Abstract
Early-life stress is a risk factor for comorbid anxiety and nicotine use. Because little is known about the factors underlying this comorbidity, we investigated the effects of adolescent stress on anxiety-like behavior and nicotine responses within individual animals. Adolescent male and female C57BL/6J mice were exposed to chronic variable social stress (CVSS; repeated cycles of social isolation + social reorganization) or control conditions from postnatal days (PND) 25–59. Anxiety-like behavior and social avoidance were measured in the elevated plus-maze (PND 61–65) and social approach-avoidance test (Experiment 1: PND 140–144; Experiment 2: 95–97), respectively. Acute nicotine-induced locomotor, hypothermic, corticosterone responses, (Experiment 1: PND 56–59; Experiment 2: PND 65–70) and voluntary oral nicotine consumption (Experiment 1: PND 116–135; Experiment 2: 73–92) were also examined. Finally, we assessed prefrontal cortex (PFC) and nucleus accumbens (NAC) synaptic transmission (PND 64–80); brain regions that are implicated in anxiety and addiction. Mice exposed to adolescent CVSS displayed increased anxiety-like behavior relative to controls. Further, CVSS altered synaptic excitability in PFC and NAC neurons in a sex-specific manner. For males, CVSS decreased the amplitude and frequency of spontaneous excitatory postsynaptic currents in the PFC and NAC, respectively. In females, CVSS decreased the amplitude of spontaneous inhibitory postsynaptic currents in the NAC. Adolescent CVSS did not affect social avoidance or nicotine responses and anxiety-like behavior was not reliably associated with nicotine responses within individual animals. Taken together, complex interactions between PFC and NAC function may contribute to adolescent stress-induced anxiety-like behavior without influencing nicotine responses.
Keywords: adolescence, social stress, anxiety, nicotine, nucleus accumbens, prefrontal cortex
INTRODUCTION
Although smoking is the leading cause of preventable death in the United States, 18% of American adults continue to smoke and use tobacco products (U.S. Surgeon General, 2012). Moreover, a strong bi-directional link exists between nicotine use and affective disorders including anxiety disorders and major depression (Fluharty et al., 2017). Increased rates of nicotine use among individuals with affective disorders may reflect the contribution of common underlying neurobiological and/or environmental factors (Rao and Chen, 2008). Affective disorders and nicotine use often emerge during adolescence which is a developmental period characterized by heightened stress responsivity and maturation of corticolimbic brain regions implicated in psychiatric disorders (Kessler et al., 2005; Andersen and Teicher, 2009; Spear, 2009; Centers for Disease Control and Prevention, 2012). Accordingly, prospective clinical studies have identified adolescent psychosocial stress exposure as an important environmental risk factor for affective disorders and the initiation of nicotine use (Compas et al., 1993; Koval et al., 2004; Finkelstein et al., 2006).
The clinical literature attributes adolescent stress effects on affective disorders and nicotine use, in part, to hypothalamic-pituitary-adrenal (HPA) axis dysregulation during adolescence (Heim and Nemeroff, 2001; de Wit et al., 2007; Rao et al., 2009). Adolescent HPA axis dysregulation may be particularly detrimental because glucocorticoid hormones modulate developmental programming of corticolimbic brain regions implicated in mood and drug-seeking behaviors (e.g., the prefrontal cortex (PFC) and nucleus accumbens (NAC)) (Pryce, 2008; Andersen and Teicher, 2009; Russo and Nestler, 2013). Thus, adolescent stress may alter the developmental trajectory of neurobiological processes involved in both anxiety and nicotine responses. Individuals may, in turn, become predisposed to develop comorbid affective disorders and nicotine use.
Social stressors are among the most prevalent and potent stress stimuli experienced by humans (Blanchard et al., 2001). Thus, rodent models of social stress provide a clinically relevant system to investigate the behavioral and neurobiological effects of human social stress (Blanchard et al., 2001; Andersen and Teicher, 2009; McCormick, 2010). Increased anxiety-like behavior and social avoidance (i.e., a common symptom of many affective disorders (Toth and Neumann, 2013)) are often reported in rats and mice exposed to adolescent social instability or social defeat stress (McCormick et al., 2007, 2008; Vidal et al., 2007; Sterlemann et al., 2008; Lukkes et al., 2009b; Green et al., 2013; Saavedra-Rodríguez and Feig, 2013; Warren et al., 2014; Iñiguez et al., 2014; Caruso et al., 2017a; Hodges et al., 2017). Adolescent social stress also has sex-specific effects on nicotine responses. Adolescent social instability did not affect males, but diminished or augmented nicotine-induced locomotor activity in late adolescent and adult female rats, respectively (McCormick et al., 2004; McCormick and Ibrahim, 2007). HPA axis dysregulation has accompanied adolescent stress-induced alterations in anxiety-like behavior, social avoidance, and nicotine responses (McCormick et al., 2007; Sterlemann et al., 2008; Iñiguez et al., 2014; Caruso et al., 2017a). Unfortunately, adolescent social stress effects on anxiety-like behaviors and nicotine responses are rarely studied within the same animals. As such, it is difficult to assess the relationship between these phenotypes and generate hypotheses about the neurobiological mechanisms underlying comorbid affective disorders and nicotine use.
In a prior study, we developed an adolescent chronic variable social stress (CVSS; repeated cycles of individual housing and exposure to novel social partners) procedure that altered nicotine responses without influencing anxiety-like behavior or social avoidance when measured in the same animal (Caruso et al., 2017b). Inbred male, but not female, BALB/cJ mice exposed to CVSS displayed augmented locomotor and HPA axis responses to nicotine during late adolescence and adulthood, respectively. Adolescent CVSS also reduced voluntary nicotine consumption in adult males. Importantly, there was no effect of CVSS on anxiety-like behavior in the elevated plus-maze (EPM) or social avoidance. Finally, individual differences in nicotine responses were not associated with anxiety-like behavior or social avoidance. Our prior findings indicate that susceptibility to the nicotine effects of CVSS could involve neurobiological or genetic factors that are distinct from those involved in susceptibility to its anxiogenic effects. However, the high anxiety phenotype exhibited by BALB/cJ mice relative to other inbred strains (e.g., low anxiety C57BL/6J mice) (Jacobson and Cryan, 2007) may have limited our ability to detect an association between anxiety-like behavior and nicotine responses in this prior study (Caruso et al., 2017b).
In the current study, we investigated the influence of CVSS on anxiety-like behavior, social avoidance, and nicotine responses in male and female C57BL/6J mice. We predicted that adolescent stress would: (1) increase anxiety-like behavior, (2) increase nicotine-induced locomotor activity and corticosterone (CORT) secretion and decrease voluntary nicotine consumption, and (3) result in a strong correlation between anxiety-like behavior and nicotine responses within individuals. These results would support the hypothesis that common neurobiological and/or environmental factors underlie susceptibility to stress-induced affective disorder and nicotine use comorbidity. Importantly, nicotine responses and stress susceptibility are highly dependent on genetic background (Marks et al., 1989; Savignac et al., 2011) and adolescent stress may exert unique effects on C57BL/6J mice relative to those reported for BALB/cJ mice (Caruso et al., 2017b). In this case, a comparison of strain differences in the effects of adolescent stress could provide insight for future studies focused on elucidating the genetic basis of susceptibility to adolescent stress-induced anxiety disorders and/or nicotine use.
Potential neurobiological mechanisms underlying the effects of adolescent CVSS were explored using ex vivo whole-cell electrophysiology. We investigated whether adolescent CVSS altered basal synaptic transmission in PFC layer 2/3 (L2/3) pyramidal neurons and NAC core neurons. These stress-susceptible corticolimbic brain regions are implicated in anxiety-like behaviors and drug-seeking processes (Heim and Nemeroff, 2001; Andersen and Teicher, 2009; Russo and Nestler, 2013). Furthermore, adolescent social stress exerts profound effects on PFC and NAC development. For example, adolescent social defeat reduced excitatory glutamatergic transmission in PFC layer 5 (L5) pyramidal neurons; a finding that is consistent with structural and molecular changes observed in the PFC following adolescent stress (Leussis and Andersen, 2008; Eiland et al., 2012; Urban and Valentino, 2016). Alterations to PFC function could influence excitatory cortical regulation of subcortical structures implicated in the behavioral effects of stress including the NAC (Britt et al., 2012; Russo and Nestler, 2013). Notably, adolescent social isolation augmented serotonin release in the NAC in vivo (Lukkes et al., 2009a) and ex vivo studies of NAC neurons have shown that serotonin inhibits presynaptic glutamate release at corticostriatal synapses (Mathur et al., 2011). Therefore, we hypothesized that CVSS would result in a net decrease in basal excitatory synaptic transmission in PFC and NAC neurons.
EXPERIMENTAL PROCEDURES
Animals
Male and female C57BL/6J mice (Stock 000664, The Jackson Laboratory, Bar Harbor, ME) were bred at The Pennsylvania State University. A total of 121 mice (67 females and 54 males) from 25 litters were used in the current study. Pups remained with the dam until weaning on postnatal day (PND) 21. Following weaning mice were housed with 3–4 same-sex cagemates in polycarbonate cages (28 cm × 17 cm × 12 cm) with corn-cob bedding in a temperature and humidity-controlled vivarium. Mice were maintained on a reverse 12-h light-dark schedule (lights on at 13:00 h) with ad libitum food and water. All procedures followed the National Institute of Health guide and were approved by the Pennsylvania State University IACUC committee.
Experimental design
Experiment 1
In Experiment 1, we assessed whether adolescent CVSS alters late adolescent acute nicotine responses and adult anxiety-like behavior, social avoidance, and voluntary nicotine consumption (Table 1; n = 10–21/group). Specifically, acute nicotine-induced changes in body temperature and locomotor activity were measured during late adolescence (PND 56–59) and voluntary oral nicotine consumption was measured during adulthood (PND 116–135). Adult anxiety-like behavior and social avoidance were measured using the elevated plus-maze (EPM; PND 62–65) and social approach-avoidance test (SAAT; PND 140–144), respectively.
Table 1.
Experimental design
| Experiment | Subjects | Outcome Measure | Age at Testing (PND) |
|
|---|---|---|---|---|
| 1 | CON = | 10 male | Acute nicotine responses | 56 – 59 |
| 19 female | Elevated plus-maze | 62 – 65 | ||
| *CVSS = | 11 male | Voluntary nicotine consumption | 116 – 135 | |
| 21 female | Social approach-avoidance test | 140 – 144 | ||
|
| ||||
| 2 | CON = | 12 male | Elevated plus-maze | 61 – 63 |
| 8 female | §Acute nicotine responses | 65 – 70 | ||
| *CVSS = | 11 male | Voluntary nicotine consumption | 73 – 92 | |
| 9 female | Social approach-avoidance test | 95 – 97 | ||
|
| ||||
| 3 | CON = | 5 male | Electrophysiology | 64 – 80 |
| 5 female | ||||
| *CVSS = | 5 male | |||
| 5 female | ||||
CON = control; CVSS = chronic variable social stress, PND = postnatal day.
CVSS exposure occurred from PND 25–59.
Acute nicotine-induced corticosterone secretion was measured 30 and 90 min after saline/nicotine injection.
Experiment 2
In Experiment 2, acute nicotine responses were assessed in a separate group of experimental mice during adulthood because prior studies have shown that adolescent social stress affects nicotine responses differently when tested during adolescence vs. adulthood (McCormick et al., 2004; McCormick and Ibrahim, 2007; Caruso et al., 2017b). In Experiment 2, we tested whether adolescent CVSS alters adult nicotine responses, anxiety-like behavior, and social avoidance (Table 1; n = 9–12/group). Adult anxiety-like behavior and social avoidance were measured on the EPM (PND 61–63) and SAAT (PND 95–97), respectively. Acute nicotine-induced changes in body temperature, locomotor activity, and plasma CORT levels (PND 65–70) as well as voluntary oral nicotine consumption (PND 73–92) were measured in adulthood.
