.3) were first normalized to the DARTEL template (created as described in Section 2.3). Subsequently, on each voxel, the asymmetry index (AI) was calculated as AI = (right − left) / (0.5 × [right + left]). Finally, the left hemispheres of the resulting AI images were discarded and the remaining right hemispheres were smoothed using a Gaussian kernel of 8 mm full-width-at-half-maximum (FWHM). These smoothed right-hemispheric AI images constituted the input for the statistical analysis (see Section 2.5). In addition, a mean template for visualization and anatomic reference was created, as detailed elsewhere (Kurth et al., 2015).
2.5 Statistical Analyses
All analyses were conducted using the general linear model with the design matrix comprising four groups, namely the 24 matched males (small brains), the 24 matched females (large brains), the 24 extreme females (small brains), and the 24 extreme males (large brains), as well as age as a nuisance variable. To assess the impact of sex and size on gray matter asymmetry, we conducted three different comparisons (contrasts 1-3), as illustrated in Figure 2. In contrast 1, we compared the 24 matched males to the 24 matched females, to reveal size-independent sex differences in brain asymmetry and establish the methodological ground truth of sex effects. In contrast 2, we compared all 48 males (extreme males; matched males) and all 48 females (extreme females; matched females), and in contrast 3 we compared all 48 large brains (extreme males; matched females) and all 48 small brains (extreme females; matched males).
Figure 2.
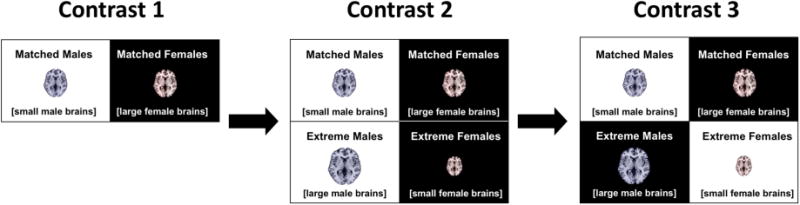
The three different comparisons (contrasts 1-3). For each contrast, black and white denote the groups being compared. Contrast 1 compares the 24 matched males and 24 matched females. Contrast 2 compares all 48 males to all 48 females. Contrast 3 compares all 48 large brains to all 48 small brains. Spatial overlap between the clusters from contrast 1 and 2 will indicate where sex differences in gray matter asymmetry are driven by sex. Spatial overlap between the clusters from contrast 2 and 3 will indicate where sex differences in gray matter asymmetry are driven by brain size.
Relating the outcomes from contrast 2 to the outcomes from contrast 1 will reveal the extent to which apparent sex differences in gray matter asymmetry are truly driven by sex (i.e., where there is spatial overlap between the clusters from contrasts 1 and 2) and not by brain size. Relating the outcomes from contrast 2 to the outcomes from contrast 3 will reveal the extent to which apparent sex differences in gray matter asymmetry are driven by brain size (i.e., where there is spatial overlap between the clusters from contrasts 2 and 3) and not by sex. Of note, these predictions are only valid if there is no significant sex-by-size interaction, which was tested as well. All analyses were corrected for multiple comparisons using non-parametric threshold-free cluster enhancement (Smith & Nichols, 2009) with 5,000 permutations and controlling the family-wise error rate at p ≤ 0.05. Results were visualized using maximum-intensity projections, and follow-up analyses were conducted on significant clusters as further described in Section 3.3.
Finally, to confirm that the described approach is sufficiently sensitive to detect significant differences between the hemispheres (asymmetries) – in addition to significant asymmetry differences between groups (which is the main focus of this study) – we conducted a supplemental analysis. For this purpose, similar as described for the main analysis, we used the smoothed AI images of the right hemisphere (see Section 2.4). However, this time, we applied two one-sample t-tests (left > right; right > left) and we focused on the combined sample (n=96), rather than any subsamples. Again, age was treated as nuisance variable and the results were corrected for multiple comparisons, as described above. The outcomes of this supplemental analysis are shown in Supplemental Figure 1.