Experiment 3
In Experiment 3, we assessed whether adolescent CVSS alters basal synaptic transmission in PFC L2/3 pyramidal neurons and NAC neurons (Table 1; n = 5/group). Basal synaptic function was measured in an ex vivo brain slice preparation using whole-cell voltage-clamp recordings in a separate experimental group of mice during adulthood (PND 64–80).
Chronic variable social stress procedure
Mice were randomly assigned to the CVSS or non-stressed control (CON) group. Littermates were evenly distributed between groups to control for litter effects. The CVSS procedure was conducted as previously described (Caruso et al., 2017a, 2017b). Beginning on PND 25, CVSS mice were individually housed for 3 days followed by 4 days of re-socialization with 1–2 unfamiliar same-sex cagemates (i.e., social reorganization). This cycle was repeated 5 times from PND 25–59. This age-range is commonly accepted as the early to late adolescent stages of development, based on age-specific behavioral, neurobiological, and pubertal changes (McCormick & Mathews, 2010). The CON mice remained with their original cagemates throughout the experiment. In order to limit differences in handling and husbandry between groups, all CON mice were placed in clean cages twice per week. On PND 59, CVSS mice were re-housed with their original cage-mates from weaning, where they remained until testing for voluntary oral nicotine intake.
Behavioral testing
Behavioral testing on the EPM, SAAT, and for acute nicotine responses was performed in a room separate from the colony room. Mice were habituated to the behavior room for at least 1 h prior to testing.
Elevated plus-maze (EPM)
Anxiety-like behavior was assessed on the EPM as previously described (Caruso et al., 2017a, 2017b). Briefly, the EPM was comprised of two open (30 × 5 cm) and two closed Plexiglas arms (30 × 14.5 × 5 cm) that were elevated 42 cm above the ground. Testing occurred under dim red lights (~30 lx) between 9:00–12:00 h. At the beginning of each test, the mouse was placed on the central platform facing a closed arm. An overhead camera recorded each mouse and behavior was scored by an automated video tracking system (ANY-maze v.4.6, Stoelting, Wood Dale, IL, USA). At the conclusion of each 5 min test, the maze was cleaned with 30% ethanol. The percent time spent on open arms (Time on open arms/[Time on open arms + time on closed arms] × 100) was used as a measure of anxiety-like behavior whereas the number of closed arm entries was used as a measure of general locomotor activity (Rodgers and Dalvi, 1997; File, 2001).
Social approach-avoidance test (SAAT)
Prior studies have reported differences in the anxiogenic effects of adolescent CVSS when tested in the social interaction test compared to a non-social test (e.g. EPM or OFT) (Vidal et al., 2007; Green et al., 2013; Saavedra-Rodríguez and Feig, 2013; Toth and Neumann, 2013). In the current study, social avoidance was measured in a modified version of the SAAT as previously described (Caruso et al., 2017b). Briefly, the SAAT was conducted in an open field arena (60 × 60 × 30 cm) made from white Plexiglas. Testing occurred between 13:00–17:00 h in a dimly lit room (~30 lx red light). Each mouse was allowed to freely explore the arena during two consecutive 2.5 min trials. Mice were placed in the home cage for 1 min between trials. During trial 1 (“Social Target Absent”) an empty circular wire mesh cage (diameter = 9 cm) was located along one wall of the arena. During trial 2 (“Social Target Present”), the empty wire mesh cage was replaced with an identical cage containing an unfamiliar same-sex adult C57BL/6J mouse. Behavior was scored by an automated video tracking system (ANY-maze v.4.6, Stoelting, Wood Dale, IL, USA). The total distance traveled (cm) and time spent in the social interaction (SI) zone (5 cm corridor surrounding the wire mesh cage) were analyzed during each trial. Social avoidance was defined as a reduction in time spent in the SI zone during the trial 2 (“Social Target present”) relative to trial 1 (“Social Target Absent”).
Acute nicotine responses
Nicotine-induced locomotor activity, body temperature, and plasma CORT secretion were assessed using a within-subjects design as part of a modified test battery (Marks et al., 1989). All animals received intraperitoneal injections of saline or nicotine on different test days (Experiment 1 – 0.5 mg/kg; Experiment 2 – 0.5 and 1.0 mg/kg; doses presented as freebase nicotine; Sigma Aldrich, St. Louis, MO, USA). The order of nicotine and saline injections were counter-balanced and testing sessions were conducted two days apart. Nicotine doses and testing times were based on published methods (Marks et al., 1989; Kamens et al., 2015; Caruso et al., 2017b). In Experiment 1, acute nicotine responses were assessed in a subset of female mice (n = 10–12/group). In Experiment 2, sample size for nicotine-induced change in locomotor activity was reduced due to a technical error (n = 2–4/group excluded).
In Experiments 1 & 2, acute nicotine effects on locomotor activity were assessed in a symmetrical Y-maze made of translucent red Plexiglas that consisted of 3 covered arms (27.5 × 8 × 10 cm). Testing occurred between 13:00–16:00 h and light intensity was low inside the Y-maze (~30 lx). Mice were given an injection and immediately placed into the center of the Y-maze, where locomotor activity was monitored with an automated video tracking system for 10 min (ANY-maze v.4.6, Stoelting, Wood Dale, IL, USA). At the conclusion of the test, mice were placed in a holding cage and body temperature was measured 15 min after injection using a TH-5 Thermalert Monitoring Thermometer with a RET-3 mouse rectal probe (Physitemp Instruments Inc., Clifton, NJ, USA) that was lubricated by peanut oil. Nicotine-induced locomotor activity was assessed by total distance traveled during two consecutive 5 min time bins. Nicotine-induced changes in locomotor activity (cm) and body temperature (°C) were used as dependent variables. Change scores were calculated as [nicotine response – saline response].
In Experiment 2, plasma CORT levels were assessed at baseline and following each saline/nicotine injection. Mice were transported in holding cages to a separate procedure room for blood collection. The mice were briefly immobilized in a broom-style restrainer 30 and 90 min following the injection, a short segment (< 1 mm) of the tail tip was cut with a scalpel, and blood was collected. Baseline samples were collected three days after the final nicotine/saline injection. All samples were collected within 3 min of initial cage disruption between 14:00–16:00 h. Near-maximal effects of nicotine on CORT secretion are observed 30 min after nicotine injection and approximate baseline levels 90 min after injection (Lutfy et al., 2006; Caruso et al., 2017b).
Two-bottle choice nicotine consumption
Voluntary oral nicotine consumption was measured in a standard 2-bottle free choice paradigm as previously described (Kamens et al., 2015; Caruso et al., 2017b). Mice were individually housed for two days prior to the start of the drinking procedure with free access to food and water to acclimate to the test environment. Water was provided in two drinking tubes (25 ml graduated cylinders fitted with drinking spouts). On day 1 of nicotine testing, one of the water tubes was replaced with a tube containing nicotine. Nicotine solutions were made of freebase nicotine diluted in tap water (Matta et al., 2007). Fluid volumes were recorded daily at ~14:00 h to monitor consumption. Every two days, the left/right location of the nicotine- and water-containing bottles was switched to avoid the development of side preference. Every four days mice were weighed and the nicotine concentration increased: days 1–4 (25 µg/ml), days 5–8 (50 µg/ml), days 9–12 (100 µg/ml), and days 13–16 (200 µg/ml). Consumption data for each nicotine concentration were adjusted for the corresponding body weight. Non-consumption-related fluid loss was recorded from drinking tubes placed on 4 empty cages to account for evaporation/leakage during the experiment. Nicotine consumption (mg/kg), nicotine preference (ml of nicotine/ total ml of fluid), and total fluid consumed (ml) were analyzed to measure nicotine consumption. These dependent variables were derived from the average of days 2 and 4 of each nicotine concentration (i.e., the second full day after the bottle side or drug concentration was changed) (Phillips et al., 1994).
Corticosterone radioimmunoassay
Blood samples were collected into heparinized capillary tubes (RAM Scientific, Yonkers, NY, USA) and stored on ice until plasma was separated and stored at −80 °C. Commercial [I125] radioimmunoassay kits (MP Biomedicals, Solon, OH, USA) were used to measure CORT levels according to the manufacturer’s instructions. Samples were assayed in duplicate. Intra- and inter-assay coefficients of variation for high and low controls were 12.7 and 7.6, respectively. Area under the curve (Pruessner et al., 2003) was calculated from the baseline, 30 min, and 90 min CORT levels to provide an integrated measure of CORT response to saline/nicotine injections. The experimenter was blinded to stress condition until all assays were completed.
Brain slice patch-clamp electrophysiology
Whole-cell voltage-clamp electrophysiological recordings were performed in the PFC (infralimbic and prelimbic regions) and NAC from acutely-prepared coronal brain slices according to landmarks based on the Allen Mouse Brain Atlas as previously described (Pleil et al., 2015; Crowley et al., 2016). Following the conclusion of CVSS, mice were deeply anesthetized with 1–2% isoflurane and decapitated. Brains were rapidly removed and placed in ice-cold high sucrose-artificial cerebrospinal fluid (aCSF) containing (in mM) 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3 and saturated with 95% O2/5% CO2. Coronal slices 300 µM in thickness containing the PFC and NAC were sectioned on a Leica VT1200 vibratome and stored in a holding chamber with 30°C, oxygenated aCSF containing (in mM) 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3, where they remained for at least 1 h before electrophysiological recordings. Slices were transferred to a submerged recording chamber for experiments, where they were perfused with heated, oxygenated aCSF at a rate of approximately 2 ml/min.
Recording electrodes (3–5 MΩ) were pulled from thin-walled borosilicate glass capillaries with a Flaming-Brown Micropipette Puller (Sutter Instruments, Novato, CA). Recordings were performed in pyramidal neurons of L2/3 of the PFC. Neuronal subtypes were not distinguished in the NAC, but recordings were most likely performed in medium spiny neurons (90–95% of all striatal neurons (Scofield et al., 2016)). Excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs, respectively) were measured in voltage-clamp mode using electrodes filled with an intracellular recording solution containing (in mM): 135 Cs-methanesulfonate, 10 KCl, 10 HEPES, 1 MgCl2, 0.2 EGTA, 4 Mg-ATP, 0.3 GTP, 20 phosphocreatine. Neurons were held at −55 mV to isolate glutamatergic synaptic transmission and record spontaneous EPSCs (sEPSCs) or +10 mV to isolate GABAergic synaptic transmission and record spontaneous IPSCs (sIPSCs) within individual neurons. Signals were digitized at 10 kHz and filtered at 6 kHz using a Multiclamp 700B amplifier and analyzed using Clampfit v.10.6 software (Molecular Devices, Sunnyvale, CA, USA). For all measures, recordings were performed in a maximum of 3–4 neurons per subregion, per mouse (minimum of 4 separate mice), and n’s reflect the number of neurons for each mouse. The experimenter was blinded to stress condition until all analysis was completed.
Statistics
All statistical analyses were performed in R (v3.3.2). Analyses of results from the EPM, SAAT, nicotine responses, and electrophysiological measures of synaptic transmission were analyzed using analysis of variance (ANOVA) or a mixed factorial ANOVA, where appropriate, with ‘Sex’, ‘Stress Condition’, ‘Nicotine Dose’, ‘Nicotine Concentration’, ‘Time', or ‘Social Target Presence’ as possible independent variables. In all statistical models, continuous litter means were included as covariates to control for litter effects. Whenever a significant main effect or interaction was identified post hoc analyses were performed using Tukey’s HSD. An α < 0.05 was considered significant for all statistical analyses including post hoc comparisons. Outliers were detected using Grubb’s outlier test (Grubbs, 1969) and data were excluded. For all statistical calculations, the number of outliers and corrected sample sizes are provided in the following format (CON Female/CVSS Female/CON Male/CVSS Male/) unless otherwise stated. Partial correlations, controlling for litter, were calculated separately for males and females in each experiment to test the relationship between anxiety-like behavior and nicotine responses within individual mice. Data are presented as mean ± standard error of the mean (SEM).