3. Results
The significant group differences are shown as red clusters in Figure 3 (top). Comparing matched males against matched females (contrast 1) revealed a significant sex difference in gray matter asymmetry in the cerebellum. Given that male and females brains were matched pair-wise for size, these significant effects constitute sex differences in brain asymmetry that are entirely independent of size differences (i.e., our ground truth). Comparing all males against all females (contrast 2) revealed a significant sex difference in gray matter asymmetry in the cerebellum, which largely2 resembles the effect as observed in contrast 1. Finally, comparing all large to all small brains (contrast 3) revealed no significant differences in gray matter asymmetry. Moreover, there was no significant sex-by-size interaction (map not shown). Altogether, this suggests that the observed sex differences in contrast 2 (i.e., males and females representative of sex-typical brain sizes) were driven by sex, and not by size. As per the anatomy toolbox (Eickhoff et al., 2005) and the cerebellar atlas (Diedrichsen, Balsters, Flavell, Cussans, & Ramnani, 2009), the cluster resulting from contrast 2 is located in lobules VIIb, VIIIb, and IX of the cerebellum (for x; y; z coordinates, see legend of Figure 3).
Figure 3.
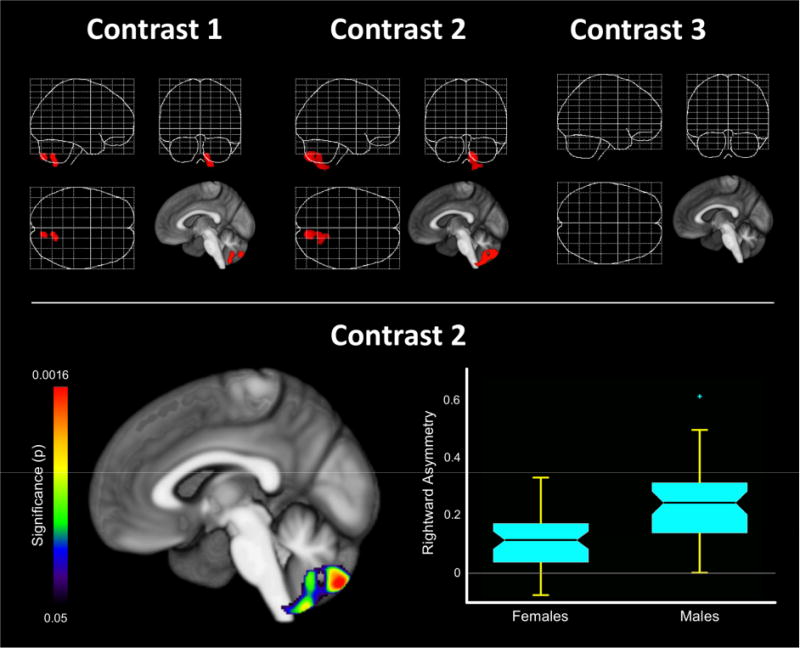
Sex differences in gray matter asymmetry. The top panel indicates the significant outcomes using the different contrasts. Contrast 1: matched males vs. matched females. Contrast 2: all males vs. all females. Contrast 3: all large brains vs. all small brains. The bottom panel (left) shows the significant cluster resulting from contrast 2 with the color bar encoding the p-value. The significance maximum (p<0.0016) was located at (x; y; z) 12; –78; –50 in symmetric template space, and at (x; y; z) 12; –77; –50 in MNI single subject space. The bottom panel (right) shows the averaged sex-specific asymmetry indices extracted from the significant cluster (median, quartiles, and 1.5 interquartile ranges). Positive values on the y-axis encode a rightward asymmetry. Altogether, the cerebellar significance cluster reflects a larger rightward asymmetry in male brains than in female brains.
As explained in detail elsewhere (Kurth et al., 2015), the significant cluster resulting from contrast 2 does not reveal anything yet with respect to the direction of the asymmetry (i.e., leftward or rightward) or the direction of the sex difference (i.e., more asymmetry in male or in female brains). Thus, we extracted the averaged asymmetry index from that significance cluster and generated sex-specific box plots (Figure 3, bottom). As shown in the box plots, the extracted asymmetry indices are positive, both in females and in males. Given the applied formula for the asymmetry index (AI = [right − left] / [0.5 × [right + left]]), positive values encode a rightward asymmetry (negative values would encode a leftward asymmetry). Thus, both females and males show a rightward asymmetry in this cerebellar cluster. However, the rightward asymmetry in males is larger than in females (indicated as larger positive values on the y-axis). Thus, we may infer that the significant sex difference in this cerebellar cluster is due to a larger rightward asymmetry in male brains than in female brains.
4. Discussion
Testing for significant sex differences in structural brain imaging is usually aimed at unveiling a sex-specific brain anatomy and therefore interpreted as such. However, it is important to consider that sex-independent factors such as brain size may produce sex differences that do not truly reflect a sex-specific characteristic, but rather a size-specific characteristic. While a few studies have examined the interplay between brain size and sex (Jancke, Merillat, Liem, & Hanggi, 2015; Jancke et al., 1997; Leonard et al., 2008; Luders, Narr, Zaidel, Thompson, & Toga, 2006; Luders, Toga, & Thompson, 2014; Sowell et al., 2007; Sullivan, Rosenbloom, Desmond, & Pfefferbaum, 2001), such research with particular respect to brain asymmetry is still largely missing. Thus, in this present study, we carefully investigated the influence of sex and brain size on gray matter asymmetry.
4.1 Comparison to Previous Studies
Our analysis revealed a significantly stronger rightward asymmetry in males compared to females within the cerebellum. These findings seem to be in close agreement with prior observations of stronger rightward asymmetries in males within the cerebellum (Fan et al., 2010) or the cerebellar vermis (Savic, 2014). On the other hand, our findings contrast with reports of significant sex differences regarding asymmetry of the Heschl’s sulcus / planum temporale (Good et al., 2001; Savic, 2014) or the middle frontal gyrus, medial occipital cortex, and thalamus (Savic, 2014). Other studies, in turn, reported a lack of significant sex differences in brain asymmetry altogether (Luders et al., 2004; Takao et al., 2011; Watkins et al., 2001). Direct comparisons between current findings and other VBM-based outcomes are rather challenging as most prior studies used some form of the simple right-left difference. Moreover, virtually all analyses were based on slightly different methods, which is partly due to significant advancements over the years in data preprocessing streams (e.g., for tissue segmentation and spatial normalization). Unfortunately, this latter aspect also limits direct comparability with the one VBM study, conducted more than a decade ago, which used the asymmetry index (Luders et al., 2004). Nonetheless, in that study no significant sex differences in gray matter asymmetry were detected. This resembles the lack of cerebral effects in the current study, where the only significant sex effect was confined to the cerebellum (but not the cerebrum). However, it still may seem somewhat surprising that we did not detect any asymmetry differences between small and large brains, especially since brain size-dependent differences in fiber connectivity and/or callosal morphology have been demonstrated previously (Hanggi et al., 2014; Jancke et al., 1997; Jancke & Steinmetz, 2003; Luders et al., 2014). It is possible though that – albeit being meaningful and measurable in other studies – such size-optimized connectivity patterns may not necessarily translate into significant differences in voxel-wise gray matter asymmetry (as examined in the current study), either because size-optimizations only affect a part of all connections and/or because altered fiber connections are not necessarily associated with localized changes in neurons or neuropil.
4.2 Possible Functional Implications
With respect to brain function, the specific location of the observed significance cluster may point to a sex difference in sensorimotor and cognitive function. That is, meta-analyses of functional MRI studies reported cerebellar lobule VIIIb to be involved primarily in sensorimotor processing (Stoodley & Schmahmann, 2009, 2010), and there seems to be a functional connectivity between lobule VIIIb and sensorimotor areas in the cerebrum (Buckner, Krienen, Castellanos, Diaz, & Yeo, 2011). A sex difference within a cerebellar region that is involved in sensorimotor processing might be related to the advantage of females in fine motor tasks (Ingram, 1975; Kimura & Vanderwolf, 1970; Michimata, Kondo, Suzukamo, Chiba, & Izumi, 2008). Lobule VIIb, on the other hand, with its connections to parietal and frontal cortices (Buckner et al., 2011; Sasaki, Oka, Matsuda, Shimono, & Mizuno, 1975), has been reported to be involved in higher-level cognitive processing as examined, for example, through the Tower-of-London task, random number generation, or complex decision making (Stoodley & Schmahmann, 2009, 2010). In addition, lobule VIIb is implicated in emotional processing (Stoodley & Schmahmann, 2009) and the phonological storage of verbal memory tasks (Chen & Desmond, 2005). Although a more recent review (Stoodley, 2012) suggests that lobule VIIb’s involvement in cognitive processes is confined to more lateral sections (current effects are located more medial), sex differences in verbal memory tasks or emotional processing have been reported (Kramer, Delis, Kaplan, O’Donnell, & Prifitera, 1997; Whittle, Yucel, Yap, & Allen, 2011). However, since no cognitive or sensorimotor measures (or any other information on potentially sex-specific expertise) are available for the present sample, the relationship between our observed sex differences in asymmetry and any functional / cognitive / behavioral implications remains merely conjecture.