RESULTS
Effects of adolescent CVSS on anxiety-like behavior and social avoidance
EPM behavior
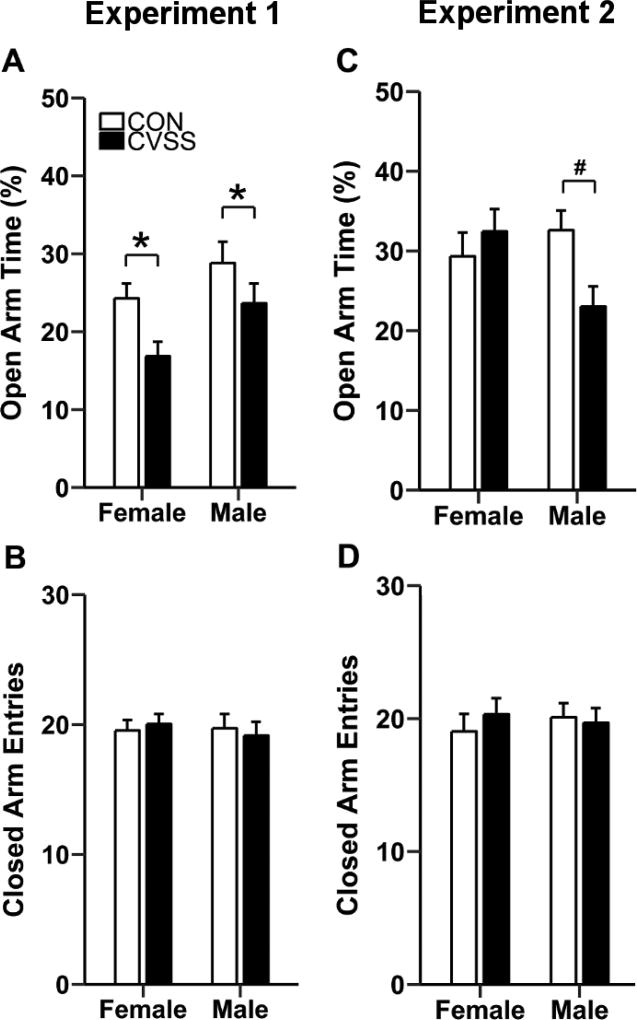
Adolescent CVSS exposure decreased percent open arm time on the EPM during adulthood (Fig. 1). In Experiment 1, relative to CON mice, CVSS mice spent less time on the open arms of the EPM (Fig. 2A; main effect of ‘Stress Condition’: F1,54= 9.72, p < 0.01; 2 outliers, 19/20/9/11). Overall, females spent less time on the open arms than males (Fig. 1A; main effect of ‘Sex’: F1,54= 5.68, p < 0.05), but there was no significant interaction with ‘Stress Condition'. There were no significant effects of ‘Stress Condition’ or ‘Sex’ on closed arm entries on the EPM (Fig. 1B; 1 outlier, 19/20/10/11).
Figure 1.
Adolescent CVSS increased anxiety-like behavior on the elevated plus-maze (EPM). In Experiment 1, (A) CVSS exposure decreased the percent time on the open arms of the EPM in males and females relative to CON mice (p < 0.01). CVSS exposure did not influence (B) the number of closed arm entries on the EPM. In Experiment 2, (C) CVSS exposure decreased the percent time spent on the open arms of the EPM in males, but not females, relative to CON males (p < 0.05). CVSS exposure did not influence the (D) number of closed arm entries on the EPM. Data are presented as mean ± SEM (Experiment 1: n = 10–21/group; Experiment 2: n = 8–12/group). *Significant main effect of ‘Stress Condition’; #Significant ‘Sex × Stress Condition’ interaction
Figure 2.
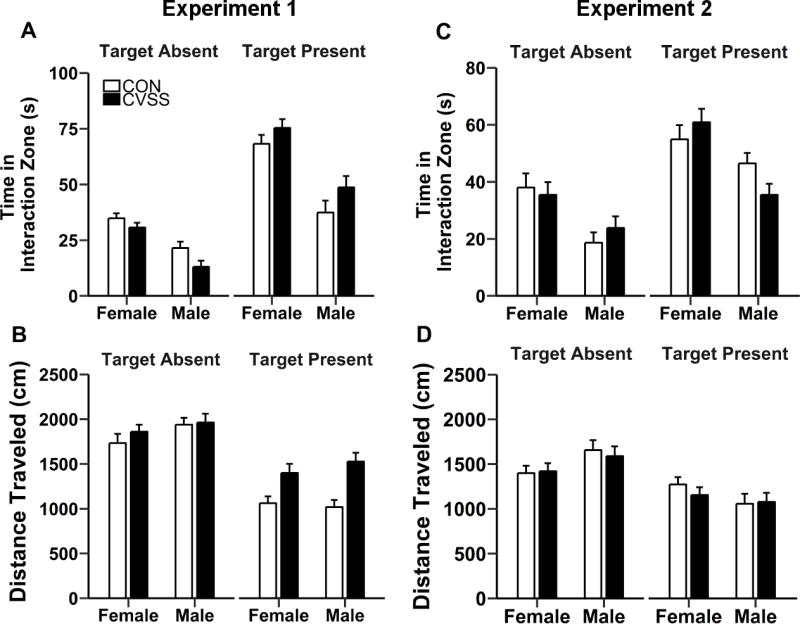
Adolescent CVSS did not influence social avoidance in the social approach-avoidance test (SAAT). In Experiment 1, there was no effect of adolescent CVSS on (A) time spent in the social interaction zone or (B) total distance traveled in the SAAT. In Experiment 2, there were no differences in (C) time spent in the social interaction zone (D) or total distance traveled between CVSS and CON mice. Data are presented as mean ± SEM (Experiment 1: n = 10–21/group; Experiment 2: n = 8–12/group).
In Experiment 2, exposure to adolescent CVSS reduced percent open arm time in a sex-dependent manner (‘Sex × Stress Condition’ interaction; F1,35= 5.52, p < 0.05; No outliers, 8/9/12/11). Post hoc analyses revealed that CVSS males spent less time on the open arms than CON males (p < 0.01), whereas no difference was observed between CVSS and CON females (Fig. 1C). There were no effects of ‘Sex’ or ‘Stress Condition’ on closed arm entries (Fig. 1D; No outliers, 8/9/12/11).
SAAT behavior
Exposure to adolescent CVSS did not influence SAAT behavior (Fig. 2). In Experiment 1, analyses of time spent in the SI zone were performed separately in males and females due to a significant ‘Social Target Presence × Sex’ interaction (F1,57= 5.72, p < 0.05; No outliers, 19/21/10/11). In males, time spent in the SI zone was greater when the social target was present compared to when the social target was absent (main effect of ‘Social Target Presence’: F1,19= 65.79, p < 0.001; 43.3 ± 3.6 vs. 17.5 ± 1.8 s, respectively). Adolescent CVSS influenced time spent in the SI zone in a trial-dependent manner (‘Social Target Presence × Stress Condition’ interaction: F1,19= 9.24, p < 0.01). However, post hoc analyses failed to identify significant differences in the time that males spent in the SI zone (Fig. 2A). Like males, female mice spent more time in the SI zone when the social target was present compared to when the social target was absent (main effect of ‘Social Target Presence’: F1,38= 164.58, p < 0.001; 70.0 ± 2.3 vs. 32.7 ± 2.3 s, respectively). There was no effect of ‘Stress Condition’ on time spent in the SI zone in females (Fig. 2A). Analyses for total distance traveled in Experiment 1 were performed separately in males and females due to a significant ‘Social Target Presence × Sex’ interaction (F1,57= 16.29, p < 0.001; No outliers, 19/21/10/11). In males, mice traveled greater distances when the social target was absent compared to when the social target was present (main effect of ‘Social Target Presence’: F1,19= 11.64, p < 0.01; 1846.4 ± 80.4 vs. 1461.1 ± 80.4 cm), but there was no effect of ‘Stress Condition’ on total distance traveled (Fig. 2B). Similar results were obtained for females (main effect of ‘Social Target Presence’: F1,38= 299.83, p < 0.0001; Social target present: 1071.4 ± 44.8 cm and Social target absent: 1906.8 ± 44.8 cm).
In Experiment 2, analyses for time spent in the SI zone were performed separately in males and females due to a significant ‘Social Target Presence × Sex × Stress Condition’ interaction (F1,32= 8.94, p < 0.01; 1 outlier; 7/7/12/11). In males, time spent in the SI zone was greater when the social target was present than when absent (main effect of ‘Social Target Presence’: F1,20= 69.93, p < 0.001; 41.6 ± 2.6 vs. 21.9 ± 2.6 s, respectively). Additionally, adolescent CVSS influenced time spent in the SI zone in a trial-dependent manner (‘Social Target Presence × Stress Condition’ interaction; F1,20= 11.16, p < 0.01). However, post hoc analyses failed to identify significant differences in the time that males spent in the SI zone (Fig. 2C). Females spent more time in the SI zone when the social target was present compared to when the social target was absent (main effect of ‘Social Target Presence’: F1,12= 35.50, p < 0.001; 57.0 ± 3.3 vs. 35.6 ± 3.2 s, respectively). There was no effect of ‘Stress Condition’ on time spent in the SI zone for females (Fig. 2C). Analyses for distance traveled in the SAAT (1 outlier; 7/7/12/11) were performed separately in males and females due to a significant ‘Social Target Presence × Sex’ interaction (F1,32= 15.10, p < 0.001). Total distance traveled was greater when the social target was absent, relative to when the social target was present, for both males (main effect of ‘Social Target Presence’: F1,20= 7.16, p < 0.05; 1395.4 ± 53.5 vs. 1211.9 ± 52.2 cm, respectively) and females (main effect of ‘Social Target Presence’: F1,12= 121.95, p < 0.001; 1644.0 ± 89.8 vs. 1069.1 ± 88.9 cm, respectively). There was no effect of ‘Stress Condition’ on total distance traveled for sex (Fig. 2D).
Effects of adolescent CVSS on acute nicotine responses and voluntary oral nicotine consumption
Acute nicotine responses
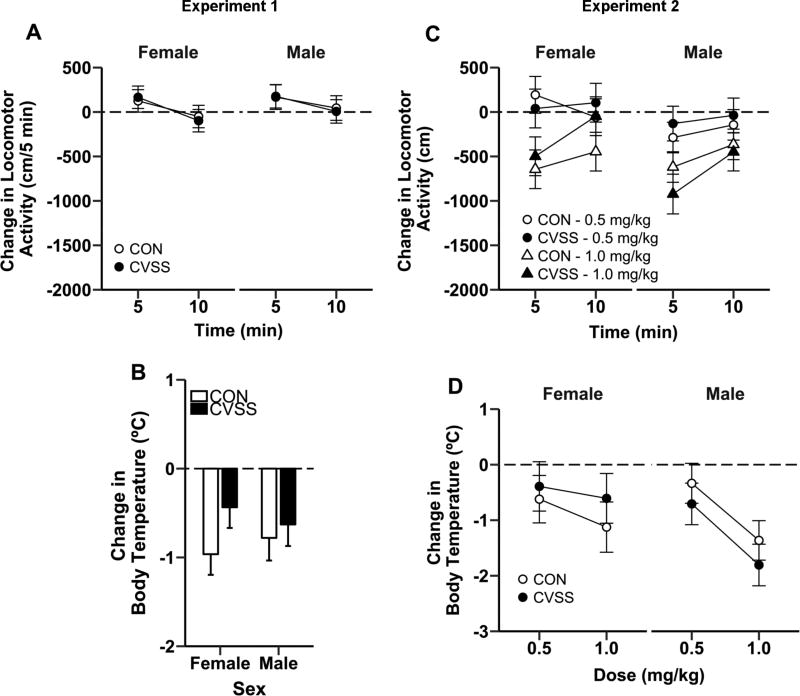
Adolescent CVSS did not influence acute nicotine-induced changes in locomotor activity or body temperature in late adolescence in Experiment 1 (Fig 3A–B; No outliers, 12/12/10/11). The nicotine-induced change in locomotor activity was significantly larger at 5 min compared to 10 min (main effect of ‘Time’: F1,41= 21.70, p < 0.001; 158.7 ± 65.4 vs. −24.3 ± 65.4 cm, respectively), but there was no effect of ‘Sex’ or ‘Stress Condition’ on nicotine-induced locomotor activity (Fig. 3A). There was no effect of ‘Sex’ or ‘Stress Condition’ for acute nicotine-induced change in body temperature (Fig. 3B).