4.3 Summary and Future Studies
The outcomes of the current study suggest the existence of true sex differences in voxel-wise gray matter asymmetry within the cerebellum. The main strength of this study is its design using a carefully compiled subsample in which male and female brains were pair-wise matched for size. This allowed establishing a “ground truth” for effects of sex as well as effects of brain size. Furthermore, we used a well-validated analysis protocol (Kurth et al., 2015), which provides a methodological framework that avoids a number of potential problems and known pitfalls in voxel-wise asymmetry analyses, and is easy to replicate in future studies. Despite these strengths, the current study has some limitations. For example, some caution is warranted in generalizing the findings as the matched subsample may be considered sex atypical in terms of brain size (male brains are on average 10-15% larger than female brains). On a related note, given the difficulty in compiling such size-matched subsamples, the sample size was rather small (24 males, 24 females) raising the potential issue of statistical power. Clearly, future studies will be needed to confirm the current results in larger samples, ideally using very large multi-site designs (Guadalupe et al., 2016) and preferably applying the same analysis protocol to allow for a direct comparability of results. Future studies may also want to obtain cognitive measures in addition to examine links between the sexual dimorphism in brain asymmetry and well-established sex differences in a number of cognitive abilities (e.g., mental rotation). Moreover, given that fetal testosterone levels have been discussed to modulate functional lateralization and brain asymmetry and also to explain sex differences in cognition, behavior and susceptibility to disorders (Geschwind & Galaburda, 1985a), futures studies including hormonal information would further advance this field of research.
Supplementary Material
Highlights.
There is a significant sex difference in voxel-wise gray matter asymmetry.
The effect was confined to the cerebellum, specifically lobules VII, VIII, and IX.
Within this region, both male and female brains show a rightward asymmetry.
However, the rightward asymmetry in male brains is larger than in female brains.
Acknowledgments
EL is funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R01HD081720) and further supported by the Cousins Center for Psychoneuroimmunology at the University of California, Los Angeles (UCLA). PT is supported by NIH grant U54 EB020403.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Either calculated before applying statistical tests or by statistically comparing the left and right hemispheres using a two-sample T-test.
The slight difference in cluster shape and size is likely due to the different numbers of subjects in contrast 1 (24 vs. 24) and in contrast 2 (48 vs. 48).
References
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. doi: S1053-8119(07)00584-8 [pii] 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. doi: jn.00339.2011 [pii] 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005;24(2):332–338. doi: 10.1016/j.neuroimage.2004.08.032. doi: S1053-8119(04)00494-X [pii] 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. doi: S1053-8119(09)00080-9 [pii] 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. doi: S1053-8119(04)00792-X [pii] 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fan L, Tang Y, Sun B, Gong G, Chen ZJ, Lin X, Liu S. Sexual dimorphism and asymmetry in human cerebellum: an MRI-based morphometric study. Brain Res. 2010;1353:60–73. doi: 10.1016/j.brainres.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Frings L, Hellwig S, Spehl TS, Bormann T, Buchert R, Vach W, Meyer PT. Asymmetries of amyloid-beta burden and neuronal dysfunction are positively correlated in Alzheimer’s disease. Brain. 2015;138(Pt 10):3089–3099. doi: 10.1093/brain/awv229. doi: awv229 [pii] 10.1093/brain/awv229. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985a;42(5):428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: II. A hypothesis and a program for research. Arch Neurol. 1985b;42(6):521–552. doi: 10.1001/archneur.1985.04060060019009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 2012;3(1):19. doi: 10.1186/2042-6410-3-19. doi: 2042-6410-3-19 [pii] 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC. Brain connectivity: gender makes a difference. Neuroscientist. 2011;17(5):575–591. doi: 10.1177/1073858410386492. doi: 1073858410386492 [pii] 10.1177/1073858410386492. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. S1053-8119(01)90857-2 [pii] [DOI] [PubMed] [Google Scholar]
- Guadalupe T, Mathias SR, vanErp TG, Whelan CD, Zwiers MP, Abe Y, Francks C. Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanggi J, Fovenyi L, Liem F, Meyer M, Jancke L. The hypothesis of neuronal interconnectivity as a function of brain size-a general organization principle of the human connectome. Front Hum Neurosci. 2014;8:915. doi: 10.3389/fnhum.2014.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. doi: 1316909110 [pii] 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram D. Motor asymmetries in young children. Neuropsychologia. 1975;13(1):95–102. doi: 10.1016/0028-3932(75)90052-4. doi: 0028-3932(75)90052-4 [pii] [DOI] [PubMed] [Google Scholar]
- Jancke L, Merillat S, Liem F, Hanggi J. Brain size, sex, and the aging brain. Hum Brain Mapp. 2015;36(1):150–169. doi: 10.1002/hbm.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Schlaug G, Huang Y, Steinmetz H. Asymmetry of the planum parietale. Neuroreport. 1994;5(9):1161–1163. doi: 10.1097/00001756-199405000-00035. [DOI] [PubMed] [Google Scholar]
- Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cereb Cortex. 1997;7(1):48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Jancke L, Steinmetz H. Anatomical brain asymmetries and their relevance for functional asymmetries. In: Hugdahl K, Davidson RJ, editors. The asymmetrical brain. London: The MIT Press, Cambridge MA; 2003. pp. 187–230. [Google Scholar]
- Kimura D, Vanderwolf CH. The relation between hand preference and the performance of individual finger movements by left and right hands. Brain. 1970;93(4):769–774. doi: 10.1093/brain/93.4.769. [DOI] [PubMed] [Google Scholar]
- Kovalev VA, Kruggel F, von Cramon DY. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. Neuroimage. 2003;19(3):895–905. doi: 10.1016/s1053-8119(03)00140-x. doi: S105381190300140X [pii] [DOI] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Kaplan E, O’Donnell L, Prifitera A. Developmental sex differences in verbal learning. Neuropsychology. 1997;11(4):577–584. doi: 10.1037//0894-4105.11.4.577. [DOI] [PubMed] [Google Scholar]
- Kurth F, Gaser C, Luders E. A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM) Nat Protoc. 2015;10(2):293–304. doi: 10.1038/nprot.2015.014. doi: nprot.2015.014 [pii] 10.1038/nprot.2015.014. [DOI] [PubMed] [Google Scholar]
- Kurth F, Jancke L, Luders E. Sexual dimorphism of Broca’s region: More gray matter in female brains in Brodmann areas 44 and 45. J Neurosci Res. 2017;95(1-2):626–632. doi: 10.1002/jnr.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Towler S, Welcome S, Halderman LK, Otto R, Eckert MA, Chiarello C. Size matters: cerebral volume influences sex differences in neuroanatomy. Cereb Cortex. 2008;18(12):2920–2931. doi: 10.1093/cercor/bhn052. doi: bhn052 [pii] 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Gaser C, Jancke L, Schlaug G. A voxel-based approach to gray matter asymmetries. Neuroimage. 2004;22(2):656–664. doi: 10.1016/j.neuroimage.2004.01.032. S1053811904000758 [pii] [DOI] [PubMed] [Google Scholar]
- Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci. 2009;29(45):14265–14270. doi: 10.1523/JNEUROSCI.2261-09.2009. doi: 29/45/14265 [pii] 10.1523/JNEUROSCI.2261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW. Hemispheric asymmetries in cortical thickness. Cereb Cortex. 2006;16(8):1232–1238. doi: 10.1093/cercor/bhj064. doi: bhj064 [pii] 10.1093/cercor/bhj064. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006;17(11):1103–1106. doi: 10.1097/01.wnr.0000227987.77304.cc. 00001756-200607310-00005 [pii] [DOI] [PubMed] [Google Scholar]
- Luders E, Toga AW. Sex differences in brain anatomy. Prog Brain Res. 2010;186:3–12. doi: 10.1016/B978-0-444-53630-3.00001-4. doi: B978-0-444-53630-3.00001-4 [pii] 10.1016/B978-0-444-53630-3.00001-4. [DOI] [PubMed] [Google Scholar]