Figure 3.
Adolescent CVSS did not influence acute nicotine effects on locomotor activity or body temperature during late adolescence or adulthood. There was no difference in the nicotine-induced (0.5 mg/kg, i.p.) change in (A) locomotor activity or (B) body temperature between CVSS and CON mice during late adolescence in Experiment 1. Similarly, there was no effect of adolescent CVSS exposure the nicotine-induced (0.5 or 1.0 mg/kg, i.p.) change in (C) locomotor activity or (D) body temperature during adulthood in Experiment 2. Data are presented as mean ± SEM of the nicotine response minus the saline response. Positive values indicate nicotine-induced locomotor stimulation and negative values indicate nicotine-induced locomotor depression and/or hypothermia (Experiment 1: n = 10–21/group; Experiment 2: n = 5–10/group).
There was no effect of adolescent CVSS on nicotine-induced changes in locomotor activity or body temperature in Experiment 2 (Fig. 3C–D). Analyses for nicotine-induced change in locomotor activity were performed for each nicotine dose separately due to a significant ‘Time × Nicotine Dose’ interaction (F1,68= 26.80, p < 0.001). There were no significant effects of ‘Time’, ‘Sex’, or ‘Stress Condition’ on nicotine-induced change in locomotor activity following treatment with 0.5 mg/kg nicotine (Fig. 3C; No outliers, 7/6/10/8). Treatment with 1.0 mg/kg nicotine resulted in a significantly larger change in locomotor activity at 5 min compared to the nicotine-induced change at 10 min (main effect of ‘Time’: F1,23= 14.15, p < 0.01; −682.1 ± 89.9 vs. −340.0 ± 88.2 cm, respectively; No outliers, 6/6/10/6). There were no effects of ‘Sex’ or ‘Stress Condition’ on nicotine-induced change in locomotor activity following treatment with 1.0 mg/kg nicotine (Fig. 3C). There were no significant effects of ‘Sex’ or ‘Stress Condition’ on nicotine-induced change in body temperature (Fig. 3D; 0.5 mg/kg: No outliers, 8/7/11/10; 1.0 mg/kg: 3 outliers, 7/7/10/11), but treatment with 1.0 mg/kg nicotine resulted in a significantly larger reduction in body temperature compared to treatment with 0.5 mg/kg nicotine (main effect of ‘Nicotine Dose’: F1,27= 8.14, p < 0.01; −1.2 ± 0.2 vs. −0.5 ± 0.2 °C, respectively).
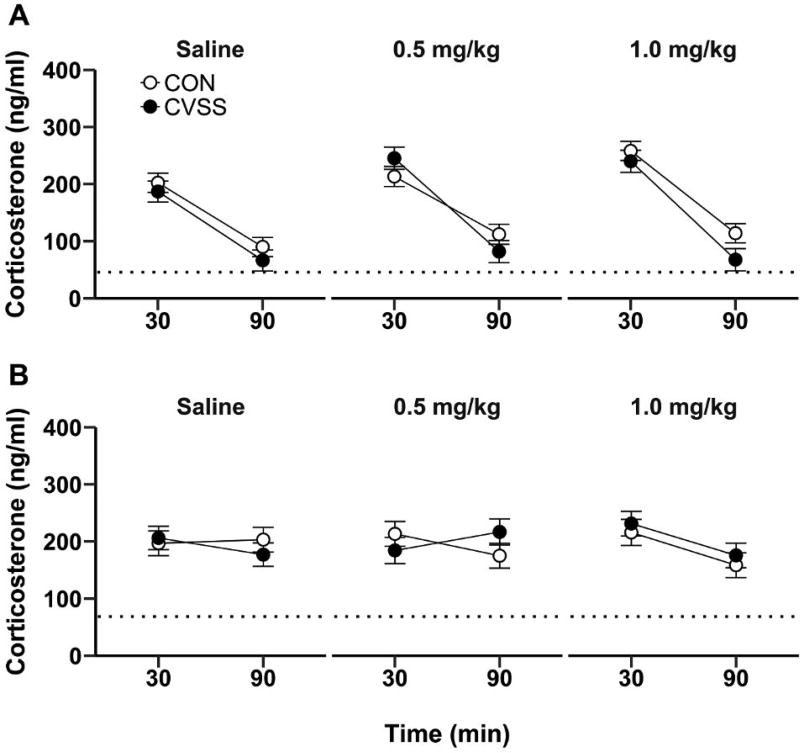
There was no effect of adolescent CVSS on adult CORT levels at baseline or following nicotine injection in Experiment 2 (Fig. 4). At baseline, females had higher plasma CORT than males (main effect of ‘Sex’: F1,32= 5.68, p < 0.05; 68.6 ± 7.3 vs. 45.6 ± 6.3 ng/ml, respectively; 1 outlier, 7/9/12/9). There was no effect of ‘Stress Condition’ or ‘Sex × Stress Condition’ interaction for baseline plasma CORT levels. Analyses for nicotine-induced CORT secretion were performed separately for males and females at each nicotine dose due to a significant ‘Nicotine Dose × Time × Sex × Stress Condition’ interaction (F2,162= 3.06, p < 0.05). In males, plasma CORT levels were consistently higher at 30 min (saline: No outliers, CON: 12/CVSS: 10; 0.5 mg/kg: No outliers, CON: 11/CVSS: 9; 1.0 mg/kg: No outliers, CON: 12/CVSS: 9), relative to 90 min (saline: No outliers, CON: 12/CVSS: 10; 0.5 mg/kg: No outliers, CON: 11/CVSS: 9; 1.0 mg/kg: No outliers, CON: 12/CVSS: 9), for all nicotine doses (Fig. 4A; main effect of ‘Time’ for saline: F1,20= 137.16, p < 0.001, 0.5 mg/kg nicotine: F1,18= 51.55, p < 0.001, 1.0 mg/kg nicotine: F1,19= 51.66, p < 0.001). Furthermore, there was no effect of ‘Stress Condition’ on plasma CORT levels following saline or nicotine injections (Fig. 4A). In females, there was no effect of ‘Time’ on plasma CORT levels following saline (30 min: No outlier, CON: 8/CVSS: 9; 90 min: No outliers, CON: 8/CVSS: 9) or 0.5 mg/kg nicotine (30 min: No outlier, CON: 8/CVSS: 7; 90 min: No outlier, CON: 8/CVSS: 7). However, plasma CORT levels were higher at 30 min (No outliers, CON: 7/CVSS: 8), relative to 90 min (No outliers, CON: 8/CVSS: 8), following 1.0 mg/kg nicotine injection (Fig. 4B; main effect of ‘Time’: F1,13= 10.02, p < 0.01). Finally, there was no significant effect of ‘Stress Condition’ on plasma CORT levels following the saline or nicotine injections (Fig. 4B).
Figure 4.
Adolescent CVSS did not influence acute nicotine-induced glucocorticoid secretion during adulthood. There were no differences in plasma corticosterone response to saline or nicotine (0.5 or 1.0 mg/kg, i.p.) injections in adult (A) male or (B) female mice. Dotted lines indicate mean baseline plasma corticosterone levels. Data are presented as mean ± SEM (n = 8–12/group).
Two-bottle choice nicotine consumption
There was no effect of adolescent CVSS on adult nicotine consumption or preference in Experiments 1 or 2 (Table 2). In Experiment 1, mice consumed more nicotine when the 100 µg/ml concentration was available compared to 25 and 200 µg/ml nicotine (main effect of ‘Nicotine Concentration’: F3,163= 8.16, p < 0.001; 25 µg/ml [No outliers, 19/21/10/11]: 2.4 ± 0.2 mg/kg; 50 µg/ml [1 outlier, 19/21/10/10]: 3.2 ± 0.4 mg/kg; 100 µg/ml [6 outliers, 18/20/7/10]: 4.6 ± 0.5 mg/kg, 200 µg/ml [1 outlier, 19/20/10/11]: 2.9 ± 0.5 mg/kg; Post hoc: p < 0.05). Additionally, females consumed more nicotine than males (main effect of ‘Sex’: F1,56= 3.95, p = 0.05; 3.6 ± 0.3 vs. 2.9 ± 0.4 mg/kg, respectively). Nicotine preference decreased when the 100 and 200 µg/ml nicotine concentrations were available compared to 25 µg/ml nicotine (main effect of ‘Nicotine Concentration’; F3,171= 27.82, p < 0.001; 25 µg/ml: 0.4 ± 0.03 mg/kg; 50 µg/ml: 0.3 ± 0.03 mg/kg; 100 µg/ ml: 0.3 ± 0.03 mg/kg, 200 µg/ml: 0.1 ± 0.02 mg/kg; Post hoc: p < 0.01; No outliers, 19/21/10/11). There was no effect of ‘Stress Condition’ on nicotine consumption or nicotine preference (Table 2). There were no effects of ‘Nicotine Concentration’ or ‘Stress Condition’ on total fluid consumption (25 µg/ml: No outliers, 19/21/10/11; 50 µg/ml: 1 outlier, 19/20/10/11; 100 µg/ ml: No outlier, 19/21/10/11; 200 µg/ml: 1 outlier, 19/20/10/11) but females consumed more total fluid than males (main effect of ‘Sex’: F1,56= 5.43, p < 0.05; 6.0 ± 0.1 vs. 5.6 ± 0.2 ml, respectively).
Table 2.
Adolescent CVSS did not influence oral nicotine consumption in Experiments 1 and 2.
| Female | Male | ||||
|---|---|---|---|---|---|
|
|
|||||
| CON | CVSS | CON | CVSS | ||
| Experiment 1 | |||||
| Nicotine Consumption (mg/kg): | |||||
| 25 µg/ml | 2.5 ± 0.3 | 2.9 ± 0.3 | 2.3 ± 0.4 | 1.7 ± 0.4 | |
| 50 µg/ml | 2.9 ± 0.3 | 3.7 ± 0.6 | 2.5 ± 0.9 | 3.3 ± 0.9 | |
| 100 µg/ml | 5.7 ± 0.8 | 4.7 ± 0.8 | 4.1 ± 1.3 | 3.7 ± 1.1 | |
| 200 µg/ml | 3.9 ± 0.7 | 2.4 ± 0.7 | 2.2 ± 1.1 | 3.1 ± 1.0 | |
| Nicotine Preference: | |||||
| 25 µg/ml | 0.40 ± 0.4 | 0.43 ± 0.04 | 0.44 ± 0.06 | 0.32 ± 0.06 | |
| 50 µg/ml | 0.27 ± 0.05 | 0.32 ± 0.05 | 0.25 ± 0.08 | 0.35 ± 0.07 | |
| 100 µg/ml | 0.30 ± 0.05 | 0.19 ± 0.05 | 0.37 ± 0.07 | 0.22 ± 0.07 | |
| 200 µg/ml | 0.17 ± 0.03 | 0.10 ± 0.03 | 0.12 ± 0.05 | 0.12 ± 0.04 | |
|
| |||||
| Experiment 2 | |||||
| Nicotine Consumption (mg/kg): | |||||
| 25 µg/ml | 2.4 ± 0.5 | 2.5 ± 0.4 | 1.6 ± 0.4 | 1.5 ± 0.4 | |
| 50 µg/ml | 3.7 ± 0.8 | 4.7 ± 0.8 | 2.6 ± 0.7 | 1.9 ± 0.7 | |
| 100 µg/ml | 8.1 ± 2.1 | 9.5 ± 1.9 | 8.4 ± 1.7 | 9.2 ± 1.8 | |
| 200 µg/ml | 5.2 ± 2.04 | 5.4 ± 1.8 | 4.3 ± 1.5 | 5.7 ± 1.7 | |
| Nicotine Preference: | |||||
| 25 µg/ml | 0.27 ± 0.07 | 0.37 ± 0.06 | 0.28 ± 0.05 | 0.26 ± 0.06 | |
| 50 µg/ml | 0.26 ± 0.06 | 0.36 ± 0.06 | 0.21 ± 0.05 | 0.16 ± 0.05 | |
| 100 µg/ml | 0.38 ± 0.08 | 0.38 ± 0.07 | 0.41 ± 0.06 | 0.42 ± 0.07 | |
| 200 µg/ml | 0.12 ± 0.05 | 0.12 ± 0.05 | 0.10 ± 0.04 | 0.18 ± 0.04 | |
Data (mean ± SEM) represent average 24 h nicotine consumption and preference for nicotine. (Experiment 1: n = 10–21/group; Experiment 2: n = 8–12/group).
In Experiment 2, nicotine consumption was greatest when the 100 µg/ml (1 outlier, 8/9/11/11) concentration was available compared to 25 µg/ml (No outliers, 8/9/12/11), 50 µg/ml (No outliers, 8/9/12/11), and 200 µg/ml nicotine (2 outliers, 7/9/12/10) and consumption of 25 µg/ml nicotine was lower than all other concentrations (main effect of ‘Nicotine Concentration’; F3,105=20.49, p < 0.001; 25 µg/ml: 2.0 ± 0.2 mg/kg, 50 µg/ml: 3.3 ± 0.4 mg/kg, 100 µg/ml: 8.8 ± 0.9 mg/kg, 200 µg/ml: 5.3 ± 0.9 mg/kg; Tukey’s HSD: p < 0.05). Additionally, females consumed more nicotine than males (main effect of ‘Sex’: F1,35= 4.62, p < 0.05; 5.3 ± 0.6 vs. 4.4 ± 0.5 mg/kg, respectively). Nicotine preference followed an inverted-U shaped dose-response curve. Mice preferred nicotine more when the 100 µg/ml (No outliers, 8/9/12/11) concentration was available relative to 50 µg/ml (No outliers, 8/9/12/11) and 200 µg/ml nicotine (No outliers, 8/9/12/11) and preference for the 200 µg/ml concentration was lower than all other nicotine concentrations (main effect of ‘Nicotine Concentration’: F3,108= 11.03, p < 0.001; 25 µg/ml: 0.3 ± 0.03 mg/kg, 50 µg/ml: 0.3 ± 0.03 mg/kg, 100 µg/ml: 0.4 ± 0.03 mg/kg, 200 µg/ml: 0.1 ± 0.02 mg/kg; Tukey’s HSD: p < 0.05). There was no effect of ‘Stress Condition’ on nicotine consumption or nicotine preference (Table 2). There were no effects of ‘Nicotine Concentration’, ‘Sex’, or ‘Stress Condition’ on total fluid consumption (data not shown).
Correlation analyses
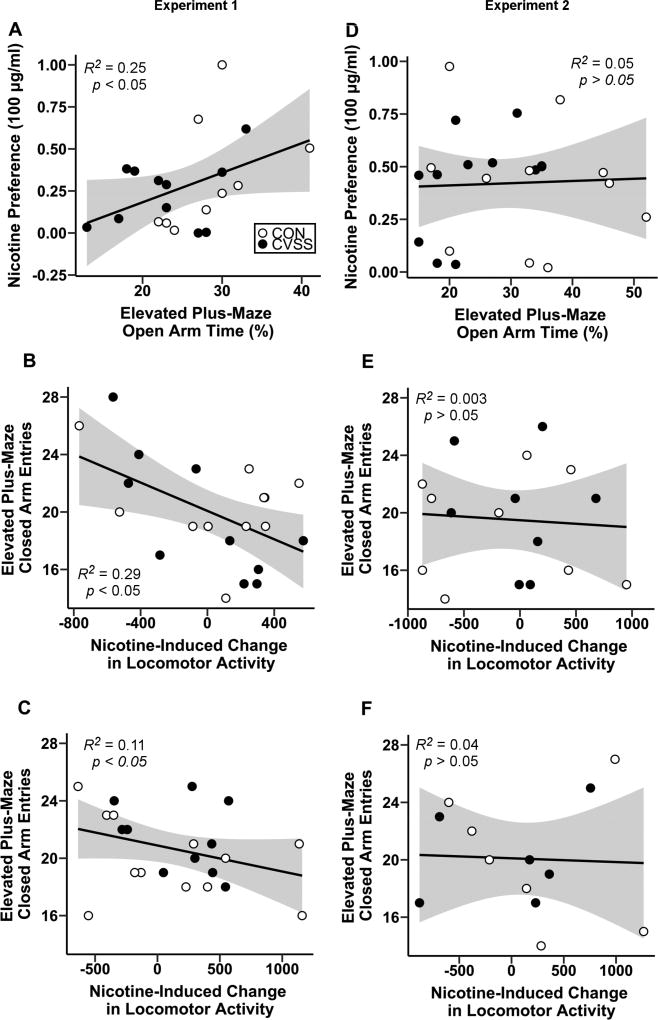
Correlation analyses were performed to assess the relationship between individual differences in anxiety and nicotine behaviors. In Experiment 1, males that spent more time on the open arms displayed greater preference for the 100 µg/ml nicotine concentration (Fig. 5A; R2= 0.25, df= 14, p < 0.05). Furthermore, mice that entered the closed arms of the EPM more frequently also exhibited greater nicotine-induced (0.5 mg/kg) locomotor depression (Fig. 5B–C; Males: R2= 0.29, df= 17, p < 0.05; Females: R2= 0.11, df= 20, p < 0.05). There were no additional associations between anxiety-like behavior on the EPM and nicotine responses in males or females. In Experiment 2, individual differences in (Fig. 5D) time spent on the open arms and closed arm entries on the EPM (Fig. 5E–F) were not associated with adult nicotine responses. However, exploratory analyses revealed several associations between nicotine-induced CORT responses, oral nicotine consumption, and nicotine-induced changes in locomotor activity. In males, greater nicotine-induced (0.5 mg/kg) CORT responses were associated with greater nicotine consumption (R2 = 0.24, df= 15, p < 0.05) and preference (R2 = 0.31, df= 16, p < 0.01) for 200 µg/ml nicotine. Additionally, males that had greater nicotine-induced (0.5 mg/kg) CORT responses displayed greater nicotine-induced locomotor depression following 0.5 mg/kg nicotine (5 min: R2 = 0.29, df= 11, p < 0.01; 10 min: R2 = 0.22, df= 11, p < 0.05) and 1.0 mg/kg nicotine (5 min: R2 = 0.38, df= 10, p < 0.01) injections.
Figure 5.
Linear relationship between anxiety-like behavior, exploratory locomotion on the EPM, and nicotine responses. In Experiment 1, (A) adult males that exhibited greater preference for 100 µg/ml nicotine spent less time on the open arms of the EPM. (B) Males and (C) females that exhibited greater nicotine-induced (0.5 mg/kg, i.p.) locomotor depression during late adolescence entered the closed arms of the EPM more frequently during adulthood. In Experiment 2, (D) preference for 100 µg/ml nicotine was not associated with time spent on the open arms of the EPM. Furthermore, nicotine-induced locomotor depression during adulthood was not associated with closed arm entries on the EPM for (E) males or (F) females. Shaded regions signify 95% confidence intervals.
Experiment 3: The effects of adolescent CVSS on basal synaptic function in the PFC and NAC
PFC
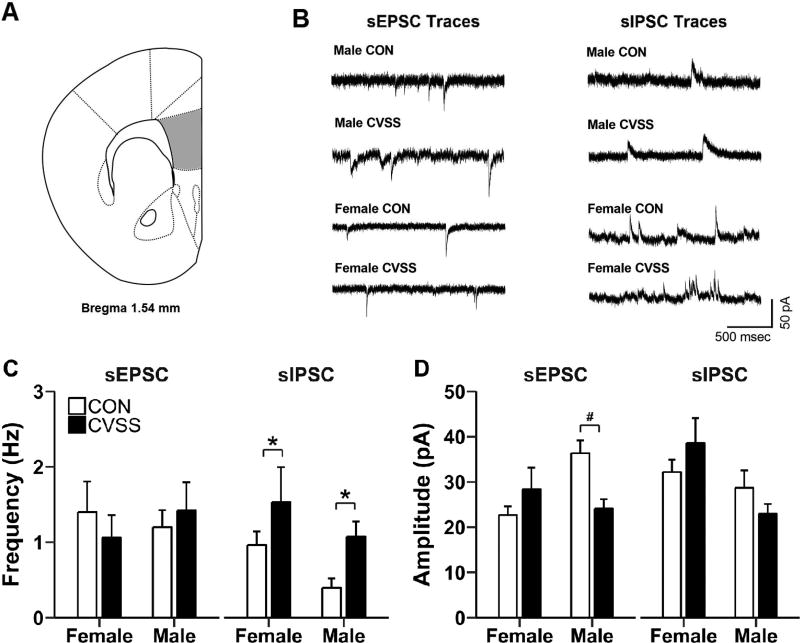
Exposure to adolescent CVSS increased inhibitory and decreased excitatory synaptic transmission in L2/3 pyramidal neurons of the PFC (Fig. 6C–D). Neurons from CVSS mice had higher sIPSC frequency compared to CON mice (Fig. 7C; main effect of ‘Stress Condition’: F1,20= 5.07, p < 0.05; 1 outlier, 5/6/6/7) and there was a tendency for neurons from females to have higher sIPSC frequency than males (Fig. 6C; main effect of ‘Sex’: F1,20= 3.34, p < 0.08). There was no effect of ‘Sex’ or ‘Stress Condition’ on sEPSC frequency (No outliers, 5/6/7/8). Adolescent CVSS influenced sEPSC amplitude in a sex-dependent manner (‘Sex × Stress Condition’ interaction: F1,22= 8.53, p < 0.01; No outliers, 5/6/7/8). Specifically, neurons from CVSS males had lower sEPSC amplitude than CON males (Fig. 6D; p < 0.05), but there was no difference between neurons from CVSS and CON females. Finally, neurons from females had greater sIPSC amplitude than males (main effect of ‘Sex’: F1,20= 7.16, p < 0.05; 35.4 ± 2.8 vs. 25.8 ± 2.6 pA, respectively; 1 outlier, 5/6/6/7), but there was no effect of ‘Stress Condition’ on sIPSC amplitude.
Figure 6.