- Luders E, Toga AW, Thompson PM. Why size matters: differences in brain volume account for apparent sex differences in callosal anatomy: the sexual dimorphism of the corpus callosum. Neuroimage. 2014;84:820–824. doi: 10.1016/j.neuroimage.2013.09.040. doi: S1053-8119(13)00976-2 [pii] 10.1016/j.neuroimage.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta JC, Woods R, Iacoboni M, Sicotte N, Yaden K, Tran M, Toga AW. The myth of the normal, average human brain–the ICBM experience: (1) subject screening and eligibility. Neuroimage. 2009;44(3):914–922. doi: 10.1016/j.neuroimage.2008.07.062. doi: S1053-8119(08)00908-7 [pii] 10.1016/j.neuroimage.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michimata A, Kondo T, Suzukamo Y, Chiba M, Izumi S. The manual function test: norms for 20- to 90-year-olds and effects of age, gender, and hand dominance on dexterity. Tohoku J Exp Med. 2008;214(3):257–267. doi: 10.1620/tjem.214.257. doi: JST.JSTAGE/tjem/214.257 [pii] [DOI] [PubMed] [Google Scholar]
- Ringo JL. Neuronal interconnection as a function of brain size. Brain Behav Evol. 1991;38(1):1–6. doi: 10.1159/000114375. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4(4):331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging. 2013;31(3):366–375. doi: 10.1016/j.mri.2012.06.007. doi: S0730-725X(12)00217-2 [pii] 10.1016/j.mri.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Oka H, Matsuda Y, Shimono T, Mizuno N. Electrophysiological studies of the projections from the parietal association area to the cerebellar cortex. Exp Brain Res. 1975;23(1):91–102. doi: 10.1007/BF00238732. [DOI] [PubMed] [Google Scholar]
- Savic I. Asymmetry of cerebral gray and white matter and structural volumes in relation to sex hormones and chromosomes. Front Neurosci. 2014;8:329. doi: 10.3389/fnins.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373(6515):607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. doi: S1053-8119(08)00297-8 [pii] 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17(7):1550–1560. doi: 10.1093/cercor/bhl066. doi: bhl066 [pii] 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. doi: S1053-8119(08)00972-5 [pii] 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. doi: S0010-9452(09)00326-8 [pii] 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A. Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiol Aging. 2001;22(4):603–611. doi: 10.1016/s0197-4580(01)00232-9. doi: S0197458001002329 [pii] [DOI] [PubMed] [Google Scholar]
- Takao H, Abe O, Yamasue H, Aoki S, Sasaki H, Kasai K, Ohtomo K. Gray and white matter asymmetries in healthy individuals aged 21-29 years: a voxel-based morphometry and diffusion tensor imaging study. Hum Brain Mapp. 2011;32(10):1762–1773. doi: 10.1002/hbm.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Narr KL, Thompson PM, Luders E. Brain Asymmetry: Evolution. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 2. Oxford: Academic Press; 2009. pp. 303–311. [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4(1):37–48. doi: 10.1038/nrn1009. nrn1009 [pii] [DOI] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Evans AC. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11(9):868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yucel M, Yap MB, Allen NB. Sex differences in the neural correlates of emotion: evidence from neuroimaging. Biol Psychol. 2011;87(3):319–333. doi: 10.1016/j.biopsycho.2011.05.003. doi: S0301-0511(11)00138-4 [pii] 10.1016/j.biopsycho.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB. Sexually-dimorphic patterns of cortical asymmetry, and the role for sex steroid hormones in determining cortical patterns of lateralization. Psychoneuroendocrinology. 1998;23(5):519–547. doi: 10.1016/s0306-4530(98)00019-5. doi: S0306453098000195 [pii] [DOI] [PubMed] [Google Scholar]
- Yucel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, Pantelis C. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb Cortex. 2001;11(1):17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Langemann C, Amunts K, Morosan P, Palomero-Gallagher N, Roland PE. Quantitative analysis of sulci in the human cerebral cortex: development, regional heterogeneity, gender difference, asymmetry, intersubject variability and cortical architecture. Hum Brain Mapp. 1997;5(4):218–221. doi: 10.1002/(SICI)1097-0193(1997)5:4<218::AID-HBM2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