Exposure to adolescent CVSS altered synaptic transmission in L2/3 pyramidal neurons of the prefrontal cortex (PFC). (A) Illustration of coronal half section of PFC; gray area depicts the region where recordings were performed. (B) Representative traces of sEPSC and sIPSC from CVSS and CON mice. (C) Exposure to CVSS increased sIPSC frequency relative to CON mice (p < 0.05). (D) Exposure to CVSS reduced sEPSC amplitude in males, but not females, relative to CON mice (p < 0.01). Data are presented as mean ± SEM (n = 5–8/group). *Significant main effect of ‘Stress Condition’; #Significant ‘Sex × Stress Condition’ interaction
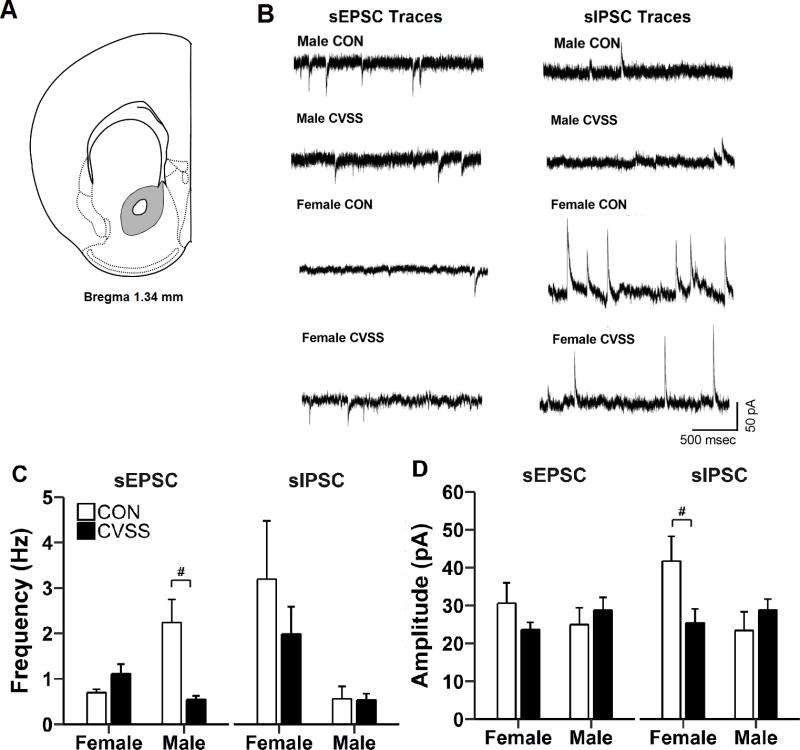
Figure 7.
Exposure to adolescent CVSS altered synaptic transmission nucleus accumbens (NAC) neurons. (A) Illustration of coronal half section of NAC; gray area depicts the region where recordings were performed. (B) Representative traces of sEPSC and sIPSC from CVSS and CON mice. Exposure to CVSS decreased (C) sEPSC frequency in males, but not females, and (D) sIPSC amplitude in females, but not males, relative to CON mice (p < 0.001 and p < 0.05, respectively). Data are presented as mean ± SEM (n = 5–8/group). #Significant ‘Sex × Stress Condition’ interaction
NAC
Adolescent CVSS exposure decreased excitatory synaptic transmission in NAC neurons of males and decreased inhibitory synaptic transmission in NAC neurons of females (Figure 7C–D). Adolescent CVSS influenced sEPSC frequency in NAC neurons in a sex-dependent manner (‘Sex × Stress Condition’ interaction: F1,19= 16.06, p < 0.001; No outliers, 5/7/5/6). Specifically, neurons from CVSS males had lower sEPSC frequency than CON males (Fig. 7C; p < 0.001), but there was no difference between neurons from CVSS and CON females. Females had higher sIPSC frequency than males (main effect of ‘Sex’: F1,17= 8.78, p < 0.01; 2.6 ± 0.5 vs. 0.5 ± 0.5 Hz, respectively; No outliers, 65/5/5/6), but there was no effect of ‘Stress Condition’ on sIPSC frequency. There was no effect of ‘Sex’ or ‘Stress Condition’ on sEPSC amplitude (No outliers, 5/7/5/6), but adolescent CVSS influenced sIPSC amplitude in NAC neurons in a sex-dependent manner (‘Sex × Stress Condition’ interaction: F1,17= 5.55, p < 0.05; No outliers, 6/5/5/6). Specifically, neurons from CVSS females had lower sIPSC amplitude than CON females (Fig. 7D; p < 0.05), but no there was no difference between neurons from CVSS and CON males.
DISCUSSION
Exposure to chronic variable social stress (CVSS) in adolescence resulted in increased anxiety-like behavior on the EPM in C57BL/6J mice. However, there was no effect of adolescent CVSS on nicotine responses. The current study was the first, to our knowledge, to characterize adolescent stress effects on basal synaptic function in both the PFC and NAC (i.e., brain regions that govern emotional and drug-seeking behaviors). In addition to effects on anxiety-like behavior in the EPM, adolescent CVSS also resulted in sex-specific alterations in PFC and NAC synaptic transmission. Although these neuronal adaptations are not directly linked with adolescent CVSS-induced anxiety-like behavior, our results demonstrate that exposure to chronic stress during adolescence leads to alterations in synaptic activity within stress-susceptible corticolimbic brain regions implicated in anxiety.
Adolescent CVSS increases adult anxiety-like behavior in C57BL/6J mice
Following the conclusion of adolescent stress exposure, adult CVSS mice displayed increased anxiety-like behavior by spending less time on the open arms of the EPM compared to CON males. This difference was not due to a reduction in general locomotor activity because adolescent CVSS did not affect the number of closed arm entries. These results are consistent with findings from clinical studies in human adolescents and preclinical rodent models which indicate that exposure to psychosocial stress during adolescence can increase vulnerability to developing affective disorders such as anxiety disorders (Koval et al., 2004; Vidal et al., 2007; McCormick et al., 2008; Sterlemann et al., 2008; Lukkes et al., 2009b; Green et al., 2013; Saavedra-Rodríguez and Feig, 2013; Warren et al., 2014; Iñiguez et al., 2014; Caruso et al., 2017a; Hodges et al., 2017). Although the results from Experiment 1 revealed a main effect of stress, reflecting a change in both sexes, a post hoc analysis of only the males from this experiment provided similar results. Experiment 1 CVSS males spent less time on the open arms than CON males (Main effect of ‘Stress Condition’ F1,18= 6.82, p < 0.05; CVSS = 23.5 ± 1.8 vs. CON = 30.5 ± 1.9). These data suggest that the long-lasting effects on anxiety-like behavior are reproducible in male mice. However, replication of the current findings with additional measures of anxiety-like behavior will be essential if strong conclusions are to be made. The effect of adolescent CVSS on female anxiety-like behavior was inconsistent. Adolescent CVSS increased female anxiety-like behavior on the EPM in Experiment 1, but not Experiment 2. Methodological differences between experiments may contribute to these inconsistent results. Acute nicotine responses were measured during late adolescence in Experiment 1 (prior to EPM testing) and adulthood in Experiment 2. Brief adolescent nicotine exposure augments adult anxiety- and depression-like behavior (Iñiguez et al., 2009; Slawecki et al., 2017). Additionally, a few minutes of nicotine exposure, at concentrations similar to those in human smokers, causes a long-lasting facilitation of excitatory PFC inputs to the basolateral amygdala (BLA) (Jiang et al., 2013). Therefore, the differences observed between females in Experiment 1 and 2 could reflect sensitization to the anxiogenic effects of brief adolescent nicotine exposure, but further experiments are required to test this hypothesis.
Social avoidance in the SAAT is thought to reflect social withdrawal, a common symptom in human psychiatric disorders such as social anxiety disorder and depression (Toth and Neumann, 2013). Several prior studies have demonstrated reduced social interaction following adolescent social stress (Vidal et al., 2007; Lukkes et al., 2009b; Green et al., 2013; Saavedra-Rodríguez and Feig, 2013; Iñiguez et al., 2014; Warren et al., 2014; Hodges et al., 2017). However, as was seen in a prior study with BALB/cJ mice (Caruso et al., 2017b), adolescent CVSS did not influence male or female SAAT behavior in the current study. SAAT behavior was measured 1–3 months after the conclusion of stress, so it is possible that the effects of adolescent CVSS dissipate over time. However, this seems unlikely because our results were similar in Experiments 1 and 2 and prior studies have demonstrated social avoidance up to 2 months after social stress concluded (Saavedra-Rodríguez and Feig, 2013). Alternatively, effects of adolescent social instability may only be detectable in social interaction tests that quantify unfettered social interactions [e.g., social play/allogrooming] with an unconfined stimulus animal (Green et al., 2013; Saavedra-Rodríguez and Feig, 2013; Hodges et al., 2017). Green and colleagues (2013) suggest that the demands of interacting with an unpredictable freely moving stimulus animal may be necessary to evoke social anxiety-like responses in animals exposed to adolescent social instability.
Adolescent CVSS does not influence nicotine behaviors in C57BL/6J mice
Prospective clinical studies have suggested that exposure to psychosocial stress during adolescence is an important risk factor for the initiation of nicotine use (Koval et al., 2004; Finkelstein et al., 2006) and these effects may be attributed to elevated levels of stress-related glucocorticoid hormones (Pomerleau and Pomerleau, 1991; de Wit et al., 2007; Rao et al., 2009). Glucocorticoids contribute to many of the pharmacological effects of nicotine, including those related to nicotine reinforcement and dependence, thereby promoting susceptibility to initiate use (Pauly et al., 1992; Fagen et al., 2007). In the current study, we found no effect of adolescent CVSS on acute nicotine-induced locomotor activity, hypothermia, or CORT responses in male and female C57BL/6J mice during late adolescence or adulthood. Similarly, there was no effect of adolescent social instability or chronic social defeat on nicotine-induced locomotor activity or nicotine self-administration (McCormick et al., 2004; McCormick and Ibrahim, 2007; Cruz et al., 2008; Zou et al., 2014). In contrast, adolescent CVSS augmented nicotine-induced (0.5 mg/kg) locomotor activity, without influencing hypothermia, in late adolescent male BALB/cJ mice (Caruso et al., 2017b). Taken together results suggest that, under certain conditions, adolescent social stress can alter the pharmacological effects of nicotine.
The precise conditions under which adolescent social stress alters nicotine responses are complex and likely depend on several factors. For example, acute nicotine stimulates locomotor activity in adolescent rodents but causes locomotor depression in adults (Yuan et al., 2015) and we observed similar age-related differences in nicotine-induced locomotor activity in Experiments 1 and 2. Interestingly, adolescent social instability diminished or augmented nicotine-induced (0.5 mg/kg) locomotor activity when assessed in late adolescent and adult female Long-Evans rats, respectively (McCormick et al., 2004; McCormick and Ibrahim, 2007). Notably, adolescent social instability did not influence female nicotine-induced locomotor activity at a lower dose (0.25 mg/kg) or at any dose in males (McCormick et al., 2004, 2005; McCormick and Ibrahim, 2007). Furthermore, a pharmacological stressor, yohimbine, escalated nicotine self-administration at lower doses in adolescent female Long-Evans rats compared to males (Li et al., 2014). Thus, age, sex, genetic background, and nicotine dose can moderate the impact of adolescent stress on nicotine responses.
Voluntary oral nicotine consumption was also measured during adulthood to assess potentially long-lasting effects on adolescent CVSS on nicotine behaviors. In the current study, adolescent CVSS had no effect on voluntary oral nicotine consumption in either experiment. Again these results are in contrast to those for BALB/cJ males, in which adolescent CVSS reduced voluntary oral nicotine consumption (Caruso et al., 2017b). Only one additional study, to our knowledge, has investigated adolescent stress effects on nicotine intake and found that acute foot-shock stress reduced nicotine’s reinforcing efficacy in self-administering adolescent male Wistar rats (Zou et al., 2014). Curiously, the same stressor enhanced the acquisition of nicotine place preference in adolescent male Sprague-Dawley rats (Brielmaier et al., 2012). These data from rats, along with our findings in C57BL/6J and BALB/cJ mice, provide evidence that a gene × environment interaction influences nicotine responses.
Association between anxiety-like behaviors and nicotine responses
Given the strong bi-directional link between stress-related affective disorders and nicotine use (Fluharty et al., 2017), we examined the relationship between anxiety-like behaviors and nicotine responses within individual animals. In Experiment 1, individual differences in anxiety-like behavior on the EPM (e.g., time spent on the open arms) were associated with increased preference for 100 µg/ml nicotine in males. However, there were no additional associations between anxiety-like behavior and nicotine responses and this relationship was not found in Experiment 2. Similarly, there was no association between anxiety-like behavior and nicotine responses when tested in BALB/cJ mice (Caruso et al., 2017b). As such, there is little overlap between the divergent anxiety- or nicotine-related phenotypes displayed by stressed C57BL/6J and BALB/cJ mice, respectively. In future studies, it will be important to investigate whether genetic background moderates susceptibility to stress-induced affective disorders or nicotine use through unique neurobiological processes.
In Experiment 2, but not Experiment 1, mice that exhibited greater sensitivity to nicotine-induced (0.5 mg/kg) locomotor depression during late adolescence also displayed greater exploratory locomotion on the EPM (e.g., closed arm entries) during adulthood. Although these associations are relatively weak, they replicate prior findings in BALB/cJ mice (Caruso et al., 2017b). Interestingly, hypoactive NAC dopamine activity can reduce novelty-evoked exploratory locomotion (Ikemoto and Panksepp, 1999). A similar phenotype results from gain/loss-of-function mutations in nicotinic acetylcholine receptor subunits that modulate NAC dopamine release (Cui et al., 2003; Grady et al., 2007; Drenan et al., 2008). These similarities are intriguing in light of developmental changes in nicotinic receptor-dependent regulation of NAC dopamine release during adolescence (Yuan et al., 2015).
There are several potential explanations for the differences in the relationship between EPM behavior and nicotine responses in Experiments 1 and 2. First, the mice in Experiment 1 were treated with nicotine during late adolescence, prior to EPM testing, as opposed to nicotine treatment during adulthood in Experiment 2. Prior nicotine exposure can result in long-lasting alterations in anxiety- and depression-like behavior (Iñiguez et al., 2009; Slawecki et al., 2017). Moreover, the acute and long-term effects of initial nicotine exposure differ when it occurs during adolescence vs. adulthood (Yuan et al., 2015). Therefore, the animal’s age during testing and/or its initial nicotine exposure could impact the relationship between nicotine responses and EPM behavior. Additional studies will be required to disentangle the role of these experimental parameters in the relationship between anxiety-like behavior and nicotine responses.
Adolescent CVSS alters synaptic transmission in stress-susceptible corticolimbic brain regions
Clinical neuroimaging studies have demonstrated an association between reduced PFC function following exposure to early life stress and vulnerability to anxiety, depression, and/or drug abuse (Heim and Nemeroff, 2001; Andersen and Teicher, 2009). Consistent with these findings, adolescent CVSS increased sIPSC frequency in PFC L2/3 pyramidal neurons of both males and females. Relative to males, we also found greater sIPSC amplitude in the PFC of females, but there was no effect of adolescent CVSS. The absence of stress-induced changes to sIPSC amplitude suggests a presynaptic alteration such as an increase in the probability of GABA release or number of GABA release sites. Similar results were demonstrated in the PFC of male rats exposed to chronic variable stress during adulthood (McKlveen et al., 2016). Increased presynaptic GABA release was associated with a selective loss of glucocorticoid receptor-mediated inhibition of parvalbumin interneurons and increased GABAergic innervation of L5 pyramidal neurons (McKlveen et al., 2016). Parvalbumin-positive axon terminals are pruned to adult levels during adolescent PFC development (Anderson et al., 1995). The high level of cortical glucocorticoid receptor expression during adolescence (Pryce, 2008) may render this developmental process vulnerable to the effects of adolescent stress in both sexes.
Adolescent CVSS also resulted in a sex-specific reduction in glutamatergic transmission in the PFC. Adolescent CVSS decreased sEPSC amplitude, with no effect on frequency, in male PFC neurons such that it was comparable to females. This decrement in putative postsynaptic glutamate responses could reflect reduced dendritic branching and/or spine density which has been observed previously following adolescent stress (Leussis and Andersen, 2008; Eiland et al., 2012). Alternatively, downregulation of excitatory amino acid receptors (e.g., AMPA or NMDA receptors) could result in a similar effect (Novick et al., 2016). Consistent with our findings, adolescent social defeat reduced excitatory synaptic transmission in PFC L5 pyramidal neurons. In this study, stressed males displayed miniature EPSC frequency levels comparable to females (Urban and Valentino, 2016). L2/3 pyramidal neurons provide feedforward excitation to both striatum-projecting and thalamus-projecting L5 pyramidal neurons (Hirai et al., 2012). Stress-induced alterations to miniature EPSC frequency in L5 neurons could reflect a reduction in glutamatergic transmission from L2/3 synaptic inputs (Urban and Valentino, 2016). Given the roles of the PFC and ventral striatum in affective disorders and/or drug abuse (Heim and Nemeroff, 2001; Andersen and Teicher, 2009; Russo and Nestler, 2013), it would be interesting to determine whether adolescent stress selectively disrupts excitatory activity in projection-specific PFC L2/3–L5 pyramidal neuron circuits.
Stress-induced PFC hypofunction would be expected to impair excitatory cortical control over subcortical structures that mediate the behavioral effects of stress (Heim and Nemeroff, 2001; Andersen and Teicher, 2009; Russo and Nestler, 2013). The NAC is one such region that receives dense glutamatergic inputs from the PFC (Britt et al., 2012). In the current study, we found sex-specific alterations in NAC neurons following adolescent CVSS. In females, the amplitude, but not frequency, of sIPSCs was reduced to male-like levels following adolescent CVSS. The behavioral implications of reduced putative postsynaptic inhibitory transmission are unclear but results suggest that stress would augment NAC output in females. In contrast, the frequency, but not amplitude, of sEPSCs was reduced to female-like levels in males exposed to adolescent CVSS. This reduction in putative presynaptic excitatory transmission could reflect diminished activity at PFC glutamate terminals in the NAC (Britt et al., 2012), but this alteration may not contribute to adolescent CVSS-induced anxiety-like behavior. Optogenetic stimulation of PFC–BLA, but not PFC–NAC, glutamate projections control anxiety-like behavior (Vialou et al., 2014) and we found no effect of adolescent CVSS on synaptic transmission in the BLA (data not shown).
Alternatively, individual differences in anxiety-like behavior are associated with ventral striatal serotonin levels and stress-induced serotonin release was augmented in the NAC in vivo following exposure to adolescent social isolation (Schwarting et al., 1998; Lukkes et al., 2009a). Within the NAC, serotonin induced long-term depression at corticostriatal synapses which was expressed as a reduced probability of presynaptic glutamate release (Mathur et al., 2011). Finally, antagonism of corticotropin-releasing factor receptors in the dorsal raphe nucleus, a major source of serotonergic innervation to the NAC, abrogated the anxiety-like behavior caused by adolescent social isolation (Lukkes et al., 2009b). Taken together, these findings suggest that reduced excitatory synaptic activity observed in the NAC of CVSS males could be the result of increased serotonergic transmission following adolescent stress. This alteration may be sufficient to increase anxiety-like behavior.
Conclusion
The human clinical literature suggests that chronic stress may predispose individuals to develop comorbid affective disorders and nicotine use through a common underlying mechanism. In the current study, we found that adolescent chronic variable social stress increased anxiety-like behavior on the elevated plus-maze in adult C57BL/6J mice without influencing social avoidance or nicotine responses. These results are the opposite of those previously found in BALB/cJ males (Caruso et al., 2017b). Further, neither study produced considerable evidence of a linear relationship between nicotine responses, anxiety-like behavior, or social avoidance within individual animals. These strain-dependent effects of adolescent stress suggest that genetic background moderates susceptibility to stress-induced affective disorders and nicotine use. Additional studies with alternate measures of anxiety- and depression-like behavior and nicotine responses are necessary to further address this hypothesis. We also identified sex-specific alterations in basal synaptic transmission in the PFC and NAC – brain regions that are implicated in anxiety and addiction. Future research identifying mechanisms by which adolescent stress results in divergent effects on anxiety-like behavior or nicotine responses may identify unique biological mediators of stress-induced anxiety or nicotine use reported in the human literature.
Highlights.
Adolescent social stress increased anxiety-like behavior in C57BL/6J mice
Adolescent social stress did not influence nicotine responses
Stress decreased excitatory transmission in the prefrontal cortex of male mice
Stress decreased excitatory transmission in the nucleus accumbens core of male mice
Stress decreased inhibitory transmission in the nucleus accumbens core of female mice
Acknowledgments
We thank T.B. Fetherston, L.W. Gallaher, C.N. Miller, C.J. Silva, T.L. Pascoe, G.M. Gavitt, A.L. Brittingham, K.M. Czajkowski, L.A. Cannon, N.Q. Singh, and C.E. Peck for their outstanding technical assistance during data collection. Funding for this research was provided by NIH grants K01 AA019447 (HMK), R21 MH092667 (SAC), The Broadhurst Career Development Professorship for the study of Health Promotion and Disease Prevention (HMK), The Penn State Institute for Neuroscience (SAC), and The Penn State Social Science Research Institute (SAC). The funding sources had no role in data collection, analysis and interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- BLA
basolateral amygdala
- CON
control
- CORT
corticosterone
- CVSS
chronic variable social stress
- EPM
elevated plus-maze
- EPSC
excitatory postsynaptic current
- HPA
hypothalamic-pituitary-adrenal
- IPSC
inhibitory postsynaptic current
- L2/3
layer 2/3
- L5
layer 5
- NAC
nucleus accumbens
- PFC
prefrontal cortex
- PND
postnatal day
- SAAT
social approach-avoidance test
- SEM
standard error of the mean
- sEPSC
spontaneous excitatory postsynaptic current
- SI
social interaction
- sIPSC
spontaneous inhibitory postsynaptic current
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare no conflict of interest.
Author contributions
MJC, HMK, SAC, and NAC designed the experiments. MJC, DER, JIC, SAC, NAC, and HMK performed the experiments. MJC analyzed the data, interpreted the results, and drafted the manuscript. HMK, SAC, NAC, and BL edited the manuscript. All authors approved the final version of the manuscript.
References
- Andersen SL, Teicher MH. Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neuroscience and Biobehavioral Reviews. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of the monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiology & Behavior. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. Available at: http://www.sciencedirect.com/science/article/pii/S0031938401004498. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Effects of acute stress on acquisition of nicotine conditioned place preference in adolescent rats: A role for corticotropin-releasing factor 1 receptors. Psychopharmacology. 2012;219:73–82. doi: 10.1007/s00213-011-2378-1. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. Available at: http://dx.doi.org/10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso MJ, Kamens HM, Cavigelli SA. Exposure to chronic variable social stress during adolescence alters affect-related behaviors and adrenocortical activity in adult male and female inbred mice. Developmental Psychobiology. 2017a;59:679–687. doi: 10.1002/dev.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso MJ, Reiss DE, Caulfield JI, Thomas JL, Baker AN, Cavigelli SA, Kamens HM. Adolescent Chronic Variable Social Stress influences exploratory behavior and nicotine responses in male, but not female, BALB/cJ mice. Brain Research Bulletin. 2017b doi: 10.1016/j.brainresbull.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Preventing Tobacco Use Among Youth and Young Adults: A report of the Surgeon General. Atlanta, GA: 2012. [Accessed July 16, 2017]. Available at: https://www.surgeongeneral.gov/library/reports/preventing-youth-tobacco-use/factsheet.html. [PubMed] [Google Scholar]
- Compas BE, Orosan PG, Grant KE. Adolescent stress and coping: Implications for psychopathology during adolescence. Journal of Adolescence. 1993;16:331–349. doi: 10.1006/jado.1993.1028. [DOI] [PubMed] [Google Scholar]
- Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, McCall NM, Yu W, Schools ZL, Krashes MJ, Lowell BB, Whistler JL, Bruchas MR, Kash TL. Dynorphin Controls the Gain of an Amygdalar Anxiety Circuit. Cell Reports. 2016;14:2774–2783. doi: 10.1016/j.celrep.2016.02.069. Available at: http://dx.doi.org/10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, DeLucia R, Planeta CS. Effects of chronic stress on nicotine-induced locomotor activity and corticosterone release in adult and adolescent rats. Addiction Biology. 2008;13:63–69. doi: 10.1111/j.1369-1600.2007.00080.x. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel Ja, McIntosh JM, Boulter J, Collins AC, Heinemann SF. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Vicini L, Childs E, Sayla MA, Terner J. Does stress reactivity or response to amphetamine predict smoking progression in young adults? A preliminary study. Pharmacology, Biochemistry and Behavior. 2007;86:312–319. doi: 10.1016/j.pbb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, Marks MJ, Lester HA. In Vivo Activation of Midbrain Dopamine Neurons via Sensitized, High-Affinity α6* Nicotinic Acetylcholine Receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagen ZM, Mitchum R, Vezina P, McGehee DS. Enhanced Nicotinic Receptor Function and Drug Abuse Vulnerability. Journal of Neuroscience. 2007;27:8771–8778. doi: 10.1523/JNEUROSCI.2017-06.2007. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.2017-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behavioural Brain Research. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Finkelstein DM, Kubzansky LD, Goodman E. Social Status, Stress, and Adolescent Smoking. Journal of Adolescent Health. 2006;39:678–685. doi: 10.1016/j.jadohealth.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Fluharty M, Taylor AE, Grabski M, Munafo MR. The association of cigarette smoking with depression and anxiety: A systematic review. Nicotine & Tobacco Research. 2017;19:3–13. doi: 10.1093/ntr/ntw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochemical Pharmacology. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male long-evans rats. Developmental Psychobiology. 2013;55:849–859. doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Morishima M, Karube F, Kawaguchi Y. Specialized Cortical Subnetworks Differentially Connect Frontal Cortex to Parahippocampal Areas. Journal of Neuroscience. 2012;32:1898–1913. doi: 10.1523/JNEUROSCI.2810-11.2012. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.2810-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges TE, Baumbach JL, Marcolin ML, Bredewold R, Veenema AH, McCormick CM. Social instability stress in adolescent male rats reduces social interaction and social recognition performance and increases oxytocin receptor binding. Neuroscience. 2017;359:172–182. doi: 10.1016/j.neuroscience.2017.07.032. Available at: http://dx.doi.org/10.1016/j.neuroscience.2017.07.032. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B, Warren BL. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress (Amsterdam, Netherlands) 2014;17:247–255. doi: 10.3109/10253890.2014.910650. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24689732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolaños-Guzmán CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2692426&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: A review. Behavior Genetics. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Jiang L, Emmetsberger J, Talmage DA, Role LW. Type III Neuregulin 1 Is Required for Multiple Forms of Excitatory Synaptic Plasticity of Mouse Cortico-Amygdala Circuits. Journal of Neuroscience. 2013;33:9655–9666. doi: 10.1523/JNEUROSCI.2888-12.2013. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.2888-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Miyamoto J, Powers MS, Ro K, Soto M, Cox R, Stitzel Ja, Ehringer Ma. The β3 subunit of the nicotinic acetylcholine receptor: modulation of gene expression and nicotine consumption. Neuropharmacology. 2015;99:639–649. doi: 10.1016/j.neuropharm.2015.08.035. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0028390815300800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Berglund P, Demler O. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–768. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Koval JJ, Pederson LL, Chan SSH. Psychosocial variables in a cohort of students in grades 8 and 11: A comparison of current and never smokers. Preventive Medicine. 2004;39:1017–1025. doi: 10.1016/j.ypmed.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Leussis M, Andersen SL. Is Adolescence a Sensitive Period for Depression? Behavioral and Neuroanatomical Findings From a Social Stress Model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Li S, Zou S, Coen K, Funk D, Shram MJ, Lê AD. Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addiction Biology. 2014;19:156–164. doi: 10.1111/j.1369-1600.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009a;158:845–855. doi: 10.1016/j.neuroscience.2008.10.036. Available at: http://dx.doi.org/10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Vuong S, Scholl JL, Oliver H, Forster GL. Corticotropin-Releasing Factor Receptor Antagonism within the Dorsal Raphe Nucleus Reduces Social Anxiety-Like Behavior after Early-Life Social Isolation. Journal of Neuroscience. 2009b;29:9955–9960. doi: 10.1523/JNEUROSCI.0854-09.2009. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Brown MC, Nerio N, Aimiuwu O, Tran B, Anghel A, Friedman TC. Repeated stress alters the ability of nicotine to activate the hypothalamic-pituitary-adrenal axis. Journal of Neurochemistry. 2006;99:1321–1327. doi: 10.1111/j.1471-4159.2006.04217.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacology, Biochemistry and Behavior. 1989;33:667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- Mathur BN, Capik NA, Alvarez VA, Lovinger DM. Serotonin Induces Long-Term Depression at Corticostriatal Synapses. Journal of Neuroscience. 2011;31:7402–7411. doi: 10.1523/JNEUROSCI.6250-10.2011. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.6250-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McCormick CM. An animal model of social instability stress in adolescence and risk for drugs of abuse. Physiology & Behavior. 2010;99:194–203. doi: 10.1016/j.physbeh.2009.01.014. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Ibrahim FN. Locomotor activity to nicotine and Fos immunoreactivity in the paraventricular nucleus of the hypothalamus in adolescent socially-stressed rats. Pharmacology, Biochemistry and Behavior. 2007;86:92–102. doi: 10.1016/j.pbb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Merrick A, Secen J, Helmreich DL. Social instability in adolescence alters the central and peripheral hypothalamic-pituitary-adrenal responses to a repeated homotypic stressor in male and female rats. [Accessed January 14, 2014];Journal of neuroendocrinology. 2007 19:116–126. doi: 10.1111/j.1365-2826.2006.01515.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17214874. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Hormones and Behavior. 2004;46:458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. [Accessed January 14, 2014];Hormones and behavior. 2005 48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15919386. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. [Accessed November 16, 2012];Behavioural brain research. 2008 187:228–238. doi: 10.1016/j.bbr.2007.09.005. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17945360. [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, Ghosal S, Mahbod P, Packard BA, Myers B, Baccei ML, Herman JP. Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biological Psychiatry. 2016;80:754–764. doi: 10.1016/j.biopsych.2016.03.2101. Available at: http://dx.doi.org/10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick AM, Mears M, Forster GL, Lei Y, Tejani-Butt SM, Watt MJ. Adolescent social defeat alters N-methyl-d-aspartic acid receptor expression and impairs fear learning in adulthood. Behavioural Brain Research. 2016;304:51–59. doi: 10.1016/j.bbr.2016.02.013. Available at: http://dx.doi.org/10.1016/j.bbr.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Grun EU, Collins AC. Tolerance to nicotine following chronic treatment by injections: A potential role for corticosterone. Psychopharmacology. 1992;108:33–39. doi: 10.1007/BF02245282. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. [Accessed February 25, 2017];Alcoholism, clinical and experimental research. 1994 18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7978106. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 2015;99:735–749. doi: 10.1016/j.neuropharm.2015.06.017. Available at: http://dx.doi.org/10.1016/j.neuropharm.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Research on stress and smoking: progress and problems. British Journal of Addiction. 1991;86:599–603. doi: 10.1111/j.1360-0443.1991.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pryce CR. Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: Inter-species and intra-species differences. Brain Research Reviews. 2008;57:596–605. doi: 10.1016/j.brainresrev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Rao U, Chen L-A. Neurobiological and psychosocial processes associated with depressive and substance-related disorders in adolescents. Current drug abuse reviews. 2008;1:68–80. doi: 10.2174/1874473710801010068. Available at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.332.6592&rep=rep1&type=pdf. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen CL, London ED, Poland RE. Contribution of hypothalamic-pituitary-adrenal activity and environmental stress to vulnerability for smoking in adolescents. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2721–2732. doi: 10.1038/npp.2009.112. Available at: http://www.nature.com.proxy.uba.uva.nl:2048/npp/journal/v34/n13/full/npp2009112a.html\n http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2784160&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neuroscience and Biobehavioral Reviews. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler E. The brain reward circuitry in mood disorders. Nature Reviews Neuroscience. 2013;14:609–625. doi: 10.1038/nrn3381. Available at: http://www.nature.com/doifinder/10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodríguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biological Psychiatry. 2013;73:44–53. doi: 10.1016/j.biopsych.2012.06.035. Available at: http://dx.doi.org/10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Finger BC, Pizzo RC, O’Leary OF, Dinan TG, Cryan JF. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience. 2011;192:524–536. doi: 10.1016/j.neuroscience.2011.04.054. Available at: http://dx.doi.org/10.1016/j.neuroscience.2011.04.054. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Thiel CM, Müller CP, Huston JP. Relationship between anxiety and serotonin in the ventral striatum. Neuroreport. 1998;9:1025–1029. doi: 10.1097/00001756-199804200-00013. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9601661. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith ACW, Roberts-Wolfe D, Kalivas PW. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacological reviews. 2016;68:816–871. doi: 10.1124/pr.116.012484. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27363441\n http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4931870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell AK, El Khoury A, Mathe AA, Ehlers CL. Increased CRF-like and NPY-like immunoreactivity in adult rats exposed to nicotine during adolescence: Relation to anxiety-like and depressive-like behavior. Neuropeptides. 2017;39:369–377. doi: 10.1016/j.npep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and psychopathology. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Müller MB, Schmidt MV. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. [Accessed January 11, 2014];Hormones and behavior. 2008 53:386–394. doi: 10.1016/j.yhbeh.2007.11.001. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18096163. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell and Tissue Research. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- U.S. Surgeon General. Surgeon General’s Report, Smoking and Tobacco Use. [Accessed July 15, 2017];2012 Available at: http://www.cdc.gov/tobacco/datastatistics/sgr/2012/
- Urban KR, Valentino RJ. Age-and Sex-Dependent Impact of Repeated Social Stress on Intrinsic and Synaptic Excitability of the Rat Prefrontal Cortex. Cerebral Cortex. 2016:1–10. doi: 10.1093/cercor/bhw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler E. Prefrontal Cortical Circuit for Depression- and Anxiety-Related Behaviors Mediated by Cholecystokinin: Role of FosB. Journal of Neuroscience. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J, de Bie J, Granneman RA, Wallinga AE, Koolhaas JM, Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. [Accessed January 14, 2014];Physiology & behavior. 2007 92:824–830. doi: 10.1016/j.physbeh.2007.06.004. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17651767. [DOI] [PubMed] [Google Scholar]
- Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, Parise EM, Bolaños-Guzmán CA. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Developmental Neuroscience. 2014;36:250–260. doi: 10.1159/000362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. The Journal of Physiology. 2015;593:3397–3412. doi: 10.1113/JP270492. Available at: http://doi.wiley.com/10.1113/JP270492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Funk D, Shram MJ, Lê AD. Effects of stressors on the reinforcing efficacy of nicotine in adolescent and adult rats. Psychopharmacology. 2014;231:1601–1614. doi: 10.1007/s00213-013-3314-3. [DOI] [PubMed] [Google Scholar]